Introduction
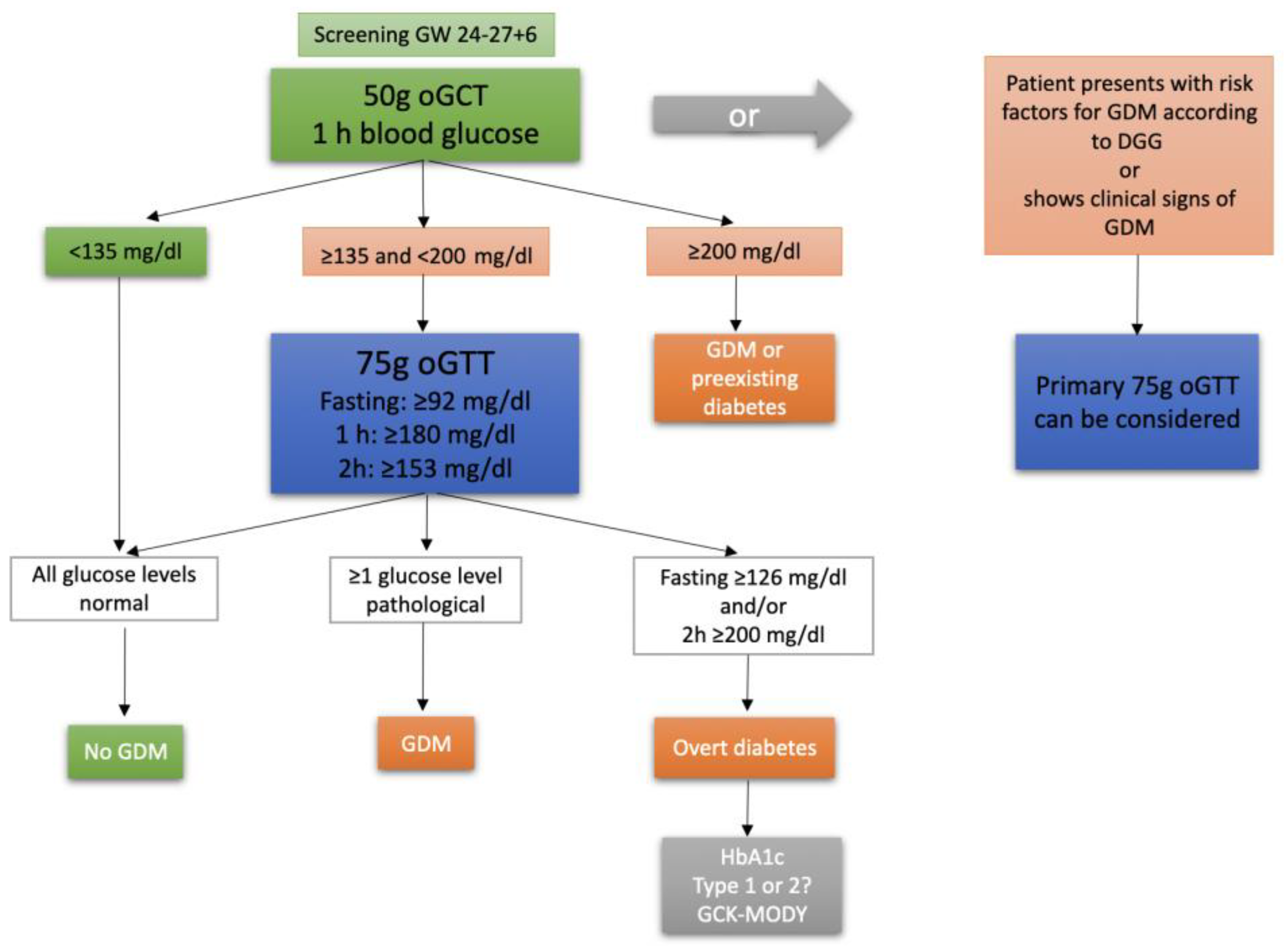
Gestational diabetes mellitus (GDM) is a common complication during pregnancy. In Germany, the incidence of GDM rose from 4,6% of all hospital deliveries in 2013 to 6,8% in 2018 (1). GDM is defined as an impairment of glucose tolerance that has been diagnosed for the first-time during pregnancy (2). With its prevalence rising and its well-known associations with other various pregnancy complications, such as preeclampsia, cesarean section (CS), macrosomia, shoulder dystocia, childbirth injury, postpartum hemorrhage or premature birth, understanding GDM fully is key to improve prenatal care and minimize the risks for mother and child (3-7). GDM is in general still treated as a homogenous disease during pregnancy, although research has shown that a more differentiated approach might be needed, as phenotypical subtypes of the condition seem to be associated with different perinatal outcomes. A possible approach that has been suggested is to focus on the extent of insulin sensitivity and insulin secretion impairment or to differentiate between the two (8-11). Another, possibly more practicable, approach is to differentiate GDM subtypes based on the glucose levels observed in the 3-point 75g oral glucose tolerance test (oGTT), which is conducted in the late second to early third trimester of pregnancy and comprises blood glucose measurements fasting, one and two hours after ingestion of a 75g glucose solution (
Figure 1) (2). The HAPO study demonstrated an association of maternal plasma glucose levels with large for gestational age (LGA) offspring, primary CS, shoulder dystocia or birth injury, preeclampsia and other adverse outcomes (12). Therefore, the correlation of 75g oGTT glucose values and fetomaternal outcome has been the subject of several studies (5, 13-15). The aim of our study is to corroborate and add evidence to the recent findings by assessing the characteristics of GDM subtypes (isolated fasting hyperglycemia=GDM-IFH, isolated post-load hyperglycemia=GDM-IPH, combined hyperglycemia=CH based on oGTT glucose values) and their respective risk of adverse maternal and fetal outcomes.
Material and Methods
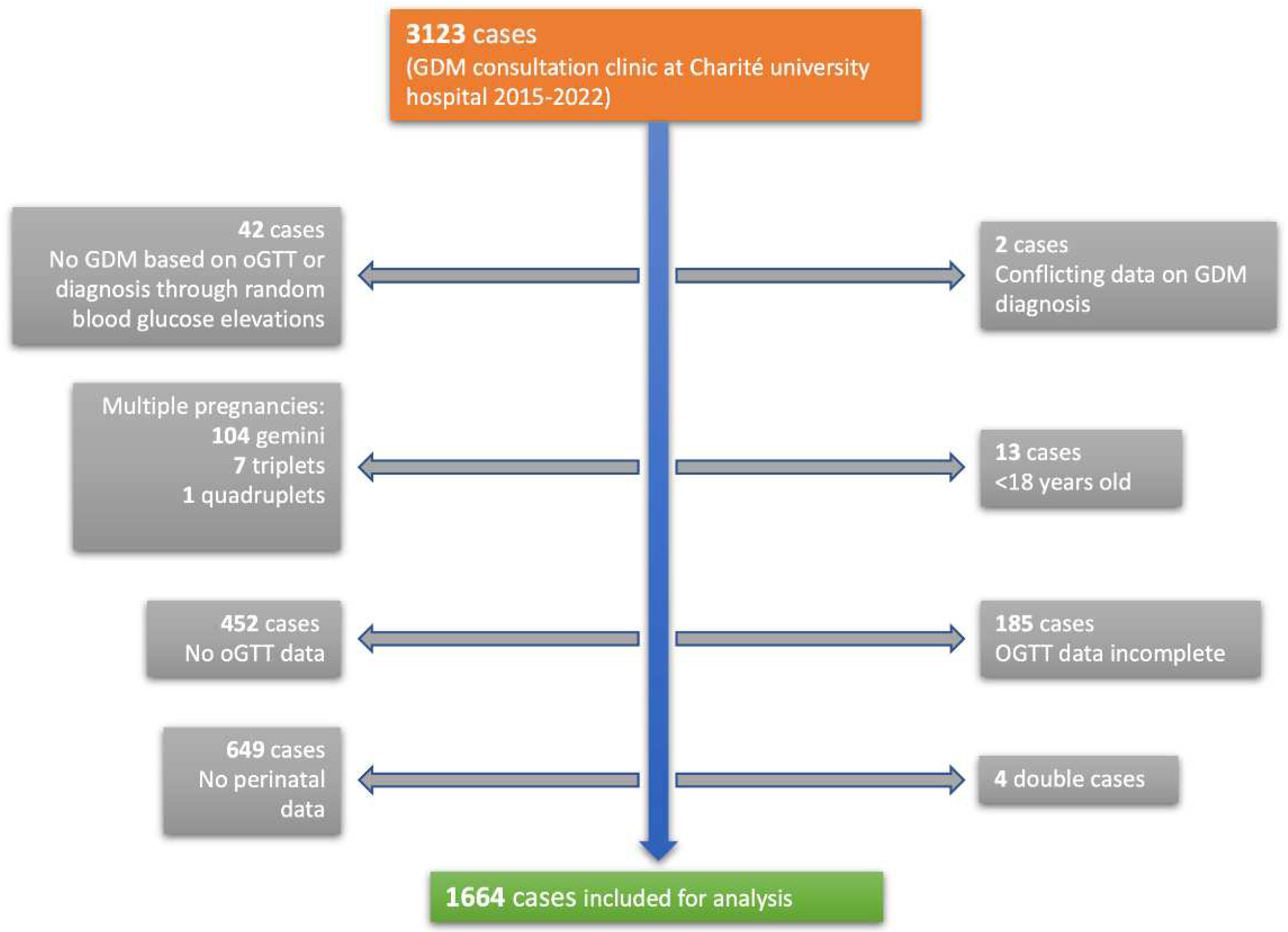
Obstetric data from 3123 pregnant women, visiting an expert gestational diabetes consultation clinic at Charité University Hospital from January 2015 to September 2022, was collected and analyzed anonymously. The Charité University Hospital is a tertiary perinatal center in the metropole region of Berlin, Germany. 1664 patients were eligible for the analysis (
Figure 2). Inclusion criteria were women ≥ 18 years with singleton pregnancies who were screened prior with a pathological glucose response in the 75g oGTT and were subsequently diagnosed with GDM. Only women who delivered their babies at Charité university hospital were analyzed. Exclusion criteria were multiple pregnancies, age < 18 years, missing or incomplete oGTT data, missing perinatal data and inconclusive documentation of GDM diagnosis. Women who were diagnosed through random elevated blood glucose levels were excluded as well. Patients were categorized into three different groups using the three blood glucose values from the 75g oGTT. Thresholds for pathological glucose levels were ≥ 92 mg/dl fasting, ≥ 180 mg/dl one hour after glucose application and ≥ 153 mg/dl two hours after according to IADPSG criteria (16). An elevation in fasting glucose only, which was measured immediately before application of the glucose solution, was considered isolated fasting hyperglycemia (GDM-IFH). If just one or both postprandial glucose values were elevated, this was considered isolated post-load hyperglycemia (GDM-IPH) and an elevation in fasting glucose and at least one of the postprandial glucose values was categorized as combined hyperglycemia (GDM-CH). The primary aim of the analysis was to assess the likelihood of delivering via cesarean section based on the glucose values of the oGTT that was performed in the second trimester. The secondary objective was to analyze maternal and fetal outcome parameters, such as vaginal operative birth, shoulder dystocia, perineal tear grade 3° and the need for episiotomy, blood loss and postpartum bleeding, and preeclampsia. Fetal outcomes included gestational age at delivery, birth weight and percentiles, fetal growth abnormalities such as intrauterine growth retardation (IUGR), small for gestational age (SGA), LGA (defined as growth ≥ 95th percentile) and low fetal weight (defined as growth < 30th percentile), premature delivery before 37 gestational weeks (GW), intrauterine fetal demise (IUFD) and the need for intensive neonatal care admission after delivery. Cord pH levels and base excess were evaluated.
This study received ethical approval from the ethics committee of Charite university hospital on February 6th, 2023 (EA2/255/22).
Data analysis was performed using SPSS Statistics by IBM (version 28.0.1.0). Categorical variables were compared among the subgroups using Chi-square-tests and binomial logistic regression, and numbers and percentages were reported. Binomial logistic regression was conducted for outcome variables which showed significant differences among the subgroups and included subtype (categorical), preconceptional BMI (< 18.5=underweight, 18.5-23.9=normal weight, 24-27.9=overweight, 28-31.9=obese, ≥ 32=severely obese), age as a continuous variable and parity (nullipara vs. ≥ primipara) as co-variates. Odds ratios and 95% confidence intervals were calculated and reported. Metric variables were compared using one-way ANOVA with subsequent post-hoc-analysis (Tukey and Games-Howell) and mean and standard deviation (SD) were reported. Results were considered statistically significant if the p-value was <0,05.
Results
Baseline Characteristics
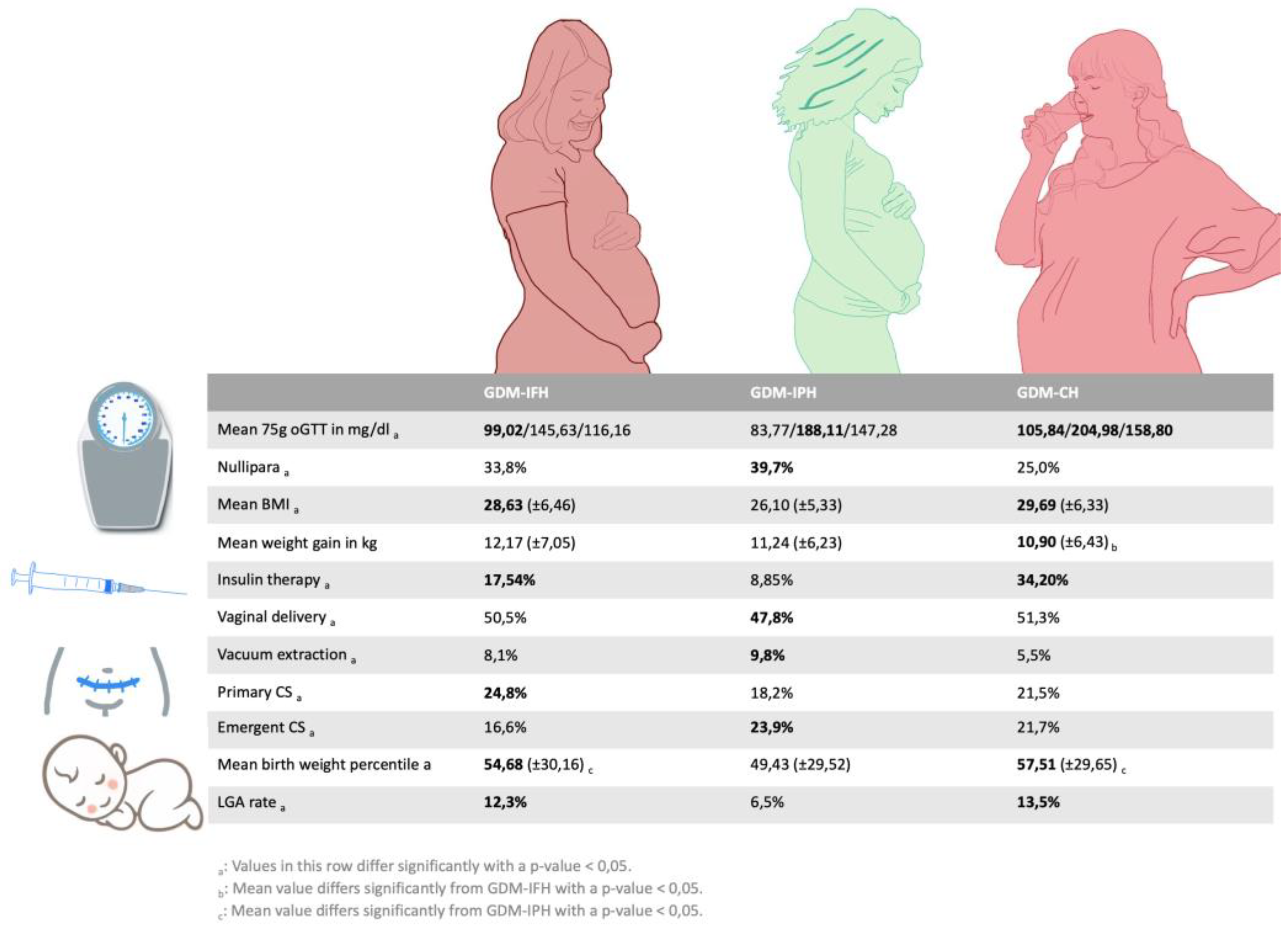
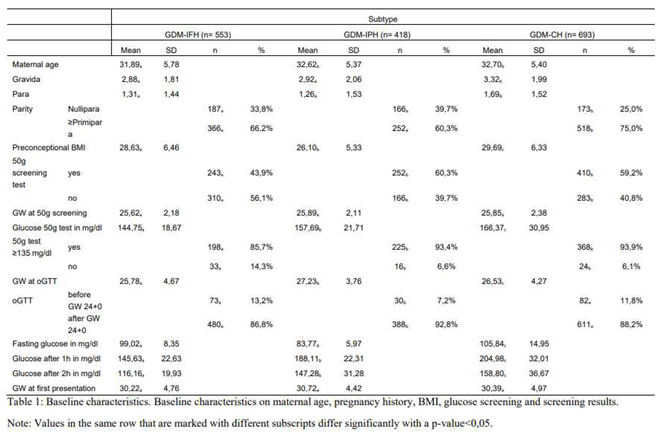
Of 1664 patients, 553 were classified as GDM-IFH (33,2%), 418 as GDM-IPH (25,1%) and 693 as GDM-CH (41,6%) (
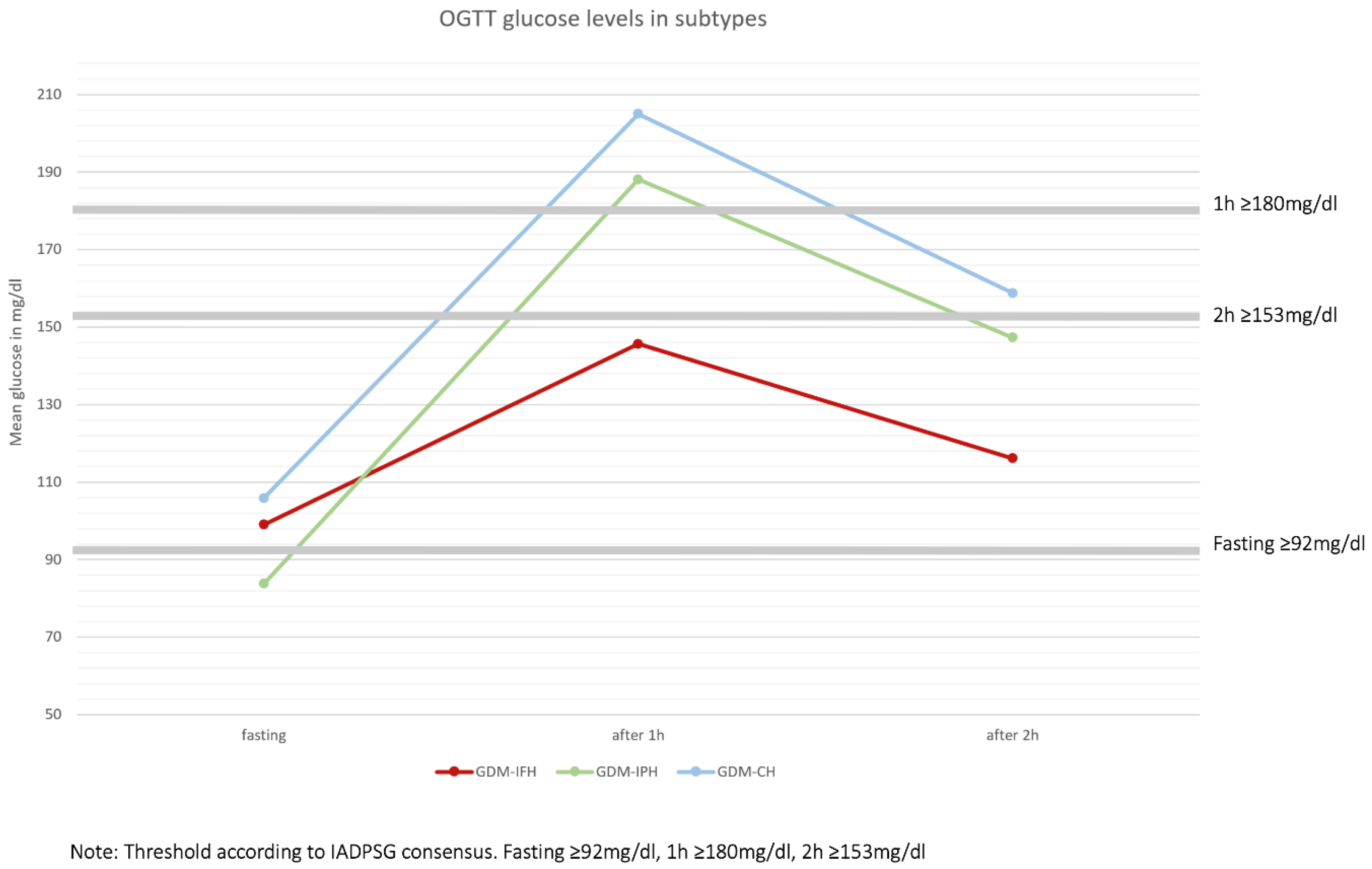
Figure 1). Mothers from the GDM-IFH group were on average significantly younger (IFH: 31,89, IPH: 32,62, CH: 32,70, p=0,024). Mean Gravidity (IFH: 2,88, IPH: 2,92, CH: 3,32, p<0,001) and parity (IFH: 1,31, IPH: 1,26, CH: 1,69, p<0,001) were significantly higher in the GDM-CH group. The rate of nulliparous women was significantly higher in the GDM-IPH group (IFH: 33,8%, IPH: 39,7%, CH: 25,0%, p<0,001). The preconceptional BMI differed significantly between all the subgroups: GDM-IPH women displayed the lowest mean BMI compared to GDM-CH women (IFH: 28,63, IPH: 26,10, CH: 29,69, p<0,001). The 50g oGCT was performed on average at 26 GW, with no significant difference between the groups (IFH: 25,62 GW, IPH: 25,89 GW, CH: 25,89 GW, p=0,362). The GDM-IFH group was less likely to receive the 50g oGCT before the 75g oGTT than the other subgroups (IFH: 43,9%, IPH: 60,3%, CH: 59,2%, p<0,001) and less likely to show a pathological glucose response in the test (IFH: 85,7%, IPH: 93,4%, CH: 93,9%, p<0,001). Mean glucose levels in the 50g oGCT were 144,75 mg/dl in the GDM-IFH, 157,69 mg/dl in the GDM-IPH and 166,37 mg/dl in the GDM-CH group (p<0,001). Women with GDM-IFH received the 75g oGTT earlier than women in the GDM-IPH or GDM-CH groups (Mean: IFH: 25,78 GW, IPH: 27,23 GW, CH: 26,53 GW, p<0,001). In the GDM-IFH and GDM-CH groups the 75g oGTT was performed before GW 24 more often than in the GDM-IPH group (13,2%, 11,8% vs. 7,2%; p=0,009). Mean 75g oGTT glucose levels before glucose intake, 1h after and 2h after were 99.02 mg/dl, 145.63 mg/dl and 116.16 mg/dl in women with GDM-IFH, 83.77 mg/dl, 188.11 mg/dl and 147.28 mg/dl in women with GDM-IPH and 105.84 mg/dl, 204.98 mg/dl and 158.80 mg/dl in women with GDM-CH, respectively (
Figure 3). The mean initial presentation at the GDM consultation clinic was at 31 GW (IFH: 30,22 GW, IPH: 30,72 GW, CH: 30,39 GW, p=0,270). A complete overview of the results is provided in Table 1.
Maternal Outcome
As provided in Table 2, the GDM-IFH group displayed the highest mean weight gain (IFH: 12,17 kg, IPH: 11,24 kg, CH: 10,90 kg, p=0,008). GDM-IFH (17,5%, OR 1,946 [1,277-2,967], p=0,002) as well as GDM-CH women were more likely to require insulin therapy (34,2%, OR 4,317 [2,915-6,392], p<0,001) compared to GDM-IPH women (8,9%). GDM-CH patients revealed a higher likelihood of insulin therapy (OR 2,218 [1,674-2,937], p<0,001) compared to GDM-IFH. The use of long-acting insulin only was significantly more common in the GDM-IFH and GDM-CH groups (IFH: 13,0%, IPH: 5,3%, CH: 19,5%, p<0,001) and the rate of combined insulin therapy higher in the GDM-CH group (IFH: 3,8%, IPH: 1,4%, CH: 12,8%, p<0,001). Two patients received treatment with metformin in combination with insulin.
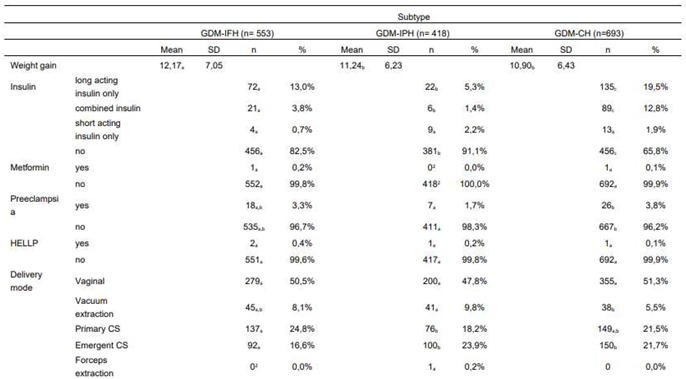
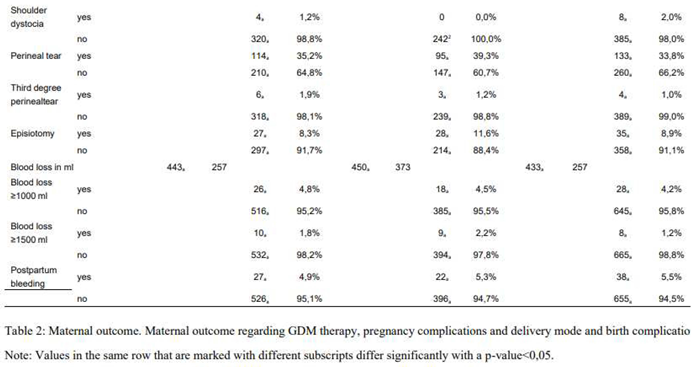
The analysis showed no significant difference between rates of CS (primary and emergent, p=0,811). However, there was a significant difference focusing on primary and emergent CS specifically. GDM-IFH women were most likely to deliver via planned primary CS compared to GDM-IPH (IFH: 24,8%, IPH: 18,2%, CH: 21,5%, p=0,047). The odds of delivering via primary CS were significantly increased only in GDM-IFH women (OR 1,376 [1,042-1,815], p=0,024) vs. GDM-CH patients. Women categorized as GDM-IPH (23,9%, OR 1,643 [1,173-2,302], p=0,004) or GDM-CH (21,7%, OR 1,48 [1,094-2,003], p=0,011) were at higher risk of an emergent CS compared to GDM-IFH women (16,6%). The rate of instrumental delivery differed significantly with 10% in GDM-IPH patients vs. 5,5% in GDM-CH patients (IFH: 8,1%, p=0,016). There were no significant differences concerning the rates of shoulder dystocia (IFH: 1,2%, IPH: 0,0%, CH: 2,0%, p=0,081), episiotomy (IFH: 8,3%, IPH: 11,6%, CH: 8,9%, p=0,389), third degree perineal tear (IFH: 1,9%, IPH: 1,2%, CH: 1,0%, p=0,620), preeclampsia (IFH: 3,3%, IPH: 1,7%, CH: 3,8%, p=0,143) and HELLP syndrome (IFH: 0,4%, IPH: 0,2%, CH: 0,1%, p=0,739). The analysis of blood loss as a continuous variable showed no significant difference (p=0,643), as well as the incidence of blood loss ≥ 1000 ml (p=0,867) and ≥ 1500 ml (p=0,400). The risk of postpartum bleeding was similar throughout the subgroups (p=0,893).
Fetal outcome
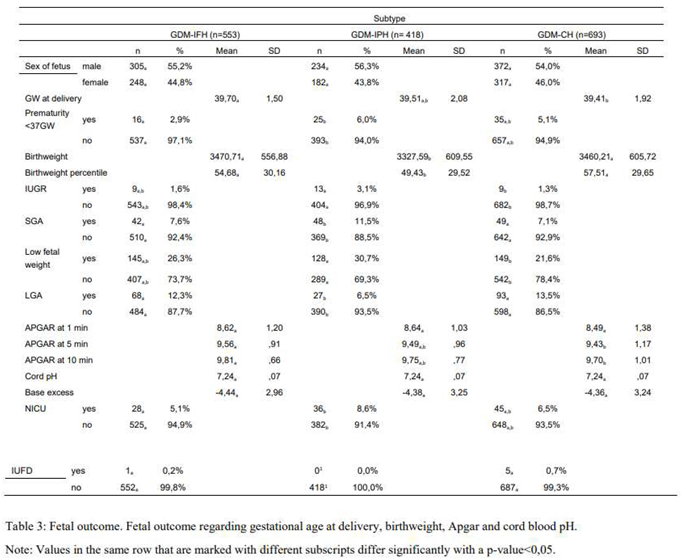
The distribution of the fetus's sex did not differ significantly between the groups (p=0,760). The mean gestational age at delivery was slightly higher in the GDM-IFH group than in the GDM-CH group (IFH: 39,70 GW, IPH: 39,51 GW, CH: 39,41 GW, p=0,008). The rates of premature delivery before 37 GW were similar among the subtypes (p=0,054). Neonates of GDM-IFH and GDM-CH mothers displayed a significantly higher mean birthweight (IFH: 3470,71g, IPH: 3327,59g, CH: 3460,21g, p<0,001) as well as birth weight percentiles (IFH: 54,68, IPH: 49,43, CH: 57,51, p<0,001) compared to neonates of GDM-IPH women. APGAR scores at 1 minute (p=0,088), 5 minutes (p=0,110) and 10 minutes after delivery (p=0,061), arterial cord pH values (p=0,446) and base excess (p=0,906) did not differ significantly among the subgroups. GDM-IFH (12,3%, OR 1,657 [1,020-2,692], p=0,041) and GDM-CH women (13,5%, OR 1,671 [1,046-2,668], p=0,032) were at higher risk of delivering neonates that were LGA compared to GDM-IPH (6,5%). The SGA rate was significantly higher among GDM-IPH women (IFH: 7,6%, IPH: 11,5%, CH: 7,1%, p=0,027). In the logistic regression analysis SGA did not reach statistical significance. However, GDM-IPH women (30,7%, OR 1,379 [1,029-1,847], p=0,031) displayed an association with low fetal weight (< 30th percentile) in comparison to GDM-CH women (21,6%, IFH: 26,3%). There was no significant difference in neonatal intensive care admission (p=0,086) and the rate of IUFD (p=0,104) (Table 3).
Effects of covariates on fetomaternal outcome
Nulliparous women displayed smaller odds of primary CS (OR 0,435 [0,320-0,593], p<0,001), but were at higher risk of delivering via emergent CS (OR 3,534 [2,700-4,625], p<0,001). Their likelihood of operative vaginal delivery was higher as well (OR 6,353 [4,100-9,843], p<0,001). Nulliparity increased the odds of delivering offspring that had low fetal weight (OR 1,583 [1,238-2,025], p<0,001) or was SGA (OR 2,155 [1,484-3,129], p<0,001), while it reduced the odds of delivering LGA neonates (OR 0,548 [0,365-0,823], p=0,004). Preconceptional BMI was associated with emergent CS (p=0,001), vaginal operative delivery (p=0,007) and LGA (p<0,001). A BMI categorized as overweight, obese or severely obese increased the likelihood of emergent CS (OR 1,451 [1,011-2,083], 1,585 [1,070-2,349] and 2,019 [1,385-2,941], respectively) and delivering an LGA fetus (OR 2,020 [1,145-3,565], 2,112 [1,172-3,805] and 3,639 [2,110-6,276], respectively). Underweight mothers (OR 2,911 [1,148-7,382]) were at significantly higher risk of delivering via vacuum extraction. Severely obese women (OR 0,425 [0,217-0,832]) on the other hand were at lower risk compared to normal weight women. Maternal age was associated with primary (OR 1,042 [1,019-1,067], p<0,001 for the increase of 1 year) as well as emergent CS (OR 1,031 [1,007-1,055], p=0,010 for the increase of 1 year).
Discussion
Our analysis was able to distinguish three different types of metabolic phenotypes with GDM based on the 75g oGTT levels. Each group revealed specific associations regarding the baseline characteristics as well as fetomaternal outcomes (
Figure 4).
GDM-IFH women were the youngest but had the highest mean weight gain. GDM-CH women were the oldest and displayed the highest number of previous deliveries. Both groups had significantly higher preconceptional BMI than the GDM-IPH group.
GDM-IFH and GDM-CH women presented an overall higher rate of well-established GDM risk factors, such as higher maternal age (17) or BMI (18). This may explain, why GDM-IFH and GDM-CH women received the 75g oGTT more frequently before 24 GW. The rate of a previous GDM was not assessable through our dataset, although it is an important contributing factor for the development of GDM (19).
Our results revealed a strong association of GDM-IFH and GDM-CH with the requirement of insulin therapy, which goes along with recent studies (14, 20-23). Kotzaeridi et al. affirmed a “worse metabolic profile”, higher BMI and a higher requirement for glucose-lowering medications in women with elevated fasting glucose, especially GDM-CH women (15).
The GDM-IFH and GDM-CH mothers delivered offspring with significantly higher birth weight as well as birth weight percentiles and increased odds of being LGA at the time of delivery compared to the GDM-IPH group. This aligns with findings of numerous studies, which previously demonstrated that maternal fasting glucose is strongly associated with LGA and higher birth weight (5, 12, 13). Uvena-Celebrezze et al. were able to establish a correlation of maternal fasting glucose and neonatal fat mass in a study that used self-monitoring of glucose levels (24). Zawiejska et al. showed that maternal fasting hyperglycemia was associated with birthweight ≥ 4000 g (14).
The higher rate of LGA in GDM-IFH and GDM-CH groups possibly contributes to an increased rate of primary CS in these groups. The rates of primary CS were highest in the GDM-IFH group, however in the binomial logistic regression only the association of GDM-IFH with primary CS in comparison to GDM-CH reached statistical significance.
The risk of emergent CS was significantly higher in the GDM-IPH and GDM-CH groups compared to GDM-IFH, whereas we found no significant difference among the subgroups regarding cesarean section in general.
GDM-IPH women presented with a lower preconceptional BMI, were more likely nulliparous and required insulin therapy less often.
While we found a higher rate of vaginal operative deliveries in women with GDM-IPH, there was no association of the subtypes and vaginal operative deliveries in the logistic regression analysis. However, the odds of a vaginal operative delivery were significantly increased if women were underweight and/or nulliparous. This goes along with a recent analysis, that was able to demonstrate an association of vacuum extraction and nulliparity (25). Ramos et al examined women requiring operative delivery assistance and found a decreased likelihood of vaginal operative delivery in women with prepregnancy obesity (26).
The rate of SGA was significantly higher in women of the GDM-IPH group; however, SGA did not reach statistical significance in the logistic regression. This may be explained by a strong association of nulliparity and SGA we found in our analysis, as well as a significantly higher rate of nulliparous women in the GDM-IPH group.
Previous studies found correlations of post-load hyperglycemia and gestational hypertension, hyperbilirubinemia and preterm delivery, whereas preterm delivery did not differ significantly among our subgroups and gestational hypertension and hyperbilirubinemia were not evaluated in this study (12-14).
Of note, IUFD and shoulder dystocia did not reach statistical significance, but all reported cases in our sample occurred either in women of the GDM-IFH or GDM-CH subgroups. Several other studies have previously shown associations of maternal fasting glucose and LGA or macrosomia with shoulder dystocia (27). They found fetal macrosomia to be a mediating factor between maternal fasting hyperglycemia and shoulder dystocia. A meta-analysis by Farrar et al. showed associations of fasting as well as post-load glucose levels with shoulder dystocia, although an increase in fasting glucose was more strongly associated (5).
A limitation of this study was the lack of healthy controls, as we collected the data solely from the gestational diabetes consultation without a control group. The inclusion of normal glucose tolerant women in previous studies, such as Kotzaeridi et al., has provided further insight and the advantage that findings regarding different pathological glucose response patterns could be put in context with a physiological glucose response (15). Additionally, the sample sizes for the individual analyses of variables were not the same throughout the study, due to sporadically missing data. On the other hand, an important advantage is the large sample size and amount of different baseline and outcome parameters that were assessed in this study.
Conclusion
To conclude, we did observe significant differences between the GDM subtypes regarding their underlying characteristics and the course of their diagnostic process and were able to identify subtypes that were at higher risk of certain adverse perinatal outcomes. Women categorized as GDM-IFH or GDM-CH were more likely in need of any kind of insulin therapy, displayed a higher BMI and their offspring had higher birthweight, birth weight percentiles and was more likely a LGA fetus, while neonates of women with GDM-IPH were at increased risk of low fetal weight. GDM-IFH was associated with primary CS, while GDM-IPH and GDM-CH were associated with emergent CS.
This analysis suggests that the categorization based on the 75g oGTT glucose levels could be a practicable approach to adapt the prenatal care of women with GDM based on their risk factors. In the future, prospective studies taking the maternal risk factors into account should be conducted, possibly including interventions for women at risk.
Author Contributions
Conceptualization, Josefine Koenigbauer; Methodology, Josefine Koenigbauer, Paul Rostin and Selina Balke; Formal Analysis, Selina Balke; Investigation, Josefine Koenigbauer and Selina Balke; Data curation, Petra Weid and Selina Balke; Original Draft Preparation, Selina Balke; Writing - Review & Editing, Josefine Koenigbauer and Wolfgang Henrich; Visualization, Selina Balke, Josefine Koenigbauer and Laura Fangmann; Supervision, Wolfgang Henrich and Josefine Koenigbauer.
Funding
This research received no external funding.
Ethical approval
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Charité university hospital on February 6th, 2023 (EA2/255/22).
Informed consent statement
Informed consent was waived due to the retrospective nature of this study. Data was kept anonymized.
Data availability
The data presented in this study are available on request from the corresponding author.
Conflicts of interest
The authors declare no conflict of interest.
Abbreviations and Acronyms
GDM = gestational diabetes mellitus
oGTT = oral glucose tolerance test
oGCT= oral glucose challenge test
CS= cesarean section
GDM-IFH= gestational diabetes with isolated fasting hyperglycemia
GDM-IPH= gestational diabetes with isolated postprandial hyperglycemia
GDM-CH= gestational diabetes with combined hyperglycemia
LGA= large for gestational age
IUGR= intrauterine growth retardation
SGA= small for gestational age
GW= gestational week
IUFD= intrauterine fetal death
References
- Reitzle L, Schmidt C, Heidemann C, Icks A, Kaltheuner M, Ziese T, et al. Gestational diabetes in Germany: Development of screening participation and prevalence. J Health Monit. 2021;6(2):3-18.
- Schäfer-Graf UM, Gembruch U, Kainer F, Groten T, Hummel S, Hösli I, et al. Gestational Diabetes Mellitus (GDM) - Diagnosis, Treatment and Follow-Up. Guideline of the DDG and DGGG (S3 Level, AWMF Registry Number 057/008, February 2018). Geburtshilfe Frauenheilkd. 2018;78(12):1219-31.
- Shand AW, Bell JC, McElduff A, Morris J, Roberts CL. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998-2002. Diabet Med. 2008;25(6):708-15.
- Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. 2010;27(4):436-41. [CrossRef]
- Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. Bmj. 2016;354:i4694.
- Waters TP, Dyer AR, Scholtens DM, Dooley SL, Herer E, Lowe LP, et al. Maternal and Neonatal Morbidity for Women Who Would Be Added to the Diagnosis of GDM Using IADPSG Criteria: A Secondary Analysis of the Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes Care. 2016;39(12):2204-10. [CrossRef]
- Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. Bmj. 2022;377:e067946. [CrossRef]
- Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130-9.
- Powe CE, Allard C, Battista MC, Doyon M, Bouchard L, Ecker JL, et al. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes Care. 2016;39(6):1052-5. [CrossRef]
- Liu Y, Hou W, Meng X, Zhao W, Pan J, Tang J, et al. Heterogeneity of insulin resistance and beta cell dysfunction in gestational diabetes mellitus: a prospective cohort study of perinatal outcomes. J Transl Med. 2018;16(1):289. [CrossRef]
- Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia. 2019;62(11):2118-28. [CrossRef]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002.
- Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care. 2010;33(12):2524-30. [CrossRef]
- Zawiejska A, Wender-Ozegowska E, Radzicka S, Brazert J. Maternal hyperglycemia according to IADPSG criteria as a predictor of perinatal complications in women with gestational diabetes: a retrospective observational study. J Matern Fetal Neonatal Med. 2014;27(15):1526-30. [CrossRef]
- Kotzaeridi G, Blätter J, Eppel D, Rosicky I, Linder T, Geissler F, et al. Characteristics of gestational diabetes subtypes classified by oral glucose tolerance test values. Eur J Clin Invest. 2021;51(9):e13628. [CrossRef]
- Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-82. [CrossRef]
- Teede HJ, Harrison CL, Teh WT, Paul E, Allan CA. Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol. 2011;51(6):499-504. [CrossRef]
- Torloni MR, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203. [CrossRef]
- Syngelaki A, Pastides A, Kotecha R, Wright A, Akolekar R, Nicolaides KH. First-Trimester Screening for Gestational Diabetes Mellitus Based on Maternal Characteristics and History. Fetal Diagn Ther. 2015;38(1):14-21. [CrossRef]
- González-Quintero VH, Istwan NB, Rhea DJ, Tudela CM, Flick AA, de la Torre L, et al. Antenatal factors predicting subsequent need for insulin treatment in women with gestational diabetes. J Womens Health (Larchmt). 2008;17(7):1183-7. [CrossRef]
- Pertot T, Molyneaux L, Tan K, Ross GP, Yue DK, Wong J. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care. 2011;34(10):2214-6.
- Wong VW, Jalaludin B. Gestational diabetes mellitus: who requires insulin therapy? Aust N Z J Obstet Gynaecol. 2011;51(5):432-6.
- Barnes RA, Wong T, Ross GP, Jalaludin BB, Wong VW, Smart CE, et al. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia. 2016;59(11):2331-8. [CrossRef]
- Uvena-Celebrezze J, Fung C, Thomas AJ, Hoty A, Huston-Presley L, Amini SB, et al. Relationship of neonatal body composition to maternal glucose control in women with gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2002;12(6):396-401.
- Cohen G, Schreiber H, Shalev Ram H, Ovadia M, Shechter-Maor G, Biron-Shental T. Can We Predict Feto-Maternal Adverse Outcomes of Vacuum Extraction? Geburtshilfe Frauenheilkd. 2022;82(11):1274-82.
- Ramos SZ, Waring ME, Leung K, Amir NS, Bannon AL, Moore Simas TA. Attempted and Successful Vacuum-Assisted Vaginal Delivery by Prepregnancy Body Mass Index. Obstet Gynecol. 2017;129(2):311-20. [CrossRef]
- Athukorala C, Crowther CA, Willson K. Women with gestational diabetes mellitus in the ACHOIS trial: risk factors for shoulder dystocia. Aust N Z J Obstet Gynaecol. 2007;47(1):37-41. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).