Submitted:
19 April 2023
Posted:
19 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
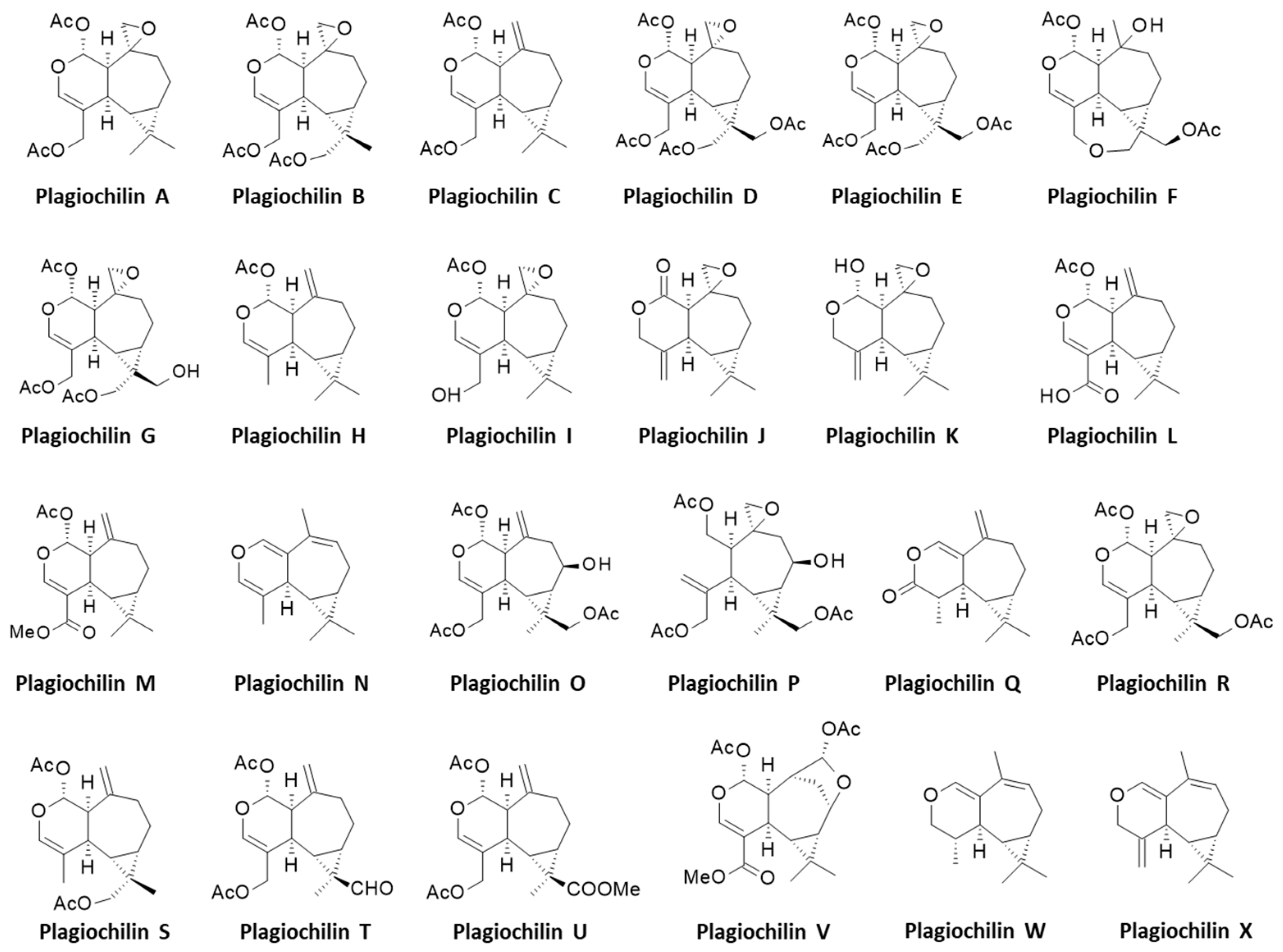
2.1. Molecular structure and software.
2.2. Molecular modeling procedure.
3. Results
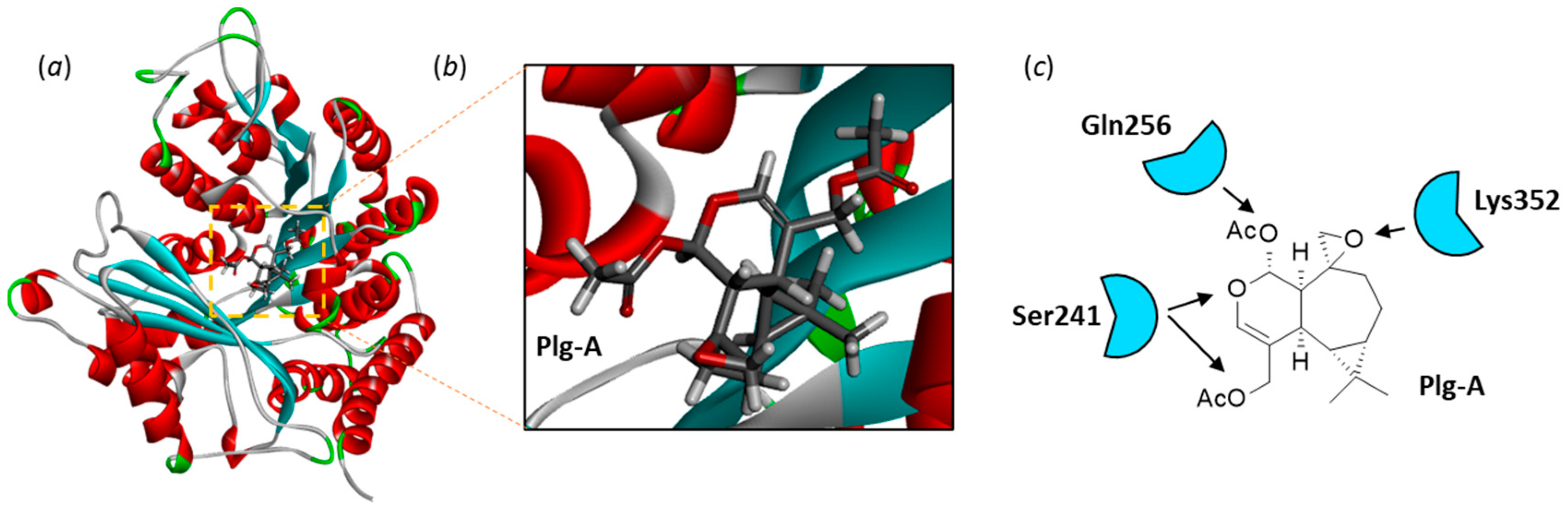
3.1. Comparative docking of plagiochilins to α-tubulin
3.2. Drug design implications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frangedakis, E., Shimamura, M., Villarreal, J.C., Li, F.W., Tomaselli, M., Waller, M., Sakakibara, K., Renzaglia, K.S., Szövényi, P. The hornworts: morphology, evolution and development. New Phytol. 2021, 229, 735-754. [CrossRef]
- Drobnik, J., Stebel, A. Four Centuries of Medicinal Mosses and Liverworts in European Ethnopharmacy and Scientific Pharmacy: A Review. Plants (Basel). 2021, 10, 1296. [CrossRef]
- Commisso, M., Guarino, F., Marchi, L., Muto, A., Piro, A., Degola, F. Bryo-Activities: A Review on How Bryophytes Are Contributing to the Arsenal of Natural Bioactive Compounds against Fungi. Plants (Basel). 2021, 10, 203. [CrossRef]
- Asakawa, Y., Nagashima, F., Ludwiczuk, A. Distribution of Bibenzyls, Prenyl Bibenzyls, Bis-bibenzyls, and Terpenoids in the Liverwort Genus Radula. J. Nat. Prod. 2020, 83, 756-769.
- Ludwiczuk, A., Asakawa, Y. Bryophytes as a source of bioactive volatile terpenoids - A review. Food Chem. Toxicol. 2019, 132, 110649. [CrossRef]
- Asakawa, Y., Toyota, M., Takemoto, T. Plagiochilide et plagiochiline a, secoaromadendrane-type sesquiterpenes de la mousse, plagiochila yokogurensis (plagiochilaceae). Tetrahedron Lett. 1978, 19, 1553-1556. [CrossRef]
- Asakawa, Y., Toyota, M., Takemoto, T. La plagiochiline a et la plagiochiline b, les sesquiterpenes du type secoaromadendrane de la mousse, Plagiochila hattoriana. Phytochemistry 1978, 17, 1794. [CrossRef]
- Asakawa, Y., Toyota, M., Takemoto, T., Suire, C. Plagiochilines C, D, E and F, four novel secoaromadendrane-type sesquiterpene hemiacetals from Plagiochila asplenioides and Plagiochila semidecurrens. Phytochemistry 1979, 18, 1355-1357. [CrossRef]
- Fukuyama, Y., Toyota, M., Asakawa, Y. Ent-kaurene diterpene from the liverwort Plagiochila pulcherrima. Phytochemistry 1988, 27, 1425-1427. [CrossRef]
- Aponte, J.C., Yang, H., Vaisberg, A.J., Castillo, D., Málaga, E., Verástegui, M., Casson, L.K., Stivers, N., Bates, P.J., Rojas, R., Fernandez, I., Lewis, W.H., Sarasara, C., Sauvain, M., Gilmanv, R.H., Hammond, G.B. Cytotoxic and anti-infective sesquiterpenes present in Plagiochila disticha (Plagiochilaceae) and Ambrosia peruviana (Asteraceae). Planta Medica 2010, 76, 705-707. [CrossRef]
- Asakawa, Y., Inoue, H., Toyota, M., Takemoto, T. Sesquiterpenoids of fourteen Plagiochila species. Phytochemistry 1980, 19, 623-2626. [CrossRef]
- Bailly, C. Discovery and anticancer activity of the plagiochilins from the liverwort genus Plagiochila. Life 2023, 13, 758. [CrossRef]
- Asakawa, Y. Chemosystematics of the hepaticae. Phytochemistry 2004, 65, 623-669. [CrossRef]
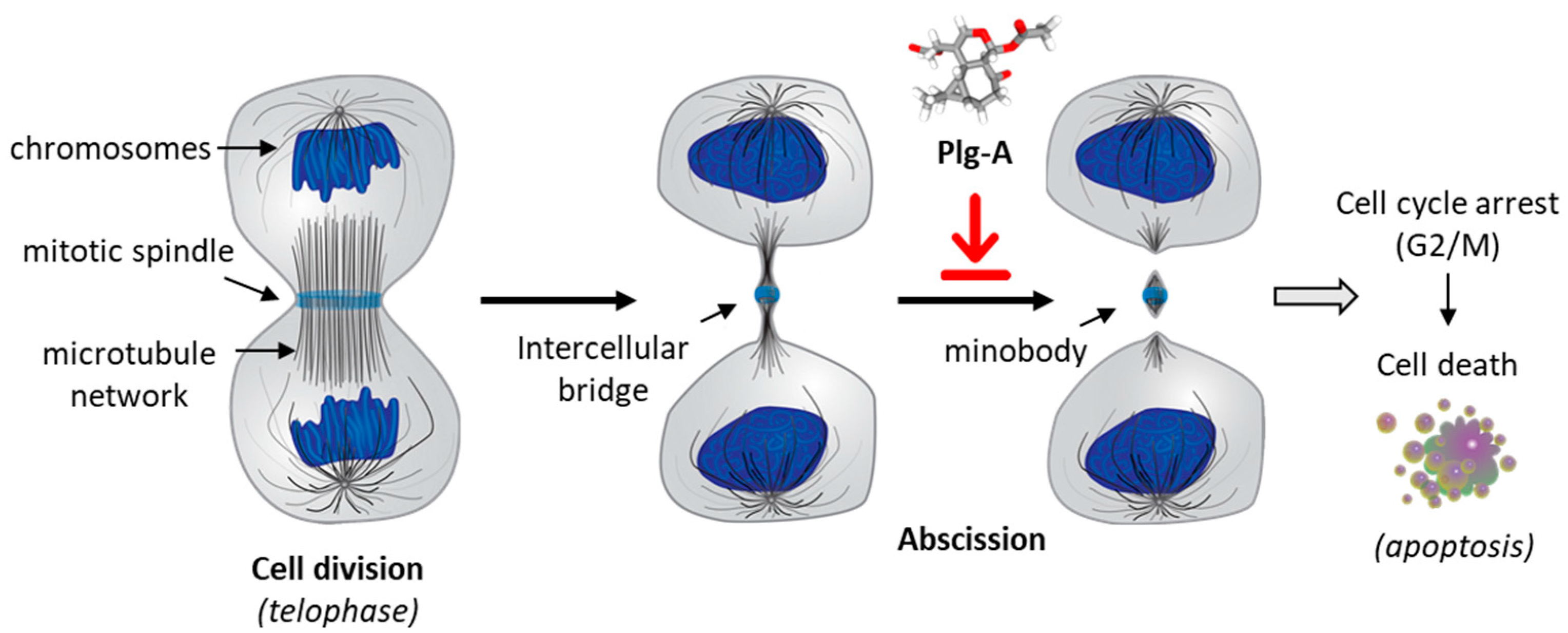
- Stivers, N.S., Islam, A., Reyes-Reyes, E.M., Casson, L.K., Aponte, J.C., Vaisberg, A.J., Hammond, G.B., Bates, P.J. Plagiochiline A Inhibits Cytokinetic Abscission and Induces Cell Death. Molecules 2018, 23, 1418. [CrossRef]
- McNeely, K.C., Dwyer, N.D. Cytokinetic Abscission Regulation in Neural Stem Cells and Tissue Development. Curr. Stem Cell Rep. 2021, 7, 161-173. [CrossRef]
- Peterman, E., Prekeris, R. The postmitotic midbody: Regulating polarity, stemness, and proliferation. J. Cell. Biol. 2019, 218, 3903-3911. [CrossRef]
- Huang, D.S., Wong, H.L., Georg, G.I. Synthesis and Cytotoxicity Evaluation of C4- and C5-Modified Analogues of the α,β-Unsaturated Lactone of Pironetin. ChemMedChem. 2017, 12, 520-528.
- Coulup, S.K., Georg, G.I. Revisiting microtubule targeting agents: α-Tubulin and the pironetin binding site as unexplored targets for cancer therapeutics. Bioorg. Med. Chem. Lett. 2019, 29, 1865-1873. [CrossRef]
- Toyota, M., Tanimura, K., Asakawa, Y. Cytotoxic 2,3-secoaromadendrane-type sesquiterpenoids from the liverwort Plagiochila ovalifolia. Planta Med. 1998, 64, 462-464.
- Toyota, M., Nakamura, I., Huneck, S., Asakawa, Y. Sesquiterpene esters from the liverwort Plagiochila porelloides. Phytochemistry 1994, 37, 1091-1093. [CrossRef]
- Yang, J., Wang, Y., Wang, T., Jiang, J., Botting, C.H., Liu, H., Chen, Q., Yang, J., Naismith, J.H., Zhu, X., Chen, L. Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule. Nature Commun. 2016, 7, 12103. [CrossRef]
- Dundas, J., Ouyang, Z., Tseng, J., Binkowski, A., Turpaz, Y., Liang, J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006, 34, W116-8. [CrossRef]
- Tian, W., Chen, C., Lei, X., Zhao, J., Liang, J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363-W367. [CrossRef]
- Jorgensen, W.L., Tirado-Rives, J. Monte Carlo versus Molecular Dynamics for conformational sampling. J Phys. Chem. 1996, 100, 14508-14513.
- Jorgensen, W.L., Tirado-Rives, J. Molecular modeling of organic and biomolecular systems using BOSS and MCPRO. J. Comput. Chem. 2005, 26, 1689-1700.
- Jorgensen, W.L., Ulmschneider, J.P., Tirado-Rives, J. Free energies of hydration from a generalized Born model and an ALL-atom force field. J. Phys. Chem. B 2004, 108, 16264-16270.
- Jones, G., Willett, P., Glen, R.C., Leach, A.R., Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727-748. [CrossRef]
- Meziane-Tani, M., Lagant, P., Semmoud, A., Vergoten, G. 2006. The SPASIBA force field for chondroitin sulfate: vibrational analysis of D-glucuronic and N-acetyl-D-galactosamine 4-sulfate sodium salts. J. Phys. Chem. A 2006, 110, 11359-11370. [CrossRef]
- Vergoten, G., Mazur, I., Lagant, P., Michalski, J.C., Zanetta, J.P. 2003. The SPASIBA force field as an essential tool for studying the structure and dynamics of saccharides. Biochimie 2003, 85, 65-73. [CrossRef]
- Lagant, P., Nolde, D., Stote, R., Vergoten, G., Karplus M. Increasing Normal Modes Analysis Accuracy: The SPASIBA Spectroscopic Force Field Introduced into the CHARMM Program. J. Phys. Chem. A 2004, 108, 4019-4029. [CrossRef]
- Homans, S.W. A molecular mechanical force field for the conformational analysis of oligosaccharides: comparison of theoretical and crystal structures of Man alpha 1-3Man beta 1-4GlcNAc. Biochemistry 1990, 29, 9110-9118. [CrossRef]
- Söderström, L., Hagborg, A., von Konrat, M., et al. World checklist of hornworts and liverworts. PhytoKeys 2016, 59, 1-828. [CrossRef]
- Heinrichs, J., Hentschel, J., Feldberg, K., Bombosch, A., Schneider, H. Phylogenetic biogeography and taxonomy of disjunctly distributed bryophytes. J. System. Evol. 2009, 47, 497-508. [CrossRef]
- Asakawa, Y., Toyota, M., Takemoto, T. Three ent-secoaromadendrane-type sesquiterpene hemiacetals and a bicyclogermacrene from Plagiochila ovalifolia and Plagiochila yokogurensis. Phytochemistry 1980, 19, 2141-2145. [CrossRef]
- Asakawa, Y., Toyota, M., Takemoto, T., Kubo, I., Nakanishi, K. Insect antifeedant secoaromadendrane-type sesquiterpenes from Plagiochila species. Phytochemistry 1980, 19, 2147-2154. [CrossRef]
- Asakawa, Y., Ludwiczuk, A., Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52-80. [CrossRef]
- Wang, S., Liu, S.S., Lin, Z.M., Li, R.J., Wang, X.N., Zhou, J.C., Lou, H.X. Terpenoids from the Chinese liverwort Plagiochila pulcherrima and their cytotoxic effects. J. Asian Nat. Prod. Res. 2013, 15, 473-481.
- Vergoten, G., Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296. [CrossRef]
- Blay, G., Cardona, L., García, B., Lahoz, L., Pedro, J.R. Synthesis of plagiochilin N from santonin. J. Org. Chem. 2001, 66, 7700-7705. [CrossRef]
- Sabovljević, M.S., Ćosić, M.V., Jadranin, B.Z., Pantović, J.P., Giba, Z.S., Vujičić, M.M., Sabovljević, A.D. The Conservation Physiology of Bryophytes. Plants (Basel) 2022, 11, 1282. [CrossRef]
- Pandey, S., Alam, A. Bryo-Pharmaceuticals: An Emerging Era of Pharmaceutical Products. In: Advanced Pharmacological Uses of Medicinal Plants and Natural Products. 2020. Chapter 14, pages 269-284. [CrossRef]
- Pannequin, A., Quetin-Leclercq, J., Costa, J., Tintaru, A., Muselli, A. First Phytochemical Profiling and In-Vitro Antiprotozoal Activity of Essential Oil and Extract of Plagiochila porelloides. Molecules 2023, 28, 616. [CrossRef]
- Qiao, Y.N., Jin, X.Y., Zhou, J.C., Zhang, J.Z., Chang, W.Q., Li, Y., Chen, W., Ren, Z.J., Zhang, C.Y., Yuan, S.Z., Lou, H.X. Terpenoids from the Liverwort Plagiochila fruticosa and Their Antivirulence Activity against Candida albicans. J. Nat. Prod. 2020, 83, 1766-1777. [CrossRef]
- Nagashima, F., Sekiguchi, T., Takaoka, S., Asakawa, Y. Terpenoids and aromatic compounds from the New Zealand liverworts Plagiochila, Schistochila, and Heteroscyphus species. Chem. Pharm. Bull. (Tokyo). 2004, 52, 556-560. [CrossRef]
- Bringmann, G., Mühlbacher, J., Reichert, M., Dreyer, M., Kolz, J., Speicher, A. Stereochemistry of isoplagiochin C, a macrocyclic bisbibenzyl from liverworts. J. Am. Chem. Soc. 2004, 126, 9283-9290. [CrossRef]
- So, M.L., Grolle, R. Studies on Plagiochila sect. Plagiochila (Hepaticae) in East and South Asia. J. Bryol. 2000, 22, 17-28.
- Matsuo, A., Ono, K., Hamasaki, K., Nozaki, H. Phaeophytins from a cell suspension culture of the liverwort Plagiochila ovalifolia. Phytochemistry. 1996, 42, 427-430. [CrossRef]
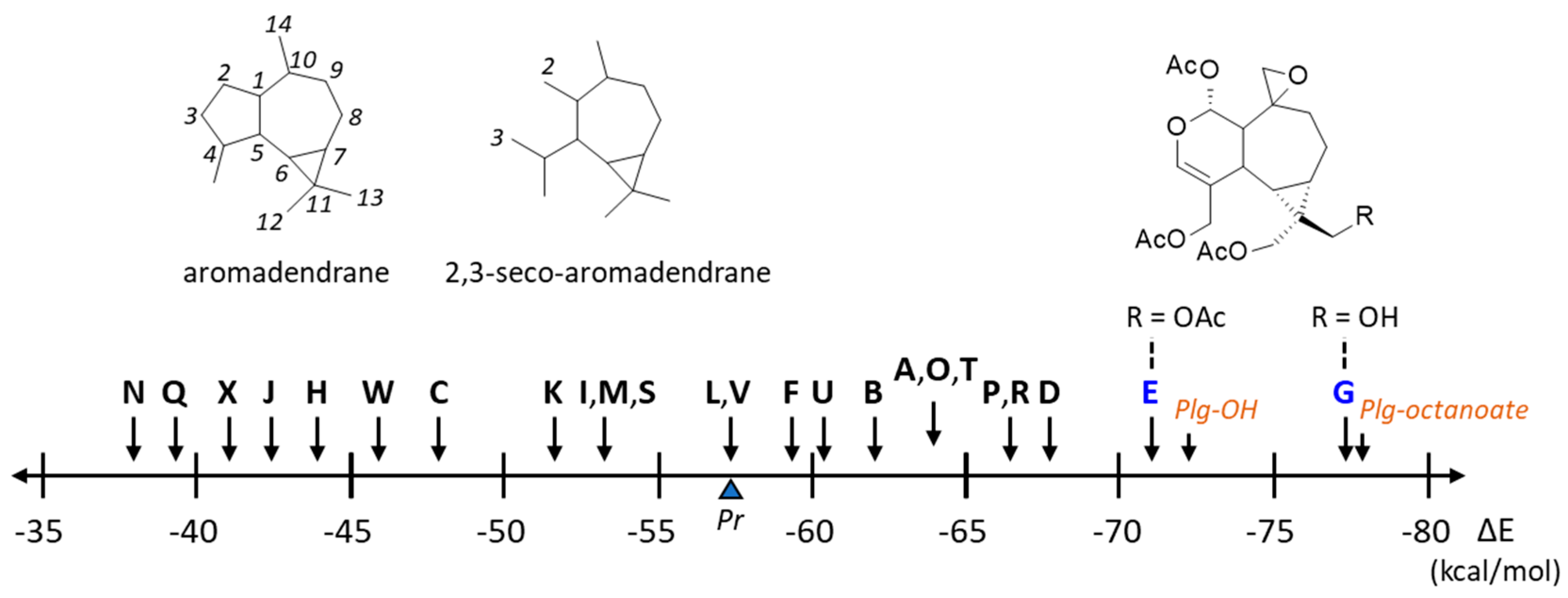

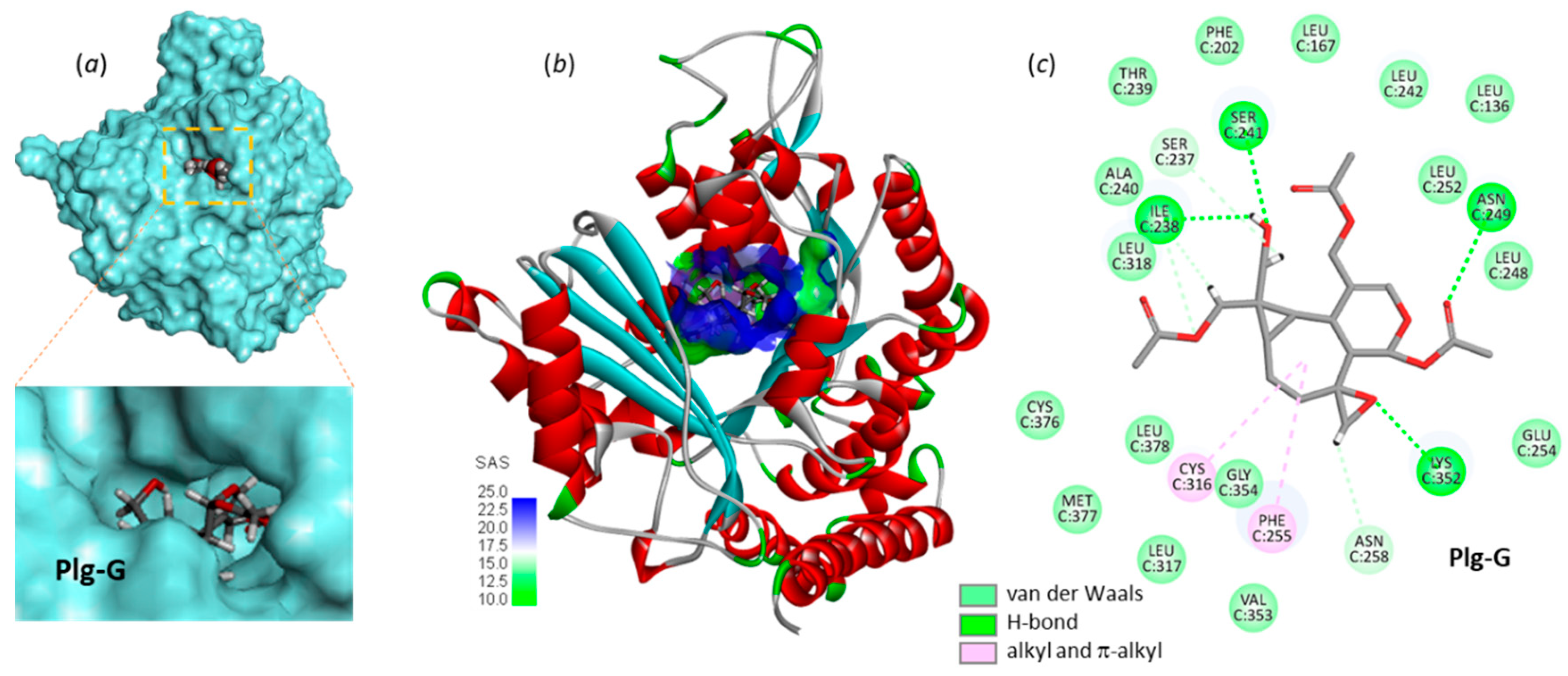
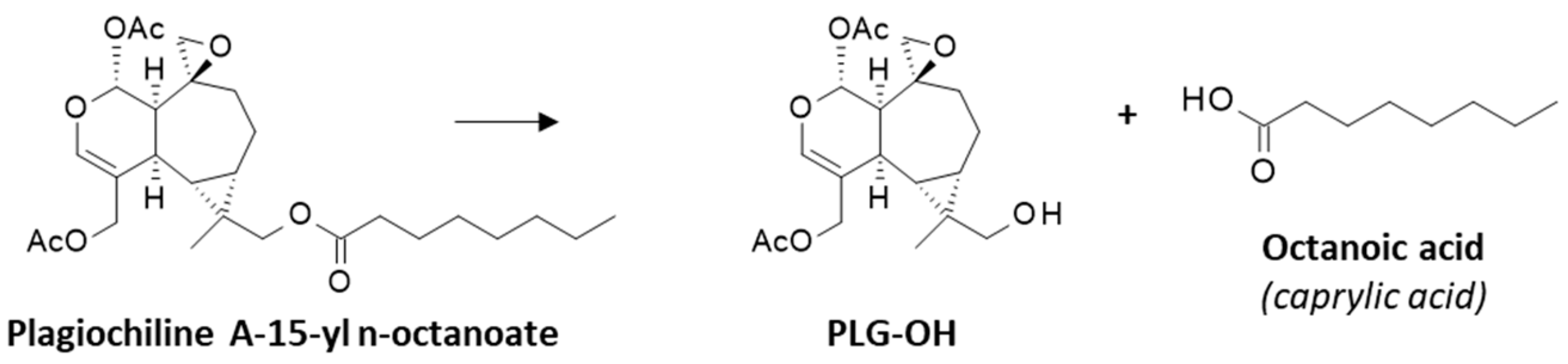

| Compounds | ΔE (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|
| Plagiochilin A | -63.80 | -19.90 |
| Plagiochilin B | -62.30 | -26.00 |
| Plagiochilin C | -47.80 | -18.55 |
| Plagiochilin D | -67.30 | -19.10 |
| Plagiochilin E | -71.00 | -17.20 |
| Plagiochilin F | -59.30 | -23.15 |
| Plagiochilin G | -77.00 | -26.00 |
| Plagiochilin H | -44.00 | -21.30 |
| Plagiochilin I | -53.25 | -16.80 |
| Plagiochilin J | -42.40 | -18.40 |
| Plagiochilin K | -52.10 | -15.50 |
| Plagiochilin L | -57.60 | -22.70 |
| Plagiochilin M | -53.70 | -21.20 |
| Plagiochilin N | -37.90 | -15.10 |
| Plagiochilin O | -64.40 | -23.70 |
| Plagiochilin P | -66.85 | -24.00 |
| Plagiochilin Q | -39.30 | -17.30 |
| Plagiochilin R | -66.50 | -25.50 |
| Plagiochilin S | -53.40 | -20.70 |
| Plagiochilin T | -63.10 | -23.30 |
| Plagiochilin U | -60.40 | -20.90 |
| Plagiochilin V | -57.45 | -16.10 |
| Plagiochilin W | -46.60 | -19.90 |
| Plagiochilin X | -41.30 | -20.00 |
| PlgA-octanoate | -77.50 | -28.40 |
| Plg-OH | -72.40 | -22.20 |
| Pironetin | -57.32 | -24.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





