1. Introduction
Thyroid hormones (TH) are essential for human brain development and functionality [
1]. The active form, 3,5,3′-triiodothyronine (T3), modulates gene expression by directly binding to specific nuclear receptors, while indirect genomic actions mediated by T4 (3,5,3′,5′-tetraiodothyronine or thyroxine) or T3 by means of its binding to different receptors at the cytoplasmic membrane, cytoplasm, and mitochondria have also been described [
2,
3]. Experimental studies in rodents have established that TH availability and action in the central nervous system (CNS) are tightly controlled by two main mechanisms: local deiodination of TH, which activates and inactivates TH inside the brain [
4,
5], and TH transport across the cell membranes [
6,
7,
8]. Several TH transporters have been described, and an earlier review by our group provided a detailed summary of these transporters, their functions, and their clinical implications [
8]. Briefly, there are two widely accepted transporters with physiological functions in the CNS: the monocarboxylate transporter 8 (MCT8) [
6] and the organic anion-transporting polypeptide 1C1 (OATP1C1) [
7], belonging to the MCT and OATP families respectively, and both included in the major facilitator superfamily (MFS), which elements show a double bundle of six transmembrane domains joined with a large intracellular loop. MCT8 has a high affinity for T4 and its nuclear active form T3 [
6] while OATP1C1 transports T4, with the highest affinity among all known TH transporters [
8], as well as the iodothyronine product of its deiodination, reverse T3 (rT3) [
7]. OATP1C1 also transports other substances such as metabolites of steroid hormones, including 17-(β-D-glucuronic acid) estradiol, but virtually no T3 [
7].
Mutations in the MCT8 gene (
SLC16A2) and in the OATP1C1 gene (
SLCO1C1) can cause a psychomotor developmental delay in humans. MCT8 deficient patients show a specific TH peripheral blood profile with high T3, low T4, low rT3, and normal to slightly elevated thyrotropin (TSH), whereas OATP1C1 deficient patients present hypothyroid symptoms such as intolerance to cold but with a normal thyroid function test in serum. MCT8 mutations located on the X chromosome are linked to the rare disease known as MCT8 deficiency or Allan-Herndon-Dudley Syndrome (AHDS) [
9,
10]. Aside from severe cognitive impairment, AHDS patients present noticeable neuromotor disturbances, such as central hypotonia, pyramidal signs such as Babinski (referable to failure in cortical commands), and extrapyramidal signs such as dystonia, choreoathetosis, and hypokinesia (referable to failure of the basal ganglia inhibitory-excitatory commands) [
11,
12,
13]. General delay in myelination or myelin dysplasia has been shown in AHDS patients by magnetic resonance imaging (MRI) [
10,
11,
14,
15,
16,
17,
18], and histopathological examinations [
19,
20]. But in some cases, MRI also shows various basal ganglia lesions such as high T1 signals in the bilateral globus pallidus [
14], low T2 signals in the bilateral globus pallidus, which were designated as calcifications by a computed tomography scan [
21], high T2 signals in the bilateral basal ganglia [
16], high T2 signal in the bilateral putamen [
22], or low T1 and high T2 signals in the left putamen region [
23]. The first and sole patient with a functional mutation in OATP1C1 was reported in 2018 [
24], a 15.5-year-old girl showing gradual deterioration of cognitive and motor domains, with gait apraxia, myoclonic-like movements in the hands, scoliosis, and spasticity of the lower limbs. Brain imaging with MRI and positron emission tomography–computed tomography demonstrated grey and white matter degeneration and severe glucose hypometabolism [
24].
Patients with TH transporters deficiency suffer from severe motor disturbances that can be attributed to dysfunction of the cerebellum and its related nuclei, dysfunctions of the cortical command, and dysfunctions of the basal ganglia. The study of the expression of TH transporters in those systems is relevant to understanding the physio-pathogenesis of the disease. In previous work, we have discussed the alterations of the cerebellum in AHDS patients [
20] as a possible underlying factor for motor disturbances. We have also discussed the possible implications of the lack of expression of MCT8 and OATP1C1 in cortical projection neurons and interneurons of adult human and monkey brain [
25]. Here we focus on the distribution of TH transporters in the basal ganglia.
Basal ganglia are a group of interconnected subcortical nuclei that are primarily responsible for the modulation of motor control. To simplify the anatomy of the basal ganglia and related nuclei, these are separated into three compartments [
26]: 1)
input nuclei, which refers to the dorsal striatum (consisting of the caudate nucleus (Cd) and the putamen (Put)) and the ventral striatum (accumbens nucleus, not covered in this article); 2)
output nuclei, consisting of the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr); and 3)
intrinsic nuclei, consisting of the external segment of the globus pallidus (GPe), the substantia nigra pars compacta (SNc), and the subthalamic nucleus (STN). Input nuclei receive information from various sources, primarily the motor cortex, the centromedian-parafascicular nucleus (CM-Pf) of the thalamus, and the substantia nigra; intrinsic nuclei relay information from input nuclei to output nuclei; and output nuclei send basal ganglia processed information to the motor thalamus, mainly the ventral anterior and ventral lateral nuclei (VA/VL), which in turn are connected to the motor cortex. The classical basal ganglia model depicts how the commands that lead to a coordinated sequence of muscle contractions produce a movement flow from the motor cortex through the basal ganglia and back to the cortex. Briefly, the cortico-spinal motor command for muscle contraction is relayed by collaterals of the pyramidal pathway to the striatum in the basal ganglia. These nuclei contain the representation of the whole final movement scenario (because they receive connections from all the association cortical areas) and generate new orders that need to go through several synapses in the intrinsic and output nuclei and in the thalamus to prepare the cortex for the next sequence of motor commands, either inhibiting the previous command or exciting the output of the new command. The motor thalamus is a key intermediate element in this mechanism: it is an excitatory pathway for the motor cortex (and movement) unless it is inhibited by the basal ganglia, similarly to the way a jockey controls a horse with the reins. This allows the preparation of the cortex for sequential and fast release of cortical motor commands one after the other, and thence a coordinated sequence of muscle contractions for movement. Disruptions in the basal ganglia network cause several movement disorders, mainly including: 1) hypokinetic (akinesia and bradykinesia), 2) hyperkinetic (ballism, chorea, and athetosis), and 3) dystonia (characterized by prolonged muscle spasms and abnormal postures) [
27,
28].
The cortex delivers the information to the striatum (caudate nucleus and putamen which have the same cytoarchitecture) via the corticostriatal pathway. This mainly ends in one kind of neuron, the medium-sized spiny neurons (MSN) which are GABAergic projection neurons, and in other interneurons that in turn modulate MSN [
26]. In all striatal subdivisions, the inhibitory projection of MSN is the origin of the two main circuits to the thalamus [
29]: a) the direct pathway formed by the inhibitory axons of MSN to the GPi and SNr (two output nuclei), which projections to the thalamus are inhibitory, resulting in a disinhibition of the motor thalamus and facilitation of the thalamocortical action for movement, and b) the indirect pathway formed by the inhibitory axons of MSN to the GPe (an intrinsic nucleus), which in turn sends inhibitory axons to another intrinsic nucleus, the STN, resulting in disinhibition of the STN. The STN in turn projects an excitatory connection back to the GPi and SNr which in turn activate inhibitory projections to the motor thalamus, thus resulting in a deactivation of the thalamocortical pathway and a dis-facilitation of the motor command. MSN that originate one or the other pathway are distinguished by their expression of dopamine receptors. MSN that express dopamine receptor type 1 (DRD1) are called D1 receptor-expressing MSN (D1-MSN) and start the direct pathway, while MSN that express dopamine receptor type 2 (DRD2) are called D2 receptor-expressing MSN (D2-MSN) and start the indirect pathway [
26,
30]. Additionally, there is a local striatal microcircuitry formed by cholinergic and several types of GABAergic interneurons that influence MSN activity both directly and indirectly through influences on corticostriatal, thalamostriatal, and nigral dopaminergic afferents [
31]. Some groups of neostriatal GABAergic interneurons can be identified by their expression of calcium-binding proteins such as Calbindin-D-28K (CALB), Calretinin (CALR) or Parvalbumin (PARV) [
32] and of neuropeptides such as Somatostatin (SOM) [
33] and nitric oxide [
33,
34].
All the projection neurons in the intrinsic and output nuclei of the basal ganglia-thalamic circuits are GABAergic [
35,
36,
37] except those in the SNc which are dopaminergic [
37] and in the STN [
38] and the VA/VL of the thalamus which are glutamatergic [
39]. All of them can also be identified by their expression of PARV in GPe, GPi, STN, and SNr [
35,
36,
37,
38] and
core thalamocortical cells of VA/VL or CALB in
matrix thalamocortical cells of the VA/VL [
40,
41], or dopamine in SNc [
37].
Syndromes caused by deficiency of the TH transporters MCT8 and OATP1C1 [
11,
12,
24], evolve with clinical disorders that point to lesions in the basal ganglia and disruptions in both direct and indirect circuitries. It is then reasonable to hypothesize that the neurons involved in basal ganglia circuits might express these transporters and that their absence causes alterations in the balance of excitation/inhibition in the striatum microcircuitry and in the activity balance between the neurons of the direct and indirect circuits, contributing to the clinical picture described. However, there is no previous information regarding the precise cellular location of MCT8 and OATP1C1 in these circuits. Previous
in situ hybridization studies have demonstrated mRNA expression of
Mct8 in the adult mouse basal ganglia [
42,
43], but there is no reference to specific cell types. Human multiple tissues northern blots [
7] have shown high expression of OATP1C1 mRNA in the striatum, and moderate expression in other nuclei of the basal ganglia and thalamus. The large-scale human study Genotype-Tissue Expression (GTEx) project [
44] shows that the median value of the MCT8 gene
SLC16A2 transcripts per million (TPM) is moderately high in basal ganglia among other brain structures while the TPM values of the OATP1C1 gene
SLCO1C1 are extremely high in comparison with other tissues. Analyses of human brain transcriptome data sets show high MCT8 expression in the caudate nucleus and nucleus basalis of Meynert [
45]. To date, the study of the protein distribution of these TH transporters in adult human basal ganglia and thalamus has been very unspecific, with a lack of information defining the precise filiation of the transporter-expressing neurons.
Even though further procedures for TH transporters deficiency syndromes are being developed [
46], effective treatments for the neurological alterations remain to be investigated. It has become critical to determine the anatomical distribution of MCT8 and OATP1C1, as well as the exact nature of the cells and elements that express them, to model the etiopathogenesis of transporter-deficient syndromes and to advance in the development of therapeutic strategies.
In the present study, we analyzed the distribution of MCT8 and OATP1C1 in the projection neurons and interneurons in input, intrinsic and output nuclei of the basal ganglia of adult humans and monkeys, and in their blood-brain barriers, aiming at further exploring the role of MCT8- and OATP1C1- expressing cells in this important system of motor modulation and control.
We found that TH transporters MCT8 and OATP1C1 are widely expressed in all projection neurons of basal ganglia and related nuclei that participate in direct and indirect motor pathways, suggesting that a lack of function of these transporters in the basal ganglia circuits would have a significant impact on the motor system modulation, leading to clinically severe movement impairment.
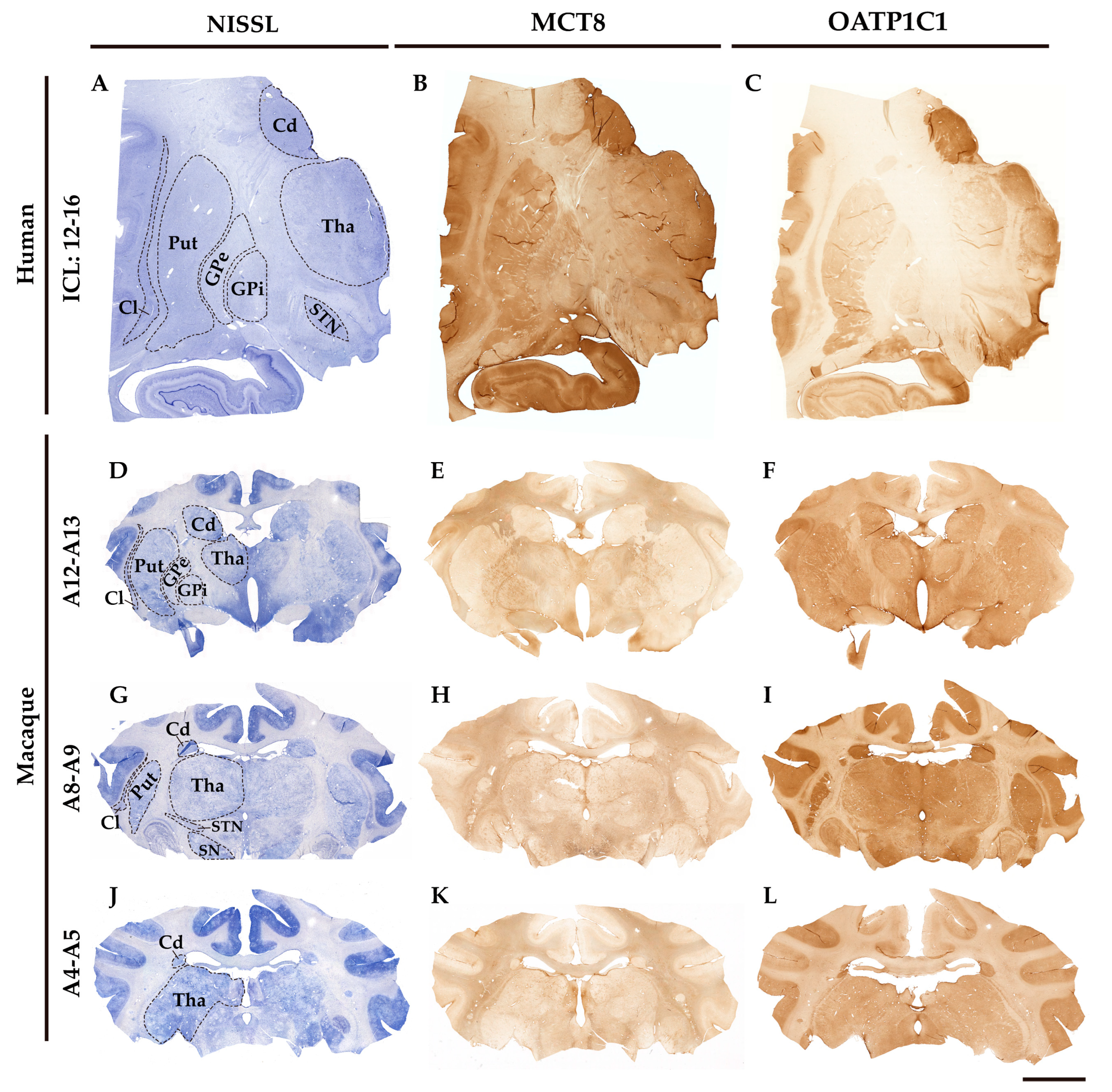
Figure 1.
MCT8 and OATP1C1 expression profiles in human and macaque coronal sections of the thalamus and basal ganglia. Compositions show representative brightfield photomicrographs taken from Nissl-stained adjacent sections (left) to MCT8 (middle) and OATP1C1 (right) immunostaining in human (
A-
C) and macaque (
D-
L), showing that both markers identify the architecture and outlines of thalamic and basal ganglia nuclei. The section level is indicated at the left side according to the atlases Mai, J. K., et al., 2015 (human) [
47] and Winters, W. D., et al., 1969 (macaque) [
48]. ICL, Inter-Commissural Line, (
A-
C) refers to the distance from the center of the anterior commissure. Cd: caudate nucleus, Cl: claustrum, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, Put: putamen, SN: substantia nigra, STN: subthalamic nucleus, Tha: thalamus. Scale bar = 8000 μm (A-C) and 9000 μm (D-L).
Figure 1.
MCT8 and OATP1C1 expression profiles in human and macaque coronal sections of the thalamus and basal ganglia. Compositions show representative brightfield photomicrographs taken from Nissl-stained adjacent sections (left) to MCT8 (middle) and OATP1C1 (right) immunostaining in human (
A-
C) and macaque (
D-
L), showing that both markers identify the architecture and outlines of thalamic and basal ganglia nuclei. The section level is indicated at the left side according to the atlases Mai, J. K., et al., 2015 (human) [
47] and Winters, W. D., et al., 1969 (macaque) [
48]. ICL, Inter-Commissural Line, (
A-
C) refers to the distance from the center of the anterior commissure. Cd: caudate nucleus, Cl: claustrum, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, Put: putamen, SN: substantia nigra, STN: subthalamic nucleus, Tha: thalamus. Scale bar = 8000 μm (A-C) and 9000 μm (D-L).
Figure 2.
Expression of MCT8 and OATP1C1 in blood vessels and brain barriers in the human and macaque basal ganglia and adjacent choroid plexus. (A) Representative brightfield photomicrograph shows immunostaining for MCT8 in the human putamen. Note that MCT8 immunopositive signal is observed along the capillary wall (red arrowhead), fibers (green arrowhead), and “bump-on-a-log” morphology pericyte-like cell (white arrowhead). (B-H) Representative confocal microscope compositions from multiple-stained sections for MCT8 (green), the endothelial marker UEA-I (red), and the vascular and pericyte biomarker PDGFR-β (purple) in human and macaque caudate nucleus. Merged image (E) shows the colocalization of all signals. (B-D) Coexpression of MCT8, UEA-I, and PDGFR-β is observed in a vessel while coexpression of MCT8 and PDGFR-β but not UEA-I is observed in a capillary-associated pericyte (white arrowheads) in humans. (F-H) Coexpression of MCT8 and PDGFR-β in a vessel and pericyte-like cell (white arrowheads) in macaques. Counterstaining with DAPI (blue) shows nuclei of all cells. (I-J) Representative brightfield photomicrographs show immunostaining for MCT8 (I) and OATP1C1 (J) in the macaque choroid plexus at the lateral ventricle. Black arrowheads point to ependymocytes. Cd: caudate nucleus, Put: putamen, PDGFR-β: platelet-derived growth factor receptor-β, UEA-I: Ulex europaeus agglutinin-I. Scale bar = 10 μm (A-H) and 50 μm (I-J).
Figure 2.
Expression of MCT8 and OATP1C1 in blood vessels and brain barriers in the human and macaque basal ganglia and adjacent choroid plexus. (A) Representative brightfield photomicrograph shows immunostaining for MCT8 in the human putamen. Note that MCT8 immunopositive signal is observed along the capillary wall (red arrowhead), fibers (green arrowhead), and “bump-on-a-log” morphology pericyte-like cell (white arrowhead). (B-H) Representative confocal microscope compositions from multiple-stained sections for MCT8 (green), the endothelial marker UEA-I (red), and the vascular and pericyte biomarker PDGFR-β (purple) in human and macaque caudate nucleus. Merged image (E) shows the colocalization of all signals. (B-D) Coexpression of MCT8, UEA-I, and PDGFR-β is observed in a vessel while coexpression of MCT8 and PDGFR-β but not UEA-I is observed in a capillary-associated pericyte (white arrowheads) in humans. (F-H) Coexpression of MCT8 and PDGFR-β in a vessel and pericyte-like cell (white arrowheads) in macaques. Counterstaining with DAPI (blue) shows nuclei of all cells. (I-J) Representative brightfield photomicrographs show immunostaining for MCT8 (I) and OATP1C1 (J) in the macaque choroid plexus at the lateral ventricle. Black arrowheads point to ependymocytes. Cd: caudate nucleus, Put: putamen, PDGFR-β: platelet-derived growth factor receptor-β, UEA-I: Ulex europaeus agglutinin-I. Scale bar = 10 μm (A-H) and 50 μm (I-J).
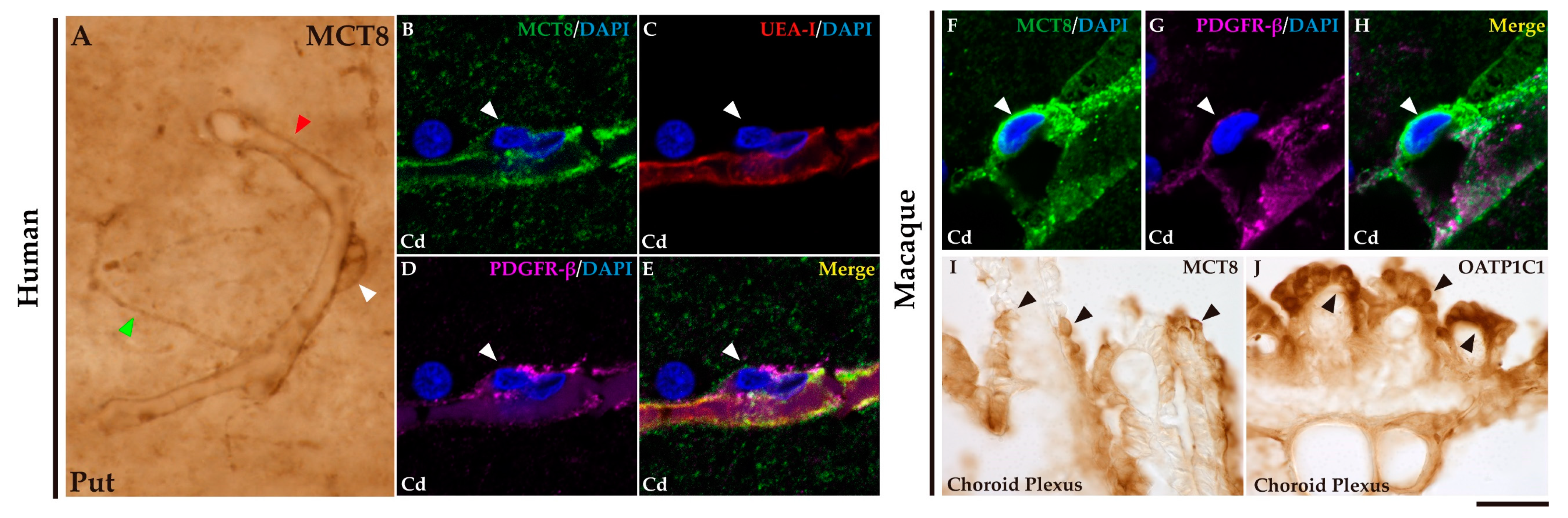
Figure 3.
Expression of MCT8 and OATP1C1 in neurons of the human and macaque striatum. (A) The diagram summarizes the basal ganglia motor circuits. The striatum (caudate nucleus and putamen) serves as the input nuclei of the basal ganglia, receiving the information primarily from the motor cortex. (B-I) Representative brightfield photomicrographs show immunostaining for MCT8 in the human (B) and macaque (C) caudate nucleus and in the human (F) and macaque (G) putamen. OATP1C1 immunostaining is also shown in human (D) and macaque (E) caudate nucleus and the human (H) and macaque (I) putamen. Note that MCT8 and OATP1C1 are observed in neurons of different sizes; and that the MCT8 signal, but not the OATP1C1 signal, is observed in capillaries (red arrowheads). Black arrows point to large-sized neurons with immunopositive signal in the soma and dendrites. Black arrowheads point to medium/small-sized immunopositive neural cells. Cd/Put: caudate nucleus and putamen, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 25 μm (B), 33 μm (C-E), 29 μm (F) and 38 μm (G-I).
Figure 3.
Expression of MCT8 and OATP1C1 in neurons of the human and macaque striatum. (A) The diagram summarizes the basal ganglia motor circuits. The striatum (caudate nucleus and putamen) serves as the input nuclei of the basal ganglia, receiving the information primarily from the motor cortex. (B-I) Representative brightfield photomicrographs show immunostaining for MCT8 in the human (B) and macaque (C) caudate nucleus and in the human (F) and macaque (G) putamen. OATP1C1 immunostaining is also shown in human (D) and macaque (E) caudate nucleus and the human (H) and macaque (I) putamen. Note that MCT8 and OATP1C1 are observed in neurons of different sizes; and that the MCT8 signal, but not the OATP1C1 signal, is observed in capillaries (red arrowheads). Black arrows point to large-sized neurons with immunopositive signal in the soma and dendrites. Black arrowheads point to medium/small-sized immunopositive neural cells. Cd/Put: caudate nucleus and putamen, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 25 μm (B), 33 μm (C-E), 29 μm (F) and 38 μm (G-I).
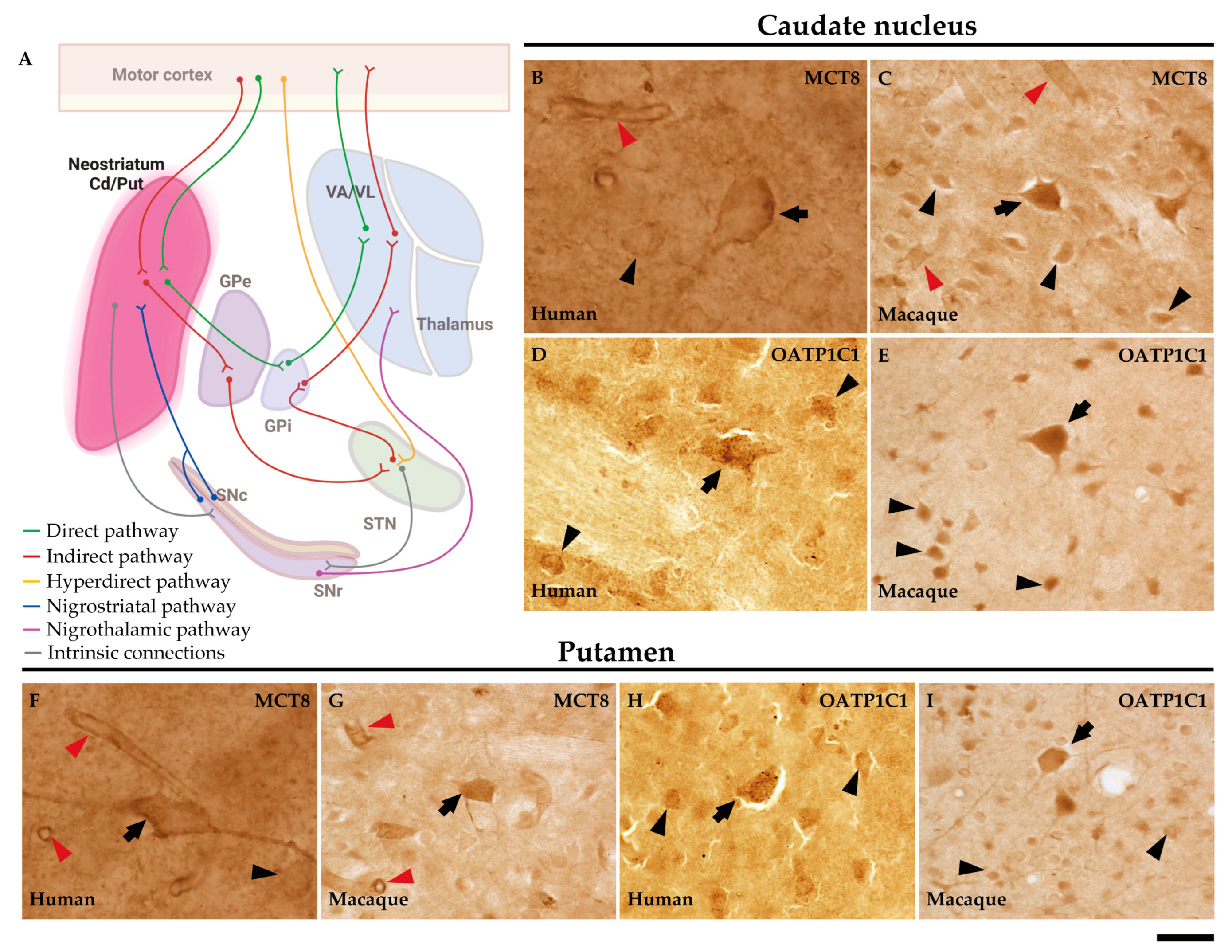
Figure 4.
Expression of MCT8 and OATP1C1 in D1-MSN (direct pathway medium-sized spiny neurons) in human and macaque striatum. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and for the D1-MSN marker DRD1 (red) in caudate nucleus or putamen. Merged images (right side) show the colocalization of both signals. Coexpression of MCT8 and DRD1 is observed in human (A-C) and macaque (D-F) striatum. Coexpression of OATP1C1 and DRD1 is observed in human (G-I) and macaque (J-L) striatum. Counter-staining with DAPI (blue) shows nuclei of all cells. Note that in humans the MCT8 signal is located mainly at the cell membrane, while in macaques it is located at the membrane and in the cytoplasm. Cd: caudate nucleus; DRD1: Dopamine receptor type 1; D1-MSN: D1 receptor-expressing medium-sized spiny neurons; Put: putamen. Scale bar = 50 μm.
Figure 4.
Expression of MCT8 and OATP1C1 in D1-MSN (direct pathway medium-sized spiny neurons) in human and macaque striatum. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and for the D1-MSN marker DRD1 (red) in caudate nucleus or putamen. Merged images (right side) show the colocalization of both signals. Coexpression of MCT8 and DRD1 is observed in human (A-C) and macaque (D-F) striatum. Coexpression of OATP1C1 and DRD1 is observed in human (G-I) and macaque (J-L) striatum. Counter-staining with DAPI (blue) shows nuclei of all cells. Note that in humans the MCT8 signal is located mainly at the cell membrane, while in macaques it is located at the membrane and in the cytoplasm. Cd: caudate nucleus; DRD1: Dopamine receptor type 1; D1-MSN: D1 receptor-expressing medium-sized spiny neurons; Put: putamen. Scale bar = 50 μm.
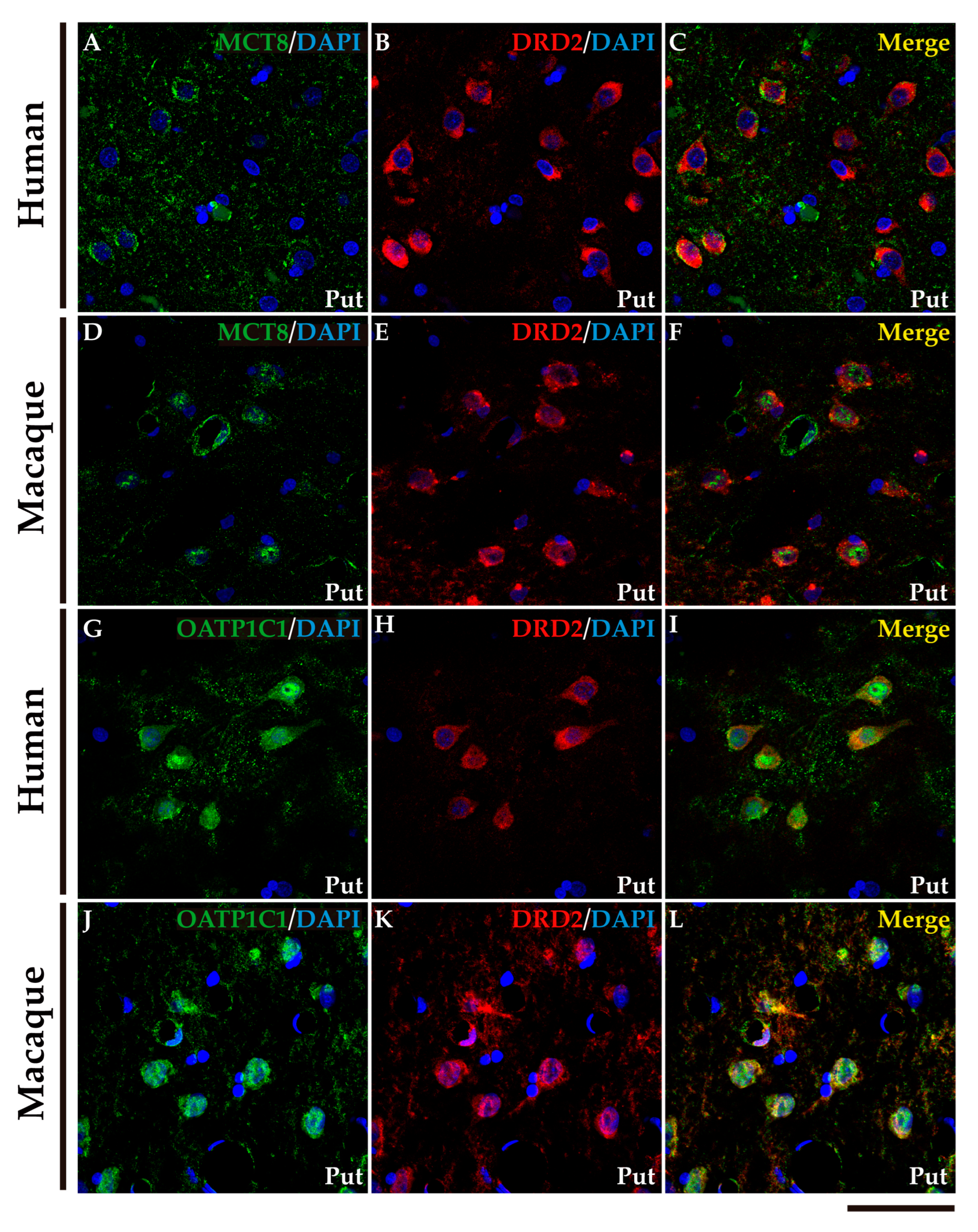
Figure 5.
Expression of MCT8 and OATP1C1 in D2-MSN (indirect pathway medium-sized spiny neurons) in human and macaque striatum. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and for the D2-MSN marker DRD2 (red) in the putamen. Merged images (right side) show the colocalization of the two signals. Coexpression of MCT8 and DRD2 is observed in human (A-C) and macaque (D-F) striatum. Coexpression of OATP1C1 and DRD2 is observed in human (G-I) and macaque (J-L) striatum. Counter-staining with DAPI (blue) shows nuclei of all cells. Note that in humans the MCT8 signals are located mainly at the cell membrane, while in macaques it is also located in the cytoplasm. D2-MSN: D2 receptor-expressing medium-sized spiny neurons; DRD2: Dopamine receptor type 2; Put: putamen. Scale bar = 50 μm.
Figure 5.
Expression of MCT8 and OATP1C1 in D2-MSN (indirect pathway medium-sized spiny neurons) in human and macaque striatum. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and for the D2-MSN marker DRD2 (red) in the putamen. Merged images (right side) show the colocalization of the two signals. Coexpression of MCT8 and DRD2 is observed in human (A-C) and macaque (D-F) striatum. Coexpression of OATP1C1 and DRD2 is observed in human (G-I) and macaque (J-L) striatum. Counter-staining with DAPI (blue) shows nuclei of all cells. Note that in humans the MCT8 signals are located mainly at the cell membrane, while in macaques it is also located in the cytoplasm. D2-MSN: D2 receptor-expressing medium-sized spiny neurons; DRD2: Dopamine receptor type 2; Put: putamen. Scale bar = 50 μm.
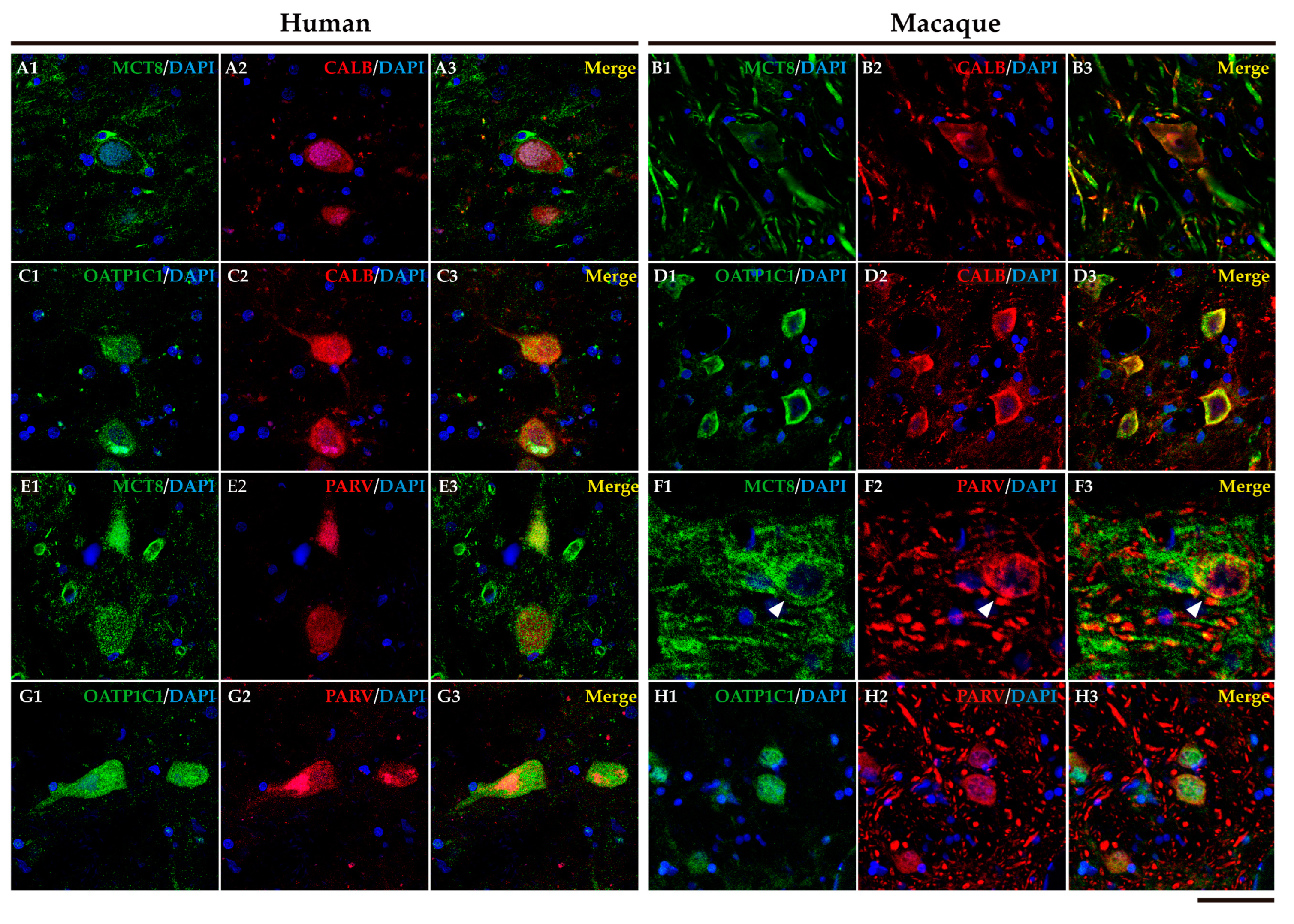
Figure 6.
Expression of MCT8 in different subpopulations of neostriatal interneurons. Representative confocal microscope compositions of human (left) and macaque (right) double-stained sections for MCT8 and several markers to visualize different subtypes of interneurons. Merged images show the colocalization of both signals. (A1-B3) Coexpression of MCT8 (green) with the interneuron marker PARV (red) in fast-spiking GABAergic interneurons in the human and macaque putamen. (C1-D3) Coexpression of MCT8 (green) with the interneuron marker CALB (red) in human putamen and macaque caudate nucleus. Note that MCT8 expression was mainly seen at the cell membrane as well as in the neuropil. (E1-F3) Coexpression of MCT8 (green) with the interneuron marker CALR (red) in the human caudate nucleus and macaque putamen. (G1-H3) Coexpression of MCT8 (green) with nNOS (red) in nitrergic interneurons and persistent low-threshold spiking interneurons in the human and macaque putamen. (I1-J3) Coexpression of MCT8 (green) with SOM (red) in persistent low-threshold spiking interneurons in the human caudate nucleus and macaque putamen. (K1-L3) Coexpression of MCT8 (green) with ChAT (red) at the soma and processes of cholinergic interneurons (tonically active interneurons) in the human and macaque caudate nucleus. Note that the coexpression of all these markers with MCT8 is only partial. Counter-staining with DAPI (blue) shows nuclei of all cells. White arrowheads point to double-labeled interneurons. CALB: Calbindin-D-28K, CALR: Calretinin, Cd: caudate nucleus, ChAT: Choline Acetyltransferase, nNOS: Neuronal nitric oxide synthase, PARV: Parvalbumin, Put: putamen, SOM: Somatostatin. Scale bar = 25 μm.
Figure 6.
Expression of MCT8 in different subpopulations of neostriatal interneurons. Representative confocal microscope compositions of human (left) and macaque (right) double-stained sections for MCT8 and several markers to visualize different subtypes of interneurons. Merged images show the colocalization of both signals. (A1-B3) Coexpression of MCT8 (green) with the interneuron marker PARV (red) in fast-spiking GABAergic interneurons in the human and macaque putamen. (C1-D3) Coexpression of MCT8 (green) with the interneuron marker CALB (red) in human putamen and macaque caudate nucleus. Note that MCT8 expression was mainly seen at the cell membrane as well as in the neuropil. (E1-F3) Coexpression of MCT8 (green) with the interneuron marker CALR (red) in the human caudate nucleus and macaque putamen. (G1-H3) Coexpression of MCT8 (green) with nNOS (red) in nitrergic interneurons and persistent low-threshold spiking interneurons in the human and macaque putamen. (I1-J3) Coexpression of MCT8 (green) with SOM (red) in persistent low-threshold spiking interneurons in the human caudate nucleus and macaque putamen. (K1-L3) Coexpression of MCT8 (green) with ChAT (red) at the soma and processes of cholinergic interneurons (tonically active interneurons) in the human and macaque caudate nucleus. Note that the coexpression of all these markers with MCT8 is only partial. Counter-staining with DAPI (blue) shows nuclei of all cells. White arrowheads point to double-labeled interneurons. CALB: Calbindin-D-28K, CALR: Calretinin, Cd: caudate nucleus, ChAT: Choline Acetyltransferase, nNOS: Neuronal nitric oxide synthase, PARV: Parvalbumin, Put: putamen, SOM: Somatostatin. Scale bar = 25 μm.
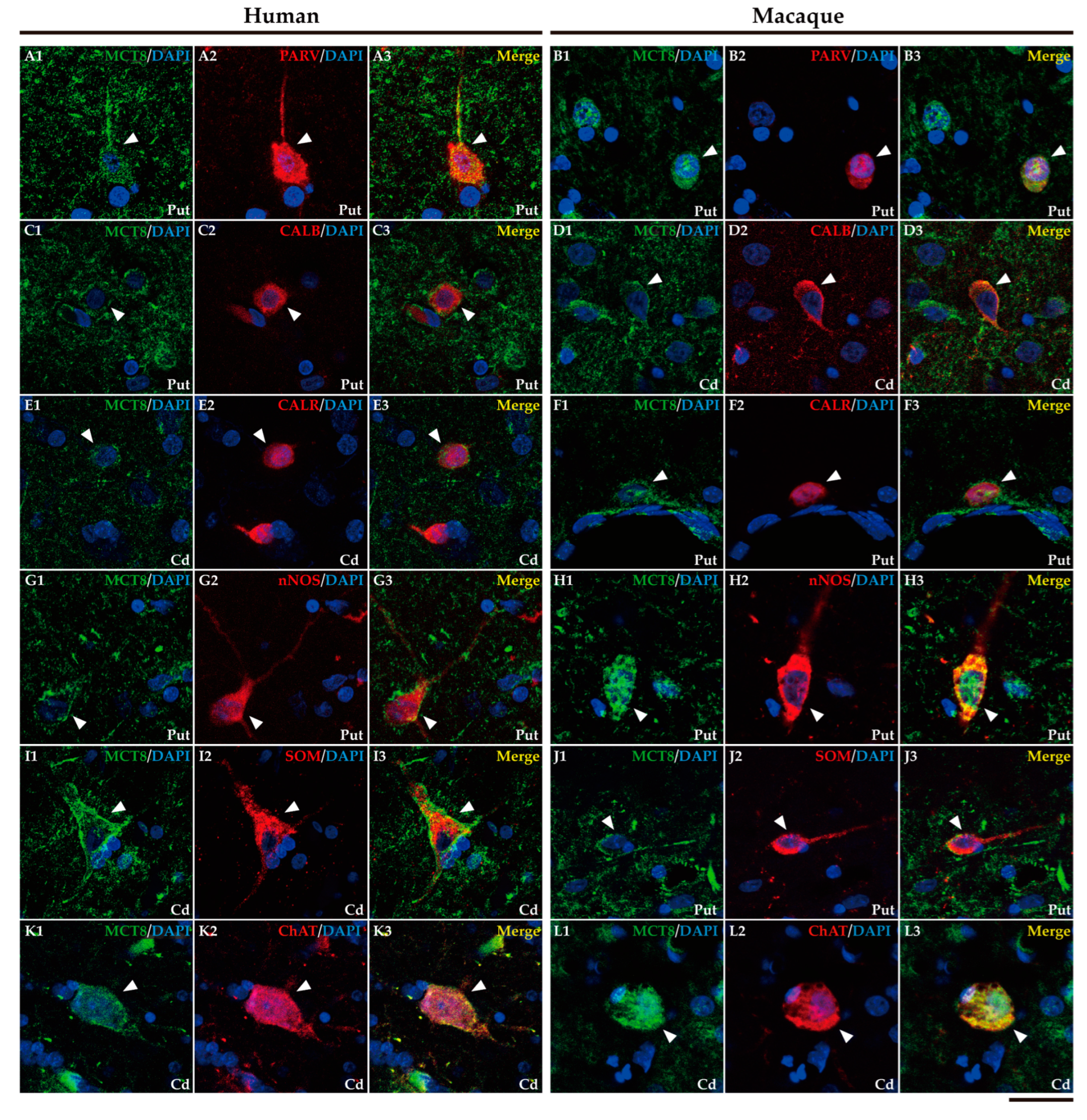
Figure 7.
Expression of OATP1C1 in different subpopulations of interneurons in the striatum. Representative confocal microscope compositions of human (left) and macaque (right) double-stained sections for OATP1C1 and several markers to visualize different subsets of interneurons. Merged images show the colocalization of the two signals. (A1-B3) Coexpression of OATP1C1 (green) with the marker PARV (red) in fast-spiking GABAergic interneurons in the human and macaque caudate nucleus. (C1-D3) Coexpression of OATP1C1 (green) with the interneuron marker CALB (red) in the human and macaque caudate nucleus. (E1-F3) Coexpression of OATP1C1 (green) with the interneuron marker CALR (red) in human and macaque putamen. (G1-H3) Coexpression of OATP1C1 (green) with nNOS (red) in nitrergic interneurons and persistent low-threshold spiking interneurons in human putamen and macaque caudate nucleus. (I1-J3) Coexpression of OATP1C1 (green) with SOM (red) in persistent low-threshold spiking interneurons in the human and macaque caudate nucleus. (K1-L3) Coexpression of OATP1C1 (green) with ChAT (red) at the soma and processes of cholinergic interneurons (tonically active interneurons) in the human putamen and macaque caudate nucleus. Counter-staining with DAPI (blue) shows nuclei of all cells. White arrowheads point to double-labeled interneurons. CALB: Calbindin-D-28K, CALR: Calretinin, Cd: caudate nucleus, ChAT: Choline Acetyltransferase, nNOS: Neuronal nitric oxide synthase, PARV: Parvalbumin, Put: putamen, SOM: Somatostatin. Scale bar = 25 μm.
Figure 7.
Expression of OATP1C1 in different subpopulations of interneurons in the striatum. Representative confocal microscope compositions of human (left) and macaque (right) double-stained sections for OATP1C1 and several markers to visualize different subsets of interneurons. Merged images show the colocalization of the two signals. (A1-B3) Coexpression of OATP1C1 (green) with the marker PARV (red) in fast-spiking GABAergic interneurons in the human and macaque caudate nucleus. (C1-D3) Coexpression of OATP1C1 (green) with the interneuron marker CALB (red) in the human and macaque caudate nucleus. (E1-F3) Coexpression of OATP1C1 (green) with the interneuron marker CALR (red) in human and macaque putamen. (G1-H3) Coexpression of OATP1C1 (green) with nNOS (red) in nitrergic interneurons and persistent low-threshold spiking interneurons in human putamen and macaque caudate nucleus. (I1-J3) Coexpression of OATP1C1 (green) with SOM (red) in persistent low-threshold spiking interneurons in the human and macaque caudate nucleus. (K1-L3) Coexpression of OATP1C1 (green) with ChAT (red) at the soma and processes of cholinergic interneurons (tonically active interneurons) in the human putamen and macaque caudate nucleus. Counter-staining with DAPI (blue) shows nuclei of all cells. White arrowheads point to double-labeled interneurons. CALB: Calbindin-D-28K, CALR: Calretinin, Cd: caudate nucleus, ChAT: Choline Acetyltransferase, nNOS: Neuronal nitric oxide synthase, PARV: Parvalbumin, Put: putamen, SOM: Somatostatin. Scale bar = 25 μm.
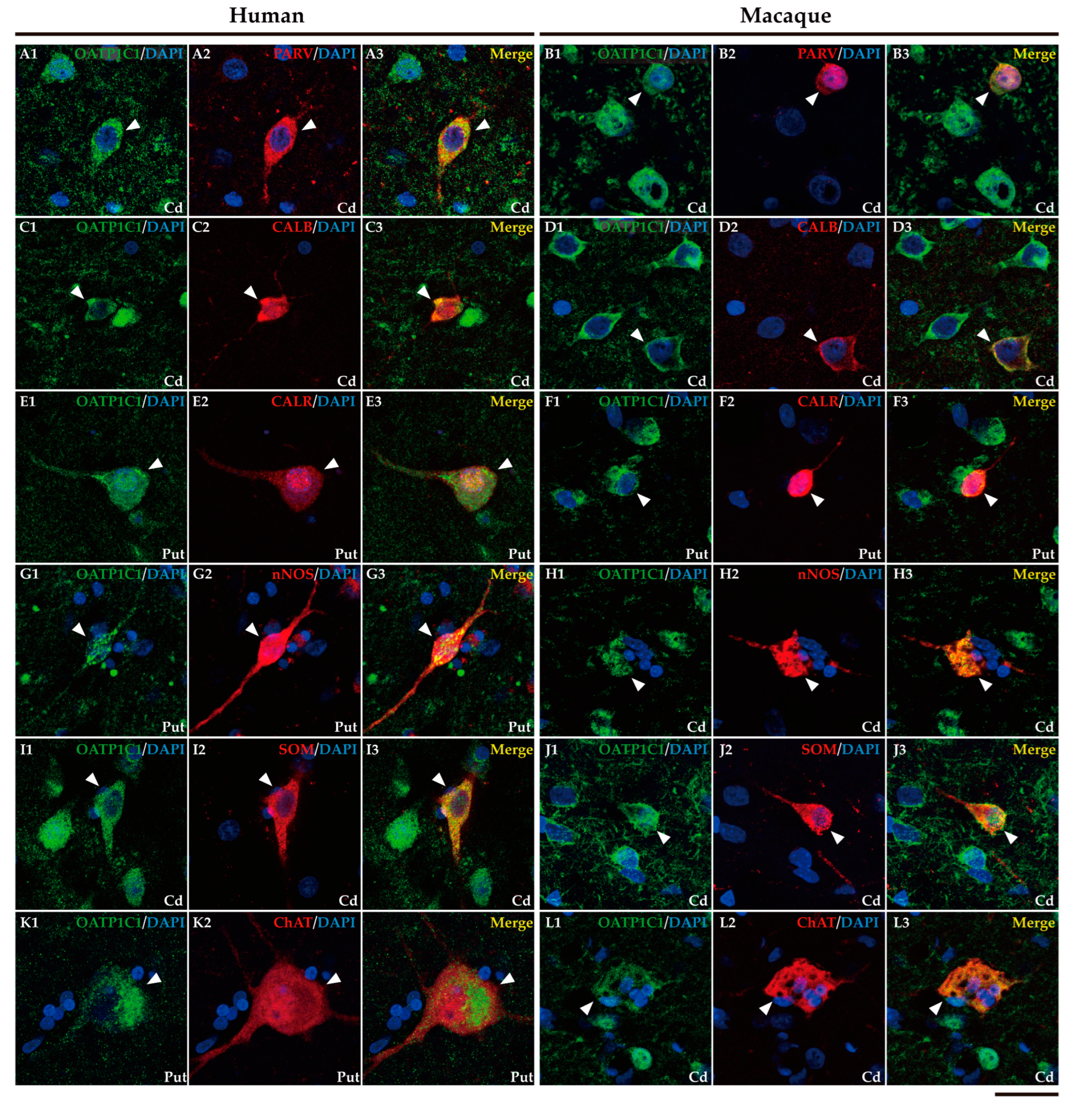
Figure 8.
Expression of MCT8 and OATP1C1 in neurons of the output nuclei of the basal ganglia in humans and macaques. (A) The diagram summarizes the basal ganglia motor circuits. GPi and SNr serve as output nuclei of the basal ganglia. The axons from GPi innervate the VA/VL of the thalamus. Representative brightfield photomicrographs show immunostaining for MCT8 at the human (B) and macaque (C) GPi, and at the human (F) and macaque (G) SNr. OATP1C1 immunostaining is also shown at the human (D) and macaque (E) GPi and at the human (H) and macaque (I) SNr. Black arrows point to neurons with immunopositive signal. Note that MCT8, but not OATP1C1, is highly expressed in capillaries (red arrowheads). Both MCT8 and OATP1C1 staining can be observed in fibers (green arrowheads). GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 33 μm (B-E) and 38 μm (F-I).
Figure 8.
Expression of MCT8 and OATP1C1 in neurons of the output nuclei of the basal ganglia in humans and macaques. (A) The diagram summarizes the basal ganglia motor circuits. GPi and SNr serve as output nuclei of the basal ganglia. The axons from GPi innervate the VA/VL of the thalamus. Representative brightfield photomicrographs show immunostaining for MCT8 at the human (B) and macaque (C) GPi, and at the human (F) and macaque (G) SNr. OATP1C1 immunostaining is also shown at the human (D) and macaque (E) GPi and at the human (H) and macaque (I) SNr. Black arrows point to neurons with immunopositive signal. Note that MCT8, but not OATP1C1, is highly expressed in capillaries (red arrowheads). Both MCT8 and OATP1C1 staining can be observed in fibers (green arrowheads). GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 33 μm (B-E) and 38 μm (F-I).
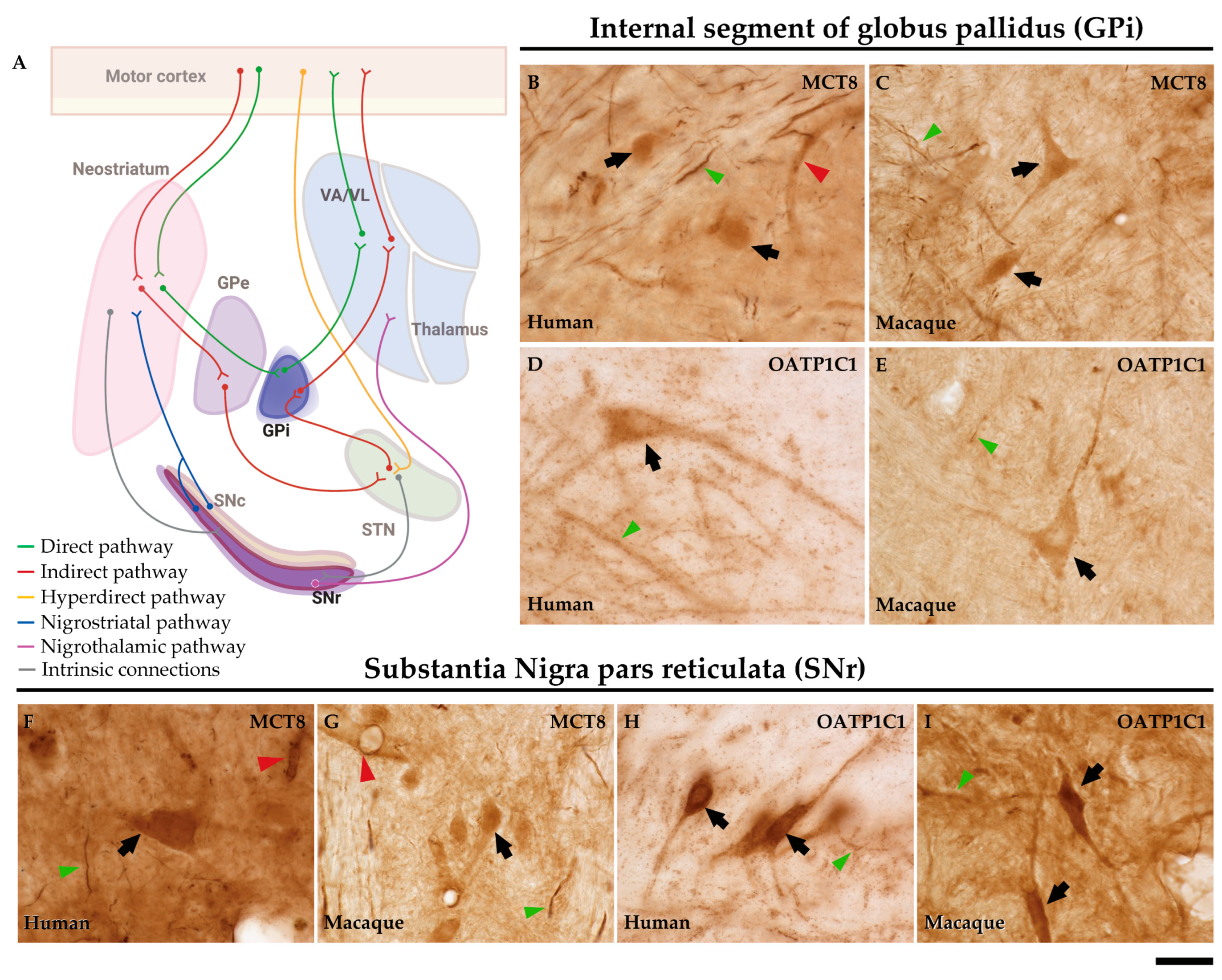
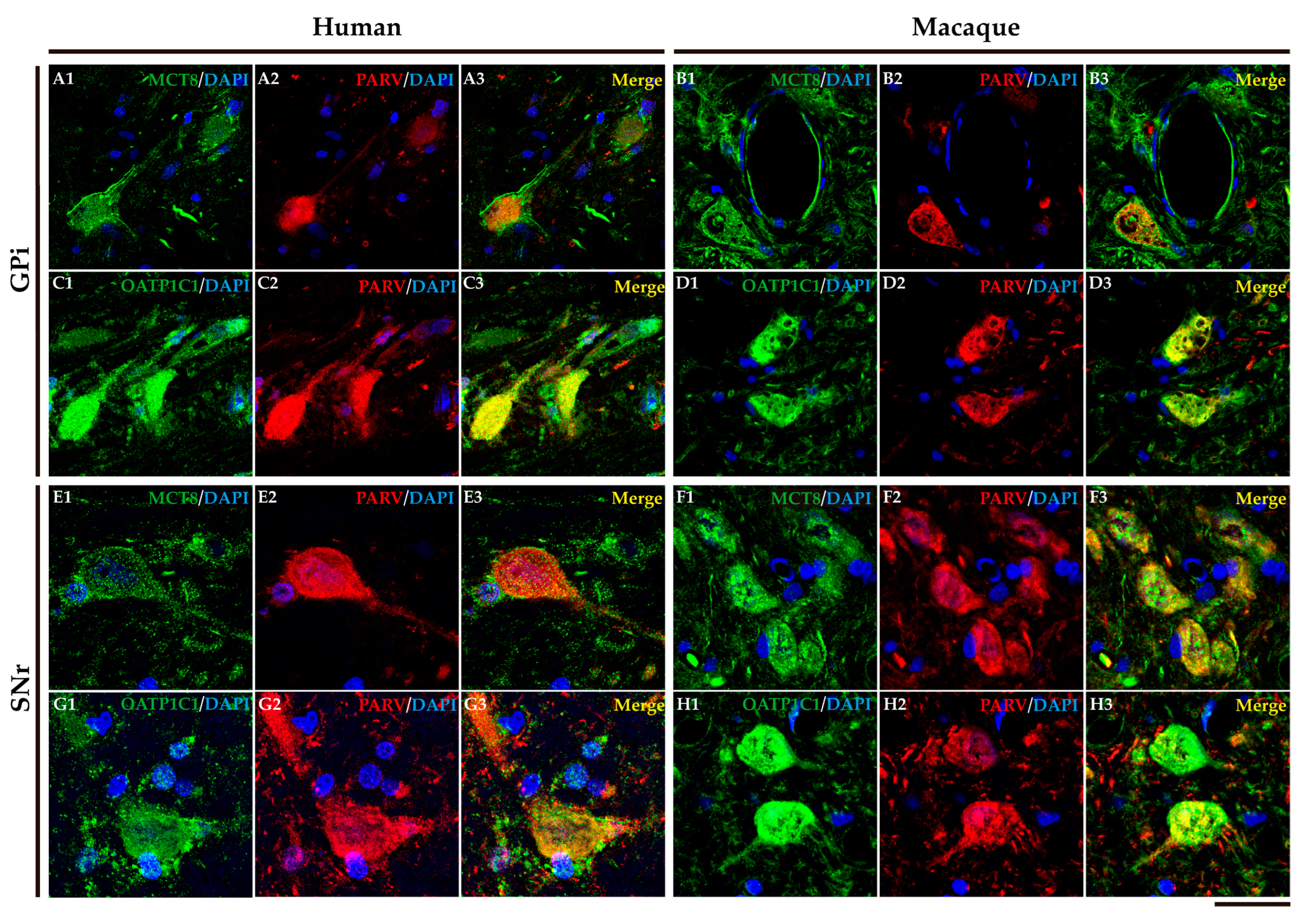
Figure 9.
Expression of MCT8 and OATP1C1 in GPi and SNr neurons. Representative confocal microscope compositions of human and macaque sections double-stained for MCT8 (green) or OATP1C1 (green) and for the neuron marker PARV, which is specific for GABAergic neurons in the GPi and SNr. Merged images show the colocalization of both markers. MCT8 and PARV are coexpressed in the human (A1-A3) and macaque (B1-B3) GPi and in the human (E1-E3) and macaque (F1-F3) SNr. OATP1C1 and PARV are coexpressed in the human (C1-C3) and macaque (D1-D3) GPi and in the human (G1-G3) and macaque (H1-H3) SNr. Counter-staining with DAPI (blue) shows nuclei of all cells. GPi: internal segment of globus pallidus, PARV: Parvalbumin, SNr: substantia nigra pars reticulata. Scale bar = 50 μm (A1-D3), 25 μm (E1-H3).
Figure 9.
Expression of MCT8 and OATP1C1 in GPi and SNr neurons. Representative confocal microscope compositions of human and macaque sections double-stained for MCT8 (green) or OATP1C1 (green) and for the neuron marker PARV, which is specific for GABAergic neurons in the GPi and SNr. Merged images show the colocalization of both markers. MCT8 and PARV are coexpressed in the human (A1-A3) and macaque (B1-B3) GPi and in the human (E1-E3) and macaque (F1-F3) SNr. OATP1C1 and PARV are coexpressed in the human (C1-C3) and macaque (D1-D3) GPi and in the human (G1-G3) and macaque (H1-H3) SNr. Counter-staining with DAPI (blue) shows nuclei of all cells. GPi: internal segment of globus pallidus, PARV: Parvalbumin, SNr: substantia nigra pars reticulata. Scale bar = 50 μm (A1-D3), 25 μm (E1-H3).
Figure 10.
Expression of MCT8 and OATP1C1 in neurons of the intrinsic nuclei of the human and macaque basal ganglia. (A) In the motor pathway, GPe, SNc, and STN serve as the intrinsic nuclei of the basal ganglia. GPe is primarily, but not exclusively, used as a relay station between the striatum and the STN. Besides the classical GPe projections, STN also receives projections from the cortex (hyperdirect pathway) and other sources. The projections from SNc innervate the striatum and form the nigrostriatal pathway. Representative brightfield photomicrographs show immunostaining for MCT8 in the GPe of humans (B) and macaques (C), STN of humans (F) and macaques (G), and SNc of humans (J) and macaques (K). OATP1C1 immunostaining is also shown in the GPe of humans (D) and macaques (E), STN of humans (H) and macaques (I), and SNc of humans (L) and macaques (M). Black arrows point to neurons with immunopositive signal. Note that the MCT8 signal, but not the OATP1C1 signal, is highly expressed in capillaries (red arrowheads). Both MCT8 and OATP1C1 staining can be observed in fibers. In addition, an OATP1C1-immunopositive Corpora amylacea (yellow arrowhead) can be observed in panel D. GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 33 μm (B-E) and 38 μm (F-M).
Figure 10.
Expression of MCT8 and OATP1C1 in neurons of the intrinsic nuclei of the human and macaque basal ganglia. (A) In the motor pathway, GPe, SNc, and STN serve as the intrinsic nuclei of the basal ganglia. GPe is primarily, but not exclusively, used as a relay station between the striatum and the STN. Besides the classical GPe projections, STN also receives projections from the cortex (hyperdirect pathway) and other sources. The projections from SNc innervate the striatum and form the nigrostriatal pathway. Representative brightfield photomicrographs show immunostaining for MCT8 in the GPe of humans (B) and macaques (C), STN of humans (F) and macaques (G), and SNc of humans (J) and macaques (K). OATP1C1 immunostaining is also shown in the GPe of humans (D) and macaques (E), STN of humans (H) and macaques (I), and SNc of humans (L) and macaques (M). Black arrows point to neurons with immunopositive signal. Note that the MCT8 signal, but not the OATP1C1 signal, is highly expressed in capillaries (red arrowheads). Both MCT8 and OATP1C1 staining can be observed in fibers. In addition, an OATP1C1-immunopositive Corpora amylacea (yellow arrowhead) can be observed in panel D. GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 33 μm (B-E) and 38 μm (F-M).

Figure 11.
Expression of MCT8 and OATP1C1 in different subpopulations of neurons in intrinsic nuclei of the human and macaque basal ganglia. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and the neuron markers PARV (red), and TYH (red). Merged images show the colocalization of both signals. MCT8 and PARV are coexpressed in the GPe in humans (A1-A3) and macaques (B1-B3), and in the STN in humans (E1-E3) and macaques (F1-F3). MCT8 and TYH are coexpressed in SNc in humans (I1-I3) and macaques (J1-J3). OATP1C1 and PARV are coexpressed in the GPe in humans (C1-C3) and macaques (D1-D3) and in the STN in humans (G1-G3) and macaques (H1-H3). OATP1C1 and TYH are coexpressed in the SNc in humans (K1-K3) and macaques (L1-L3). Counter-staining with DAPI (blue) shows nuclei of all cells. GPe: external globus pallidus, PARV: Parvalbumin, SNc: substantia nigra pars compacta, STN: subthalamic nucleus, TYH: Tyrosine hydroxylase. Scale bar = 50 μm.
Figure 11.
Expression of MCT8 and OATP1C1 in different subpopulations of neurons in intrinsic nuclei of the human and macaque basal ganglia. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and the neuron markers PARV (red), and TYH (red). Merged images show the colocalization of both signals. MCT8 and PARV are coexpressed in the GPe in humans (A1-A3) and macaques (B1-B3), and in the STN in humans (E1-E3) and macaques (F1-F3). MCT8 and TYH are coexpressed in SNc in humans (I1-I3) and macaques (J1-J3). OATP1C1 and PARV are coexpressed in the GPe in humans (C1-C3) and macaques (D1-D3) and in the STN in humans (G1-G3) and macaques (H1-H3). OATP1C1 and TYH are coexpressed in the SNc in humans (K1-K3) and macaques (L1-L3). Counter-staining with DAPI (blue) shows nuclei of all cells. GPe: external globus pallidus, PARV: Parvalbumin, SNc: substantia nigra pars compacta, STN: subthalamic nucleus, TYH: Tyrosine hydroxylase. Scale bar = 50 μm.
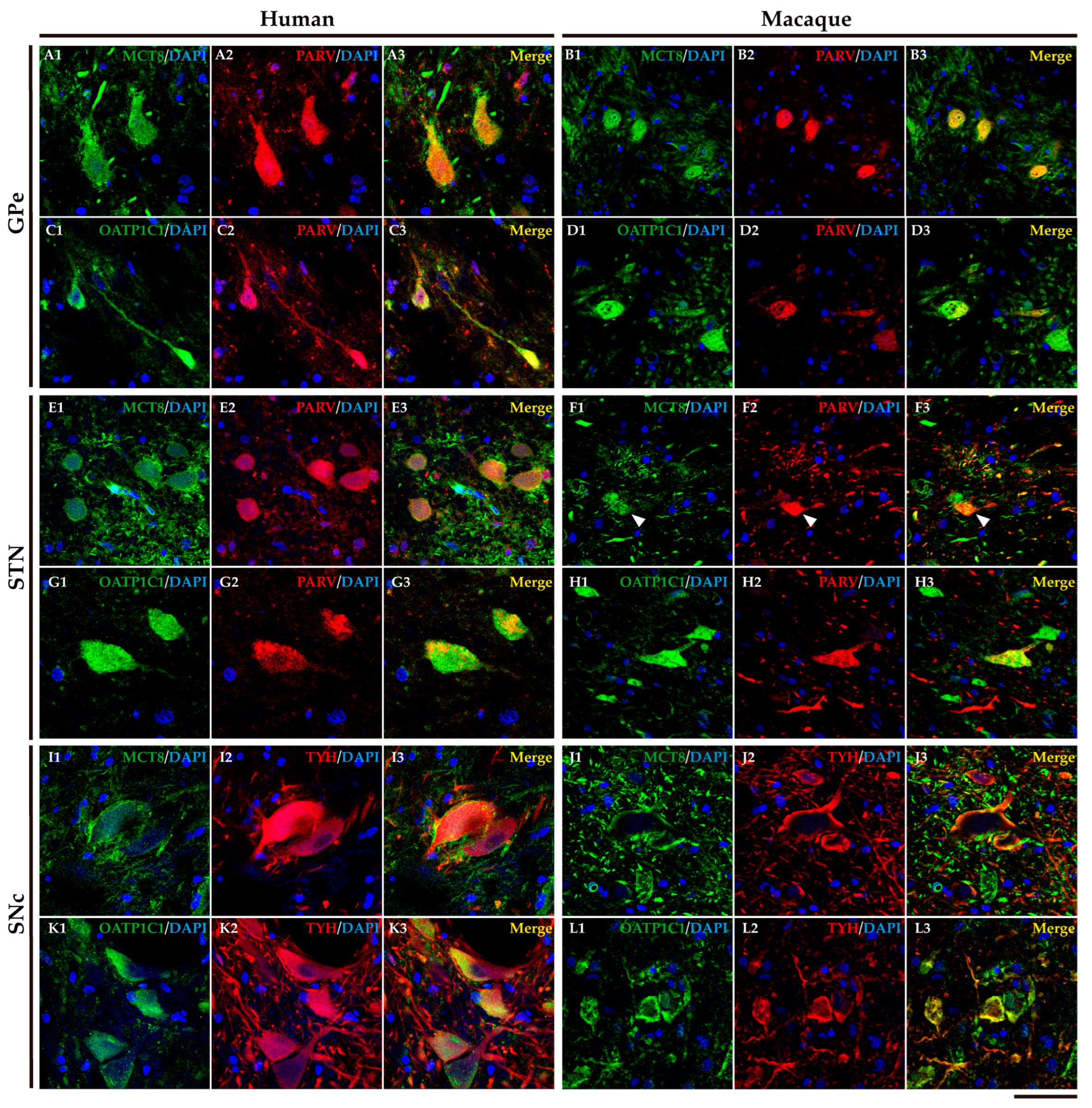
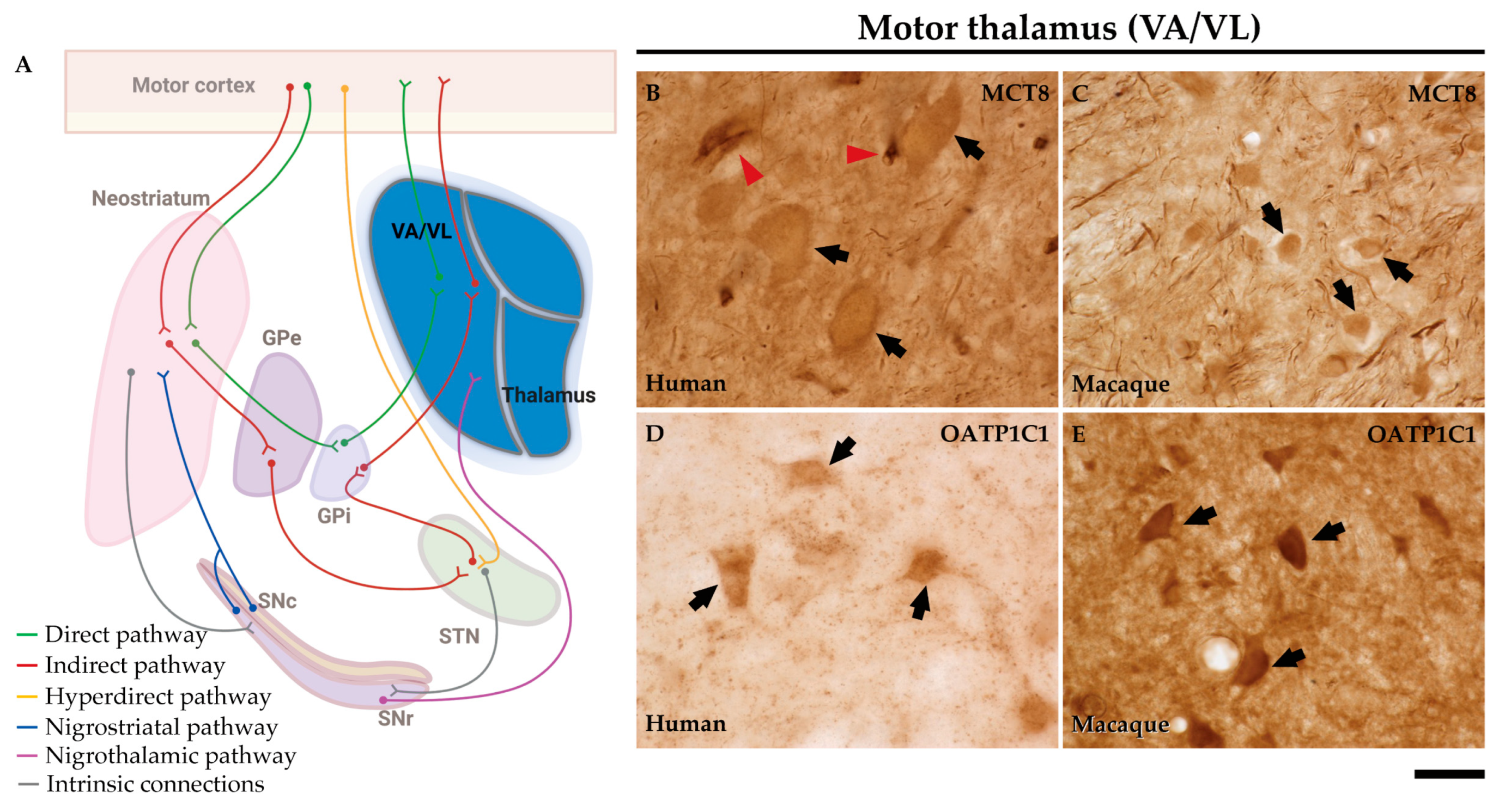
Figure 12.
Expression of MCT8 and OATP1C1 in neurons of the motor thalamus. (A) The diagram shows that the pathway to the cortex leaves from the GPi to the motor cortex with a relay in the VA/VL of the motor thalamus. Representative brightfield photomicrographs show immunostaining for MCT8 and OATP1C1 in the human (B and D) and macaque (C and E) VA/VL. Black arrows point to neurons with immunopositive signals. Note that the MCT8 signal is highly expressed in capillaries (red arrowheads) and fibers, while OATP1C1 can be observed in fibers but rarely in capillaries. GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 33 μm (B-E).
Figure 12.
Expression of MCT8 and OATP1C1 in neurons of the motor thalamus. (A) The diagram shows that the pathway to the cortex leaves from the GPi to the motor cortex with a relay in the VA/VL of the motor thalamus. Representative brightfield photomicrographs show immunostaining for MCT8 and OATP1C1 in the human (B and D) and macaque (C and E) VA/VL. Black arrows point to neurons with immunopositive signals. Note that the MCT8 signal is highly expressed in capillaries (red arrowheads) and fibers, while OATP1C1 can be observed in fibers but rarely in capillaries. GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus. Scale bar = 33 μm (B-E).
Figure 13.
Expression of MCT8 and OATP1C1 in matrix cells and core cells of the human and macaque motor thalamus. Representative confocal microscope compositions of brain sections double stained for MCT8 (green) or OATP1C1 (green) and the matrix cells marker, CALB (red), or core cells marker, PARV (red). Merged images show the colocalization of both signals. MCT8 is coexpressed with CALB in the human (A1-A3) and macaque (B1-B3) VA/VL, and with PARV in the human (E1-E3) and macaque (F1-F3) VA/VL (white arrowheads). OATP1C1 is coexpressed with CALB in the human (C1-C3) and macaque (D1-D3) VA/VL, and with PARV in the human (G1-G3) and macaque (H1-H3) VA/VL. Counterstaining with DAPI (blue) shows nuclei of all cells. CALB: Calbindin-D-28K, PARV: Parvalbumin. Scale bar = 50 μm (A1-E3 and G1-H3), 25 μm (F1-F3).
Figure 13.
Expression of MCT8 and OATP1C1 in matrix cells and core cells of the human and macaque motor thalamus. Representative confocal microscope compositions of brain sections double stained for MCT8 (green) or OATP1C1 (green) and the matrix cells marker, CALB (red), or core cells marker, PARV (red). Merged images show the colocalization of both signals. MCT8 is coexpressed with CALB in the human (A1-A3) and macaque (B1-B3) VA/VL, and with PARV in the human (E1-E3) and macaque (F1-F3) VA/VL (white arrowheads). OATP1C1 is coexpressed with CALB in the human (C1-C3) and macaque (D1-D3) VA/VL, and with PARV in the human (G1-G3) and macaque (H1-H3) VA/VL. Counterstaining with DAPI (blue) shows nuclei of all cells. CALB: Calbindin-D-28K, PARV: Parvalbumin. Scale bar = 50 μm (A1-E3 and G1-H3), 25 μm (F1-F3).
Figure 14.
Expression of MCT8 and OATP1C1 in the nucleus basalis of Meynert. Representative brightfield photomicrographs show immunostaining for MCT8 (A) and OATP1C1 (E) in the human nucleus basalis of Meynert with insets that show the exact location (red points) of the images shown. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and the cholinergic neuron marker ChAT (red) in the macaque nucleus basalis of Meynert. Merged images show the colocalization of both signals. (B-D) Coexpression of MCT8 and ChAT in the macaque nucleus basalis of Meynert. (F-H) Coexpression of OATP1C1 and ChAT in the macaque nucleus basalis of Meynert. Note that most neurons coexpress both signals. Counterstaining with DAPI (blue) shows nuclei of all cells. ac: anterior commissure, Cd: caudate nucleus, ChAT: Choline acetyltransferase, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, LV: lateral ventricle, Put: putamen. Scale bar = 50 μm (A, E) and 110 μm (B-D and F-H).
Figure 14.
Expression of MCT8 and OATP1C1 in the nucleus basalis of Meynert. Representative brightfield photomicrographs show immunostaining for MCT8 (A) and OATP1C1 (E) in the human nucleus basalis of Meynert with insets that show the exact location (red points) of the images shown. Representative confocal microscope compositions from double-stained sections for MCT8 (green) or OATP1C1 (green) and the cholinergic neuron marker ChAT (red) in the macaque nucleus basalis of Meynert. Merged images show the colocalization of both signals. (B-D) Coexpression of MCT8 and ChAT in the macaque nucleus basalis of Meynert. (F-H) Coexpression of OATP1C1 and ChAT in the macaque nucleus basalis of Meynert. Note that most neurons coexpress both signals. Counterstaining with DAPI (blue) shows nuclei of all cells. ac: anterior commissure, Cd: caudate nucleus, ChAT: Choline acetyltransferase, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, LV: lateral ventricle, Put: putamen. Scale bar = 50 μm (A, E) and 110 μm (B-D and F-H).
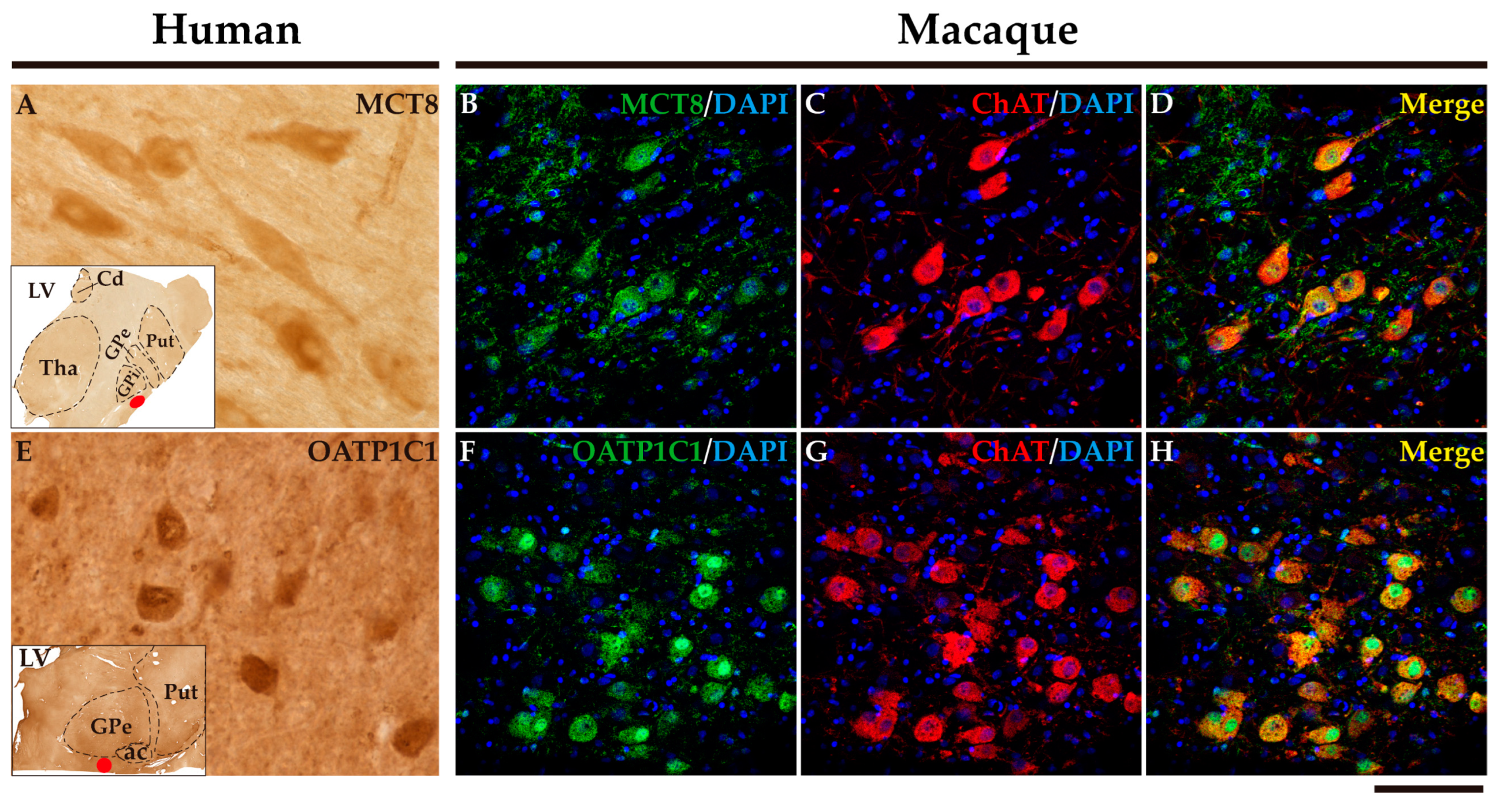
Figure 15.
Model of TH transporters in human and primate neostriatum microcircuitry based on our findings. The left panel summarizes the main circuits of the basal ganglia, and the right panel depicts the local microcircuit that occurs in the neostriatum (black frame on the left panel) with the expression of TH transporters in its components and their specific neurotransmitters. Left: the neocortex projects directly to the striatum (caudate and putamen) cells via the corticostriatal pathway (glutamatergic). MSN (most of the striatum cells) and some types of interneurons receive this excitatory cortical input. MSN are GABAergic projection neurons. The CM-Pf nuclei of the thalamus provide other major excitatory glutamatergic input to MSN via the thalamostriatal pathway. Additionally, the striatum receives the projections of dopaminergic cells from the SNc via the nigrostriatal pathway in MSN and in other types of interneurons. Interneurons are either cholinergic or GABAergic and participate in the local microcircuitry that modulates MSN activity. The MSN integrate all this information and produce two different signaling pathways: the direct pathway, in which MSN send inhibitory projections to the GPi, which in turn disinhibits the thalamus activating the thalamocortical system and therefore movement, and the indirect pathway, where MSN first inhibit the GPe, followed by disinhibition of the STN, which then excites GPi inhibiting the thalamus and thence movement. Finally, the VA/VL nuclear complex of the Thalamus is the primary target of the GPi, relaying the modulatory effects of the basal ganglia to the upper motor neurons in the cortex. The SNr sends axons to the thalamus and to the superior colliculus. Right: The right-hand diagram shows the distribution of the transporters MCT8 (orange) and OATP1C1 (green) in all types of cells of the striatal local microcircuitry in the human and macaque. TH are involved in modulating the output of MSN and the activity of all the neurons related in the corticostriatal, thalamostriatal, and nigrostriatal pathways, controlling movement. Ach: acetylcholine, CM-Pf: centromedian-parafascicular nuclei, FSI: fast-spiking GABAergic interneurons, GABA: γ-Aminobutyric acid, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, MSN: medium-sized spiny neurons, PLTS: persistent and low-threshold spiking interneurons, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, TAN: tonically active interneurons, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus (created with biorender.com).
Figure 15.
Model of TH transporters in human and primate neostriatum microcircuitry based on our findings. The left panel summarizes the main circuits of the basal ganglia, and the right panel depicts the local microcircuit that occurs in the neostriatum (black frame on the left panel) with the expression of TH transporters in its components and their specific neurotransmitters. Left: the neocortex projects directly to the striatum (caudate and putamen) cells via the corticostriatal pathway (glutamatergic). MSN (most of the striatum cells) and some types of interneurons receive this excitatory cortical input. MSN are GABAergic projection neurons. The CM-Pf nuclei of the thalamus provide other major excitatory glutamatergic input to MSN via the thalamostriatal pathway. Additionally, the striatum receives the projections of dopaminergic cells from the SNc via the nigrostriatal pathway in MSN and in other types of interneurons. Interneurons are either cholinergic or GABAergic and participate in the local microcircuitry that modulates MSN activity. The MSN integrate all this information and produce two different signaling pathways: the direct pathway, in which MSN send inhibitory projections to the GPi, which in turn disinhibits the thalamus activating the thalamocortical system and therefore movement, and the indirect pathway, where MSN first inhibit the GPe, followed by disinhibition of the STN, which then excites GPi inhibiting the thalamus and thence movement. Finally, the VA/VL nuclear complex of the Thalamus is the primary target of the GPi, relaying the modulatory effects of the basal ganglia to the upper motor neurons in the cortex. The SNr sends axons to the thalamus and to the superior colliculus. Right: The right-hand diagram shows the distribution of the transporters MCT8 (orange) and OATP1C1 (green) in all types of cells of the striatal local microcircuitry in the human and macaque. TH are involved in modulating the output of MSN and the activity of all the neurons related in the corticostriatal, thalamostriatal, and nigrostriatal pathways, controlling movement. Ach: acetylcholine, CM-Pf: centromedian-parafascicular nuclei, FSI: fast-spiking GABAergic interneurons, GABA: γ-Aminobutyric acid, GPe: external segment of globus pallidus, GPi: internal segment of globus pallidus, MSN: medium-sized spiny neurons, PLTS: persistent and low-threshold spiking interneurons, SNc: substantia nigra pars compacta, SNr: substantia nigra pars reticulata, STN: subthalamic nucleus, TAN: tonically active interneurons, VA/VL: ventral anterior and ventral lateral nuclei of the thalamus (created with biorender.com).
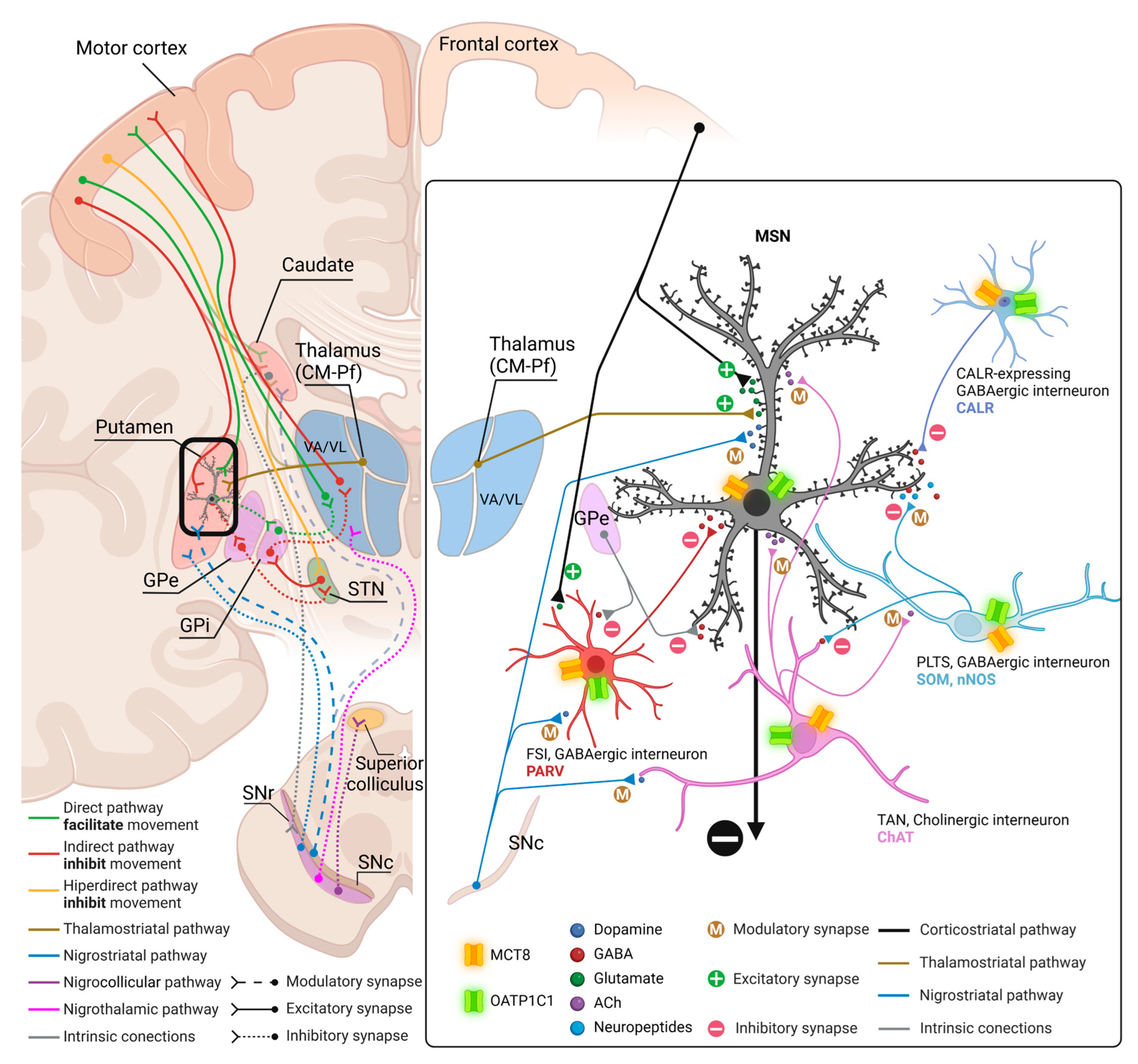
Table 1.
Clinical data of the human donors.
Table 1.
Clinical data of the human donors.
| Sex |
Age (y) |
Postmortem interval (h) |
Brain weight (g) |
Cause of death |
| Male |
29 |
4 |
1500 |
Lung tumor |
| Male |
32 |
3 |
1420 |
Hemorrhagic gastroenteritis |
| Male |
54 |
12 |
1350 |
Aortic aneurysm |
| Male |
59 |
<24 |
1020 |
Pneumonia |
| Male |
86 |
<24 |
- |
- |
| Male |
97 |
9 |
1238 |
Septic shock |
| Female |
98 |
6 |
1168 |
- |
Table 2.
Data of monkey brain tissue.
Table 2.
Data of monkey brain tissue.
| Species |
Age (y) |
Sex |
| M. fascicularis |
3 |
Female |
| Saimiri sciureus |
3 |
Female |
| M. fascicularis |
5 |
Male |
| M. fascicularis |
5 |
Male |
| M. fascicularis |
5 |
Female |
| M. fascicularis |
5 |
Male |
| M. fascicularis |
5 |
Male |
| M. fascicularis |
5 |
Female |
| M. fascicularis |
5 |
Male |
| M. mulatta |
7 |
Male |