INTRODUCTION
Psoriasis is an autoimmune inflammatory disease that causes the cells of the last layer of the skin, the keratinocytes, to grow much faster than normal, every three or four days instead of 28 days, as occurs with the cells of a healthy person, giving rise to psoriatic plaques. This disease affects almost 138 million people worldwide, the prevalence of this type of disease worldwide is about 2 to 3%, its peak incidence is between 20 and 50 years of age, it causes itching or pain in patches of thickened, reddened and silvery scaly skin that appear on different parts of the body.1 This disease does not respect age, sex, origin, socioeconomic or cultural factors, its peak incidence is between 20 and 50 years of age.2-3 The cause of psoriasis is unclear, but it is known to involve immune stimulation of epidermal keratinocytes; T cells appear to play a central role. A family history is common, and some genes and human leukocyte antigens (Cw6, B13, B17) have been associated with psoriasis.4-5 There are different types of psoriasis: Erythroderma: skin redness is very intense and covers a large area. Guttate (guttate or guttural): small red to pink patches appear on the skin. Inverse: redness and skin irritation occur in the armpits, groin and between overlying skin.6 Plaque: Thick, red patches of skin covered by silvery to white scales. It is the most common type of psoriasis and affects about 80% of sufferers. Pustular: white blisters surrounded by red, irritated skin7-8.
Treatment depends on the type of lesion and its complications; retinol, corticosteroids, vitamins and salicylates, phototherapy, cyclosporine, immunosuppressants, cytosines, monoclonal antibodies and others are used. Preventive hygienic, dietary and sanitary measures are recommended9.
Psoriasis presents many complications that affect the quality of life of patients with genetic, environmental and lifestyle predisposition.10 The lack of response to treatments causes patients to visit several specialists due to their physical discomfort, psychological suffering that sometimes leads them to attempt suicide, since their physical appearance generates rejection in society, affecting their family, work and economic life.11 There are different single or combined treatments approved by regulatory agencies such as the FDA and the EMEA, but they are also harmful in the medium and long term, presenting resistance and abandonment of conventional treatments, in addition to their high economic cost, especially the moderate and severe form.12-13 This disease is characterized by relapses and remissions that affect the quality of personal, family, work and social life, including severe depressive disorders14 There are also natural treatments alone or in combination already marketed, which are offered as “home remedies “15. There is no topical preparation using salicylic acid, animal lard, sublimated sulfur, triple aluminum sulfate, Vaseline and essential oil of Laurel. For the treatment of psoriasis, since these products have anti-inflammatory, soothing, disinfectant, antimicrobial and healing properties, we set out to conduct a pre-experimental study with a topical cream containing these natural compounds. It was first applied on healthy subjects to evaluate hypersensitivity and then on patients to evaluate its effectiveness by comparing before and after treatment. Therefore, our hypothesis was: Will the topical cream elaborated with natural products reduce the clinical signs characteristic of Psoriasis?
METHODOLOGY
Place of preparation of the combined and complete formulas: “La Salud” Pharmacy (FLS), Guayaquil. Address: Urdesa Central, Bálsamos Norte, #425 y calle 6ta (Plaza Dañín) , Guayaquil, Ecuador
Equipment to be used in the Pharmacy Laboratory “La Salud”: Morter. Beaker of 1000 ml. Beaker of 250 ml. and Computer and printer.
Preparation of complete formula: Pulverize the alum in a mortar and pestle, crush the sulfur and salicylic acid, and mix all powders in a 1000 ml Beaker. In a 250 ml. In a 250 ml. beaker, add the petroleum jelly, the laurel oil and add it to the 100 ml. beaker. Finally add the lard and mix until a homogeneous mass is obtained.
Quality control: Analyzing the organoleptic properties (color, odor, appearance and consistency), physical properties (determination of the consistency), physical properties (determination of extensibility) of the final products.
Healthy subjects: was performed intra-domiciliary 2019, using the safety test in 100 healthy volunteers, combining 5 formulations with the different compounds of the research formula, administered to 5 groups of 20 people each. No adverse effects were observed in the healthy volunteers.
Patients with mild to moderate psoriasis: quasi-experimental clinical trial no randomized study without control group, from February to December 2022 in the outpatient clinic of the Dermatology Service of the Hospital “Teodoro Maldonado Carbo” in the city of Guayaquil.
| Hypersensitivity test in 100 healthy subjects with the 5 formulations and complete |
| Fórmule # 1 |
Fórmule # 2 |
Fórmule # 3 |
Fórmule # 4 |
Fórmule # 5 |
Fórmule # 6 (complete phase II) |
| Promoter code: ICD10.L40.0-001 |
Promoter code: ICD10.L40.0-002 |
Promoter code: ICD10.L40.0-003 |
Promoter code: ICD10.L40.0-004 |
Promoter code: ICD10.L40.0-005 |
Promoter code: ICD10.L40.0-006 |
| 20 healthy subjects |
20 healthy subjects |
20 healthy subjects |
20 healthy subjects |
20 healthy subjects |
60 patients |
| Salicylic acid |
Salicylic acid |
sulphur |
sulphur |
Salicylic acid |
Salicylic acid |
| sublimed alum |
sulphur |
sublimed alum |
Salicylic acid |
sublimed alum |
sublimed alum |
| solid vaseline |
solid vaseline |
solid vaseline |
pork fat |
pork fat |
pork fat |
| bay laurel oil |
|
|
bay laurel oil |
|
solid vaseline |
|
|
|
|
|
bay laurel oil |
|
|
|
|
|
sulphur |
| Developed by: E.Benites |
|
|
|
|
The universe was 270 patients from the Dermatology service of the TMC Hospital. The sample selection was made by calculating the sample size giving 120 patients with psoriasis with a margin of error of 5% and a confidence level of 95%. We selected 60 patients with plaque psoriasis who met the inclusion criteria and administered the complete formula.
Inclusion criteria.–Patients receiving medical attention in Dermatology at the Teodoro Maldonado Carbo Hospital of IESS Patients diagnosed with mild and moderate Psoriasis in any of its locations with or without treatment. Over 18 years old. Patients who do not have malignant tumor pathology in skin, autoimmune or other disabling pathologies.
Exclusion criteria. - Patients who do not receive care in Dermatology at the Teodoro Maldonado Carbo Hospital of IESS. Patients corresponding to other skin pathologies. Patients under 18 years of age. Patients suffering from psychiatric disorders due to other pathologies that could interfere in the data base and evaluation of quality of life. Patients with malignant tumor pathology in skin, autoimmune or other disabling pathologies.
Methodology. – Quasi-experimental clinical trial no randomized procedure was prospective, observational, descriptive, differential analytical. For the interpretation of results, the statistical programs Excel and SPSS - MODELER v.18 were used, using tables, graphs for the interpretation and observation of the results.
The city of Guayaquil has a humid tropical climate with a temperature of 32oC +/- 5, this climatic factor affects the frequency of care in the Dermatology department of the TMC Hospital. The patients were selected according to the scheduled medical attention schedule, 95% are recidivists treated with conventional systemic, oral and biologic drugs among others. All 60 patients voluntarily signed the informed consent approved by the Independent Ethics Committee of the LV Hospital.
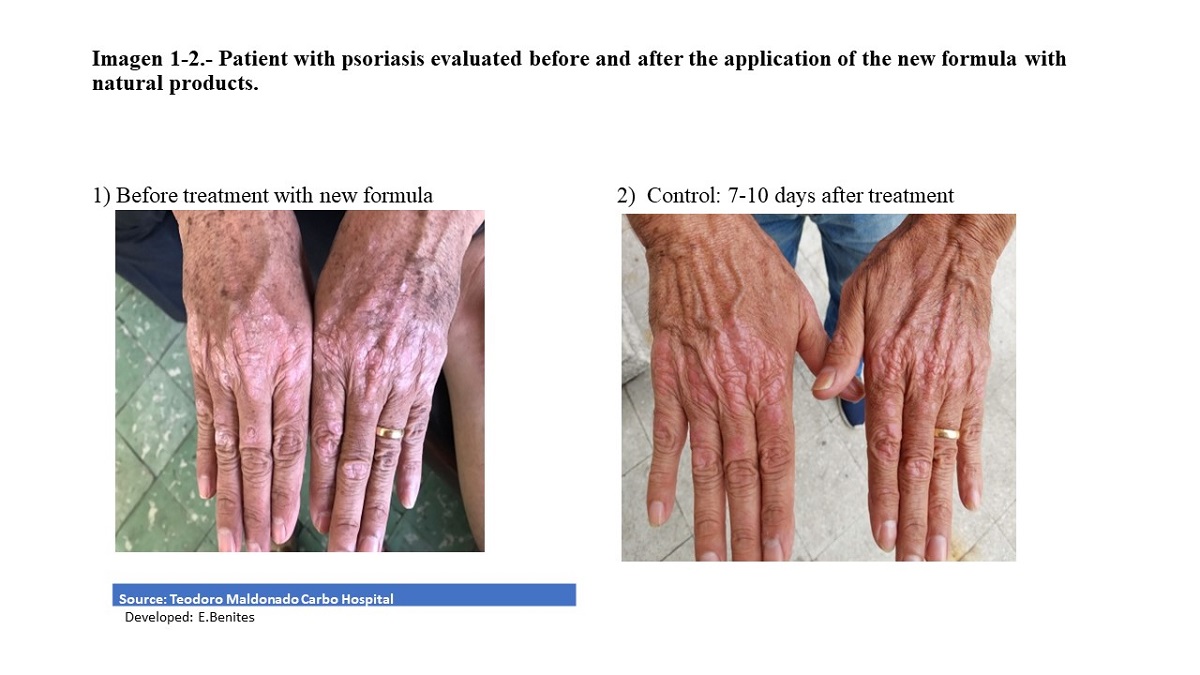
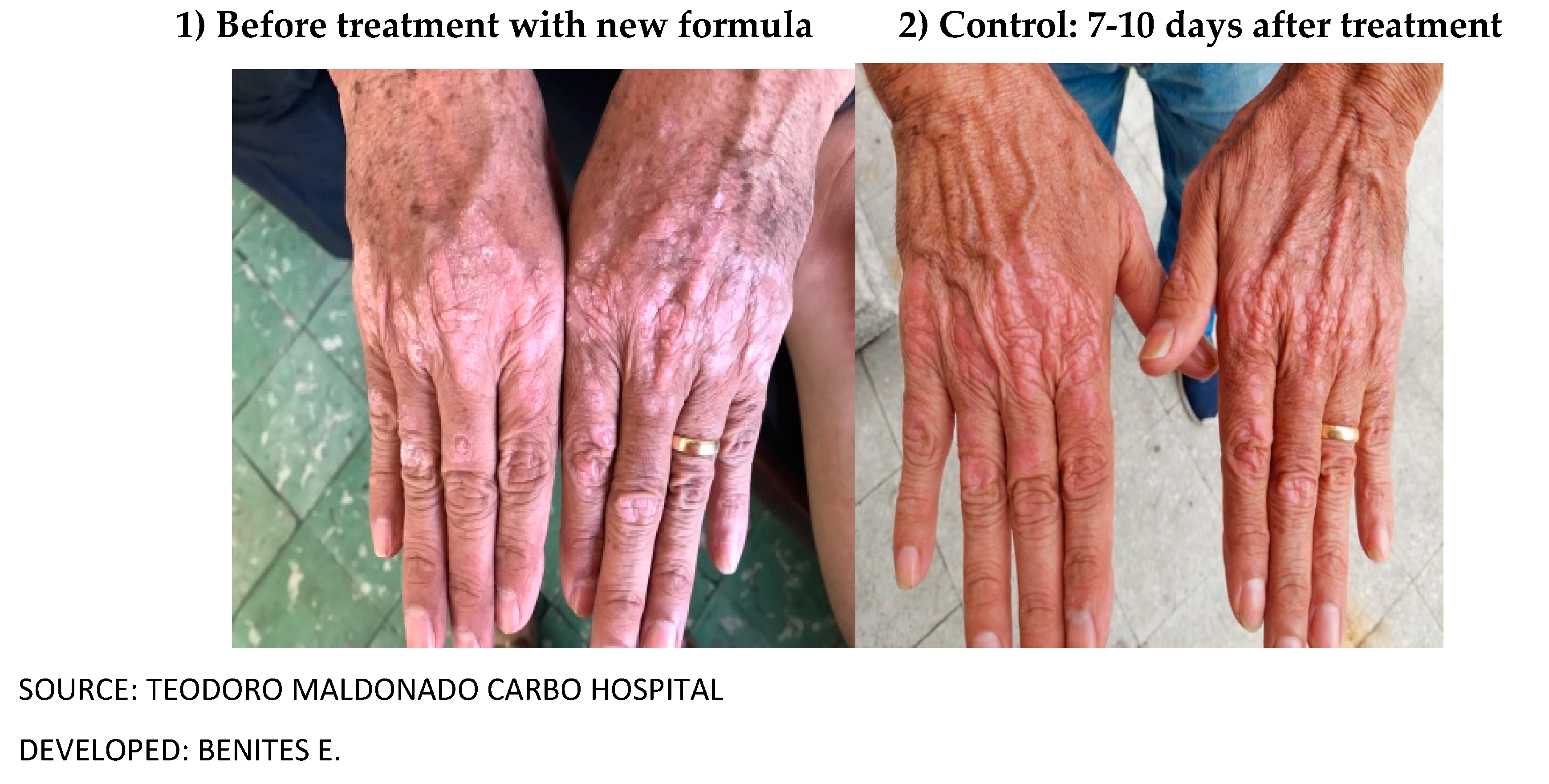
In our study, the pre-experimental treatment consisted of applying the topical cream on the erythematous desquamative lesions every 8 hours, Loratadine at night and Vit D, good personal hygiene. The effectiveness of the treatment was evaluated 10 days after the beginning of the application of the cream with the new formula of natural products.
The evaluation criteria were based on the PASI (Index for evaluating the degree of severity of the lesions.) and the BSA (Surface area affected) , determining the area of skin involvement of the patient with mild and moderate psoriasis. The body was divided by sectors giving a higher score according to the percentage of the affected area. The severity of the lesions was evaluated considering erythema, desquamation, infiltration and thickness, giving each one a scale between 0-4 points for the calculation of the PASI severity index before and after treatment. Quantitative and qualitative variables of corresponding clinical signs and symptoms were used. The database for this study is in the Figshare and Dryad repository.
Ethical and Legal Aspects: Ethical aspects have been considered, such as non-disclosure of patient names, safeguarding the principle of confidentiality, and informed consent was requested and approved by the Ethics Committee on Human Subjects (CEISH) of the Luis Vernaza Hospital in Guayaquil. The database of the clinical histories of the selected patients will be used only for the project in question and for statistical purposes to test the hypothesis of our study.
Limitation. - TMC Hospital was declared a sentinel hospital for Covid 19 for the years 2020 and 2021. As of January 2022, medical attention was normalized in the outpatient clinic of that hospital, although the first 4 months the attention quota was limited, it was normalized in July of that year, being able to continue with the sampling of the 60 non-randomly selected volunteer patients, for the application of the topical cream made with natural products.
OUTCOMES
Of the 100 healthy subjects, 99% did not present any clinical symptom of hypersensitivity, in terms of habits, only 27% were passive smokers, they did not report alcohol and only 2% reported having consumed marijuana.
Baseline characteristics of the sample to be studied at the start of treatment we had: With the new product 66% are male and 44% female, mean age is 48.78 years (12-84), weight 69kg and height 1mt 60 cm, BMI 24.5, Glucose 86, urea 24.8, BP 120/70 and Leucocytes 6.58. (
Table 1)
Baseline characteristics of the sample to be studied at the start of treatment we had: With the new product 66% are male and 44% female, mean age is 48.78 years (12-84), weight 69kg and height 1mt 60 cm, BMI 24.5, Glucose 86, urea 24.8, BP 120/70 and Leucocytes 6.58.
Table 2)
In terms of occupation, among the patients with the new product, 71% are clerks and 10% pensioners, 6.7% professionals, 5% operatives, 3.3% professor and 3.3% housewives, As for the ethnic groups, among the patients with the new product, 50% was mixed, 20-25% was indigenous, blacks 10-12%, whites 3-5%
In erythema before and after treatment with the new formulation, Significance=0.05 T-value from Student’s t-table= 1.96 Calculated t-value = 19.144, is greater than 1.96 we accept H1: The average erythema assessment before treatment with the new formulation differs from the average erythema assessment after treatment with the new formulation. P value= 0.000
Interpretation: The assessment of erythema before treatment with the new formulation differed after treatment.
In infiltrate before and after treatment with the new formulation, Significance=0.05 Student’s t-table t-value= 1.96 Calculated t-value = 6.725, is greater than 1.96 we accept H1: The average evaluation of the infiltrate before treatment with the new formulation differs from the average evaluation of the infiltrate after treatment with the new formulation. P value= 0,000
Interpretation: The assessment of the infiltrate before treatment with the new formulation has changed after treatment.
In desquamation before and after treatment with the new formulation, Significance=0.05 T-value from Student’s t-table= 1.96 Calculated t-value = 12.475., is greater than 1.96 we accept H1: The average evaluation of the infiltrate before treatment with the new formulation differs from the average evaluation of the infiltrate after treatment with the new formulation. P value= 0.000
Interpretation: The assessment of the desquamation before treatment with the new formulation differed after treatment.
In itching before and after treatment with the new formulation, Significance=0.05 Student’s t-table t-value= 1.96 Calculated t-value = 10.863 is greater than 1.96 we accept H1: The average evaluation of itch before treatment with the new formulation differs to the average evaluation of itch after treatment with the new formulation. P value= 0.000
Interpretation: The assessment of itch before treatment with the new formulation differed after treatment.
DISCUSSION
Baseline characteristics of the 60 patients to be studied at the beginning of the treatment were: 66% male and 44% female, mean age 48.78 years (12-84), weight 69kg and height 1mt 60 cm, BMI 24.5, Glucose 86, urea 24.8, BP 120/70 and Leucocytes 6.58.
Regarding occupation, among the patients 71% are office workers and 10% pensioners, 6.7% professionals, 5% workers, 3.3% teachers and 3.3% housewives, most of them are of mixed race. Overweight and the humid tropical climate of the city are negative factors for this type of injury.
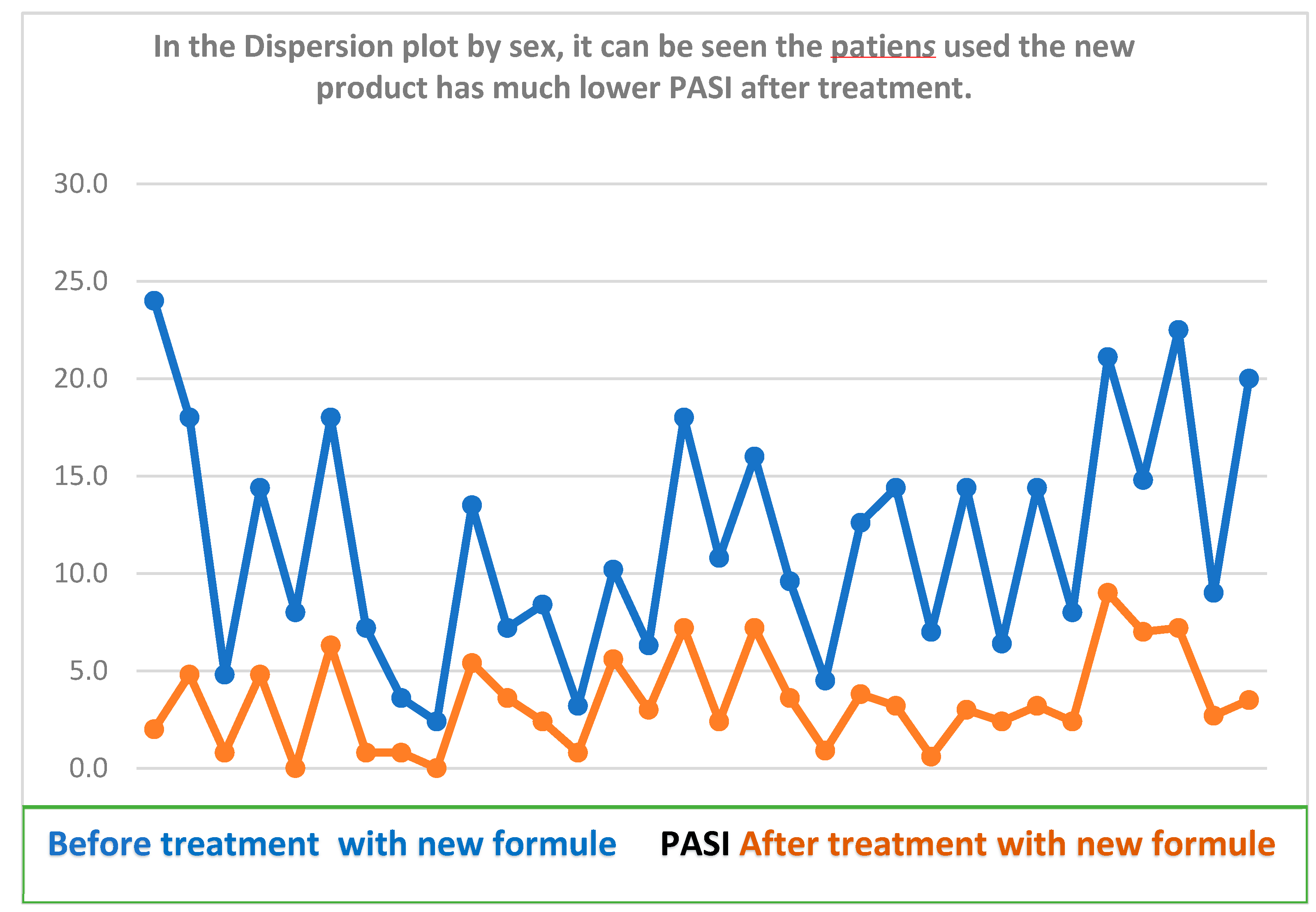
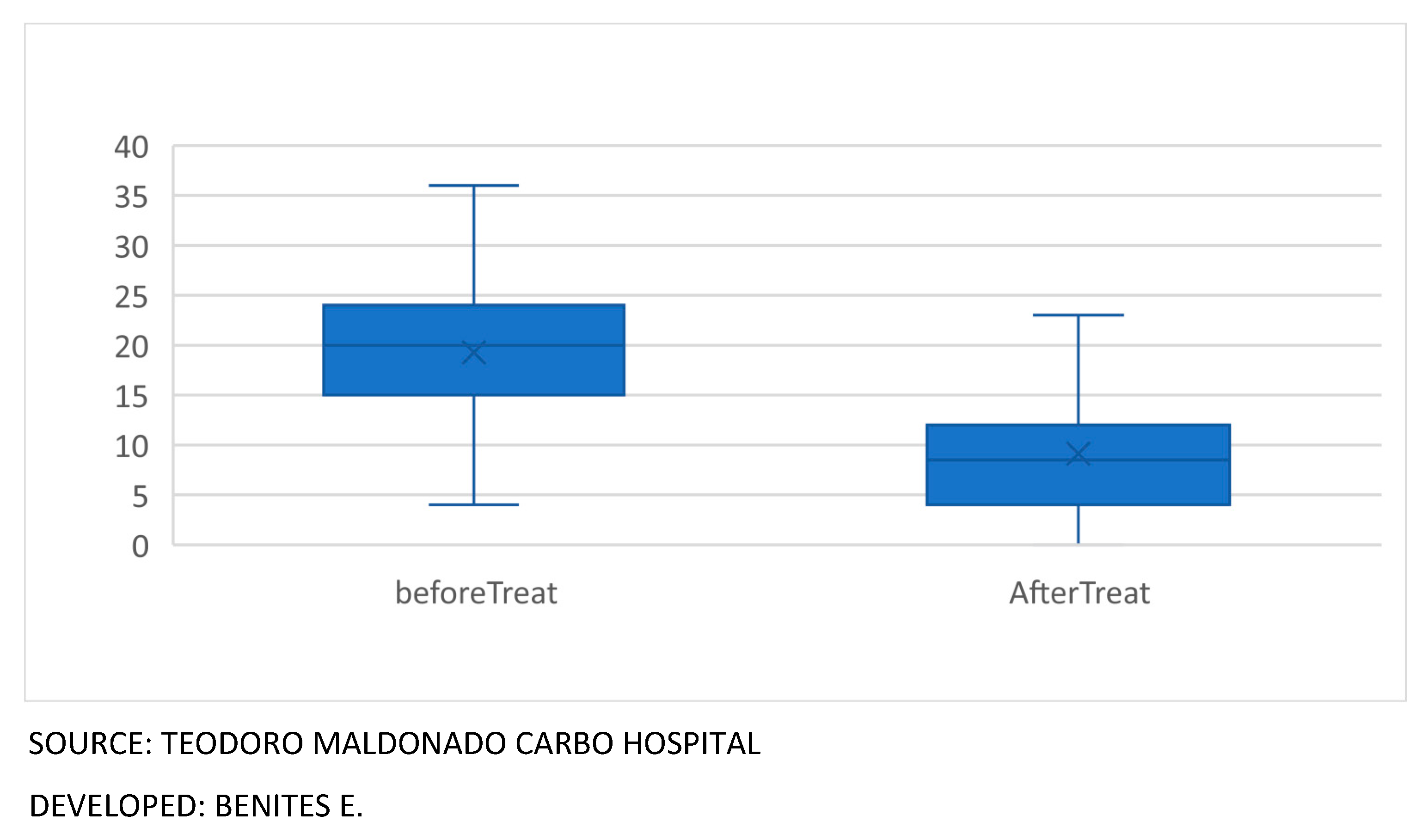
When evaluating the new formula before and after treatment in the 60 patients, with the clinical variables of erythema, infiltrate, desquamation and pruritus, PASI index and BSA, the T Student test for paired samples was used, showing the following behavior in the t values for paired samples, erythema 7. 590, infiltrate 10.573, desquamation 7.311, pruritus 3.615, PASI 2.631, BSA 4.411, presented a P value = 0.000 which showed that the new formula had greater variation after treatment. No published studies were found that use the composition of a cream containing these six natural products for the treatment of Psoriasis, there are publications of these components separately, which are used in skin lesions such as: salicylic acid16 Vaseline17, triple aluminum sulfate18 Sulfur sublimate19, essential oil of laurel20 that intervene other components in its formulation.
This study demonstrated our hypothesis in which the topical cream elaborated with natural products such as: animal butter, sublimated sulfur, triple aluminum sulfate, petroleum jelly and essential oil of laurel and salicylic acid, did reduce the clinical signs characteristic of psoriasis, demonstrating its efficacy and improving the dose response in the regeneration of the skin in patients with mild and moderate psoriasis.
This topical cream is considered as an alternative and complementary therapy in the medium and long term for this type of disease, since it did not show secondary reactions in phase I in healthy subjects, and in patients its effectiveness was demonstrated in this study (
Figure 3)
It is important to continue with population studies in Ecuador by regions: coast, highlands, east-Amazon and island region or Galapagos, whose climatic diversity and lifestyles are different, to evaluate its effectiveness and whether the environmental factor affects the response to treatment of this topical cream made with natural products in mild and moderate psoriasis.
Author Contributions
Benites E, Carrillo E, Heras M, have had full access to the study database and assume responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and designs: Benites E, Carrillo E. Manuscript drafting_ Benites E, Carrillo E. Critical revision of manuscript_ Benites E, Carrillo E, Heras M. Analysis and interpretation of the important intellectual content: All authors. Statistical analysis: Benites E. Funding obtained: The authors did not receive financial support for the authorship of the research and/or publication of this article. Administrative, technical or material support: Benites E, Carrillo E, Heras M. Study supervision: Benites E, Carrillo E,
Additional contribution
The authors are grateful for the collaboration of the Teodoro Maldonado Carbo Hospital in obtaining the database, Dr. Mario Paredes MSc, of the Catholic University in Guayaquil and Dr. José Moleón of the Granada University in Spain, Gregory Celis MD.MSC, Central University in Quito for helping us in the critical revision of the manuscript, all of them accepted to be named in this work.
Conflicts of interests
All authors have critically read this manuscript and have made their revisions and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and they have now all approved this final version for submission. All authors agree to be accountable for the future integrity of this study.
This material is original research has not been previously published and has not been submitted for publication elsewhere while under consideration. All authors of this original article have not conflict of interest to declare.
Conflict of interest disclosures
Not reported.
List of Abbreviations
HTMC: Hospital “Teodoro Maldonado Carbo”
Cream Topic: New therapeutic natural produc cream
CEISH: Ethics Committee on Human Subjects
PASI: Índice de evaluación del grado de severidad de las lesiones.
BSA:Area de superficie afectada
Cw6, B13, B17: Antígenos leucocitarios humanos
WHO World Health Organization
ICD-10 L40 the international classification of psoriasis
TEST SF-36V2 Feeling of well-being sum of subjective “feel-good” sensations.
SPSS-MODLER v.18: IBM® SPSS® Modeler 18.0
References
- Takagi Y, Shimizu M, Morokuma Y, Miyaki M, Kiba A, Matsuo K, Isoda K, Mizutani H A new formula for a mild body cleanser: sodium laureth sulphate supplemented with sodium laureth carboxylate and lauryl glucoside. Int J Cosmet Sci. 2014 ;36(4):305-311.
- Menter, A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016 Jun;22 (8 Suppl:s216-24.
- Raychaudhuri, SK; Maverakis, E; Raychaudhuri, SP (2014 Apr-May). «Diagnosis and classification of psoriasis». Autoimmun Rev 13 (4-5): 490-5.
- Chiricozzi A, Romanelli P, Volpe E, Borsellino G,Romanelli M. Scanning the Immunopathogenesis of Psoriasis. Int J Mol Sci. 2018;. https://doi.org/10.3390/ijms19010179. [CrossRef]
- Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, et al. The emerging role of IL-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J Invest Dermatol. 2012; 133:17-26.
- Flor García A., Martínez Valdivieso L,Menéndez Ramos F, Barrera Hernandez D,mejía Recuero M, Barrera Hernández D. Actualización en el tratamiento de la psoriasis. Boletín Farmaco-terapéutico de Castilla-La mancha. 2013. Vol XIV No1 1-9.
- Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PloS one 2012, 7(12): 5293.
- Marc Juliá Manresa, Juan Antonio Romero Moreno: Tratamientos tópicos de la psoriasis: actualización. Med Cutan Iber Lat Am 2005; 33(4); 147-157.
- Armario JC, Fernández Vozmediano JM. Actualización en el tratamiento para la psoriasis (XI). Nuevas perspectivas terapéuticas. Actualidad Dermatológica 2001; 40:637.
- Armario JC, Fernández Voz mediano JM. Actualización en el tratamiento para la psoriasis (XI). Nuevas perspectivas terapéuticas. Actualidad Dermatológica 2001; 40:637.
- Marc Juliá Manresa, Juan Antonio Romero Moreno: Tratamientos tópicos de la psoriasis: actualización. Med Cutan Iber Lat Am 2005; 33(4); 147-157.
- Armstrong AW, Bagel J, Van Voorhees AS, Robertson AD, Yamauchi PS.Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol 2015; 151: 432–438.
- Raychaudhuri SK, Maverakis E, Raychaudhuri SP Diagnosis and classification of psoriasis, Autoimmun Rev. 2014;13(4-5):490-495.
- Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-4.
- Cohen, B. E., Martires, K. J., y Roger. S.Psoriasis and the Risk of Depression in the US Population. National Health and Nutrition Examination Survey. JAMA Dermatology, 2012; 152, 73-70. https://medlineplus.gov/spanish/druginfo/meds/a607072-es.html.
- Teresa J Kelechi 1, Sally Stroud, The four ‘Vs’ for foot care. Vaseline, vegetable shortening, vinegar and Vicks VapoRub. Adv Nurse Practice. 2004 Jun;12(6):67-70,84. [PubMed,]. https://www.msdmanuals.com/es-ec/hogar/trastornos-de-la-piel/tratamiento-de-los-trastornos-cut%C3%A1neos/tratamiento-de-los-trastornos-cut% C3%A1neos.
- Aditya K Gupta 1, Karyn Nicol, The use of sulfur in dermatology Review J Drugs Dermatol . 2004 Jul-Aug;3(4):427-31 [PubMed,].
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol 2017; 177: 628–636.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).