Submitted:
18 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
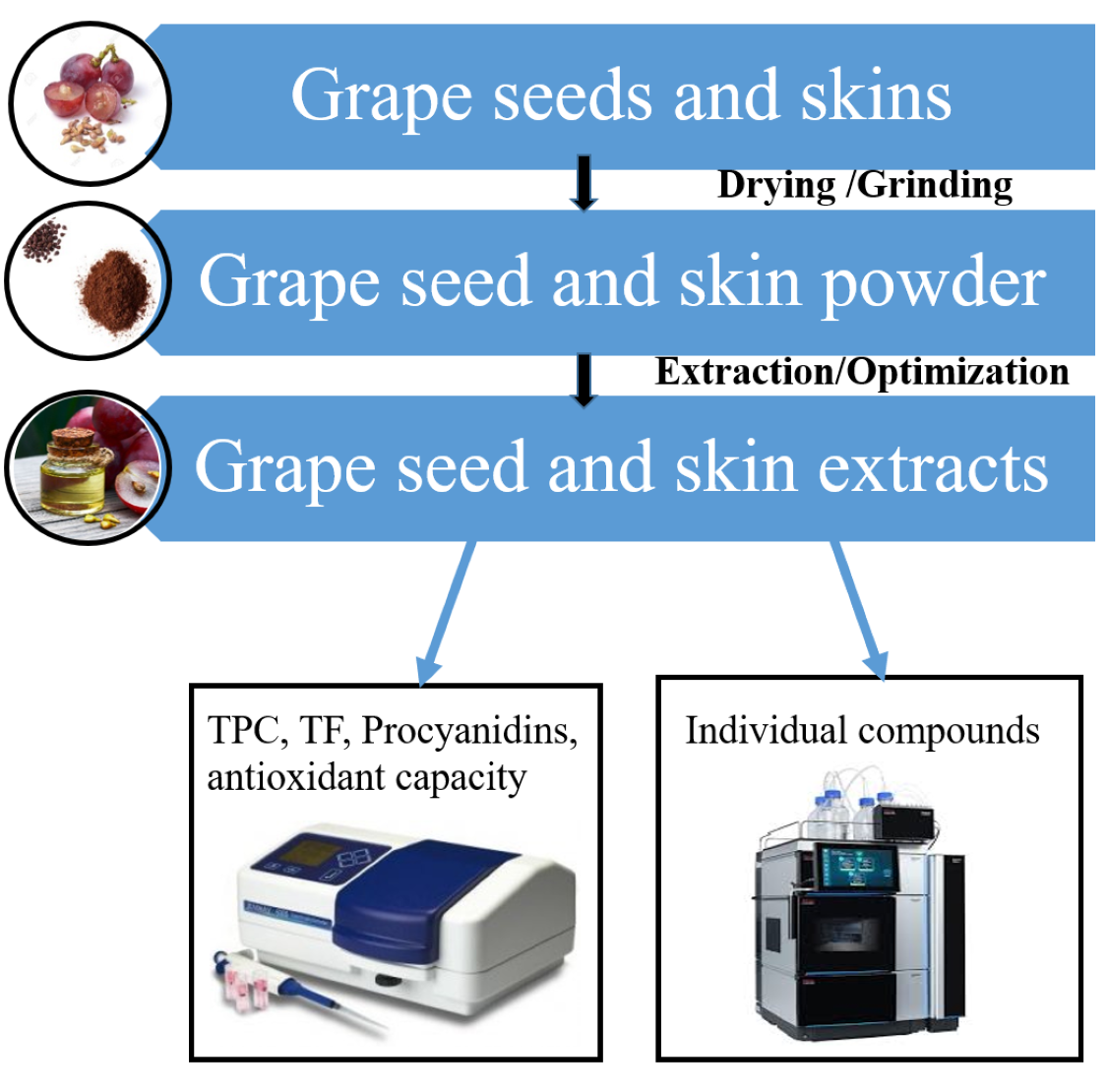
1. Introduction
2. Results and Discussions
2.1. Comparison of total phenolic parameters of grape skin and seed extracts
| Grape extract | Parameters | Pinot Noir | Cabernet Sauvignon | Marselan | Tamyanka |
|---|---|---|---|---|---|
| Skin | Yeld, % | 5.52 ± 0.51 | 13.25 ± 0.52 | 15.42 ± 0.55 | 10.02 ± 0.52 |
| TPC, mg GAE/g DW | 45.05 ± 0.85 | 42.32 ± 0.32 | 56.17 ± 0.41 | 36.28 ± 0.29 | |
| TF, mg QE/g DW | 4.41 ± 0.12 | 6.45 ± 0.12 | 6.70 ± 0.16 | 2.64 ± 0.11 | |
| PC, mg CE/g DW | 3.31 ± 0.15 | 3.65 ± 0.13 | 4.52 ± 0.14 | 1.23 ± 0.10 | |
| TA, mg CGE/g DW | 1.21 ± 0.10 | 3.34 ± 0.12 | 3.94 ± 0.15 | 0.015 ± 0.08 | |
| AA, mg/g DW | 3.17 ± 0.16 | 2.72 ± 0.11 | 2.76 ± 0.11 | 3.53 ± 0.14 | |
| Seed | Yield, % | 12.05 ± 0.77 | 16.02 ± 0.65 | 18.34 ± 0.66 | 15.13 ± 0.63 |
| TPC, mg GAE/g DW | 111.22 ± 1.28 | 88.22 ± 0.72 | 103.24 ± 1.11 | 79.06 ± 0.65 | |
| TF, mg QE/g DW | 51.50 ± 0.30 | 45.95 ± 0.14 | 52.01 ± 0.34 | 40.05 ± 0.18 | |
| PC, mg CE/g DW | 170.45 ± 2.52 | 157.22 ± 2.10 | 152.18 ± 2.05 | 31.44 ± 0.23 | |
| TA, mg CGE/g DW | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.062 ± 0.01 | no | |
| AA, mg/g DW | 11.07 ± 0.25 | 3.01 ± 0.11 | 2.71 ± 0.13 | 4.88 ± 0.13 |
| Standard compounds | Wave Length, nm | Conc. range, mg/L | Equations | R2 | LOD mg/L | LOQ mg/L | RSD %RT (n = 5) |
|---|---|---|---|---|---|---|---|
| GA | 280 | 1.0–10.0 | y = 19.497x − 0.5612 | 0.9999 | 0.25 | 0.75 | 0.14 |
| (+)-C | 280 | 10.0–50.0 | y = 2.202x + 0.8166 | 0.9992 | 2.59 | 7.84 | 0.27 |
| (-)-EC | 280 | 10.0–100.0 | y = 1.732x + 4.7597 | 0.9995 | 4.80 | 14.55 | 0.17 |
| (-)-EGCG | 280 | 2.0–10.0 | y = 12.648x + 5.035 | 0.9991 | 0.98 | 2.99 | 0.18 |
| B1 | 280 | 10.0–30.0 | y = 2.534x − 13.617 | 0.9986 | 1.75 | 5.30 | 0.26 |
| B2 | 280 | 10.0–20.0 | y = 3.901x − 9.72 | 0.9966 | 4.32 | 13.10 | 0.23 |
| B3 | 280 | 2.5 –100.0 | y = 9.254x + 1.58 | 0.9998 | 0.26 | 0.78 | 0.26 |
| C1 | 280 | 2.0–10.0 | y = 9.490x + 0.81 | 0.9999 | 0.22 | 0.66 | 0.18 |
| O | 280 | 1.0–5.9 | y = 27.966x − 24.586 | 0.9991 | 0.31 | 0.94 | 0.46 |
| Q3G | 360 | 1.9–8.0 | y = 11.416x − 13.243 | 0.9999 | 0.13 | 0.38 | 0.50 |
| R | 320 | 0.7–5.6 | y = 4.928x − 2.062 | 0.9979 | 0.40 | 1.23 | 0.11 |
| Q | 360 | 0.7–5.6 | y = 15.663x − 8.784 | 0.9993 | 0.22 | 0.65 | 0.79 |
2.2. Validation of HPLC method
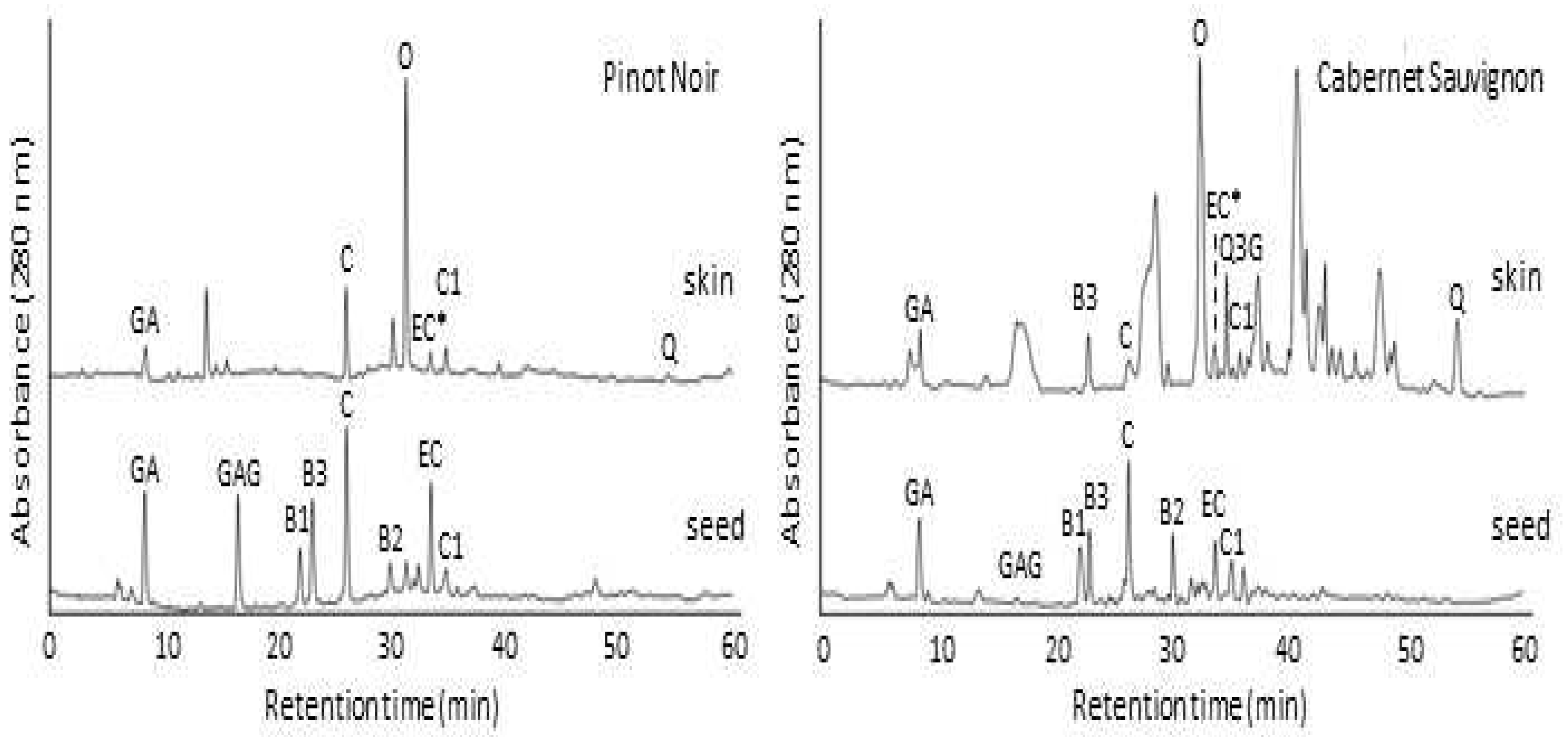
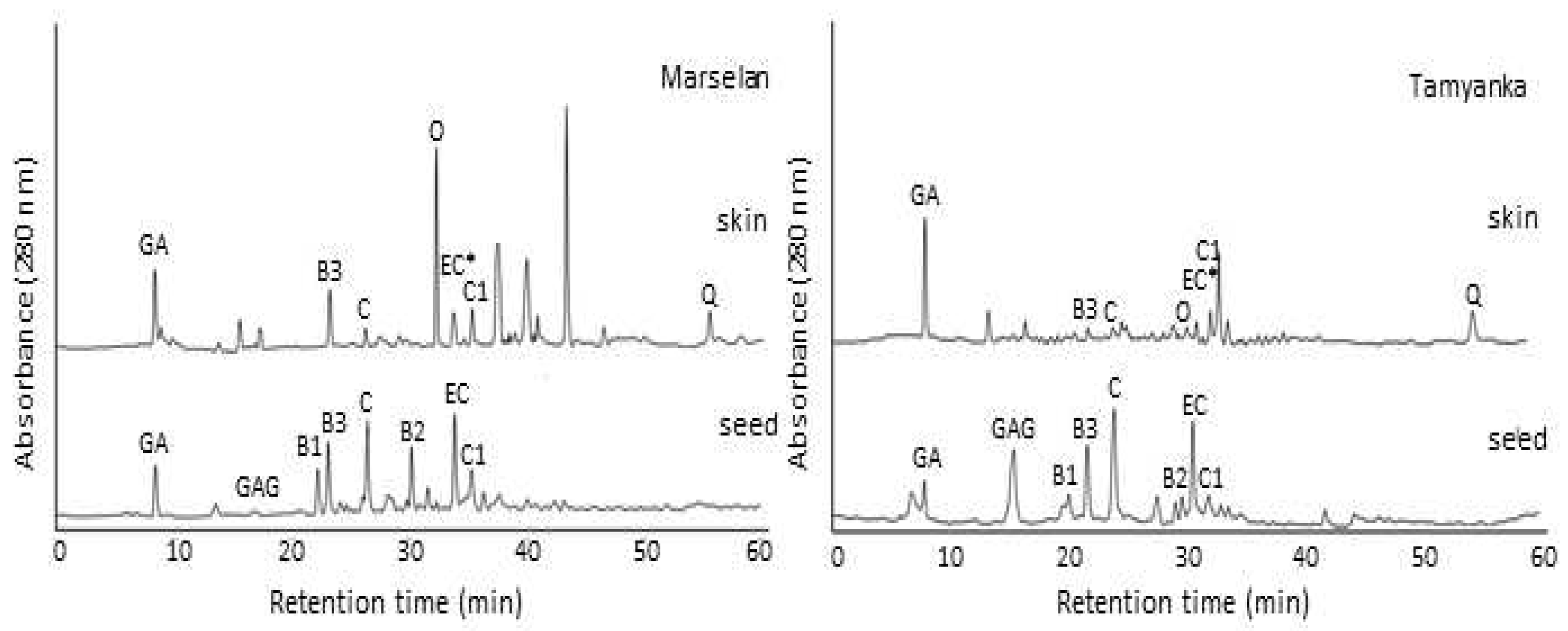
2.3. Comparison of HPLC identification of phenolic compounds in grape skin and seed extracts
| Grape extract | Polyphenols | Pinot Noir | Cabernet Sauvignon | Marselan | Tamyanka |
|---|---|---|---|---|---|
| Skins | Gallic acid | 0.30 ± 0.12 | 0.37 ± 0.13 | 0.65 ± 0.18 | 1.11 ± 0.54 |
| Procyanidin B3 | - | 0.65 ± 0.19 | 0.97 ± 0.23 | 0.39 ± 0.11 | |
| (+)-Catechin | 5.86 ± 0.97 | 1.51 ± 0.69 | 2.23 ± 0.61 | 0.44 ± 0.14 | |
| Oenin | 2.21 ± 0.63 | 2.66 ± 0.85 | 2.54 ± 0.71 | 0.99 ± 0.31 | |
| (-)-EC*(Epicatechin+Epigallocatechin gallat) | 0.45 ± 0.15 | 0.54 ± 0.13 | 0.47 ± 0.15 | 0.21 ± 0.09 | |
| Quercetin 3-gallate | - | 1.84 ± 0.78 | - | - | |
| Procyanidin C1 | 0.32 ± 0.12 | 0.39 ± 0.14 | 0.42 ± 0.14 | 0.12 ± 0.08 | |
| Quercetin | 0.67 ± 0.34 | 1.25 ± 0.37 | 1.15 ± 0.55 | 0.45 ± 0.15 | |
| Seeds | Gallic acid | 0.61 ± 0.23 | 0.44 ± 0.30 | 0.42 ± 0.31 | 0.35 ± 0.15 |
| Gallic acid glucoside | 0.88 ± 0.32 | 0.13 ± 0.07 | 0.05 ± 0.01 | 0.64 ± 0.02 | |
| Proanthocyanidin B1 | 8.81 ± 1.09 | 8.82 ± 1.17 | 7.57 ± 0.65 | 7.05 ± 0.73 | |
| Proanthocyanidin B3 | 2.90 ± 0.39 | 0.95 ± 0.37 | 1.31 ± 0.93 | 1.69 ± 0.73 | |
| (+)-Catechin | 12.16 ± 0.98 | 9.17 ± 0.73 | 8.06 ± 0.51 | 7.35 ± 0.50 | |
| Proanthocyanidin B2 | 4.06 ± 0.41 | 5.17 ± 0.21 | 5.17 ± 0.99 | 3.46 ± 0.62 | |
| (-)-Epicatechin | 10.16 ± 1.09 | 5.94 ± 0.45 | 14.27 ± 0.64 | 4.89 ± 0.31 | |
| Proanthocyanidin C1 | 0.34 ± 0.11 | 0.55 ± 0.21 | 0.68 ± 0.32 | 0.14 ± 0.07 |
| Grape Skins | PC B1, mg/g | PC B2, mg/g | PC B3, mg/g | PC C1, mg/g | C, mg/g | EC, mg/g | Q, mg/g | Reference |
|---|---|---|---|---|---|---|---|---|
| Cabernet Sauvignon | 0.012 ** | 0.001 ** | 0.027 ** | - | 0.017 ** | 0.006 ** | 0.048 ** | [27] |
| Merlot | 0.002 ** | 0.021 ** | 0.035 ** | - | 0.025 ** | 0.013 ** | 0.031 ** | [27] |
| Cabernet Sauvignon | - | - | - | - | 1.41 | 1.27 | 0.006 | [38] |
| Cabernet Sauvignon | - | - | - | - | - | - | 0.23 | [32] |
| Grenache | 0.62 | 0.41 | 0.27 | 0.46 | 0.76 | 0.28 | - | [25] |
| Carignan | 0.74 | 0.43 | 0.28 | 0.53 | 1.01 | 0.35 | - | [25] |
| Pinot Noir | - | - | - | - | 0.13 | - | 0.15 | [32] |
| Pinot Noir | 0.30 | 0.28 | - | - | 0.61 | 0.34 | 1.54 | [28] |
| Pinot Noir | - | - | - | 0.32 | 5.86 | 0.45 * | 0.67 | Current study |
| Cabernet Sauvignon | - | - | 0.65 | 0.39 | 1.51 | 0.54 * | 1.25 | Current study |
| Marselan | - | - | 0.97 | 0.42 | 2.23 | 0.47 * | 1.15 | Current study |
| Tamyanka | - | - | 0.39 | 0.12 | 0.44 | 0.21 * | 0.45 | Current study |
2.4. Comparison of antioxidant capacity of skin and seed extracts
| Grape extract | Parameters | Pinot Noir | Cabernet Sauvignon | Marselan | Tamyanka |
|---|---|---|---|---|---|
| skins | DPPH, µM TE/g DW | 75.77 ± 1.12 | 81.23 ± 0.73 | 89.74 ± 0.78 | 14.22 ± 0.18 |
| ABTS, µM TE/g DW | 87.61 ± 1.25 | 99.05 ± 0.88 | 109.31 ± 1.01 | 58.23 ± 0.41 | |
| FRAP, µM TE/g DW | 11.28 ± 0.29 | 35.68 ± 0.45 | 29.05 ± 0.28 | 10.03 ± 0.34 | |
| CUPRAC, µM TE/g | 12.53 ± 0.32 | 14.41 ± 0.17 | 18.93 ± 0.22 | 11.64 ± 0.18 | |
| seeds | DPPH, µM TE/g DW | 579.33 ± 4.15 | 435.25 ± 3.3 | 597.23 ± 4.12 | 245.6 ± 3.23 |
| ABTS, µM TE/g DW | 2203.51 ± 10.25 | 2246.23 ± 11.33 | 2273.92 ± 12.32 | 1907.24 ± 9.56 | |
| FRAP, µM TE/g DW | 142.76 ± 2.35 | 97.15 ± 0.82 | 115.22 ± 1.10 | 67.45 ± 0.75 | |
| CUPRAC, µM TE/g | 68.18 ± 0.98 | 64.38 ± 0.52 | 72.34 ± 0.81 | 32.23 ± 0.29 |
| Time, min | Eluent A, % | Eluent B, % |
|---|---|---|
| 0.0–5.0 | 90 | 10 |
| 5.1–20.0 | 80 | 20 |
| 20.1–25.0 | 75 | 25 |
| 25.1–50.0 | 70 | 30 |
| 50.1–80.0 | 55 | 45 |
3. Materials and Methods
3.1. Materials
3.2. Preparation of grape skin and seed extracts
3.3. Spectrophotometric methods for determination of skin and seed polyphenols
3.4. Spectrophotometric methods for determination of antioxidant capacity of skin and seed extracts
3.5. HPLC assays for determination of phenolic composition of skin and seed extracts
3.6. Data processing of HPLC method
3.7. Statistical Analysis
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The Study of Antioxidant Components in Grape Seeds. Molecules 2020, 25, 3736. [Google Scholar] [CrossRef]
- Garcia-Cabezon, C.; Teixeira, G.G.; Dias, L.G.; Salvo-Comino, C.; García-Hernandez, C.; Rodriguez-Mendez, M.L.; Martin-Pedrosa, F. Analysis of Phenolic Content in Grape Seeds and Skins by Means of a Bio-Electronic Tongue. Sensors 2020, 20, 4176. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Cebova, M.; Jurikova, T.; Mlcek, J.; Anna Adamkova, A.; Nedomova, S.; Mojmir Baron, M.; Sochor, J. Health Effects of Grape Seed and Skin Extracts and Their Influence on Biochemical Markers. Molecules 2020, 25, 5311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Du, C.G. Could grape-Based food supplements prevent the development of chronic kidney disease? Crit. Rev. Food Sci. Nutr. 2020, 60, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Sanna, G.; Manca, G.; Franco, M.A.; Porcu, A. Variability in flavonol content of grapes cultivated in two mediterranean islands (sardinia and corsica). J. Food Compos. Anal. 2010, 23, 580–585. [Google Scholar] [CrossRef]
- Mohamedshah, Z.; Chadwick-Corbin, S.; Wightman, J.D.; Ferruzzi, M.G. Comparative assessment of phenolic bioaccessibility from 100% grape juice and whole grapes. Food Funct. 2020, 11, 6433–6445. [Google Scholar] [CrossRef] [PubMed]
- Ćurko, N.; Tomašević, M.; Bubalo, M.C.; Gracin, L.; Redovniković, I.R.; Ganić, K.K. Extraction of Proanthocyanidins and Anthocyanins from Grape Skin by Using Ionic Liquids. Food Technol. Biotechnol. 2017, 55, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem X. 2021, 12, 100149. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery by-products: Extraction Techniques and their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Esselen, M.; Barth, S.W. Food-borne topoisomerase inhibitors: Risk or benefit. Adv. Mol. Toxicol. 2014, 8, 123–171. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of Catechin, Epicatechin, and Rutin: Optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci. Tecnol. 2007, 41, 235. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkeviciu, M.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.W. Quercetin-3-glucoside extracted from apple pomace induces cell cycle arrest and apoptosis by increasing intracellular ROS levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef]
- Nouri, A.; Salehi-Vanani, N.; Heidarian, E. , Antioxidant, anti-inflammatory and protective potential of gallic acid against paraquat-induced liver toxicity in male rats. Avicenna J Phytomed. 2021, 11, 633–644. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A role of gallic acid in oxidative damage diseases: A comprehensive review. Nat. prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; Shen, J.-Q.; Rajput, S.A.; Li, J.-H. Resveratrol (RV): A pharmacological review and call for further research. Biomed.Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.; Torres, C.; Oliveira, A.; Pereira, J.E.; Amaral, S.A.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control. 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Ghafoor, K.; Uslu, N.; Özcan, M.; Juhaimi, F.; Babiker, E.; Ahmed, I.; Azmi, I. Influence of grape variety on bioactive compounds, antioxidant activity, and phenolic compounds of some grape seeds grown in Turkey. J. Food Process. Preserv. 2020, 44, e14980. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Göksel, Z.; Erdogan, S.; Öztürk, A.; Atak, A.; Özer, C. Antioxidant activity and phenolic content of seed, skin and pulp parts of 22 grape (Vitis vinifera L.) cultivars (4 common and 18 registered or candidate for registration). J. Food Proc. Preserv. 2014, 39, 1682–1691. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed]
- Guaita, M.; Bosso, A. Polyphenolic characterization of grape skins and seeds of four Italian red cultivars at harvest and fermentative maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.L.C.; Gascuena, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Benmeziane, F.; Cadot, Y. Identification of proanthocyanidins compounds in skins of some table grape Vitis vinifera varieties from Algeria grown in Mediterranean climate by highperformance liquid chromatography. Adv Food Technol Nutr Sci Open J. 2018, SE, S45–S50. [Google Scholar] [CrossRef]
- Benmeziane, F.; Cadot, Y. Quantitative analysis of proanthocyanidins (tannins) from cardinal grape (Vitis vinifera) skin and seed by RP-HPLC, Nor. Afr. J. Food Nutr. Res. 2019, 3, 201–205. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.-L.; Haroutounin, A.S. Bioactive non-colored polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Maria-Rizelio, V.; De Souza, A.; Gonçalves, S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Sovak, M. Grape extract, resveratrol, and its analogs: A review. J. Med. Food 2001, 4, 93–105. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J Food Sci Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J Funct. Foods. 2020, 67, 103861. [Google Scholar] [CrossRef]
- Abharzanjani, F.; Mina Hemmati, M. Protective effects of Quercetin and Resveratrol on aging markers in kidney under high glucose condition: In vivo and in vitro analysis. Mol Biol Rep. 2021, 48, 5435–5442. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Melilli, G.M.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Castro-Lopeza, L.; Castillo-Sanchez, G.; Dıaz-Rubio, L.; Cordova-Guerrero, I. Total content of phenols and antioxidant activity of grape skins and seeds Cabernet Sauvignon cultivated in Valle de Guadalupe, Baja California, Mexico. In Proceedings of the BIO Web of Conferences; 2019. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Matei, C.; Deacinu, M.; Stanciuc, A.M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 33, 110787. [Google Scholar] [CrossRef]
- Sofi, F.R.; Raju, S.V.; Lakshmisha, L.P.; Ratankumar, S.R. Antioxidant and antimicrobial properties of grape and papaya seed extracts and their application on the preservation of Indian mackerel (Rastrelliger kanagurta) during ice storage. J. Food Sci. Tech. 2016, 53, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981 https://. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




