Submitted:
17 April 2023
Posted:
18 April 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental site and cultivars used
2.2. Sample Preparation and methods of analysis
2.3. Data Analysis
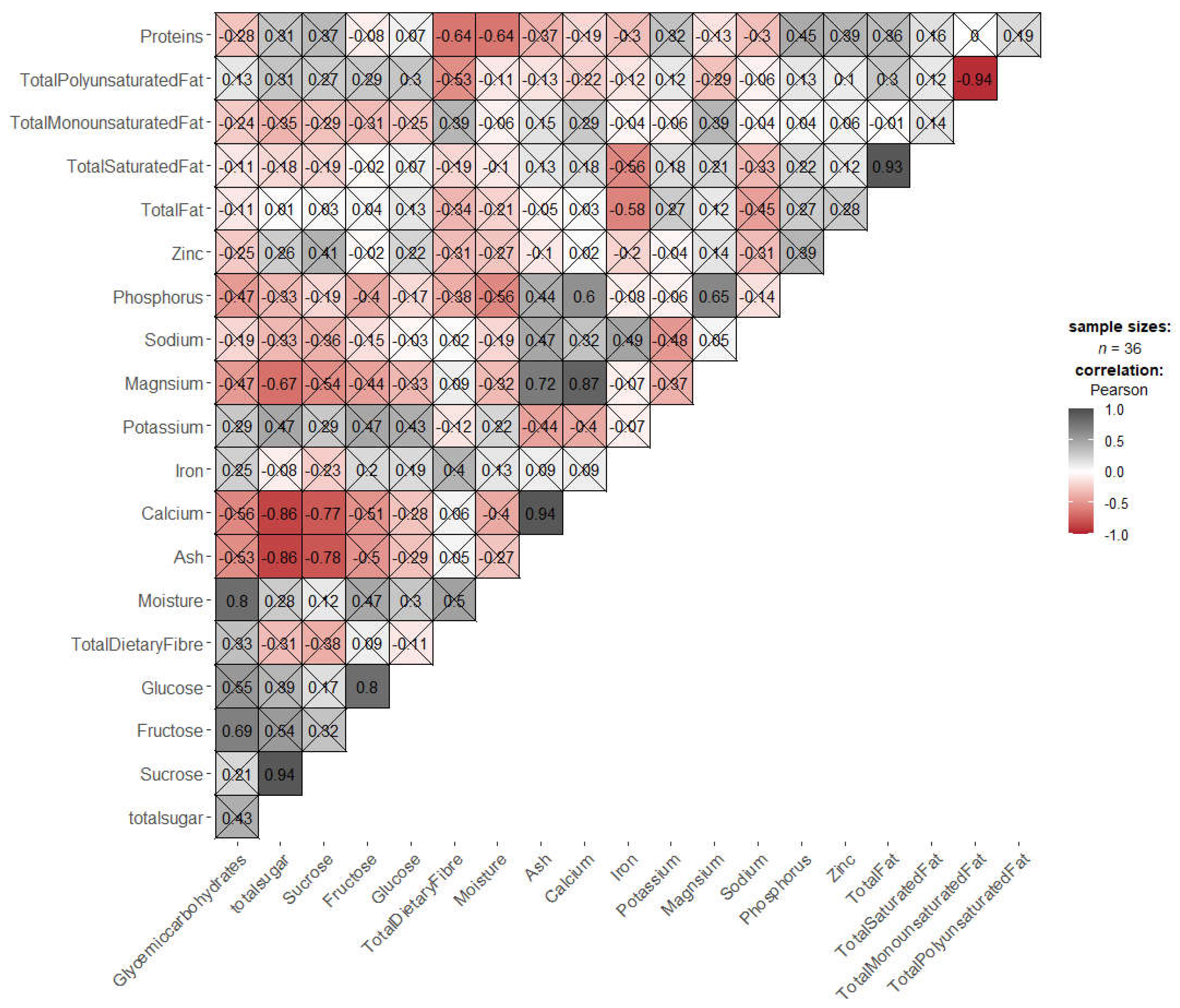
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karthy, E. S., Ranjitha, P.,Mohankumar, A., 2009. Antimicrobial potential of plant seed extracts against Multidrug Resistant Methicillin Resistant Staphylococcus aureus (MDR - MRSA). International Journal of Biology 1, 34-40. [CrossRef]
- Tshabalala, T., Ncube, B., Madala, N. E., Nyakudya, T. T., Moyo, H. P., Sibanda, M.,Ndhlala, A. R., 2019. Scribbling the cat: A Case of the “miracle” plant, Moringa oleifera. Plants 8, 510. [CrossRef]
- Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J.,Bertoli, S., 2015. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. International Journal of Molecular Sciences 16, 12791-12835. [CrossRef]
- Zheng, Y., Sun, H., Zhang, Y., Wu, J. 2019. Evaluation of the adaptability, productivity, and leaf powder quality of eight Moringa oleifera cultivars introduced to a dry-hot climate of Southwest China. Industrial Crops & Products 128, 199–205. [CrossRef]
- Habtemariam, S., 2016. The African Moringa is to change the lives of millions in Ethiopia and far beyond. Asian Pacific Journal of Tropical Biomedicine 6, 355-356. [CrossRef]
- Ndhlala, A., Mulaudzi, R., Ncube, B., Abdelgadir, H., du Plooy, C.,Van Staden, J., 2014. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 19, 10480. [CrossRef]
- Mucina, L.,Rutherford, M. C. (Eds) 2006. The Vegetation of South Africa, Lesotho and Swaziland. Pretoria, South Africa: South African National Biodiversity Institute.
- Acocks, J. P. H. 1988. Veld types of South Africa. Pretoria, South Africa: Department of Agriculture Technical Services.
- Panagos, M. D., Westfall, R. H., van Staden, J. M.,Zacharias, P. J. K., 1998. The plant communities of the Roodeplaat Experimental Farm, Gauteng, South Africa and the importance of classification verification. South African Journal of Botany 64, 44-61. [CrossRef]
- AOAC, 2000. Association of Analytical Chemists. Official methods of analysis. AOAC International: Gaithersburg, ML, USA.
- AOAC 2003. Official Methods of Analysis of the Association of Analytical Chemists International, 17th edn. AOAC International: Gaithersburg, ML, USA.
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T.,Smith, F., 1956. Colorimetric method for determination of sugars related substance. Analytical Chemistry 28, 350-356. [CrossRef]
- Langemeier, J. M.,Rogers, D. E., 1995. Rapid method for sugar analysis of doughs and baked products. Cereal Chem. 72, 349-351.
- R Core Team, 2020.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org.
- Omale, J., Adeyemi, A. R.,Omajali, J. B., 2010. Phytoconstituents, proximate and nutrient investigations of Saba florida (Benth.) from Ibaji forest. International Journal of Nutrition and Metabolism 2, 88-92.
- Mathlouthi, M., 2001. Water content, watter activity, water structure and stability of foodstuffs. Food Control 12, 409 – 417. [CrossRef]
- Pravina, P., Sayaji, D., Avinash, M., 2013. Calcium and its Role in Human Body. International Journal of Research in Pharmaceutical and Biomedical Sciences 4, 659-668.
- Fahey, J. W., 2005. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties. Trees for Life Journal 1, 5.
- Ramachandran, C., Peter, K. V.,Gopalakrishnan, P. K., 1980. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Economic Botany 34, 276-283. [CrossRef]
- Nyakudya, T. T., Tshabalala, T., Dangarembizi, R., Erlwanger, K. H., Ndhlala, A. R., 2020. The Potential Therapeutic Value of Medicinal Plants in the Management of Metabolic Disorders. . Molecules 25, 2669. [CrossRef]
- Makita, C., Madala, N. E., Cukrowska, E., Abdelgadir, H., Chimuka, L., Steenkamp, P., Ndhlala, A. R., 2017. Variation in pharmacologically potent rutinoside-bearing flavonoids amongst twelve Moringa oleifera Lam. Cultivars. South African Journal of Botany 112, 270-274. [CrossRef]
| Cultivar | Country of Origin | Source of seeds |
|---|---|---|
| Limpopo | South Africa | Local domesticated cultivar from Limpopo Province, South Africa |
| CHM | South Africa | Silver Hill, KwaZulu-Natal Province, South Africa |
| SH | South Africa | Silver Hill, KwaZulu-Natal Province, South Africa |
| TOT4893 | Thailand | World Vegetable Centre (Taiwan) |
| TOT4951 | Thailand | World Vegetable Centre (Taiwan) |
| TOT4977 | Thailand | World Vegetable Centre (Taiwan) |
| TOT 5028 | Thailand | World Vegetable Centre (Taiwan) |
| TOT5077 | Thailand | World Vegetable Centre (Taiwan) |
| TOT5169 | Thailand | World Vegetable Centre (Taiwan) |
| TOT5330 | Thailand | World Vegetable Centre (Taiwan) |
| TOT7266 | Thailand | World Vegetable Centre (Taiwan) |
| TOT4100 | Taiwan | World Vegetable Centre (Taiwan) |
| TOT4880 | USA | World Vegetable Centre (Taiwan) |
| Cultivar | Total Sugar | Total energy | Glycemic carbohydrates | Fructose | Glucose | Lactose | Maltose | Sucrose | Total Dietary Fibre |
| g/100g | KJ/100g | g/100g | g/100g | g/100g | g/100g | g/100g | g/100g | g/100g | |
| CHM | 5.70 ± 0.33cd | 612 ± 0.707d | 6.00 ± 0.27efg | 1.10 ± 0.37ab | 1.30 ± 0.48a | <0.3 | <0.3 | 3.30 ± 0.27c | 43.30 ± 0.03d |
| Limpopo | 7.90 ± 0.45ab | 741 ± 4.240a | 8.00 ± 0.35a | 1.00 ± 0.33b | 1.50 ± 0.55a | <0.3 | <0.3 | 5.40 ± 0.44b | 40.80 ± 0.02h |
| SH | 5.70 ± 0.33cd | 666 ± 3.540bc | 7.50 ± 0.34b | 1.30 ± 0.42a | 1.40 ± 0.51a | <0.3 | <0.3 | 3.00 ± 0.01cd | 42.70 ± 0.08f |
| TOT4100 | 4.90 ± 0.28e | 588 ± 0.707e | 5.00 ± 0.23hi | 1.30 ± 0.42a | 1.10 ± 0.40a | <0.3 | <0.3 | 2.50 ± 0.21cde | 45.80 ± 0.03a |
| TOT4880 | 4.80 ± 0.27e | 616 ± 4.240f | 5.30 ± 0.24gh | 0.70 ± 0.23b | 0.60 ± 0.21a | <0.3 | <0.3 | 3.50 ± 0.28c | 44.80 ± 0.04c |
| TOT4893 | 4.70 ± 0.27e | 555 ± 7.07d | 5.10 ± 0.23hi | 0.70 ± 0.23b | 1.00 ± 0.40a | <0.3 | <0.3 | 3.00 ± 0.24cd | 43.30 ± 0.03d |
| TOT4951 | 6.70 ± 0.38bc | 670 ± 0.707b | 7.00 ± 0.31cd | 1.10 ± 0.37ab | 1.10 ± 0.40a | <0.3 | <0.3 | 6.00 ± 0.50ab | 41.30 ± 0.02g |
| TOT4977 | 4.70 ± 0.27e | 651 ± 5.660c | 5.00 ± 0.23hi | 0.90 ± 0.30b | 1.10 ± 0.40a | <0.3 | <0.3 | 2.70 ± 0.23cde | 42.90 ± 0.03e |
| TOT5028 | 3.00 ± 0.17f | 526 ± 4.240g | 4.10 ± 0.18ij | 0.40 ± 0.13bc | 0.90 ± 0.32a | <0.3 | <0.3 | 1.70 ± 0.14ef | 45.70 ± 0.03a |
| TOT5077 | 3.00 ± 0.17f | 552 ± 0.707f | 3.00 ± 0.13j | 0.30 ± 0.10c | 1.10 ± 0.39a | <0.3 | <0.3 | 1.50 ± 0.13f | 45.40 ± 0.08b |
| TOT5169 | 5.50 ± 0.31ed | 532 ± 0.707g | 6.30 ± 0.28de | 1.20 ± 0.40ab | 1.40 ± 0.51a | <0.3 | <0.3 | 2.90 ± 0.24cd | 45.80 ± 0.05a |
| TOT5330 | 5.90 ± 0.34cd | 658 ± 3.540bc | 6.00 ± 0.27efg | 1.30 ± 0.42a | 1.60 ± 0.58a | <0.3 | <0.3 | 3.00 ± 0.24cd | 40.80 ± 0.02h |
| TOT7266 | 5.90 ± 0.34cd | 589 ± 2.830e | 6.00 ± 0.27efg | 1.00 ± 0.33b | 1.30 ± 0.48a | <0.3 | <0.3 | 3.60 ± 0.30c | 42.70 ± 0.03f |
| Cultivar | Moisture | Ash | Total Saturated Fat | Total Monounsaturated Fat | Total Polyunsaturated Fat | Cholesterol | Protein |
| % | g/100g | g/100g | g/100g | g/100g | mg/100g | g/100g | |
| CHM | 7.50 ± 0.01e | 15.80 ± 0.06d | 0.81 ± 0.04ef | 0.12 ± 0.06c | 1.22 ± 0.02d | <0.9 | 24.4 ± 0.04f |
| Limpopo | 6.90 ± 0.04l | 12.30 ± 0.04h | 1.20 ± 0.03a | 0.16 ± 0.07c | 1.56 ± 0.02a | <0.9 | 28.9 ± 0.06a |
| SH | 7.00 ± 0.04g | 13.70 ± 0.04g | 0.98 ± 0.08bc | 1.08 ± 0.06ab | 0.28 ± 0.01c | <0.9 | 27.8 ± 0.06b |
| TOT4100 | 7.40 ± 0.02f | 15.10 ± 0.05e | 1.04 ± 0.02b | 0.12 ± 0.06c | 1.20 ± 0.02c | <0.9 | 23.6 ± 0.02g |
| TOT4880 | 8.20 ± 0.01a | 16.90 ± 0.06c | 1.00 ± 0.03bc | 1.00 ± 0.05ab | 0.32 ± 0.01c | <0.9 | 23.2 ± 0.04h |
| TOT4893 | 7.30 ± 0.04g | 15.60 ± 0.07d | 0.94 ± 0.03bcd | 0.18 ± 0.06c | 1.22 ± 0.02c | <0.9 | 25.7 ± 0.04d |
| TOT4951 | 7.10 ± 0.01h | 15.00 ± 0.05ef | 0.94 ± 0.02bcd | 0.11 ± 0.03c | 1.34 ± 0.02c | <0.9 | 25.8 ± 0.05cd |
| TOT4977 | 7.40 ± 0.04f | 14.80 ± 0.05f | 1.17 ± 0.04a | 1.20 ± 0.03a | 0.38 ± 0.01b | <0.9 | 25.2 ± 0.04e |
| TOT5028 | 7.60 ± 0.03d | 18.20 ± 0.07a | 0.94 ± 0.02bcd | 0.92 ± 0.05b | 0.30 ± 0.01d | <0.9 | 22.4 ± 0.02i |
| TOT5077 | 7.80 ± 0.01c | 16.90 ± 0.06c | 0.93 ± 0.01cd | 0.91 ± 0.06b | 0.32 ± 0.01d | <0.9 | 23.2 ± 0.02h |
| TOT5169 | 8.10 ± 0.04b | 17.50 ± 0.06b | 0.99 ± 0.02bc | 0.92 ± 0.06b | 0.32 ± 0.01c | <0.9 | 20.8 ± 0.04k |
| TOT5330 | 7.50 ± 0.01e | 15.80 ± 0.05d | 0.94 ± 0.03bcd | 0.16 ± 0.05c | 1.23 ± 0.02c | <0.9 | 25.8 ± 0.06cd |
| TOT7266 | 7.50 ± 0.04e | 17.50 ± 0.06b | 0.75 ± 0.01f | 0.13 ± 0.04c | 1.03 ± 0.02e | <0.9 | 21.6 ± 0.03j |
| Cultivar | calcium | Cadmium | Iron | Lead | Magnesium | Mercury | Sodium | Potassium | Phosphorus | Zinc |
| mg/1000g | mg/1000g | mg/1000g | mg/1000g | mg/1000g | mg/1000g | mg/1000g | mg/1000g | mg/1000g | mg/1000g | |
| CHM | 40654 ± 116d | <0.01 | 382 ± 0.679b | <0.04 | 4722 ± 9.7j | <0.01 | 14.7 ± 0.01f | 11550 ± 0.891b | 3397 ± 6.97i | 22.9 ± 4.47a |
| Limpopo | 31363 ± 90h | <0.01 | 243 ± 0.438k | <0.04 | 4549 ± 9.3k | <0.01 | 9.1 ± 0.01k | 10390 ± 0.368d | 3808 ± 7.81d | 26.5 ± 5.18a |
| SH | 32652 ± 93g | <0.01 | 346 ± 0.622g | <0.04 | 3460 ± 7.1l | <0.01 | 29 ± 0.04c | 11916 ± 0.523a | 2620 ± 5.37m | 17.5 ± 3.42a |
| TOT4100 | 40161 ± 115e | <0.01 | 181 ± 0.325e | <0.04 | 4901 ± 10.1i | <0.01 | 16.8 ± 0.03d | 8839 ± 0.679f | 3308 ± 6.79j | 20.5 ± 4.00a |
| TOT4880 | 48150 ± 138c | <0.01 | 290 ± 0.523i | <0.04 | 7998 ± 16.4b | <0.01 | 9 ± 0.01k | 5742 ± 0.438k | 3969 ± 8.13c | 23.5 ± 4.58a |
| TOT4893 | 37785 ± 108d | <0.01 | 424 ± 0.750f | <0.04 | 5337 ± 10.9g | <0.01 | 11.8 ± 0.01h | 8438 ± 0.622g | 4863 ± 9.97a | 22.1 ± 4.31a |
| TOT4951 | 41871 ± 120ef | <0.01 | 234 ± 0.410l | <0.04 | 7354 ± 15.1c | <0.01 | 3.1 ± 0l | 6225 ± 0.325j | 4712 ± 9.66b | 27.0 ± 5.28a |
| TOT4977 | 42320 ± 121f | <0.01 | 405 ± 0.721h | <0.04 | 7133 ± 14.6d | <0.01 | 9.8 ± 0.01j | 8047 ± 0.523h | 3732 ± 7.65e | 22.2 ± 4.33a |
| TOT5028 | 51893 ± 148a | <0.01 | 244 ± 0.438c | <0.04 | 5568 ± 11.4f | <0.01 | 30.6 ± 0.04b | 2387 ± 0.750m | 3695 ± 7.58f | 24.7 ± 4.82a |
| TOT5077 | 47814 ± 137c | <0.01 | 512 ± 0.919j | <0.04 | 6749 ± 13.8e | <0.01 | 16.2 ± 0.01e | 6410 ± 0.410i | 3048 ± 6.25k | 23.0 ± 4.48a |
| TOT5169 | 53008 ± 151b | <0.01 | 496 ± 0.891d | <0.04 | 8108 ± 16.6a | <0.01 | 13.5 ± 0.01g | 11416 ± 0.721c | 3678 ± 7.54g | 22.4 ± 4.37a |
| TOT5330 | 42615 ± 122d | <0.01 | 203 ± 0.368i | <0.04 | 5553 ± 11.4f | <0.01 | 10.3 ± 0.01i | 9508 ± 0.438e | 3636 ± 7.45h | 22.0 ± 4.30a |
| TOT7266 | 42473 ± 121b | <0.01 | 292 ± 0.523a | <0.04 | 5160 ± 10.6h | <0.01 | 80.1 ± 0.09a | 3000 ± 0.919l | 2684 ± 5.50l | 18.7 ± 3. 65a |
| Cultivar | Folic acid | Vitamin A | Vitamin B12 | Vitamin C |
| mg/100g | µg/100g | µg/100g | mg/100g | |
| CHM | 0.94 ± 0.01d | 527 ± 3.79d | Not Detected | 275.5 ± 0.265e |
| Limpopo | 1.07 ± 0.01f | 279 ± 2.09ef | Not Detected | 364.9 ± 0.341i |
| SH | 1.12 ± 0.02f | 978 ± 3.06f | Not Detected | 276.3 ± 0.473e |
| TOT4100 | 0.09 ± 0.02a | 730 ± 2.00a | Not Detected | 283.2 ± 1.740g |
| TOT4880 | 1.05 ± 0.02e | 2277 ± 2.00e | Not Detected | 280.1 ± 0.252f |
| TOT4893 | 0.35 ± 0.03c | 758 ± 2.00c | Not Detected | 263.4 ± 0.510d |
| TOT4951 | 0.08 ± 0.02a | 832 ± 2.00a | Not Detected | 246.9 ± 0.404ab |
| TOT4977 | 1.09 ± 0.01f | 956 ± 2.69f | Not Detected | 256.8 ± 1.770c |
| TOT5028 | 0.16 ± 0.02b | 642 ± 1.43b | Not Detected | 382.3 ± 0.511j |
| TOT5077 | 1.07 ± 0.02f | 279 ± 2.31ef | Not Detected | 364.9 ± 2.070i |
| TOT5169 | 1.64 ± 0.03g | 790 ± 2.68g | Not Detected | 306.8 ± 0.503h |
| TOT5330 | 1.10 ± 0.02f | 341 ± 1.33f | Not Detected | 248.2 ± 0.306b |
| TOT7266 | 0.22 ± 0.02b | 815 ± 1.00b | Not Detected | 244.4 ± 0.436a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





