1. Introduction
Corythucha (Stål) belongs to the Hemiptera Order, Tingidae Family. The genus was first described in 1873 by Say. 70 species have been described belonging to this genus [
1]. California is home to more than a dozen species of lace bugs.
According to Gibson (1918), the host plants preferred by species of the Corythuca genus belong to the genera Alnus, Malus, Betula, Stathylea, Aesculus, Juglans, Cephalanthus, Prunus, Pyrus, Ulmus, Ribes, Quercus, Rosa, Populus, Celtis, Crataegus, Corylus, Aesculus, Tilia, Amelanchier, Carya, Rhus, Carduus, Cirsium, Lathyrus, Salix [
2].
The
Corythucha arcuata, (the oak lace bug (OLB)) species is native to North America, where it is classified as very common. Each feed on a single or a small number of closely related plant species. Alder, ash, avocado, coyote brush, birch, ceanothus, photinia, poplar, sycamore, toyon, and willow are among potential hosts [
3].
In their natural habitat, these species are not harmful, their population numbers being controlled by biotic and abiotic factors [
4]. But invasive insects do not encounter natural enemies, so they often rapidly grow in number and cause significant damage to the invaded ecosystems [
5]. To limit the spread of an invasive insect species, the invasion routes, and the spatial distribution, the intraspecific diversity must be identified [
6,
7].
OLB has internationally spread to some European, Asian, [
2] African, Australian, and Oceanian areas [
8,
9,
10,
11]. Now considered widespread in several regions of the world, this includes the Balkan Peninsula, Europe, and Turkey [
12].
The global health status of forests is now affected by several harmful insects, some of which cause significant damage with serious economic and environmental implications [
13]. Invasive insect species pose a threat to biological diversity and ecosystems [
14]. With free movement of goods (trade in products of plants of origin), global changes in the environment: the degradation of ecosystems, deforestation, climate change, and industrial pollution [
15], has led to greater impact of the spread of invasive species occupying new territories [
16].
In Europe, OLB has been reported to affect a greater number of forest ecosystems on an expanding scale [
17]. The rapid expansion of OLB in the invaded countries, and the very high attack caused to the host plants, compared to the native area of origin, are most probably due to the lack of natural enemies, pathogens, and competitors [
18].
1.1. The chronology of OLB spread in Europe
When first identifying OLB, the researchers sought more information about its status in Europe. There was no easy to go to place that brought the different sighting and chronology together. Extensive time-consuming desk research was undertaken. We hope the chronological information and map below and the density of reported species sighting will save other researchers time (
Figure 1). OLB was first reported in Europe in northern Italy (Lombardy and Piedmont regions) in May 2000, on
Quercus robur,
Q. pubescens and supposed hybrids of
Q. robur and
Q. petrea [
19,
20].
In 2002, OLB extended to the south of Switzerland, where the species was detected in April and July 2002, near Pura (Malcantone). In summer 2003, it was found on fallen oak leaves in a mixed forest near Chiasso, southern Switzerland [
21]. Researchers hypothesized that OLB arrived in the Swiss Canton of Ticino in 2001 or 2002, immigrating from Lombardy (Italy) [
22].
In 2003, OLB was recorded in Turkey for the first time in the province of Bolu and then in the provinces of Düzce, Zonguldak, Sakarya, Kocaeli, Eskişehir, Ankara, Çankırı and Bilecik [
23,
24]. Subsequent research in Turkey led to the conclusion that the species has infected 28.000 km
2 of territory [
9,
25] and is now listed as a permanently monitored invasive species [
26,
27].
In 2005, it was detected in Iran [
28]. In 2005, OLB was reported in Iran, West Azerbaijan [
28].
In Poland, it was announced for the first time in 2009 and since then other 27 alerts have been reported [
29].
In 2012, July and September, OLB was reported in Bulgaria for the first time on the leaves of
Quercus robur in Plovdiv and mixed stands formed by
Q. robur and
Q. cerris in the vicinity of Zlatidolvill near the city of Simeonovgrad in southern Bulgaria. Most likely, OLB entered Bulgaria from the Asian part of Turkey, where it is widespread [
30]. In just five years after the first registration, OLB invaded most of Bulgaria, where the attack reached approximately 85% of the infested trees [
31].
By 2013, the pest expanded its European range, entering Hungary [
32]. It was detected for the first time in Debrecen, Eastern Hungary [
33], and later it spread throughout Hungary [
34,
35,
36]. Currently, the species is being monitored, and from data collected in different research, 11 subspecies of oak have been reported as host plants. Based on these findings, experts conclude the only thing that will restrict the expansion of OLB in Europe and Asia is a lack of host trees [
37].
Also in 2013, in Croatia, OLB was confirmed in five locations on elm stands (
Ulmus minor) [
38]. In 2014, OLB expanded to over 30.000 ha through the Spačva basin, the largest and most valuable complex oak forest (
Quercus robur) in the EU. By 2015, it has spread throughout the Spačva basin, attacking 60,000 ha of forest [
39].
In 2013, Serbia reported a powerful attack in the forests of Vojvodina [
40,
41,
42].
In 2015, OLB entered Russia, found in the Krasnodar city park [
24]. Satellite images of the oak forests confirmed the OLB spread into the North-West Caucasus [
43], and extended to the south of the European part of Russia [
44,
45,
46,
47]. In 2015, the first report of OLB in Romania was published.
In 2016 (Autumn), OLB was reported in south-east Slovenia on leaves of
Quercus robur in an oak forest in the village of Zakot, near Brezice. Specialists hypothesized the spread came from Croatia, where it was previously reported in the Luznica Castle park near Zapresic, just 14 km from the park in Slovenia. The area had good rail and car traffic routes along the northern edge of the infested forest. In 2017, the spread of the species was reported in the Krakovo oak forest [
48]. More recently, monitoring studies have been set up across Slovenia, through the Life Artemis project, which engages citizens to send the coordinates of the places where they observed the pest on the trees [
49].
From 2016, was reported in other European countries: Albania, Bosnia and Herzegovina, Greece and Ukraine [
48,
50,
51,
52,
53,
54].
In 2017, in France, OLB was detected in Toulouse (Occitanie, Haute-Garonne, 31555), on 3 more species of oaks: on Hungarian oaks
Quercus frainetto, pedunculated oaks
Quercus robur and acorn oaks
Quercus petraea [
55].
In 2018, saw the first record of OLB in Slovakia in June, on leaves of
Quercus cerris near the village of Mužla, close to the Danube in southern Slovakia, and in August near Čičarovece, in eastern Slovakia. Researchers confirm the hypothesis of the spread came from Hungary, where a strong infestation was reported in several areas of the country [
56].
In 2018, OLB was discovered in the central area of Bosnia, in two localities. Specialists indicated the possibility of its spread throughout Bosnia and Herzegovina in the coming years [
54].
In 2019, Austria recorded OLB in September in south-east Styria and south Burgenland in 21 locations in the districts of Hartberg-Fürstenfeld, Leibnitz, south-east Styria and Güssing and Jennersdorf. Infestation intensity varied widely between 1% and 95% [
57]. Also, in 2019 was reported in North Macedonia [
58] and in Czech Republic [
59].
Since its accidental introduction, around the year 2000, OLB has spread rapidly over a wide geographical area and is now found in over 20 other European countries [
60]. Latest reports highlight the OLB species affects ALL Eurasian oak species and will also feed on other woody plant species [
36,
61]. The most frequently attacked trees are the Oak species of
Quercus robur,
Quercus frainetto, Quercus petraea and
Quercus cerris, [
50].
In March 2001, this invasive species was recognized as a potential threat to European oak and was declared by European and Mediterranean Organization for Plant Protection on the list of alerts an EPPO [
50].
1.2. OLB in Romania
In Romania, invasions of some non-native insect species are becoming a big problem because they represent a threat to biodiversity and the functioning of ecosystems [
62]. OLB was first observed in 2015, in the ‘Pavel Covaci’ University Botanical Garden in Macea [
51] and later, on October 20, 2015, in Arad [
63]. In just a few months, it spread to other areas of Romania [
52,
64,
65]. In August 2016, OLB was identified around the city of Bucharest [
65] expanding into Giurgiu County, in the mixed gray oak and downy oak forests and into Călărași County where the aggressive attack ranged from 60% to 100%. According to Tomescu et al. (2018) the species was present also in Băicoi, in Prahova County, central Romania, and to the west in Baciu Forest, Cluj County [
66]. During 2016, OLB was found in other west locations, in Timișoara city and Denta [
63], in Dolj county (in Craiova and Balasan) and in Ciolănești, Teleorman county [
66]. From the studies carried out between 2016-2022, by the National Institute for Research and Development in Forestry ‘Marin Dracea’ specialists [
62,
64,
65,
67,
68,
69,
70], OLB was reported in 67 of the 124 locations studied, mainly in West, Central and South part of Romania (
Figure 2). In recent years, it has been reported in more areas of Romania where the host species are
: Quercus robur, Q. petraea, Q.cerris, Q. frainetto and
Q. pubescens but also on
Ulmus minor, Tilia platyphyllos, Tilia tomentosa and
Prunus cerasifera [
66]. The studies confirm the attack on the host trees in all habitats (woods, parks, nurseries, isolated trees, etc.) where the attack intensity varied from 1% to 100% [
17,
66].
The 1% damage intensity has no impact on the tree’s leaves and corresponds to occasional adult populations of up to 10 per host plant. A large attack that can reach 100% reflects a significant number of individuals from several generations that overlap in all three development phases (eggs, nymphs of different generations and adults). Adults and nymphs feed by perforation in the leaf epidermis, resulting in chlorotic patches, where the leaf sap is damaged by the degradation of chlorophyll pigments. In a large attack, all the tree's leaves are damaged.
In 2021, in Sibiu, we identified OLB on a sessile oak tree in the inner courtyard of the Natural History Museum. In 2022, under observation, we confirm the expansion in all areas with oak and sessile oak species. Our local concern related to the necessity of issuing a warning in an attempt to prevent the spread of OLB in the county’s extensive forest ecosystems.
2. Materials and Methods
Sibiu is located in southern Transylvania, in the center of Romania. The city is crossed by the Cibin river watercourse, bordered to the north by Mureș County, to the south by Vâlcea and Argeș counties, to the east by Brașov County and to the west by Alba and Hunedoara counties. Sibiu city is in a temperate (moderate) continental climatic area. Secondary microclimatic thermal influences exist due to the ground wind direction, influenced by relief factors caused by a depression area and the Carpathian and Cindrel mountain ranges that surround the city to the south and southwest. Annual average precipitation is 662 mm, with minimum values in February (26.7 mm) and maximum in June (113 mm) [
86]. The average annual temperature is 8.9°C [
86]. The climate, relief and soil structure create favorable conditions for rich flora and fauna, with a high-density oak and sessile oak forest area that climbs from the city to the hills and slopes of the Cindrel Mountains. In exploring the OLB spread in Sibiu, we started monitoring the species from summer 2021 and throughout 2022, studying its biology, ecology and, especially, its ethology. We tracked down the OLB locations and, based on data collected from the presence of host trees in the observational sites, we created a map representing the spread of the species (
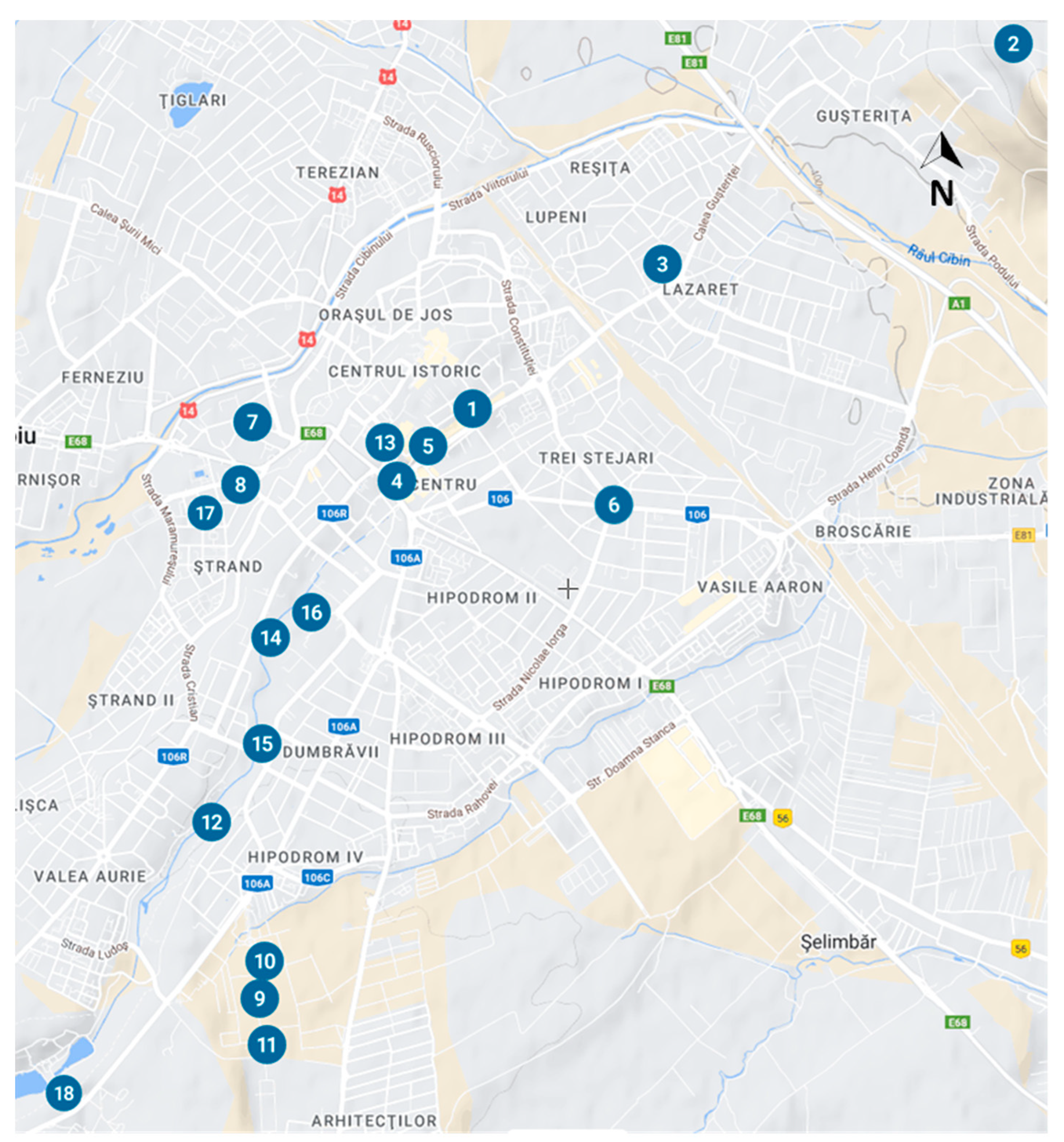
Figure 3). Through our ULBS study we believe our findings to be of scientific importance as we identify novel elements relating to the species' tolerance to minimum winter temperatures, and the number of annual generations that are impacted by the meteorological environment (temperature, humidity, and wind direction).
During August to October 2021 and February to October 2022, we conducted 18 site observations (P
1-P
18), all located in the urban area of Sibiu, not only in isolated stands but also in parks (Sub Arini park-4 sites, Astra and Cetății parks-2 sites), green areas (Natural History Museum of Sibiu, Turismului street, Cârlova street, Ștefan Cel Mare street, Ovidiu street -3 sites, city’s cemetery, Sibiu’ s Zoo) from the districts of Ștrand, Hipodrom, Gușterița and Turnișor, and on trees of Dumbrava Sibiului forest’s edge (
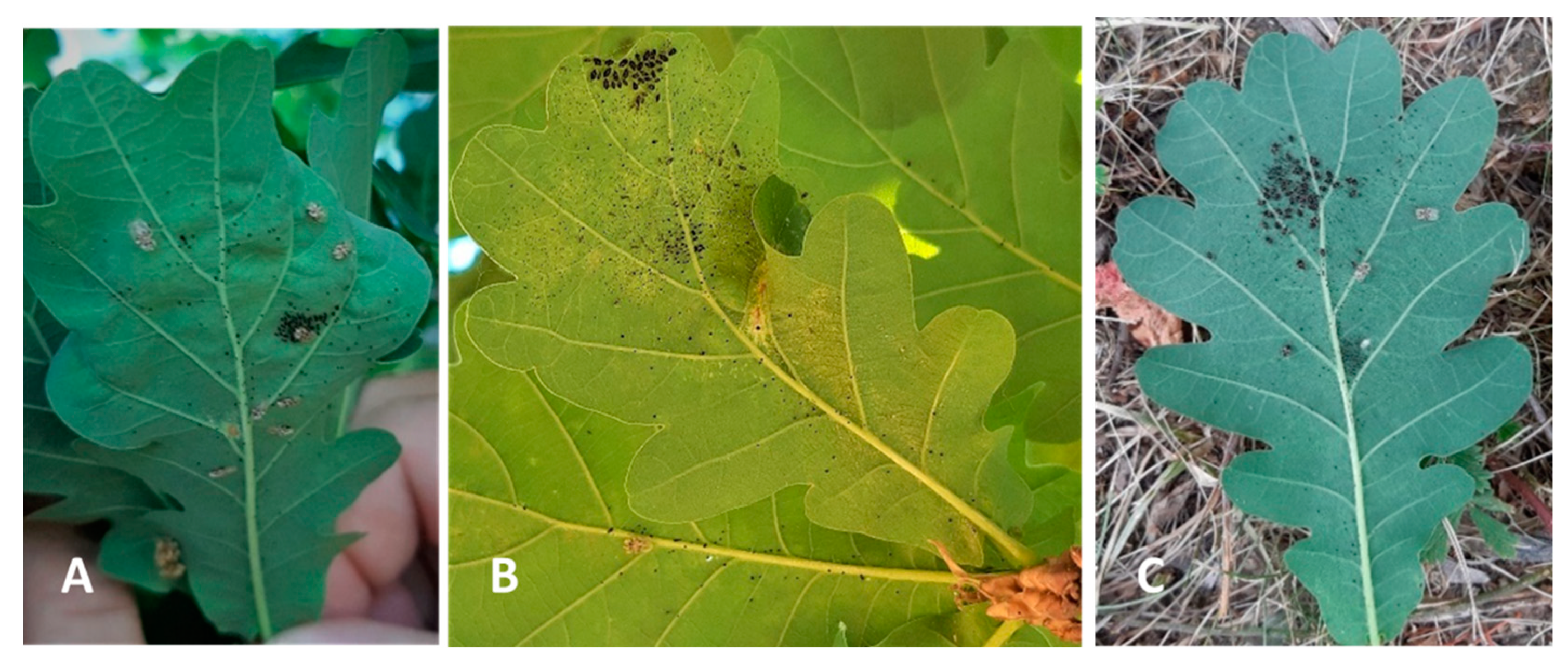
Figure 1). Data collected included the attack intensity, indicating the percentage of the attacked leaves (Figures 7–11). During May-September 2022, we observed OLB adults at distances greater than 500m from any host tree.
This involved detailed monitoring over a two-year period at the 18 sites detailed in the Study Area. We then followed a methodology studying the morphology of the insect and their extent of attack. Signs of OLB presence appear on the underside of the leaves, with yellowish to brownish spots, with the presence of adults, nymphs, or dark excreta recognized as additional signs [
9,
71,
72].
| Observation point |
Latitude |
Longitude |
Dominant forest species |
Average height (m) |
Age (years) |
| (P1)- Museum of Natural History |
45.794861 N |
24.154331 E |
Quercus petraea |
10 |
30 |
| (P2)- Gușterita Hill |
45.8130271 N |
24.1929242 E |
Quercus robur, Q. petraea |
20 |
80 |
| (P3)-Ovidiu Street |
45.8020408 N |
24.1679062 E |
Quercus robur |
15 |
20 |
| (P4)- Cetății Street |
45.7930233 N |
24.1511521 E |
Quercus robur, Q. petraea |
20 |
80 |
| (P5)- Cetății Street |
45.7930287 N |
24.1511370 E |
Quercus robur, Q. petraea |
20 |
80 |
| (P6)- Bâlea Street |
45.7901032 N |
24.1644136 E |
Quercus robur |
15 |
70 |
| (P7)- Turismului Street |
45.7942431 N |
24.1385803 E |
Quercus robur |
12 |
50 |
| (P8)- Vasile Cârlova Street |
45.7910946 N |
24.1377347 E |
Quercus robur |
10 |
30 |
| (P9)- Municipal Cemetery |
45.766123 N |
24.138881 E |
Quercus robur, Q. petraea, Q. polycarpa |
25 |
80-100 |
| (P10)- Municipal Cemetery |
45,767434 N |
24,139437 E |
Quercus robur, Q. petraea, Q. polycarpa |
25 |
80-100 |
| (P11)- Municipal Cemetery |
45.763336 N |
24.139674 |
Quercus robur, Q. petraea, Q. polycarpa |
25 |
80-100 |
| (P12)- Dumbrava Sibiu Forest |
45.774330 N |
24.135681 E |
Quercus robur, Q. petraea, Q. polycarpa |
20 |
100-120 |
| (P13)-Astra Park |
45.793181 N |
24.148019 E |
Quercus robur |
13 |
80 |
| (P14)-Sub Arini Park |
45.7835213 N |
24.1398915 E |
Quercus robur, Q. petraea, |
19 |
60-80 |
| (P15)- Sub Arini Park |
45.7782210 N |
24.1393353 E |
Q. petraea, Quercus robur |
19 |
60-80 |
| (P16)- Sub Arini Park, Silva Restaurant |
45.784750 N |
24.142833 E |
Quercus robur, Q. petraea |
19 |
60-80 |
| (P17)-Onisifor Ghibu High School |
45.789686 N |
24. 135182 E |
Quercus robur |
10 |
40 |
| (P18)- Zoo Sibiu |
45.7609454N |
24.1251612E |
Quercus robur |
20 |
600 |
To determine the attack severity, we use the degree of leaf discoloration, with the benchmark scale being the normal unaffected leaf color. The degree of attack was determined by viewing both sides of leaves affected by OLB, ensuring we excluded from the analysis, discoloration produced by other variables (attack by other insect species, diseases, or other physiological causes such as dryness) (Figures 7–11). Roughly 150 leaves were analyzed at each observation station (P1-P18), between April 1 and September 30, 2022.
Throughout the two years, ethological studies of the species were conducted, documenting the sum of temperatures at each developmental stage and the number of generations. As an invasive species, we witnessed the natural adult spread in all 18 observational districts during the summer of 2022. Adults were carried by air currents, on the clothing of locals, or by cars.
The development and evolution of OLB in Sibiu’s climatic conditions included aspects related to zoogeographical propagation. OLB spread was characterized by a complex set of factors, the most important of which were thermal and precipitation factors.
Three generations were observed between 2021 and 2022, with a single generation being composed of egg stages, five-stages of nymphs, and development to adult stage.
To understand better the period of the OLB development cycle and the number of generations in each year, we processed meteorological data (Sibiu Meteorological Station) to determine the: biological threshold of OLB, thermal constant (K), prolificacy threshold (O), thermal optimum (O1), and upper threshold (T).
The difference (tn-to) between the absolute temperature (tn) and the biological threshold (to) was used to calculate the effective temperature.
Each developmental stage and each generation required a sum of different effective temperatures, which we calculated using Blunck’s developmental equation (1914-1923), Eq.1:
where x
n—time of development, t
0—temperature of development.
In this equation, K is represented by the effective temperature sum, characteristic of the year 2021 and 2022, necessary for one generation development from the stage of egg to nymph stage, then adult.
To determine the period required for the development of one generation, we calculated the multiplication between the period of development xn and the effective temperature (tn – to).
OLBs value of thermal constant (K) was represented by the sum of the development stage thermal constants being egg laying, the appearance of nymphs and adults.
The population dynamics during the development and growth period in Sibiu’ climatic conditions, is represented by the number of generations Eq. 2, in 2021 and in 2022, which we correlated to the actual temperatures using the multiplication equation (Savescu et al., 1969).
Aridity index by Martonne was calculated annually and expressed as being the restrictive character of climatic conditions Eq. 3, for the Sibiu urban vegetation in 2021 and 2022.
Temperature variations played a central role in metabolism, metamorphosis, as OLB spread to different city areas in search of new host trees. This influenced the number of eggs laid on the leaves and the appearance of the 3 generations in the conditions of 2021-2022 (
Table 1 and
Table 2).
Additionally, the precipitation amount during the growing season was an important factor in the OLB spread. Abundant August rainy days were one factor that influenced the third generation (G
3) dynamic. In June-July 2021-2022, Sibiu had a water deficiency, which, according to Connor’s (1988) research, could affect the population feeding preferences, affirming that OLB does not feed on water deficient leaves, thereby slowing growth cycles [
83].
The monthly maximum, minimum and average temperatures, and the correlated quantity of precipitations in 2021-2022 in the city of Sibiu are listed in the tables below:
The lowest temperature months were January and February 2021 and January, February, March 2022, with average temperatures between -4.52°C and -2.53°C. The warmest months were July 2021 and 2022, with maximum temperatures between 28.61-22.82°C. Average annual rainfall in Sibiu in the climatic conditions of 2021 and 2022 varied between 283 mm and 923 mm. Average rainfall values in the driest months were between 0.12 mm and 0.94 mm, while the average rainfall in the wettest month was 3.08 mm and 3.53 mm.
As we better understood how thermal constant played a key role in the generational cycle development, we also wanted to understand how OLB handles extremely low temperatures and if the average warming of minimum temperature in Europe’s climates could be aiding OLB to overwinter and survive.
3. Results
Of the 18 observational sites (P
1-P
18) the most affected habitational type by OLB, were the trees from parks (Sub Arini, Cetății, Astra, city cemetery, Sibiu Zoo) where the extent of attack reached 80-90% at the end of vegetation period. On the sessile oak tree located in the Natural History Museum yard, the attack was 95% (observation point P
1 as presented in
Figure 1), while on other isolated trees the average attack extent was 45% (observation points P
3, P
6, P
7, P
8, P
13, and P
17 as presented in
Figure 1), up to 75% (Figure 11).
Our observational findings concluded that OLB adults preferred the following species of trees:
Q. robur, Q.pubescens Q. petraea and
Q. cerris which grow under an open canopy. Abundance occurs in areas where the trees were exposed to most light, where the leaf surface was greater and therefore the level of carbon was higher. As observed in Astra, Sub Arini and Cetății parks, but also on isolated trees from Ștrand, Hipodrom, Gușterița and Turnișor districts. Abundance density correlates to the leaf water content [
73].
3.1. Biology of the species C. arquata
Adults are relatively small, with a body length of 3-4.5mm and a width of 1-2.5mm.
The head is completely hidden by the pronotal hood, which ends just above the level of the head.
The antennae are long, slender, and covered with hairs [
2]. The first antennae segment length is at least double that of the second, the third is considerably longer than the first two together with the fourth segment shorter than the third and slightly thicker.
The pronotum is broad and covered with small spines on the outer margins.
The wing is broad at the base and the outer margin is strongly reflected anteriorly, with a tumid elevation near the inner anterior margin.
The insect has erected nervures in an irregular pattern on its hemelytra and pronotum [
2].
In spring, after wintering, OLB is found on oak leaves immediately after they appear. Adults feed on the host tree young leaf sap, highlighting their ability to overwinter. Both adults and instars (the phase between two periods of molting) feed on leaf sap by stinging the leaf epidermis and extracting the sap from their cellular tissue. Only the stylets (mandibles) which are very thin parts of their mouth, can penetrate the leaf to suck the sap to feed with it [
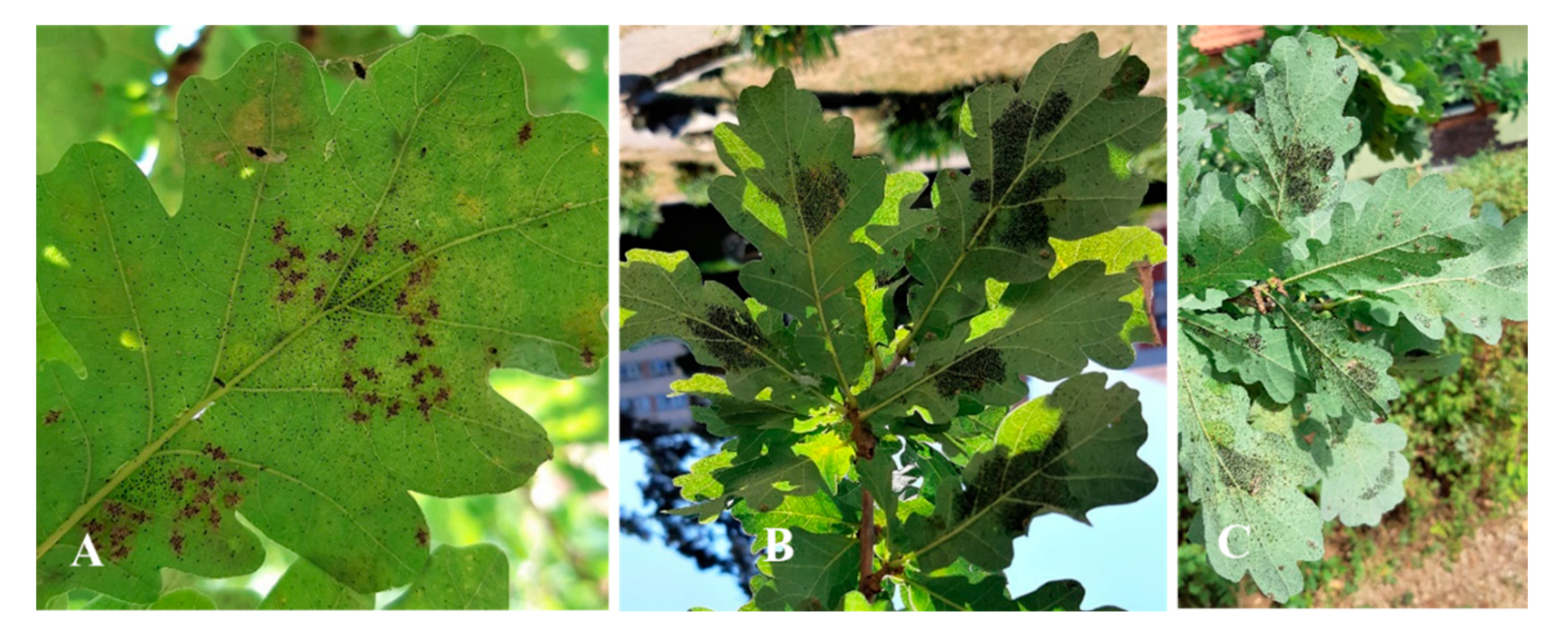
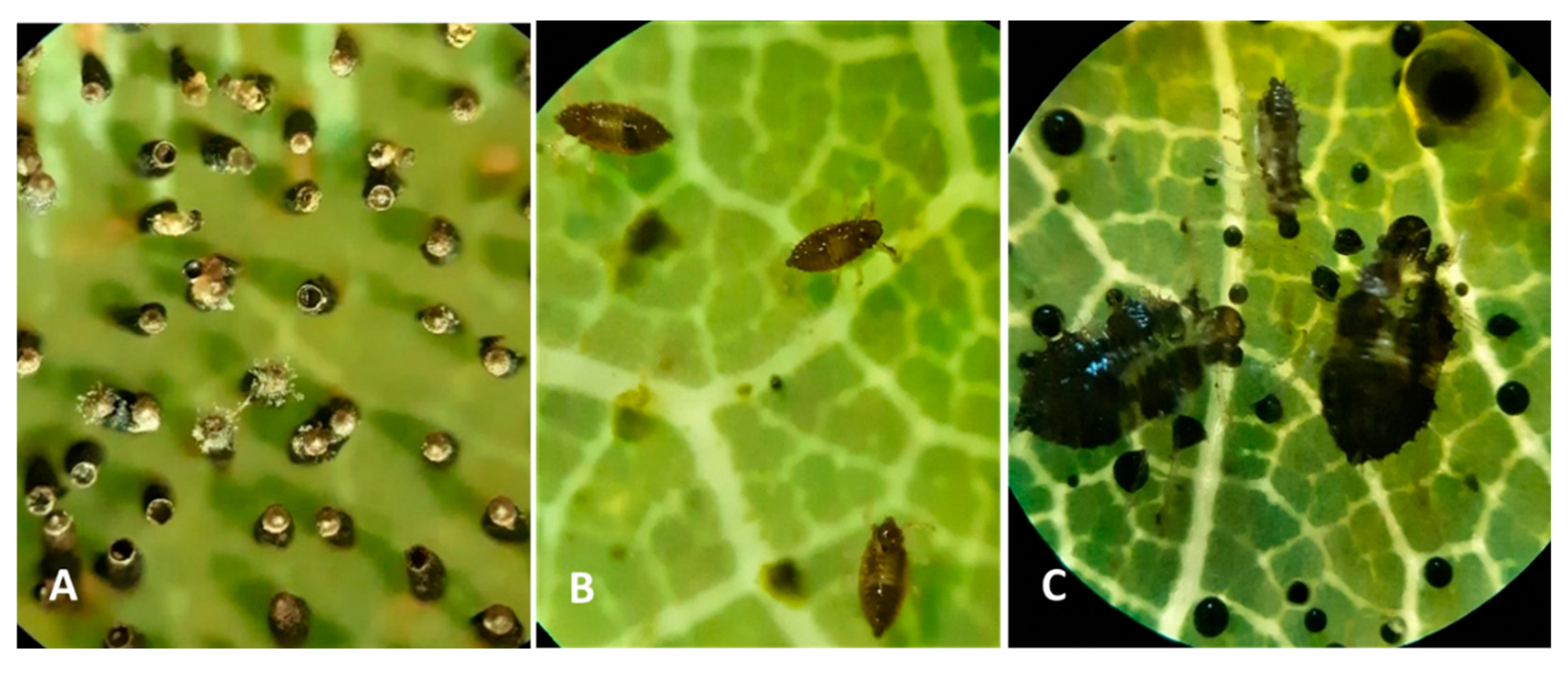
74]. Females lay long, black eggs (
Figure 2a) on the abaxial side of leaves, the number of eggs can vary from 15 up to over 100 eggs per female [
75]. According to Bernardelli (2001), the medium number of eggs laid on the trees analyzed in north Italy is around 100 eggs per cluster and during one vegetative period, 3-4 egg generations were laid. OLB growth and development from egg to adult, composed of 5 successive separate stages of instars (molting) which in Sibiu’s climatic conditions, took between 4 to 6 weeks [
74].
The OLB eggs were embedded deep in the lower side of leaves (
Figure 4a), almost to the operculum, then covered with a brown sticky excretion which rapidly hardens, forming a protective coat. After emerging and drying, the nymphs start to feed, forming clusters near the empty eggshells and adults. If disturbed, they regroup fast. Leaf chlorosis appears because after hatching from the egg clusters, nymphs feeding on the leaf sap begin immediately. Where there are several different nymph stages, feeding is intensive. This leads to complete degradation of chlorophyll pigments [
76].
At the end of 2021, following the field surveys in the months of November, December, January 2022, and February, we observed that OLB overwinters in the adult stage in, on or under bark cracks at a depth of 3-4 cm and displays gregarious behavior even in the hibernation site under the bark.
In Spring (April 21, 2021, and May 5, 2022), only a few adults were identified on leaves, (1-3 individuals).
The first generation of adults (G1) was observed at the beginning of May 2021 and at the end of April 2022.
Second generation adults (G2) were observed at the beginning of June 2021 and at the end of July 2022.
Only the adults observed at the end of August developed a third generation (G3).
In 2021-2022, the development time from nymph to adult varied from 16 to 39 days in Sibiu’s climate, compared to 13 to 27 days in the Italian region climate [
71]. Following the monitoring at the 18 sites (P
1-P
18) and examining the number of eggs on the abaxial side of leaves, we noticed the fact that the OLB’s egg is composed of two distinct regions, the basal part, which is smooth, surrounded by an unknown substance secreted by the female and operculum which is formed by a lip and an apical cap, thus being the most distinctive egg zone.
Not only the edge but also the cap is very porous [
75]. After counting, we noticed the presence of some groups of eggs, which varied between 7 and 80 eggs, being laid alongside the midrib of leaf (
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8). In Sibiu, the adults and the nymphs were observed on the abaxial side of leaf while feeding on the host tree, causing numerous dark spots, while the adaxial side of leaf presented chlorotic discolorations areas [
9,
71].
From July through October, all phases of insect development (eggs, nymphs of varying ages, and adults) were seen on the undersides of leaves. In regions P
1 to P
12 where the attack was most intense, the insect caused tree defoliation. In the absence of natural limiting conditions, it is recognized that a powerful attack reduces the resilience of host trees, making them susceptible to attack by additional diseases and/or other insect pests [
9,
30].
3.2. Ecology of species C. arquata
Temperature, precipitation, and light were ecologically significant physical elements that affected the growth of OLB in the climate of Sibiu.
As with many insects, OLB are poikilothermic creatures, with their body temperature varying in response to the thermal fluctuations of their surroundings [
77].
The temperature of hibernating adults dropped significantly during the diapause, which also explains the endurance of adults during the winter of 2021–2022, when the minimum temperature in January was -3.7
oC, in February it was -2.53
oC, and in March it was +1.79
oC when they emerged from the diapause. Thermal constants, at temperature values, at which insects undergo drastic changes in biochemical processes, are of special relevance in their life and activity and allow acquisition of knowledge of favorable and unfavorable thermal development circumstances [
78].
For OLB, the thermal constants at which the determined chemical and biochemical processes take place were calculated. Thus, it was discovered that OLB development occurred at the biological development threshold (t0) which was 0.3⁰ C (in 2021) and 0.1⁰ C (in 2022) (Equation 4 and Table 4). In Sibiu, OLB enters the hyaline diapause if temperatures fall below these parameters. In the development stage equation, was substituted this temperature value (t0) and derived the thermal constant (K), which had values of 14.2⁰ C (in 2021) and 9.85⁰ C (in 2022) (Equation 5). In Sibiu above these temperatures, OLB entered summer diapause.
Adult G1 flight occurred in early May 2022. Meteorological circumstances during the 2022 OLB growing season had a major effect, impacting its metabolic activity, abundance rate, and dispersion over Sibiu.
The air temperature (minimum, mean, and maximum) proved crucial in determining the OLB behavior [
79].
The values of De Martonne aridity index Equation 3, ranged from 21.18 in 2021 to 34.29 in 2022. It was calculated annually, and it expresses the restrictive nature of the climatic conditions on the urban vegetation of Sibiu, considering the precipitation sums of January-December 2021 and January-September 2022 and the average monthly temperature for the same periods. The calculations results are shown below:
Figure 10.
The degree of attack by adults on sessile oak's leaves: A-Eggs and adults G1 June 5, 2021 (P1); B-Eggs and adults G1 June 19, 2022 (P1); C-Oak leaf attacked by G1 June 26, 2021 (P6).
Figure 10.
The degree of attack by adults on sessile oak's leaves: A-Eggs and adults G1 June 5, 2021 (P1); B-Eggs and adults G1 June 19, 2022 (P1); C-Oak leaf attacked by G1 June 26, 2021 (P6).
Figure 11.
The attack on leaves: A-Upper side of sessile oak leaf attacked on June 30, 2022 (P1); B-Attacked on lower side of the oak leaf on July 10, 2022 (P4); C-Attacked on lower side oak leaf on July 17, 2022 (P7); D-Attacked on lower side of the oak leaf on July 20, 2022 (P11).
Figure 11.
The attack on leaves: A-Upper side of sessile oak leaf attacked on June 30, 2022 (P1); B-Attacked on lower side of the oak leaf on July 10, 2022 (P4); C-Attacked on lower side oak leaf on July 17, 2022 (P7); D-Attacked on lower side of the oak leaf on July 20, 2022 (P11).
Figure 12.
The attack on leaves: A-Lower side of the oak leaf on July 24, 2022 (P1); B-Oak leaves attacked on August 6, 2022 (P10); C-Oak leaves attacked on August 14, 2022 (P17).
Figure 12.
The attack on leaves: A-Lower side of the oak leaf on July 24, 2022 (P1); B-Oak leaves attacked on August 6, 2022 (P10); C-Oak leaves attacked on August 14, 2022 (P17).
Figure 13.
The attack extent on oak leaves by OLB nymphs and adults: A-5% August 28, 2021 (P1); B-25%; September 4, 2022 (P7); C-75% September 11, 2021 (P1); D-95% September 18, 2022 (P1).
Figure 13.
The attack extent on oak leaves by OLB nymphs and adults: A-5% August 28, 2021 (P1); B-25%; September 4, 2022 (P7); C-75% September 11, 2021 (P1); D-95% September 18, 2022 (P1).
3.3. Ethology of the species C. arquata
Our 2022 studies focused on the attacked trees, where we noticed that the intensity of the attack was far more severe compared to 2021. OLB growth was based on a complex set of physical, chemical, biochemical, and biological reactions whose speed varied according to temperature. The degree of attack at 80-90% was greater in trees at the edge of Dumbrava Sibiului forest (P
12), and the cemetery (P
9-P
11). Trees exposed to full light and with sun exposure were more affected (P
1, P
13-P
16, P
18) compared to those inside the stands, the differences of values reaching 50%. At the observational site (P
2-P
8) the interior trees were 25% attacked. The population dynamics during the vegetation period of 2021-2022 varied according to weather factors, and the climate changes during the day influenced daily activity. Some researchers have shown that the insect density on the leaves may not be associated with increased leaf nutrient concentrations, but with the amount of light, higher concentrations of carbon in the leaves (C), lignin and total phenols, and lower levels of cellulose [
80].
Our research supports this thesis. At the end of September 2021, according to our observations, OLB wintered in the adult stage, or eggs laid by the third generation. We are questioning the possibility that eggs are overwintered. Because during 2021-2022 we had three overlapped generations (G
1-G
3). More research in 2023 will seek to understand this. To study the OLB ethology under Sibiu’s climatic conditions, we analyzed the thermic constants which are specific to this species (
Table 3).
The growth and reproduction occurred at the temperature of the prolificacy threshold (O) and the shortest development time and maximum prolificacy occurred at the thermal optimum (O1) while the biological activity of the species ceased at the level of the upper threshold (T). These values calculated for each year are shown in
Table 3.
The minimum temperature at which OLB reacted in the climatic conditions of Sibiu, as well as the average temperatures specific to each generation Equation 4, are presented in
Table 4.
The OLB development constants in Sibiu’s climatic conditions were calculated using Blunck’s evolution equation (1914) elaborated by Săvescu, 1969 Equation 5, calculated by us for each year according to the following correlations:
Prolificacy threshold (O): has been represented by the temperature degree t
n whereas above these values the two genders of OLB become fertile, Equation 6. In 2021, the OLB prolificacy threshold was 5,7⁰ C, while in 2022 it has dropped to 5,3⁰ C.
Superior threshold of temperature (T): In Sibiu’s climatic conditions of 2021-2022, the superior threshold of temperature was constituted by the maximum temperature values above whom the species interrupts its development. The maximum temperature supported Equation 8, by the OLB in the climatic conditions of Sibiu in 2021 was 28.02⁰ C and in 2022 the value decreased to 27.9⁰ C.
The multiplication constants C and √X: The two coordinates of point C, where the multiplication hyperbole and the regression line of the number of generations γ in a year intersect, will be t
n and x
n Equation 9:
The multiplication equation (γ), Equation 10:
Developmental speed of the species (V), Equation 11:
4. Discussion
The desk research identified an alarming, explosive growth in OLB range and density in Europe from 2012 onward. Climatic records from the Sibiu Meteorological Office highlight a marked jump in average temperatures and lower average minimum temperatures also from the decade of 2010+, with the warmest records for Sibiu being 2022, 2019 and 2020, respectively. This supports the fact that the climate now in Europe matches the native climatic conditions.
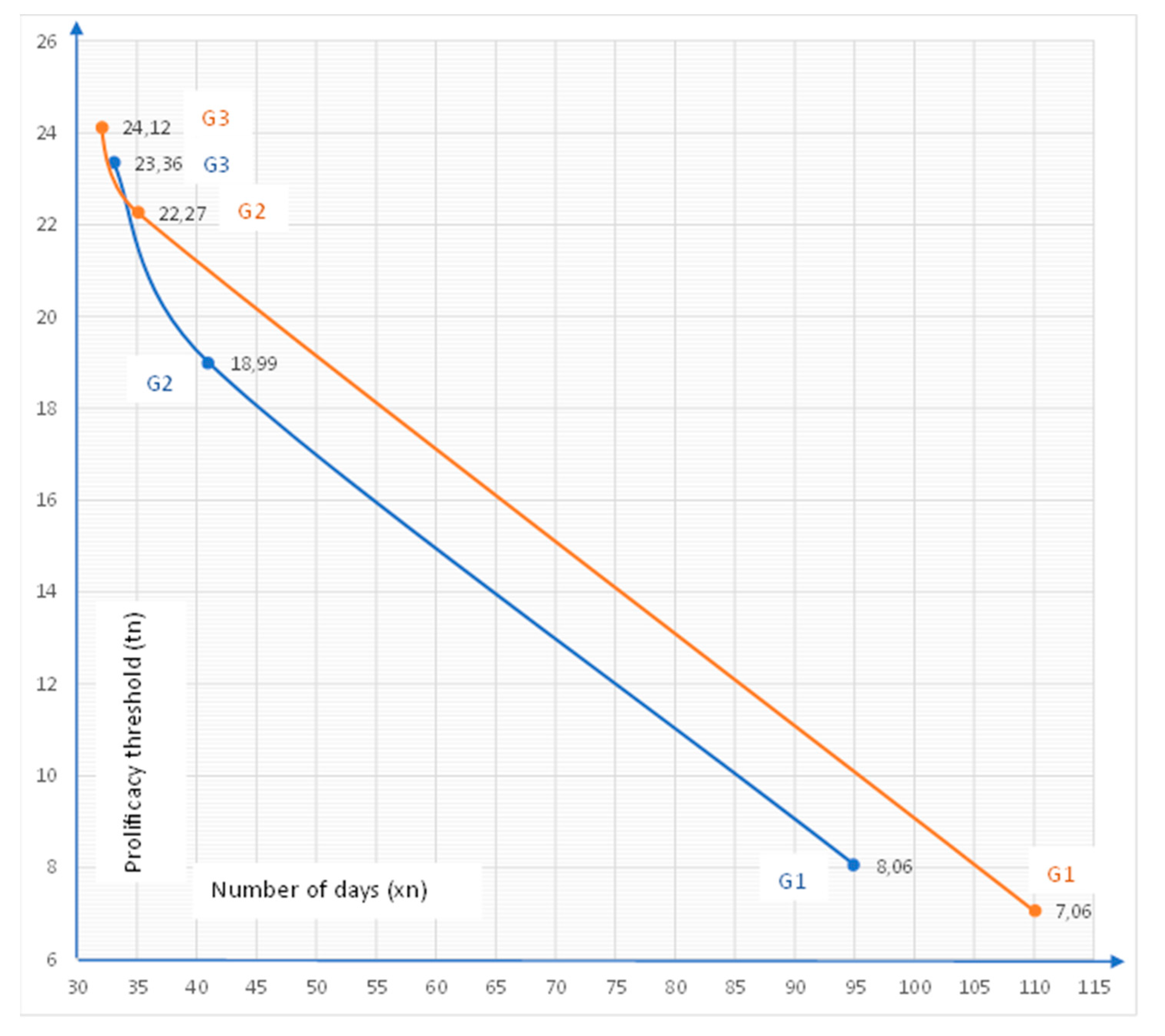
Our field research identified 3 strong generational lifecycles with the extent of attack being stronger in 2022 than 2021, suggesting a good overwintering and with the climate conditions of 2022 being beneficial to both rapid lifecycle development through 5 stages to adult and multiple generations. Other factors do come into play, such as levels of precipitation as dry leaves slow the development cycle and light as OLB prefers sunnier, light-exposed areas more than trees in the middle of the wood with less sun and greater shade. Where OLB has light population numbers and few overlapping generations of nymph, the density of attack is low. However, where population density is high and several generations of nymph all feed at the same time on the oak leaf sap, such that the attack density is high, defoliation occurs. This long-term will be a problem in the healthy development and survivability of the tree, especially if defoliation occurs early in the season. Our statistical research on thermal constant highlighted a tight correlation between temperature and speed of lifecycle development, see the graph below (
Figure 14).
Where the earlier spring, later fall and higher average temperatures with reasonable precipitation levels exist, it is feasible for four generation cycles to fully develop. We witnessed three development cycles in observation points from the Sibiu County area. OLB comes out of its overwintering phase when the average minimum temperature reaches above freezing, i.e. at 0.1c. Feeding begins immediately on the young leaves, which will be rich in sap. As can be seen in the graph, the acceleration of a lifecycle can be less than 16 days from nymph to adult (13 days recorded in Italy). Little is still known about OLB's capability to overwinter and how it achieves this. Further field research is being conducted in the 2022–2023 winter phase. We do not yet know if both forms of adult and egg survive winter, or just the adult. We do not yet know how the adult successfully overwinters.
Are adults form of OLB burying themselves in the soil at a temperature they can survive at, or is the micro-climate of the cracks in the Oak at 3cm-4cm sufficient to keep them alive? OLB has few natural predators in Europe. Chemical control has not been entirely successful and biological control has not been tested enough.
Our further research is oriented in 2023 in this direction to test deeper these control methods. The lack of hard wing cases and the delicate “lace wing” like structure means they are more susceptible to extreme cold conditions. However, the marked warming of Europe in the recent decade is a key factor in the establishment of OLB and the explosive growth in recorded sightings.
4.1. Changing Climate leading to higher temperatures
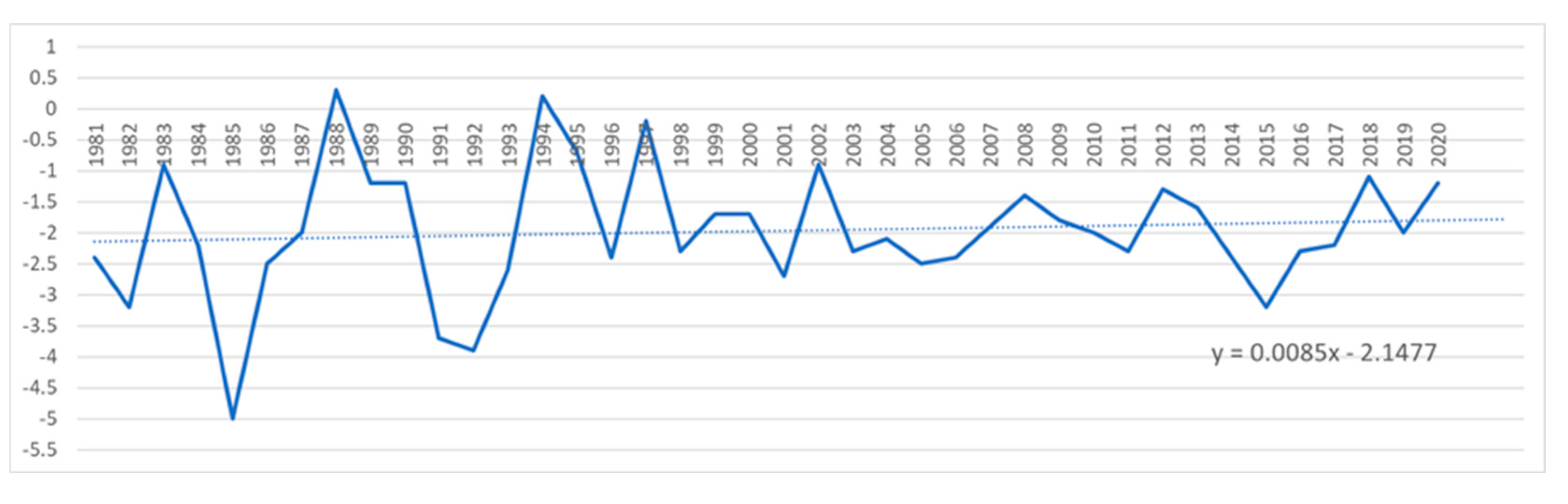
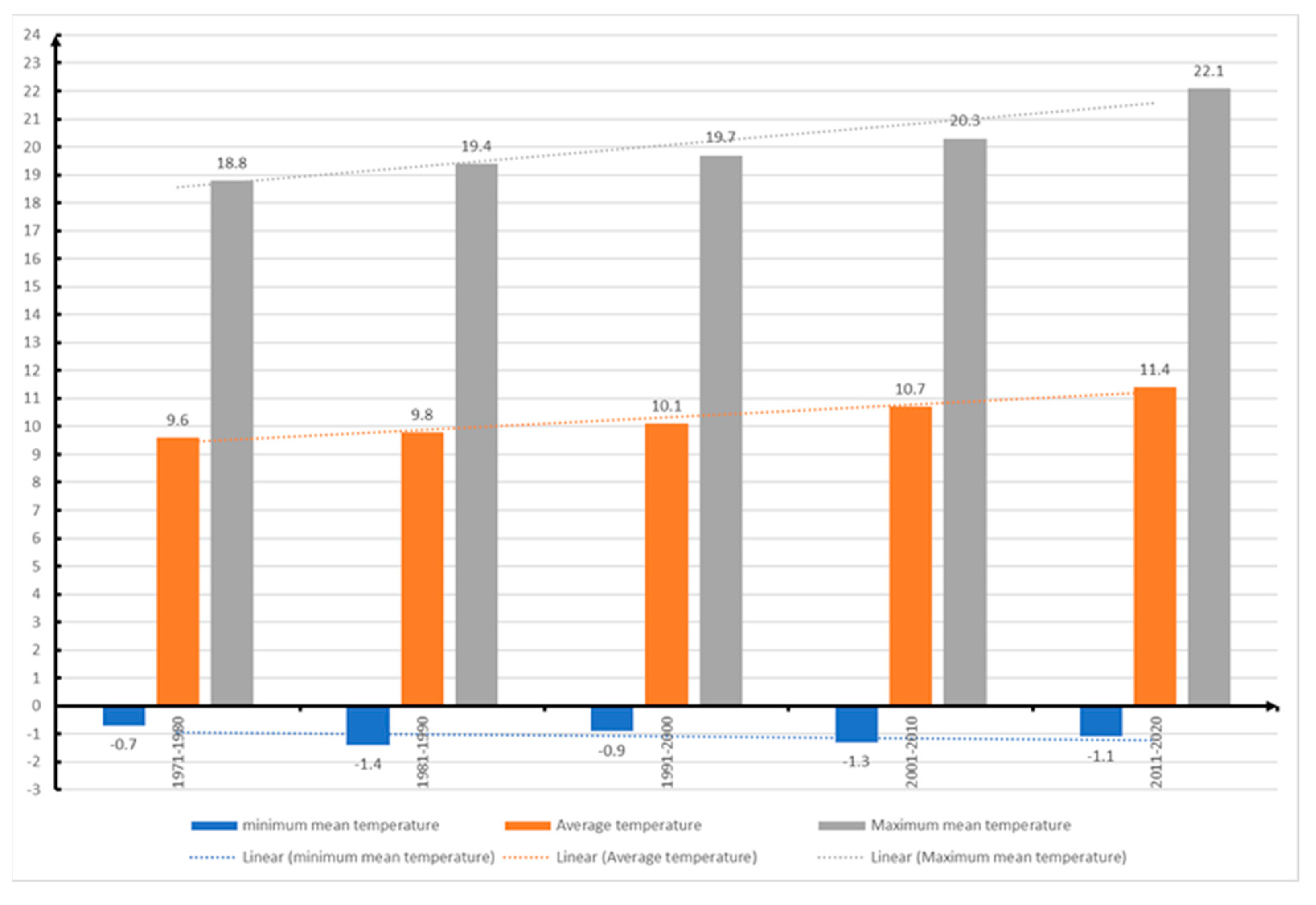
According to data from the National Meteorological Administration of Romania (ANMR), the year 2022 was considered the 3rd warmest year in the history of meteorological measurements in Romania, the average annual temperature was 11.77⁰ C, with a temperature deviation of 1.55⁰ C from the average period between 1981-2010. In the time span 1900-2022, the five warmest years were 2019, 2020, 2022, 2015 and 2007. And in the interval between 2012-2022 represents a period of 11 consecutive years with the highest temperatures in Romania (
Figure 15 and
Figure 16). This information confirms the trend of increasing air temperatures in our country. The climate changes that have led to positive temperature anomalies in Romania between 0.68⁰ C (2021) and 1.92⁰ C (2019), reported between 2012-2022, correspond to the trends reported globally, but especially in Europe [
73,
77]. Also, from the data provided by ANMR, the time period between 1991-2020, considered the current climatic reference period, registers an increase of 0.5⁰ C between multiannual mean air temperatures in Romania, compared to the time between 1981-2010. The largest temperature increases are in the last two decades (2001-2010 and 2011-2020), with frequent daily, monthly, seasonal and annual temperature records being recorded in the last twenty years [
87].
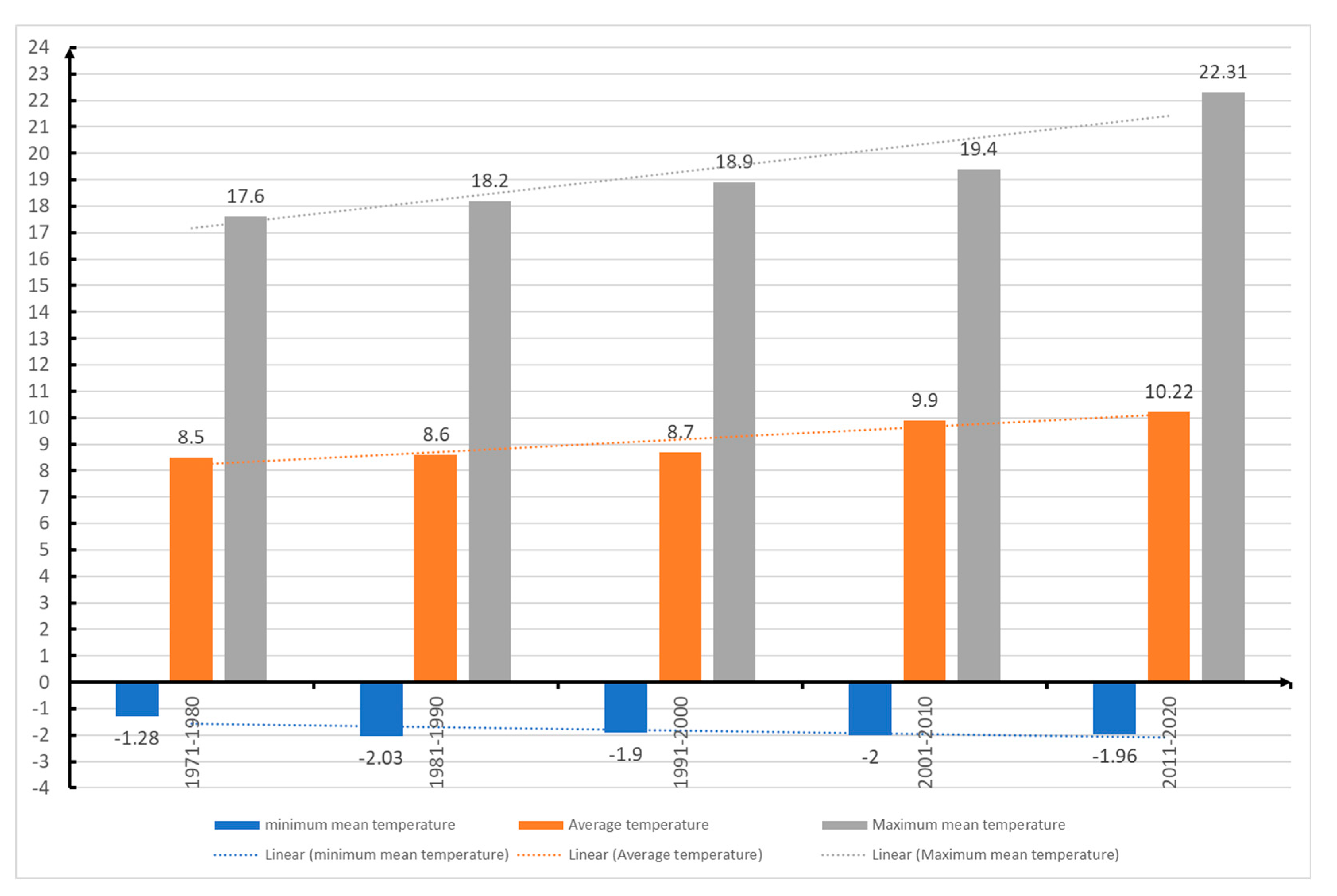
These climatic changes may be the hypothesis of the OLB invasion in our country, as the insect finds climatic conditions similar to its country of origin. The development of the species and the attack of
Quercus sp. in the climatic conditions of Sibiu (
Figure 17) and the appearance of the three generations (
Table 3) is also linked to the higher temperatures in May-August when the values were between 8.06⁰ C - 23.36⁰ C in 2021 and 7.06⁰ C - 22.27⁰ C in 2022. In 2022, positive monthly temperature deviations were recorded at the national level, the highest in decreasing order being in February and December (+3.1⁰ C), November (+2.6⁰ C), August (+2.0⁰ C), June (+1.8⁰ C), July (+1. 7⁰ C), October (+1.6⁰ C), January (+1.3⁰ C) and May (+0.4⁰ C). This explains why in 2022 the OLB needed 0.1⁰ C biological development threshold compared to 0.3⁰ C in 2021, and why the first G1 adults were reported in May.
Based on these temperatures, it can be observed that the number of days decreased between generations starting with G
1 (95 days in 2021 and 110 days in 2022), G
2 (41 days in 2021 and 32 days in 2022) and G
3 (33 days in 2021 and 35 days in 2022) directly proportional to the temperature increase between May and August (
Figure 17). The distribution of seasonal precipitation amounts in the year 2021-2022 were below climatological averages in the fall, spring and summer seasons, except in winter 2021-2022 when December recorded values above normal.
5. Conclusions
OLB was first documented in Sibiu in 2021 on an oak tree in the Natural History Museum’s courtyard (P
1). During 2021 and 2022, this tree’s leaves were marked to monitor the number of generations and determine development cycles. Another 17 places (P2-P18) were examined, where the same parameters relating to the development and generation numbers, as well as attack extent, which ranged from 25% to 95%, were monitored (
Figure 3). The species of trees attacked in Sibiu (
Quercus robur, Q. petraea, Q. polycarpa) are preferred hosts, just like the other areas reported in other countries where OLB's presence has been confirmed [
71,
72]. OLB attack intensity between those two years does not differ significantly. In the area of origin, the species survives in less extreme conditions [
71], which explains why the species has survived and thrived in the south-eastern area of Hungary from 2016 to 2017 [
34,
35] and in the similar conditions in west and southern areas of Romania [
17]. In Sibiu, the 2021 minimum annual temperature was -0.84
o C and in 2022 (January-September) was -0.62
o C. Sibiu’s climatic conditions now (in the last decade) are similar to the native area of origin, Eastern North America, [
17]. Climate Change would appear to play a very strong correlation to the establishment and now rapid growth of OLB in Sibiu and Europe. In the current context of the invasion in Sibiu, the most important step was to detect and to monitor the species, to understand the behavior of this pest, to contribute to form decisions based on scientific research on how best to control and limit the attack in the forest ecosystems in Sibiu County. During the two-year study in Sibiu, information on population abundance and environmental conditions in the area was documented, as well as the identification of factors influencing the development and reproduction of the invasive OLB populations [
70]. Following our study, we conclude that the likely origin of the OLB infestation in Sibiu could be Cluj County, located 175 km from where the species was reported in 2017 in Baciu Forest [
66]. The spread to Sibiu was possible via transportation, by wind and air currents.
As the specialists who have studied the ecology and biology of OLB agree, it can spread quickly via roads and railways, in all stages (egg, nymphs and adults); also, the insect can be transported by pedestrians, car traffic and rail traffic [
24,
31,
37,
81,
82].
The species can also be passively spread by the wind [
24,
37] and Sibiu has dominant winds (Mureşan wind that blows from Mures county), with high frequency and from a north-west direction, the mountain breeze and the so called ‘Great Wind’ (snow eater) which manifests in early spring.
As soon as the presence of OLB was detected, we sought to rapidly identify all infected areas in Sibiu County. Early detection of infected host trees and applying control precautions reduced the chance of spreading to new areas. The published information until now about the spread of OLB in Romania [
70] and other European countries can be helpful in predicting its likely future spread. In Sibiu, OLB was found mainly on oak species (
Quercus spp.), but in the future it can infest other types of trees such as elm (
Ulmus spp.), lime (
Tilia spp.) and rose (
Rosa spp.) [
37,
83,
84,
85]. In 2021-2022, damage to monitored trees (P1-P18) was caused by sap feeding by adults and nymphs, causing a decrease in photosynthesis [
42], premature defoliation and discoloration of leaves [
37,
61] and reduction of resistance to attack of other pests, diseases, and sources of pollution [
24]. Considering the adaptive capacity of OLB, we can conclude that this insect, which does not cause major damage in the native area, causes extremely severe damage in other geographical areas such as Sibiu. OLB can be considered a serious problem for both forest ecosystems and for trees in the city’s parks and gardens, as it can affect the aesthetics of trees and in the future also the production of wood [
69]. This conclusion is worrying in terms of the future of
Quercus stands in Sibiu County, which in 2022 were 95% affected (P
1) by this invasion. We therefore intend to carry out more research in the future to determine what might prevent this species from spreading farther into the Sibiu region and find ways to eradicate it. We expect that in 2023, if climatic conditions are favorable, the increase and spread of attacks will extend throughout the county in all forest ecosystems because of the lack of natural limiting factors and lack of control methods in this stage of invasion. To limit the OLB attack in Sibiu County, we suggest that there is an urgent need to develop a set of biosecurity measures as well as to support research by the relevant bodies.
Author Contributions
Conceptualization, CSM, GM, MR, MS and DS; methodology, CSM, GM.; software, CSM, GM, MR; validation, CSM, GM, MR, MS and DS; formal analysis, CSM abd GM.; investigation, CSM and DS; resources, CSM, GM, MR, MS and DS; data curation, CSM, GM, MR, MS and DS; writing—original draft preparation, CSM, GM, MR, MS and DS.; writing—review and editing, CSM, GM, MR, MS and DS visualization, CSM, GM, MR, MS and DS; supervision, CSM, GM, MR, MS and DS.; project administration, CSM; funding acquisition, CSM. All authors have read and agreed to the published version of the manuscript.
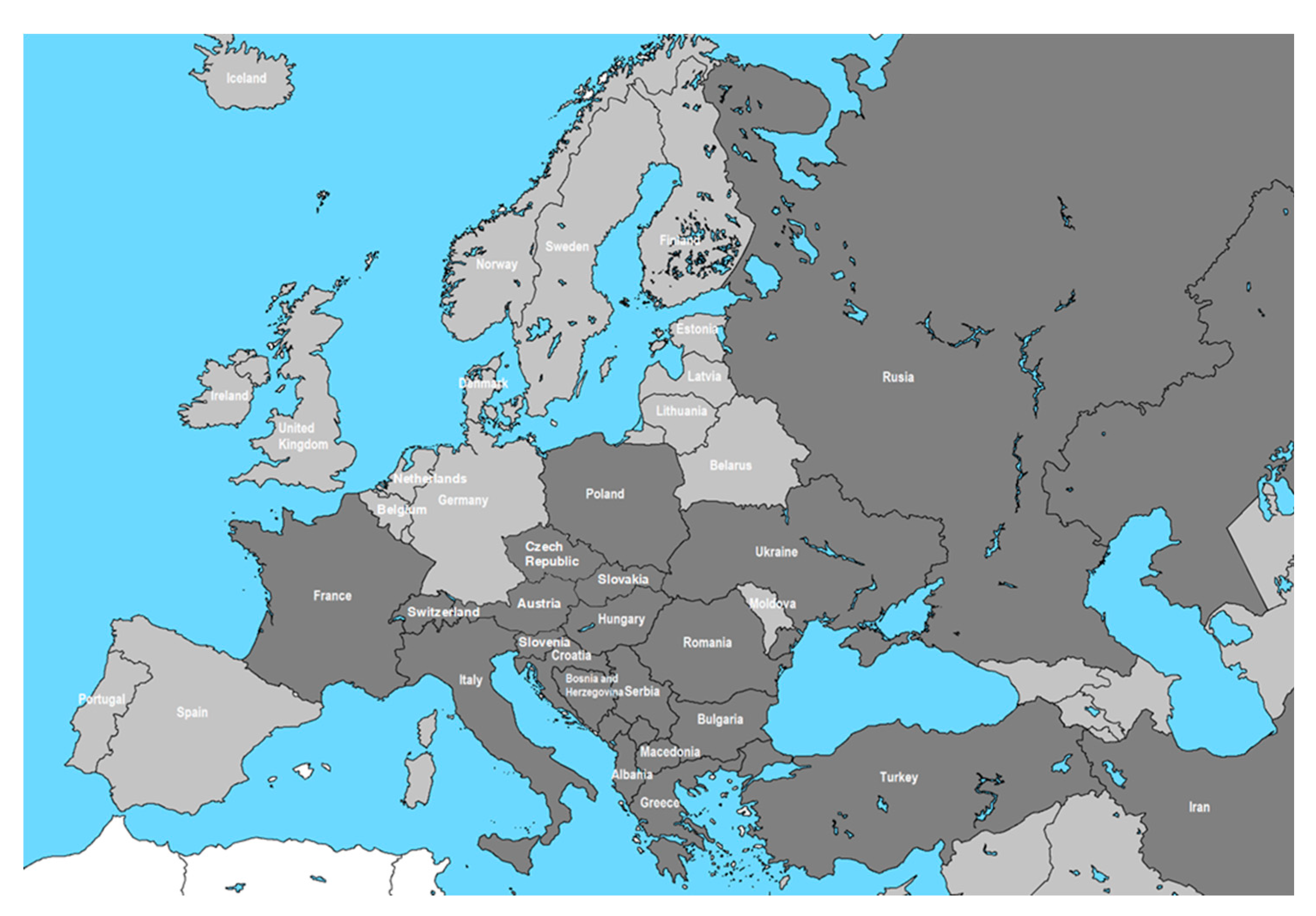
Figure 1.
Chronological spread in Europe–the dark gray highlights recorded sightings of OLB: 2000-Italy [
19,
20]; 2002- Switzerland [
21,
22]; 2003- Turkey [
9,
23,
24,
25,
26,
27,
28]; 2005- Iran [
28]; 2009-Poland [
29]; 2012-Bulgaria [
30,
31]; 2013- Hungary [
32,
34,
35,
36,
37]; 2013-Croația [
39]; 2013-Serbia [
40,
41,
42]; 2015- Russia [
24,
43,
44,
45,
46,
47]; 2015-Romania [
52,
62,
64,
65,
67,
68,
69,
70]; 2016- Albania, Bosnia and Herzegovina, Greece and Ukraine [
48,
50,
51,
53,
54]; 2017-Slovenia [
48,
49]; 2017-France [
55]; 2018- Slovakia [
56]; 2018- Bosnia [
54]; 2019- Austria [
57]; 2019- North Macedonia [
58]; 2019- Czech Republic [
59].
Figure 1.
Chronological spread in Europe–the dark gray highlights recorded sightings of OLB: 2000-Italy [
19,
20]; 2002- Switzerland [
21,
22]; 2003- Turkey [
9,
23,
24,
25,
26,
27,
28]; 2005- Iran [
28]; 2009-Poland [
29]; 2012-Bulgaria [
30,
31]; 2013- Hungary [
32,
34,
35,
36,
37]; 2013-Croația [
39]; 2013-Serbia [
40,
41,
42]; 2015- Russia [
24,
43,
44,
45,
46,
47]; 2015-Romania [
52,
62,
64,
65,
67,
68,
69,
70]; 2016- Albania, Bosnia and Herzegovina, Greece and Ukraine [
48,
50,
51,
53,
54]; 2017-Slovenia [
48,
49]; 2017-France [
55]; 2018- Slovakia [
56]; 2018- Bosnia [
54]; 2019- Austria [
57]; 2019- North Macedonia [
58]; 2019- Czech Republic [
59].
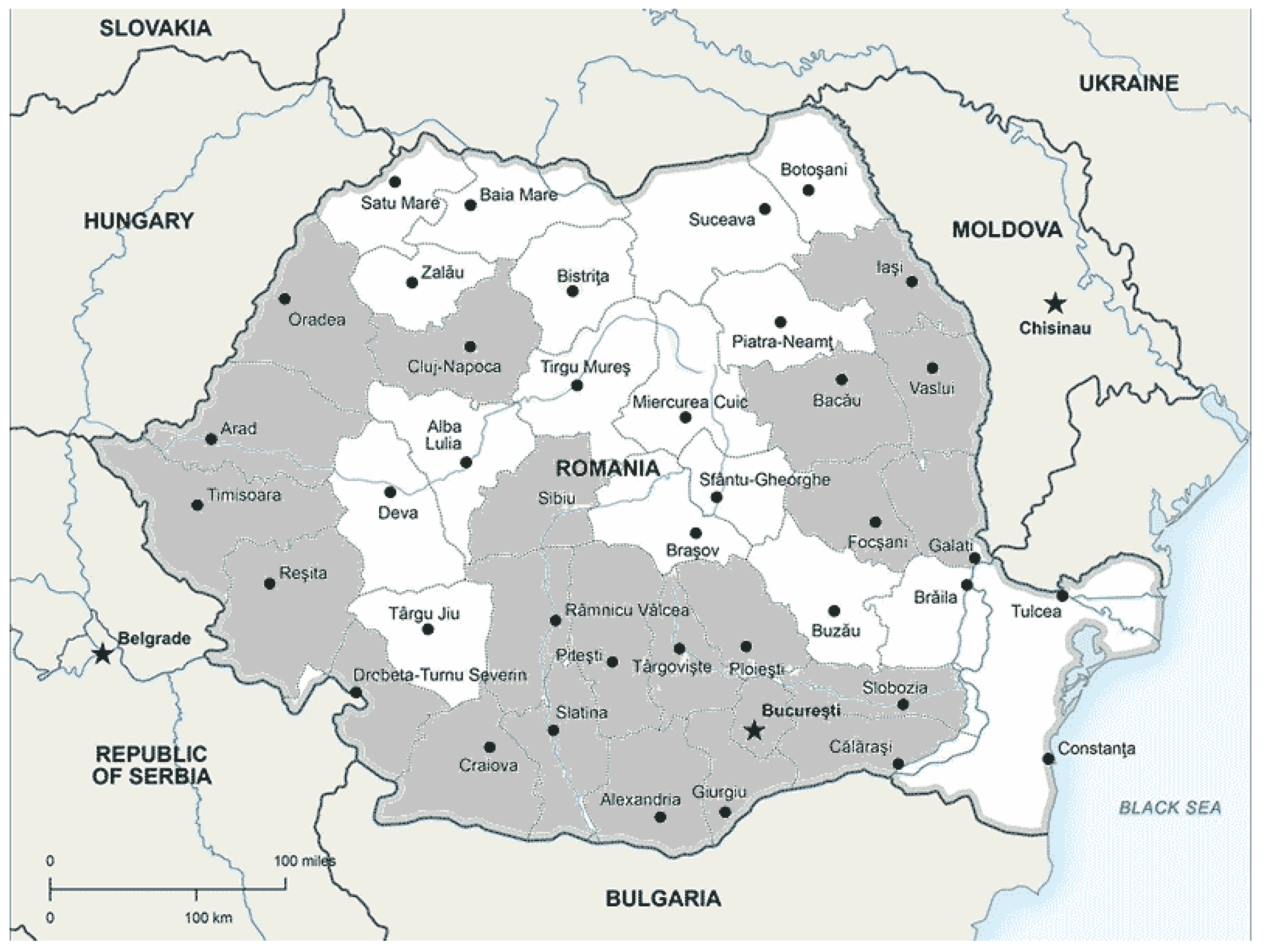
Figure 2.
Chronological spread in Romania- in the dark gray shaded areas are recorded identification of OLB: 2015-Arad [
63]; 2016- București, Teleorman [
52,
63,
64,
65,
66]; 2017-Argeș, Bacău, Bihor, Caraș Severin, Călărași, Cluj, Dâmbovița, Dolj, Galați, Giurgiu, Ialomița, Iași, Ilfov, Mededinți, Olt, Prahova, Timiș, Vaslui, Vâlcea, Vrancea [
17,
66,
68,
69,
70]; 2021-Sibiu (Stancă-Moise).
Figure 2.
Chronological spread in Romania- in the dark gray shaded areas are recorded identification of OLB: 2015-Arad [
63]; 2016- București, Teleorman [
52,
63,
64,
65,
66]; 2017-Argeș, Bacău, Bihor, Caraș Severin, Călărași, Cluj, Dâmbovița, Dolj, Galați, Giurgiu, Ialomița, Iași, Ilfov, Mededinți, Olt, Prahova, Timiș, Vaslui, Vâlcea, Vrancea [
17,
66,
68,
69,
70]; 2021-Sibiu (Stancă-Moise).
Figure 3.
Location of the 2022 study area in Sibiu with map, below detailed locations.
Figure 3.
Location of the 2022 study area in Sibiu with map, below detailed locations.
Figure 4.
Microscope image on sessile oak leaf from observation point P1 of: A-Eggs stage July 24, 2022; B-Nymph stage 2, August 21, 2022; C-excrements and nymph in stage 2 and 4, September 25, 2022.
Figure 4.
Microscope image on sessile oak leaf from observation point P1 of: A-Eggs stage July 24, 2022; B-Nymph stage 2, August 21, 2022; C-excrements and nymph in stage 2 and 4, September 25, 2022.
Figure 5.
Microscope image on oak leaf of Nymph Stage 4 from: A-Observation point P5, July 10, 2022; B-Observation point P7, July 3, 2022; C- Observation point P12, August 14, 2022.
Figure 5.
Microscope image on oak leaf of Nymph Stage 4 from: A-Observation point P5, July 10, 2022; B-Observation point P7, July 3, 2022; C- Observation point P12, August 14, 2022.
Figure 6.
Microscope image on sessile oak leaf from observation point P1: A-Eggs and nymphs G1, G2, June 26, 2022; B-Nymph stage G3, August 14, 2022; C-Nymph stage 5, G3, September 4, 2022.
Figure 6.
Microscope image on sessile oak leaf from observation point P1: A-Eggs and nymphs G1, G2, June 26, 2022; B-Nymph stage G3, August 14, 2022; C-Nymph stage 5, G3, September 4, 2022.
Figure 7.
Microscope image on an oak leaf of: A-Nymph stage 1 and 4, observation point P9, September 12, 2022; B-Nymphs stage 3, G3, observation point P6, September 29, 2022; C-sessile oak leaf, Adult G3, observation point P1, October 2, 2022.
Figure 7.
Microscope image on an oak leaf of: A-Nymph stage 1 and 4, observation point P9, September 12, 2022; B-Nymphs stage 3, G3, observation point P6, September 29, 2022; C-sessile oak leaf, Adult G3, observation point P1, October 2, 2022.
Figure 8.
Microscope image on an oak leaf of adults: A-Adults G1, observation point P4, June 26, 2022; B-Adult G2, observation point P10, July 10, 2022; C-Adult G3, observation point P12, October 18, 2022.
Figure 8.
Microscope image on an oak leaf of adults: A-Adults G1, observation point P4, June 26, 2022; B-Adult G2, observation point P10, July 10, 2022; C-Adult G3, observation point P12, October 18, 2022.
Figure 9.
Attacked Sessile oak's leaves: A-Eggs from winter February 3, 2022 (P1); B-Presence of adults on the leaves May 3, 2021 (P1); C-Presence of adults on the leaves May 15, 2022 (P1); D-Adults on oak leaves May 19, 2022 (P3).
Figure 9.
Attacked Sessile oak's leaves: A-Eggs from winter February 3, 2022 (P1); B-Presence of adults on the leaves May 3, 2021 (P1); C-Presence of adults on the leaves May 15, 2022 (P1); D-Adults on oak leaves May 19, 2022 (P3).
Figure 14.
Correlation between temperature and speed of lifecycle development.
Figure 14.
Correlation between temperature and speed of lifecycle development.
Figure 15.
Minimum temperature in Sibiu from 1981 up to 2020.
Figure 15.
Minimum temperature in Sibiu from 1981 up to 2020.
Figure 16.
Minimum temperature in Romania from 1971 up to 2020.
Figure 16.
Minimum temperature in Romania from 1971 up to 2020.
Figure 17.
Minimum temperature in Sibiu from 1971 up to 2020.
Figure 17.
Minimum temperature in Sibiu from 1971 up to 2020.
Table 1.
Average temperatures and the quantity of precipitations, during the year 2021.
Table 1.
Average temperatures and the quantity of precipitations, during the year 2021.
| Month |
T minimum (oC) |
T average (oC) |
T maximum (oC) |
Precipitations (mm) |
| January |
-3.7 |
0.36 |
4.53 |
0.12 |
| February |
-2.53 |
2.48 |
8.07 |
0.26 |
| March |
1.79 |
2.86 |
8.88 |
2.29 |
| April |
2.42 |
7.68 |
13.91 |
1.78 |
| May |
8.63 |
14.36 |
20.46 |
3.53 |
| June |
12.55 |
18.85 |
25.08 |
2.55 |
| July |
15.39 |
22.45 |
28.82 |
2.96 |
| August |
13.34 |
19.9 |
16.78 |
1.84 |
| September |
8.35 |
14.31 |
21.62 |
2.08 |
| October |
2.59 |
7.88 |
14.80 |
0.43 |
| November |
0.31 |
4.99 |
11.81 |
0.94 |
| December |
0.84 |
2.12 |
5.33 |
1.98 |
| Total (average) |
4.55 |
9.90 |
15.86 |
1.87 |
Table 2.
Average temperatures and the quantity of precipitations, during the year 2022.
Table 2.
Average temperatures and the quantity of precipitations, during the year 2022.
| Month |
T minimum (o C) |
T average (o C) |
T maximum (o C) |
Precipitations (mm) |
| January |
-4.52 |
-0.62 |
3.9 |
0.38 |
| February |
-3.13 |
1.87 |
8.51 |
0.35 |
| March |
-3.36 |
2.25 |
8.58 |
0.79 |
| April |
1.98 |
8.39 |
14.98 |
2.44 |
| May |
8.23 |
16.01 |
22.87 |
1.04 |
| June |
12.19 |
19.79 |
26.81 |
1.64 |
| July |
13.97 |
21.59 |
28.61 |
2.15 |
| August |
14.91 |
21.33 |
27.19 |
1.84 |
| September |
9.86 |
13.77 |
19.67 |
3.08 |
| Total (average) |
5.57 |
11.60 |
17.9 |
1.52 |
Table 3.
The constants of development for the species C. arcuata in the climatic conditions of Sibiu.
Table 3.
The constants of development for the species C. arcuata in the climatic conditions of Sibiu.
| Year |
Generation |
Period (day/month) |
Constant of temperature K (o C) |
Prolificacy threshold tn
|
Number of days (xn) |
Biological threshold(t0) |
| 2021 |
G1
|
23.II.-29. V. |
765.5 |
t1= 8.06 |
95 |
0.3o C |
| G2
|
29.V-8.VII |
778.9 |
t2=18.99 |
41 |
| G3
|
9.VII-11. VIII. |
770.9 |
t3=23.36 |
33 |
| 2022 |
G1
|
2.II.-23.VI. |
776.9 |
t1=7.06 |
110 |
0.1o C |
| G2
|
24.IV.-1.VII. |
771.8 |
t2=24.12 |
32 |
| G3
|
2.VII-6. VIII. |
779.3 |
t3=22.27 |
35 |
Table 4.
Average temperatures specific to each generation during 2021-2022.
Table 4.
Average temperatures specific to each generation during 2021-2022.
| Year 2021/ average temperature according to generation |
Year 2022/ average temperature according to generation |
|
|
|
|
|
|