1. Introduction
Chronic Myeloid Leukemia (CML) is a tri-phasic myeloproliferative disorder, characterized by a specific cytogenetic and molecular marker (Ph chromosome, BCR::ABL1 fusion gene) and consisting of a chronic, accelerated and blastic phases [
1]. At diagnosis, CML patients present most frequently in Chronic Phase (CP), whereas 4-5% of patients are firstly diagnosed in Accelerated Phase (AP) and only 1-2% of patients present in Blastic Phase (BP, or blastic crisis) [
1,
2]. From a physiopathology point of view, CP is the latent phase, in which often the patients are mild symptomatic or experience symptoms such as fatigue, weight loss and sweating correlated to marked leukocytosis, anemia and splenomegaly [
1,
2]. AP phase is characterized by additional genetic alterations, cytopenia and increased blasts, while BP phase that may present with myeloid, lymphoid or mixed phenotype, leads to a fatal outcome in nearly all CML patients due to resistance to current treatment strategies. Tyrosine Kinase Inhibitors (TKIs) have significantly improved the outcome of CP-CML patients by inducing a stable molecular response in the great majority of patients on continuous treatment and offering overall in about 15-20% of them the possibility of discontinuing TKI without disease recurrence. [
3]. However, the risk of progression to AP or BP phase still remains, mainly in those patients not achieving an optimal response with available first, second and third generation TKIs.
CML LSCs have been identified in the fraction CD34
+/CD38
−/Lin
− and specifically co-express CD26, which expression discriminates CML LSCs from normal HSCs and from LSCs of other myeloproliferative disorders [
4]. In our previous studies, we demonstrated that CD26
+LSCs are easily measurable in all Peripheral Blood (PB) samples of CP-CML patients at diagnosis and they are detectable during first line TKI treatment (imatinib, nilotinib, dasatinib) and during TFR, albeit with fluctuating values [
5,
6,
7]. A confirmation that CD26 represent a marker of stemness, was obtained from a study performed with a confocal laser microscope in which it has been showed that CD26 antigen is co-expressed with the protein Polycomb BMI1. Usually, BMI1 is highly expressed in CML despite a lower transcript level of BCR::ABL1; in this study it has been demonstrated that the fraction CD34
+/CD38
−/CD26
+ represent the reservoir of the BMI1 protein [
8].
Thanks to the “know how” acquired by performing these studies on CML LSCs, we developed a rapid custom-made flow cytometry tool for the measurement of CD34+/CD38−/CD26+ population in PB samples of CP-CML patients and divulgated a standardized method useful for the diagnosis of CP-CML. However, few data are available regarding the behavior of CD26+LSCs during AP or BP- CML and the role, if any, this peculiar staminal cell compartment may play in disease progression. In the present study we compared the presence and phenotypic characteristics of circulating CD26+ LSCs at diagnosis in CP-CML patients, in an AP-CML case and in 4 cases of progression to lymphoid BP.
2. Materials and Methods
The expression of CD34
+/CD38
-/CD26
+LSCs was evaluated by flow cytometry using lyophilized reagents (CD34-FITC, CD26-PE, CD38-APC, and CD45-V500 BD, Biosciences) on EDTA PB samples of patients with leukocytosis suspected of CML. Data acquisition has been done using BD FACSCanto
TM II or a BD FACSLyric
TM cytometers (BD, Biosciences). Data analysis has been performed using BD FACSDiva
TM or BD FACSuite
TM softwares (BD, Biosciences) using a strict gating strategy as previously described [
6]. All CML diagnosis have been confirmed with the measurement of BCR::ABL1 transcript levels by RT-PCR assays, according to European Leukemia Net (ELN) criteria.
3. Results
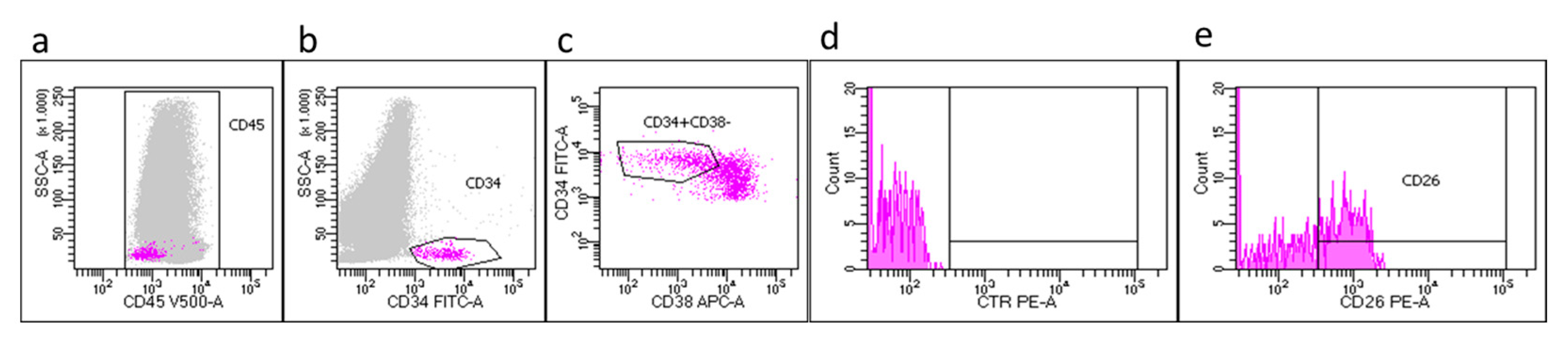
Newly diagnosed CP-CML patients
In the contest of a prospective multicenter Italian study (Prospective Flowers Study) coordinated by Siena Hematology Unit, 270 newly diagnosed CP-CML patients have been studied. Flow cytometry evaluation on PB samples have been performed, according to our previous standardized procedure, and in 100% of the patients we detected the hallmark CD34
+/CD38
-/CD26
+ population, characterized by a low expression of CD45 (CD45
dim). More in details, flow cytometry measurements performed on PB CP-CML samples at diagnosis showed that the median value of CD34
+ circulating cells, accounted for 0.36% (range 0.001-13%) of CD45
+ cells; the CD34
+/CD38
- subset, represented 17% (range 0.3-71%) of total CD34
+ cells. Within the CD34
+/CD38
− stem cell fraction, the median percentage of CD26
+ cells were 35% (range 0.07–98%).
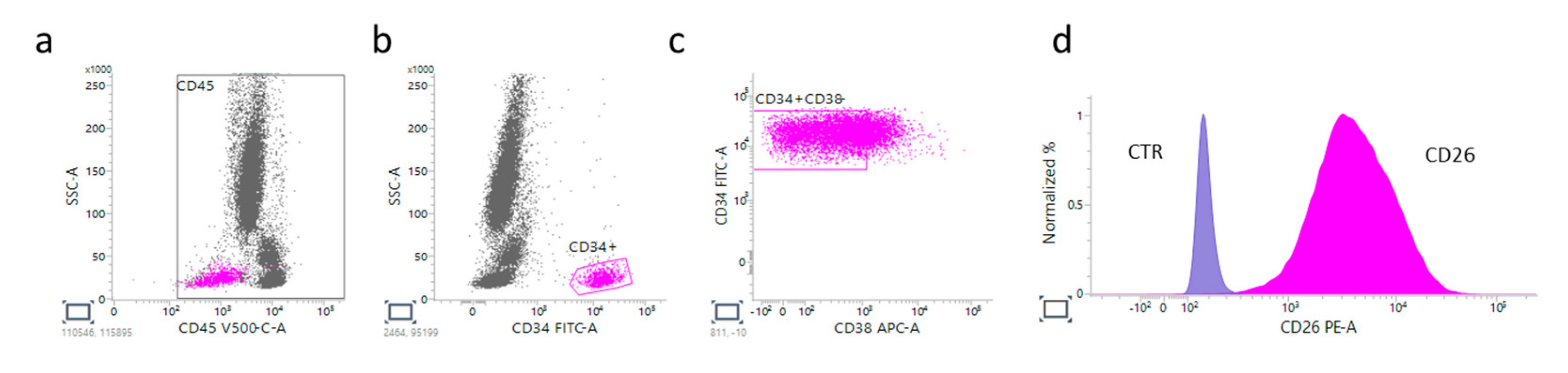
Figure 1 shows a representative CP-CML case at diagnosis in which is evident that LSCs express CD45
dim.
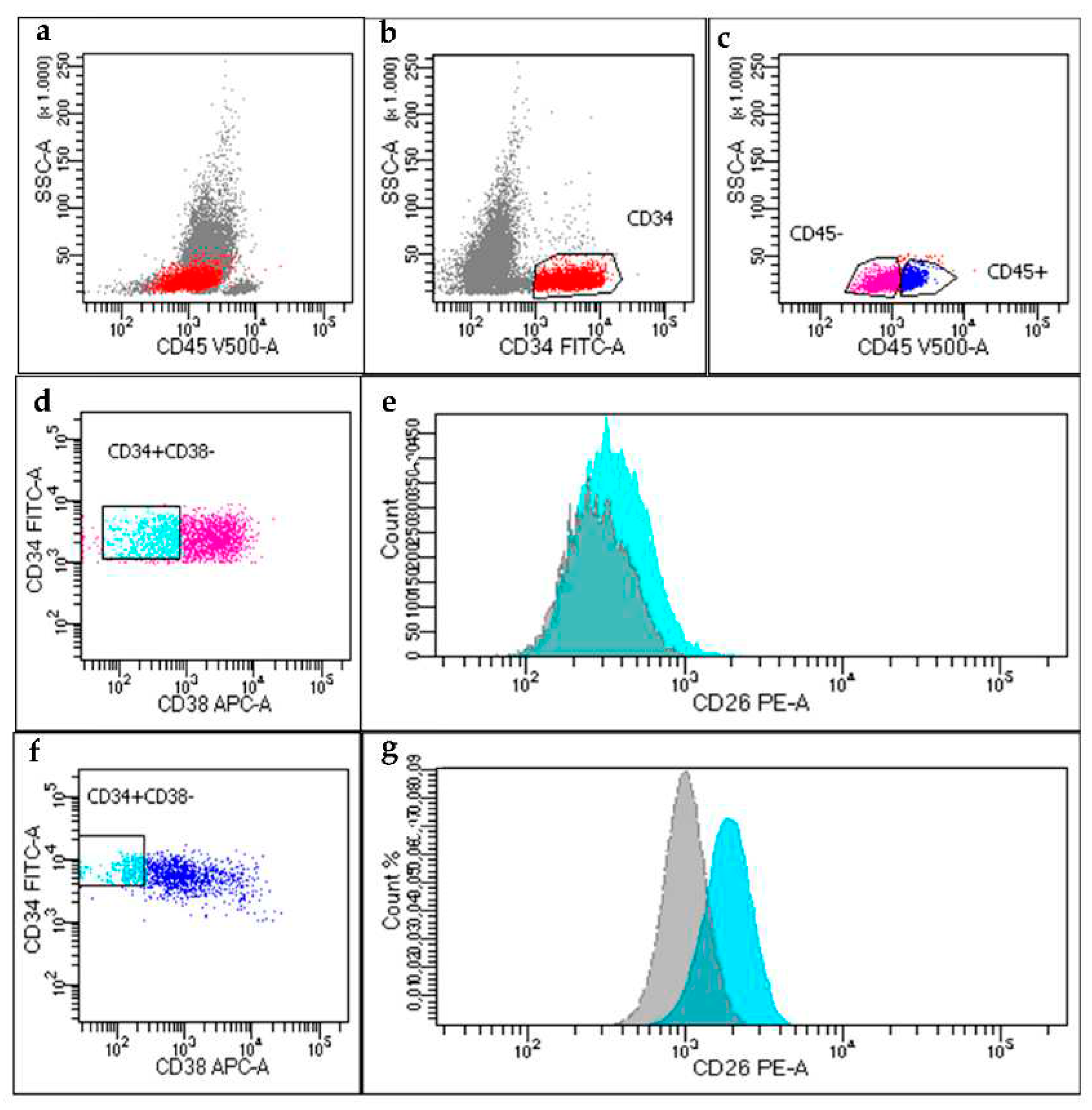
Accelerated Phase CML case
On February 2022, PB sample of a 49-years-old male, suspected new diagnosis for CML, referred to our laboratory for the search of CD26
+ LSCs. Cell blood count showed leukocytosis (WBC 68.405 x10
9/L), anemia (Hb 9.4 g/dl) and thrombocytosis (PLT 1058 x10
9/L). Surprisingly, we found a high total amount of CD34
+ cells (27% of WBCs) that was in part CD45
- (54%) and in part CD45
dim (46%). We found that 70% of the CD45
dim/CD34
+/CD38
- fraction co-expressed CD26, thus accounting for the CD26
+LSCs population. However, when we analyzed the CD26 expression within the CD45
-/CD34
+/CD38
− cell fraction (supposedly, a blastic population) we found still a detectable, yet lower expression, of CD26
+ cells (8%) (
Figure 2). The patient underwent first line dasatinib therapy and was monitored for the presence of CD26
+LSCs at 3- and 6-months during treatment. Along with the achievement of a molecular response, both flow cytometry evaluations showed the absence of CD26
+LSCs and of the blastic cell population.
Blastic Phase CML cases
We studied 4 cases of BP-CML patients. As following reported, we documented different cellular phenotypes.
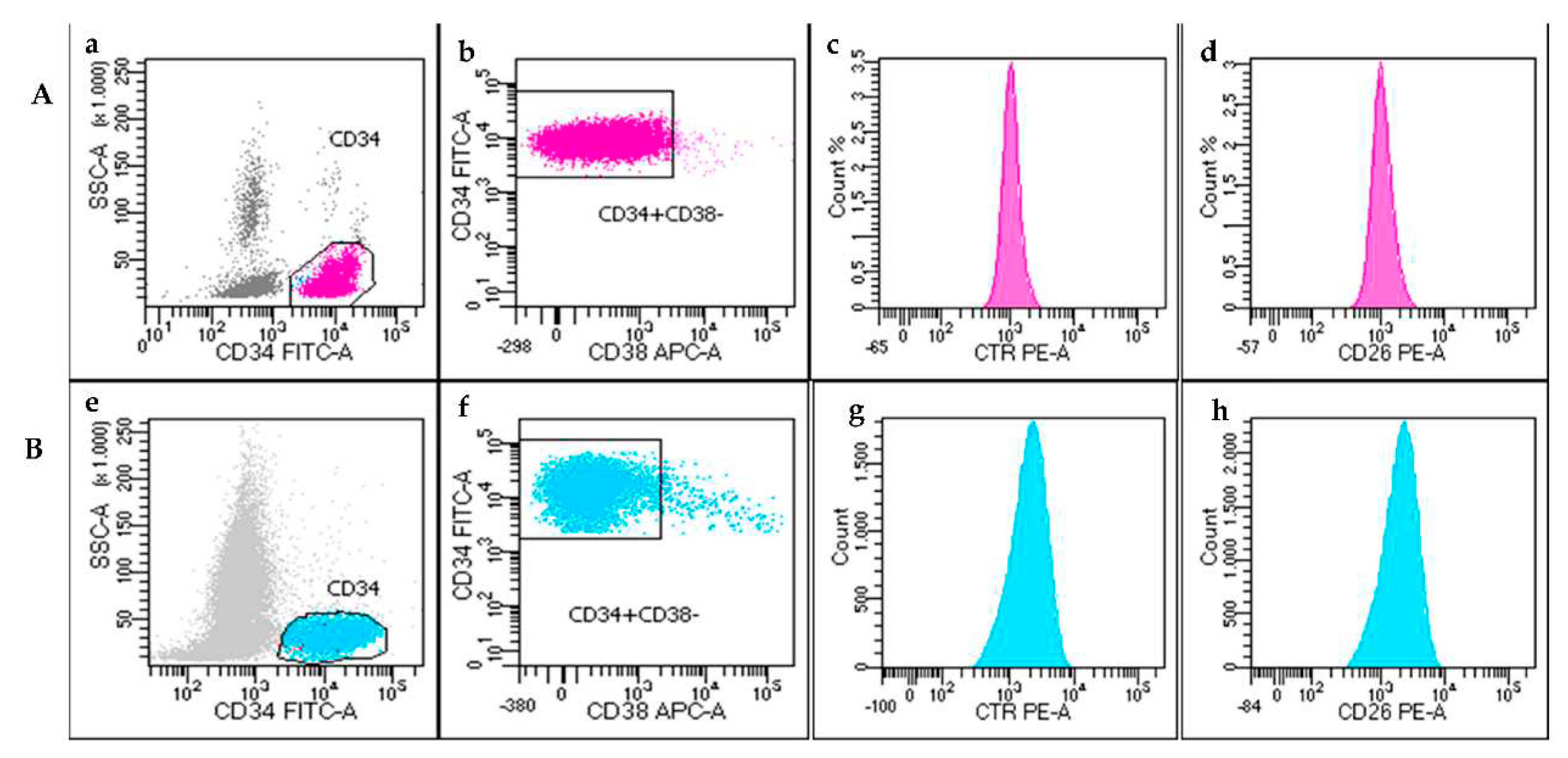
Case #1
The first BP-CML case was a 67-years-old male, with cardiovascular co-morbidities, diagnosed in August 2016 with CP-CML. Patient started treatment with second generation TKI dasatinib (100mg/die) until complete morphological and molecular remission (June 2017). However, therapy was then discontinued for onset of dyspnea and pleural effusion and, after 6 months of discontinuation, patient showed a molecular relapse (BCR::ABL1 0.2%, MR 2), and started treatment with imatinib (400mg/die). On June 2021, he presented leukocytosis at PB (WBCs 18.610x10
9/L), Hb 10.4g/dL and PLT 72x10
9/L with a suspect of a BP-CML evolution. Blastic phase was confirmed by the presence of 72% of blast cells at the PB smear, FISH and molecular assays (85% of nuclei and 62% of BCR::ABL1 transcript, respectively). Flow cytometry analysis showed a cell population CD45
-/CD34
+/CD19
+/CD10
+/TdT
+ confirming the morphological evaluation. All CD34
+ cells were CD38
- and CD26
- at both PB and BM samples (
Figure 3A). Patient was admitted to Hyper-CVAD + Ponatinib treatment.
Case #2
This case refers to an 83-years-old male who received diagnosis of CP-CML in February 2019. At that time, FISH confirmed CML diagnosis in 90% of cell studied and RT-PCR detected a p210 transcript type b2a2. The patient started treatment with first line imatinib (400 mg/die) achieving a complete cytogenetic response. A PB sample was then sent to Siena Hematology lab, in February 2021, to investigate the presence of CD26
+LSCs during TKI treatment. Unexpectedly, cell blood count was found 2x10
9/L. Immunophenotypic analysis showed the presence of CD34
+/CD38
- cells (9,3% of total cellularity in the sample), that were surprisingly negative for both CD45 and CD26 (
Figure 3B). The latter evidence suggested an evolution to BP, that was shortly confirmed by a BM analysis, performed at local center, that documented a blast quote of 36%.
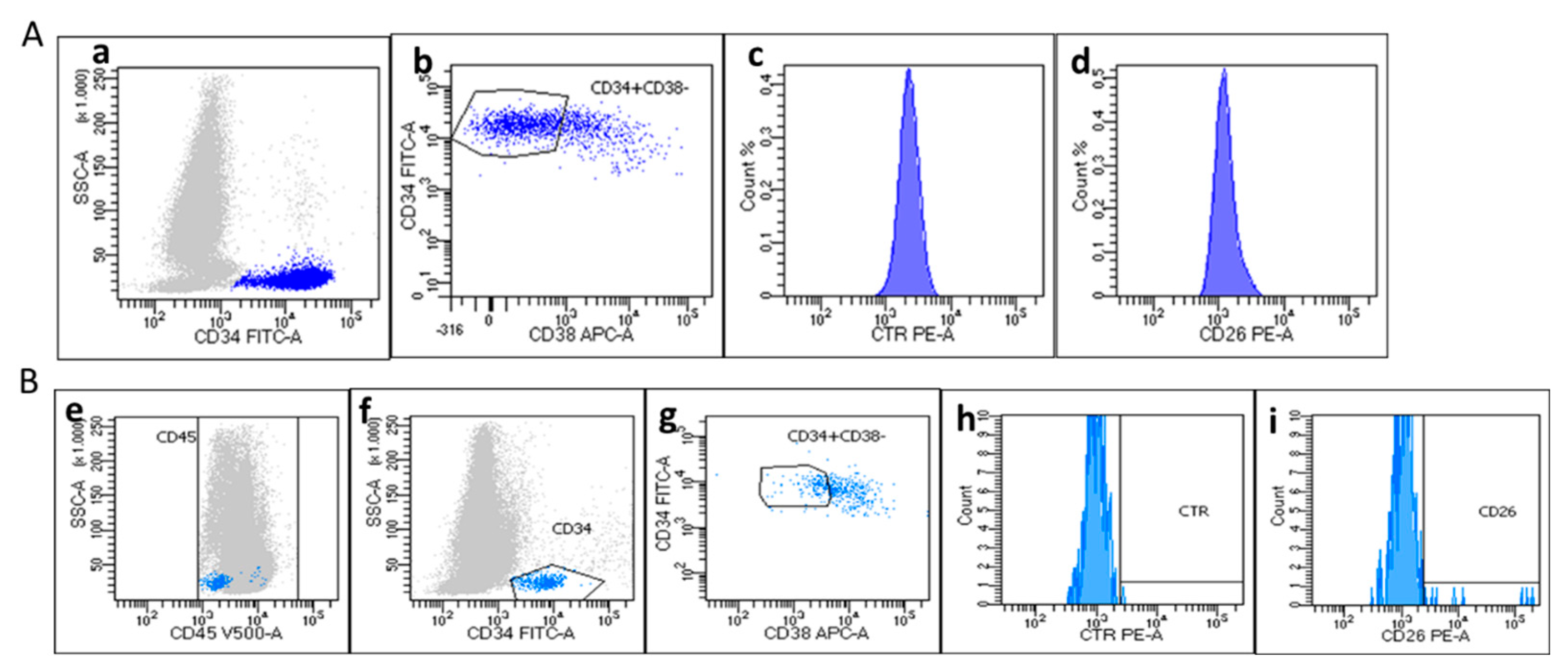
Case #3
The present case involves a 34-years-old female patient that was diagnosed with a de novo lymphoid BP-CML. Cell blood count at diagnosis was the following: WBC 62.880 x10
9/L, Hb 9.4 g/dl and PLT 51 x10
9/L. Morphological examination of blood smear showed a consistent quote of myeloid immature cells and 23% of blasts with increased nuclear-cytoplasmatic ratio and prominent nucleoli. PB flow cytometry test showed the presence of lymphoid blasts CD45
-/CD34
+/CD19
+/CD10
+/TdT
+ and RT-PCR detected the presence of p210 BCR::ABL1 transcript (b3a2). Bone marrow aspirate confirmed a B-Lymphoid BP-CML. Circulating PB CD26
+LSCs were not detected at that time (
Figure 4A). The patient underwent induction treatment with Hyper-CVAD + Ponatinib and at recovery, concomitantly with the response to treatment and PB lymphoblasts disappearance, we documented a slight, but evident population of CD34
+ (0.091% of CD45
+ cells, with CD45
dim) and CD34
+/CD38
- (12% of CD34
+) cells. CD26
+ LSCs were 24% of CD34
+/CD38
- population (
Figure 4B). Yet clearly detectable, the absolute percentage of CD26
+LSCS accounted for 0.002% of CD45+ cells.
Case #4
The present case is referred to a 68-years old male, diagnosed in a peripheral hematology center in November 2021 with CP-CML. Diagnosis of CML was confirmed by CD26+LSCs evaluation, molecular and cytogenetic analysis in our laboratory. He started treatment with Imatinib (400 mg/die) but, due to GI toxicity, he discontinued therapy for two weeks and then reduced Imatinib dosage to 200 mg/die for intolerance. Few months later, patient’s BM and PB samples were sent to Flow Cytometry Lab in Siena due to leukocytosis (WBCs 15.903x109/L) and suspicion of lymphoid blast crisis. Surprisingly, the immunophenotypic analysis showed the presence of a population CD45dim/CD34+/CD38- , similar to the phenotype of a CP-CML, co-expressing CD19 and CD10 antigens, as for a typical lymphoid blast crisis. Consequently, we searched for the expression of CD26 and, indeed, the analysis confirmed that the CD45dim/CD34+/CD38-/CD19+/CD10+ cells co-expressed this antigen.
4. Discussion
Based on these results, we can confirm that the search of PB CD26+ LSCs cells is a powerful tool for the diagnosis of CML in CP, since these cells symbolize a “signature” of CP-CML. Indeed, as demonstrated in our previous studies, and documented in our prospective study, CD26 represents a specific marker of CML LSCs, expressed on 100% of PB samples of CP-CML at diagnosis. However, we also observed an interesting and misleading/controversial immunophenotypic panel in the AP and BP-CML samples studied.
In the AP-CML case, we identified the presence of two distinct PB CD34+/CD38- populations: the first subset included CD45dim cells in which CD26 antigen was expressed (70% of CD34+/CD38- fraction) while, simultaneously, a second subset CD45-/CD34+/CD38- was present in which blast cells were predominant, but in which we documented that the CD26 was still expressed on the CD34+/CD38- population, yet in a lower percentage of cells (8%).
Regarding the four lymphoid BP-CML cases, we documented a homogeneous phenotype in 3 patients, where the blasts were negative for CD45 expression and consequently, also for CD26 antigen. Surprisingly, the case #4 was countertrend compared with others, showing a blast phenotype CD45dim/CD34+/CD38-/CD19+/CD10+ and the expression of CD26+ (99% of CD34+/CD38- cells). In this latter case lymphoid blasts displayed a phenotype CD45dim/CD34+/CD38- comparable to that of LSCs found in CP-CML at diagnosis. Even if we studied only few patients, the different CD26 expression documented in our AP- and BP-CML cases, induced to ask new questions and to formulate possible hypothesis. We confirmed, as reported by others, that CD26+LSCs are not detectable (or “disappear”?) during a classical CD45- lymphoid BP-CML. However, we documented also that a “blastic” CD45dim/CD26+LSCs population may coexist.
This preliminary, yet unique, evidence arise the question regarding the role of CD26+LSCs during blastic transformation, suggesting two possible scenarios: CD26 LSCs may “shift” phenotype, first losing CD45 expression and subsequently CD26 expression and then acquiring a blastic phenotype as it would suggest the case studied during AP and to some extent the BP case #4 ; alternatively, CD34+ blasts arise from a different subset and CD26+ LSCs cells may coexist during BP albeit surmounted by the bulk of CD34+ blasts and thus not detectable.
In conclusion, the present study reports for the first time, additional information about CD26+ expression in AP and BP CML cases, never investigated until now. However, more studies are needed to better enlighten if the CD26 antigen concur or not to the progression of CML from Chronic to Accelerated and Blast Phase.
Author Contributions
Conceptualization, M.B. and D.R.; methodology, P.P., A.Si., E.B., D.R.; clinical data collection, A.I., E.A., A.Sa, M.M. and E.Z.; writing—original draft preparation, P.P and A.Si.; writing—review and editing, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Associazione Italiana per la Ricerca sul Cancro (AIRC), grant number 20133.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Azienda Ospedaliera Universitaria Senese (protocol code n 12321_2018) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
9 authors (PP, ASi, EB, ASa, AI, MM, EZ, DR, and MB) of this publication are members of the European Reference Network for rare hematological diseases EuroBloodNet.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood 2013, 122, 872–874. [Google Scholar] [CrossRef]
- Hoffmann, V.S.; Baccarani, M.; Hasford, J.; Castagnetti, F.; Di Raimondo, F.; Casado, L.F.; Turkina, A.; Zackova, D.; Ossenkoppele, G.; Zaritskey, A.; et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia. 2017, 31, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing chronic myeloid leukemia for treatment-free remission: a proposal from the GIMEMA CML WP. Blood Adv. 2019, 3, 4280–90. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Sadovnik, I.; Ráčil, Z.; Herrmann, H.; Blatt, K.; Cerny-Reiterer, S.; Eisenwort, G.; Lion, T.; Holyoake, T.; Mayer, J. DPPIV (CD26) as a novel stem cell marker in Ph+ chronic myeloid leukaemia. Eur. J. Clin. Invest. 2014, 44, 1239–45. [Google Scholar] [CrossRef] [PubMed]
- Bocchia, M.; Sicuranza, A.; Abruzzese, E.; Iurlo, A.; Sirianni, S.; Gozzini, A.; Galimberti, S.; Aprile, L.; Martino, B.; Pregno, P.; et al. Residual Peripheral Blood CD26+ Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission. Front. Oncol. 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Raspadori, D.; Pacelli, P.; Sicuranza, A.; Abruzzese, E.; Iurlo, A.; Cattaneo, D.; Gozzini, A.; Galimberti, S.; Baratè, C.; Pregno, P.; et al. Flow Cytometry Assessment of CD26+ Leukemic Stem Cells in Peripheral Blood: A Simple and Rapid New Diagnostic Tool for Chronic Myeloid Leukemia. Cytometry B 2019, 96, 294–99. [Google Scholar] [CrossRef] [PubMed]
- Bocchia, M.; Sicuranza, A.; Pacelli, P.; Iurlo, A.; Abruzzese, E.; Galimberti, S.; Liberati, A.M.; Ferrigno, I.; Ciofini, S.; Defina, M.; et al. Peripheral Blood CD26+ Leukemia Stem Cells Monitoring in Chronic Myeloid Leukemia Patients from Diagnosis to Response to TKIs: Interim Results of a Multicenter Prospective Study (PROSPECTIVE FLOWERS). Blood. 2020, 136, 45–46. [Google Scholar] [CrossRef]
- Galimberti, S.; Grassi, S.; Baratè, C.; Guerrini, F.; Ciabatti, E.; Perutelli, F.; Ricci, F.; Del Genio, G.; Montali, M.; Barachini, S.; et al. The Polycomb BMI1 Protein Is Co-expressed With CD26+ in Leukemic Stem Cells of Chronic Myeloid Leukemia. Front Oncol. 2018, 8, 555. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).