1. Introduction
Plant propagation methods include sowing, stem cutting, and tissue culture. The sowing method has the following advantages: (1) a large number of plants can be secured at once, (2) it reduces labor, and (3) it does not require a relatively large number of auxiliary facilities as that required by a tissue culture facility. However, some species must have the conditions required for seed germination. “Seed dormancy," an innate seed property, defines the environmental conditions that must be met before the seed can germinate [
1]. Thus, to propagate plants using the sowing method, a suitable dormancy-breaking technique specific to the seeds must first be determined [
2].
Various types of seed dormancy exist depending on the life cycle of the plants, ambient environmental conditions, and their geographical distribution; seed dormancy has been studied and classified according to plant species and genera [
3]. Lang [
4] classified seed dormancy into three types: eco-dormancy, para-dormancy, and endo-dormancy. Baskin and Baskin [
3] classified seed dormancy into five types by comprehensively considering physiological and morphological factors: physiological dormancy (PD), where inhibitory compounds inside and outside the seeds prevent germination; morphological dormancy (MD), where the seeds contain underdeveloped or immature embryos; morphophysiological dormancy (MPD), which is a combination of PD and MD; physical dormancy (PY), which involves suppression of water absorption by the seeds; and combinational dormancy (PY+PD), which is a combination of PY and PD.
Berberis koreana Palibin, commonly known as Korean barberry, is a deciduous shrub of Berberidaceae, endemic to Korea. Berberidaceae members, comprising approximately 450 species, have a worldwide distribution [
5]. These plants are native to Central Asia, East Asia, and South America, with some species distributed in North America, Europe, and Africa [
6]. The Korean representatives of
Berberis comprise three species and four varieties, namely,
B. koreana Palibin,
B. koreana var.
angustipolia Nakai,
B. koreana var.
ellipsoidea Nakai,
Berberis amurensis Rupb,
B. amurensis var.
latifolia Nakai,
B. amurensis var.
quelpaertensis Nakai, and
Berberis poiretii C. K. Schneid. [
7]. The leaves of
B. koreana are toxic, but its stems and roots are used as medicine to cure stomach ailments. Traditionally, stems and roots of
B. koreana have been used in oriental medicine for their anti-inflammatory, analgesic, anti-cancer, anti-conjunctivitis, and antibacterial properties [
8,
9]. A modern component analysis study conducted in the early 2000s showed that the compound berberine extracted from the roots and stems of
B. koreana exhibited a high antioxidant effect [
10]. Moreover, depending upon the extraction method, the root and stem extracts exhibit an anti-cancer activity-enhancing effect [
11,
12]. Additionally, the fermentation of barberry extract with lactic acid bacteria and other probiotics increases the polyphenol and flavonoid contents [
13].
Berberis koreana is a useful forest biological resource unique to Korea; it has been used not only as a medicinal material but also as a source of functional foods [
14].
According to a study by Jannatizadeh and Khadivi-Khub [
15],
Berberis integerrima fruits and seeds show significant differences in seed size, weight, and length depending on the growing environment and region. Therefore, in this study, we present the external morphological measurements of
B. koreana seeds. This information can be used as reference data for research on the morphological characteristics of barberry plants in Korea.
Baskin and Baskin [
3] reported that the seeds of five
Berberis species, namely,
Berberis aristata, Berberis dictrophylla, Berberis dubia, Berberis kansuensis, and
Berberis vernae, displayed PD. Thakur et al. [
16] reported that the dormancy of
B. aristata seeds was broken under light conditions during the growth phase at 20 °C. Wang et al. [
17,
18] reported that
B. dictrophylla and
B. kansuensis seeds were subjected to low-temperature wet treatment for 80 days to break the dormancy. The dormancy of
B. dubia and
B. vernae seeds was broken when culturing at 20/15 °C growth phase after 168 days of low-temperature wet treatment [
3].
The seeds of most plants in the genus
Berberis have PD; based on the reports [
19,
20,
21,
22] that their dormancy is broken under cold stratification treatment and temperature conditions of 20 °C, it is expected that
B. koreana seeds would also have PD. We hypothesized the following: (a) B. koreana seeds will absorb water, (b) low-temperature wet treatment will break the dormancy of B. koreana seeds, and (c) hormone treatment (GA3) will break the dormancy of B. koreana seeds.
2. Materials and Methods
2.1. Experimental Materials
The seeds of B. koreana used in this study were obtained from wild plants growing near Samneung in Paju (37° 44’ 35’’ N, 126° 49’ 27’’ E), South Korea, on October 17, 2019. The harvested fruit was removed using the repair method, and the selected seeds were shade-dried for 7 days in a well-ventilated space. The seeds were then stored under refrigeration (4–5 °C) until further use.
2.2. Investigation of Internal and External Characteristics of Seeds
To investigate the external morphological characteristics of the seeds, images were acquired using a scanning electron microscope (SEM; CX-200, COXEM, Daejeon, Korea). The weight of dried seeds were measured per 1,000 dried seeds in triplicate.
To investigate the internal morphological characteristics, the seeds were cut in half using a double-edged razor (stainless steel blade, Dorco, Seoul, Korea) and photographed using a digital microscope (DVM6, Leica, Land Hessen, Germany). Changes in the embryos and endosperms were observed before and after seed germination.
2.3. Seed Disinfection and Setting
Before the start of the experiment, the seeds were surface-sterilized by soaking in 1,000 mg·L−1 Benomyl (FarmHannong, Seoul, Korea) for 24 h; the seeds were then washed three times with distilled water. The surface-sterilized seeds were placed over two sheets of filter paper (Whatman No. 1; GE Healthcare, Buckinghamshire, UK) in a Petri dish containing 5 mL of distilled water.
After GA3 and cold stratification treatments (described below), the germination percentage was investigated in a growth chamber (TGC-130H, Espec Mic Corp., Aichi, Japan), where the seeds were cultured at a constant temperature of 20 °C. The experiment was performed with four replicates and 25 seeds per treatment group. If microorganisms were present during the culture period, they were disinfected by soaking for 24 h in 1,000 mg·L−1 Benomyl (FarmHannong), followed by distilled water washes; distilled water was replenished in the Petri dishes to prevent drying of the filter paper.
The seeds were considered germinated when the radicle emergence through the seed coat was > 1 mm; germination percentage was recorded at 1-week intervals. Seeds that died due to decay during the experiment were removed immediately and excluded while calculating the germination percentage.
2.4. Water Imbibition Test
To determine whether the
B. koreana seeds were physically dormant, the moisture absorption percentage of the seeds was measured. A Petri dish containing two sheets of filter paper (Whatman No. 1) soaked in distilled water was plated with 100 seeds; three replicates were maintained. The initial weight before water absorption and the weights at 3, 6, 9, 12, 24, 36, and 48 h after settling were measured. The water absorption percentage was calculated using the following formula [
23]:
where Ws is the relative increase in weight of the seeds due to moisture absorption, Wh is the weight of seeds per hour after water supply, and Wi is the initial weight of seeds in the dried state.
2.5. Effect of Temperature on Germination: A Move-along Experiment
According to Baskin and Baskin [
24], the "move-along experiment" provides the dormancy-breaking temperature required for germination in most species. For temperature treatments, the four seasons of natural environmental conditions were set as spring (15 °C), summer (25 °C), autumn (20 °C), and winter (5 °C). The treatment groups were subjected to temperature changes from winter to spring and summer (T1: 5→15→20→25 °C) and temperature changes from summer to autumn and winter (T2: 25→20→15→5 °C). In the T1 and T2 treatment groups, dwell times at each temperature were set to 12, 4, 4, and 12 weeks (
Table 1). For all treatment groups, 25 seeds were used in four replicates, and measurements were made at 1-week intervals. In addition, to observe the changes in the embryo and endosperm based on temperature changes, the surface of the seeds was cut at 1-month intervals and photographed using a digital microscope.
2.6. Effect of Cold Stratification Experiment on Germination
For cold stratification treatment, surface-sterilized seeds placed in a Petri dish were subjected to 4 °C in a growth chamber for 0, 2, 4, 8, and 12 weeks. At the end of each low-temperature treatment duration, the Petri dishes were shifted to a 20 °C growth chamber; the germination percentage was recorded while culturing the treatment groups at 20 °C, and the experiment interval was set to 1 week. For all treatment groups, 25 seeds were used in four replicates, and measurements were made at 1-week intervals.
2.7. Experiment to Determine the Effect of GA3 on Germination
Seeds were soaked in solutions with 0 (distilled water, control), 10, 100, 500, or 1000 mg·L−1 GA3 for 24 h at room temperature and then incubated in a growth chamber at 20 °C. Germination rates were determined at 1-week intervals. For all treatment groups, 25 seeds were used in four replicates, and measurements were made at 1-week intervals.
2.8. Effect of Light Conditions on Seed Germination
For the cold stratification, GA3 treatment, and move-along experiment, similar light (12 h light/dark photoperiod) and dark conditions (24 h dark) were utilized. For all treatment groups, 25 seeds were used in four replicates, and measurements were made at 1-week intervals.
The light conditions inside the growth chamber were maintained using fluorescent lamps with 40 ± 10 μmol·m−2·s−1 PPFD. Dark conditions were maintained by wrapping the Petri dishes with aluminum foil.
2.9. Phenology of Embryo Growth, Germination, and Seedling Emergence under Natural Environmental Conditions
To observe the seasonal changes of seeds under natural environmental conditions, the ground (almost loamy sand) was dug to a depth of 5 cm and a tray was planted in the nursery field of the Baekdudaegan National Arboretum. The tray was filled with potting soil, with a mixing ratio of 64.3% cocopeat, 15% peatmoss, 2.5% nitrogen, 10% pearlite, 8% zeolite, 0.19% fertilizer, and 0.01% wetting agent. The phenology of embryo growth, germination, and seedling emergence was investigated from December 1, 2019, to August 1, 2020.
2.9.1. Embryo Growth
Approximately 400 seeds were placed in fine-mesh polyester bags filled with river sand and buried in a tray filled with potted soil. Trays were placed at ground level in the nursery field. Every 2 or 4 weeks, a bag was exhumed, and 10 seeds were randomly selected for embryo growth measurement. The seeds were cut into thin sections using a razor blade; the lengths of the seeds and embryos were measured using a digital microscope. The ratio of embryo length to seed length (E:S ratio) was calculated to correct for the positive correlation between seed and embryo lengths.
2.9.2. Germination
Four replicates of 25 seeds were sown in 8-cm plastic pots filled with potting soil and placed in trays filled with the same potting soil. Trays were placed at the ground level in the experimental garden. Seeds with emerged radicles were counted and removed every week. Seeds were considered to have “germinated” when the radicle protrusion length was at least 1 mm. Intact seeds that did not germinate were buried in the field.
2.9.3. Seedling Emergence
The timing of seedling emergence was monitored by sowing four replicates of 25 seeds at a depth of 3 cm in plastic pots filled with potting soil and placed in the trays described above. Emerged seedlings were counted and removed every week during the field experiments. The pots were covered with nets to prevent disturbance by wild animals.
2.10. Statistical Analyses
Statistical analyses were performed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). The results of the germination experiment were subjected to analysis of variance and Duncan’s multiple range test (p ≤ 0.05).
3. Results
3.1. Investigation of Internal and External Characteristics of Seeds
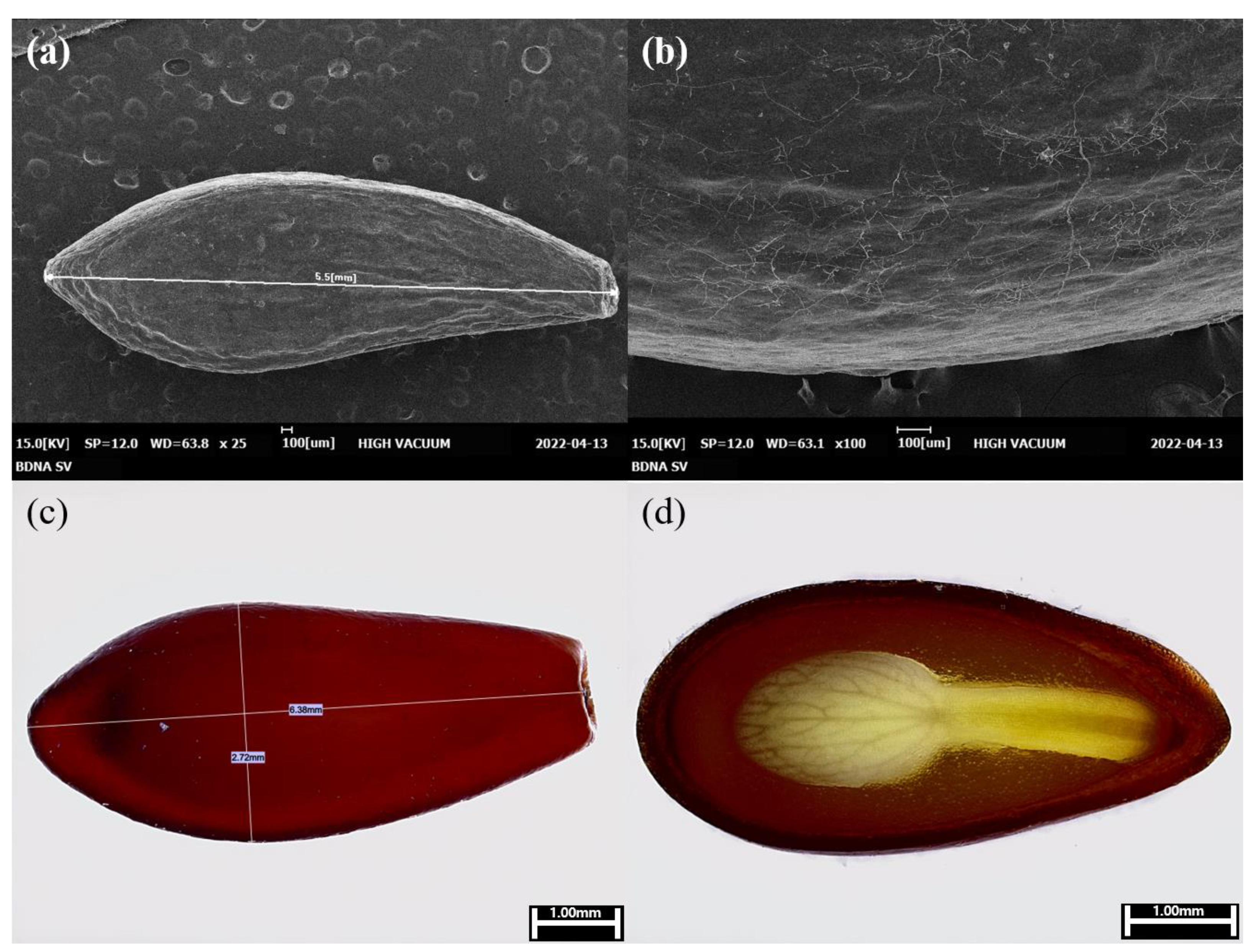
To investigate the morphological characteristics of
B. koreana seeds, they were photographed using scanning electron and digital microscopes (
Figure 1). The color of the seed coat was reddish-brown (
Figure 1c). When part of the seed coat was magnified and photographed using an electron microscope, it revealed a curvature (
Figure 1a and b). The examination of dissected seeds using a digital microscope confirmed the development of the embryo in the seed of the ripe fruit (
Figure 1d).
The mean length of the embryo was 4.00 ± 0.04 mm (mean ± standard error), and the mean seed length was 6.48 ± 0.03 mm; the E/S ratio (embryo: seed ratio) was 0.62 ± 0.05. In addition, the mean weight of 1,000 seeds was 11.514 ± 0.392 g.
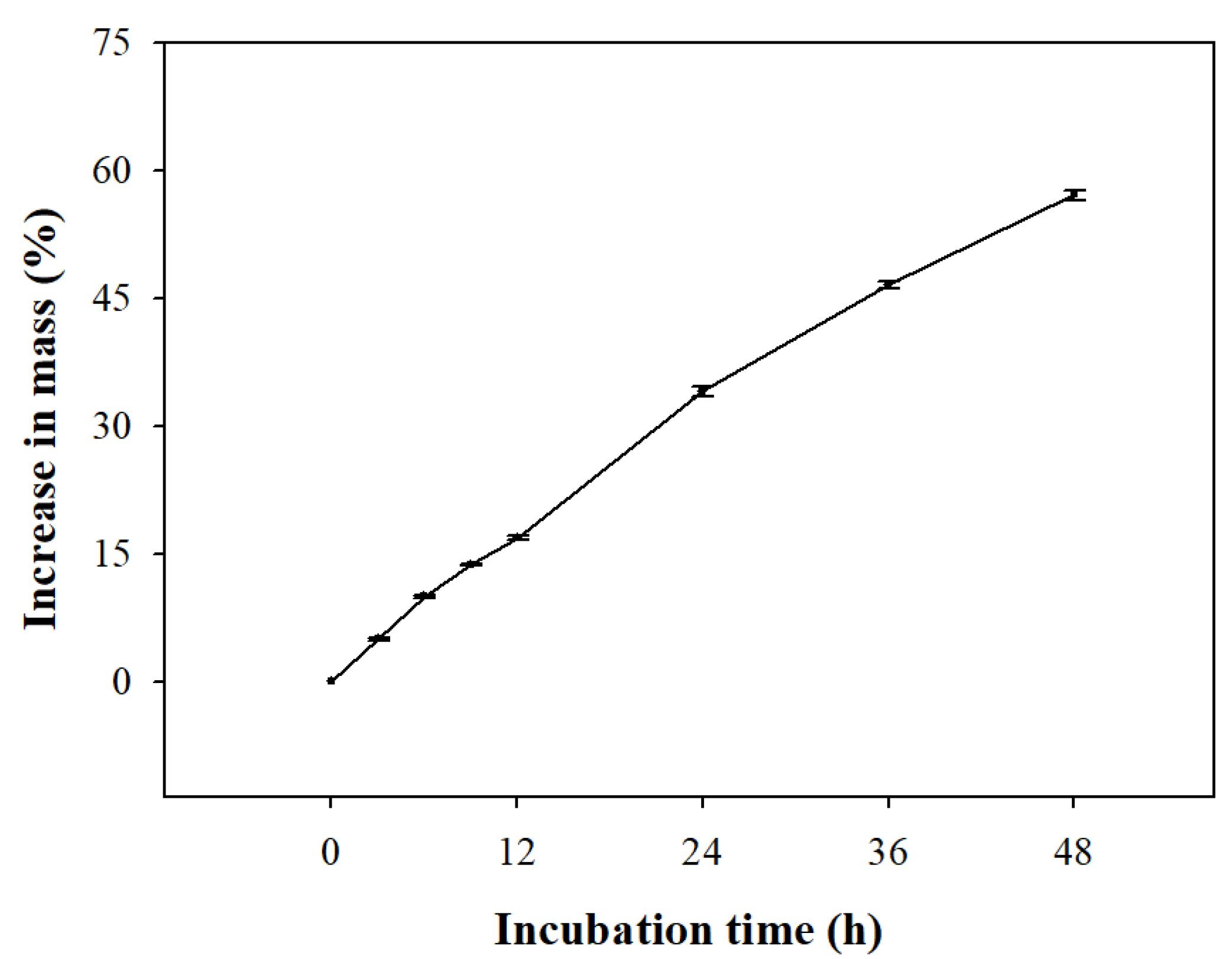
3.2. Water Imbibition Test
The water imbibition test, performed to evaluate the permeability of the
B. koreana seeds, showed that the seed weight increased by 34.10 ± 0.54% in 24 h and 57.13 ± 0.58% in 48 h (
Figure 2).
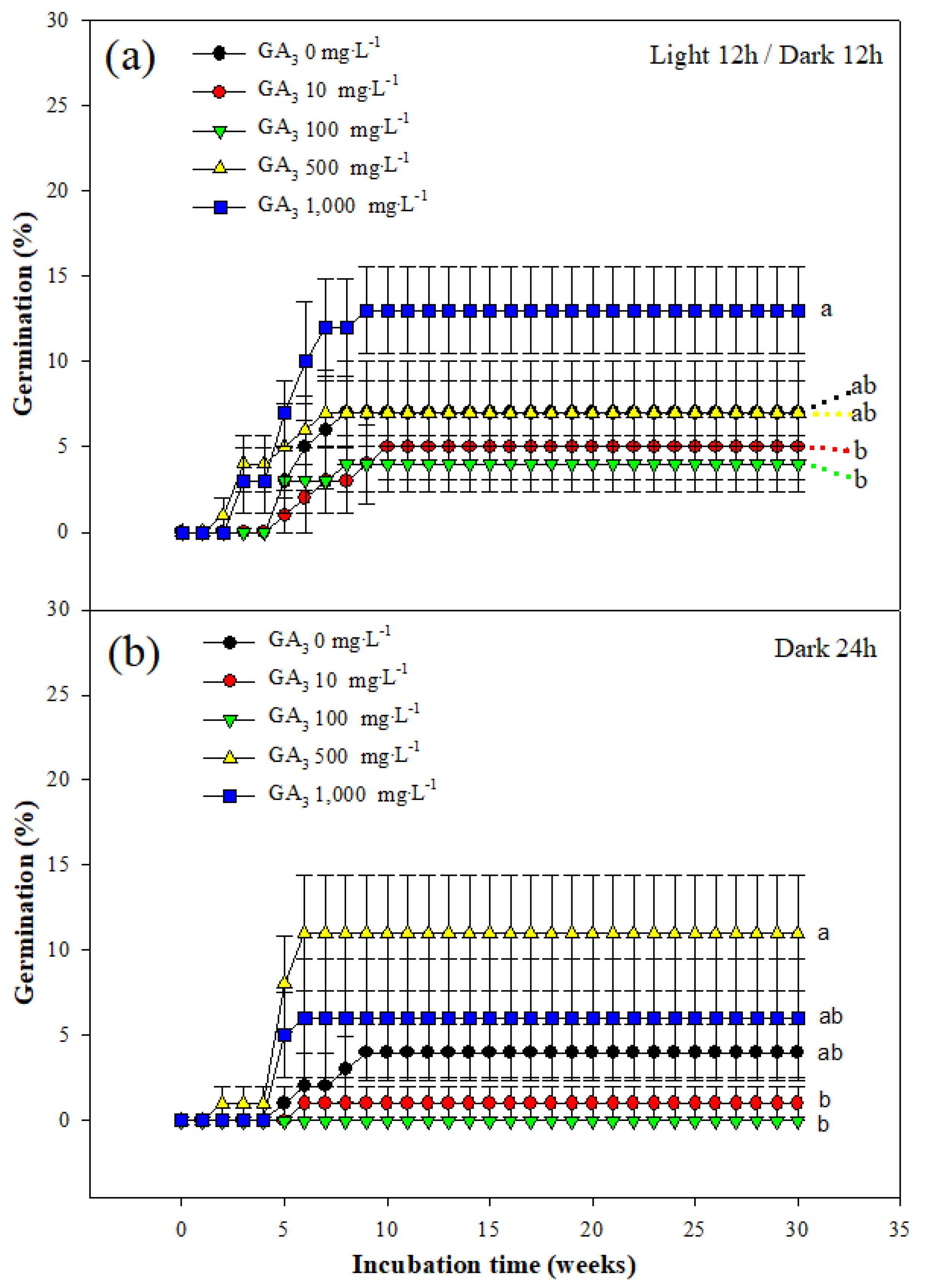
3.3. Effect of GA3 Treatment on Seed Germination
The germination percentages of
B. koreana seeds subjected to GA
3 treatment for 30 weeks at 20 °C under light/dark conditions were as follows: At GA
3 concentrations of 0, 10, 100, 500, and 1,000 mg·L
−1, the final germination percentages under light/dark cycle conditions were 7.00 ± 3.00, 5.00 ± 1.91, 4.00 ± 1.63, 7.00 ± 1.91, and 13.00 ± 2.52%, respectively, and the final germination percentages under dark conditions were 4.00 ± 1.63, 1.00 ± 1.00, 0, 11.00 ± 3.42, and 6.00 ± 3.46%, respectively. Both conditions showed a significant difference when the GA
3 concentration was 500 mg/L
−1 or higher (
Figure 3).
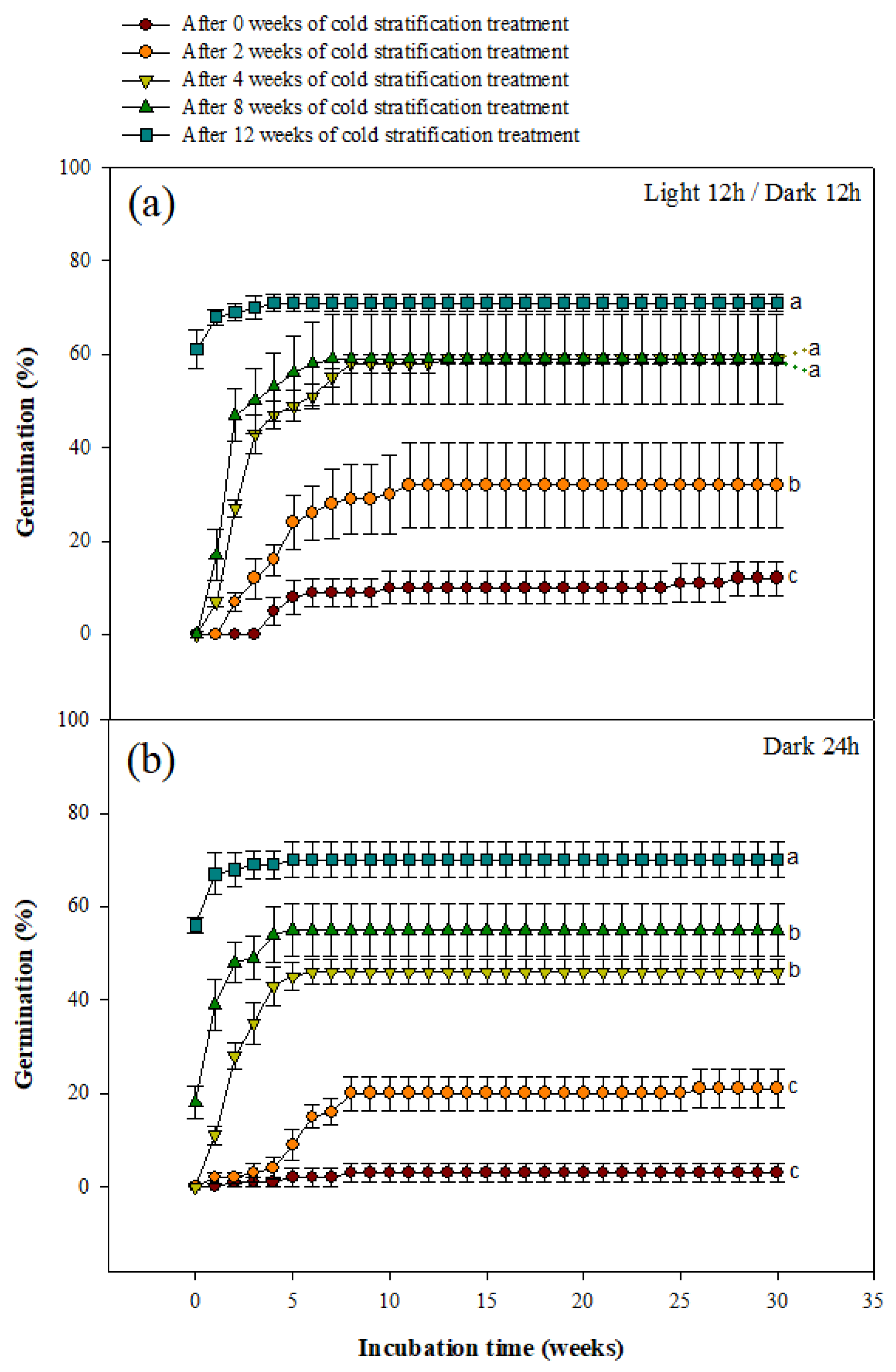
3.4. Effect of Cold Stratification Experiment on Seed Germination
Seeds treated with cold stratification for 0, 2, 4, 8, and 12 weeks were cultured in a growth chamber (light/dark conditions) at 20 °C for 30 weeks. The final germination percentage of seeds treated with cold stratification for 12 weeks was the highest at 71.00 ± 1.91%, regardless of the light conditions (
Figure 4a).
Under light conditions, the final germination percentage of seeds was the highest at 71 ± 1.91% when treated with cold stratification for 12 weeks. After the 8th week germination percentage, the germination rate at 4, 8, and 12 weeks of cold stratification was statistically identical. Under dark conditions, the final germination percentage of seeds was the highest at 70.00 ± 3.83% when treated with cold stratification for 12 weeks.
In addition, cold stratification for 12 weeks under light conditions and cold stratification for 8 and 12 weeks under dark conditions were observed to initiate germination during the cold stratification treatment period.
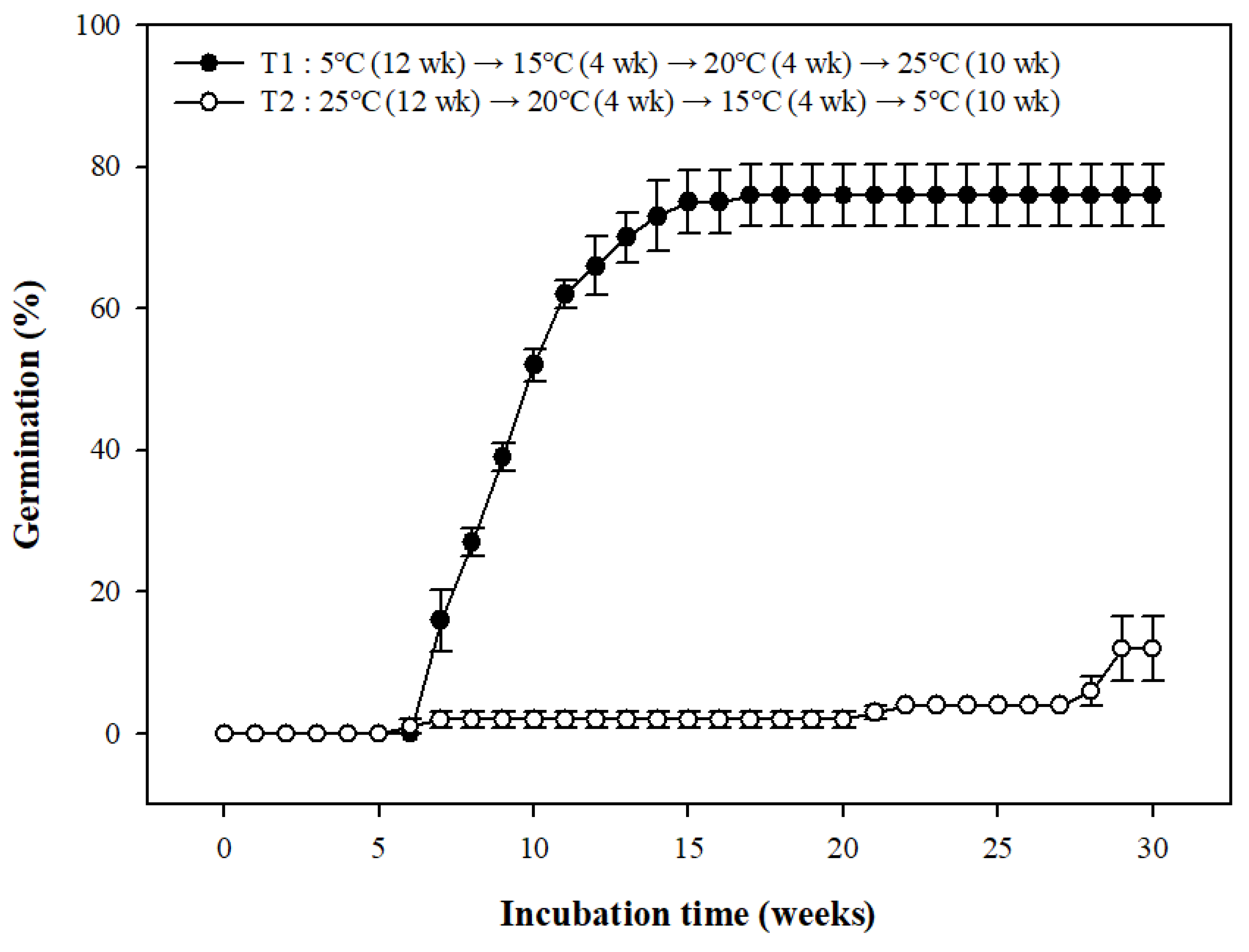
3.5. Seed Germination Based on Temperature Conditions: A Move-along Experiment
A move-along experiment indicated a final germination percentage of 76.00 ± 4.32% at T1 (5→15→20→25 °C) and 12.00 ± 4.62% at T2 (25→20→15→5 °C), indicating that temperature change from winter to spring and summer showed better results. Berberis koreana seeds germinated under T1 temperature conditions as follows: (1) the seeds started to germinate in 6 weeks under the winter temperature conditions (5 °C); the germination percentage was 66.0 ± 4.16% until 12 weeks; (2) the germination percentage increased to 73.00 ± 4.43% under early spring temperature conditions (15 °C, 13–16 weeks); (3) after changing the temperature conditions in late spring (20 °C), the final germination percentage was 76.00 ± 4.32% at 17 weeks; and (4) further germination of the seeds was not observed.
Figure 5.
Seed germination in Berberis koreana incubated under a temperature sequence beginning at 5 °C (T1) or at 25 °C (T2). Vertical error bars represent standard error (n = 4).
Figure 5.
Seed germination in Berberis koreana incubated under a temperature sequence beginning at 5 °C (T1) or at 25 °C (T2). Vertical error bars represent standard error (n = 4).
3.6. Phenology of Embryo Growth, Germination, and Seedling Emergence
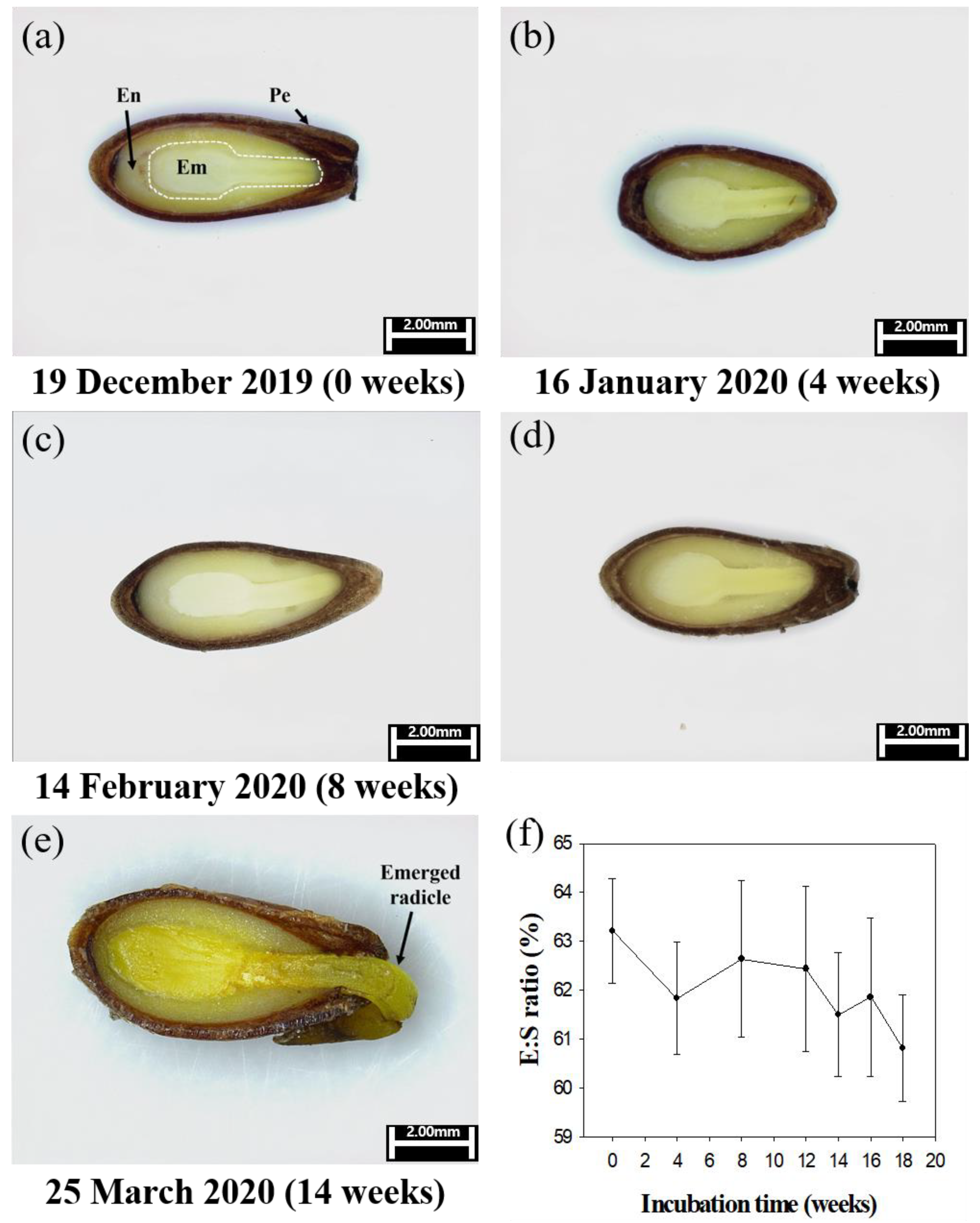
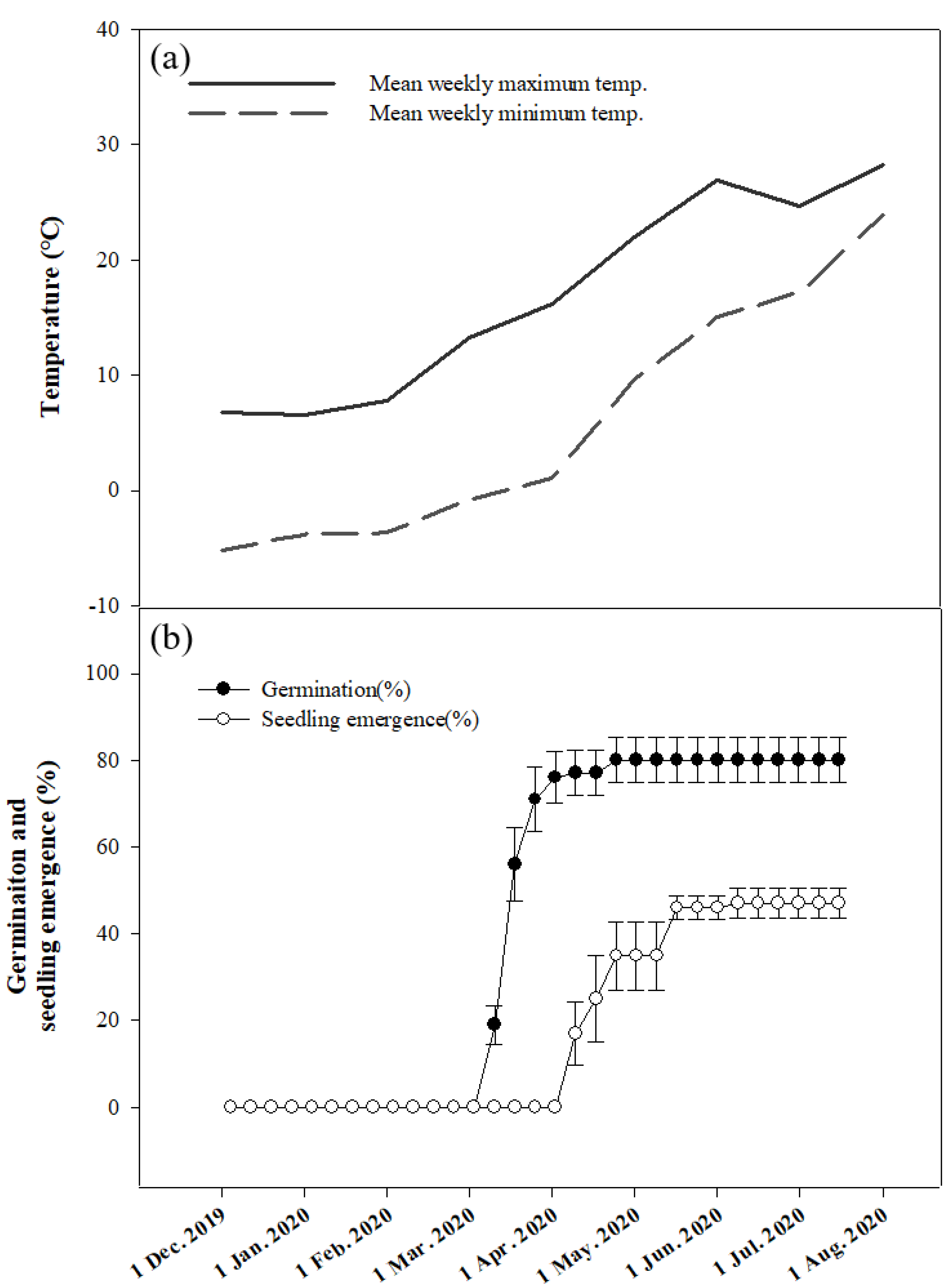
The cutting plane of seeds observed at 2–4-week intervals showed that there was no increase in the E:S ratio (
Figure 6). The E:S ratio was measured for ungerminated seeds. Germination rate measurements based on seasonal changes showed that germination began in the 13th week (March 19, 2020) and continued until the 18th week (April 23, 2020); the final germination percentage was 80.00 ± 1.65%. Seedling emergence rate measurements were observed from the 17th week (April 16, 2020) and were completed at 47.00 ± 3.42% on the 25th week (June 11, 2020;
Figure 7).
4. Discussion
Measurements of the cutting plane of
B. koreana seeds with time showed that there was no increase in the E/S ratio, and no embryo growth was observed (
Figure 6). According to Baskin and Baskin [
24], if a seed has an immature embryo, it will grow and germinate within 30 days of incubation under appropriate conditions, referred to as MD. The results of our study indicate that
B. koreana seeds do not show MD.
B. koreana seeds showed water permeability as the seed weight increased by 134% within 24 h of water imbibition (
Figure 2). According to Baskin and Baskin [
24], if the water absorption rate of the seed is ≤ 20% of its dry weight within 24 h, it is considered impermeable; this phenomenon is termed PY. Our results indicate that
B. koreana seeds do not have PY.
According to Baskin and Baskin [
25], the seeds of most
Berberis plants have PD; seeds with PD can be divided into three types: deep PD, intermediate PD, and non-deep PD. In the case of non-deep PD, it has been reported that dormancy can be broken using GA
3 treatment. GA
3 treatment of
B. koreana seeds showed that there was no correlation between the GA
3 concentration and germination percentage, regardless of the light/dark cycle or dark conditions (
Figure 3). In addition, the maximum germination percentage was 13.0%, which showed that GA
3 had limited influence on seed germination. Therefore, it is concluded that GA
3 did not affect dormancy breakage;
B. koreana seeds do not have a non-deep PD.
The germination percentage of
B. koreana seeds increased with an increase in the period of cold stratification treatment (
Figure 4), indicating that dormancy was ended by it. According to Zheng et al. [
26], among the dormant types, dormancy can be broken with 2–3 months of cold stratification treatment in the case of intermediate PD, and 3–4 months in the case of deep PD. Our results indicate that
B. koreana seeds are of the "intermediate PD" type. Similar results were obtained in previous studies conducted using seeds of the same genus [
20,
22].
However, in all experiments, there was no significant difference in the germination percentage between the seeds subjected to light/dark cycles and dark conditions. Therefore, it appears that B. koreana seeds germinate regardless of light or dark conditions. In addition, some seeds started germinating during the cold stratification treatment. From these results, it can be hypothesized that some B. koreana seeds have a life cycle that begins within the ground in the winter under natural conditions and then germinate as the temperature rises.
Seeds with PD break their dormancy with cold stratification treatment, but dormancy may also be broken using warm stratification treatment [
25]. A move-along experiment indicated an increase in the germination percentage under the T1 treatment whereas the seeds under T2 treatment showed a poor germination percentage (
Figure 5). These results indicate that the dormancy release of
B. koreana seeds is affected by cold and not warm stratification treatment.
The phenology experiment showed that most seeds germinated during a period of 6–12 weeks at 5 °C which is characteristic of winter (
Figure 7). These results suggest that natural
B. koreana seeds germinate in the ground during winter and seedling emergence occurs when the temperature rises in spring. These observations were similar to those observed in the cold stratification experiment and consistent with those of previous reports [
18,
27,
28,
29,
30].
5. Conclusions
We hypothesized that (a) B. koreana seeds will absorb water, (b) low-temperature wet treatment will break the dormancy of B. koreana seeds, and (c) hormone treatment (GA3) will break the dormancy of B. koreana seeds. Our results show that our hypotheses (a) and (b) were consistent and GA3 treatment had little effect on seed dormancy (hypothesis c). The most effective method to break the dormancy was cold stratification treatment at 5 °C for 12 weeks. Collectively, the physiological characteristics indicate that, in the natural environment, the seeds of B. koreana begin to germinate at an average temperature of 20 °C after experiencing low-temperature conditions for over 8 weeks in the soil. Thus, the seeds of B. koreana Palibin exhibit intermediate PD among the dormant seed types.
Author Contributions
Conceptualization, D.H.K., C.S.N. and D.H.L.; methodology, D.H.K., C.S.N. and D.H.L.; validation, D.H.K., C.S.N. and D.H.L.; formal analysis, D.H.K.; investigation, D.H.K. and S.G.K.; resources, S.G.K.; data curation, D.H.K.; writing—original draft preparation, D.H.K.; writing—review and editing, D.H.K., C.S.N. and D.H.L.; visualization, D.H.K.; supervision, C.S.N. and D.H.L.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea grant funded by the Korean Government (NRF-2019R1I1A2A01062559).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, S.Y.; Rhie, Y.H.; Kim, Y.J. Morphological and morphophysiological dormancy in seeds of several spring ephemerals native to Korea. Flower Res. J. 2012, 20, 193–199. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Lang, G.A. Dormancy: A new universal terminology. Horts. 1987, 22, 817–820. [Google Scholar] [CrossRef]
- Willis, J.C. A Dictionary of the Flowering Plants & Ferns, 8th ed.; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Ohwi, J. Flora of Japan; Smithsonian Institution: Washington, DC, USA, 1984. [Google Scholar]
- Lee, N.J.; Chang, C.S.; Kim, H. A morphometric study of Berberis amurensis and B. koreana. Bull. of Seoul Nat’l Univ. Arboretum. 2004, 24, 83–93. [Google Scholar]
- Yoo, S.J.; Lee, K.B.; Kwak, J.H. Studies on the seasonal variation of berberine contents in Berberis koreana. Kor. J. Pharmacogn. 1986, 17, 123–128. [Google Scholar]
- Hyun, M.S.; Woo, W.H.; Hur, J.M.; Kim, D.; Mun, Y.J. The role of ROS and p38 MAP kinase in berberine-induced apoptosis on human hepatoma Hep G2 cells. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 129–135. [Google Scholar]
- Jung, H.K.; Kim, Y.J.; Park, B.K.; Park, S.C.; Jeong, Y.S.; Hong, J.H. Antioxidative and antimicrobial activities of medicinal plant extracts for screening phytobiotic material. J. Korean Soc. Food Sci. Nutr. 2007, 36, 1235–1240. [Google Scholar] [CrossRef]
- Jin, L.; Han, J.G.; Ha, J.H.; Jeong, H.S.; Kwon, M.C.; Ahn, J.H.; Kim, J.C.; Choi, G.P.; Chung, E.K.; Lee, H.Y. Effect of immune activity on Berberis koreana Palibin by ultrahigh pressure low temperature process. Korean J. Med. Crop Sci. 2008, 16, 439–445. [Google Scholar]
- Jin, L.; Ha, J.H.; Jeong, M.H.; Chung, E.K.; Chung, A.R.; Kim, J.C.; Ahn, J.H.; Lee, H.Y. Enhancement of the antioxidant and anticancer activities of Berberis koreana bark using a low temperature and high-pressure extraction process. Korean J. Food Sci. Technol. 2009, 41, 284–291. [Google Scholar]
- Ha, J.H.; Jeong, M.H.; Seo, Y.C.; Yong, C.W.; Kim, J.S.; Kim, H.H.; Ahn, J.H.; Lee, H.Y. Enhancement of antioxidant activities of bark of Berberis koreana Palibin by lactic acid fermentation. Korean J. Med. Crop Sci. 2010, 18, 421–428. [Google Scholar]
- Ling, J.; Ha, J.; Choi, Y.; Seo, Y.; Kim, J.; Kim, Y.; Cha, S.; Kim, J.; Lee, H. Enhancement of cosmeceutical activities of Berberis koreana bark by high pressure and ultrasonification extraction processes. Korean J. Med. Crop Sci. 2011, 19, 54–65. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Khadivi-Khub, A. Morphological variability of Berberis integerrima from Iran. Erwerbs-Obstbau 2016, 58, 247–252. [Google Scholar] [CrossRef]
- Thakur, A.; Thakur, P.S.; Mehta, R. Studies on germination, viability and vigour in Indian barberry (Berberis aristata DC.)—An endangered medicinal plant species of western Himalayas. Indian For. 2006, 132, 485–492. [Google Scholar]
- Wang, J.H.; Du, G.Z.; Cui, X.L.; Zheng, X.F.; Qi, W. Germination characteristics of 61 common woody species from the eastern Qinghai-Tibet Plateau of China and their life history correlates. Zhi Sheng Xue. 2009, 33, 171–179. [Google Scholar]
- Wang, J.H.; Baskin, C.C.; Chen, W.; Du, G.Z. Variation in seed germination between populations of five sub-alpine woody species from eastern Qinghai-Tibet Plateau following dry storage at low temperatures. Ecol. Res. 2010, 25, 195–203. [Google Scholar] [CrossRef]
- Bahuguna, V.K.; Rawart, M.M.S.; Joshi, S.R. Preliminary studies on seed germination behaviour of Berberis lycium Royle—An important shrub for reclamation of wastelands in the Himalaya. Indian For. 1988, 114, 181–183. [Google Scholar]
- Sathyakumar, S.; Viswanath, S. Observations on food habits of Asiatic black bear in Kedarnath Wildlife Sanctuary, India: Preliminary evidence on their role in seed germination and dispersal. Ursus. 2003, 14, 99–103. [Google Scholar]
- Rudolf, P.O.; Buckeye, A.L. , Horse chestnut. Seeds of Woody Plants in the United States. Agric. Handb. 1974, 450, 195–199. [Google Scholar]
- Taylor, J. Propagation successes, failures and lessons learned. In Proceedings of the Conference: Native Plant Propagation and Restoration Strategies; 2002; pp. 45–54. [Google Scholar]
- Lee, S.Y.; Rhie, Y.H.; Kim, K.S. Non-deep simple morphophysiological dormancy in seeds of Thalictrum rochebrunianum, an endemic perennial herb in the Korean Peninsula. Hortic. Environ. Biotechnol. 2015, 56, 366–375. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. When breaking seed dormancy is a problem: Try a move-along experiment. Nat. Plants J. 2003, 4, 17–21. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Zheng, R.; Ma, Z.; Jiang, L.; Zhao, Z.; Shi, X.; Wang, L. Germination characteristics of plump and shriveled seeds of Tamarix ramosissima matured in different seasons. Seed Sci. Technol. 2022, 50, 21–25. [Google Scholar] [CrossRef]
- Bravo, C.; Chamorro, D.; Hiraldo, F.; Speziale, K.; Lambertucci, S.A.; Tella, J.L.; Blanco, G. Physiological dormancy broken by endozoochory: Austral parakeets (Enicognathus ferrugineus) as legitimate dispersers of calafate (Berberis microphylla) in the Patagonian Andes. J. Plant Ecol. 2020, 13, 538–544. [Google Scholar] [CrossRef]
- Khudonogova, E.; Zatsepina, O.; Polovinkina, S.; Rachenko, M.; Tyapaeva, M. Seed germination of woody and shrubby introduced species. In IOP Conf. Ser.: Earth Environ. Sci. 2019, 316, 12–21. [Google Scholar] [CrossRef]
- Larsen, S.U.; Eriksen, E.N. Delayed release of primary dormancy and induction of secondary dormancy in seeds of woody taxa caused by temperature alternations. Acta Hortic. 2004, 630, 91–100. [Google Scholar] [CrossRef]
- Belwal, T.; Bisht, A.; Bhatt, I.D.; Rawal, R.S. Influence of seed priming and storage time on germination and enzymatic activity of selected Berberis species. Plant Growth Regul. 2015, 77, 189–199. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).