1. Introduction
Despite advances in surgical techniques and adjuvant therapies, surgical resection and adequate reconstruction of malignant lesions continue to pose challenges for surgeons. Malignant lesions may initially be asymptomatic, but as they grow larger or develop into ulcers, patients begin to experience pain and other symptoms. To prevent recurrence, an appropriate safety margin must be established, which often results in larger resection sites. Consequently, factors such as the location, type of lesion, and duration of the condition can influence a patient’s prognosis.[
1]
Malignant lesions in the oral and maxillofacial region can occur in various locations, including the lips, tongue, floor of the mouth, buccal cheek mucosa, upper and lower gingiva, palate, and salivary glands. As a result, surgical modalities and defects vary according to the primary cancer site. The oral and maxillofacial area is not only essential for aesthetics but also plays functional roles in swallowing and speech. Therefore, reconstruction and restoration following the removal of malignant lesions in this region must consider not only morphological aspects but also overall functionality.[
2]
Reconstruction options following lesion removal include primary closure, free graft, local flap, remote flap, and free flap with microvascular anastomosis. For extensive malignant lesions, a combination of free grafts, local flaps, remote flaps, and free flaps with microvascular anastomosis may be used to achieve both morphological and functional restoration, depending on the components involved.[
3] However, for localized malignant lesions or areas where reconstruction is challenging, primary closure may be the preferred method due to the limited receiving area for flap-based reconstruction. Primary closure involves simply approximating the edges of the wound[
4] and is typically performed after resecting small lesions that do not compromise the anatomical structure’s movement and function.[
5]
In cases where malignant lesions have spread to hard tissues such as alveolar bone and require resection of the hard tissue, flap surgery can reconstruct both the lost hard tissue and the surrounding soft tissue, potentially restoring masticatory function. However, it is true that reconstruction using flaps in defects of soft tissues and muscles, such as the tongue and pharynx, may recover the lost volume but has limitations in restoring the inherent abilities of soft tissues and muscles, such as swallowing and pronunciation. In these cases, primary closure can be a useful alternative. However, surgeons aiming to remove lesions and reconstruct the area may be so accustomed to finding an appropriate flap depending on the size of the removed lesion, the type of tissue defect, and the functions that need to be restored that they may occasionally forget the alternative option of primary closure.
This study aims to retrospectively analyze the data of patients who underwent surgery for oral cancer at Chosun University Dental Hospital over the past 10 years, focusing specifically on the characteristics of patients who underwent primary closure. We seek to explore which patients may benefit from primary closure as a useful alternative.
2. Materials and Methods
This study’s protocol was approved by the institutional ethics committee (CUDHIRB 2005009). Written informed consent was obtained from all patients, and a retrospective study was conducted using the medical records of patients who underwent surgery for oral cancer at Chosun University Dental Hospital between January 2010 and May 2020. All surgeries were performed by a single skilled surgeon. All patients underwent an incisional biopsy during the work-up period, and panoramic radiographs and CT scans were taken. Additionally, PET CT scans were performed to determine the presence of lymph node metastasis in advance, which helped decide whether to perform neck dissection and its type before surgery. During surgery, wide excision was performed by marking the planned resection margin using a surgical guide or marking pen to ensure the margin was maintained. Furthermore, frozen biopsy was performed on the resection margin during surgery to minimize the possibility of cancer cells remaining within the safety margin. Patients were discharged when they were considered capable of carrying out everyday activities at home. For those who underwent DCIA free flap or fibula free flap grafting, they were discharged when they had sufficiently recovered and could walk. After discharge, regular follow-up visits were conducted to check for recurrence at the surgical site, and PET CT scans were performed if lymph node metastasis was suspected through routine contrast-enhanced CT scans. During this period, a total of 85 patients (42 men and 43 women) underwent malignant lesion removal in the oral and maxillofacial area. The medical records of these 85 patients were all tabulated. Data that can be obtained include gender, age, operation time, hospitalization and discharge period, surgical site, surgical method, type of flap used, existence and type of neck dissection, duration of f/u, presence or absence of recurrence, stage, and biopsy results. From January 2010 to May 2020, medical data of 16 patients who underwent primary closure out of the 85 patients with malignant lesions removed were analyzed. Factors such as patients’ TNM, stage, neck dissection, histologic type, recurrence, and period of hospitalization were investigated. The staging was according to the American Joint Committee on Cancer (AJCC, 8th edition).[
6]
Data were analyzed using the SPSS system. The Mann-Whitney test was used to determine the statistical significance between age and primary closure. The chi-square test was employed to assess the significance of gender and surgical method (primary closure and flap), and the chi-square test was also utilized to evaluate the significance of neck dissection and surgical method (primary closure and flap), and the Mann-Whitney test was used to determine the statistical significance between period of hospitalization and primary closure.
3. Results
3.1. Patient Characteristics Analysis
From January 2010 to May 2020, a total of 85 patients with malignant lesions in the maxillofacial area underwent surgical removal. Among these 85 patients, 42 were male and 43 were female, with an average age of 64.57 years (range: 15 to 87 years). The most common stage was stage I, with 26 patients, followed by 24 stage IV patients, 18 stage II patients, and 17 stage III patients. Fifty-seven patients underwent malignant lesion removal with neck dissection, and 28 patients had the procedure without neck dissection. Among the 57 patients who underwent neck dissection, 53 had selective neck dissection, and 4 had modified radical neck dissection. The most common site of malignant lesions was the lower gingiva (33 cases), followed by the upper gingiva (17), tongue (13), buccal mucosa (8), floor of the mouth (7), and palate (7) (
Table 1).
3.2. Factors Associated with Primary Closure (Table 1)
We investigated the associations between age, gender, stage, neck dissection, operation time, lesion site, and other factors to determine the patient factors associated with primary closure compared to patients with flap reconstruction. The average age of patients with primary closure was 65.25 years, with no statistically significant difference (P=0.779). Among primary closure patients, there were 11 women and 5 men, and there was no statistically significant difference between gender and primary closure (P=0.107). Of the 16 total patients, eight underwent surgery with selective neck dissection, one with modified neck dissection, and seven without neck dissection. No statistically significant difference was found between neck dissection and primary closure (P=0.307). Statistically significant differences were observed between staging and primary closure, with 13 stage I and three stage II patients using primary closure (P=0.046). Statistically significant differences were identified between operation time and primary closure, with an average of 4.02 hours (P=0.015). In patients with primary closure, the distribution of lesion sites was as follows: tongue (5), lower gingiva (3), palate (3), upper gingiva (2), floor of the mouth (2), and buccal mucosa (1); however, no significant difference was noted in primary closure by lesion site (P=0.120). The average period of hospitality for patients who underwent primary closure was 13.4 days, compared to 26.7 days for those who underwent flap surgery. This difference was statistically significant (P=0.0003).
3.3. Patient Analysis with Primary Closure
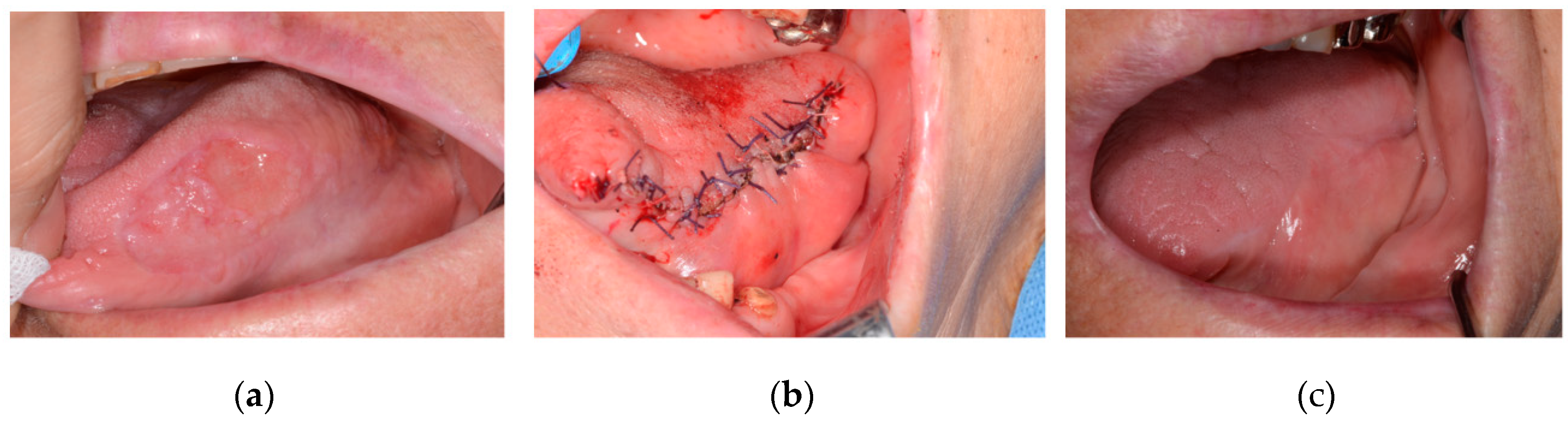
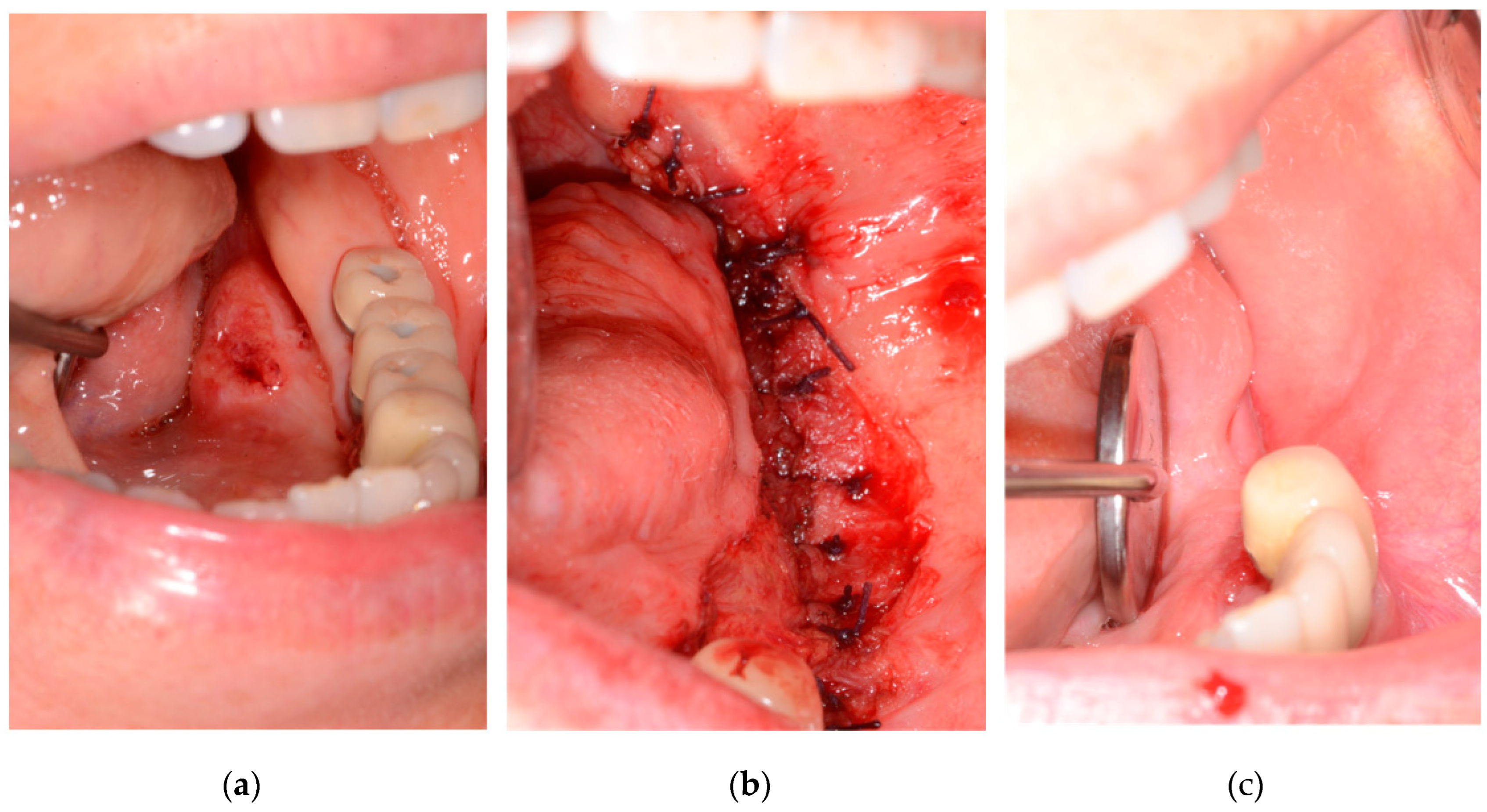
Out of the total 85 patients, 16 patients underwent primary closure and 69 had flap reconstruction. Among the 16 patients who received primary closure, 5 were male and 11 were female. The average age of patients with primary closure was 65.25 years. Thirteen of the 16 primary closure patients were stage I, and 3 were stage II. No stage III or IV patients underwent primary closure. Nine primary closure patients had malignant lesion removal with neck dissection, while seven did not. Among the nine patients who underwent primary closure with neck dissection, eight had selective neck dissection, and one had modified radical neck dissection. The distribution of malignant lesions among the 16 primary closure patients was as follows: tongue (5), lower gingiva (3), palate (3), upper gingiva (2), floor of the mouth (2), and buccal mucosa (1). Squamous cell carcinoma (SCC), mucoepidermoid carcinoma, and verrucous carcinoma were diagnosed in the 16 primary closure patients (
Figure 1 and
Figure 2). Thirteen patients were diagnosed with SCC, two with mucoepidermoid carcinoma, and one with verrucous carcinoma. Among the SCC patients, seven had a moderately differentiated histological grade, and six had a well-differentiated grade. One of the two mucoepidermoid carcinoma patients had a low-grade tumor, and the other had a high-grade tumor. In the assessment of postoperative resection margins in the 16 primary closure patients, carcinoma was not involved in 14 patients. However, dysplasia was observed in the resection margin in two patients. High-grade dysplasia was observed in the anterior, posterior, and ventral posterior resection margins of a patient with well-differentiated SCC on the right lateral border of the tongue, but no recurrence was observed during a 6-year follow-up. High-grade dysplasia was observed in the middle posterior and lower middle vestibule resection margins in a patient with moderately differentiated SCC on the right mandibular posterior gingiva, but no recurrence was observed during a 2-year follow-up (
Table 2).
3.4. Patient Analysis with Primary Closure
Sixteen patients by primary closure were followed up for an average of 38.37 months, ranging from 12 to 80 months. Recurrence was confirmed in 2 of the 16 patients. One patient with recurrence had been diagnosed with stage I and well-differentiated SCC on the upper gingiva. During the initial operation, neck dissection was not performed, but regional recurrence was observed in the left level II lymph node during follow-up. The other patient was diagnosed with stage II and moderately differentiated SCC on the floor of the mouth. During the initial operation, selective neck dissection from levels I to III was performed, and regional recurrence was observed at the right lymph node levels II and III during follow-up (
Table 2).
4. Discussion
Malignant lesions in the oral and maxillofacial areas can lead to functional and aesthetic issues after surgical removal, such as impaired mastication and pronunciation.[
7,
8,
9] To address these defects, various types of flaps are used for reconstruction, including free grafts, local flaps, remote flaps, and free flaps by microvascular anastomosis.[
10,
11] In this study, anterolateral thigh free flap, deep circumflex iliac artery flap, and radial forearm flap were used to recover the defects. Although flap reconstruction provides a basis for implants and restores continuity, it can result in problems like the loss of essential soft tissue for chewing function.[
12] In particular, tongue reconstruction may lead to a decrease in normal tongue mobility.[
13]
Using flaps to recover the functional defect on Oral and maxillofacial area, but in Fred MS McConnel’s study comparing flaps and primary closure in patients with localized tongue removal of 30% and base 60%, the use of flaps did not show a significant difference in swallow efficiency. In fact, it was confirmed that the swallowing efficiency was higher in the case of the patient who are primary closured than the patient with using the flap. Also, when comparing tongue movements to evaluate pronunciation function, there was no significant difference, but it was confirmed that the case with the primary closure scored higher than the case with the flap.[
14] In the study of Martin Canis and Fred MS MnConnel, when lesions exceeding 30% to 40% were removed, flap restoration was beneficial for functional recovery, but when lesions within the range of 20 to 30% or less were removed, it was confirmed that the use of primary closure did not have a significant difference in functional recovery.[
15,
16,
17] In addition, the loss of chewing function was confirmed in Giovanni Nicoletti’s study that it occurred in extensive resection of the mouth or anterior region or retromolar trigone, and did not occur in any size of tongue resection.[
18,
19]
In this study, primary closure was performed in patients in the early stage, Stage I and II, and in the lesions of squamous cell carcinoma, mucoepidermoid carcinoma, and verrucous carcinoma. Statistically significant correlation was confirmed between stage and Primary closure(P<0.05). This means that primary closure may be useful after removal of early stage (stage I or II) malignant lesions.
Figure 1 is a patient was followed-up after resection under the diagnosis of squamous cell carcinoma on the left lateral border of the tongue. Primary closure was performed with remained the tongue and mucosa of the floor of the mouth.
Figure 2 is a patient who was followed-up after resection under the diagnosis of squamous cell carcinoma on left floor or mouth. Primary closure was performed with mucosa of floor of mouth and buccinator muscle flap.
Neck dissection is a procedure that lymph nodes are removed when there is a suspicion of metastasis of the malignant lesion ipsilaterally or bilaterally to the area where the malignant lesion exists. It is divided into therapeutic neck dissection for therapeutic purposes when cervical metastasis is evident and prophylactic neck dissection for safer clinical symptoms and progress when there is no evidence of metastasis. Neck dissection is performed because it can remove metastatic lesions in the lymph nodes and play a crucial role in staging of lesion. Clinically, if the patient complains of pain on palpation at the ipsilateral or bilateral lymph node region in the neck of the patient’s malignant lesion, or if the patient complains of pain even without palpation, lymph node metastasis can be suspected.[
20] In this study, patients with clinical observation as well as PET-CT scans was performed neck dissection for suspected cervical metastases, and selective neck dissection (Level I,II,III) was performed in early oral cancer patients. Determination of additional treatment is depending on whether or not metastases have actually occurred through examination of the tissues removed from the neck dissection area.
In the follow-up observation of 16 patients who had been treated the defect with the primary closure, the patients did not complain of discomfort, but at the initial follow-up of the outpatient clinic after discharge, 5 patients complained of a feeling of pulling at the operation site. Patients complaining of abnormal pronunciation in functional aspects were also observed. However, during follow-up, the discomfort disappeared, and it was confirmed that there was no particular discomfort in the final follow-up record. In the case of patients who was performed neck dissection, some patients complained of more discomfort in the neck dissection area than in the malignant lesion resection area, but it was confirmed that the discomfort was resolved later. However, this evaluation itself was difficult to obtain validity because the frequency of investigation was different for each patient, and there were differences between patients who received additional treatment and those who did not.
The average length of hospitalization for each patient was 13.4 days in patients who underwent primary closure and 26.7 days in patients who underwent flap surgery, showing an average difference of about 2 weeks, and the difference between the two was statistically significant. This indicates that the patient group who underwent primary closure had a faster recovery and easier return to daily life, and there were fewer factors such as postoperative side effects that prolonged the hospitalization period. Since the grades of the lesions in the two patient groups are not the same, a simple comparison between the two would be incorrect, but if both primary closure and flap reconstruction are possible for patients, primary closure may be a sufficient alternative.
In patients with primary closure, recurrence was observed in two patients. In the case of one patient, who got the malignant lesion on the floor of mouth removed it but the recurrence of Lt. lymph node Level II was observed. For resecting recurred lesion, neck dissection was performed. In the other, the malignant lesion on the Rt. upper gingiva was removed, but the recurrence of the Rt. lymph node level II, III was observed, and a neck dissection was performed. In both cases, recurrence was in the lymph node area, and no recurrence was observed at the safety margin set by the surgeon. In patients who underwent primary closure, the safety margin area was clearly visible. Therefore, if a recurrence occurs in the margin area, it will be easier to detect than a patient reconstructed using a flap. This is considered to be easier to determine recurrence because the lesion removal site is less obscured by other flap structures.
It was confirmed that the use of primary closure was shorter in terms of operation time compared to the defect repair using flaps. Since the flap was not used, the evaluation of the primary lesion was easy, and the evaluation of recurrence was also smooth. Also most of the patients’ discomfort was the feeling of pulling due to the primary closure after removal of the lesion, and it was confirmed that it disappeared during follow-up after stitch out.
Further studies are needed to evaluate to which lesions the primary closure is useful and further patient analysis is needed. In addition, data analysis on patients who have radiation therapy prior to or after surgical treatment will also be necessary.
5. Conclusions
After removal of malignant lesions in the oral and maxillofacial area, primary closure was useful for treating early stage malignant lesions, postoperative patient discomfort was reduced, and operation time was also reduced. Considering this, early diagnosis is very important for the prognosis of patients, and it will be important to determine an appropriate surgical method after diagnosis.
Author Contributions
Conceptualization S.-Y.M.; methodology, S.-Y.M. and J.-S.C.; software, S.-Y.M. and Y.-J.J.; validation, J.-S.C. and H.-J.K.; formal analysis, J.-S.C. and Y.-J.J.; investigation, J.-S.C. and H.-J.K.; resources, S.-Y.M.; data curation, J.-S.C. and H.-J.K.; writing—original draft preparation, H.-J.K. and J.-S.C.; writing—review and editing, H.-J.K. and S.-Y.M.; visualization, S.-Y.M. and J.-S.C.; supervision, S.-Y.M.; project administration S.-Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chosun University Dental Hospital, 2022.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Chosun University Dental Hospital (CUDHIRB 2005009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Where no new data were created, or where data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anguiano, L.; Mayer, D.K.; Piven, M.L.; Rosenstein, D. A Literature Review of Suicide in Cancer Patients. Cancer Nurs. 2012, 35, E14–E26. [Google Scholar] [CrossRef] [PubMed]
- Day, T.A.; Davis, B.K.; Gillespie, M.B.; Joe, J.K.; Kibbey, M.; Martin-Harris, B.; Neville, B.; Reed, S.G.; Richardson, M.S.; Rosenzweig, S. Oral cancer treatment. Current treatment options in oncology 2003, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Engel, H.; Huang, J.J.; Lin, C.-Y.; Lam, W.; Kao, H.-K.; Gazyakan, E.; Cheng, M.-H. A Strategic Approach for Tongue Reconstruction to Achieve Predictable and Improved Functional and Aesthetic Outcomes. Plast. Reconstr. Surg. 2010, 126, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Simman, R. Wound closure and the reconstructive ladder in plastic surgery. The Journal of the American College of Certified Wound Specialists 2009, 1, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.-H.; Cho, J.-K.; Koo, B.S.; Kwon, M.; Kwon, S.K.; Kwon, S.Y.; Kim, M.-S.; Kim, J.K.; Kim, H.; Nam, I.; et al. Guidelines for the Surgical Management of Oral Cancer: Korean Society of Thyroid-Head and Neck Surgery. Clin. Exp. Otorhinolaryngol. 2019, 12, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC cancer staging manual; Springer: 2017; Volume 1024.
- Brown, J.; Rogers, S.; Lowe, D. A comparison of tongue and soft palate squamous cell carcinoma treated by primary surgery in terms of survival and quality of life outcomes. Int. J. Oral Maxillofac. Surg. 2006, 35, 208–214. [Google Scholar] [CrossRef] [PubMed]
- So, W.; Chan, R.; Chan, D.; Hughes, B.; Chair, S.; Choi, K.; Chan, C. Quality-of-life among head and neck cancer survivors at one year after treatment—A systematic review. Eur. J. Cancer 2012, 48, 2391–2408. [Google Scholar] [CrossRef] [PubMed]
- Urken, M.L.; Buchbinder, D.; Weinberg, H.; Vickery, C.; Sheiner, A.; Parker, R.; Schaefer, J.; Som, P.; Shapiro, A.; Lawson, W.; et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: A comparative study of reconstructed and nonreconstructed patients. Laryngoscope 1991, 101, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Baek, C.-H.; Jeong, H.-S.; Chung, M.K.; Son, Y.-I. Analysis of 130 Cases of Pectoralis Major Flap for the Head and Neck Reconstruction. Korean J. Otorhinolaryngol. Neck Surg. 2016, 59, 133–139. [Google Scholar] [CrossRef]
- Curtis, D.A.; Plesh, O.; Miller, A.J.; Curtis, T.A.; Sharma, A.; Schweitzer, R.; Hilsinger, R.L.; Schour, L.; Singer, M. A comparison of masticatory function in patients with or without reconstruction of the mandible. Head Neck 1997, 19, 287–296. [Google Scholar] [CrossRef]
- Schliephake, H.; Rüffert, K.; Schneller, T. Prospective study of the quality of life of cancer patients after intraoral tumor surgery. J. Oral Maxillofac. Surg. 1996, 54, 664–669. [Google Scholar] [CrossRef]
- Hsiao, H.-T.; Leu, Y.-S.; Lin, C.-C.B. Primary Closure Versus Radial Forearm Flap Reconstruction After Hemiglossectomy: Functional Assessment of Swallowing and Speech. Ann. Plast. Surg. 2002, 49, 612–616. [Google Scholar] [CrossRef] [PubMed]
- McConnel, F.M.; Pauloski, B.R.; Logemann, J.A.; Rademaker, A.W.; Colangelo, L.; Shedd, D.; Carroll, W.; Lewin, J.; Johnson, J. Functional results of primary closure vs flaps in oropharyngeal reconstruction: a prospective study of speech and swallowing. Archives of Otolaryngology–Head & Neck Surgery 1998, 124, 625–630. [Google Scholar]
- McConnel, F.M.S.; Teichgraeber, J.F.; Adler, R.K. A Comparison of Three Methods of Oral Reconstruction. Arch. Otolaryngol. Neck Surg. 1987, 113, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Weiss, B.G.; Ihler, F.; Hummers–Pradier, E.; Matthias, C.; Wolff, H.A. Quality of life in patients after resection of pT3 lateral tongue carcinoma: microvascular reconstruction versus primary closure. Head & neck 2016, 38, 89–94. [Google Scholar]
- Pauloski, B.R. Rehabilitation of dysphagia following head and neck cancer. Physical medicine and rehabilitation clinics of North America 2008, 19, 889–928. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Soutar, D.S.; Jackson, M.S.; Wrench, A.A.; Robertson, G. Chewing and Swallowing after Surgical Treatment for Oral Cancer: Functional Evaluation in 196 Selected Cases. Plast. Reconstr. Surg. 2004, 114, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pauloski, B.R.; Logemann, J.A.; Rademaker, A.W.; McConnel, F.M.S.; Heiser, M.A.; Cardinale, S.; Shedd, D.; Lewin, J.; Baker, S.R.; Graner, D.; et al. Speech and Swallowing Function After Anterior Tongue and Floor of Mouth Resection With Distal Flap Reconstruction. J. Speech, Lang. Hear. Res. 1993, 36, 267–276. [Google Scholar] [CrossRef]
- Holland, J.F.; Kufe, D.W. Holland-Frei cancer medicine 8; PMPH-USA: 2010; Volume 8.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).