Submitted:
18 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
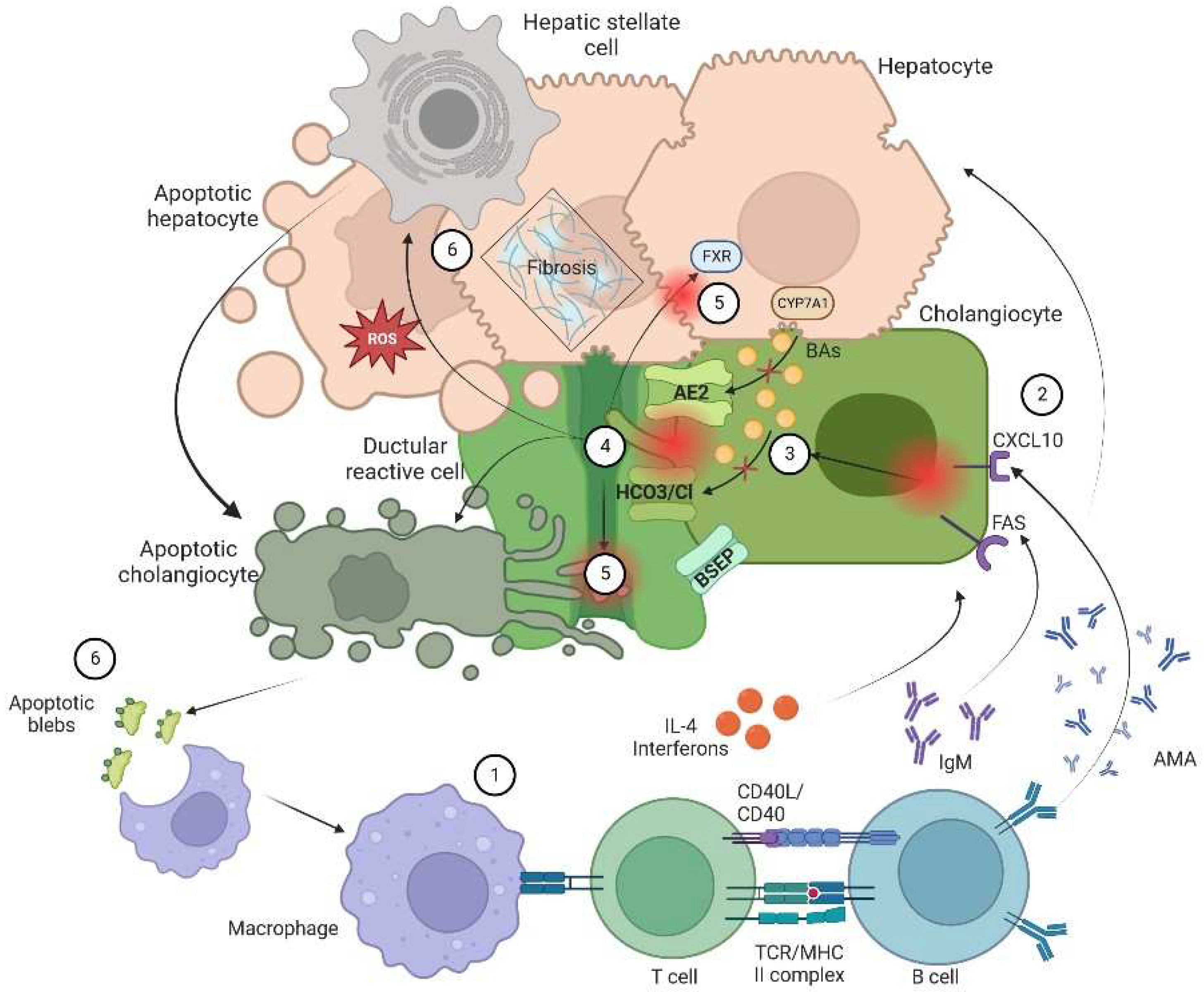
2. Pathophysiology of Cholangiopathies
2.1. Ductular Reaction
2.2. Biliary Stasis
2.3. Citotoxic Profile of Biliary Acids
2.4. Profibrotic State
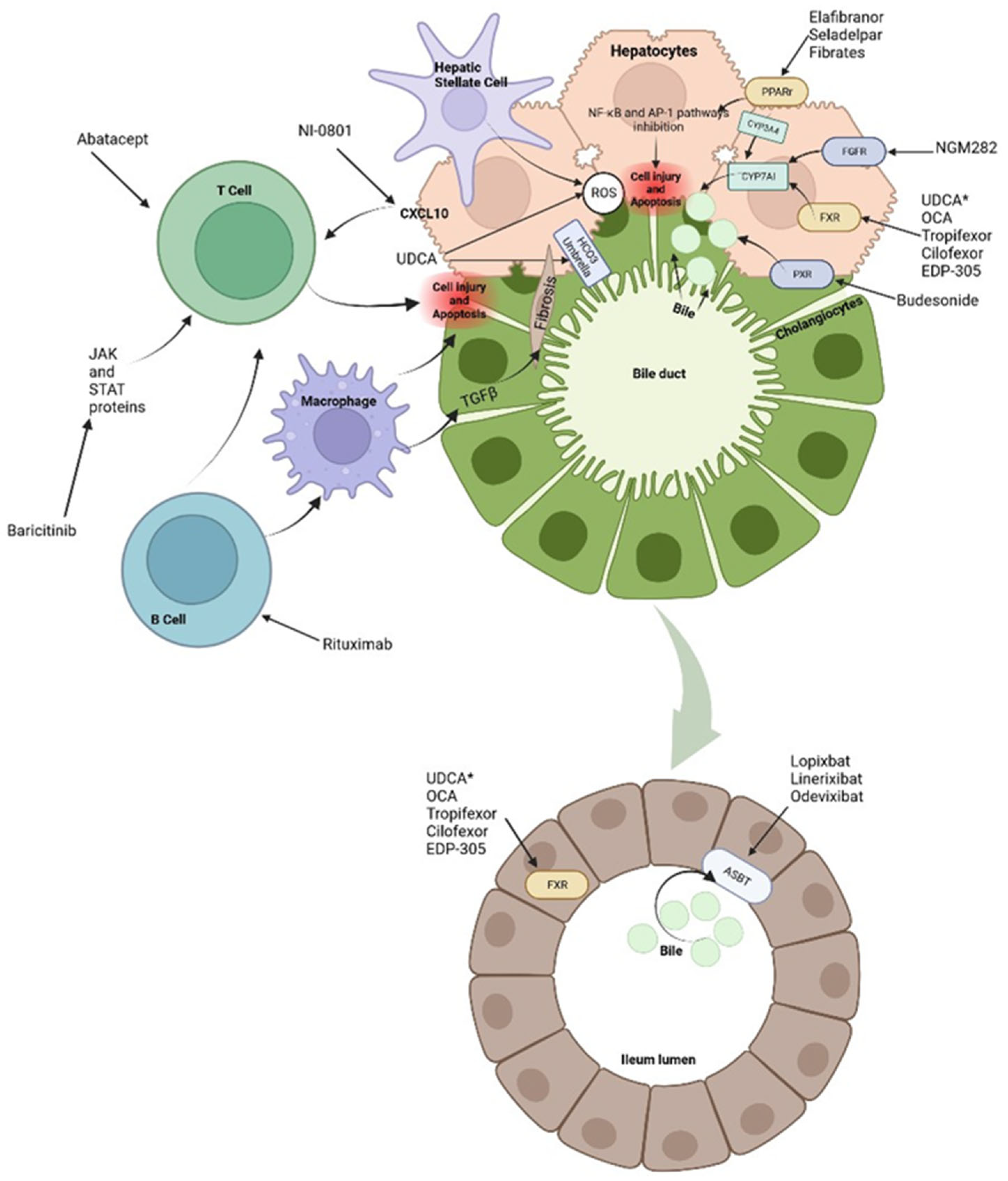
3. Therapeutic Options
3.1. Hydrophilic Bile Acids (BA): UDCA
3.2. FXR Agonists: Tropifexor (LJN-452), Cilofexor (GS-9674) and EDP-305
3.3. Fibroblast Growth Factor 19 (FGF-19) Analogs
3.4. PPAR Agonists: Fibrates, Seladelpar, Elafibranor
3.5. ASBT Inhibitors
3.6. Immune-Modulation Drugs: Corticosteroids and Biological Therapies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanjel, B.; Shim, W.S. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: A review. Biochim Biophys Acta Mol Basis 2020, 1;1866(12):165958. [CrossRef]
- Gazda, J.; Drazilova, S.; Janicko, M.; et al. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol. 2021, 19; 2021:9151525. [CrossRef]
- Tabibian, J.H.; Ali, A.H.; Lindor, K.D. Primary Sclerosing Cholangitis, Part 1: Epidemiology, Etiopathogenesis, Clinical Features, and Treatment. 2018. [Google Scholar]
- Malik, A.; Kardashian, A.A.; Zakharia, K.; et al. Preventative care in cholestatic liver disease: Pearls for the specialist and subspecialist. Liver Res 2019, 3(2):118-127. [CrossRef]
- Gerussi, A.; Restelli, U.; Croce, D. ; et tal. Cost of illness of Primary Biliary Cholangitis - a population-based study. Dig Liver Dis 2021, 53(9):1167-1170. [CrossRef]
- Yokoda, R.T.; Rodriguez, E.A. Review: Pathogenesis of cholestatic liver diseases. World J Hepatol 2020, 12(8): 423–435. [CrossRef]
- Méndez-Sánchez, N. Bile Acids in Health and Disease Foreword. Ann Hepatol 2017;16(Suppl. 1: s3-105.):s3. [CrossRef]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011 Mar;458(3):251-9. [CrossRef]
- Goldstein, J.; Levy, C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018;38(9):1520-1535. [CrossRef]
- Hirschfield, G.M.; Chazouillères, O.; Drenth, J.P.; Thorburn, D.; Harrison, S.A.; Landis, C.S.; Mayo, M.J.; Muir, A.J.; Trotter, J.F.; Leeming, D.J.; et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: A multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol 2019;1;70(3):483–93. [CrossRef]
- Méndez-Sánchez, N. Management of primary biliary cholangitis: the importance to identify patients' non-responders to standard treatment. Minerva Med 2018;109(6):407-409. [CrossRef]
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in Liver Diseases: A Review of Their Mechanisms of Action and Clinical Applications. Handb Exp Pharmacol 2019; 256:237-264. [CrossRef]
- Fabris, L.; Fiorotto, R.; Spirli, C.; et al. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol 2019; 16(8): 497–511. [CrossRef]
- Hartl, L.; Haslinger, K.; Angerer, M.; Semmler, G.; Schneeweiss-Gleixner, M.; Jachs, M.; Simbrunner, B.; Bauer, D.J.M.; Eigenbauer, E.; Strassl, R.; et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology 2022;76(6):1563-1575. [CrossRef]
- Beuers, U.; Spengler, U.; Kruis, W.; et al. Ursodeoxycholic acid for treatment of primary sclerosing cholangitis: a placebo-controlled trial. Hepatology 1992;16(3):707-14. [CrossRef]
- Beuers, U.; Trauner, M.; Jansen, P.; et al. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol 2015;62(1 Suppl):S25-37. [CrossRef]
- Poupon, R.; Chrétien, Y.; Poupon, R.E.; et al. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis?. Lancet 1987; 11;1(8537):834-6. [CrossRef]
- Poupon, R.E.; Balkau, B.; Eschwège, E.; et al. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med 1991; 30;324(22):1548-54. [CrossRef]
- Poupon, R.E.; Balkau, B.; Guéchot, J.; et al. Predictive factors in ursodeoxycholic acid-treated patients with primary biliary cirrhosis: role of serum markers of connective tissue. Hepatology 1994; 19(3):635-40. [CrossRef]
- Poupon, R.E.; Bonnand, A.M.; Chrétien, Y.; et al. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group.Hepatology. 1999; 29(6):1668-71. [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017 Jul;67(1):145-172.
- Shi, J.; Li, Z.; Zeng, X.; et al. Ursodeoxycholic acid in primary sclerosing cholangitis: meta-analysis of randomized controlled trials. Hepatol Res. 2009; 39(9):865-73. [CrossRef]
- Triantos, C.K.; Koukias, N.M.; Nikolopoulou, V.N.; et al. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011; 34(8):901-10. [CrossRef]
- Olsson, R.; Boberg, K.M.; de Muckadell, O.S.; et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005; 129(5):1464-72. [CrossRef]
- Lindor, K.D.; Kowdley, K.V.; Luketic, V.A.; et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009; 50(3):808-14. [CrossRef]
- Black, D.D.; Mack, C.; Black, D.D.; et al. A Prospective Trial of Withdrawal and Reinstitution of Ursodeoxycholic Acid in Pediatric Primary Sclerosing Cholangitis. Hepatol Commun.2019; 29;3(11):1482-1495. [CrossRef]
- Pardi, D.S.; Loftus, E.V. Jr.; Kremers, W.K.; et al. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003; 124(4):889-93. [CrossRef]
- Tung, B.Y.; Emond, M.J.; Haggitt, R.C.; et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med.2001; 16;134(2):89-95. [CrossRef]
- Lindström, L.; Boberg, K.M.; Wikman, O.; et al. High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Aliment Pharmacol Ther.2012; 35(4):451-7. [CrossRef]
- Eaton, J.E.; Silveira, M.G.; Pardi, D.S.; et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011; 106(9):1638-45. [CrossRef]
- Singh, S.; Khanna, S.; Pardi, D.S.; et al. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis.2013; 19(8). [CrossRef]
- Hansen, J.D.; Kumar, S.; Lo, W.K. Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis. Dig Dis Sci. 2013; 58(11):3079-87. [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology.2023; 1;77(2):659-702. [CrossRef]
- Floreani, A.; Gabbia, D.; de Martin, S. Update on the Pharmacological Treatment of Primary Biliary Cholangitis. Vol. 10, Biomedicines. MDPI; 2022. [CrossRef]
- Schramm, C.; Wedemeyer, H.; Mason, A.; Hirschfield, G.M.; Levy, C.; Kowdley, K. v.; et al. Farnesoid X receptor agonist tropifexor attenuates cholestasis in a randomised trial in patients with primary biliary cholangitis. JHEP Reports. 2022; 1;4(11). [CrossRef]
- Trauner, M.; Gulamhusein, A.; Hameed, B.; Caldwell, S.; Shiffman, M.L.; Landis, C.; et al. The Nonsteroidal Farnesoid X Receptor Agonist Cilofexor (GS-9674) Improves Markers of Cholestasis and Liver Injury in Patients With Primary Sclerosing Cholangitis. Hepatology. 2019; 1;70(3):788–801. [CrossRef]
- Trauner, M.; Bowlus, C.L.; Gulamhusein, A.; et al. Safety and sustained efficacy of the farnesoid X receptor (FXR) agonist cilofexor over a 96-week open-label extension in patients with PSC. Clin Gastroenterol Hepatol. 2022; 4;S1542-3565(22)00720-0. [CrossRef]
- Kowdley, K.V.; Bonder, A.; Heneghan, M.A.; et al. Final data of the phase 2a intrepid study with EDP-305, a non-bile acid farnesoid x receptor (FXR) agonist. Hepatology (Baltimore, Md.) 2020, 72(1 SUPPL), 746A-747A. [CrossRef]
- Mayo, M.J.; Wigg, A.J.; Leggett, B.A.; Arnold, H.; Thompson, A.J. ; Weltman M, et al. NGM282 for Treatment of Patients With Primary Biliary Cholangitis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Hepatol Commun 2018 Aug 30;2(9):1037-1050. [CrossRef]
- Harrison, SA.; Rinella, M.E.; Abdelmalek, M.F.; Trotter, J.F.; Paredes, A.H.; Arnold, H. L, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2018; 24;391(10126):1174–85. [CrossRef]
- Harrison, S.A.; Neff, G.; Guy, C.D.; Bashir, M.R.; Paredes, A.H.; Frias, J.P.; et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2021; 1;160(1):219-231.e1. [CrossRef]
- Botta, M.; Audano, M.; Sahebkar, A.; et al. PPAR Agonists and Metabolic Syndrome: An Established Role?. Int J Mol Sci. 2018; 19(4): 1197. [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications- a review. Nutr J.2014; 14; 13:17. [CrossRef]
- Ghonem, N.S.; Assis, D.N.; Boyer, J.L. On Fibrates and Cholestasis: A review. Hepatology. 2015; 62(2): 635–643. [CrossRef]
- Kita, R.; Takamatsu, S.; Kimura, T.; et al. Bezafibrate may attenuate biliary damage associated with chronic liver diseases accompanied by high serum biliary enzyme levels. J Gastroenterol 2006;41:686–692. [CrossRef]
- Hazzan, R.; Tur-Kaspa, R. Bezafibrate treatment of primary biliary cirrhosis following incomplete response to ursodeoxycholic acid. J Clin Gastroenterol 2010;44:371–373. [CrossRef]
- Takeuchi, Y.; Ikeda, F.; Fujioka, S.; et al. Additive improvement induced by bezafibrate in patients with primary biliary cirrhosis showing refractory response to ursodeoxycholic acid. J Gastroenterol Hepatol 2011;26:1395–1401. [CrossRef]
- Iwasaki, S.; Ohira, H.; Nishiguchi, S.; et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatol Res 2008;38:557–564. [CrossRef]
- Ohmoto, K.; Yoshioka, N.; Yamamoto, S. Long-term effect of bezafibrate on parameters of hepatic fibrosis in primary biliary cirrhosis. J Gastroenterol 2006; 41(5):502-3. [CrossRef]
- Ohira, H.; Sato, Y.; Ueno, T.; Sata, M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol 2002; 97:2147–2149. [CrossRef]
- Dohmen, K.; Mizuta, T.; Nakamuta, M.; et al. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J Gastroenterol 2004;10:894–898. [CrossRef]
- Levy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R, Clark V, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther 2011;33:235–242. [CrossRef]
- Han, X.F.; Wang, Q.X.; Liu, Y.; et al. Efficacy of fenofibrate in Chinese patients with primary biliary cirrhosis partially responding to ursodeoxycholic acid therapy. J Dig Dis 2012;13:219–224. [CrossRef] [PubMed]
- Liberopoulos, E.N.; Florentin, M.; Elisaf, M.S.; et al. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J. 2010; 4:120–126. [CrossRef]
- Zhang, H.; Li, S.; Feng, Y.; et al. Efficacy of fibrates in the treatment of primary biliary cholangitis: a meta-analysis. Clin Exp Med 2022 Nov 1. [CrossRef]
- Zhang, Y.; Li, S.; He, L.; et al. Combination therapy of fenofibrate and ursodeoxycholic acid in patients with pri mary biliary cirrhosis who respond incompletely to UDCA monotherapy: a meta-analysis. Drug Des Devel Ther. 2015; 9: 2757–2766. [CrossRef]
- Corpechot, C.; Chazouillères, O.; Rousseau, A.; et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018; 7;378(23):2171-2181. [CrossRef]
- Tanaka, A.; Hirohara, J.; Nakano, T.; et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol.2021; 75(3):565-571. [CrossRef]
- Jones, D.; Boudes, PF.; Sawin, MG.; et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study.Lancet Gastroenterol Hepatol.2017; 2(10):716-726. [CrossRef]
- Bowlus, C.L.; Galambos, M.R.; Aspinall, R.J.; et al. A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis. J Hepatol.2022; 77(2):353-364. [CrossRef]
- Kremer, A.E.; Mayo, M.J.; Hirschfield, G.; et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022; 42(1):112-123. [CrossRef]
- Zhang, L.; Huang, X.; Meng, Z.; et al. Significance and mechanism of CYP7a1 gene regulation during the acute phase of liver regeneration. Mol Endocrinol. 2009; 23(2):137-45. [CrossRef]
- Colapietro, F.; Gershwin, M.E.; Lleo, A. PPAR agonists for the treatment of primary biliary cholangitis: old and new tales. J Transl Autoimmun. 2023; 6: 100188. [CrossRef]
- Westerouen Van Meeteren, M.J.; Drenth, J.P.H.; Tjwa, E.T.T.L. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin Investig Drugs. 2020; 29(2):117-123. [CrossRef]
- Schattenberg, J.M.; Pares, A.; Kowdley, K.V.; et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021; 74(6):1344-1354. [CrossRef]
- Tao, Li.; Ren, X.; Zhai, W.; et al. Progress and Prospects of Non-Canonical NF-κB Signaling Pathway in the Regulation of Liver Diseases. Molecules. 2022; 2;27(13):4275.
- Li, F.; Patterson, A.D.; Krausz, K.W.; et al. Metabolomics reveals an essential role for peroxisome proliferator-activated receptor α in bile acid homeostasis. J Lipid Res. 2012; 53(8):1625-35. [CrossRef]
- Trial ELATIVE.
- Yang, N.; Dong, Y.Q.; Jia, G.X.; et al. ASBT(SLC10A2): A promising target for treatment of diseases and drug discovery. Biomed Pharmacother.2020; 132:110835. [CrossRef]
- Salic, K.; Kleemann, R.; Wilkins-Port, C.; et al. Apical sodium-dependent bile acid transporter inhibition with volixibat improves metabolic aspects and components of non-alcoholic steatohepatitis in Ldlr-/-.Leiden mice. PLoS One. 2019; 24;14(6): e0218459. [CrossRef]
- Kunst, R.F.; de Waart, D.R.; Wolters, F.; et al. Systemic ASBT inactivation protects against liver damage in obstructive cholestasis in mice. JHEP Rep. 2022; 27;4(11):100573. [CrossRef]
- Gonzales, E.; Hardikar, W.; Stormon, M.; et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): a randomised phase 2 study. The Lancet. 2021; 398(10311):1581-1592. [CrossRef]
- Hegade, V.S.; Kendrick, S.F.; Dobbins, R.L.; et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017; 18;389(10074):1114-1123. [CrossRef]
- Ino, H.; Endo, A.; Wakamatsu, A.; et al. Safety, Tolerability, Pharmacokinetic and Pharmacodynamic Evaluations Following Single Oral Doses of GSK2330672 in Healthy Japanese Volunteer. Clin Pharmacol Drug Dev. 2019; 8(1):70-77. [CrossRef]
- Levy, C.; Kendrick, S.; Bowlus, C.L.; et al. GLIMMER: A Randomized Phase 2b Dose-Ranging Trial of Linerixibat in Primary Biliary Cholangitis Patients with Pruritus. Clin Gastroenterol Hepatol. 2022; 4; S1542-3565(22)01021-7. [CrossRef]
- Thompson, R.; Arnell, H.; Artan, R.; et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2022; 7(9):830-842. [CrossRef]
- Deeks, E.M. Odevixibat: First Approval. Drugs. 2021; 81(15): 1781–1786. [CrossRef]
- Hempfling, W.; Grunhage, F.; Dilger, K.; et al. Pharmacokinetics and pharmacodynamic action of budesonide in early- and late-stage primary biliary cirrhosis. Hepatology. 2003; 38(1):196-202. [CrossRef]
- Al-Aqil, F.A.; Monte, M.J.; Peleteiro-Vigil, A.; et al. Interaction of glucocorticoids with FXR/FGF19/FGF21-mediated ileum-liver crosstalk. Biochim Biophys Acta Mol Basis Dis. 2018; 1864(9 Pt B):2927-2937. [CrossRef]
- Zhang, Y. ; Lu. J.; Wang, F.; et al. Combination Therapy of Ursodeoxycholic Acid and Corticosteroids for Primary Biliary Cirrhosis with Features of Autoimmune Hepatitis: A Meta-Analysis. Gastroenterol Res Pract. 2013; 2013: 490731. [CrossRef]
- Zhang, H.; Li, S.; Yang, J.; et al. A meta-analysis of ursodeoxycholic acid therapy versus combination therapy with corticosteroids for PBC-AIH-overlap syndrome: evidence from 97 monotherapy and 117 combinations. Prz Gastroenterol.2015; 10(3):148-55. [CrossRef]
- Silveira, M.G.; Lindor, K.D. Obeticholic acid and budesonide for the treatment of primary biliary cirrhosis. Expert Opin Pharmacother. 2014; 15(3):365-72. [CrossRef]
- Hempfling, W.; Grunhage, F.; Dilger, K.; et al. Pharmacokinetics and pharmacodynamic action of budesonide in early and late-stage primary biliary cirrhosis. Hepatology 2003; 38:196–202. [CrossRef]
- Hirschfield, G.M.; Beuers, U.; Kupcinskas, L.; et al. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA. J. Hepatol. 2021; 74, 321–329. [CrossRef]
- Geier, A.; Gartung, C.; Dietrich, C.G.; et al. Side effects of budesonide in liver cirrhosis due to chronic autoimmune hepatitis: influence of hepatic metabolism versus portosystemic shunts on a patients complicated with HCC. World J Gastroenterol 2003; 9: 2681–85. [CrossRef]
- Myers, R.P.; Swain, M.G.; Lee, S.S.; et al. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013; 108(6):933-941. [CrossRef]
- Tsuda, M.; Moritoki, Y.; Lian, Z.X.; et al. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and incomplete response to ursodeoxycholic acid. Hepatology. 2012; 55(2):512-21. [CrossRef]
- Hart, P.A.; Topazian, M.D.; Witzig, T.E.; et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: The Mayo Clinic experience. Gut. 2012; 62,1607-1615. [CrossRef]
- Yamada, Y.; Hoshino, K.; Fuchimoto, Y.; et al. Rituximab induction to prevent the recurrence of PSC after liver transplantation – The lessons learned from ABO-Incompatible living donor liver transplantation. Transplant Direct. 2018; 4(2): e342. [CrossRef]
- Bowlus, C.L. ; Yang, G-X.; Liu, C.H.; et al. Therapeutic trials of biologics in primary biliary cholangitis: an open label study of abatacept and review of the literature. J Autoimmun. 2019; 101:26–34. [CrossRef]
- Gordon, S.C; Trudeau, S.; Regev, A.; et al. Baricitinib and primary biliary cholangitis. J. Transl. Autoimmun. 2021; 4, 100107. [CrossRef]
- Hirschfield, G.M.; Gershwin, M.E.; Strauss, R.; et al. Ustekinumab for patients with primary biliary cholangitis who have an inadequate response to ursodeoxycholic acid: a proof-of-concept study. Hepatology. (2016); 64:189–99. [CrossRef]
- Lynch, K.D.; Chapman, R.W.; Keshav, S.; et al. Effects of vedolizumab in patients with primary sclerosing cholangitis and inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2019); 18:179–87. e6. [CrossRef]
- De, Graaf K.L.; Lapeyre, G.; Guilhot, F.; et al. NI-0801, an anti-chemokine (C-X-C motif) ligand 10 antibody, in patients with primary biliary cholangitis and an incomplete response to ursodeoxycholic acid. Hepatol. Commun. 2018; 2, 492–503. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




