Submitted:
18 April 2023
Posted:
19 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
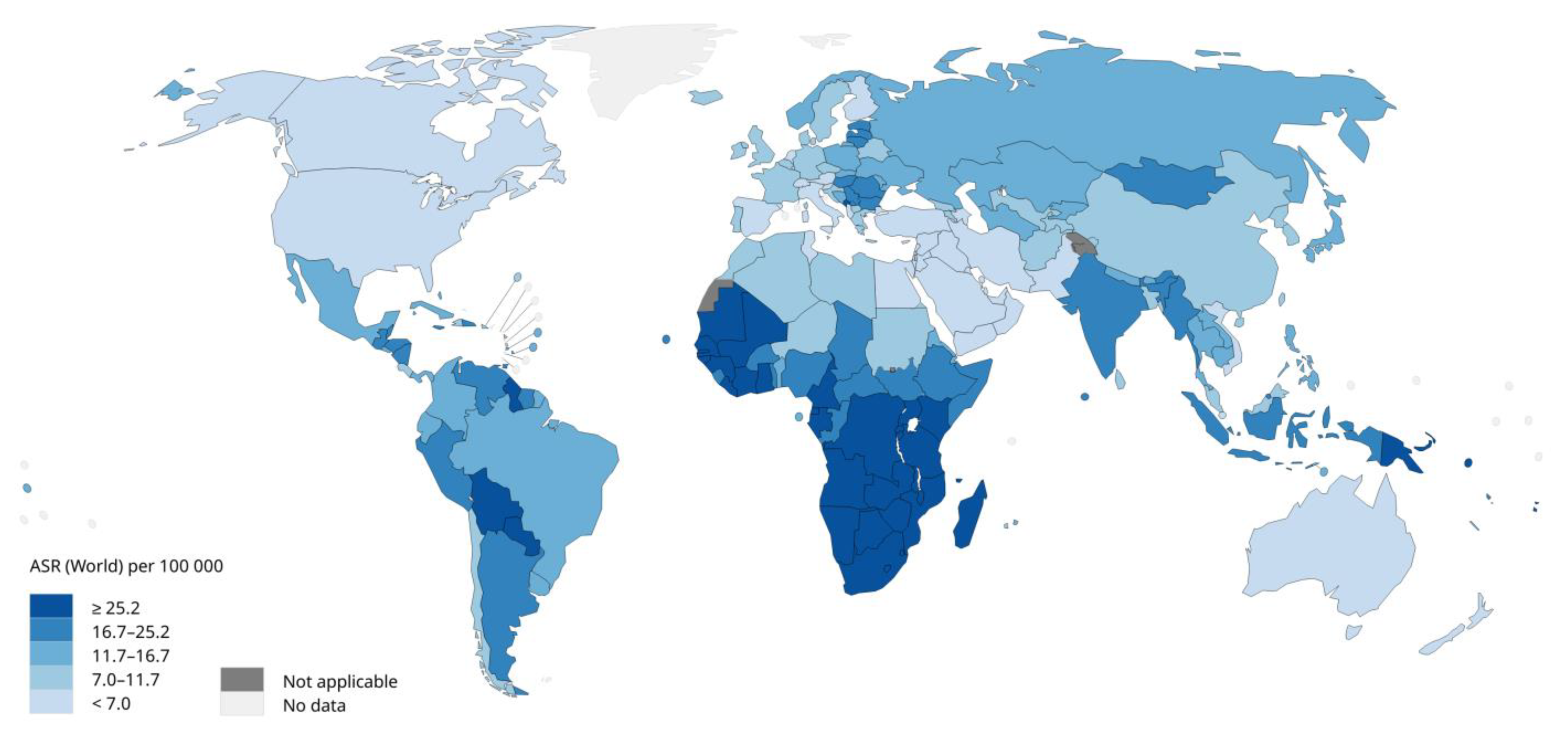
2. Epidemiology
3. Transmission
4. Clinical Manifestation
4.1. Benign
4.2. Malignant
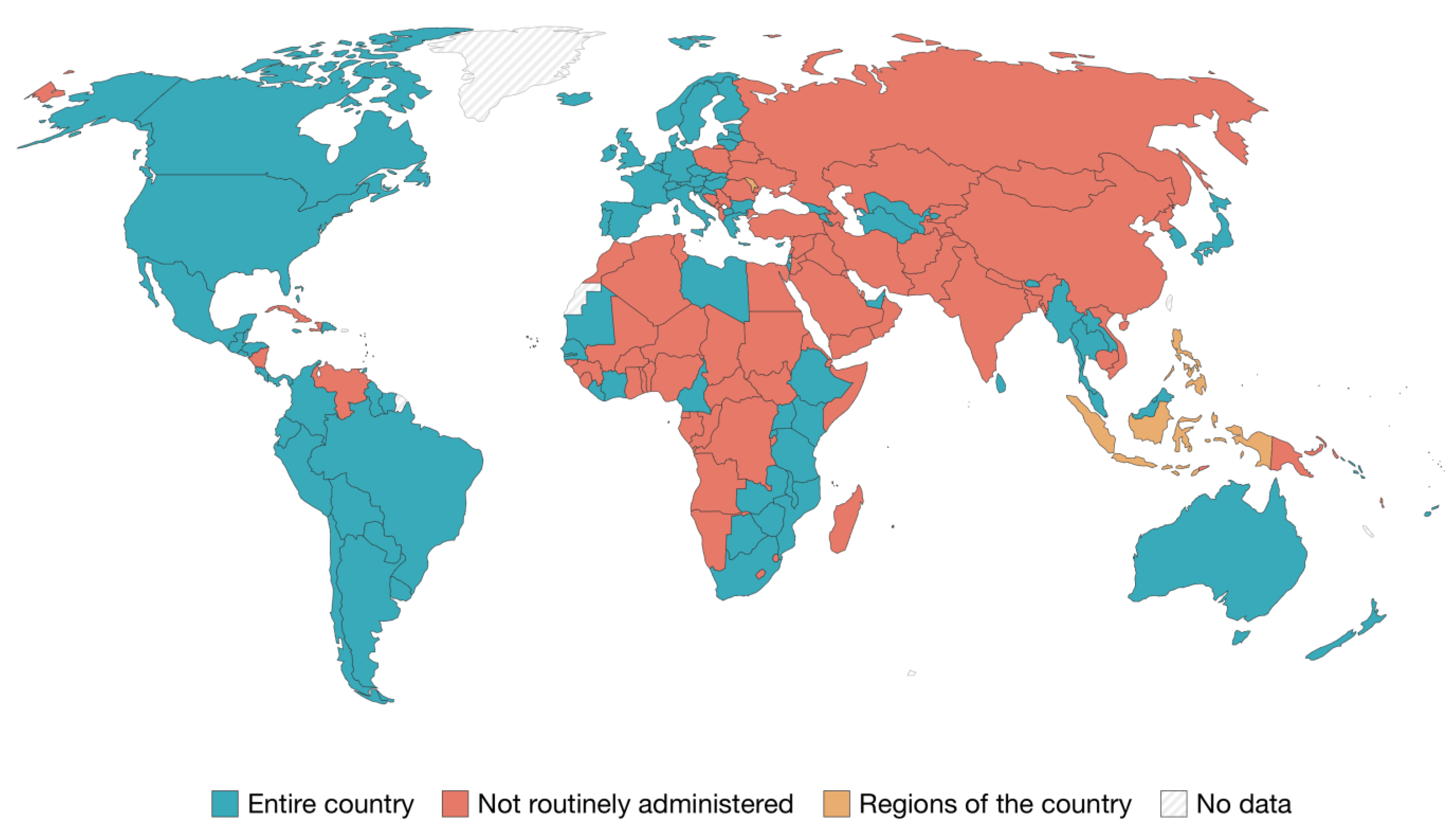
5. Prevention
5.1. Primary prevention
5.2. Secondary prevention
5.3. Tertiary prevention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nøhr, B.; Kjaer, S.K.; Soylu, L.; Jensen, A. High-risk human papillomavirus infection in female and subsequent risk of infertility: a population-based cohort study. Fertil Steril. 2019, 111, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019, 16, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Rosalik, K.; Tarney, C.; Han, J. Human Papilloma Virus Vaccination. Viruses 2021, 13, 1091. [Google Scholar] [CrossRef] [PubMed]
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 2017, 772, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med J Islam Repub Iran 2021, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 2, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A. HPV testing as a screen for cervical cancer. BMJ. 2015, 30, h2372. [Google Scholar] [CrossRef]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J Surg Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Ryndock, E.J.; Meyers, C. A risk for non-sexual transmission of human papillomavirus? Expert Rev Anti Infect Ther. 2014, 12, 1165–70. [Google Scholar] [CrossRef]
- Iorga, L.; Dragos, Marcu, R.; Cristina Diaconu, C.; et al. Penile carcinoma and HPV infection (Review). Exp Ther Med. 2020, 20, 91–96. [Google Scholar] [CrossRef]
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann Ig. 2018, 30, 28–32. [Google Scholar] [PubMed]
- Sabeena, S.; Bhat, P.; Kamath, V.; Arunkumar, G. Possible non-sexual modes of transmission of human papilloma virus. J Obstet Gynaecol Res. 2017, 43, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front Public Health. 2021, 20, 552028. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Benedetti Panici, P.; Bogani, G. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, MS.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; Velandia-González, M.; Pastore, R.; Gacic-Dobo, M.; Bloem, P. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 202, 144, 106399. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shao, H.; Zhang, T.; Pu, J.; Tang, C. Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program. Vaccines 2022, 10, 1054. [Google Scholar] [CrossRef]
- Elst, L.; Albersen, M. HPV Vaccination: Does It Have a Role in Preventing Penile Cancer and Other Preneoplastic Lesions? Semin Oncol Nurs. 2022, 38, 151284. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front Immunol. 2022, 27, 805695. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, H.; Liu, J.; Zhang, Z.; Fang, W.; Yang, Y.; Hong, S.; Xian, W.; Ma, Y.; Zhou, T.; Zhang, Y.; Zhao, H.; Huang, Y.; Zhang, L. Incidence and risk factors of second primary cancer after the initial primary human papillomavirus related neoplasms. MedComm 2020, 1, 400–409. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021, 155 (Suppl 1), 28–44. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer, World Health Organization. Incidence, prevalence and mortality rates (World) in 2020. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=23&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=0&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D (accessed on 11/02/23).
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The role of viral infection on vaginal microbiota. J Med Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, G.E.; Ricciardi, W. Verso un mondo HPV free: strategie internazionali, da implementare a livello nazionale, per l’eliminazione del cancro cervicale: il valore della prevenzione e della vaccinazione anti-HPV negli adolescenti Da: I Numeri del Cancro in Italia. AIOM-AIRTUM,2022. Available online: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf.
- Pimple, S.; Mishra, G. Cancer cervix: Epidemiology and disease burden. Cytojournal. 2022, 29, 21. [Google Scholar] [CrossRef] [PubMed]
- Efua Sackey, M.; Markey, K.; Grealish, A. Healthcare professional's promotional strategies in improving Human papillomavirus (HPV) vaccination uptake in adolescents: A systematic review. Vaccine. 2022, 26, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Sasidharanpillai, S.; Ravishankar, N.; Kamath, V.; Bhat, P.V.; Bhatt, P.; Arunkumar, G. Prevalence of Human Papillomavirus (HPV) DNA among Men with Oropharyngeal and Anogenital Cancers: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2021, 22, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.; Gray, P.; Louvanto, K.; Vänskä, S. In 30 years, gender-neutral vaccination eradicates oncogenic human papillomavirus (HPV) types while screening eliminates HPV-associated cancers. Expert Rev Vaccines. 2022, 21, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Carlander, A.F.; et al. A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses. 2021, 13, 1326. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Gabutti, G.; d'Anchera, E.; De Motoli, F.; Savio, M.; Stefanati, A. Human Papilloma Virus Vaccination: Focus on the Italian Situation. Vaccines (Basel). 2021, 9, 1374. [Google Scholar] [CrossRef]
- Zamani, M.; et al. The current epidemic of HPV-associated oropharyngeal cancer: an 18-year Danish population-based study with 2,169 patients. Eur. J. Cancer. 2020, 134, 52–59. [Google Scholar] [CrossRef]
- Del Mistro, A.; et al. Age-independent increasing prevalence of human papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci. Rep. 2020, 10, 1–10. [Google Scholar]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Seedat, R.Y. Juvenile-Onset Recurrent Respiratory Papillomatosis Diagnosis and Management - A Developing Country Review. Pediatric Health Med Ther. 2020, 11, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; Sant'Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Mastora, E.; Kitsou, C.; Evangelou, T.; Zikopoulos, A.; Zagorianakou, N.; Georgiou, I. Presence of HPV 16 and HPV 18 in Spermatozoa and Embryos of Mice. In Vivo. 2021, 35, 3203–3209. [Google Scholar] [CrossRef]
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Perinatal transmission of human papilomavirus DNA. Virol J. 2009, 21, 83. [Google Scholar] [CrossRef]
- Casalegno, J.S.; Le Bail Carval, K.; Eibach, D.; Valdeyron, M.L.; Lamblin, G.; Jacquemoud, H.; Mellier, G.; Lina, B.; Gaucherand, P.; Mathevet, P.; Mekki, Y. High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PLoS One. 2012, 7, e48137. [Google Scholar] [CrossRef]
- Palma, S.; Gnambs, T.; Crevenna, R.; Jordakieva, G. Airborne human papillomavirus (HPV) transmission risk during ablation procedures: A systematic review and meta-analysis. Environ Res. 2021, 192, 110437. [Google Scholar] [CrossRef]
- Sawchuk, W.S.; Weber, P.J.; Lowy, D.R.; Dzubow, L.M. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. Journal of the American Academy of Dermatology. 1989, 21, 41–9. [Google Scholar] [CrossRef]
- Garden, J.M.; O'Banion, M.K.; Bakus, A.D.; Olson, C. Viral disease transmitted by laser-generated plume (aerosol). Archives of dermatology 2002, 138, 1303–1307. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp Ther Med. 2020, 20, 186. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chang, Y.C.; Liu, H.W.; Chu, T.Y. Long-term persistence of human papillomavirus in environments. Gynecol Oncol. 2011, 121, 148–151. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G. Papillomavirus. In: Global Water Pathogen Project. Rose JB and Jiménez-Cisneros B (eds) (Meschke JS and Girones R (eds) Part 3 Viruses). Michigan State University, E. Lansing, MI, Unesco, 2016.
- Symonds, E.M. Viruses in raw sewage and their potential to indicate fecal pollution in coastal environments. Graduate School Theses and Dissertations 2008.
- Cantalupo, P.G.; Calgua, B.; Zhao, G.; Hundesa, A.; Wier, A.D.; Katz, J.P.; et al. Raw sewage harbors diverse viral populations. mBio 2021, 2, e00180–11. [Google Scholar] [CrossRef] [PubMed]
- Bibby, K.; Peccia, J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ- mental Science and Technology 2013, 47, 1945–1951. [Google Scholar] [CrossRef]
- Fratini, M.; Di Bonito, P.; La Rosa, G. Oncogenic papillomavirus and polyomavirus in water environments: Is there a potential for waterborne transmission? Food Environ Virol. 2014, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Della Libera, S.; Petricca, S.; Iaconelli, M.; Sanguinetti, M.; Graffeo, R.; Accardi, L.; La Rosa, G. A large spectrum of alpha and beta papillomaviruses are detected in human stool samples. J Gen Virol. 2015, 96, 607–613. [Google Scholar] [CrossRef]
- Dediol, I.; Buljan, M.; Vurnek-A Ivkoviä, M.; Bulat, V.; A Itum, M.; A Ubriloviä, A. Psychological burden of anogenital warts. J Eur Acad Dermatol Venereol. 2009, 23, 1035–8. [Google Scholar] [CrossRef]
- Tyros, G.; Mastraftsi, S.; Gregoriou, S.; Nicolaidou, E. Incidence of anogenital warts: epidemiological risk factors and real-life impact of human papillomavirus vaccination. Int J STD AIDS. 2021, 32, 4–13. [Google Scholar] [CrossRef]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Araujo Neto, C.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir Med. 2017, 126, 116–121. [Google Scholar] [CrossRef]
- Seedat, R.Y.; Dikkers, F.G. Global epidemiology of HPV-associated recurrent respiratory papillomatosis and effect of vaccination. Future Virology 2022, 17, 265–268. [Google Scholar]
- McLaughlin-Drubin, M.E.; Crum, C.P.; Münger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci U S A. 2011, 108, 2130–5. [Google Scholar] [CrossRef]
- Singh, R.K. Diffuse Non-Genital Cutaneous Warts. The American journal of tropical medicine and hygiene 2021, 106, 378–379. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, S.; Fernández-Campos, F.; Alonso, C.; Limón, D.; Halbaut, L.; Garduño-Ramirez, M.L.; Calpena, A.C.; Mallandrich, M. Topical Mucoadhesive Alginate-Based Hydrogel Loading Ketorolac for Pain Management after Pharmacotherapy, Ablation, or Surgical Removal in Condyloma Acuminata. Gels 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, K.B.; McCready, T.A. Condyloma Acuminata. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2022.
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV integration and its progression to cervical cancer. Infect Genet Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef]
- Bañuelos-Villegas, E.G.; Pérez-yPérez, M.F.; Alvarez-Salas, LM. Cervical Cancer, Papillomavirus, and miRNA Dysfunction. Front Mol Biosci. 2021, 8, 758337. [Google Scholar] [CrossRef]
- Merz, J.; Bossart, M.; Bamberg, F.; Eisenblaetter, M. Revised FIGO Staging for Cervical Cancer - A New Role for MRI. Rofo. 2020, 192, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology. 2021, 88, 187–197. [Google Scholar] [CrossRef]
- Schlenker, B.; Schneede, P. The Role of Human Papilloma Virus in Penile Cancer Prevention and New Therapeutic Agents. Eur Urol Focus. 2019, 5, 42–45. [Google Scholar] [CrossRef]
- Iorga, L.; Dragos Marcu, R.; Cristina Diaconu, C.; Maria Alexandra Stanescu, A.; Pantea Stoian, A.; Liviu Dorel Mischianu, D.; Surcel, M.; Bungau, S.; Constantin, T.; Boda, D.; Fekete, L.; Gabriel Bratu, O. Penile carcinoma and HPV infection (Review). Exp Ther Med. 2020, 20, 91–96. [Google Scholar] [CrossRef]
- Kuasne, H.; Barros-Filho, M.C.; Busso-Lopes, A.; Marchi, F.A.; Pinheiro, M.; Muñoz, J.J.; Scapulatempo-Neto, C.; et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 2017, 8, 15294–15306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chien, C.Y.; Huang, T.L.; Chiu, T.J.; Wang, Y.M.; Fang, F.M.; Li, S.H. Low p16 Cytoplasmic Staining Predicts Poor Treatment Outcome in Patients with p16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma Receiving TPF Induction Chemotherapy. Biomedicines 2023, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Bird, S.W.; Bustamante, J.P.; Leon, L.E.; Nieto, P.A.; Addae, K.; et al. A novel sequencing-based vaginal health assay combining self-sampling, HPV detection and genotyping, STI detection, and vaginal microbiome analysis. PLoS One 2019, 14, e0215945. [Google Scholar] [CrossRef] [PubMed]
- Eun, T.J.; Perkins, R.B. Screening for Cervical Cancer. The Medical clinics of North America 2020, 104, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. The Journal of infectious diseases 2021, 224, S367–S378. [Google Scholar] [CrossRef] [PubMed]
- Gualano, M.R.; Bert, F.; Voglino, G.; Buttinelli, E.; D'Errico, M.M.; De Waure, C.; Di Giovanni, P.; Fantini, M.P.; Giuliani, A.R.; Marranzano, M.; Masanotti, G.; Massimi, A.; Nante, N.; Pennino, F.; Squeri, R.; Stefanati, A.; Signorelli, C.; Siliquini, R.; Collaborating Group. Attitudes towards compulsory vaccination in Italy: Results from the NAVIDAD multicentre study. Vaccine 2018, 36, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, Z.; Rafiei, A.; Valadan, R.; Ashrafi, H.; Pasandi, M.; Kardan, M. Designing a potent L1 protein-based HPV peptide vaccine: a bioinformatics approach. Computational biology and chemistry. 2020, 85, 107209. [Google Scholar] [CrossRef]
- Soca Gallego, L.; Dominguez, A.; Parmar, M. Human Papilloma Virus Vaccine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 2017, 772, 3–12. [Google Scholar] [CrossRef]
- Panwar, K.; Godi, A.; Cocuzza, C.E.; Andrews, N.; Southern, J.; Turner, P.; Miller, E.; Beddows, S. Multiplex Human Papillomavirus L1L2 virus-like particle antibody binding assay. MethodsX. 2022, 9, 101776. [Google Scholar] [CrossRef]
- Godi, A.; Panwar, K.; Haque, M.; Cocuzza, C.E.; Andrews, N.; Southern, J.; Turner, P.; Miller, E.; Beddows, S. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine. 2019, 37, 2455–2462. [Google Scholar] [CrossRef]
- Roser, M.; Ortiz-Ospina, E.-. "Which countries include human papillomavirus (HPV) vaccines in their vaccination schedules?, 2021". (accessed on: 02/03/23). Available online: https://ourworldindata.org/grapher/human-papillomavirus-vaccine-immunization-schedule?country=BFA~ROU~ARM.
- Brotherton, J.M.; Malloy, M.; Budd, A.C.; Saville, M.; Drennan, K.T.; Gertig, D.M. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: observational cohort of young women in Australia. Papillomavirus Research 2015, 1, 59–72. [Google Scholar] [CrossRef]
- Wang, W.V.; Kothari, S.; Khoury, H.; Niccolai, L.; Garland, S.M.; Sundström, K.; de Pouvourville, G.; Bonanni, P.; Chen, Y.T.; Franco, E.L. A review of data systems for assessing the impact of HPV vaccination in selected high-income countries. Expert Rev Vaccines. 2023, 22, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Dorji, T.; Tshomo, U.; Gyamtsho, S.; Tamang, S.T.; Wangmo, S.; Pongpirul, K. Gender-neutral HPV elimination, cervical cancer screening, and treatment: Experience from Bhutan. Int J Gynecol Obstet 2022, 156, 425–429. [Google Scholar] [CrossRef]
- Dykens, J.A.; Peterson, C.E.; Holt, H.K.; Harper, D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front Public Health. 2023, 11, 1067299. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human Papillomavirus Vaccination in South Africa: Programmatic Challenges and Opportunities for Integration With Other Adolescent Health Services? Front Public Health. 2022, 10, 799984. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Liu, F.; Lian, G.; Li, S.; He, Q.; Li, T. Human papillomavirus vaccination coverage and knowledge, perceptions and influencing factors among university students in Guangzhou, China. Hum Vaccin Immunother. 2021, 17, 3603–3612. [Google Scholar] [CrossRef]
- Simms, K.T.; Hanley, S.J.B.; Smith, M.A.; Keane, A.; Canfell, K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020, 5, e223–e234. [Google Scholar] [CrossRef]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019, 68, 698–702. [Google Scholar] [CrossRef]
- Nogueira-Rodrigues, A.; Flores, M.G.; Macedo Neto, A.O.; Braga, L.A.C.; Vieira, C.M.; de Sousa-Lima, R.M.; de Andrade, D.A.P.; Machado, K.K.; Guimarães, A.P.G. HPV vaccination in Latin America: Coverage status, implementation challenges and strategies to overcome it. Front Oncol. 2022, 12, 984449. [Google Scholar] [CrossRef]
- Athanasiou, A.; Bowden, S.; Paraskevaidi, M.; Fotopoulou, C.; Martin-Hirsch, P.; Paraskevaidis, E.; Kyrgiou, M. HPV vaccination and cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2020, 65, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Biundo, E.; Courcier, S.; Damm, O.; Launay, O.; Maes, E.; Marcos, C.; Matthews, S.; Meijer, C.; Poscia, A.; Postma, M.; Saka, O.; Szucs, T.; Begg, N. A report on the status of vaccination in Europe. Vaccine. 2018, 36, 4979–4992. [Google Scholar] [CrossRef]
- Nguyen-Huu, N.H.; Thilly, N.; Derrough, T.; Sdona, E.; Claudot, F.; Pulcini, C.; Agrinier, N. ; HPV Policy working group. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine. 2020, 38, 1315–1331. [Google Scholar] [PubMed]
- Italian Communication campaign on HPV vaccination. Available at: [https://www.salute.gov.it/portale/vaccinazioni/dettaglioCampagneVaccinazioni.jsp?lingua=italiano& menu=campagne&p=dacampagne&id=167] (Accessed on: 28/03/23).
- Giuliano, A.R.; Nyitray, A.G.; Albero, G. Male circumcision and HPV transmission to female partners. Lancet. 2011, 377, 183–4. [Google Scholar] [CrossRef] [PubMed]
- Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization; 2020. Available at: https://www.who.int/publications/i/item/9789240014107 (Accessed on 29/03/23).
- Sami, J.; Lemoupa Makajio, S.; Jeannot, E.; et al. Smartphone-Based Visual Inspection with Acetic Acid: An Innovative Tool to Improve Cervical Cancer Screening in Low-Resource Setting. Healthcare (Basel). 2022, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Khairkhah, N.; Bolhassani, A.; Najafipour, R. Current and future direction in treatment of HPV-related cervical disease. J Mol Med (Berl). 2022, 100, 829–845. [Google Scholar] [CrossRef]
- Cooper, D.B.; Dunton, C.J. Colposcopy. 2022 Jul 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Bhatla, N.; Singhal, S. Primary HPV screening for cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2020, 65, 98–108. [Google Scholar] [CrossRef]
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: Acting on evidence to change the current paradigm. Preventive medicine 2017, 98, 5–14. [Google Scholar] [CrossRef]
- Ebisch, R.M.F.; Rijstenberg, L.L.; Soltani, G.G.; et al. Adjunctive use of p16 immunohistochemistry for optimizing management of CIN lesions in a high-risk human papillomavirus-positive population. Acta Obstet Gynecol Scand. 2022, 101, 1328–1336. [Google Scholar] [CrossRef]
- Stoler, M.H.; Wright, T.C. Jr.; Ferenczy, A.; Ranger-Moore, J.; Fang, Q.; Kapadia, M.; Ridder, R. Routine Use of Adjunctive p16 Immunohistochemistry Improves Diagnostic Agreement of Cervical Biopsy Interpretation: Results From the CERTAIN Study. Am J Surg Pathol. 2018, 42, 1001–1009. [Google Scholar] [CrossRef]
- Profozić, Z.; Meštrović, T.; Savić, I.; Profozić, V. Prevalence of HPV Infection in Croatian Men during a 12-year Period: a Comparative Study of External Genital and Urethral Swabs. Cent Eur J Public Health. 2016, 24, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.J.; Ma, J.H.; Zhang, F.L.; Pan, F.; Zhao, D.; Zhang, X.Y. [HPV infection of the external genitalia in men whose female partners have cervical HPV infection]. Zhonghua Nan Ke Xue. 2018, 24, 516–519. [Google Scholar] [PubMed]
- Luttmer, R.; Dijkstra, M.G.; Snijders, P.J.F.; et al. Presence of human papillomavirus in semen of healthy men is firmly associated with HPV infections of the penile epithelium. Fertil Steril. 2015, 104, 838–844e8. [Google Scholar] [CrossRef] [PubMed]
- Tuan, L.A.; Prem, K.; Pham, Q.D.; Toh, Z.Q.; Tran, H.P.; Nguyen, P.D.; et al. Anal human papillomavirus prevalence and risk factors among men who have sex with men in Vietnam. Int J Infect Dis. 2021, 112, 136–143. [Google Scholar] [CrossRef]
- Shapiro, G.K. HPV Vaccination: An Underused Strategy for the Prevention of Cancer. Curr Oncol. 2022, 29, 3780–3792. [Google Scholar] [CrossRef] [PubMed]
- Kisling, L.A.; M Das, J. Prevention Strategies. 2022 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
- Swedish, K.A.; Factor, S.H.; Goldstone, S.E. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012, 54, 891–8. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Cantor, S.B.; Fenwick, E.; Chiao, E.Y.; Nyitray, A.G.; Stier, E.A.; Goldstone, S.E.; Wilkin, T.; Chhatwal, J. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: The time is now. Vaccine. 2017, 35, 5102–5109. [Google Scholar] [CrossRef]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- Michalczyk, K.; Misiek, M.; Chudecka-Głaz, A. Can Adjuvant HPV Vaccination Be Helpful in the Prevention of Persistent/Recurrent Cervical Dysplasia after Surgical Treatment? -A Literature Review. Cancers (Basel). 2022, 14, 4352. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Bogani, G.; Cavallari, E.N.; Palaia, G.; Perniola, G.; Ralli, M.; et al. HPV Vaccination after Primary Treatment of HPV-Related Disease across Different Organ Sites: A Multidisciplinary Comprehensive Review and Meta-Analysis. Vaccines (Basel). 2022, 10, 239. [Google Scholar] [CrossRef]
- Kin Cho Goon, P.; Scholtz, L.U.; Sudhoff, H. Recurrent respiratory papillomatosis (RRP)-time for a reckoning? Laryngoscope Investig Otolaryngol. 2017, 2, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Swedish, K.A.; Goldstone, S.E. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014, 9, e93393. [Google Scholar] [CrossRef] [PubMed]
- Goon, P.; Sauzet, O.; Schuermann, M.; Oppel, F.; Shao, S.; Scholtz, L.U.; Sudhoff, H.; Goerner, M. Recurrent Respiratory Papillomatosis (RRP)-Meta-analyses on the use of the HPV vaccine as adjuvant therapy. NPJ Vaccines. 2023, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Husein-ElAhmed, H. Could the human papillomavirus vaccine prevent recurrence of ano-genital warts?: a systematic review and meta-analysis. Int J STD AIDS. 2020, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Jung, A.C.; Fakhry, C. Primary, secondary and tertiary prevention of human papillomavirus-driven head and neck cancers. Eur J Cancer. 2017, 78, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; Wentz, A.; Posner, M.R.; Gross, N.D.; Haddad, R.I.; Gillison, M.L.; et al. Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol 2015, 1, 907e15. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Qualliotine, J.R.; Zhang, Z.; Agrawal, N.; Gaykalova, D.A.; Bishop, J.A.; et al. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratifica- tion and recurrence of HPV-associated oropharyngeal cancer. Cancer Prev Res (Phila) 2016, 9, 135e41. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, K.R.; Li, G.; Hussey, C.S.; Vo, J.T.; Wei, Q.; Zhao, C.; et al. Circulating human papillomavirus DNA as a marker for disease extent and recurrence among patients with oropharyngeal cancer. Cancer 2015, 121, 3455e64. [Google Scholar] [CrossRef]
- Lin, J.C.; Wang, W.Y.; Chen, K.Y.; Wei, Y.H.; Liang, W.M.; Jan, J.S.; et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 2004, 350, 2461e70. [Google Scholar] [CrossRef]
- Twu, C.W.; Wang, W.Y.; Liang, W.M.; Jan, J.S.; Jiang, R.S.; Chao, J.; et al. Comparison of the prognostic impact of serum anti-EBV anti- body and plasma EBV DNA assays in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2007, 67, 130e7. [Google Scholar] [CrossRef]
- Chrysostomou, A.C.; Stylianou, D.C.; Constantinidou, A.; Kostrikis, LG. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses. 2018, 10, 729. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




