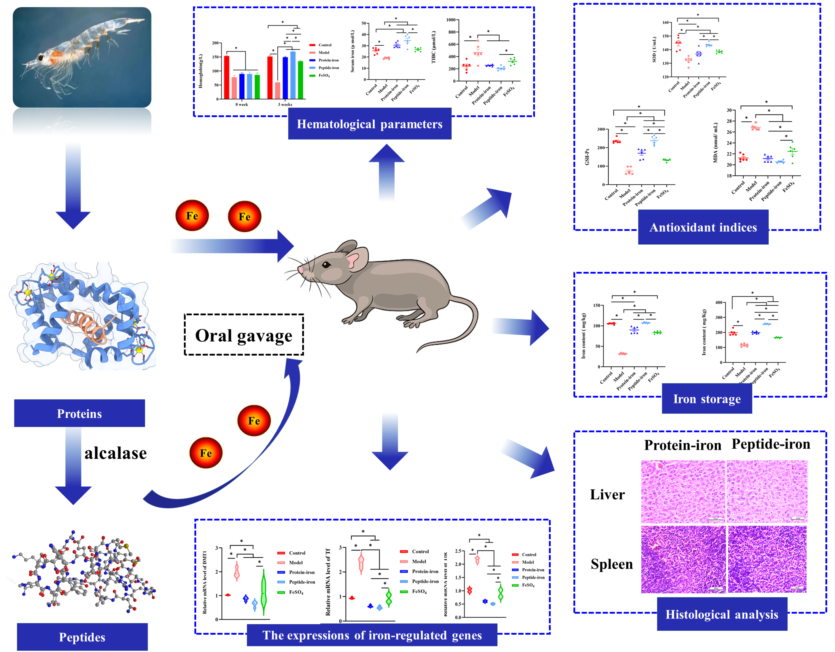
1. INTRODUCTION
Many biological processes rely on iron, including oxygen transport and energy metabolism [
1]. Iron deficiency can cause iron deficiency anemia (IDA), which is one of the most frequent nutritional deficiencies, afflicting millions of people around the world, especially infants, children, and pregnant women [
2]. In addition, iron deficiency can also increase oxidative stress [
3], which may be part of the pathogenesis of IDA patients [
4].
Ferric iron is generally accepted to be the most prevalent form of non-heme iron. Cytochrome b on the apical surface of intestinal epithelial cells reduces ferric iron to ferrous form [
5]. Ferrous ions are further transported to intestinal epithelial cells
via DMT1[
6]. Iron is transported throughout the body
via transferrin from the sites of absorption [
7]. Cells uptake iron
via transferrin-bound Ferric iron
via the transferrin receptor (TfR1) [
8]. The bioavailability of dietary iron is very low, ranging from 5% to 18% [
9]. Intestinal iron absorption is influenced by the solubility and exchange ability of iron from the food matrix [
10]. In order to address this pressing public health issue, many iron supplements have been developed and studied [
11,
12]. As an iron supplement, FeSO
4 is allowed in many countries under pharmaceutical and food additive regulations [
13]. However, they have the adverse reactions of affecting the intestinal lumen and the intestinal mucosal area by reason of free iron dependent on the production of free radicals [
14,
15]. Therefore, it need reduce the interaction of free iron with cells in the gastrointestinal tract during digestion and absorption. In addition to reducing the toxicity of free iron, it is also capable of increasing its solubility and bioavailability. Absorption of intestinal iron is generally considered to be the only factor controlling iron levels [
16]. The body maintains homeostasis of iron through upregulation or downregulation of iron absorption mechanisms, and it regulates the absorption and never excretes iron [
17]. Hence, enhanced intestinal iron absorption is crucial for improving blood and peripheral tissue iron levels during IDA.
In recent years, iron supplements containing iron-chelating proteins or peptides have been discovered. For an illustration, iron deficiency anemia induced DMT1, TfR gene mRNA levels were significantly reduced by lentil-derived protein–iron complexes [
18]. Ovalbumin can enhance recovery of the hematocrit, hemoglobin, transferrin saturation level and the hepatic iron content of IDA rats [
19]. Desalted duck egg white peptides-ferrous chelate significantly increased hematological parameters in IDA rats [
20]. Peptides from barley proteins can increase iron uptake and ferritin levels in Caco-2 cells [
21]. Our previous study found that Antarctic krill peptides obtained by enzymolysis of Antarctic krill powder with trypsin were rich in aspartic acid and glutamic acid, which could enhance iron transport in Caco-2 cells after simulated gastrointestinal digestion [
22]. However, the effect of Antarctic krill peptides on promoting iron absorption
in vivo has not been reported. In addition, whether there is a difference for
in vivo iron absorption enhancement of Antarctic krill peptides compared with their original proteins, needs to be investigated in more depth.
Antarctic krill proteins are rich in essential amino acids, and its biological value is higher than that of milk protein and other animal proteins [
23]. In order to explore which is more effective for the recovery of iron deficiency anemia between Antarctic krill protein-iron complex and peptide-iron complex, this study prepared Antarctic krill proteins and peptides from Antarctic krill powder and evaluated the effects of Antarctic krill proteins and peptides on iron bioavailability, expression of iron-regulated genes, and
in vivo antioxidant capacity.
2. MATERIALS AND METHODS
2.1. Extraction of Antarctic krill proteins and preparation of Antarctic krill peptides
The extraction method of Antarctic krill proteins was referred to Gao et al [
24]. The Antarctic krill meat was added to the distilled water with constant stirring and mixing. The pH of the mixture was adjusted to 11.5 and continually stirred at 4 °C for 2 h, followed by centrifugation for 10 min at 4500 r/min to obtain the supernatant. Thereafter, the supernatant was extracted for 2 h at 4 °C and pH 4.6 to collect the precipitate. The precipitate was frozen dried and labeled as Antarctic Krill proteins.
In addition, the method of Antarctic krill peptides was according to the following study with minor modifications [
25]. First, Antarctic krill proteins was dissolved in double distilled water, followed by hydrolyzation with alcalase (50 °C, pH 8.5) at a dose of 5000 U/g protein. After 3 h of hydrolysis, the enzyme was inactivated by heating at 100 for 10 min in a water bath. The mixture was centrifuged at 10,000 g for 20 min at 4 °C to obtain the supernatant and stored at −20 °C for later analysis.
2.2. Preparation of iron complexes with Antarctic krill proteins or peptides
According to our previous
method [
25]
, freeze-dried Antarctic krill proteins or peptides were dissolved in deionized water at a concentration of 30 mg/mL, followed by the addition of FeSO
4 to a final concentration of 50 mM. The mixed system was shaken and reacted for 30 min at 25 °C and pH 6.0.
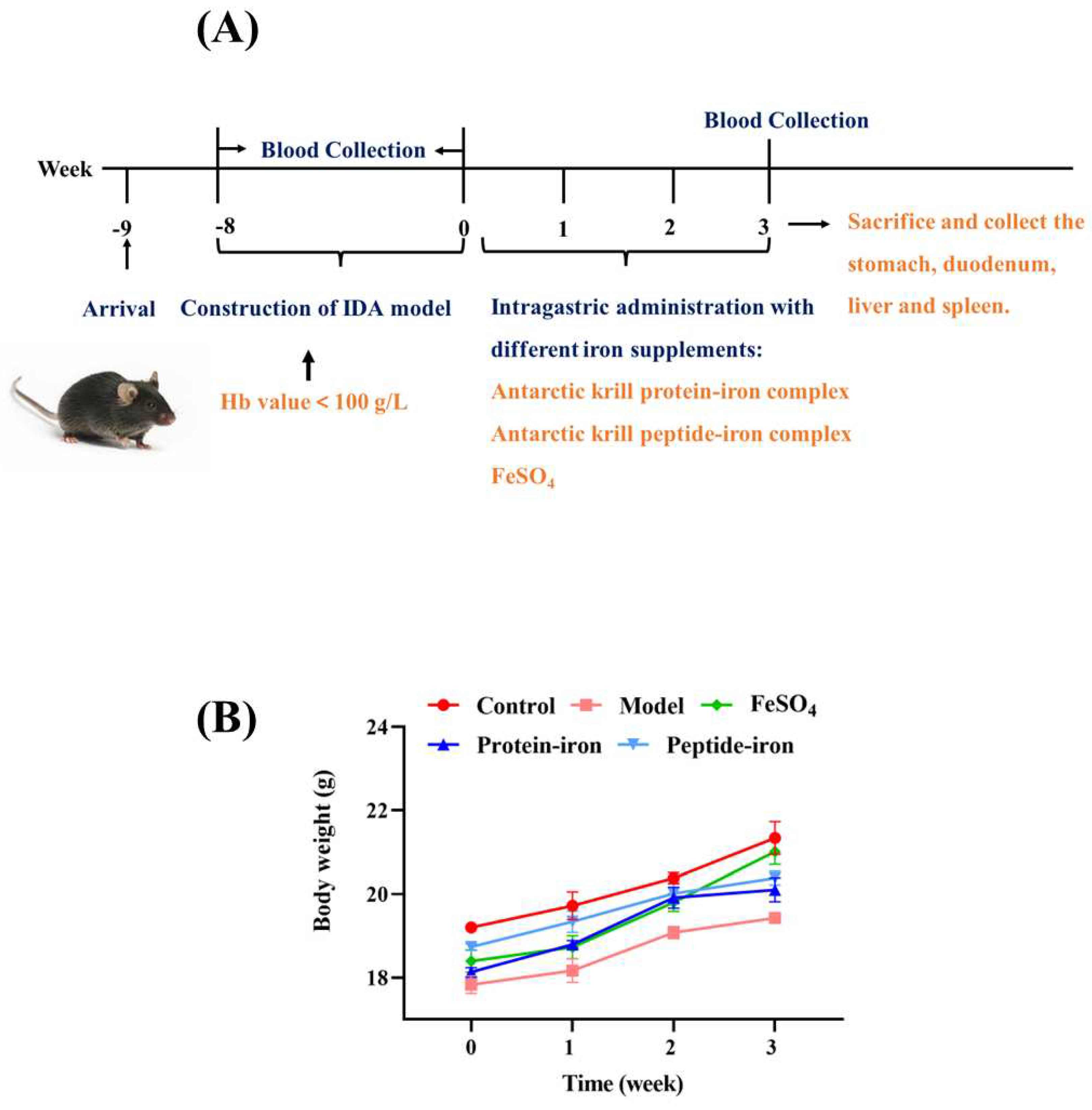
2.3. Animal care and treatment
Three-weeks old female C57/B6 mice weighting 13±5 g was obtained from Liaoning Changsheng Biotechnology Co., Ltd. (Benxi, Liaoning, China). Standard environmental conditions (22±2 °C, 60% ± 5% humidity, 12 hours of darkness and light cycle, and continuous distilled deionized water) were maintained for mice. Animal ethics approval was granted by Animal Ethics Committee of Dalian Polytechnic University (protocol number: DLPU2022024). A control group of ten mice was fed a standard diet containing 45 ppm iron throughout the experiment. In other experiments, mice were fed an iron-deficient diet containing 12 ppm iron for 8 weeks in order to obtain IDA mice models. To conduct routine blood tests, blood was collected using orbital venipuncture after 8 weeks The hemoglobin (Hb) value of less than 100 g/L was taken as indicative of an IDA. The IDA mice were randomly divided into four groups of ten mice each after establishing the model (0 day of iron supplement): the model group (iron-deficient diet); the positive control group was administered FeSO4 (2 mg Fe/kg BW), and the other two groups given Antarctic krill protein-iron complex and peptide-iron complex respectively. The same dose of iron was used in both groups (2 mg Fe/kg BW). Deionized water was administered only to mice in the control group and blank group. During a 3-week period of intragastric administration, food intake and body weight were measured weekly.
2.4. Hematological test
The hemoglobin (Hb), serum iron (SI) concentration and total iron binding capacity (TIBC) levels were measured using kits (Nanjing Jiancheng Bioengineering Inst., Nanjing, China).
2.5. relative biological value and Hb regeneration efficiency
In order to determine the Hb iron pool (mg), we assumed a total blood volume of 6.7% BW and a Hb iron concentration of 0.335% [
26]. The related formula is as follows:
2.6. Analysis of iron content in liver and spleen
0.1 g liver and spleen were digested with nitric acid/perchloric acid (20: 1, v/v) at 300 °C for 30–40 min until the solution became clear. The content of iron was determined using ICP-OES (PerkinElmer, Waltham, USA).
2.7. Real-time (RT) PCR analysis
A quantitative real-time polymerase chain reaction (RT-PCR) was used to determine liver DMT1, Tf, and TfR expression and mRNA levels. The GAPDH was used as the internal reference. Gene expression levels were measured using the ΔΔCt method in relation to 𝛽-actin levels.
Table 1 displays the primer sequences. Quantitative RT-PCR was performed using three biological samples and three technical replications.
2.8. The detection of the SOD, GSH-PX and MDA levels in the gastric tissue
0.1 g of mice gastric tissue was taken, followed by homogenization with ice saline, and centrifugation at 5000 rpm for 15 min at 4 °C. The kits (Nanjing Jiancheng Bioengineering Institute, Nanjing City, China) were used to determine the levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) in gastric tissue.
2.9. Histopathological observation
Firstly, the liver and spleen of each group of mice were soaked in 10% formalin. Tissue samples were then dehydrated with an ethanol gradient, cleared with xylene, followed by being embedded in paraffin. Afterwards, tissue samples were stained with hematoxylin and eosin. Slices were observed with an optical microscope with a × 400 magnification.
2.10. Statistical analysis
Data from experiments were represented as the mean ± standard deviation (SD). Comparison between any two groups was performed with one-way analysis of variance (ANOVA), followed by Duncan’s multiple comparison tests. P < 0.05 was used to determine statistically significant differences between groups.
3. RESULTS AND DISCUSSION
3.1. The growth of IDA mice during iron supplementation
In this experiment, an iron-deficient diet was used to establish the IDA mouse model and then the therapeutic effect of Antarctic krill peptide-iron complex and protein-iron complex on IDA mice was explored, and the mouse serum was obtained (
Figure 1A). During this period, the body weight was measured regularly. The mice in the model group lost weight significantly after 8 weeks of iron-deficient diet feeding (
P < 0.05) as compared with those in the control group (
Figure 1B). Additionally, there was no significant difference in body weight between groups treated with iron-deficient diet (
P > 0.05). Many studies have reported that IDA can affect the growth of mice [
12,
27], which is consistent with the experimental result. Moreover, IDA mice were in poor health during the development of iron deficiency model, presented by pale skin, rough hair, slow growth and loss of appetite. Estimates of these parameters also got worse as the feeding time increased due to lack of iron intake. Similar results were found by Liu et al [
28]and Zielińska-Dawidziak et al [
29]. During iron supplementation, a significant improvement in their health was observed. At the end of the iron supplementation (3 weeks), the treatment groups reported significant increases in body weight compared with the model group (
P < 0.05), and there were no significant differences between the Antarctic krill protein-iron complex group and the peptide-iron complex group (
P > 0.05). The results showed that iron supplementation promoted the growth of mice compared with the IDA group, and Antarctic krill protein-iron complex and peptide-iron complex relieved IDA symptoms more effectively than FeSO
4.
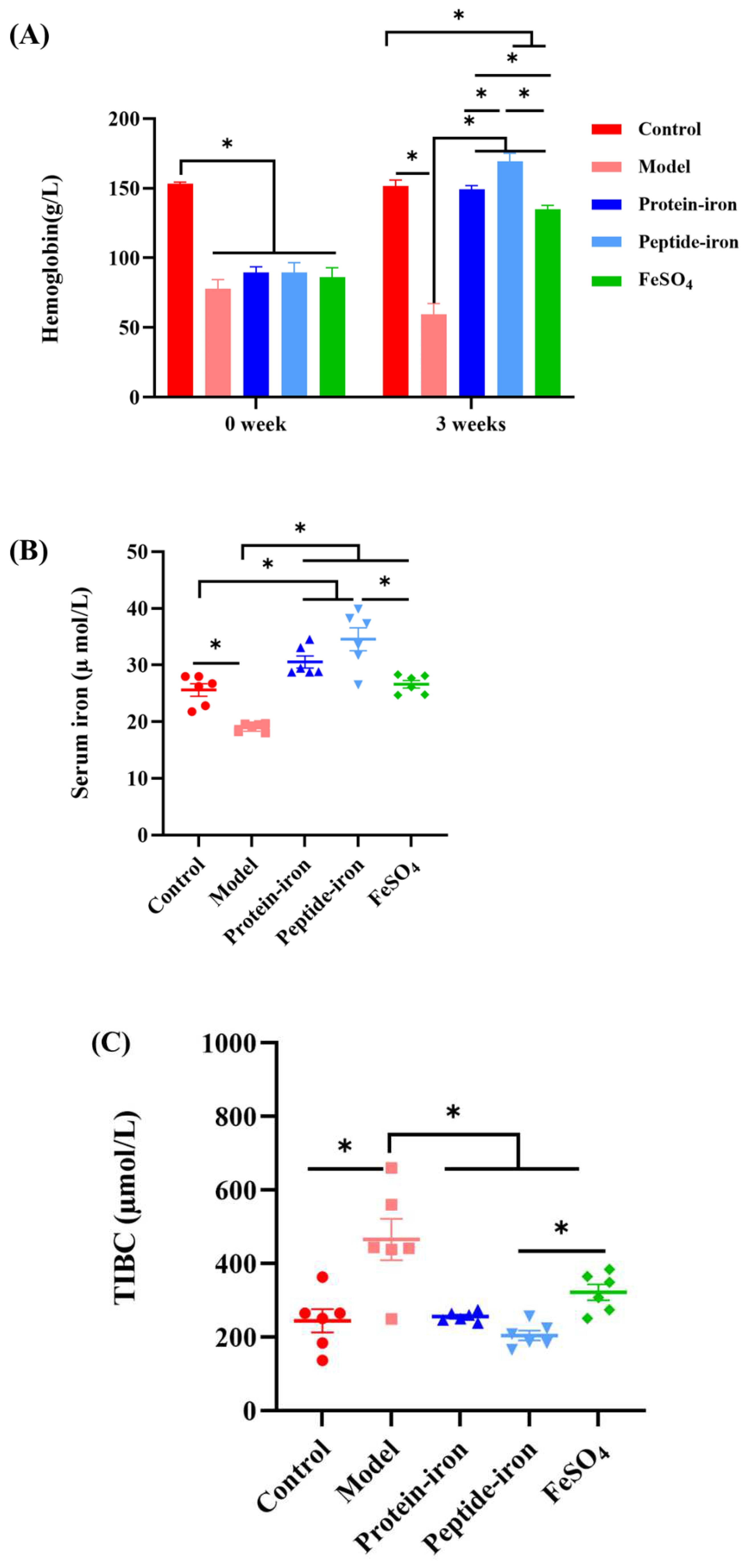
3.2. Hematological parameters analysis
In this study, we examined hematological indicators associated with iron status, including Hb, SI and TIBC. Hb levels before and after iron supplement are shown in
Figure 2A. IDA can cause a decrease in Hb [
30]. Due to dietary iron deficiency, the Hb levels in the IDA group were significantly lower than those in the control group (
P<0.05). This is because an iron deficiency causes the functional iron level in the blood to decrease, which decreases Hb levels [
31]. The Hb content in the iron-supplemented groups and the IDA group were not significantly different at the beginning of oral administration (
P > 0.05). Following iron supplementation, the Hb levels of the mice in the iron-supplemented groups were dramatically increased, and gradually approached to the control group. Among them, the Hb concentration of the Antarctic krill peptide-iron complex group (169.23±5.87 g/L) and the protein-iron complex group (149.19±2.78 g/L) were significantly higher than that of the FeSO
4 group (135.13±2.56 g/L) (
P < 0.05). Indeed, the Hb levels would return to near normal levels with improved iron status [
32].
The determination of SI and TIBC is of great significance to the clinical diagnosis of IDA [
33]. SI reflects iron absorption in the intestine by measuring the amount of circulating iron bound to transferrin [
34]. It can be seen from
Figure 2B that iron deficiency anemia has the greater impact on SI concentration in mice, resulting in lower SI concentration (19.05±0.73 µmol/L). However, Antarctic krill peptide-iron complex and protein-iron complex significantly increased SI concentrations in IDA mice (
P < 0.05). In the iron supplementation group, the mice in the Antarctic krill peptide-iron complex group had the higher SI concentration (34.55±4.96 µmol/L), followed by the Antarctic krill protein-iron complex group (30.54±2.57 µmol/L). The mice in the FeSO
4 group had the lower serum iron concentration (26.60±1.65 µmol/L).
TIBC reflects the ability of blood to bind iron with transferrin, and it often increases when iron deficiency is present [
35]. It was shown in
Figure 2C that IDA mice had significantly higher TIBC levels (
P < 0.05) (465.92±56.30 µmol/L). After iron supplementation, the TIBC level in anemic mice significantly decreased (
P < 0.05). The TIBC levels of mice in the Antarctic krill peptide-iron complex group, the protein-iron complex group and the FeSO
4 group were nearly to the control group. Moreover, as compared to the control group, there were no significant differences between the Antarctic krill peptide iron complex group and the protein-iron complex group (
P > 0.05). In contrast, there was significant difference between the Antarctic krill peptide-iron complex group and the FeSO
4 group (
P < 0.05). In addition, among in the iron supplementation group, the TIBC levels of mice in the Antarctic krill peptide-iron complex group (204.95±13.31 µmol/L) was lower than that of the Antarctic krill protein-iron complex group (255.86±5.35 µmol/L) and the FeSO
4 group (322.04±21.64 µmol/L) (
P < 0.05).
The results exhibited that there was a significant improvement in Hb, SI, and TIBC levels after administration of the Antarctic krill peptide-iron complex and protein-iron complex. Specifically, the Antarctic krill peptide-iron complex showed a better therapeutic effect. This may be related to the structure and amino acid composition of Antarctic krill proteins and peptides. Wang et al found that after Antarctic krill powder was hydrolyzed by trypsin, and Asp, Glu and His content on Antarctic krill peptides are positively correlated with iron-binding activity [
22]. It has been shown that peptides rich in these amino acids promote iron absorption [
36,
37]. Therefore, Antarctic krill peptide-iron complex can be used as an effective iron supplement.
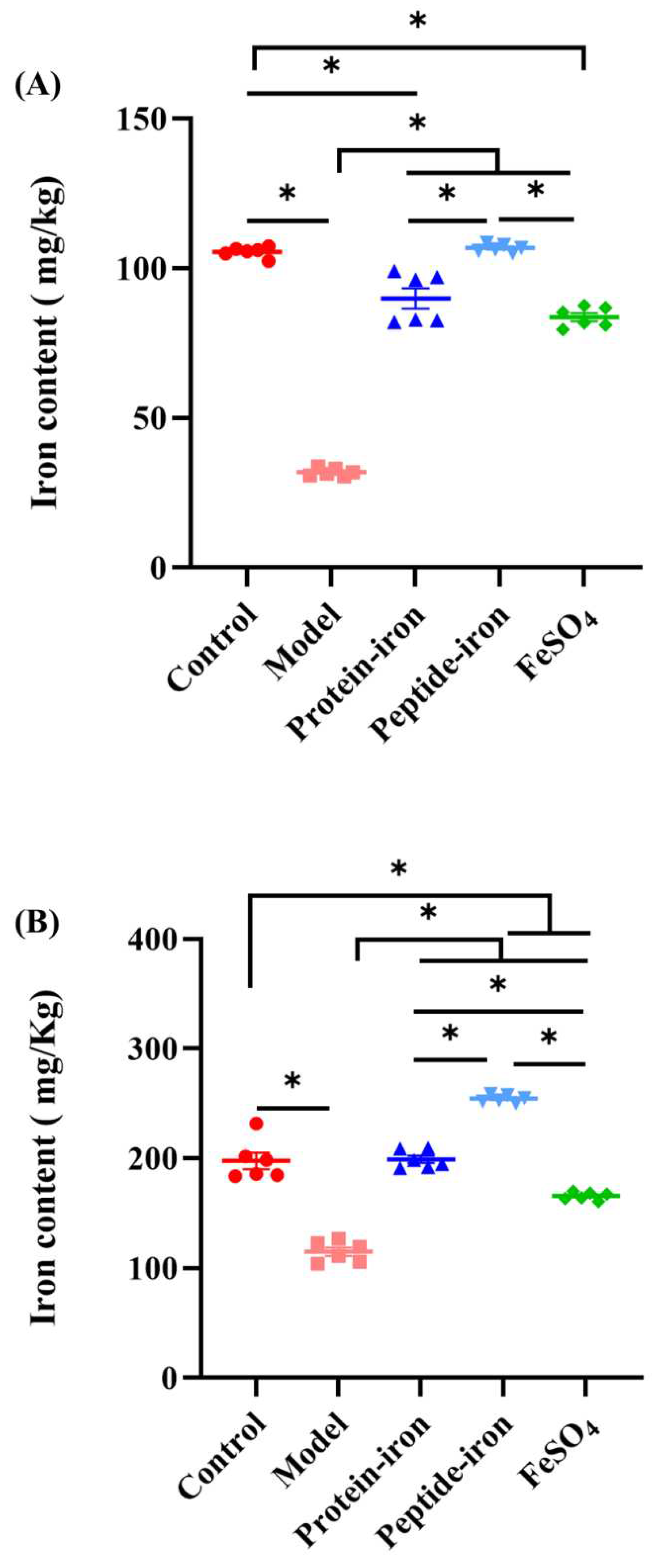
3.3. Iron content in the liver and spleen of mice
Figure 3 shows the accumulation of iron in the liver and spleen. As shown in
Figure 3A, the iron content of liver in the IDA mice (31.93±0.52 mg/kg) was significantly lower than those in the control group (105.49±0.68 mg/kg) (
P < 0.05). The iron content of liver in the Antarctic krill peptide-iron complex group (106.766±0.53 mg/kg) gradually near the control group after iron supplementation, and there was no significant difference between each of them (
P > 0.05). Moreover, the iron content of liver in the Antarctic krill peptide-iron complex group was significantly higher than that in the Antarctic krill protein-iron complex group (89.94±3.37 mg/kg) and the FeSO
4 group (83.67±1.34 mg/kg) (
P < 0.05), and the difference between the Antarctic krill protein-iron complex group and the FeSO
4 group was not significant (
P > 0.05). In addition, the iron content in the spleen has a similar trend to that in the liver. The iron content of spleen in the Antarctic krill peptide-iron complex group (254.54±1.35 mg/kg) was significantly higher than those in the control group (197.74±7.46 mg/kg), the Antarctic krill protein-iron complex group (199.26±3.30 mg/kg), and the FeSO
4 group (165.80±1.43 mg/kg) (
Figure 3B). The difference between the Antarctic krill protein-iron complex group and the control group was not significant (
P > 0.05), but the iron content of spleen in the Antarctic krill protein-iron complex group higher than that of the FeSO
4 group.
The liver and spleen are major iron storage tissues and play a vital role in iron homeostasis [
38]. As a rule, IDA mice use iron stored in the liver and spleen for transport to maintain serum iron balance [
39]. Therefore, IDA mice generally had lower levels of liver iron than the other groups of mice. This is consistent with the results observed by Zhang et al [
40]. Study has confirmed that in order to compensate for iron deficiency and Hb reduction, the body activates haemolytic states under iron deficiency, which lead to hypertrophy of the liver and spleen [
30]. This phenomenon has been observed in our experiment [
41]. In addition, the amino acid composition in the diet may also affect the accumulation of iron in the liver and spleen. Ma et al demonstrated that glutamate-rich peptides reduce the production of transferrin, which favors iron storage in the liver and spleen [
41]. This may be the reason why peptide-iron complex are more favorable for iron storage than protein-iron complex.
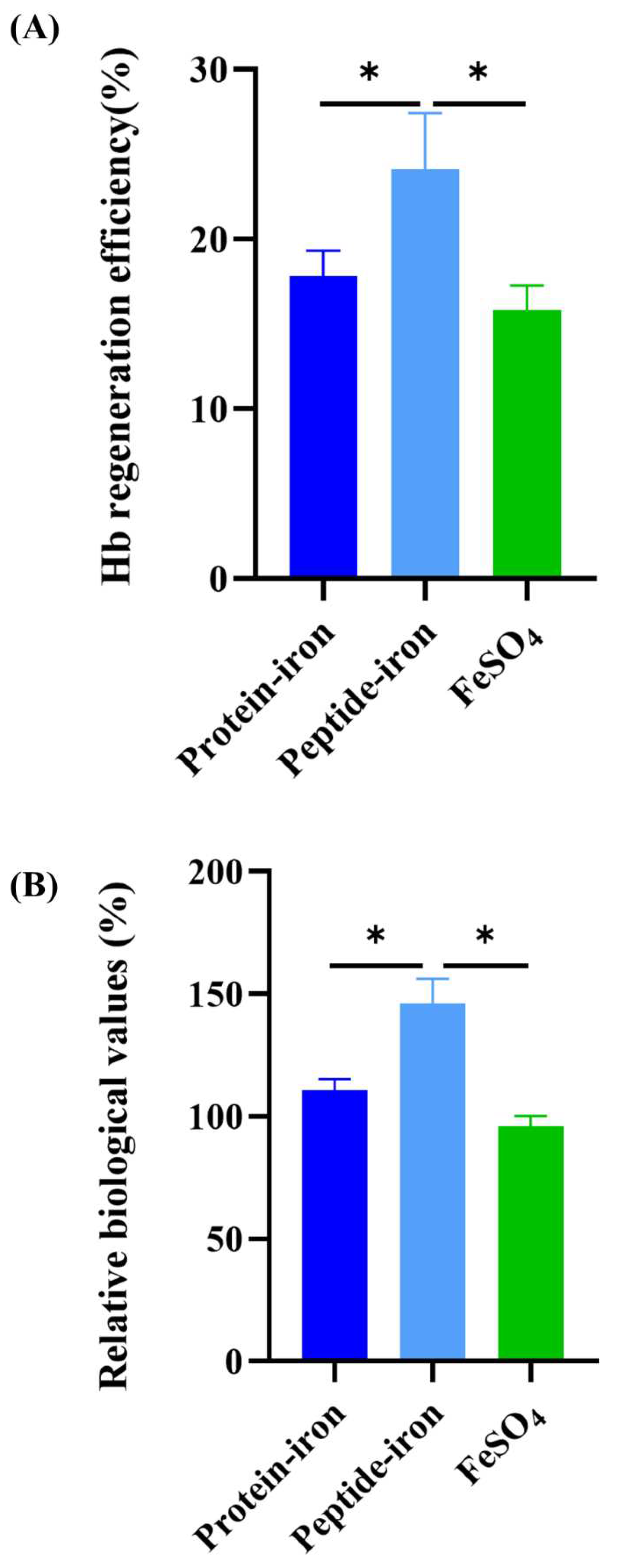
3.4. Hemoglobin regeneration efficiency and relative biological value
The Hb regeneration efficiency is shown in
Figure 4A. Oral administration of the Antarctic krill peptide-iron complex (24.10±3.33%) was the most efficient than that of the protein-iron complex (17.82±1.52%). The Hb regeneration efficiency of the FeSO
4 group was lower (15.80±1.46%). There was no significant difference in the Hb regeneration efficiency between the Antarctic krill protein-iron complex group and the FeSO
4 group (
P>0.05). Using the Hb regeneration efficiency of FeSO
4 as a reference (100%), the relative biological value of the iron-supplemented group was calculated (
Figure 4B). The relative bioavailability of iron in the Antarctic krill peptide-iron complex group (152.53±21.05%) was significantly higher than those in the protein-iron complex group (112.75±9.60%) (
P<0.05). Interestingly, the relative biological value of the Antarctic krill peptide-iron complex group was 39.78% higher than that of the protein-iron complex group. Combined with the results of the hematological indicators, iron content of liver and spleen, and hemoglobin regeneration efficiency, the Antarctic krill peptide-iron complex is a better iron supplement compared with protein-iron complex.
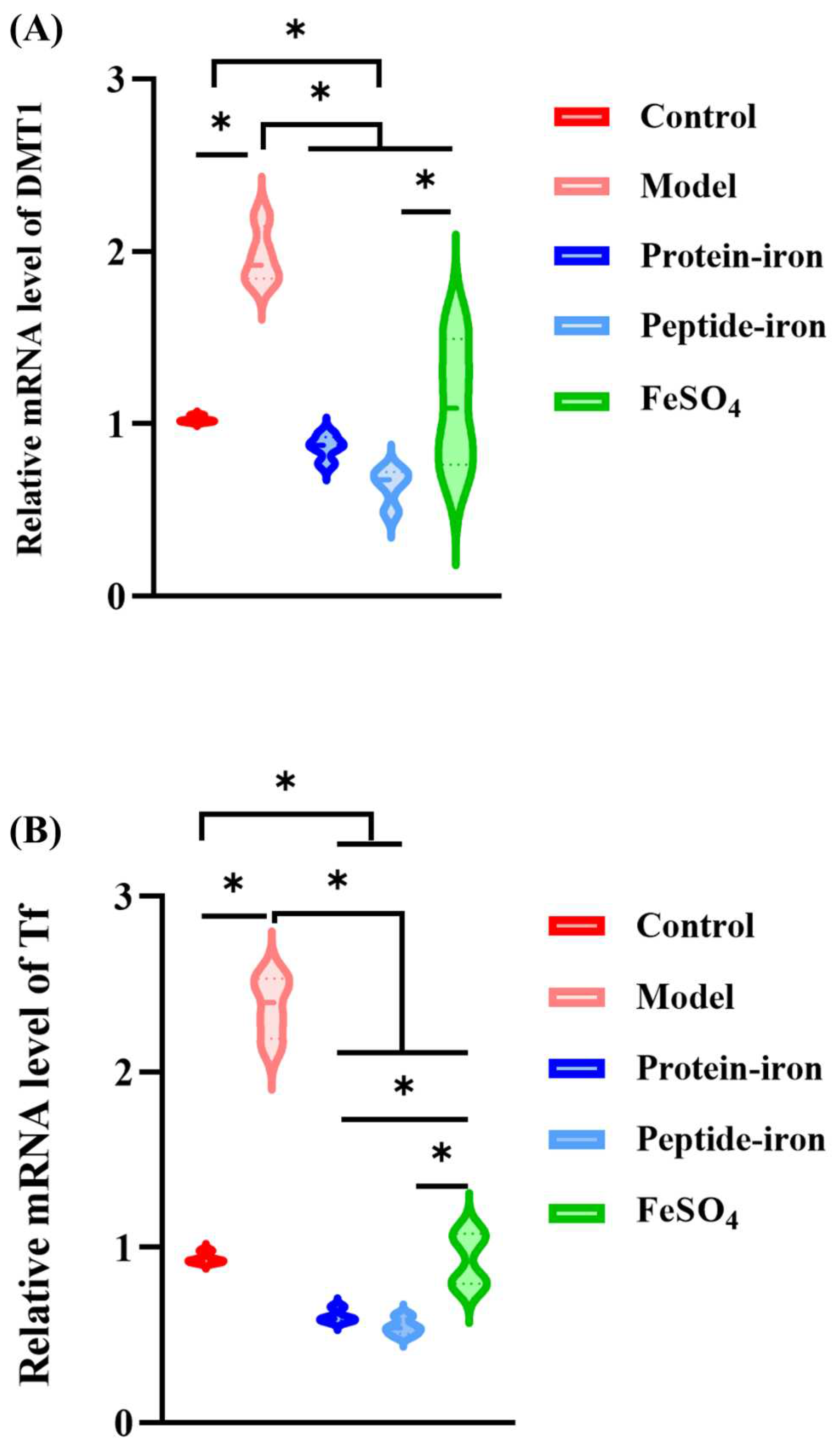
3.5. Expression of iron-regulated genes
The transmembrane protein DMT1 transports non-heme iron across the apical membrane of enterocytes [
42]. Researchers have found that rats fed iron-deficient diets had significantly higher renal DMT1 levels, while rats fed iron-enriched diets showed significantly lower levels [
43]. As shown in
Figure 5A, there was a significant increase in DMT1 mRNA levels in the model group (
P < 0.05) compared with those in the other groups. The decrease in DMT1 mRNA level indicated an increase in iron absorption from the intestinal segment. In this study, after gavage of FeSO
4, Antarctic krill protein-iron complex, and peptide-iron complex to mice, there was a return to normal levels of gene expression for DMT1. The ranking of the DMT1 gene expression levels is Antarctic krill peptide-iron complex<protein-iron complex<FeSO
4.
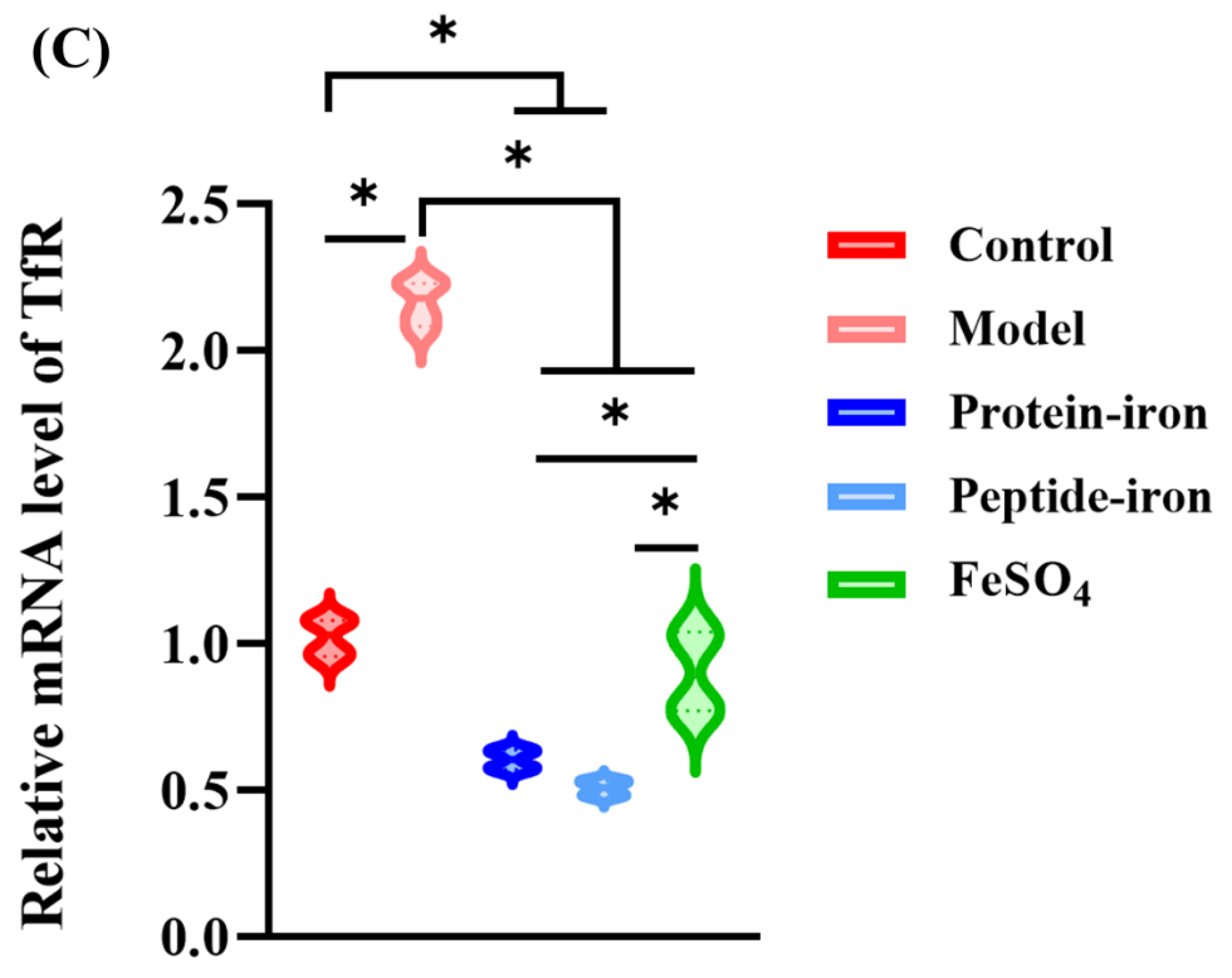
The main source of iron for most tissues is iron-loaded Tf. Tf originates in the liver and transports iron from the site of absorption to almost all tissues. In iron-deficient states, its synthesis is markedly increased by unknown mechanisms. Most cells facilitate iron uptake by Tf bound ferric iron
via TfR [
44]. Tf with high iron binds to TfR on the surface of red blood cells and enters the cell through pinocytosis [
45,
46]. As shown in
Figure 5B,5C, as compared with the control group, the level of Tf and TfR mRNA increased significantly in the model group (
P < 0.05). Several studies have shown an inverse correlation between Tf and its mRNA in response to iron deficiency [
47], which is consistent with our findings. In addition, the Tf and TfR genes expression levels of the FeSO
4 group, the Antarctic krill protein-iron group, and the peptide-iron group decreased to the normal levels by oral iron supplementation, in which the Tf and TfR gene expression levels decreased more rapidly after oral administration of the Antarctic krill protein-iron complex and the peptide-iron complex as compared to those in the FeSO
4 group, indicating an increase in the absorption of iron by the intestine. Moreover, no significant difference on the Tf and TfR gene expression levels between the Antarctic krill protein-iron group and the peptide-iron group (
P > 0.05).
The absorption mechanism of inorganic ferrous ions was not similar to that of organic complex. There is a possibility that iron from Antarctic krill protein-iron complex, peptide-iron complex and FeSO4 enters the common matrix for efflux into the blood. Regulation of DMT1, Tf and TfR mRNA expression revealed good bioavailability of Antarctic krill protein-iron complex, peptide-iron complex and FeSO4 in mice. The Antarctic krill protein-iron complex and peptide-iron complex groups had lower DMT1, Tf and TfR mRNA levels in liver compared with the FeSO4 group at the same doses. Furthermore, the Antarctic krill peptide-iron complex group had lower DMT1, equal Tf and TfR mRNA levels compared with the Antarctic krill protein-iron complex group at the same doses, indicating that the Antarctic krill peptide-iron complex is easier to be absorbed by the small intestine than the protein-iron complex and FeSO4.
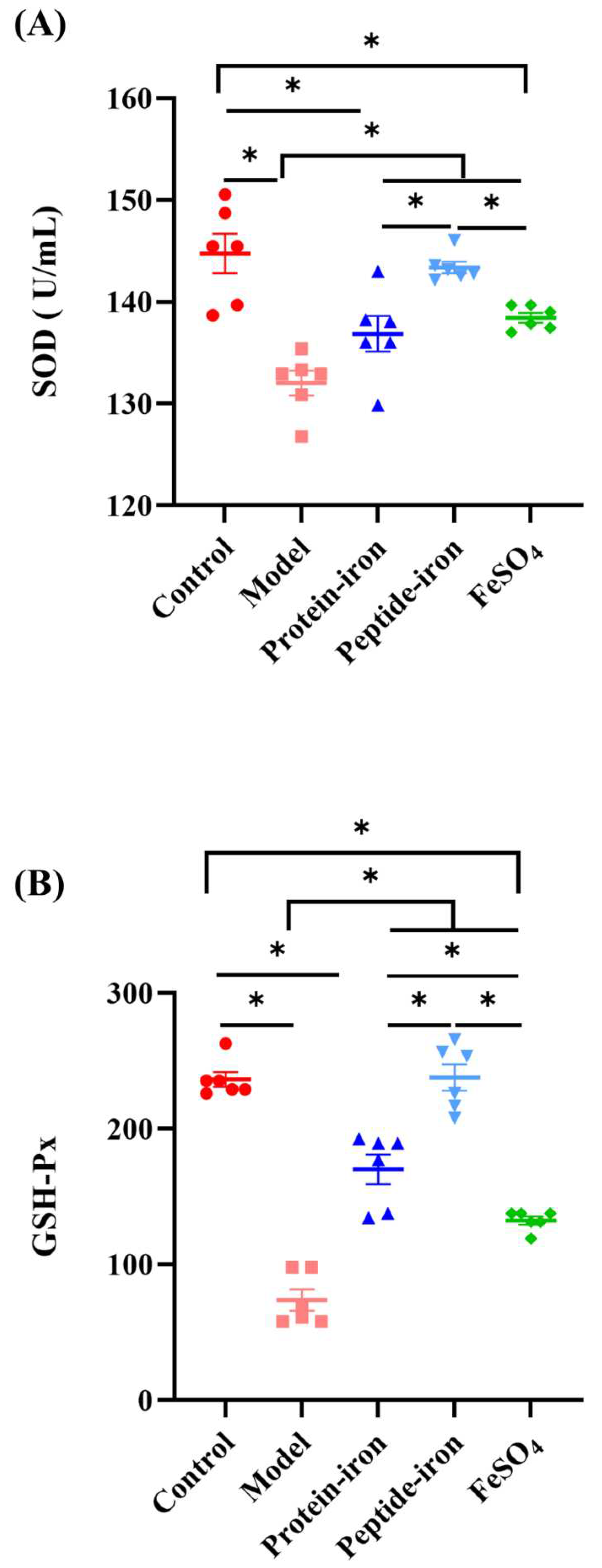
3.6. In vivo antioxidant activities
Cells and their structures can be disrupted by accumulating reactive oxygen species (ROS). In the presence of superoxide dismutase (SOD), superoxide anion was catalyzed it to hydrogen peroxide (H
2O
2), which is then disintegrated into H
2O by catalase [
48]. In addition, glutathione peroxidase acts directly as an antioxidant by reacting with superoxide radicals, peroxyl radicals and singlet oxygen molecules [
49]. A variety of cellular functions are regulated by these enzymes, and they protect cells against oxidative damage in organisms. Studies have reported that iron deficiency anemia-induced oxidative stress can damage gastric mucosal tissue [
50,
51]. In order to explore the protective effect of Antarctic krill protein-iron complex and peptide-iron complex on the gastric mucosa of IDA mice, the SOD activity, the glutathione peroxidase (GSH-Px) activity and the malondialdehyde (MDA) concentration of gastric tissue were determined.
It can be seen from
Figure 6A that the SOD activity of anemic mice (132.03±2.95 U/mL) was significantly lower than that of normal mice or iron-supplemented mice. The activity of the SOD activity of the Antarctic krill protein-iron group (136.85±4.27 U/mL) was comparable to that in the FeSO
4 group (138.44±1.17 U/mL) and the difference was not significant (
P > 0.05). The SOD activity of the mice in the Antarctic krill peptide-iron group (143.38±1.39 U/mL) was higher (
P < 0.05). Nevertheless, the difference was not significant as compared with those in the control group (
P > 0.05). As shown in
Figure 6B, as compared with those in the normal group or iron-supplemented groups, the activity of GSH-Px of the anemic mice (73.85± 19.07) was the lowest (
P < 0.05). In addition, the GSH-Px activity of the mice was higher in the Antarctic krill peptide-iron complex group (237.84±23.93) compared with those in the control group and there was no significant difference (
P > 0.05). Moreover, among the iron-supplemented mice, the GSH-Px activity of mice in the FeSO
4 group (132. 42±7.14) was lower. In general, decreased levels of SOD and GSH-Px activity are the main manifestation of iron deficiency anemia [
52]. Our study suggests that iron deficiency anemia reduces the activity of antioxidant enzyme. Gavage administration of the Antarctic krill protein-iron complex, the peptide-iron complex and FeSO
4 significantly increased the activity of antioxidant enzyme and gradually returned to near-normal levels. As compared with traditional FeSO
4 treatment, Antarctic krill protein-iron complex treatment and peptide-iron complex treatment were more effective, in which Antarctic krill peptide-iron complex had higher
in vivo antioxidant activity. This showed that the antioxidant activity of the enzymatic hydrolyzate obtained by proteolysis was improved.
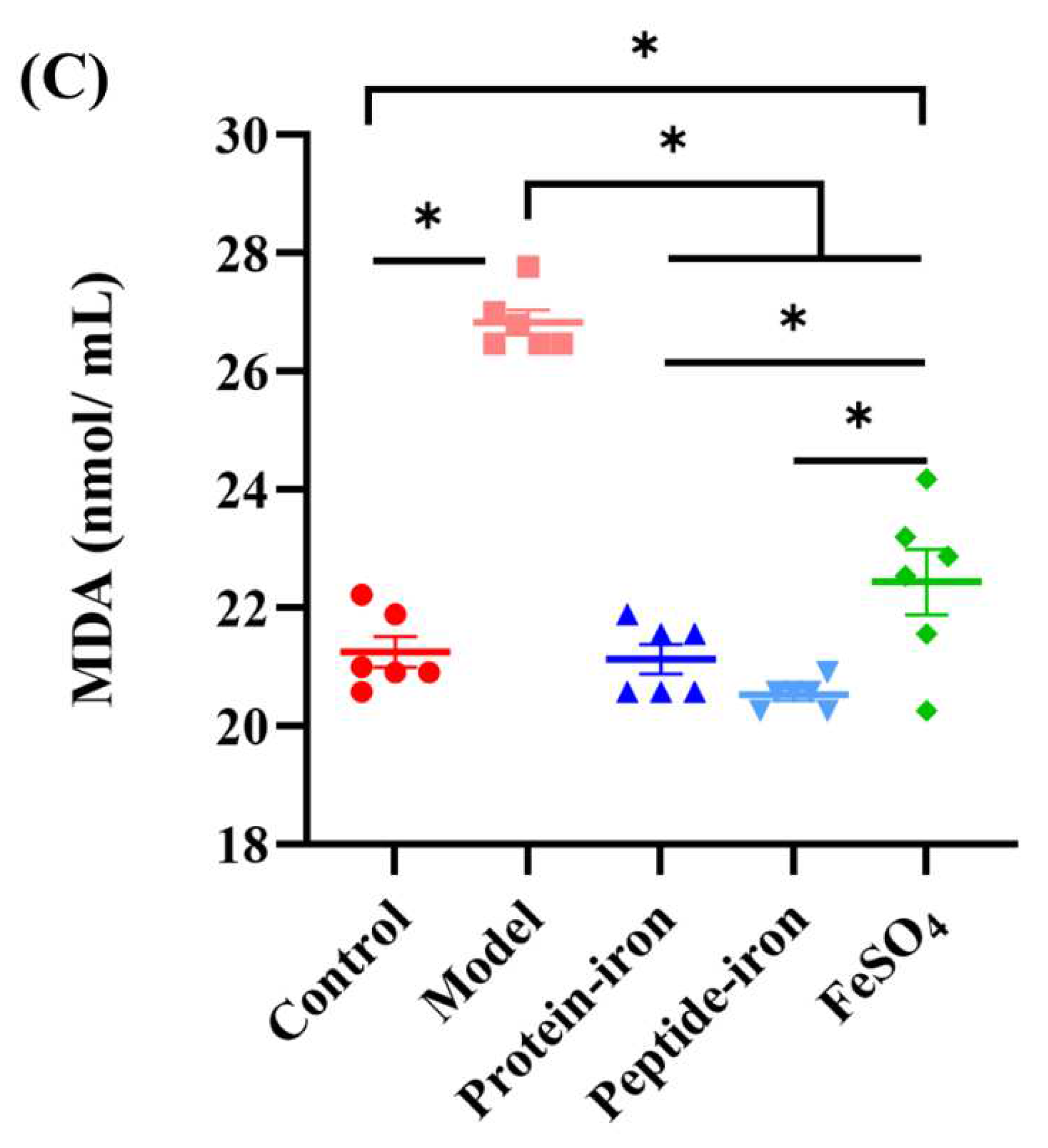
As a product of lipid peroxidation, malonaldehyde (MDA) is typically used to estimate the extent of lipid peroxidation in biological systems [
53]. Oral iron supplementation has not been extensively studied despite extensive literature on iron and lipid peroxidation. In this study, the average concentration of MDA in normal mice was 21.26±0.64 nmol/mL (
Figure 6C). The concentration of MDA was significantly increased in anemic mice (26.83±0.51 nmol/mL) (
P < 0.05). Furthermore, the concentration of MDA in the Antarctic krill protein-iron complex group (21.13±0.11 nmol/mL) and the peptide-iron complex group (20.53±0.25 nmol/mL) was significantly lower than that in the IDA group (
P < 0.05), but not significantly different from the normal mice (
P > 0.05). In general, the Antarctic krill protein-iron complex and the peptide-iron complex could enhance the activity of antioxidant enzymes, reduce the malondialdehyde level in the IDA mice, in which the Antarctic krill peptide-iron complex exhibited higher
in vivo antioxidant activity than that of the protein-iron complex.
Oxidative stress can be caused by dysregulation of iron pathways. The main cause of oxidative stress is the generation of hydroxyl radicals and the oxidation of lipids [
54]. Several studies have demonstrated that animal proteins and/or its antioxidative peptides may reduce oxidative stress by hydrolyzation [
55]. Rahimpour et al also confirmed that Antarctic krill hydrolyzate has certain antioxidant activity [
56]. Ferrous iron chelators form coordination bonds with ferrous ion, which lowers the redox potential of the ferrous ion, thereby stabilizing the oxidized form of ferrous ion. This property gives ferrous iron chelators effective secondary antioxidant capacity [
57]. Since the iron-binding activity of Antarctic krill hydrolysates gradually increased with the time of hydrolysis [
22], the antioxidant capacity of the Antarctic krill peptide-iron complex was higher than that of the protein-iron complex.
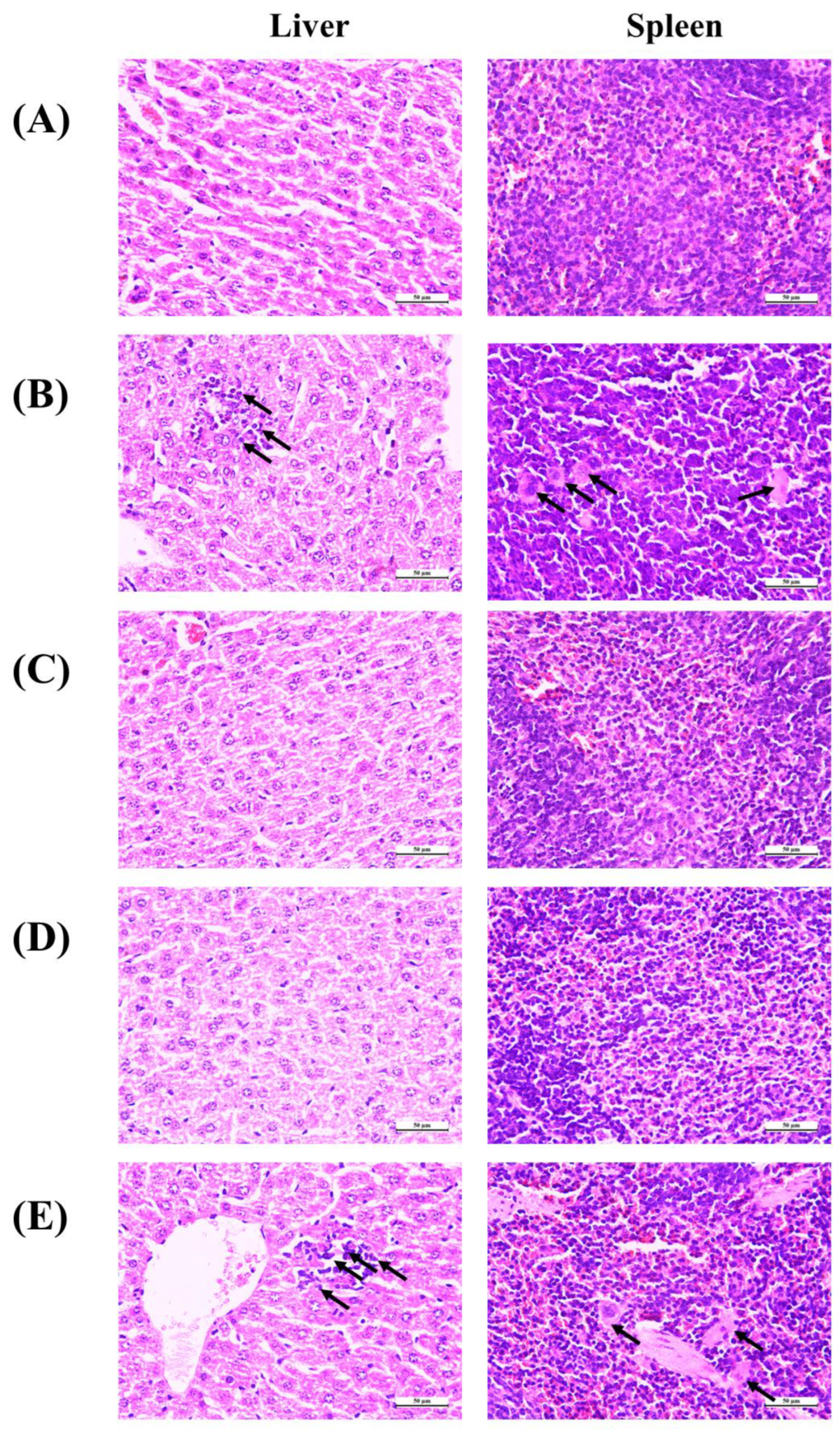
3.7. Histopathological observation
For this purpose of investigating the protective effect of Antarctic krill proteins and peptides on the tissue damage of liver and spleen caused by IDA, HE staining was used in this experiment and the paraffin sections of liver and spleen were observed under a microscope. As shown in
Figure 7, histological evaluation of the liver showed iron-deficiency anemia-induced hepatic injury, including monocyte infiltration in the portal area and fine hemosiderin pigment deposited in individual macrophages (black arrows). In addition, there is a phenomenon of reunion. After treatment with Antarctic krill peptide-iron complex or protein-iron complex, no evidence of organ damage or inflammation was observed in liver tissue from mice. Moreover, the liver tissue of mice treated with FeSO
4 showed agglomerated particles. As for the spleen tissue, the microscope of the control group (normal) showed that the spleen cell structure was intact. Spleen injury caused by iron deficiency anemia is manifested by the obvious appearance of tangible body macrophages and inflammatory cells (black arrows). Moreover, tangible body macrophages were observed in liver tissue of mice treated with FeSO
4. Antarctic krill protein-iron complex group and peptide-iron complex groups showed normal spleen tissue structure without detectable lesions. FeSO
4 causes splenic lesions in the FeSO
4 group, confirming its toxic effects.
It has been shown that iron deficiency can cause oxidative stress, which is closely related to the production of reactive oxygen species (ROS) [
58]. Highly reactive ROS damage cells by oxidizing and destroying biomacromolecules. Diet may be an important regulator of cellular redox status and protect cells from oxidative stress-induced apoptosis. FeSO
4 can aggravate oxidative stress in vivo and its histopathological damage [
59]. In this study, administration of Antarctic krill peptide-iron complex or protein-iron complex significantly reduced the extent of iron deficiency-induced macroscopic and microscopic spleen tissue damage.
In summary, the Antarctic krill peptide-iron complex was found to be more effective than the protein-iron complex and FeSO4 in iron absorption and utilization by evaluating the body weight, blood parameters, iron content in the liver and spleen of the mice. Results indicated that Antarctic krill peptide-iron complex increased significantly the hemoglobin, serum iron and iron content in the liver and spleen in iron-deficiency anemia mice (P < 0.05) compared with Antarctic krill protein-iron complex. Despite the gene expressions of divalent metal transporter 1, transferrin and transferrin receptor could be better regulated by Antarctic krill peptide-iron complex and protein-iron complex, the relative bioavailability of iron in the Antarctic krill peptide-iron complex group (152.53±21.05%) was significantly higher than in the protein-iron complex (112.75±9.60%) (P<0.05). Moreover, Antarctic krill peptide-iron complex could enhance the antioxidant enzyme activities of superoxidase dismutase and glutathione peroxidase in gastric tissue, reduce the malondialdehyde level in IDA mice compared with the protein-iron complex, reducing the cell damage caused by IDA. These findings suggest that Antarctic krill peptide-iron complex is an effective source of iron supplementation for IDA mice. Further study is needed to investigate mechanism of Antarctic krill peptide-iron complex for improving iron deficiency.
Author Contributions
Conceptualization, S.Y.L. and N.S.; Data curation, S.J.H.; Formal analysis, S.J.H.; Funding acquisition, N.S.; Investigation, S.J.H. and Q.F.; Methodology, S.J.H., X.Q.H., H.W.X and N.S.; Project administration, N.S.; Resources, N.S.; Supervision, N.S.; Validation, N.S.; Visualization, L.C.; Writing – original draft, S.J.H..; Writing – review & editing, S.Y.L., and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31972008) and the LiaoNing Revitalization Talents Program of China (XLYC2007078).
Institutional Review Board Statement
Animal ethics approval was granted by Animal Ethics Committee of Dalian Polytechnic University (protocol number: DLPU2022024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the results of this study are included in the present article.
Conflict of Interest
The authors have no competing financial or other conflicts of interest to declare.
References
- Sangkhae V, Nemeth E. Regulation of the Iron Homeostatic Hormone Hepcidin[J]. Adv Nutr 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-deficiency anemia[J]. New England journal of medicine 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Ross E, M. Evaluation and treatment of iron deficiency in adults[J]. Nutrition in clinical care 2002, 5, 220–224. [Google Scholar] [CrossRef]
- Vives Corrons J L, Miguel-García A, Pujades M A, et al. Increased susceptibility of microcytic red blood cells to in vitro oxidative stress[J]. Eur J Haematol 1995, 55, 327–331. [Google Scholar] [CrossRef]
- Sharp P, Srai S K. Molecular mechanisms involved in intestinal iron absorption[J]. World journal of gastroenterology: WJG 2007, 13, 4716. [Google Scholar] [CrossRef]
- Garrick M D, Dolan K G, Horbinski C, et al. DMT1: a mammalian transporter for multiple metals[J]. Biometals 2003, 16, 41–54. [Google Scholar] [CrossRef]
- Conrad M E, Umbreit J N, Moore E G. Iron absorption and transport[J]. The American journal of the medical sciences 1999, 318, 213–229. [Google Scholar] [CrossRef]
- Scotland P B, Heath J L, Conway A E, et al. The PICALM protein plays a key role in iron homeostasis and cell proliferation[J]. PLoS One 2012, 7, e44252. [Google Scholar]
- Hurrell R, Egli I. Iron bioavailability and dietary reference values[J]. The American journal of clinical nutrition 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Jahan T A, Vandenberg A, Glahn R P, et al. Iron Fortification and Bioavailability of Chickpea (Cicer arietinum L.) Seeds and Flour[J]. Nutrients, 2019, 11(9).
- Tang N, Chen L-Q, Zhuang H. Effects of heme iron enriched peptide on iron deficiency anemia in rats[J]. Food & function 2014, 5, 390–399. [Google Scholar]
- Wang F-R, Xie Z-G, Ye X-Q, et al. Effectiveness of treatment of iron deficiency anemia in rats with squid ink melanin–Fe[J]. Food & function 2014, 5, 123–128. [Google Scholar]
- Tolkien Z, Stecher L, Mander A P, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis[J]. PloS one 2015, 10, e0117383. [Google Scholar]
- Bries A, Wang C, Wels B, et al. Assessment of Gastrointestinal Symptoms and Non-transferrin Bound Iron After Oral Ferrous Sulfate and Iron-enriched Aspergillus Oryzae Supplementation in Women (P24-039-19)[J]. Current Developments in Nutrition, 2019, 3(Supplement_1): nzz044. P24-039-19.
- Huda T, Dibley M, Arifeen S E, et al. Assessing the Efficacy of Bovine Lactoferrin to Correct Iron Deficiency Anemia in Non-pregnant Non-lactating Women: A Randomized Controlled Trial (FS08-02-19)[J]. Current Developments in Nutrition, 2019, 3(Supplement_1): nzz044. FS08-02-19.
- Strbak O, Balejcikova L, Kmetova M, et al. Quantification of Iron Release from Native Ferritin and Magnetoferritin Induced by Vitamins B(2) and C[J]. Int J Mol Sci, 2020, 21(17).
- [17] Bhattacharya P T, Misra S R, Hussain M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review[J]. Scientifica (Cairo) 2016, 2016, 5464373. [Google Scholar]
- Evcan E, Gulec S. The development of lentil derived protein–iron complexes and their effects on iron deficiency anemia in vitro[J]. Food & Function 2020, 11, 4185–4192. [Google Scholar]
- Kobayashi Y, Wakasugi E, Yasui R, et al. Egg Yolk Protein Delays Recovery while Ovalbumin Is Useful in Recovery from Iron Deficiency Anemia[J]. Nutrients 2015, 7, 4792–4803. [Google Scholar] [CrossRef]
- Li B, He H, Shi W, et al. Effect of duck egg white peptide-ferrous chelate on iron bioavailability in vivo and structure characterization[J]. Journal of the Science of Food and Agriculture 2019, 99, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Eckert E, Lu L, Unsworth L D, et al. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins[J]. Journal of Functional Foods 2016, 25, 291–301. [Google Scholar] [CrossRef]
- Wang T, Lin S, Cui P, et al. Antarctic krill derived peptide as a nanocarrier of iron through the gastrointestinal tract[J]. Food Bioscience 2020, 36, 100657. [Google Scholar]
- Li Y, Peng Z, Tan L, et al. Structural and functional properties of soluble Antarctic krill proteins covalently modified by rutin[J]. Food Chemistry 2022, 379, 132159. [Google Scholar] [CrossRef]
- Gao C, Wang F, Yuan L, et al. Physicochemical property, antioxidant activity, and cytoprotective effect of the germinated soybean proteins[J]. Food Science & Nutrition 2019, 7, 120–131. [Google Scholar]
- Sun N, Cui P, Jin Z, et al. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates[J]. Food Chemistry 2017, 230, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Gao F, Guo W, Zeng M, et al. Effect of microalgae as iron supplements on iron-deficiency anemia in rats[J]. Food & function 2019, 10, 723–732. [Google Scholar]
- He H, Huang Q, Liu C, et al. Effectiveness of AOS–iron on iron deficiency anemia in rats[J]. RSC advances 2019, 9, 5053–5063. [Google Scholar] [CrossRef] [PubMed]
- Liu J-Y, Zhang Y, You R-X, et al. Polysaccharide isolated from Angelica sinensis inhibits hepcidin expression in rats with iron deficiency anemia[J]. Journal of medicinal food 2012, 15, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Dawidziak M, Hertig I, Piasecka-Kwiatkowska D, et al. Study on iron availability from prepared soybean sprouts using an iron-deficient rat model[J]. Food chemistry 2012, 135, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Linberg R, Conover C D, Shum K L. Hemoglobin based oxygen carriers: how much methemoglobin is too much?[J]. Artificial Cells, Blood Substitutes, and Biotechnology 1998, 26, 133–148. [Google Scholar] [CrossRef]
- Matsumoto N, Ikeda H, Shigefuku R, et al. Hemoglobin decrease with iron deficiency induced by daclatasvir plus asunaprevir combination therapy for chronic hepatitis C Virus genotype 1b[J]. Plos one 2016, 11, e0151238. [Google Scholar]
- Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients[J]. Acta haematologica 2004, 112, 126–128. [Google Scholar] [CrossRef]
- Yamanishi H, Iyama S, Yamaguchi Y, et al. Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity[J]. Clinical chemistry 2003, 49, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Zhuo Z, Fang S, Hu Q, et al. Digital gene expression profiling analysis of duodenum transcriptomes in SD rats administered ferrous sulfate or ferrous glycine chelate by gavage[J]. Scientific Reports 2016, 6, 37923. [Google Scholar] [CrossRef]
- Sánchez-Rivera L, Martínez-Maqueda D, Cruz-Huerta E, et al. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides[J]. Food Research International 2014, 63, 170–181. [Google Scholar] [CrossRef]
- Hu S J, Lin S Y, Liu Y, et al. Exploration of iron-binding mode, digestion Kinetics, and iron absorption behavior of Antarctic Krill-derived heptapeptide-iron complex[J]. FOOD RESEARCH INTERNATIONAL, 2022, 154.
- Storcksdieck S, Bonsmann G, Hurrell R. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources[J]. Journal of food science 2007, 72, S019–S029. [Google Scholar]
- Giorgi G, Roque M E. Immunohistochemical studies on duodenum, spleen and liver in mice: distribution of ferroportin and prohepcidin in an inflammation model[J]. International Journal of Morphology 2011, 29, 747–753. [Google Scholar] [CrossRef]
- Huh M, Shin M, Lee Y, et al. Effect of soybean hull iron on growth, iron bioavailability, and behavioral function in anemic rats induced by iron deficiency during gestation or lactation[J]. Nutrition Research 1999, 19, 1749–1761. [Google Scholar] [CrossRef]
- Zhang X-G, Wei G-X, Wang W-N, et al. Effects of Fe-YM1504 on iron deficiency anemia in rats[J]. Food & function 2016, 7, 3184–3192. [Google Scholar]
- Ma X, Liu C, Song W, et al. Evaluating the efficacy of a ferrous-ion-chelating peptide from Alaska pollock frame for the improvement of iron nutritional status in rats[J]. Food & Function 2019, 10, 4888–4896. [Google Scholar]
- Akashi K, Nagashima Y, Tabata T, et al. Immunochemical analysis of iron transporters and M2 macrophages in ovarian endometrioma and clear cell adenocarcinoma[J]. Mol Clin Oncol 2021, 15, 159. [Google Scholar] [CrossRef]
- Jiang B, Liu G, Zheng J, et al. Hephaestin and ceruloplasmin facilitate iron metabolism in the mouse kidney[J]. Sci Rep 2016, 6, 39470. [Google Scholar] [CrossRef] [PubMed]
- Aigner E, Weiss G, Datz C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver[J]. World J Hepatol 2015, 7, 177–188. [Google Scholar]
- Kleven M D, Jue S, Enns C A. Transferrin Receptors TfR1 and TfR2 Bind Transferrin through Differing Mechanisms[J]. Biochemistry 2018, 57, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Sanyear C, Butthep P, Eamsaard W, et al. Iron homeostasis in a mouse model of thalassemia intermedia is altered between adolescence and adulthood[J]. PeerJ 2020, 8, e8802. [Google Scholar] [CrossRef] [PubMed]
- Han J, Day J R, Connor J R, et al. Gene expression of transferrin and transferrin receptor in brains of control vs. iron-deficient rats[J]. Nutritional neuroscience 2003, 6, 1–10. [Google Scholar]
- Mukherjee S, Banerjee S K, Maulik M, et al. Protection against acute adriamycin-induced cardiotoxicity by garlic: Role of endogenous antioxidants and inhibition of TNF-α expression[J]. BMC Pharmacology 2003, 3, 16. [Google Scholar]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes[J]. Biochimica et Biophysica Acta (BBA)-General Subjects 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva-Frank T S, Weiss G, Koppenol W H, et al. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress[J]. Free Radical Biology and Medicine 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- 51. Wang T, Zhou YT, Chen XN, Zhu AX, Wu BH. Remote ischemic postconditioning protects against gastric mucosal lesions in rats. World J Gastroenterol. 9519.
- Yoo J-H, Maeng H-Y, Sun Y-K, et al. Oxidative status in iron-deficiency anemia[J]. Journal of Clinical Laboratory Analysis 2009, 23, 319–323. [Google Scholar] [CrossRef]
- Li J L, Wang Q Y, Luan H Y, et al. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha[J]. J Biomed Sci 2012, 19, 32. [Google Scholar]
- Khalid S, Ahmad S I. Correction of iron deficiency anemia in pregnancy and its effects on Superoxide dismutase[J]. Pakistan Journal of Pharmaceutical Sciences, 2012, 25(2).
- Ding Y, Ko S-C, Moon S-H, et al. Protective effects of novel antioxidant peptide purified from alcalase hydrolysate of velvet antler against oxidative stress in chang liver cells in vitro and in a zebrafish model in vivo[J]. International Journal of Molecular Sciences 2019, 20, 5187. [Google Scholar] [CrossRef]
- Rahimpour A, Heidarzadehpilehrood R, Aghel M, et al. Bioinformatics Analysis of MicroRNA Profiles Unveils Novel Biological Markers of Alzheimer’s Disease[J]. Neurochemical Journal 2022, 16, 334–342. [Google Scholar] [CrossRef]
- Gordon, M. The mechanism of antioxidant action in vitro[J]. Food antioxidants, 1990: 1-18.
- Ghoneum M, Abdulmalek S, Pan D. Reversal of age-associated oxidative stress in mice by PFT, a novel kefir product[J]. International Journal of Immunopathology and Pharmacology 2020, 34, 2058738420950149. [Google Scholar]
- Toblli J E, Cao G, Angerosa M. Ferrous sulfate, but not iron polymaltose complex, aggravates local and systemic inflammation and oxidative stress in dextran sodium sulfate-induced colitis in rats[J]. Drug design, development and therapy 2015, 9, 2585. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).