1. Introduction
Human T-lymphotropic virus type 1, also known as human T-leukemia virus (HTLV-1), was the first human retrovirus to be isolated and associated with disease before HIV [
1]. HTLV-1 is transmitted through either i) blood, through blood transfusions in areas where screening is not performed, or through the exchange of needles and syringes containing contaminated blood; ii) from mother-to-child during pregnancy, at birth or mainly through breastfeeding; iii) through unprotected sexual intercourse [
2].
In the city of Salvador, Brazil, a region where HTLV-1 infection is endemic, a population-based study showed that a higher prevalence of HTLV-1 infection in women that increases with age. In 2002, approximately 10% of women over 50 years of age were found to infected with HTLV-1 [
3]. In addition, sexual intercourse has been described as the main route of transmission in this city’s general population [
4].
The presence of HTLV-1 has been detected in semen and cervicovaginal secretions from infected individuals. Although greater efficacy of male-to-female sexual transmission has been reported, female-to-male transmission also occurs and should therefore not be neglected [
5,
6,
7,
8]. HTLV-1 mainly infects CD4+ T lymphocytes, as well as CD8+ T lymphocytes to a lesser extent [
9]. The virus integrates into the cell genome in the form of a provirus. Infected T cells produce few free virus particles; therefore, quantification of the integrated virus (provirus) is expressed as proviral load (PVL) [
10]. In general, the proportion of lymphocytes is higher in the seminal fluid than in the vaginal fluid of healthy individuals [
10], which may partly explain the greater effectiveness of male-to-female HTLV-1 transmission.
We have previously shown higher levels of Th1, Th2, and Th17 inflammatory cytokines in the vaginal fluid from HTLV-1-infected women compared to that of uninfected women [
11]. In this study, we aimed to quantify PVL in the vaginal fluid of HTLV-1-infected women and then investigate correlations with PVL in peripheral blood mononuclear cells (PBMC).
2. Materials and Methods
2.1. Patients and study design
This was a cross-sectional observational study conducted at the Integrative Multidisciplinary HTLV Center (CHTLV) of the Bahiana School of Medicine and Public Health (EBMSP) in Salvador, Bahia, Brazil [
12]. Women infected with HTLV-1 were consecutively recruited during medical consultations and referred for gynecological examinations. Relevant inclusion and exclusion criteria are described elsewhere [
13]. Briefly, HTLV-1-infected women (positivity on ELISA and Western blot) aged 20 to 50 years who had been sexually active in the past four weeks were included. Patients were classified as asymptomatic (no signs of myelopathy) or diagnosed with HAM/TSP (HTLV-associated myelopathy/Tropical Spastic Paraparesis) according to the criteria established by Castro Costa et al. [
14].
2.2. Ethical Approval
The present study protocol was approved by the Institutional Research Board of EBMSP (CAAE 33098414.4.0000.5544). All procedures were planned and carried out in accordance with the ethical principles established by the Declaration of Helsinki [
15]. All included women signed a term of informed consent.
2.3. Sample Collection
Clinical and demographic data were collected using a standardized form. Whole blood samples were collected in EDTA tubes and peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation and cryopreserved until use. A complete gynecologic examination and the collection of cervicovaginal specimens were performed by a single trained gynecologist [
13]. Cotton swabs were used to collect fluid from the ectocervix, endocervix, and vaginal walls for PVL measurement. Samples obtained for evaluation of vaginal PVL were then placed in tubes containing 400 µl hydroxymethyl ethylene diamine tetra-acetic acid (Tris-EDTA) solution and stored at -20°C. For cytopathological and vaginal microbiota analysis, Papanicolaou smear samples were collected from the ectocervix and endocervix using an Ayres spatula and cytobrush, respectively. The collected samples were fixed in absolute alcohol for further processing.
2.4. Sample Analysis
Measurement of HTLV-1 PVL was performed in cervicovaginal cells and PBMCs. DNA was extracted using spin column DNA extraction system (Qiagen, Hilden, Germany). HTLV-1 proviral load was quantified using a real-time TaqMan polymerase chain reaction (PCR) method, as described previously [
16]. Briefly, SK110/SK111 primers were used to amplify a 186 bp fragment of the pol gene and dual TaqMan probe (5′-FAM/5′ VIC and 3′-TAMRA) was located at 4,829–4,858 bp of the HTLV-1 reference sequence (HTLVATK). Albumin DNA was used as an endogenous reference. The value of HTLV-1 proviral load was reported as the [(HTLV-1 average copy number)/(albumin average copy number)] × 2 × 10
6 and expressed as the number of HTLV-1 copies per 10
6 cells in both blood and vaginal fluid samples, as previously described [
11,
17]. Results were analyzed using 7500 v2.0.1 software (Applied Biosystems). Cell abnormalities detected by Papanicolaou smear tests were classified in accordance with the Bethesda System using light microscopy [
18].
2.5. Statistical evaluations
Quantitative sociodemographic variables without normal distributions, such as family income, educational level, age of partner, number of partners and parity, are presented as medians with 25th and 75th percentiles. Age, a quantitative variable with normal distribution, is presented as mean with standard deviation. The qualitative variables skin color and marital status are expressed as simple frequencies/proportions. PVL comparisons were evaluated using the nonparametric Wilcoxon Signed Rank Test, expressed as median values and 25th and 75th percentiles. Spearman’s rank correlation coefficient was used to analyze the relationship between proviral load in vaginal fluid compared to PBMCs. Differences in cervicovaginal cytopathology and vaginal microbiota profiles were assessed using the Chi-squared test. p-values <0.05 were considered statistically significant. All analyses were performed using GraphPad software version 9.5 and SPSS software version 17.0 for Windows.
3. Results
Fifty-six women were studied, 43 of whom were asymptomatic and 13 diagnosed with HAM/TSP. Almost half of the patients (46.4%) self-reported black skin color. The mean patient age was 35.9 (± 7.2) years. Most participants reported being married or in a stable relationship (73.2%) with a median partner age of 42 (33.5-47.5) years. The median number of lifetime sexual partners was 4 (3-13.5) (
Table 1).
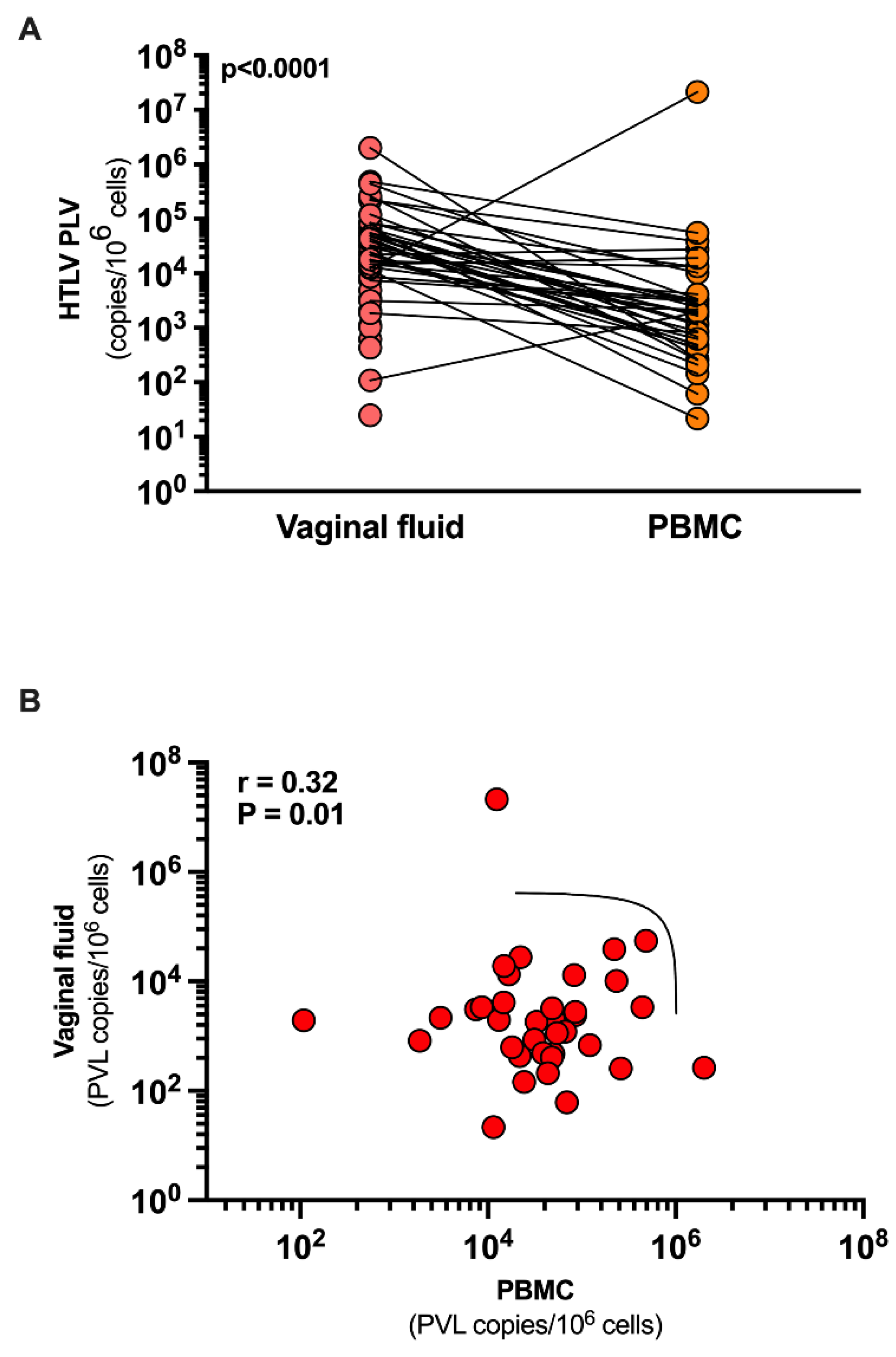
Of the 56 patients included, PVL was detectable in the vaginal fluid of 36 (64.3%) women compared to 54 women in PBMCs (96.5%). In two women, PVL was undetectable in both the vaginal fluid and PBMCs. PVL levels were statistically lower in vaginal fluid: median 451.9 copies/10
6 cells (IQR: 0 - 2,672), compared to PBMCs: median 23,264 copies/10
6 (IQR: 7,420 - 64,371) (p< 0.0001). A positive correlation was observed between PVL in vaginal fluid and PBMCs, R=0.32 (p=0.01) (
Figure 1).
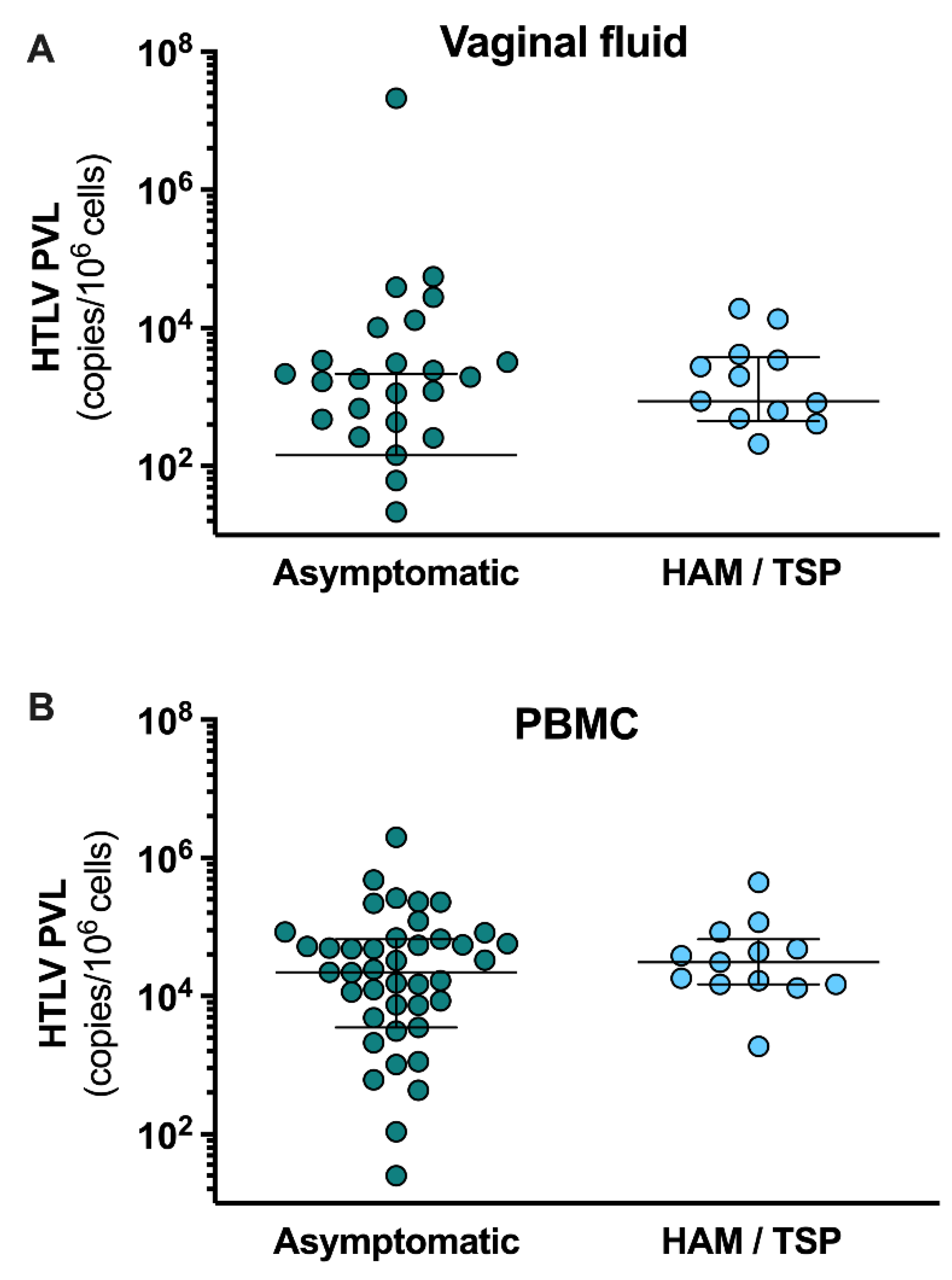
PVL levels in PBMCs from asymptomatic carriers (22,155 copies/10
6 cells [IQR: 3,521 - 66,538]) were similar to those in patients with HAM / TSP (31,080 copies/10
6 cells [IQR: 14,799 - 66,420]), (p = 0.30) (
Figure 2). Regarding PVL in cervicovaginal fluid, no statistical differences (p = 0.62) were found between asymptomatic women (144.6 copies/10
6 cells [IQR: 0 - 2,168]) and carriers of HAM / TSP (869 copies/10
6 cells [IQR: 447.9 - 3,760]) (
Figure 2). PVL was not detected in the vaginal fluid of 20 women, 19 of whom were asymptomatic carriers. Moreover, cytopathologic findings and vaginal microbiota were similar between these groups (
Table 2).
An additional assessment of the cervicovaginal environment was performed comparing women with detectable and undetectable HTLV-1 PVL in vaginal fluid. No statistical differences were found between the groups with regard to neoplasia in the cervicovaginal cytopathology. One patient among those with detectable PVL had atypical squamous cells of undetermined significance (ASC-US). Women with undetectable PVL presented a higher frequency of
Lactobacillus spp. (p = 0.004) and a lower frequency of Coccus / Bacillus (p = 0.001). The frequency of
Gardnerella vaginalis /
Mobiluncus spp. and
Candida spp. were similar between the groups (
Table 3).
4. Discussion
The results of the present study demonstrate a direct correlation between HTLV-1 PVL in vaginal fluid and PVL in PBMCs. Interestingly, a 50-fold difference in PVL levels were found in vaginal fluid compared to peripheral blood. Of note, PVL in vaginal fluid was undetectable in one-third of the women evaluated, almost all (n=19/20) of whom were asymptomatic. To our knowledge, this was the first study that aimed to quantify and correlate HTLV-1 PVL in vaginal fluid and PBMCs.
The detection of HTLV-1 DNA in cervicovaginal secretion samples was previously reported in 68% of sex workers infected with the virus in Peru, which was further associated with cervicitis [
19]. In addition, a study in Gabon evaluated women with HTLV-1 DNA in vaginal fluid, demonstrating the local production of anti-HTLV-1 antibodies, suggesting an immune response in the vaginal mucosa [
20]. Our work has previously shown that HTLV-1-infected women exhibit an activated immune response in the vaginal mucosa, as reflected by higher concentrations of cytokines, such as IL-2, TNF, IL-10, IL-4, and IL-17, in cervical fluid compared to uninfected women. Cytokine levels in vaginal fluid have not been found to correlate with HTLV-1 PVL in cervicovaginal fluid or peripheral blood mononuclear cells in asymptomatic patients [
11]. In addition, cytokine analyses revealed no differences between women with detectable or undetectable PVL (data not shown).
The role of PVL as a predictor for the development of HTLV-1-associated diseases has been suggested, as patients with HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM / TSP) and adult T-cell lymphoma/leukemia (ATLL) present higher PVL in PBMCs than asymptomatic carriers [
17,
21]. Patients with isolated neurological changes, peripheral neuropathy, and neurogenic bladder may also have higher PVL than asymptomatic patients [
22,
23]. Regarding correlations between PVL in PBMCs and other body fluids, a previous study indicated a positive correlation between PVL in PBMCs and saliva, with higher mean PVL found in patients diagnosed with HAM/TSP compared to asymptomatic carriers, suggesting the possible influence of systemic inflammatory symptoms on oral health as well as potential transmission via saliva [
24]. A similar correlation was found between PVL in peripheral blood and cerebrospinal fluid (CSF) by Lezin et al. in 2005, who further demonstrated an relevant association with the patients’ neurological symptoms [
25]. A study conducted in Japan found that higher HTLV-1 PVL in the blood could be indicative of more severe lung involvement, which was also closely correlated with lymphocyte counts and increased PVL in bronchoalveolar fluid [
26]. Thus, the presence of HTLV-1 PVL in body compartments may be directly related to localized inflammation and cytokine production, in addition to the presence of diseases associated with HTLV-1. Herein, HTLV-1 PVL was found to be much less detectable in the vaginal fluid of asymptomatic women, which correlated with lower PVL in the PBMCs from these patients. This may be due to the presence of HTLV-1-infected cells in vaginal fluid being related to the natural process of vaginal transudation, which allows lubrication through vasodilation-transudation reactions [
27,
28], or localized activation of the immune response in the vaginal environment. Importantly, cytopathologic findings and cytokine levels in vaginal fluid were not observed to differ between women with detectable and undetectable PVL. Moreover, cytopathologic findings were similar between asymptomatic carriers and women diagnosed with HAM/TSP.
The present study, it was not possible to determine the factors for correlation of HTLV-1 PVL between PBMC and vaginal fluid. This interference between the systemic immune response and the immune response activated in the vaginal environment could explain the higher viral concentrations of HTLV-1 in different mucous membranes. However, this still requires in-depth studies, including those on CD4+ and CD8+ T cell counts.
Regarding vaginal microbiota, significantly higher levels of
Lactobacillus spp. were found in women with undetectable PVL in vaginal fluid, which is likely not clinically relevant considering that high levels are a common finding in the microbiota [
29]. In addition, Coccus/Bacillus was more abundant in women with detectable PVL in vaginal fluid. While these pathogens may be associated with bacterial vaginosis, they are also present in healthy women [
30,
31]. Zunt et al. associated cervicitis with increased cervical shedding of HTLV-1 and sexual transmission [
19]. However, as confirmatory testing was not performed on the present sample, it was impossible to confirm the species of pathogens found and their possible association with cervicitis, which represents a limitation regarding this association.
The presence of HTLV-1 in the vaginal fluid of infected women may have implications on the route of viral transmission. Sexual transmission of HTLV-1 can occur both from male-to-female as well as vice versa [
7]. Overall, the frequency of CD4+ T lymphocytes, the main cells targeted by HTLV-1, is higher in the semen than in the vaginal fluid of healthy individuals [
10]. However, although no studies have attempted to quantify HTLV-1 PVL in the semen of infected individuals, it has been speculated that higher PVL in semen may explain the presumed greater efficacy of male-to-female sexual transmission [
32]. A report on serodiscordant couples suggested a greater risk of sexual HTLV-1 transmission to a seronegative partner when the infected partner presented higher HTLV-1 PVL in PBMCs compared to couples in which transmission did not occur [
7]. On the other hand, the presence of HTLV-1 in vaginal fluid may also be relevant to the vertical transmission of HTLV-1, which is especially important considering that no consensus exists regarding the preferred route of delivery (vaginal versus cesarian) in HTLV-1-infected women. Although less common, transmission from mother-to-child occurs in up to 5% of women who have not breastfed their infants [
33,
34,
35]. Therefore, it is of utmost importance to determine whether women with higher HTLV-1 PVL may be more likely to transmit the virus to newborns during pregnancy or delivery. To date, the use of antiretroviral drugs has not been recommended during pregnancy for HTLV-1-infected women.
5. Conclusions
Our results support the notion that PVL in peripheral blood may correlate with PVL in vaginal fluid, which is a relevant factor not only for the sexual route of female-to-male HTLV-1 transmission, but could also have repercussions on vertical transmission, particularly in the context of vaginal delivery.
Author Contributions
BG-C and MFRG: conceptualization. AAF, PRTGF, ALLM, THAA, LLG, ESB, JPLA and MFRG: data curation. AAF, PRTGF, ALLM, THAA, LLG, ESB, JPLA, BG-C and MFRG: formal analysis and methodology. All authors involved in investigation, writing—original draft, review, and editing, contributed to the article, and approved the submitted version.
Funding
This work was supported by the Coordination of Superior Level Staff Improvement-Brazil (CAPES) - Finance Code 001, The Bahia State Research Support Foundation (FAPESB) - BOL0525/2019 and National Foundation for the Development of Private Higher Education (FUNADESP), grants 9600140 and 9600141. Maria Fernanda R. Grassi and Bernardo Galvão-Castro are research fellows of CNPq (308167/2021-0 and 473667/2012-6, respectively).
Institutional Review Board Statement
The present study protocol was approved by the Institutional Research Board of EBMSP (CAAE 33098414.4.0000.5544). All procedures were planned and carried out in accordance with the ethical principles established by the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would also like to thank Andris K. Walter for critical analysis, English language revision, and manuscript copyediting assistance.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415-9. [CrossRef]
- Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol. 2012 Nov 15;3:388. [CrossRef]
- Dourado I, Alcantara LC, Barreto ML, da Gloria Teixeira M, Galvão-Castro B. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr. 2003 Dec 15;34(5):527-31. [CrossRef]
- Nunes D, Boa-Sorte N, Grassi MF, Taylor GP, Teixeira MG, Barreto ML, Dourado I, Galvão-Castro B. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS One. 2017 Feb 3;12(2):e0171303. [CrossRef]
- Murphy EL. The clinical epidemiology of human T-lymphotropic virus type II (HTLV-II). J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 Suppl 1:S215-9. [CrossRef]
- Manns A, Hisada M, La Grenade L. Human T-lymphotropic virus type I infection. Lancet. 1999 Jun 5;353(9168):1951-8. [CrossRef]
- Roucoux DF, Wang B, Smith D, Nass CC, Smith J, Hutching ST, Newman B, Lee TH, Chafets DM, Murphy EL; HTLV Outcomes Study Investigators. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. J Infect Dis. 2005 May 1;191(9):1490-7. [CrossRef]
- Pereira CCC, La-Roque DG de L, Albuquerque R dos S, Silva IC, Covre L de SC, Nobre AFS, Reis M de NL dos, Assis IM de, Souza JD de, Moraes SS de, Santos PFSL, Silva LBL da, Almeida D de S, Sousa MS de. Human T-lymphotropic virus (HTLV) research in cervical-vaginal discharge samples from women, in Belém, Pará, Brazil. RSD. 2021 Apr.1 ;10(4):e9410413867. [CrossRef]
- Yasunaga Ji, Sakai T, Nosaka K, Etoh Ki, Tamiya S, Koga S, Mita S, Uchino M, Mitsuya H, Matsuoka M. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood. 2001 May 15;97(10):3177-83. [CrossRef]
- Pique C, Jones KS. Pathways of cell-cell transmission of HTLV-1. Front Microbiol. 2012 Oct 24;3:378. [CrossRef]
- Firmino AA, Martins ALL, Gois LL, Paixão TS, Batista EDS, Galvão-Castro B, Grassi MFR. Evaluation of the cervicovaginal environment in asymptomatic Human T-cell lymphotropic virus type 1 infected women. Braz J Infect Dis. 2019 Jan-Feb;23(1):27-33. [CrossRef]
- Galvão-Castro B, Grassi MFR, Galvão-Castro AV, Nunes A, Galvão-Barroso AK, Araújo THA, Rathsam-Pinheiro RH, Nunes CLX, Ribeiro A, Lírio M, Gonçalves NL, Rangel SL, Dias CMCC, Ozores DP, Dubois-Mendes SM, Lima I, Silva ALP, de Jesus WLA, Santos FLN, de Oliveira JGR, de Moraes YVP, de Jesus AO, Daltro F, Boa-Sorte N, Castro-Lima H, Soliani MLC. Integrative and Multidisciplinary Care for People Living With Human T-Cell Lymphotropic Virus in Bahia, Brazil: 20 Years of Experience. Front Med (Lausanne). 2022 Jun 7;9:884127. [CrossRef]
- Lopes Martins AL, Rios Grassi MF, de Aquino Firmino A, Lacerda Araujo JP, Paixao TS, Galvão-Castro B, Boa-Sorte N. Human T-Lymphotropic Virus-1-Associated Myelopathy/Tropical Spastic Paraparesis Is Associated With Sexual Dysfunction in Infected Women of Reproductive Age. Sex Med. 2018 Dec;6(4):324-331. [CrossRef]
- De Castro-Costa CM, Araújo AQ, Barreto MM, Takayanagui OM, Sohler MP, da Silva EL, de Paula SM, Ishak R, Ribas JG, Rovirosa LC, Carton H, Gotuzzo E, Hall WW, Montano S, Murphy EL, Oger J, Remondegui C, Taylor GP. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses. 2006 Oct;22(10):931-5. [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191-4. [CrossRef]
- Dehée A, Césaire R, Désiré N, Lézin A, Bourdonné O, Béra O, Plumelle Y, Smadja D, Nicolas JC. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J Virol Methods. 2002 Apr;102(1-2):37-51. [CrossRef]
- Grassi MF, Olavarria VN, Kruschewsky Rde A, Mascarenhas RE, Dourado I, Correia LC, de Castro-Costa CM, Galvão-Castro B. Human T cell lymphotropic virus type 1 (HTLV-1) proviral load of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients according to new diagnostic criteria of HAM/TSP. J Med Virol. 2011 Jul;83(7):1269-74. [CrossRef]
- Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, Young N; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002 Apr 24;287(16):2114-9. [CrossRef]
- Zunt JR, Dezzutti CS, Montano SM, Thomas KK, Alarcón JO, Quijano E, Courtois BN, Sánchez JL, Campos P, Gotuzzo E, Guenthner PC, Lal RB, Holmes KK. Cervical shedding of human T cell lymphotropic virus type I is associated with cervicitis. J Infect Dis. 2002 Dec 1;186(11):1669-72. [CrossRef]
- Bélec L, Georges-Courbot MC, Georges A, Mohamed AS, Londos-Gagliardi D, Hallouin MC, Hocini H, Guillemain B. Cervicovaginal synthesis of IgG antibodies to the immunodominant 175-199 domain of the surface glycoprotein gp46 of human T-cell leukemia virus type I. J Med Virol. 1996 Sep;50(1):42-9. [CrossRef]
- Okayama A, Stuver S, Matsuoka M, Ishizaki J, Tanaka G, Kubuki Y, Mueller N, Hsieh CC, Tachibana N, Tsubouchi H. Role of HTLV-1 proviral DNA load and clonality in the development of adult T-cell leukemia/lymphoma in asymptomatic carriers. Int J Cancer. 2004 Jul 1;110(4):621-5. [CrossRef]
- Santos SB, Oliveira P, Luna T, Souza A, Nascimento M, Siqueira I, Tanajura D, Muniz AL, Glesby MJ, Carvalho EM. Immunological and viral features in patients with overactive bladder associated with human T-cell lymphotropic virus type 1 infection. J Med Virol. 2012 Nov;84(11):1809-17. [CrossRef]
- Tanajura D, Castro N, Oliveira P, Neto A, Muniz A, Carvalho NB, Orge G, Santos S, Glesby MJ, Carvalho EM. Neurological Manifestations in Human T-Cell Lymphotropic Virus Type 1 (HTLV-1)-Infected Individuals Without HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis: A Longitudinal Cohort Study. Clin Infect Dis. 2015 Jul 1;61(1):49-56. [CrossRef]
- Lins L, de Carvalho VJ, de Almeida Rego FF, Azevedo R, Kashima S, Gallazi VN, Xavier MT, Galvão-Castro B, Alcantara LC Jr. Oral health profile in patients infected with HTLV-1: clinical findings, proviral load, and molecular analysis from HTLV-1 in saliva. J Med Virol. 2012 Sep;84(9):1428-36. [CrossRef]
- Lezin A, Olindo S, Oliere S, Varrin-Doyer M, Marlin R, Cabre P, Smadja D, Cesaire R. Human T lymphotropic virus type I (HTLV-I) proviral load in cerebrospinal fluid: a new criterion for the diagnosis of HTLV-I-associated myelopathy/tropical spastic paraparesis? J Infect Dis. 2005 Jun 1;191(11):1830-4. [CrossRef]
- Mori S, Mizoguchi A, Kawabata M, Fukunaga H, Usuku K, Maruyama I, Osame M. Bronchoalveolar lymphocytosis correlates with human T lymphotropic virus type I (HTLV-I) proviral DNA load in HTLV-I carriers. Thorax. 2005 Feb;60(2):138-43. [CrossRef]
- Masters WH, Johnson VE. eds. Human Sexual Response. New York: Little, Brown and Company, 1966.
- Woodard TL, Diamond MP. Physiologic measures of sexual function in women: a review. Fertil Steril. 2009 Jul;92(1):19-34. [CrossRef]
- Amabebe E, Anumba DOC. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front Med (Lausanne). 2018 Jun 13;5:181. [CrossRef]
- Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014 Aug 1;210(3):338-43. [CrossRef]
- Coudray MS, Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020 Feb;245:143-148. [CrossRef]
- Kajiyama W, Kashiwagi S, Ikematsu H, Hayashi J, Nomura H, Okochi K. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986 Nov;154(5):851-7. [CrossRef]
- Kinoshita K, Amagasaki T, Hino S, Doi H, Yamanouchi K, Ban N, Momita S, Ikeda S, Kamihira S, Ichimaru M, et al. Milk-borne transmission of HTLV-I from carrier mothers to their children. Jpn J Cancer Res. 1987 Jul;78(7):674-80. PMID: 2887539.
- Caterino-de-Araujo A, de los Santos-Fortuna E. No evidence of vertical transmission of HTLV-I and HTLV-II in children at high risk for HIV-1 infection from São Paulo, Brazil. J Trop Pediatr. 1999 Feb;45(1):42-7. [CrossRef]
- Kashiwagi K, Furusyo N, Nakashima H, Kubo N, Kinukawa N, Kashiwagi S, Hayashi J. A decrease in mother-to-child transmission of human T lymphotropic virus type I (HTLV-I) in Okinawa, Japan. Am J Trop Med Hyg. 2004 Feb;70(2):158-63. PMID: 14993627.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).