Submitted:
18 April 2023
Posted:
19 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
3. Discussion
3.1. Сhemical composition of the sea buckthorn varieties analysed
3.2. Antimicrobial activity of sea buckthorn fruits and puree
4. Materials and Methods
4.1. Materials
4.2. Chemical Materials
4.3. Content of biologically active substances
4.3.1. Ascorbic acid content
4.2.2. Organic acids
4.2.3. Carotenoid content
4.4. Physicochemical Analysis
4.5. Antimicrobial activity
4.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bal, L.M.; Meda, V.; Naik, S.N.; Satya, S. Sea Buckthorn Berries: A Potential Source of Valuable Nutrients for Nutraceuticals and Cosmeceuticals.
- Dong, R.; Su, J.; Nian, H.; Shen, H.; Zhai, X.; Xin, H.; Qin, L.; Han, T. Chemical Fingerprint and Quantitative Analysis of Flavonoids for Quality Control of Sea Buckthorn Leaves by HPLC and UHPLC-ESI-QTOF-MS. Journal of Functional Foods 2017, 37, 513–522. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic Compounds Extracted by Acidic Aqueous Ethanol from Berries and Leaves of Different Berry Plants. Food Chemistry 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Sharma, U. K.; Sharma, K.; Sharma, N.; Sharma, A.; Singh, H. P.; Sinha, A. K. Microwave-Assisted Efficient Extraction of Different Parts of Hippophae Rhamnoides for the Comparative Evaluation of Antioxidant Activity and Quantification of Its Phenolic Constituents by Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC). J. Agric. Food Chem. 2008, 56, 374–379. [Google Scholar] [CrossRef]
- Beveridge, T.; Li, T. S. C.; Oomah, B. D.; Smith, A. Sea Buckthorn Products: Manufacture and Composition. J. Agric. Food Chem. 1999, 47, 3480–3488. [Google Scholar] [CrossRef]
- Jaśniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae Rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F. V.; Focşan, M.; Rugină, D. O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2019, 12. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E.; Ozdemir, O.; Sengul, M. The Genotypic Effects on the Chemical Composition and Antioxidant Activity of Sea Buckthorn (Hippophae Rhamnoides L.) Berries Grown in Turkey. Scientia Horticulturae 2007, 115, 27–33. [Google Scholar] [CrossRef]
- Zhang W.; Yan J.; Duo J.; Ren B.; Guo J. Preliminary study of biochemical constitutions of berry of sea buckthorn growing in Shanxi province and their changing trend; Proceedings of the International Symposium on Sea Buckthorn (H. rhamnoides L.); Xi’an, China. 19–23 October 1989; pp. 96–105.
- Solà Marsiñach, M.; Cuenca, A. P. The Impact of Sea Buckthorn Oil Fatty Acids on Human Health. Lipids Health Dis 2019, 18. [Google Scholar] [CrossRef]
- Sandulachi, E.; Bulgaru, V.; Ghendov-Mosanu, A.; Sturza, R. Controlling the Risk of Bacillus in Food Using Berries. FNS 2021, 12, 557–577. [Google Scholar] [CrossRef]
- Sandulachi, E.; Cojocari, D.; Balan, G.; Popescu, L.; Ghendov-Moșanu, A.; Sturza, R. Antimicrobial Effects of Berries on Listeria Monocytogenes. FNS 2020, 11, 873–886. [Google Scholar] [CrossRef]
- Sturza, R.; Sandulachi, E.; Cojocari, D.; Balan, G.; Popescu, L.; Ghendov-Mosanu, A. ANTIMICROBIAL PROPERTIES OF BERRY POWDERS IN CREAM CHEESE. 2019. [CrossRef]
- Cojocari, D.; Sturza, R.; Sandulachi, E.; Macari, A.; Balan, G.; Ghendov-Mosanu, A. INHIBITING OF ACCIDENTAL PATHOGENIC MICROBIOTA IN MEAT PRODUCTS WITH BERRY POWDERS. 2019. [CrossRef]
- Kohli, K. New Insights towards Implications of Sea Buckthorn Oil in Human Health: A Review. IPCM 2018, 2. [Google Scholar] [CrossRef]
- Mohan Gupta, S.; K Gupta, A.; Ahmed, Z. Antibacterial and Antifungal Activity in Leaf, Seed Extract and Seed Oil of Seabuckthorn (Hippophae Salicifolia D. Don) Plant. J Plant Pathol Microbiol 2011, 02. [Google Scholar] [CrossRef]
- Schubertová, S.; Krepsová, Z.; Janotková, L.; Potočňáková, M.; Kreps, F. Exploitation of Sea Buckthorn Fruit for Novel Fermented Foods Production: A Review. Processes 2021, 9. [Google Scholar] [CrossRef]
- Kallio, H.; Yang, W.; Liu, P.; Yang, B. Proanthocyanidins in Wild Sea Buckthorn ( Hippophaë Rhamnoides ) Berries Analyzed by Reversed-Phase, Normal-Phase, and Hydrophilic Interaction Liquid Chromatography with UV and MS Detection. J. Agric. Food Chem. 2014, 62, 7721–7729. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A.; Rudzińska, M.; Oszmiański, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea Buckthorn ( Hippophaë Rhamnoides L.) Berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A. C.; Bunea, A.; Pripon Furtuna, F. R.; Olah, N. K.; Madden, R. H.; Corcionivoschi, N. Phytochemical Composition and Biological Activity of Berries and Leaves from Four Romanian Sea Buckthorn (Hippophae Rhamnoides L.) Varieties. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Gâtlan, A.-M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. IJERPH 2021, 18. [Google Scholar] [CrossRef]
- Terechuk, L.; Starovoytova, K.; Ivanova, S.; Sergeeva, I. Obtaining Functional Products from Sea Buckthorn Berries. In Proceedings of the 2nd International Conference on Education Science and Social Development (ESSD 2019); Atlantis Press: Changsha, China, 2019. China. [CrossRef]
- Yang, B.; Kallio, H. P. Fatty Acid Composition of Lipids in Sea Buckthorn ( Hippophaë Rhamnoides L.) Berries of Different Origins. J. Agric. Food Chem. 2001, 49, 1939–1947. [Google Scholar] [CrossRef]
- Yue, X.-F.; Shang, X.; Zhang, Z.-J.; Zhang, Y.-N. Phytochemical Composition and Antibacterial Activity of the Essential Oils from Different Parts of Sea Buckthorn (Hippophae Rhamnoides L.). Journal of Food and Drug Analysis 2017, 25, 327–332. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn ( Hippophae Rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Qadir, M. I.; Abbas, K.; Younus, A.; Shaikh, R. S. Report - Antibacterial Activity of Sea Buckthorn (Hippophae Rhamnoides L.) against Methicillin Resistant Staphylococcus Aureus (MRSA). Pak J Pharm Sci 2016, 29, 1711–1713. [Google Scholar] [PubMed]
- Smida, I.; Pentelescu, C.; Pentelescu, O.; Sweidan, A.; Oliviero, N.; Meuric, V.; Martin, B.; Colceriu, L.; Bonnaure-Mallet, M.; Tamanai-Shacoori, Z. Benefits of Sea Buckthorn ( Hippophae Rhamnoides ) Pulp Oil-based Mouthwash on Oral Health. J Appl Microbiol 2019, 126, 1594–1605. [Google Scholar] [CrossRef]
- Jeong, J. H. Antioxidant and Antimicrobial Activities of Extracts from a Medicinal Plant, Sea Buckthorn. J. Korean Soc. Appl. Biol. Chem. 2009, 53, 33–38. [Google Scholar] [CrossRef]
- Ivanišová, E.; Blašková, M.; Terentjeva, M.; Grygorieva, O.; Vergun, O.; Brindza, J.; Kačániová, M. Biological Properties of Sea Buckthorn (Hippophae Rhamnoides L.) Derived Products [Pdf]. Acta Sci Pol Technol Aliment 2020, 19, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M. E.; Elfakir, C. Antimicrobial, Antioxidant and Phytochemical Investigations of Sea Buckthorn (Hippophaë Rhamnoides L.) Leaf, Stem, Root and Seed. Food Chemistry 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Upadhyay, N. K.; Yogendra Kumar, M. S.; Gupta, A. Antioxidant, Cytoprotective and Antibacterial Effects of Sea Buckthorn (Hippophae Rhamnoides L.) Leaves. Food and Chemical Toxicology 2010, 48, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Richa Arora. Antimicrobial Activity of Seed, Pomace and Leaf Extracts of Sea Buckthorn (Hippophae Rhamnoides L.) against Foodborne and Food Spoilage Pathogens. Afr. J. Biotechnol. 2012, 11. [Google Scholar] [CrossRef]
- Kasparaviciene, G.; Briedis, V.; Ivanauskas, L. [Influence of sea buckthorn oil production technology on its antioxidant activity]. Medicina (Kaunas) 2004, 40, 753–757. [Google Scholar]
- Sandulachi, E.; Macari, A.; Cojocari, D.; Balan, G.; Popa, S.; Turculet, N.; Ghendov-Mosanu, A.; Sturza, R. ANTIMICROBIAL PROPERTIES OF SEA BUCKTHORN GROWNIN THE REPUBLIC OF MOLDOVA. JES 2022, 29, 164–175. [Google Scholar] [CrossRef]
- Michalak, M.; Paradowska, K. ; Zielińska, A. Selected plant oils as a source of carotenoids for the applications in cosmetology Postępy Fitoterapii 2018 No.1 pp.10-17.
- Mendelová, A.; Mendel, Ľ.; Czako, P.; Mareček, J. Evaluation of Carotenoids, Polyphenols Content and Antioxidant Activity in the Sea Buckthorn Fruit. Potr. S. J. F. Sci. 2016, 10, 59–64. [Google Scholar] [CrossRef]
- Beveridge, T. Chemical composition and some physical properties. In Sea Buckthorn (Hippophaë rhamnoides L.): Production and Utilization, 2003, 79-88. Ottawa, ON: NRC Research Press.
- R. Yakimishen; S. Cenkowski; W. E. Muir. OIL RECOVERIES FROM SEA BUCKTHORN SEEDS AND PULP. Applied Engineering in Agriculture 2005, 21, 1047–1055. [Google Scholar] [CrossRef]
- Lõugas, T. Study on Physico-Chemical Properties and Some Bioactive Compounds of Sea Buckthorn (Hippophae Rhamnoides L.); Thesis on natural and exact sciences; TUT Press: Tallinn, 2006. [Google Scholar]
- Kumar, R.; Kumar, G. P.; Chaurasia, O.; Bala Singh, S. Phytochemical and Pharmacological Profile of Seabuckthorn Oil: A Review. Research J. of Medicinal Plant 2011, 5, 491–499. [Google Scholar] [CrossRef]
- Munkhbayar, D.; Ariuntungalag, J.; Delgersuuri, G.; Badamkhand, D. Enzymatic Technology for Sea Buckthorn Oil Extraction and Its Biochemical Analysis. Mong. J. Chem. 2014, 15, 62–65. [Google Scholar] [CrossRef]
- Beveridge, T.; Harrison, J. E.; Drover, J. Processing Effects on the Composition of Sea Buckthorn Juice from Hippophae Rhamnoides L. Cv. Indian Summer. J. Agric. Food Chem. 2002, 50, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Yong Hoon Lee, Hee Joo Jang, Kun Hee Park, Seon-Hee Kim, Jung Kyu Kim, Jin-Chul Kim, Tae Su Jang, Ki Hyun Kim Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives Plants 2021, 10(5), 860. I. [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J. G.; Walker, R. P. The Organic Acids That Are Accumulated in the Flesh of Fruits: Occurrence, Metabolism and Factors Affecting Their Contents – a Review. rchsh 2015, XXI (2), 97–128. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.-H.; Drincovich, M. F.; Farinelli, D.; Lara, M. V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R. P. Stone Fruits: Growth and Nitrogen and Organic Acid Metabolism in the Fruits and Seeds—A Review. Front. Plant Sci. 2020, 11, 572601. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cui, Y.; Feng, G. Studies on the fruit character and biochemical compositions of some forms within Chinese sea buckthorn (Hippophae rhamnoids ˙ subsp. sinensis). In Proceedings of the International Symposium on Sea Buckthorn (H. rhamnoides L.), Xi’an, China, 19–23 October 1989; pp. 106–113.
- Kallio, K.; Yang, B.R.; Tahvonen, R.; Hakala, M. Composition of sea buckthorn berries of various origins. In Proceedings of the International Symposium on Sea Buckthorn (Hippophae rhamnoids L.), Beijing, China, 29 August 1999. [Google Scholar]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Molecular Aspects of Medicine 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Roshan Kumar Shah, Aniket Idate, Poorva Sharma Review on Antimicrobial Properties of Sea Buckthorn Hippophae Rhamnoides L International Journal of All Research Education and Scientific Methods (IJARESM), ISSN: 2455-6211 Volume 8, Issue 12, December-2020. 2.
- Merhan, O. The Biochemistry and Antioxidant Properties of Carotenoids. In Carotenoids; Cvetkovic, D. J., Nikolic, G. S., Eds.; InTech, 2017. [CrossRef]
- Kopsell, D.; Kopsell, D. Accumulation and Bioavailability of Dietary Carotenoids in Vegetable Crops. Trends in Plant Science 2006, 11, 499–507. [Google Scholar] [CrossRef]
- Gammone, M.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Marine Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Stephen Clark, in xPharm: The Comprehensive Pharmacology Reference, 2007.
- Kasparaviciene, G.; Briedis, V.; Ivanauskas, L. [Influence of sea buckthorn oil production technology on its antioxidant activity]. Medicina (Kaunas) 2004, 40, 753–757. [Google Scholar]
- Šnē, E.; Galoburda, R.; Segliņa, D. Sea Buckthorn Vegetative Parts – A Good Source of Bioactive Compounds. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences 2013, 67, 101–108. [Google Scholar] [CrossRef]
- Siriangkhawut, W. Electrochemical Analysis of Ascorbic Acid in Commercial Fruit Juices and Drinks. Asian J. Chem. 2014, 26, 6487–6491. [Google Scholar] [CrossRef]
- Ferey, L.; Delaunay, N. Food Analysis on Electrophoretic Microchips. Separation & Purification Reviews 2016, 45, 193–226. [Google Scholar] [CrossRef]
- Wang, M.; Qu, F.; Shan, X.-Q.; Lin, J.-M. Development and Optimization of a Method for the Analysis of Low-Molecular-Mass Organic Acids in Plants by Capillary Electrophoresis with Indirect UV Detection. Journal of Chromatography A 2003, 989, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rovio, S.; Sirén, K.; Sirén, H. Application of Capillary Electrophoresis to Determine Metal Cations, Anions, Organic Acids, and Carbohydrates in Some Pinot Noir Red Wines. Food Chemistry 2011, 124, 1194–1200. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K. N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC INTERNATIONAL, 22nd ed.; Latimer, G. W., Ed.; Oxford University PressNew York, 2023. [CrossRef]
- Hudzicki, J. (2016) Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology, Washington DC, 23.
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M. E.; Elfakir, C. Antimicrobial, Antioxidant and Phytochemical Investigations of Sea Buckthorn (Hippophaë Rhamnoides L.) Leaf, Stem, Root and Seed. Food Chemistry 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]


| Sea buckthorn species | Samples | Chemical indicators (CI), mg/100g | ||||||
|---|---|---|---|---|---|---|---|---|
| Carotenoid content (CC), | Ascorbic acid content (AAC), | Organic acids (OA), | ||||||
| mean | min-max | mean | min-max | malic | citric | succinic | ||
| Clara | 42 | 7.88±0.54 | 5.72±0.56 -11.13±0.53 | 151.63±0.88 | 99.08±0,41 -193.85±1.36 | 11,90±0.004 | 0,20±0.001 | 1,1±0.001 |
| Dora | 42 | 23.16±0.52 | 18.50±0.43 -48.92±0.61 | 254.16±1.95 | 198.68±1,64 -322.11±2.26 | 5,80±0.002 | 0,08±0.001 | 0,36±0.001 |
| Cora | 42 | 7.13±0.50 | 1.79±0.43 - 4.92±0.57 | 113.14±0.81 | 74.36±0,60 -241.83±1.02 | 9,60±0.002 | 0,09±0.001 | 0,72±0.001 |
| Mara | 42 | 13.93±0.15 | 7.37±0.17 - 18.77±0.13 | 278.71±2.03 | 248.52±1.78 -373.38±2.29 | 13,40±0.002 | 0,32±0.01 | 0,03±0.001 |
| Average fruit values | 17,03±0.43 | 1.79±0.43-48.92±0.61 | 199.34±1.42 | 74.36±0,60-373.38±2.29 | 10,18±0.002 | 0,17±0.002 | 0,55±0.002 | |
| Indicator tested | Sea buckthorn species | |||
|---|---|---|---|---|
| Clara | Dora | Cora | Mara | |
| TDM,% | ||||
| Min–Max | 16.22±0,02…20.37±0.08 | 14.19±0.09… 20.50±0.03 | 17.22±0.07…24.54±0.09 | 16.71±0.05…24.20±0.06 |
| Mean | 17.34±0.04 | 16.74±0.06 | 17.22±0.08 | 18.78±0.05 |
| TA,% | ||||
| Min–Max | 2.57±0,04... 4.73±0.25 | 2.15±0,05...5.55±0,00 | 3.59±0,23...8.76±0,00 | 4.52±0,10...7.21±0.14 |
| Mean | 3.25±14 | 3.22±0.03 | 4.94±0.12 | 5.92±0.12 |
| pH | ||||
| Min–Max | 2.77±0.03 ... 3.00±0.07 | 2.87±0.02...2.99±0.03 | 2.66±0.01..2.87±0.05 | 2.73±0.02...2.98±0.02 |
| Mean | 2.88±-.0.05 | 3.00±0.02 | 2.76±0.03 | 2.84±0.02 |
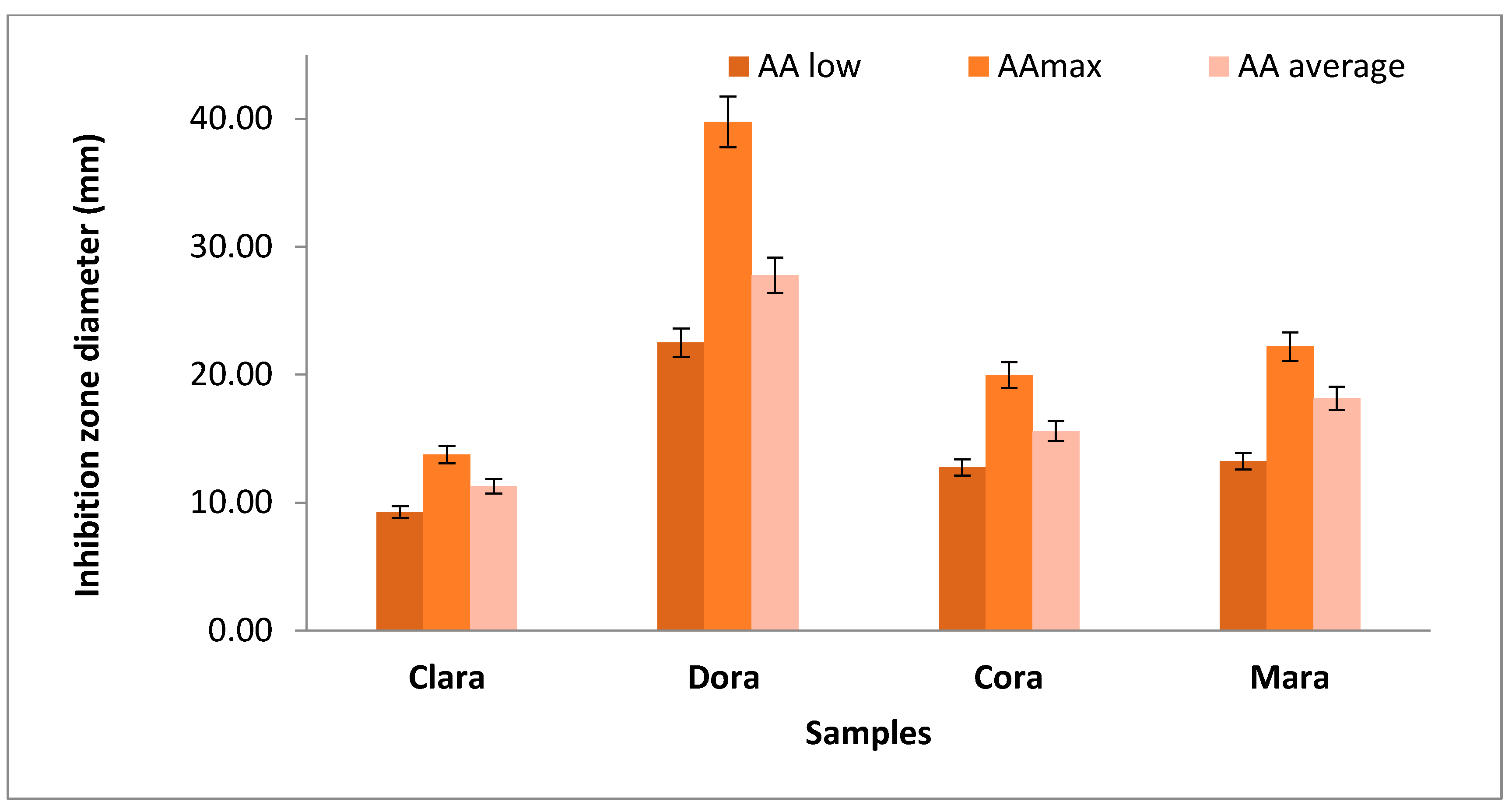
| Sea buckthorn species | Fruit mass, g | Antibacterial activity (AA) (Diameter of inhibition zone in mm) | Pearson coefficient Pc = f(AAB. pumilus and PCI) |
|||||
|---|---|---|---|---|---|---|---|---|
| mean | min–max | TDM | CC | AAC | TA | pH | ||
| Whole sea buckthorn fruits | ||||||||
| Clara | 0.28 ….0.30 | 13.10 | 7.60 ….15.40 | |||||
| 0.10* | 4.52 | 3.70 …5.50 | 0.8520 | 0.8488 | 0.5738 | 0.9762 | - 0.9524 | |
| 0.25* | 11.29 | 9.25 …13.75 | 0.8525 | 0.8473 | 0.5727 | 0.9766 | - 0.9534 | |
| Dora | 0.16 … 0.19 | 13.33 | 10.80 …17.50 | |||||
| 0.10* | 11.11 | 9.00 …15.91 | 0.9791 | 0.9791 | 0.9791 | 1.0000 | - 0.9628 | |
| 0.25* | 27.78 | 22.50 …39.77 | 0.9758 | 0.9758 | 0.9758 | 1.0000 | - 0.9758 | |
| Cora | 0.16 …0.19 | 11.03 | 9.70 …11.90 | |||||
| 0,10* | 6.25 | 5.11 …7.19 | 0.7154 | 0.7179 | 0.9689 | 0.9689 | - 0.9952 | |
| 0,25* | 15.61 | 12.76…17.97 | 0.8417 | 0.8443 | 0.9766 | 0.9927 | - 0.8429 | |
| Mara | 0.16 … 0.20 | 12.83 | 10.60 …14.20 | |||||
| 010* | 7.26 | 5.30 …8.88 | 0.9261 | 0.9209 | 0.8714 | 0.9706 | - 0.9280 | |
| 0.25* | 18.16 | 13.25 …22.19 | 0.9254 | 0.9202 | 0.8704 | 0.9704 | - 0.9273 | |
| Sea buckthorn puree | ||||||||
| Clara | 0.14…0,15 | 21.50 | 20.00…23.60 | |||||
| 0.10* | 15.00 | 13.33…16.86 | 0.8232 | 0.8526 | 0.5174 | 0.9883 | - 0.9588 | |
| 0.25* | 37.50 | 33.33…42.14 | 0.8233 | 0.8528 | 0.5177 | 0.9888 | - 0.9886 | |
| Dora | 0.07 …0.12 | 18.07 | 17.60…17.60 | - 0.9582 | ||||
| 0.10* | 17.48 | 15.00…26.14 | 0.9577 | 0.9577 | 0.9577 | 1.000 | - 0.9582 | |
| 0.25* | 43.71 | 37.50…62.86 | 0.9578 | 0.9578 | 0.9578 | 1.000 | - 0.9583 | |
| Cora | 0.09 …0.17 | 24.10 | 20.80…27.50 | |||||
| 0.10* | 19.54 | 16.18…26.67 | 0.7174 | 0.7552 | 0.7178 | 0.7061 | - 0.7720 | |
| 0.25* | 48.85 | 40.44…66.67 | 0.7176 | 0.7554 | 0.7781 | 0.7062 | - 0.7720 | |
| Mara | 0.10 | 19.30 | 17.20…21.40 | |||||
| 0.10* | 19.30 | 17.20…21.40 | 0.9035 | 0.9940 | 0.9035 | 0.7780 | - 0.9047 | |
| 0.25* | 48.25 | 44.75…53.50 | 0.9035 | 0.9940 | 0.9035 | 0.7780 | - 0.9450 | |
| Properties | Investigation method | Target microorganisms | Source |
|---|---|---|---|
| Antibacterial activity | Standard disc diffusion method | Staphylococcus aureus | Muhammad Imran Qadir et al. [26] |
| Antimicrobial | Streptococcus gordonii, Porphyromonas gingivalis, Actinomyces viscosus and Candida albicans. | Smida et al. [27] | |
| antimicrobial activity | Candida albicans, Pichia jadinii, Bacillus subtilis and Staphilococcus aureus | Jeong, J.H et al. [28] | |
| Antiviral, antibacterial activity, fungal strains | Inhibition zone diameter, IS(50) | Staphilococcus aureus, Haemophilus influenzae, Streptococcus agalactiae, Streptococcus pyogenes, Streptococcus pneumoniae | Heikki Kallio]. [18] |
| Antibacterial property | Diffusion method, Minim inhibition concentration (MIC) |
Escherichia coli, Salmonella enterica.Yersinia enterocolitica, Bacillus thuringiensis, Listeria monocytogenes, Stapylococcus aureus | Ivanišová, E. et al. [29] |
| Antibacterial property andfungal strains | gram positive (Bacillus cereus, Enterococcus durans, Enterococcus faecalis, Staphilococcus aureus), gram negative (Aeromonas hydrophila, Bacillus subtilus, Escherichia coli, Pseudomonas aeruginosa, Pseudomonas fluorescens, Pichia jadinii, Salmonella enterica, Salmonella typhimurium, Yersinia enterolitica) bacteria and yeast (Candida albicans). |
Jeong, J.H et al. [28]; Michel et al. [30]; Upadhyay et al. [31]; Arora et al. [32]; |
|
| Antimicrobial activity | Escherichia coli | Giedre Kasparaviciene [33] | |
| Antimicrobial activity | Inhibition zone, Minim inhibition concentration (MIC) | Staphylococcus aureus, Bacillus subtilis, Salmonella Typhimurium, Escherichia coli, Candida albicans | Sandulachi et al. [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





