1. Introduction
Electrochemical (EC) transducers need to provide high charge transfer rates, low background currents, ease of functionalization and wide EC window, justifying the increasing interest in the nanoallotropic forms of carbon for this purpose 1–3. However, the rapid, facile, reproducible and low cost synthesis of many nanocarbons is still very difficult, involving multiple step protocols, hindering its widespread application. Also, their miniaturization is difficult, delaying the production of compact multiplexed devices for simultaneous detection of multiple biomarkers in minimal sample volumes. Among nanocarbons, graphene is especially appealing for EC biosensors due to its unique charge transport characteristics combined with low dimensionality (comparable to most biomolecules) and with superior EC performance 4,5, allowing analyte detection at the ng/mL range or even lower 6. Its low-cost and controlled synthesis is still a challenge in many cases but a new process was developed to obtain a 3D porous graphene-based material on a polymer sheet via IR, VIS or UV laser beam irradiation of polyimide (PI) sheets at atmospheric conditions, known as laser-induced graphene (LIG) 7. LIG is electrically conductive, flexible, easy, and cheap to produce and amenable to mass production. Miniaturized patterns can be formed during synthesis avoiding the need for posterior lithographic processing. Besides PI, this method also permits graphene synthesis directly on unconventional substrates such as cork 8, paper 9, wood and even food 10, constituting an interesting alternative for device development on sustainable and low cost materials that usually are not compatible with standard microfabrication techniques. LIG-based biosensors have been extensively studied in the last years and many articles highlight their merits in this regard 5,11–13. In general, it is pointed out that the relatively low sheet resistance of a few tens of Ω/sq, the large surface area provided by the porous arrangement of interconnected graphene flakes, and the graphene electrocatalytic activity towards many EC processes, turn LIG into a high-performance material when it comes to lowering limits of detection (LoD) and augmenting sensitivity for a wide variety of analytes.
On the other hand, optical fibres (OFs) have also been applied to biosensing, mainly exploring surface plasmon resonance (SPR) employing metal nanofilms or nanoparticles (NPs)14–17. Their flexibility, small diameter and low-weight combined with immunity to electromagnetic interference, remote sensing capabilities, multi-point and multiplexing capacity are crucial for probing very small or inaccessible places, or conditions where EC sensors alone are unreliable. Indeed, pure EC sensors suffer from electromagnetic noise and interference in chemically complex matrices that affect their performance at low analyte concentrations 16, also requiring precise pH and temperature data for quantification.
The above considerations raise the interest in combining both approaches into a synergistic hybrid device for a lab-on-fibre approach that could carry interest for biomedical applications. The advantages in terms of sensing include a broaden applicability, namely in hard to reach places, multiparametric monitoring (biomarkers, temperature, pH, among others), in-situ optical/EC counterproofing, and complementary detection ranges provided by the dual mode; all these contained in multiplexed “smart” OFs. Although some fundamental studies with ITO 18,19 and sputtered Au 16 electrodes on OFs have been reported, these hybrid sensors remain relatively unexplored. In the literature, Au-coated OFs were applied to the coupled EC/SPR detection of trace lead ions16 with improved selectivity and lower LoD compared to standard chromatography or EC sensors. Last, but not least, such hybrid arrangement would allow to use one signal type as excitation and the other as detection, covering a wide range of options.
Concerning LIG on OF, Hou et. al. have shown that LIG scribed on PI-coated hollow core OF can be used efficiently as a relative humidity sensor20. A recent work by our group 21 has shown that LIG produced on PI-coated OFs does not interfere with the intrinsic optical spectral characteristics of sensors inscribed in the OF core but increases its refractive index sensitivity which is very useful for biosensing applications. LIG on OF can be produced at lower costs compared with other electrode materials used in conjunction with OFs, such as sputtered Au and ITO, whilst minimizing the use of scarce, unsustainable or toxic nanomaterials. However, the EC functionality of LIG on OF is still to be demonstrated.
In this work, we show state-of-the-art performance of LIG sensors laser-scribed on OFs on the non-enzymatic EC detection and quantification of dopamine (DA), an important neurotransmitter and hormone controlling or affecting several body functions and processes. DA is a catecholamine that plays a significant role both as a neurotransmitter and as a hormone, actuating in the cardiovascular, central nervous, renal and hormonal systems22. It is deeply related to the motor skills, pleasure, motivation and reward sensations, as well as to humor and learning mechanisms. Several behavioral and medical conditions are related with DA deficiency or surplus, such as neuroendocrine tumors, hypertension or cardiac insufficiency, drug abuse, Parkinson, Alzheimer, schizophrenia, stress or depression22. It is thus unsurprising that DA is monitored in in-vivo studies in order to better understand its physiological roles as well as to study the process of specific drug development.
In the present study, it was shown that LIG sensors on OFs quantify DA at physiologically relevant concentration and provide excellent electroanalytical performance, comparable to LIG produced in PI sheets. This work paves the way for affordable bio platforms in lab-on-fibre configuration capable of synergistic label-free EC-optical operation and sets a framework for further development.
2. Materials and Methods
2.1. Materials and reagents
The PI-coated OFs were of the type SM1500(4.2/125)P.001, acquired from Fibercore (SMF with 4.2 µm of core diameter, 125 µm total diameter and 30 µm PI coating thickness). Silver ink (Electrodag 1415) was obtained from Agar Scientific, as well as the insulating resin (Lacomit). Pt NPs aqueous dispersion (3 nm, 99.99%, 1000 ppm in H2O), Potassium hexacyanoferrate(II) trihydrate (>99%) and Nafion 117 solution (5% in a mixture of lower aliphatic alcohols and H2O) were supplied from Merck, and DA hydrochloride (>99%), uric acid (>99%) and ascorbic acid (>99.7%) were purchased from Alfa Aesar. Phosphate Buffer Saline (PBS) tablets (pH = 7.4, 1 tablet per 200 ml provides a solution with 0.137 M NaCl, 0.027 M KCl, and 10 mM of phosphate buffer) were acquired from Fisher Bioreagent. Deionized (DI) water was obtained from a MilliQ water purification system, with a resistivity of 18.2 MΩ.cm. All reagents were used as received without further purification steps.
2.2. Synthesis apparatus and electrode fabrication
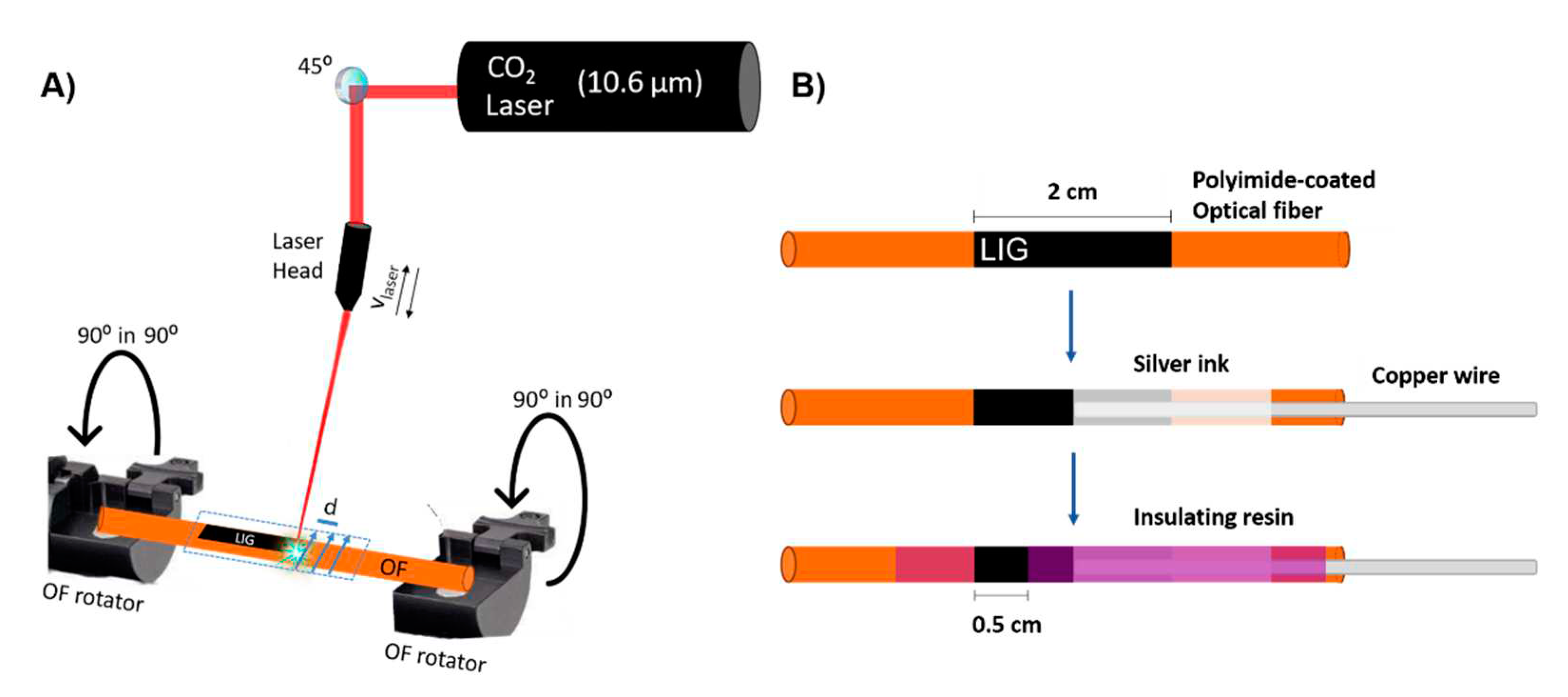
In order to produce LIG on OFs, a continuous CO
2 laser with wavelength of 10.6 µm and maximum power output of 60 W (RedSails Technologies) was used. The laser beam exits through a laser head that can move linearly in a
xy plane, so it can pattern any kind of 2D design on the substrate, in this case a silica OF coated with PI (
Figure 1a). The scribing movement is conditioned by several adjustable parameters, such as the spacing between consecutive lines of laser scribing (d), scanning speed (v
laser) and laser power (P). In this work, d = 0.08 mm and v
laser = 150 mm.s
−1 were used for all LIG electrodes. The laser scanning was done transversely to the OF axe. Since the PI irradiated in this case is not a sheet but rather a coating surrounding the OF, a platform that straps and rotates the OF in its longitudinal axis was employed in order to produce LIG all around the OF circumference. The OF was rotated by 90º before each laser irradiation. A power meter (S350C model from Thorlabs) was used to measure the optical power from the laser. The distance between the laser head and the OF was equal to the laser head lens focal length, 1.8 cm (in focus operation).
Following LIG production, the OFs were thoroughly rinsed in DI water, left to dry overnight and electrical contacts were subsequently established (
Figure 1b). The final LIG electrode exhibit 0.5 cm in length, corresponding to ~ 0.196 cm
2 of exposed active area.
Pt NPs and a Nafion selective membrane were employed seeking enhanced DA sensitivity and selectivity against the most problematic interfering molecules in biological fluids, namely ascorbic acid (AA) and uric acid (UA). Pt NPs deposition on the electrochemically active area of LIG was accomplished by dropcasting 100 µL of Pt NPs aqueous dispersion with a micropipette and let it evaporate overnight. Nafion deposition was performed by dipcoating the OFs in Nafion 117 solution for 10 seconds followed by evaporation overnight.
2.3. Morphological, Structural and Elemental Characterization
Scanning electron microscopy (SEM) images were acquired using a TESCAN Vega 3SB instrument, in the secondary electron mode, at high voltages of 25 keV. Energy-dispersive X-ray spectroscopy (EDX) spectra were acquired to identify chemical elements and impurities present on laser irradiated OFs, as well as to verify if Pt NPs were successfully dropcasted and adsorbed by LIG electrodes. Room temperature micro-Raman spectra were acquired via a Jobin Yvon HR800 instrument (Horiba, Japan), using the retro dispersion configuration, with a He-Cd laser (Kimmon Japan) with a wavelength of 442 nm and 1 mW power. The laser beam was focused on the OFs using a long focus distance objective (Olympus, 50x, 0.5 NA). A neutral-density filter was used to avoid modification of graphene with light intensity, as it reduces the laser intensity in all wavelengths. The LIG OFs were placed, once at a time, in a xy-plane holder. Between spectrum acquisitions, the cylindrical LIG OF was rotated manually 180◦ (turned upside down) so that two different parts of the LIG coating around the OF could be checked for uniformity and overall quality. A 600 grooves/mm grating was used to disperse the radiation to the Peltier cooled CCD sensor. Gauss-Lorentz functions were employed for the fitting of Raman bands using the Labspec software.
2.4. Electrochemical Characterization and Dopamine Electroanalysis
For EC measurements, a three-electrode setup was employed using a homemade 3D printed cell, where LIG on OFs were the working electrode (WE), a platinum wire the counter electrode (CE) and a standard calomel electrode (SCE, 3.9 M KCl) the reference electrode (RE). The OFs were placed through orifices of the cell lid, so that they could be suspended and dipped 3.5 cm in the electrolyte solution. For EC characterization via cyclic voltammetry (CV) measurements, the electrolyte solution consisted in 5 mM of potassium ferrocyanide - [Fe(CN)6]4- - as the active redox probe, in a 50 ml of Phosphate Buffer Saline (PBS) solution with pH = 7.4. All measurements were performed inside a faraday cage.
Differential pulse voltammetry (DPV) was employed for DA electroanalysis in the presence of ascorbic acid and uric acid interferents. Two distinct sets of DPV parameters were previously studied in DA-PBS solution and ultimately the set of conditions providing lower LoD was chosen for further studies. For all the EC measurements the electrolyte volume was 35 mL. A preconcentration step to boost sensitivity towards DA was employed in order to explore the different charge states of DA and AA/UA interferents. Prior to the DPVs, the electrodes were conditioned in PBS buffer by cycling the potential in the -1 to 1 V window at 50 mV/s for 20 cycles.
3. Results and Discussion
3.1. Morphological, Structural and Electrochemical Characterization
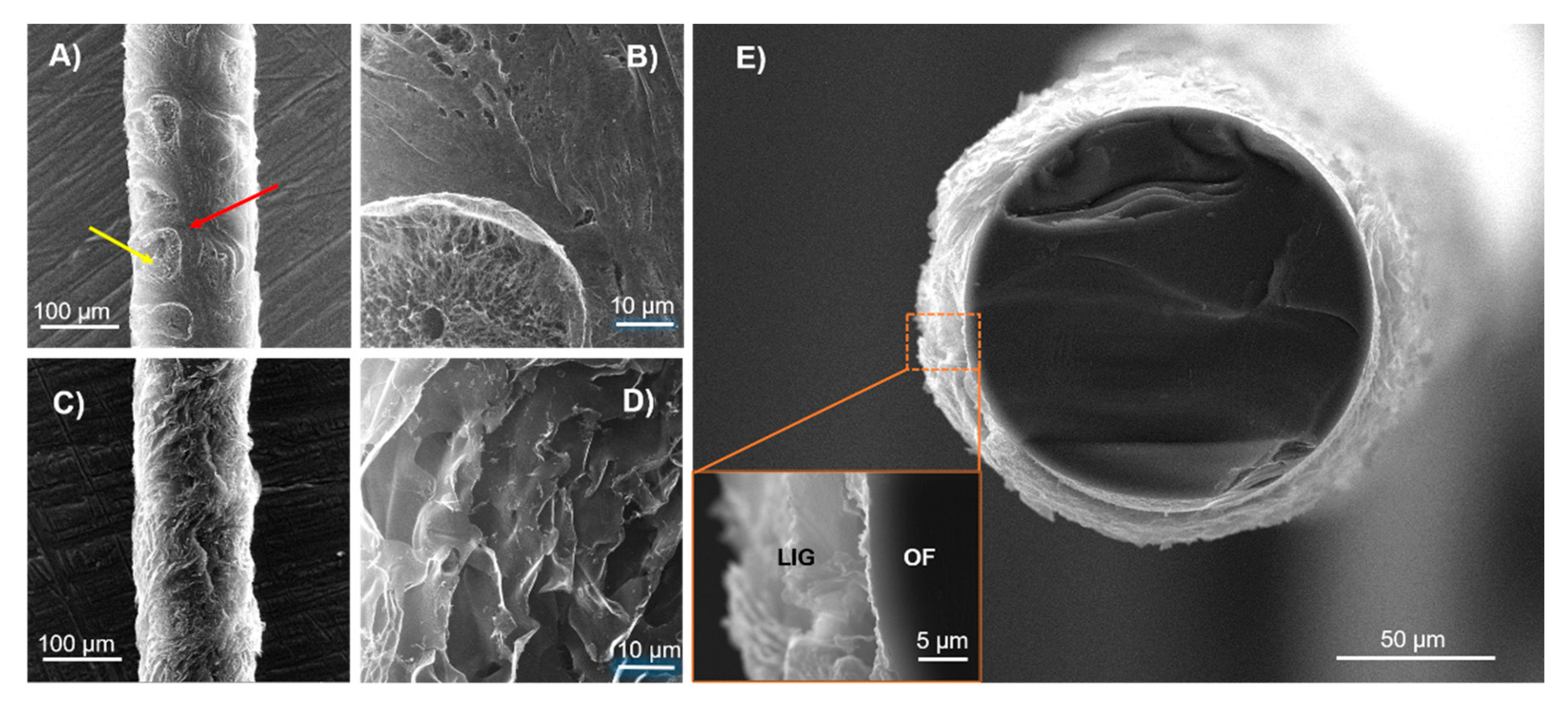
Figure 2 shows the SEM images of PI-coated OFs processed at two different laser powers, 2.5 W and 4 W. At 2.5 W, it is possible to observe two different morphologies. Pinpointed areas highlighted by an yellow arrow in
Figure 2a, show a porous structure with a 3D arrangement of graphene multi-layers, characteristic of LIG, as confirmed by Raman spectroscopy, Figure 3. However, this transformation of PI into LIG is not uniform, as some areas pinpointed in red that appear in between them do not present the typical microstructure of LIG (
Figure 2b). Thus, 2.5 W lasing power for the specific focusing conditions and scribing system employed in this study seems to be close to the threshold lasing power to attain the photothermal conversion of PI into LIG. In contrast, LIG formed at 4 W laser power denote superior homogeneity and all PI has been transformed into a laminated and porous microstructure (Figures 2c and 2d). This is corroborated by cross-section imaging of
Figure 2e, showing LIG formed all around the OF circumference and deep into the cladding interface. No unprocessed PI appears in between the LIG layer and the OF cladding. It is thus clear that increasing the lasing power from 2.5 to 4 W increases the overall LIG homogeneity and overall porosity, in accordance with previous findings on these matters
23.
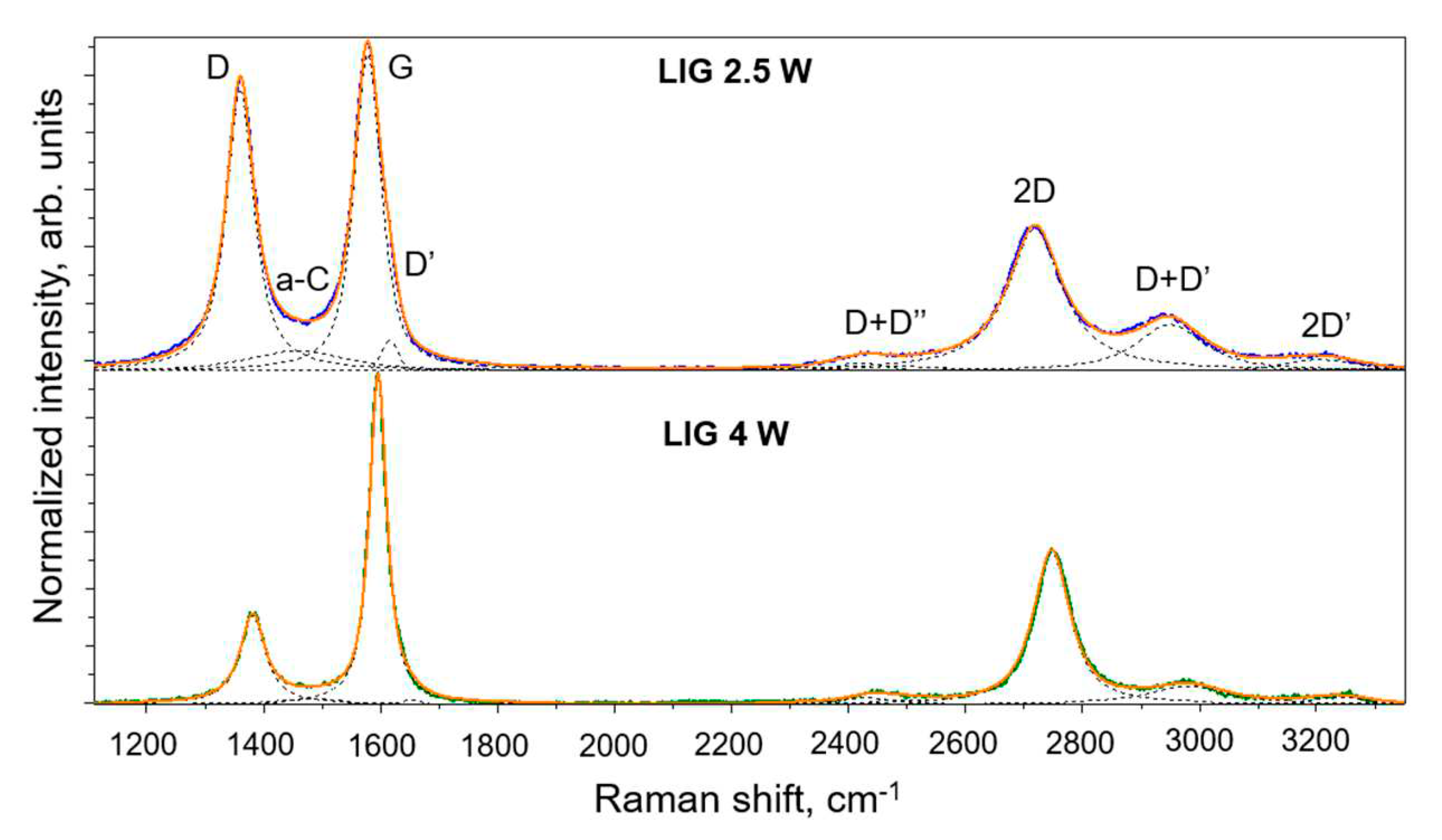
Raman spectroscopy was used to compare the structural quality of the LIG electrodes processed under 2.5 and 4 W lasing powers, and representative results are shown in
Figure 3. The Raman spectra of LIG is dominated by the D, G and 2D (D overtone) bands, along with some other smaller bands, D’ and 2D’, as well as the combined overtones D+D’ and D+D”. The G peak at ∼ 1580 cm
−1 corresponds to phonon scattering of the stretching mode of the sp
2 carbon bonds (E
2g vibrational mode), indicating graphitization. The 2D overtone at ∼ 2740 cm
−1 refers to the scattering of two Brillouin zone boundary phonons, with symmetric momenta, of the breathing modes of perfect aromatic rings. For single-layer graphene (SLG), the 2D peak is very sharp, symmetric and has increased intensity, compared to bilayer or few-layer graphene, where the peak will broaden and shift in position as well
24. The ratio between the 2D and G bands, I
2D/I
G, allows to qualitatively infer on the number of graphene layers present in LIG. A I
2D/I
G≈ 2 is an usual feature for SLG
24, whereas in LIG that ratio is about 0.3 to 0.4 and the 2D band is significantly broadened, indicating the presence of uncorrelated multilayer graphene.
The D band at ∼ 1370 cm
−1 , not allowed by momentum selection-rules in pure graphene, arises from one-phonon scattering involving the breathing mode of aromatic rings, requiring defective or bent sp
2 carbon bonds for its activation
25. Its presence therefore indicates a significant concentration of defects in the graphene structure. Along with the D- and G-band full width at half-maximum (FWHM), the I
D/I
Gratio can be used to study structural aspects of the multilayer graphene. The I
D/I
Gratio scales with disorder for sp
2 phases obeying the Tuinstra-Konig relation, valid for sp
2 carbon phases presenting well defined and intense G bands at ∼ 1580-1590 cm
−1 26. Clearly, both the D and G bands’ FWHM and the I
D/I
Gratio are larger for LIG produced at 2.5 W (57 cm
−1 and 55 cm
−1 for the D and G band fwhm, respectively, and an I
D/I
Gratio of 0.89) compared to LIG produced at 4 W (52 cm
−1 and 36 cm
−1 for the D and G bands’ FWHM, respectively, and an I
D/I
Gratio of 0.27), clearly indicating that the latter presents lower defect density and higher crystallinity. Moreover, the a-C band at ∼ 1450-1500 cm
−1, related to the presence of amorphized carbon phases, is much more intense for LIG produced at 2.5 W. This analysis corroborates the SEM observations suggesting that 2.5 W laser power is indeed near the threshold for LIG formation, since at lower laser powers only amorphized carbon phases are produced instead of LIG. Note that the spectrum presented for 2.5 W LIG corresponds to the regions indicated by the yellow arrow in
Figure 2. Raman spectra acquired from the regions pinpointed by the red arrows (not shown) still denote the typical fingerprint of LIG, though the bands are further broadened and the I
2D/I
G intensity ratio is typically lower.
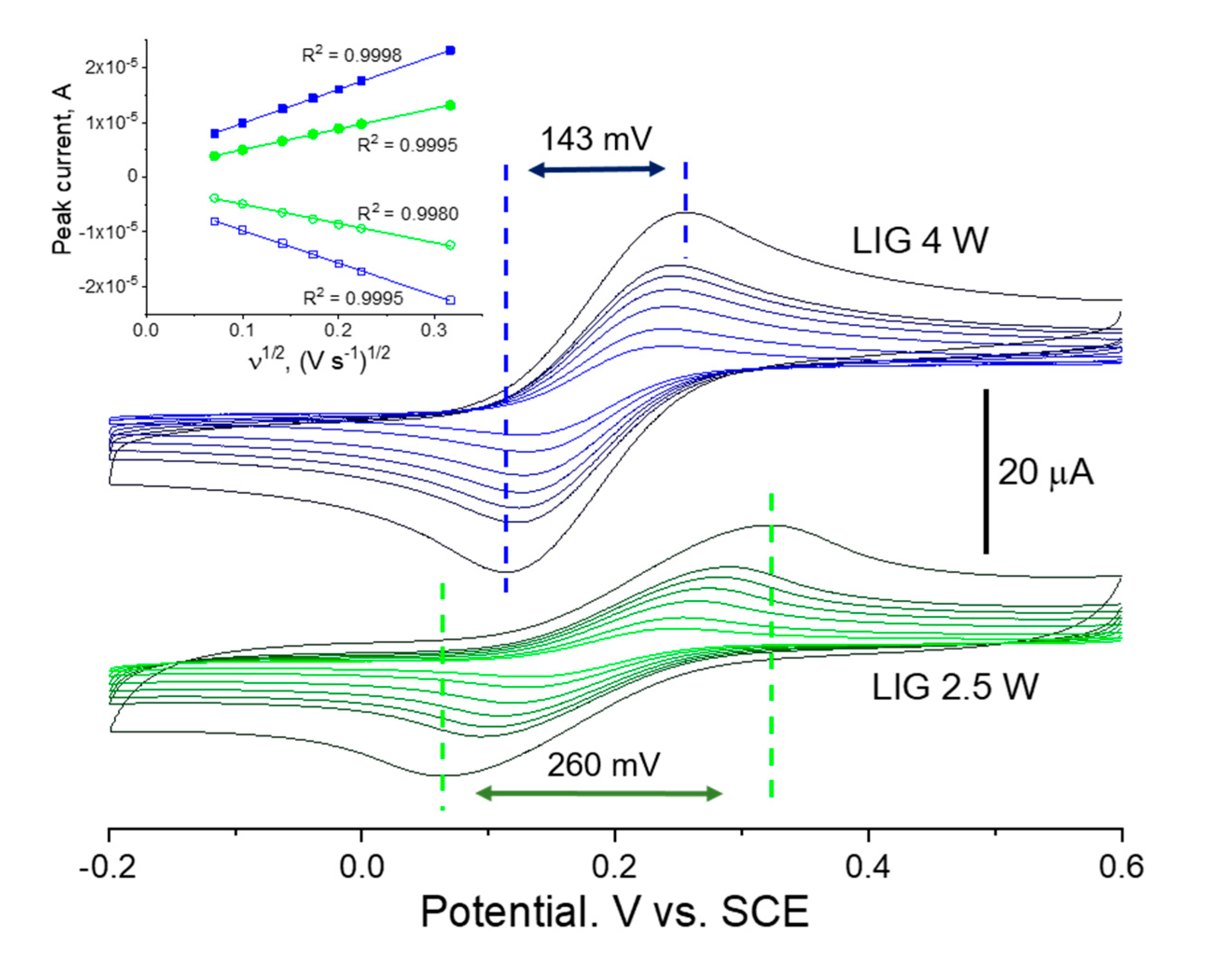
These differences in terms of morphology and structure are obviously reverberated into the EC response of each. This is clearly seen in the cyclic voltammetry studies of
Figure 4 employing a standard, “well-behaved” probe, [Fe(CN)
6]
3-/4- in PBS buffer.
For both 4W and 2.5 W LIG on OFs, the redox waves are well defined and the current response increases with increasing scan rates as expected. Faster scan rates provide less time for reactions to take place at the electrode’s surface and decrease the diffusion layer thickness, which in turn increases the mass transport of ions and the current signal
27. Specifically, for a one-electron reversible electron transfer process limited by a semi-infinite diffusion regime, the peak current
ip (A) is linearly proportional to the square root of the scan rate ν (V s
−1), according to the Randles-Ševčík relation (at 25 °C)
27
where
C (mM) is the [Fe(CN)
6]
4- bulk concentration in mM (5 mM),
Aeff (cm
2) is the electrode’s electrochemically active area, and
D (cm
2 s
−1) is the [Fe(CN)
6]
4- diffusion coefficient in aqueous solution
28 (
cm
2 s
−1). This is clearly the case of both 2.5 W and 4 W LIG in the studied range of scan rates, for which the anodic and cathodic peak currents show a symmetric linear dependence with the square root of the scan rate, with all fittings having coefficient of determination R
2 above 0.998. The voltammograms differ however in the magnitude of peak currents and in the separation of the anodic and cathodic peak potentials. Indeed, as exemplified for 100 mV/s, LIG produced on OFs at 4W LIG maintains a 143 mV peak-to-peak separation, about 120 mV less that LIG produced at 2.5 W lasing power. This undoubtedly indicates that the 4 W material is characterized by faster electron transfer kinetics compared to that of 2.5 W. The electrochemically active area can be derived via the slope of the linear regression of anodic branches and via equation 1, resulting in 1.09 mm
2 and 1.78 mm
2 for the 2.5 W and 4 W LIG electrodes, respectively, for a geometrical exposed area of
c.a. 1.96 mm
2. The severely diminished active area of the 2.5 W LIG electrode compared to the geometrical area is in accordance with the analysis of LIG morphology in
Figure 2. These results indicate that LIG electrodes produced at 4 W present superior EC characteristics for biosensing purposes and were thus selected for the detection and quantification of DA.
3.2. Dopamine (DA) Electroanalysis
DA is easily electro-oxidized into dopamine-o-quinone (C
8H
9NO
2), in a two proton-2 electron reversible reaction:
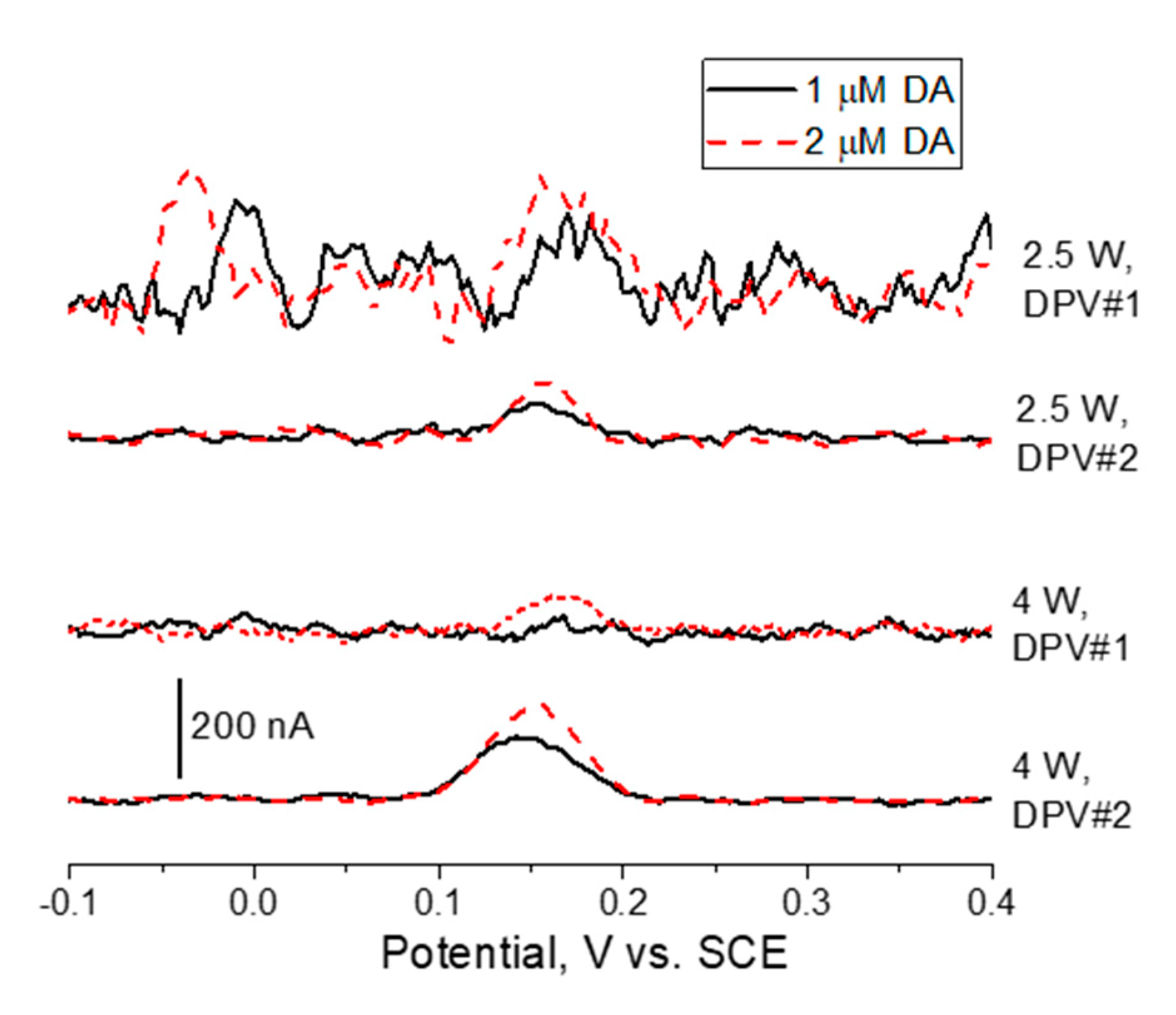
Figure 5 shows differential pulse voltammetry (DPV) measurements in DA-containing PBS buffer after blank baseline subtraction. DA oxidation reaction (eq. 2) peaks at about 0.15 V vs. SCE. It is clear that the LIG electrodes produced at 4W laser power permit higher DA oxidation currents when compared to its 2.5 W counterpart. In addition, for both DPV parameter sets tested, the signal to noise ratio is higher for 4W LIG. Hence, lower LoD and higher sensitivity is attained for these sensors. This is in agreement with the arguments of the previous morphological, structural and EC characterization using ferro/ferricyanide redox probes. The conductive and highly porous 3D graphene arrangement obtained at 4W lasing power provides high surface area and fast charge transfer for reversible redox reactions to take place. On the contrary, at 2.5 W laser power a non-homogeneous and amorphized graphene phase is obtained with obvious impact in charge transfer kinetics and electroanalytical capabilities.
As regards to the two different parameter set for DPV, it is clear that DPV#2 allows for both higher differential currents and signal-to-noise ratio. The combination of 2.5 W lasing and DPV#1 hampers DA detection at 2 μM, whereas 4W lasing plus DPV#2 allows for a clear DA oxidation wave at 1 μM. The last set was therefore employed for the subsequent DA quantification tests.
Other molecules present in biological fluids at relevant concentrations, such as ascorbic acid (AA) and uric acid (UA), are also electrooxidized at electrode potentials that can interfere with the DA electrooxidation, either directly by overlapping oxidation waves or indirectly via undesired interactions of AA/UA interferents (or their oxidation by-products) with DA. Uric acid (C
5H
4N
4O
3) is electrooxidized into diimide quinonoid (C
5H
2N
4O
3) according to
On the other hand, ascorbic acid (C
6H
8O
6) is electrooxidized into dehydroascrobate (C
6H
6O
6), also a two proton-two electron process:
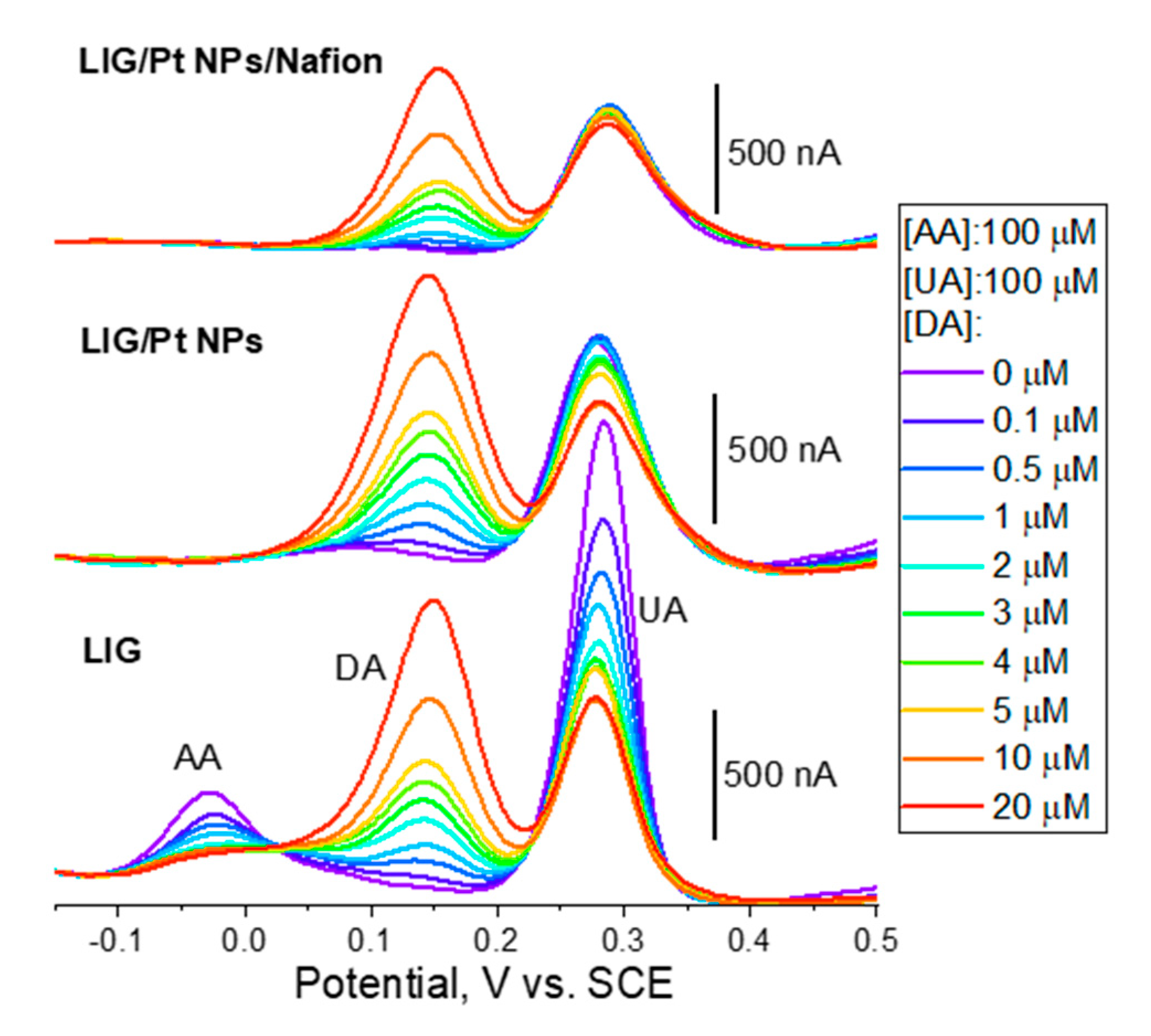
Figure 6 shows the DPV measurements in PBS (pH 7.4) employing LIG electrodes on OFs. Importantly, regardless the electrode architecture employed (LIG, LIG/Pt NPs and LIG/Pt NPs/Nafion) the measured LoD is 100 nM, which is adequate for measuring DA in several biological fluids, such as in urine (maximum reference value for a “healthy” adult of 3300 nM/24h)
29 or abnormal concentrations in extracellular fluid (> c.a. 100 nM)
30. The measured LoDs are inclusively similar to those found for LIG produced on PI sheets (see
Table 2). Sensitivities are comparable to other LIG sensors produced on PI sheets and employing Pt NPs, although one order of magnitude lower compared to the high record in the literature for PI-LIG
5.
AA electrooxidation is usually a less reversible reaction compared to DA and UA. This is readily observed in the DPV using bare LIG electrodes (bottom DPVs in
Figure 6), where peak currents for UA oxidation (c.a. 280 mV vs. SCE) are four-fold those of AA oxidation (c.a. – 25 mV vs. SCE) for the same concentration (100 μM) and identical to the peak current obtained for DA at a concentration of 4 μM. This underlines the electrocatalytic effect of LIG towards DA oxidation favoring it against AA and UA interferents. This phenomenon has been attributed to the π-π stacking and electrostatic attraction between graphene delocalized π electrons and DA aromatic rings.
34,35 UA also possesses an aromatic ring in its structure, whereas AA does not. Moreover, pyrrolic-N has been shown to be present in LIG samples produced on PI sheets, which can facilitate DA oxidation via hydrogen bonds with the DA hydroxyl or amine groups.
36,37 On the other hand, the negatively charged AA and UA are repulsed electrostatically by graphene, contributing to the natural selectivity of LIG towards DA. Finally, the employed preconcentration step at -0.3 V vs. SCE for 3 minutes further contributes to this discrimination taking advantage of the opposite intrinsic charge of positive DA molecules and negative AA and UA molecules.
Interestingly, when Pt NPs are dropcasted into the LIG electrode, the DPV profile changes dramatically. Interestingly, AA oxidation wave at c.a. -25 mV disappears so that Pt NPs are effective in preventing direct AA interference in DA quantification. It is important to note that the Pt NPs are dispersed in DI water and dropcasted into LIG surface, so that this behavior cannot be attributed to any effect related to the interaction of the dispersing media and the LIG surface. In fact, a similar effect has been reported in the literature for reduced graphene oxide decorated with Pt NPs of similar size as used herein
38. On the other hand, despite a small increase in sensitivity (see
Table 2), Pt NPs seem not to induce a decisive enhancement in the electroanalytical response. In fact, DA-UA peak-to-separations remain similar when incorporating Pt NPs as only a small 4 mV increase is observed. These observations contradict the findings of another work where planar LIG/Pt NPs electrodes produced on PI sheets
32 were characterized in AA-DA-UA ternary mixtures. In that work, the incorporation of Pt NPs is seen to improve the electroanalytical response of LIG via a clear enhancement in sensitivity and lower LoD, which is attributed to the remarkable electrocatalytic properties of Pt NPs. Possible explanations on this apparent contradiction may rely on the different sizes of NPs employed, their concentration at the electrode surface, the specific physical properties and surface chemistry of each LIG type and/or dissimilar DPV measurement parameters. The presence of Pt NPs on LIG was accessed via multi-point EDX measurements (not shown) and resulted in an average concentration of
at%.
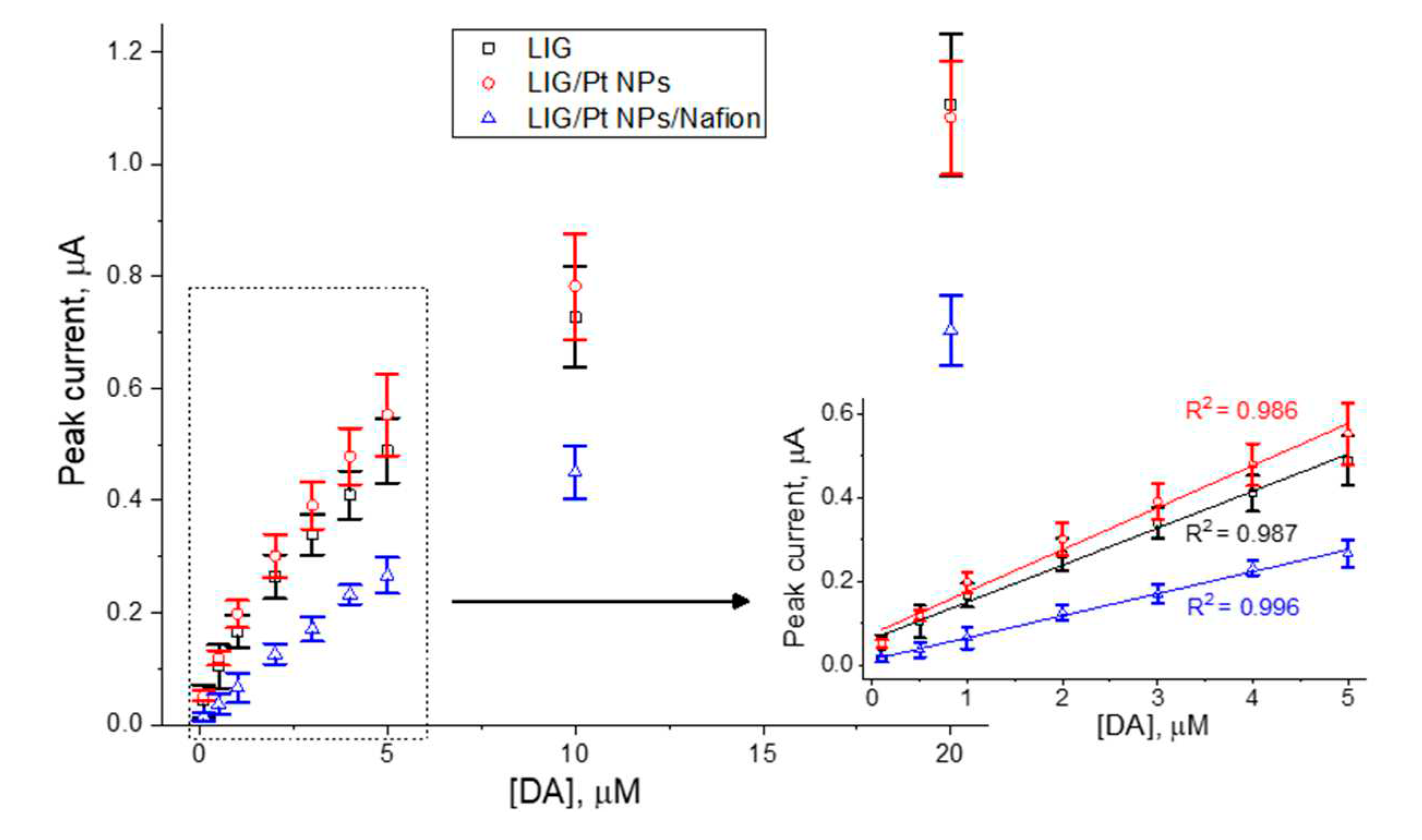
Regarding the bare LIG electrodes, it is clear that, with the successive measurements in AA-DA-UA ternary mixtures, the intensity of the AA and UA peaks are decreasing. It is also clear that albeit an initial quasi-linear response of the sensor at lower DA concentrations (
Figure 7), a progressive signal saturation occurs. Similarly to the findings in the literature regarding LIG on PI sheets
5, this is likely caused by electrode fouling via deposition of reagent molecules and/or oxidation byproducts. It is widely accepted that DA electrochemical mechanisms and reaction pathways are complex
39. However, there is also a general agreement in the literature that a chemical step follows the initial electrooxidation step presented in equation 2, probably a Michael addition of the primary amine group to the aromatic ring
39. This, in addition with possible intermolecular polymerization reactions, may result in the progressive formation of deposits that partially inactivate the electrode surface, and hence to the diminishing of subsequent oxidation currents. This is readily and especially observable in the bare LIG electrode, where UA and AA oxidation currents are diminished severely as DPVs are performed.
The complexity of DA electrochemistry and the wide variety of electrode materials employed may be the reason why different explanations exist for similar features encountered in DA electroanalysis. For instance, a report in the literature employing ZnO nanowire electrodes states that, at lower concentrations, DA surface coverage proceeds via monolayer adsorption, whereas for higher concentrations the surface monolayer starts saturating and secondary multilayer formation occurs40. The interaction with electrode surface by these higher/ranking adsorption layers is partially screened by the former, leading to a change in electrode response into a second linear regime40. This surface coverage model is also concomitant with the progressive hindering of AA and UA oxidation waves. A thorough investigation in these matters is out of the scope of this work, where the obtained data points were chosen in order to privilege DA concentrations up to 5 μM as this constitutes the more useful range for clinical and medical applications. The higher concentration range data points are insufficient to check if indeed it constitutes a second linear region.
When Nafion, an anionic polymer, is dropcasted on top of the LIG/PtNPs-coated OFs, further changes to the electroanalytical response are observable. Sensitivity to DA is lowered compared to LIG/Pt NPs, which is expected due to the presence of Nafion. Despite being permeable to cationic forms (such as DA), it still constitutes a physical barrier to diffusion of DA. On the other hand, UA oxidation wave is still visible in a ratio to the DA wave similar to the case of the LIG/Pt NPs. This is somewhat unexpected since UA is negatively charged at pH 7.4 and its diffusion into the LIG/Pt NPs surface should have been blocked by the anionic polymer. This effect was also observed in the literature when developing uric acid sensors41. In both cases, one possible explanation could lie on the insufficient Nafion coverage of the sensor surface, which is especially prone to occur in highly porous materials such as LIG. It is however interesting to note that, especially up of 5 μM DA, UA oxidation currents are much more stable compared to the remaining, and a higher coefficient of determination (R2=0.996) of DA calibration curve is achieved. This indicates that, in this concentration range, fouling is being suppressed by the use of Nafion, which is of extreme relevance if continuous and long-term measurements are intended.
4. Conclusions
Laser induced graphene produced phototermally on optical fibers via CO2 laser irradiation of polyimide-coated OFs is suitable for dopamine electroanalysis considering the physiologically ranges of interest. The measured LoD of 100 nM and minimization of fouling effects and interference from Ascorbic acid and Uric acid via deposition of Pt NPs and Nafion are adequate for in vivo measurements, also taking in account the proven biocompatibility and antimicrobial properties of LIG42, Pt NPs43 and Nafion44. These results support further work on the exploitation of these cost-effective hybrid LIG/OF platforms for electrochemical/optical biosensors/bioactuators for a wide variety of scenarios. Possible future applications include fiberscopes employing OF imaging probes with in-situ label-free EC capabilities, as well as highly versatile combination of electrochemistry and optogenetics at high selectivity and usable spatial and temporal resolutions, critical aspects in the study of biologic functions.
Author Contributions
conceptualization N.F.S.; methodology C. M. and N.F.S.; Formal analysis N.F.S.; results validation N.F.S. and C.M.; investigation L. L. F, R. A. R., A.J.S.F. and N. F. S.; visualization L.L.F., R.A.R. and N. F.S.; Resources N.F.S., C.M. and F.M.C.; writing-original draft by N.F.S.; writing-review and editing L.L.F., R.A.R., F. M. C., A. J. S. F., C. M. and N. F. S.; supervision N. F. S. and C.M.; Project administration F. M. C.. All valued authors read carefully and agreed to publish version of the manuscript.
Funding
This work was developed within the scope of the projects i3N (LA/P/0037/2020, UIDB/50025/2020, and UIDP/50025/2020) and DigiAqua (PTDC/EEI-EEE/0415/2021), financed by national funds through the (Portuguese Science and Technology Foundation/MCTES (FCT I.P.), Portugal).
Data Availability Statement
Statement data set available with last author.
Acknowledgments
N. F. Santos acknowledges FCT I.P. for the research action 2022.04595.CEECIND (GraFiberSens project). C. Marques acknowledges the research actions CEECIND/00034/2018 (iFish project) / 2021.00667.CEECIND (iAqua project), by FCT I.P, Portugal.
Conflicts of Interest
All authors confirmed that they have no conflict of interest to declare.
References
- Coroş, M.; Pruneanu, S.; Staden, R.-I. S. Review—Recent Progress in the Graphene-Based Electrochemical Sensors and Biosensors. J. Electrochem. Soc. 2019, 167, 037528. [Google Scholar] [CrossRef]
- Santos, N. F.; Pereira, S. O.; Fernandes, A. J. S.; Vasconcelos, T. L.; Fung, C. M.; Archanjo, B. S.; Achete, C. A.; Teixeira, S. R.; Silva, R. F.; Costa, F. M. Physical Structure and Electrochemical Response of Diamond–Graphite Nanoplatelets: From CVD Synthesis to Label-Free Biosensors. ACS Appl. Mater. Interfaces, 2019; acsami.9b00352. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J. A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C. K.; Latiff, N. M.; Loo, A. H.; Wong, C. H. A.; Eng, A. Y. S.; Bonanni, A.; Pumera, M. Graphene and Its Electrochemistry-an Update. Chemical Society Reviews. Royal Society of Chemistry May 7, 2016, pp 2458–2493. [CrossRef]
- Santos, N. F.; Pereira, S. O.; Moreira, A.; Girão, A. V; Carvalho, A. F.; Fernandes, A. J. S.; Costa, F. M. IR and UV Laser-Induced Graphene: Application as Dopamine Electrochemical Sensors. Adv. Mater. Technol. 2021, 6, 2100007. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, S.; Wu, J.; Yu, Y.; Li, R.; Eda, S.; Chen, J.; Feng, G.; Lawrie, B.; Hu, A. Bisphenol A Sensors on Polyimide Fabricated by Laser Direct Writing for Onsite River Water Monitoring at Attomolar Concentration. ACS Appl. Mater. Interfaces 2016, 8, 17784–17792. [Google Scholar] [CrossRef]
- Ye, R.; James, D. K.; Tour, J. M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A. F.; Fernandes, A. J. S.; Martins, R.; Fortunato, E.; Costa, F. M. Laser-Induced Graphene Piezoresistive Sensors Synthesized Directly on Cork Insoles for Gait Analysis. Adv. Mater. Technol. 2020. [Google Scholar] [CrossRef]
- Kulyk, B.; Pereira, S. O.; Fernandes, A. J. S.; Fortunato, E.; Costa, F. M.; Santos, N. F. Laser-Induced Graphene from Paper for Non-Enzymatic Uric Acid Electrochemical Sensing in Urine. Carbon N. Y. 2022, 197, 253–263. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S. P.; Arnusch, C. J.; Tour, J. M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Simsek, M.; Wongkaew, N. Carbon Nanomaterial Hybrids via Laser Writing for High-Performance Non-Enzymatic Electrochemical Sensors: A Critical Review. Anal. Bioanal. Chem. 2021, 413, 6079–6099. [Google Scholar] [CrossRef]
- Li, G. Direct Laser Writing of Graphene Electrodes. J. Appl. Phys. 2020, 127. [Google Scholar] [CrossRef]
- Pereira, S. O.; Santos, N. F.; Carvalho, A. F.; Fernandes, A. J. S.; Costa, F. M. Electrochemical Response of Glucose Oxidase Adsorbed on Laser-Induced Graphene. Nanomater. 2021, Vol. 11, Page 1893 2021, 11, 1893. [Google Scholar] [CrossRef]
- Soares, M. S.; Silva, L. C. B.; Vidal, M.; Loyez, M.; Facão, M.; Caucheteur, C.; Segatto, M. E. V.; Costa, F. M.; Leitão, C.; Pereira, S. O.; et al. Label-Free Plasmonic Immunosensor for Cortisol Detection in a D-Shaped Optical Fiber. Biomed. Opt. Express 2022, 13, 3259. [Google Scholar] [CrossRef]
- Soares, M. S.; Vidal, M.; Santos, N. F.; Costa, F. M.; Marques, C.; Pereira, S. O.; Leitão, C. Immunosensing Based on Optical Fiber Technology: Recent Advances. Biosensors 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Lao, J.; Zhang, X.; Liu, Y.; Cai, S.; Gonzalez-Vila, A.; Li, K.; Huang, Y.; Yuan, Y.; Caucheteur, C.; et al. Electrochemical Plasmonic Fiber-Optic Sensors for Ultra-Sensitive Heavy Metal Detection. J. Light. Technol. 2019, 37, 3495–3502. [Google Scholar] [CrossRef]
- Liu, X.; Singh, R.; Li, M.; Li, G.; Min, R.; Marques, C.; Zhang, B.; Zhang, B.; Kumar, S. Plasmonic Sensor Based on Offset-Splicing and Waist-Expanded Taper Using Multicore Fiber for Detection of Aflatoxins B1 in Critical Sectors. Opt. Express, Vol. 31, Issue 3, pp. 4783-4802 2023, 31, 4783–4802. [Google Scholar] [CrossRef] [PubMed]
- Tramarin, L.; Casquel, R.; Gil-Rostra, J.; González-Martínez, M. Á.; Herrero-Labrador, R.; Murillo, A. M. M.; Laguna, M. F.; Bañuls, M. J.; González-Elipe, A. R.; Holgado, M. Design and Characterization of ITO-Covered Resonant Nanopillars for Dual Optical and Electrochemical Sensing. Chemosensors 2022, 10. [Google Scholar] [CrossRef]
- Niedziałkowski, P.; Białobrzeska, W.; Burnat, D.; Sezemsky, P. Study on Combined Optical and Electrochemical Analysis Using Indium-Tin-Oxide-Coated Optical Fiber Sensor. 2019, 1–8. [CrossRef]
- Hou, M.; Wang, N.; Chen, Y.; Ou, Z.; Chen, X.; Shen, F.; Jiang, H. Laser-Induced Graphene Coated Hollow-Core Fiber for Humidity Sensing. Sensors Actuators B Chem. 2022, 359, 131530. [Google Scholar] [CrossRef]
- Martins, L.; Kulyk, B.; Theodosiou, A.; Ioannou, A.; Moreirinha, C.; Kalli, K.; Santos, N.; Costa, F.; Pereira, S. O.; Marques, C. Laser-Induced Graphene from Commercial Polyimide Coated Optical Fibers for Sensor Development. Opt. Laser Technol. 2023, 160, 109047. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and Sensors for Dopamine Detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Duy, L. X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J. M. Laser-Induced Graphene Fibers. Carbon N. Y. 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Ferrari, A. C.; Meyer, J. C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K. S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A. C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron-Phonon Coupling, Doping and Nonadiabatic Effects. Solid State Commun. 2007, 143, (1–2). [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J. L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Bard, A. J.; Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, 1980. [Google Scholar]
- Konopka, S. J.; McDuffie, B. Diffusion Coefficients of Ferri- and Ferrocyanide Ions in Aqueous Media, Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Davidson, D. F. Elevated Urinary Dopamine in Adults and Children. Ann. Clin. Biochem. 2005, 42 Pt 3, 200–207. [Google Scholar] [CrossRef]
- O’Neill, R. D. Microvoltammetric Techniques and Sensors for Monitoring Neurochemical Dynamics in Vivo. A Review. Analyst 1994, 119, 767–779. [Google Scholar] [CrossRef]
- Hong, Q.; Yang, L.; Ge, L.; Liu, Z.; Li, F. Direct-Laser-Writing of Three-Dimensional Porous Graphene Frameworks on Indium-Tin Oxide for Sensitive Electrochemical Biosensing. Analyst 2018, 143, 3327–3334. [Google Scholar] [CrossRef]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H. N. Highly Efficient Laser Scribed Graphene Electrodes for On-Chip Electrochemical Sensing Applications. Adv. Electron. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z. A.; Desprez, V.; Kilmartin, P. A.; Travas-Sejdic, J. Sensitive, Selective, Disposable Electrochemical Dopamine Sensor Based on PEDOT-Modified Laser Scribed Graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Krishnan, S. K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H. S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8781. [Google Scholar] [CrossRef]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly Sensitive and Selective Detection of Dopamine in the Presence of Ascorbic Acid at Graphene Oxide Modified Electrode. Sensors Actuators, B Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Li, S. M.; Yang, S. Y.; Wang, Y. S.; Lien, C. H.; Tien, H. W.; Hsiao, S. T.; Liao, W. H.; Tsai, H. P.; Chang, C. L.; Ma, C. C. M.; et al. Controllable Synthesis of Nitrogen-Doped Graphene and Its Effect on the Simultaneous Electrochemical Determination of Ascorbic Acid, Dopamine, and Uric Acid. Carbon N. Y. 2013, 59, 418–429. [Google Scholar] [CrossRef]
- Ding, X.; Bai, J.; Xu, T.; Li, C.; Zhang, H. M.; Qu, L. A Novel Nitrogen-Doped Graphene Fiber Microelectrode with Ultrahigh Sensitivity for the Detection of Dopamine. Electrochem. commun. 2016, 72, 122–125. [Google Scholar] [CrossRef]
- Xu, T. Q.; Zhang, Q. L.; Zheng, J. N.; Lv, Z. Y.; Wei, J.; Wang, A. J.; Feng, J. J. Simultaneous Determination of Dopamine and Uric Acid in the Presence of Ascorbic Acid Using Pt Nanoparticles Supported on Reduced Graphene Oxide. Electrochim. Acta 2014, 115, 109–115. [Google Scholar] [CrossRef]
- Randviir, E. P. A Cross Examination of Electron Transfer Rate Constants for Carbon Screen-Printed Electrodes Using Electrochemical Impedance Spectroscopy and Cyclic Voltammetry. Electrochim. Acta 2018, 286, 179–186. [Google Scholar] [CrossRef]
- Yue, H. Y.; Huang, S.; Chang, J.; Heo, C.; Yao, F.; Adhikari, S.; Gunes, F.; Liu, L. C.; Lee, T. H.; Oh, E. S.; et al. ZnO Nanowire Arrays on 3D Hierachical Graphene Foam: Biomarker Detection of Parkinson’s Disease. ACS Nano 2014, 8, 1639–1646. [Google Scholar] [CrossRef]
- Mundaca-Uribe, R.; Bustos-Ramírez, F.; Zaror-Zaror, C.; Aranda-Bustos, M.; Neira-Hinojosa, J.; Peña-Farfal, C. Development of a Bienzymatic Amperometric Biosensor to Determine Uric Acid in Human Serum, Based on Mesoporous Silica (MCM-41) for Enzyme Immobilization. Sensors Actuators, B Chem. 2014, 195, 58–62. [Google Scholar] [CrossRef]
- Singh, S. P.; Li, Y.; Be’Er, A.; Oren, Y.; Tour, J. M.; Arnusch, C. J. Laser-Induced Graphene Layers and Electrodes Prevents Microbial Fouling and Exerts Antimicrobial Action. ACS Appl. Mater. Interfaces 2017, 9, 18238–18247. [Google Scholar] [CrossRef]
- Jan, H.; Gul, R.; Andleeb, A.; Ullah, S.; Shah, M.; Khanum, M.; Ullah, I.; Hano, C.; Abbasi, B. H. A Detailed Review on Biosynthesis of Platinum Nanoparticles (PtNPs), Their Potential Antimicrobial and Biomedical Applications. J. Saudi Chem. Soc. 2021, 25, 101297. [Google Scholar] [CrossRef]
- Kim, G.; Kim, H.; Kim, I. J.; Kim, J. R.; Lee, J. I.; Ree, M. Bacterial Adhesion, Cell Adhesion and Biocompatibility of Nafion Films. J. Biomater. Sci. Polym. Ed. 2009, 20, 1687–1707. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).