Submitted:
19 April 2023
Posted:
20 April 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Pathophysiology
The Burden of 22q11.2 in Pediatrics
Clinical Symptomatology
Immune Deficiency and Immune Dysregulation
Diagnosis
Therapeutic Approach
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cortes-Martin J, Lopez Penuela N, Sanchez-Garcia JC, Montiel-Troya M, Diaz-Rodriguez L, Rodriguez-Blanque R. Deletion syndrome 22q11.2: a systematic review. Children. 2022, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Fomin ABF, Pastorino AC, Kim CA, Pereira AC, Carneiro-Sampaio M, Abe Jacob CM. DiGeorge syndrome: a not so rare disease. Clinics. 2010, 65, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Palmer LD, Butcher NJ, Boot E, Hodgkinson KA, Heung T, Chow EWC, et al. Elucidating the diagnostic odyssey of 22q11.2 deletion syndrome. Am J Med Genet. 2018, 176, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Kersseboom R, Brooks A, Weemaes C. Educational paper: Syndromic forms of primary immunodeficiency. Eur J Pediatr. 2011, 170, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Demaret Bardou ML, Teixeira Henriques M, Sevciovic Grumach A. Inborn errors of immunity associated with characteristic phenotypes. J Pediatr. 2021, 97, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Szczawińska-Popłonyk A, Begier K, Dorota A, Dąbrowska M, Gałecka D, Wawrzeniak K, Wróblewski K. Syndromic immunodeficiencies: a pediatrician’s perspective on selected diseases. Allergol Immunopathol. 2020, 49, 117–136. [Google Scholar]
- Hsu P, Ma A, Wilson G, Williams M, Curotta J, Munns CF, et al. CHARGE syndrome: a Review. J Paediatr Child Health. 2014, 50, 504–511.
- Trider CL, Arra-Robar A, van Ravensvaaij-Arts C, Blake K. Developing a CHARGE syndrome checklist: Health supervision across the lifespan (from head to toe). Am J Med. Genet. 2017, 173A, 684–691. [Google Scholar]
- Szczawińska-Popłonyk A, Popłonyk N, Niedziela M, Sowińska-Seidler A, Sztromwasser P, Jamsheer A, Obara-Moszyńska M. Case report: The cardio-facio-cutaneous syndrome due to a novel germline mutation in MAP2K1: a multifaceted disease with immunodeficiency and short stature. Front Pediatr. 2022, 10, 990111. [Google Scholar] [CrossRef]
- Pierpont ME, Magoulas PL, Adi S, Kavamura MI, Neri G, Noonan J, et al. Cardio-facio-cutaneous syndrome: clinical features, diagnosis and management guidelines. Pediatrics. 2014, 134, 1149–1162. [Google Scholar] [CrossRef]
- Bucciol G, Pillay B, Casas-Martin J, Delafontaine S, Proesmans M, Lorent N, et al. Systemic inflammation and myelofibrosis in a patient with Takenouchi-Kosaki syndrome due to CDC42 Tyr64Cys mutation. J Clin Immunol. 2020, 40, 567–570. [Google Scholar] [CrossRef]
- Martinelli S, Crumbach OHF, Pantaleoni F, Coppola S, Amin E, Pannone L, et al. Functional dysregulation of CDC42 causes diverse developmental phenotypes. Am J Hum Genet. 2018, 102, 309–320. [Google Scholar] [CrossRef]
- Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndrome. Lancet. 2007, 370, 1443–1452. [Google Scholar] [CrossRef]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2016, 1, 15071. [Google Scholar]
- Gavril EC, Popescu R, Nuca I, Ciobanu CG, Butnariu LI, Rusu C, et al. Different types of deletions created by low-copy repeats sequences location in 22q11.2 deletion syndrome: Genotype-phenotype correlation. Genes. 2022, 13, 2083. [Google Scholar] [CrossRef] [PubMed]
- Burnside, RD. 22q11.21 deletion syndromes: A Review of proximal, central, and distal deletions and their associated features. Cytogenet Genet Res 2015;146:89-99. [CrossRef]
- Funato, N. Craniofacial phenotypes and genetics of DiGeorge syndrome. J Dev Biol. 2022, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Gao S, Moreno M, Eliason S, Cao H, Li X, Yu W, et al. TBX1 protein interactions and microRNA-96-5 regulation controls cell proliferation during craniofacial and dental development: Implications for 22q11.2 deletion syndrome. Hum Mol Genet. 2015, 24, 2330–2348. [Google Scholar] [CrossRef] [PubMed]
- Du Q, de la Morena MT, Van Oers NSC. The genetics and epigenetics of 22q11.2 deletion syndrome. Front Genet. 2020, 10, 1365. [Google Scholar] [CrossRef]
- Rooney K, Levy MA, Haghshenas S, Kerkhof J, Rogaia D, Tedesco MG, et al. Identification of a DNA methylation episignature in the 22q11.2 deletion syndrome. Int J Mol Sci. 2021, 22, 8611. [Google Scholar] [CrossRef] [PubMed]
- Costain G, Chow EW, Silversides CK, Basset AS. Sex differences in reproductive fitness contribute to preferential maternal transmission of 22q11.2 deletions. J Med Genet. 2011, 48, 819–824. [Google Scholar] [CrossRef]
- Van L, Heung T, Graffi J, Ng E, Malecki S, Van Mil S, et al. All-cause mortality and survival in adults with 22q11.2 deletion syndrome. Genet Med. 2019, 21, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Palmer LD, McManus Z, Heung T, McAlpine G, Blagojevic C, Corral M, et al. Reproductive outcomes in adults with 22q11.2 deletion syndrome. Genes. 2022, 23, 2126. [Google Scholar]
- Kruszka P, Addissie YA, McGinn DE, Porras AR, Biggs E, Share M, et al. 22q11.2 deletion syndrome in diverse populations. Am J Med Genet. 2017, 173, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Mc Donald-McGinn, D. 22q11.2 deletion syndrome: a tiny piece leading to a big picture. Nat Rev Dis Primers. 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Jackson O, Crowley TB, Sharkus R, Smith R, Jeong S, Solot C, et al. Palatal evaluation and treatment in 22q11.2 deletion syndrome. Am J Med Genet A. 2019, 179, 1184–1195. [Google Scholar] [CrossRef]
- Seselgyte R, Swan MC, Birch MJ, Kangesu L. Velopharyngeal incompetence in children with 22q11.2 deletion syndrome: velar and pharyngeal dimensions. J Craniofac Surg. 2021, 32, 578–580. [Google Scholar] [CrossRef]
- Claynen I, Engchuan W, Hestand MS, Heung T, Holleman AM, Johnston HR, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q.11.2 deletion syndrome. Mol Psychiatry. 2021, 26, 4496–4510. [Google Scholar] [CrossRef]
- Van L, Boot E, Basset AS. Update on the 22q11.2 deletion syndrome and its relevance to schizophrenia. Curr Opin Psychiatry. 2017, 30, 191–196. [Google Scholar] [CrossRef]
- Putotto C, Pugnaloni F, Unolt M, Maiolo S, Trezzi M, Digilio MC, et al. 22q11.2 deletion syndrome: impact of genetics on the treatment of conotruncal heart defects. Children. 2022, 9, 722.
- Calcagni G, Pugnaloni F, Digilio MC, Unolt M, Putotto C, Niceta M, et al. Cardiac defects and genetic syndromes: old incertainities and new insights. Genes. 2021, 12, 1047. [Google Scholar] [CrossRef]
- Goldmuntz, E. 22q11.2 deletion syndrome and congenital heart disease. Am J Med Genet. 2020, 184, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Tan M, Wang X, Liu H, Peng X, Yang Y, Yu H, et al. Genetic diagnostic yield and novel causal genes of congenital heart disease. Front Genet. 2022, 13, 941364. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf E, Khalek N, Johnson MP, Gebb J, Coleman B, Crowley TB, et al. Expanding the fetal phenotype: prenatal sonographic findings and perinatal outcomes in a cohort of patients with a confirmed 22q11.2 deletion syndrome. Am J Med Genet. 2018, 176, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- McDonald R, Dodgen A, Goyal S, Gossett JM, Shinkawa T, Uppu S. C., et al. Impact of 22q11.2 deletion on the postoperative course of children after cardiac surgery. Pediatr Cardiol. 2013, 34, 341–347. [Google Scholar] [CrossRef]
- Cuturilo G, Drakulic D, Jovanovic I, Ilic S, Kalanj J, Vulicevic I, et al. The impact of 22q11.2 microdeletion on cardiac surgery postoperative outcome. Pediatr Cardiol. 2017, 38, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Lewyllie A, Roosenboom J, Indecleeft K, Claes P, Swillen A, Devriendt K, et al. A comprehensive craniofacial study of 22q11.2 deletion syndrome. J Dent Res. 2017, 96, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- AlQarbi MA, Alharbi A, Merdad L. Dental management of a patient with 22q11.2 deletion syndrome (22q11.2DS). BMJ Case Rep. 2018, 2018, bcr2018225765. [Google Scholar]
- Wong DH, Rajan S, Hallett KB, Manton DJ. Medical and dental characteristics of children with 22q11.2 deletion syndrome at the Royal Children’s Hospital, Melbourne. Int J Paediatr Dent. 2021, 31, 682–690. [Google Scholar] [CrossRef]
- Cardenas-Nieto D, Forero-Castro M, Esteban-Perez C, Martinez-Lozano J, Briceno-Balcazar I. The 22q11.2 microdeletion in pediatric patients with cleft lip, palate, or both and congenital heart disease: a systematic review. J Pediatr Genet. 2020, 9, 1–8.
- Kirschner RE, Baylis AL. Surgical considerations in 22q11.2 deletion syndrome. Clin Plast Surg. 2014, 41, 271–272. [Google Scholar] [CrossRef]
- Failla S, You P, Rajakumar C, Dworschak-Stokan A, Doyle PC, Husein M. Characteristics of velopharyngeal dysfunction in 22q11.2 deletion syndrome: a retrospective case-control study. J Otolaryngol Head Neck Surg. 2020, 49, 54. [Google Scholar] [CrossRef]
- Abe Y, Hirade T, Koike D, Matama C, Kato F. Laryngeal web with 22q11.2 deletion syndrome. Int J Pediatr Adolesc Med. 2022, 9, 182–184. [Google Scholar] [CrossRef]
- Komasińska P, Szczawińska-Popłonyk A, Jończyk-Potoczna K, Bręborowicz A. Congenital atresia of the larynx and esophagus in a girl with 22q11.2 deletion—a case report. Polish J Pediatr. 2017, 92, 335–341. [Google Scholar]
- Hankey PB, Ghulmiyyah J, Yeh HW, Tracey N, Arganbright J. Airway anomalies in patients with 22q11.2 deletion syndrome: a scoping review. Int J Pediatr Otorhinolaryngol. 2022, 163, 11373.
- Verheij E, Speleman L, Mink van der Molen AB, Thomeer HGXM. Congenital respiratory tract disorders in 22q11.2 deletion syndrome. Int J Pediatr Otorhinolaryngol. 2018, 104, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Huang RY, Shapiro NL. Structural airway anomalies in patients with DiGeorge syndrome: a current review. Am J Otolaryngol. 2000, 21, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Ebert B, Morrell N, Zavala H, Chinnadurai S, Tibesar R, Barnett Roby B. Percutaneous enteral feeding in patients with 22q11.2 deletion syndrome. Cleft Palate Craniofac. 2022, 59, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Unolt M, Versacci P, Anaclerio S, Lambiase C, Calgagni G, Trezzi M, et al. Congenital heart diseases and cardiovascular abnormalities in 22q11.2 deletion syndrome: From well-established knowledge to new frontiers. Am J Med Genet. 2018, 176, 2087–2098.
- Yi JJ, Tang SX, McDonald-McGinn DM, Calkins ME, Whinna DA, Souders MC, et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet 2014, 165B, 137–147.
- Rayannavat A, Levitt Katz LE, Crowley TB, Lessig M, Grand K, Goldmuntz E, et al. Association of hypocalcemia with congenital heart disease in 22q11.2 deletion syndrome. Am J Med. Genet A. 2018, 176, 2099–2103. [Google Scholar] [CrossRef]
- Zhao Y, Diacou A, Johnston HR, Mousfee FI, McDonald-McGinn DM, McGinn D, et al. Complete sequence of the 22q11.2 allele in 1,053 subjects with 22q11.2 deletion syndrome reveals modifiers of conotruncal heart defects. Am J Hum Genet. 2020, 106, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Mlynarski EE, Xie M, Taylor D, Sheridan MB, Guo T, Racedo SE, et al. Rare copy number variants and congenital heart defects in the 22q11.2 deletion syndrome. Hum Genet. 2016, 135, 273–285. [Google Scholar] [CrossRef]
- Quach TT, Stratton HS, Khanna R, Kolattukudy PE, Honorrat J, Meyer K, et al. Intellectual disability: dendritic anomalies and emerging genetic perspectives. Acta Neuropathol. 2021, 141, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Hopkins SE, Cadehumbe M, Blaine Crowley T, Zackai EH, Bilaniuk LT, McDonald-McGinnDM, et al. Neurologic challenges in 22q11.2 deletion syndrome. Am J Med Genet A. 2018, 176, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Bagautdinova J, Zoller D, Schaer M, Padula MC, Mancini V, Schneider M, et al. Altered cortical thickness development in 22q11.2 deletion syndrome and association with psychotic symptoms. Mol Psychiatry. 2021, 26, 7671–7678. [Google Scholar] [CrossRef] [PubMed]
- Mudigoudar B, Nune S, Fulton S, Dayyat E, Wheeless JW. Epilepsy in 22q11.2 deletion syndrome: a case series and literature review. Pediatr Neurol. 2017, 76, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bayat M, Bayat A. Neurologic manifestations of 22q11.2 deletion syndrome. Neurol Sci. 2022, 43, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Osley O, Evans NA, Fernandes-Carriba S, Smearman EL, Rockers K, Morrier MJ, et al. Examining the overlap between autism spectrum disorders and 22q11.2 deletion syndrome. Int J Mol Sci. 2017, 18, 1071. [Google Scholar] [CrossRef]
- Fiksinski AM, Schneider M, Zinstok J, Baribeau D, Chawner SJRA, Vorstman JAS. Neurodevelomental trajectories and psychiatric morbidity: lessons learned from the 22q11.2 deletion syndrome. Curr Psychiatry Rep. 2021, 23, 13. [Google Scholar] [CrossRef]
- Swillen, A. The importance of understanding cognitive trajectories: the case of 22q11.2 deletion syndrome. Curr Opin Psychiatry. 2016, 29, 133–137. [Google Scholar] [CrossRef]
- Biria M, Tomescu MI, Custo A, Cantonas LM, Song KW, Schneider M, et al. Visual processing deficits in 22q11.2 deletion syndrome. Neuroimage Clin. 2017, 17, 976–986. [Google Scholar]
- Francisco AA, Foxe JJ, Horsthuis DJ, DeMaio D, Molholm S. Assessing auditory processing endophenotypes associated with schizophrenia in individuals with 22q11.2 deletion syndrome. Transl Psychiatry. 2020, 10, 85. [Google Scholar] [CrossRef]
- Casteels I, Casaer P, Gewillig M, Swillen A, Devriendt K. Ocular findings in children with a microdeletion in chromosome 22q11.2. Eur J Pediatr. 2008, 167, 751–755. [Google Scholar] [CrossRef]
- Gokturk G, Topcu-Yilmaz P, Bozkurt B, Yildrim MS, Guner SN, Sayar EH, et al. Ocular findings in children with 22q11.2 deletion syndrome. J Pediatr Ophthalmol Strabismus. 2016, 53, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Levy-Shraga Y, Gothelf D, Goichberg Z, Katz U, Somech R, Pinhas-Hamiel O, et al. Growth characteristics and endocrine abnormalities in 22q11.2 deletion syndrome. Am J Med Genet. 2017, 9999, 1–7. [Google Scholar]
- Choi JH, Shin YL, Kim GH, Seo EJ, Kim Y, Park IS, et al. Endocrine manifestations of chromosome 22q11.2 microdeletion syndrome. Horm Res. 2005, 63, 294–299. [Google Scholar]
- Cheung ENM, George SR, Costain GA, Andrade DM, Chow EWC, Silversides CK, et al. Prevalence of hypocalcemia and its associated features in 22q11.2 deletion syndrome. Clin Endocrinol. 2014, 81, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Cheung EN, George SR, Andrade DM, Chow EW, Silversides CK, Bassett AS. Neonatal hypocalcemia, neonatal seizures and intellectual disability in 22q11.2 deletion syndrome. Genet Med. 2014, 16, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Arganbright JM, Tracy M, Feldt M, Narayanan S, Mahadev A, Noel-McDonnell J. Postoperative hypocalcemia following non-cardiac surgical procedures in children with 22q11.2 deletion syndrome. Genes. 2022, 13, 1905. [Google Scholar] [CrossRef]
- Jang C, Ge J, Zhang R, Chen C, Yi L, Shen L, et al. The correlation between severity of postoperative hypocalcemia and perioperative mortality in chromosome 22q11.2 microdeletion (22q11.2DS) patient after cardiac-correction surgery: a retrospective analysis. Heart Surg Forum. 2022, 23, 549–555. [Google Scholar]
- Fujii S, Nakanishi T. Clinical manifestations and frequency of hypocalcemia in 22q11.2 deletion syndrome. Pediatr Int. 2015, 57, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi EM, Kanaka-Gantenbaum C. Autoimmune thyroide disease in specific genetic syndromes in childhood and adolescence. Front Endocrinol. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Ricci S, Sarli WA, Lodi L, Canessa C, Lippi F, Azzari C, et al. Characterization of autoimmune thyroid disease in cohort of 73 pediatric patients affected by 22q11.2 deletion syndrome: longitudinal single-centre study. Genes. 2022, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Shugar AI, Shapiro JM, Cytrynbaum C, Hedges S, Weksberg R, Fishman L. An increased prevalence of thyroid disease in children with 22q11.2 deletion syndrome. Am J Med Genet A. 2015, 167, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Ueda Y, Uraki S, Inaba H, Nakashima S, Ariyasu H, Iwakura H, et al. Grave’s disease in pediatric and elederly patients with 22q11.2 deletion syndrome. Intern Med. 2017, 56, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Brown JJ, Datta V, Browning MJ, Swift PGF. Graves’ disease in DiGeorge syndrome: patient report with a review of endocrine autoimmunity associated with 22q.11.2 deletion. J Pediatr Endocrinol Metab. 2004, 17, 1575–1579. [Google Scholar]
- Stagi S, Lapi E, Gambineri E, Salti R, Genuardi M, Colarusso G, et al. Thyroid function and morphology in subjects with microdeletion of chromosome 22q11 (del(22)q11). Clin Endocrinol. 2010, 72, 839–844. [Google Scholar] [CrossRef]
- Elder DA, Kaiser-Rogers K, Aylsworth AS, Calikoglu AS. Type I diabetes mellitus in a patient with 22q11.2 deletion syndrome. Am J Med. Genet. 2001, 101, 17–19. [Google Scholar] [CrossRef]
- Lima K, Abrahamsen TG, Wolff AB, Husebye E, Alimohammadi M, Kampe O, et al. Hypoparathyroidism and autoimmunity in the 22q11.2 deletion syndrome. Eur J Endocrinol. 2011, 165, 345–352. [Google Scholar] [CrossRef]
- Van L, Heung T, Malecki SL, Fenn C, Tyrer A, Sanches R, et al. 22q11.2 microdeletion and risk for type 2 diabetes. EClinicalMedicine. 2020, 26, 100528. [Google Scholar] [CrossRef]
- Blagojevic C, Heung T, Malecki S, Ying C, Cancelliere S, Hegele RA, et al. Hypertriglyceridemia in young adults with a 22q11.2 microdeletion. Eur J Endocrinol. 2022, 187, 91–99. [Google Scholar] [CrossRef]
- Voll SL, Boot E, Butcher NJ, Cooper S, Heung T, Chow EWC, et al. Obesity in adults with 22q11.2 deletion syndrome. Genet Med. 2017, 19, 204–208. [Google Scholar] [CrossRef]
- Sullivan, KE. Chromosome 22q11.2 deletion syndrome and DiGeorge syndrome. Immunol Rev. 2019, 287, 186–201. [Google Scholar] [CrossRef]
- Gennery, AR. Immunological aspects of 22q11.2 deletion syndrome. Cell Mol Life Sci. 2012, 69, 17–27. [Google Scholar] [CrossRef]
- Dar N, Gothlef D, Korn D, Frisch A, Weizman A, Michaelovsky E, et al. Thymic and bone marrow output in individuals with 22q11.2 deletion syndrome. Pediatr Res. 2015, 77, 579–585. [Google Scholar] [CrossRef]
- Gul KA, Overland T, Osnes L, Baumbusch LO, Pettersen RD, Lima K, et al. Neonatal levels of T-cell receptor excision circles (TREC) in patients with 22q11.2 deletion syndrome and later disease features. J Clin Immunol. 2015, 35, 408–415. [Google Scholar] [CrossRef]
- Collins C, Sharpe E, Silber A, Kulke S, Hsieh EWY. Congenital athymia: genetic etiologies, clinical manifestations, diagnosis, and treatment. J Clin Immunol. 2021, 41, 881–895. [Google Scholar] [CrossRef]
- Guris DI, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell. 2006, 10, 81–92. [Google Scholar] [CrossRef]
- Shah SS, Lai SY, Ruchelli E, Kazahaya K, Mahboubi S. Retropharyngeal aberrant thymus. Pediatrics. 2001, 108, 94. [Google Scholar] [CrossRef]
- Grudzień K, Kuzaj J, Dębicka M, Kwiatkowski S, Milczarek O. Retropharyngeal ectopic thymus in a pediatric patient with 22q11.2 deletion syndrome. Cureus. 2023, 15, e33350. [Google Scholar]
- Crowley B, Ruffner M, McDonald-Mc Ginn DM, Sullivan KE. Variable immune deficiency related to deletion size in chromosome 22q11.2 deletion syndrome. Am J Med Genet. 2018, 176, 2082–2086. [Google Scholar] [CrossRef]
- Giardino G, Borzacchiello C, De Luca M, Romano R, Prencipe R, Cirillo E, et al. T-cell immunodeficiencies with congenital alterations of thymic development: genes implicated and differential immunological and clinical features. Front Immunol. 2020, 11, 1837. [Google Scholar] [CrossRef]
- Davies, EG. Immunodeficiency in DiGeorge syndrome and options for treating cases with complete athymia. Front Immunol. 2013, 4, 322. [Google Scholar] [CrossRef]
- Patel K, Akhter J, Kobrynski L, Gathmann MA, Davis O, Sullivan KE. Immunoglobulin deficiencies: the B lymphocyte side of DiGeorge syndrome. J Pediatr. 2012, 161, 950–953. [Google Scholar] [CrossRef]
- Zemble R, Luning Prak E, McDonald K, McDonald-McGinn D, Zackai E, Sullivan K. Secondary immunologic consequences in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/Velocardiofacial syndrome). Clin Immunol. 2010, 136, 409–418. [Google Scholar] [CrossRef]
- Derfalvi B, Maurer K, McDonald-McGinn DM, Zackai E, Meng W, Luning Prak ET, et al. B cell development in chromosome 22q11.2 deletion syndrome. Clin Immunol. 2016, 163, 1–9. [Google Scholar] [CrossRef]
- Montin D, Marolda A, Licciardi F, Robasto F, Di Cesare S, Ricotti E, et al. Immunophenotype anomalies predict the development of autoimmune cytopenia in 22q11.2 deletion syndrome. J Allergy Clin Immunol Pract. 2019, 7, 2369–2376. [Google Scholar] [CrossRef]
- Wahrmann S, Kainulainen L, Kyto V, Lempainen J. Childhood manifestations of 22q11.2 deletion syndrome: a Finnish nationwide register-based cohort study. Acta Paediatr. 2023. epub ahead of print. [CrossRef]
- Di Cesare S, Puliafito P, Ariganello P, Marcovecchio GE, Mandolesi M, Capolino S, et al. Autoimmunity and regulatory T cells in 22q11.2 deletion syndrome patients. Pediatr Allergy Immunol. 2015, 26, 578–594. [Google Scholar]
- Fernando-Martinez S, Lorente R, Gurbindo D, De Jose MA, Leal M, Munoz-Fernandez MA, et al. Low thymic output, peripheral homeostatsis deregulation, and hastened regulatory T cells differentiation in children with 22q11.2 deletion syndrome. J Pediatr. 2014, 164, 882–889. [Google Scholar] [CrossRef]
- Montin D, Marolda A, Licciardi F, Robasto F, Di Cesare S, Ricotti E. Immunophenotype anomalies predict the development of autoimmune cytopenia in 22q11.2 deletion syndrome. J Allergy Clin Immunol Pract. 2019, 7, 2369–2376. [Google Scholar] [CrossRef]
- Pinnaro CT, Henry T, Major HJ, Parida M, DesJardin LE, Manak JR, et al. Candidate modifier genes for immune function in 22q11.2 deletion syndrome. Mol Genet Genomic Med. 2020, 8, e1057. [Google Scholar] [CrossRef]
- Oliveira LM, Teixeira FME, Sato MN. Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflamm. 2018, 2018, 3067126. [Google Scholar]
- Cancrini C, Puliafito P, Digilio MC, Soresina A, Martino S, Rondelli R, Consolini R, et al. Clinical features and follow-up in patients with 22q11.2 deletion syndrome. J Pediatr. 2014, 164, 1475–1480. [Google Scholar] [CrossRef]
- Deshpande DR, Demirdag YY, Marsh RA, Sullivan KE, Orange JS. Relationship between severity of T cell lymphopenia and immune dysregulation in patients with DiGeorge syndrome (22q11.2 deletion and/or related TBX1 mutations: a USIDNET study. J Clin Immunol. 2021, 41, 29–37. [Google Scholar] [CrossRef]
- Mahe P, Nagot N, Portales P, Lozano C, Vincent T, Sarda P, et al. Risk factors of clinical dysimmune manifestations in a cohort of 86 children with 22q11.2 deletion syndrome: a retrospective study in France. Am J Med Genet. 2019, 179A, 2207–2213. [Google Scholar]
- Ciano-Petersen NL, Hamad-Cueto O, Drissi-Reyes H, Dona-Diaz A, Garcia-Martin G. Case report: Autoimmune psychosis in 22q11.2 deletion syndrome. Front Immunol. 2021, 12, 708625. [Google Scholar] [CrossRef]
- Jesenak M, Zelieskova M, Repko M, Banovcin P. Successful treatment of severe allergic asthma with omalizumab in a girl with DiGeorge syndrome. Centr Eur J Immunol. 2020, 45, 361–363. [Google Scholar] [CrossRef]
- Morsheimer M, Brown Whitehorn TF, Heimall J, Sullivan KE. The immune deficiency of chromosome 22q11.2 deletion syndrome. Am J Med Genet. 2017, 9999, 1–7. [Google Scholar]
- Maggadottir SM, Sullivan KE. The diverse clinical features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome). J Allergy Clin Immunol Pract. 2013, 1, 589–594. [Google Scholar] [CrossRef]
- Stevens T, van der Werff Ten Bosch J, De Rademaeker M, Van Den Bogaert A, van den Akker M. Risk of malignancy in 22q11.2 deletion syndrome. Clin Case Rep. 2017, 5, 486–490. [Google Scholar] [CrossRef]
- Itoh S, Ohno T, Kakizaki S, Ichinohasama R. Epstein-Barr virus-positive T-cell lymphoma cells having chromosome 22q11.2 deletion: an autopsy report of DiGeorge syndrome. Hum Patol. 2011, 42, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Hong R, Shen V, Rooney C, Hughes DP, Smith C, Comoli P, et al. Correction od DiGeorge anomaly with EBV-induced lymphoma by transplantation of organ-cultured thymus and Epstein Barr-specific cytotixic T lymphocytes. Clin Immunol. 2001, 91, 54–61. [Google Scholar]
- Pongpruttipan T, Cook JR, Reyes-Mugica M, Spahr E, Swerdlow SH. Pulmonary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue associated with granulomatous inflammation in a child with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome). J Pediatr. 2012, 161, 954–958. [Google Scholar] [CrossRef] [PubMed]
- McDonald-McGinn DM, Reilly A, Wallgren-Pettersson C, Hoyme HE, Yang SP, Adam MP, et al. Malignancy in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Am J Med Genet A. 2006, 140, 906–909. [Google Scholar]
- Murray JC, Donahue DJ, Malik SI, Dzurik YB, Braly EZ, Dougherty MJ, et al. Temporal lobe pleomorphic xanthoastrocytoma and acquired BRAF mutation in an adolescent with the constitutional 22q11.2 deletion syndrome. J Neurooncol. 2011, 102, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Digilio MC, Angioni A, De Santis M, Lombardo A, Giannotti A, Dallapicola B, et al. Spectrum of clinical variability in familial deletion 22q11.2: from full manifestation to extremely mild clinical anomalies. Clin Genet. 2003, 63, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Karbarz, M. Consequences of 22q11.2 microdeletion on the genome, individual and population level. Genes. 2020, 11, 977. [Google Scholar] [CrossRef]
- Motahari Z, Moody SA, Maynard TM, LaMantia AS. In the line-up: deleted genes associated with DiGeorge/22q11.2 deletion syndrome: are they all suspects? J Neurodev Disord. 2019, 11, 7. [Google Scholar] [CrossRef]
- Giacomelli M, Kumar R, Soresina A, Tamassia N, Lorenzini T, Moratto D, et al. Reduction of CRKL expression in patients with partial DiGeorege syndrome is associated with impairment of T cell functions. J Allergy Clin Immunol. 2016, 138, 229–240. [Google Scholar] [CrossRef]
- Guo T, Chung JH, Wang T, McDonald-McGinn DM, Kates WR, Hawuła W, et al. Histone modifier genes alter conotruncal heart phenotypes in 22q11.2 deletion syndrome. Am J Hum Genet. 2015, 97, 869–877. [Google Scholar] [CrossRef]
- Leon LE, Benavides F, Espinoza K, Vial C, Alvarez P, Palomares M, et al. Partial microduplication in the histone acetyltransferase complex member KANSL1 is associated with congenital heart defects in 22q11.2 microdeletion syndrome patients. Nature. 2017, 7, 1795. [Google Scholar]
- Mlynarski EE, Sheridan MB, Xie M, Guo T, Racedo SE, McDonald-McGinn DM, et al. Copy-number variation of the glucose transporter gene SLC2A3 and congenital heart defects in the 22q11.2 deletion syndrome. Am J Hum Genet. 2015, 96, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Halder A, Jain M, Chaudhary I, Gupta N, Kabra M. Fluorescence in situ hybridization (FISH) using non-commercial probes in the diagnosis of clically suspected microdeletion syndromes. Indian J Med Res. 2013, 138, 135–142. [Google Scholar]
- Maran S, Faten SA, Lim SHE, Lai KS, Ibrahim WPW, Ankathil R, et al. Screening of 22q11.2 DS using multiplex ligation as alternative diagnostic method. Biomed Res Int. 2020, 6945730. [Google Scholar]
- Monteiro FP, Vieira TP, Sgardioli IC, Molck MC, Damiano AP, Souza J, et al. Defining new guidelines for screening the 22q11.2 deletion based on a clinical and dysmorphologic evaluation of 194 individuals and review of the literature. Eur J Pediatr. 2013, 172, 927–945. [Google Scholar] [CrossRef] [PubMed]
- Barry JC, Blaine Crowley T, Jyonouchi S, Heimall J, Zackai EH, Sullivan KE, et al. Identification of 22q11.2 deletion syndrome via newborn screening for severe combined immunodeficiency. J Clin Immunol. 2017, 37, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf E, Khalek N, Johnson MP, Gebb J, Coleman B, Crowlet TB, et al. Expanding the fetal phenotype: prenatal sonographic findings and perinatal outcomes in a cohort of patients with a confirmed 22q11.2 deletion syndrome. Am J Med. Genet A. 2018, 176, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Grati FR, Gross SJ. Noninvasive screening by cell-free DNA for 22q11.2 deletion: benefits, limitations, and challenges. Prenat Diagn. 2019, 39, 70–80. [Google Scholar] [CrossRef]
- Bevilacqua E, Jani CC, Chaoui R, Suk EKA, Palma-Dias R, Ko TM, et al. Performance of targeted cell-free DNA prenatal test for 22q11.2 deletion in a large clinical cohort. Ultrasound Obstet Gynecol. 2021, 58, 597–602. [Google Scholar] [CrossRef]
- Dar P, Jacobsson B, Clifton R, Egbert M, Malone F, Wapner RJ, et al. Cell-free DNA screening for prenatal detection of 22q11.2 deletion syndrome. Am J Obstet Gynecol. 2022, 227, 79. [Google Scholar]
- Kagan KO, Hoopmann M, Pfaff T, Prodan N, Wagner P, Schmid M, et al. First trimester screening for common trisomies and microdeletion 22q11.2 syndrome using cell-free DNA: a prospective clinical study. Fetal Diagn Ther. 2020, 47, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Biggs SE, Gilchrist B, May KR. Chromosome 22q11.2 deletion (DiGeorge syndrome): immunologic features, diagnosis, and management. Curr Allergy Asthma Rep. 2023, 23, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Davies, EG. Immunodeficiency in DiGeorge syndrome and options for treating cases with complete athymia. Front Immunol. 2013, 4, 322. [Google Scholar] [CrossRef] [PubMed]
- Davies EG, Cheung M, Gilmour K, Maimaris J, Curry J, Furmanski A, et al. Thymus transplantation for complete DiGeorge syndrome: European experience. J Allergy Clin Immunol. 2017, 140, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Caka C, Cimen O, Kahyaoglu, Tezcan I, Cagdas D. Selective IgM deficiency: follow-up and outcome. Pediatr Allergy Immunol. 2021, 32, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Kung SJ, Gripp KW, Stephan MJ, Fairchok MP, McGeady SJ. Selective IgM deficiency and 22q11.2 deletion syndrome. Ann Allergy Asthma Immunol. 2007, 99, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ozen S, Akcal O, Taskirdi I, Haci AI, Karaca NE, et al. 22q11.2 deletion syndrome: 20 years of experience from two pediatric immunology units and review of clues for diagnosis and disease management. Allergol Immunopathol. 2021, 49, 95–100. [Google Scholar] [CrossRef]
- Nissan E, Katz U, Levy-Shraga Y, Frizinsky S, Carmel E, Gothelf D, et al. Clinical features in a large cohort of patients with 22q11.2 deletion syndrome. J Pediatr. 2021, 238, 215–220. [Google Scholar] [CrossRef]
- Habel A, Herriot R, Kumararatne D, Allgrove J, Baker K, Baxendale H, et al. Toward a safety net for management of 22q11.2 deletion syndrome: guidelines for our times. Eur J Pediatr. 2014, 173, 757–765.
- Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013, 1, 573–582. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk A, Bręborowicz A, Samara H, Ossowska L, Dworacki G. Impaired antigen-specific immune response to vaccines in children with antibody production defects. Clin Vaccine Immunol. 2015, 22, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter AM, Jakob K, Klein NP. , Dekker CL, Edwards KM, Halsey NA, et al. Live vaccine use and safety in DiGeorge syndrome. Pediatrics. 2014, 13, 946–954. [Google Scholar]
- Lim SM, Shin JH, Baek JY, Lee JY, Kang JM, Ahn JG. Safety of live immunization in DiGeorge syndrome: a retrospective single-center study in Korea, 2005-2021. Vaccines. 2022, 10, 2163. [Google Scholar]
- Berkhout A, Preece K, Varghese V, Prasad V, Heussler H, Clark J, et al. Optimising immunisation in children with 22q11 microdeletion. Ther Adv Vaccines Immunother. 2020, 8, 2515135520957139. [Google Scholar]
- Morrow B, McDonald-McGinn DM, Emanuel BS, Vermeesch JR, Scambler PJ. Molecular Genetics of 22.q11.2 deletion syndrome. Am J Med Genet. 2018, 176, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Mustillo PJ, Sullivan KE, Chinn IK, Notarangelo LD, Haddad E, Davies EG, et al. Clinical practice guidelines for the immunological management of chromosome 22q11.2 deletion syndrome and other defects in thymic development. J Clin Immunol. 2023, 43, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Cohen JL, Crowley TB, McGinn D, McDougall C, Unolt M, Lambert MC, et al. 22q and two: 22q11.2 deletion syndrome and coexisting conditions. Am J Med Genet. 2018, 176, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- McGovern PE, Crowley TB, Zackai EH, Burrows E, McDonald-McGinn DM, Nance ML. Surgical insights and management in patients with 22q11.2 deletion syndrome. Pediatr Surg Int. 2022, 38, 899–905. [Google Scholar] [CrossRef]
- Abu-Ghname A, Perdanasari AT, Raj S, Seema J, Wilson KT, Maricevich RS. Access to multidisciplinary care for patients with 22q11.2 deletion syndrome: Identifying breakdowns in the screening process. J Craniofac Surg. 2020, 31, 428–431. [Google Scholar] [CrossRef]
- Boot E, Oskarsdottir S, Loo JCY, Crowley TB, Orchanian-Cheff A, Andrade DM, et al. Updated clinical practice recommendations for managing adults with 22q11.2 deletion syndrome. Genet Med. 2023, 25, 100344. [Google Scholar] [CrossRef]
| Systemic involvement | Phenotypic features | Frequency in 22q11.2 DS |
|---|---|---|
| Facial dysmorphism | Elongated face Hooded eyelids Upslanted palpebral fissures Epicanthus Wide nasal bridge Long nose with a bulbous tip Narrow alar base Short philtrum Small mouth Micrognathia and retrognathia Low-set small ears |
80-99% |
| Ocular findings | Posterior embryotoxon Tortous retinal vessels Refractive errors Strabismus Amblyopia |
7-70% |
| Dentition | Delayed teeth eruption Agenesis of permanent dentition Supernumerary teeth Enamel hypoplasia Impaired enamel calcification |
2.5% |
| Palatal anomalies | Velopharyngeal insufficiency and hypotonia Cleft palate Submucous cleft palate Bifid uvula |
69-100% |
| Laryngeal anomalies | Glottic web Laryngeal stenosis Laryngeal cleft Laryngomalacia Vocal fold anomalies |
25-43% |
| Lower airway anomalies | Tracheo and bronchomalacia Tracheal stenosis Short trachea with reduced tracheal rings Aberrant tracheal bronchus Tracheoesophageal fistula |
21% |
| Cardiovascular anomalies | Interrupted aortic arch type B Truncus arteriosus Tetrealogy of Fallot Conoventricular septal defect Isolated aortic arch anomaly Double outlet right ventricle Transposision of the great arteries Hypoplastic left heart syndrome Valvar pulmonary stenosis |
49-83% |
| Genitourinary anomalies | Renal agenesis Multicystic dysplastic kidney Hydronephrosis Duplicated collecting system Absent uterus Hypospadias, cryptorchidism |
33% |
| Gastrointestinal anomalies | Gastroesophageal reflux Esophageal atresia Impaired swallowing Hirschprung disease Imperforate anus |
30% |
| Central nervous system anomalies | Cerebral atrophy Polymicrogyria Atrophy of the hippocampus Cerebellar atrophy |
8% |
| Endocrine anomalies | Thyroid gland aplasia/hypoplasia Retrocarotid and retroesphageal thyroid extension Inhomogeneous thyroid structure Parathyroid gland dysfunction Growth hormone deficiency |
65% |
| Skeletal and muscular anomalies | Cervical spine anomalies Thoracic vertebral anomalies Arachnodactyly, Camptodactyly, Syndactyly Hammer toes Skull malformations Diaphragmatic hernia |
17-19% |
| Immune disorders | Athymia Thymic hypoplasia Ectopic thymus |
75% |
| Genetic modifier | Role | Phenotypic expression |
|---|---|---|
|
CRKL (CRK like proto-oncogene adaptor protein) |
Activates the RAS and JUN kinase signaling pathways, mediates transduction of intracellular signals | Development of organs originating from the neural crest, thymus, parathyroid glands, craniofacial structures, T lymphocytes, cardiac outflow region |
|
SLC2A3 aka GLUT3 (Solute carrier family 2 member 3) |
Facilitated glucose transporter | Conotruncal heart region, aortic arch |
|
KANSL1 (KAT8 regulatory NSL complex subunit 1) |
Histone acetyltransferase complex member | Developmment of aortic arch, semilunar valve, cardiac septa, pulmonary artery |
|
JMJD1C (jumonji domain containing 1C) |
Chromatin expression modification, histone demethylation | Pharyngeal apparatus, cardiac outflow region |
|
RREB1 (Ras responsive element binding protein 1) |
Chromatin expression modification, histone demethylation | Conotruncal heart region |
|
SEC24C (SEC24 family member C) |
Role in transporting proteins from the endoplasmic reticulum to the Golgi apparatus | Embryonic development, cardiac outflow region |
|
MINA (MYC induced nuclear antigen) |
Chromatin expression modification, histone demethylation | Cardiac development |
|
KDM7A (Lysine-specific demethylase 7A) |
Chromatin expression modification, histone demethylation | Cardiac development |
|
DGCR 8 (DiGeorge syndrome critical region 8) |
miRNAs and lnRNAs regulation | Embryo development, Immune, naurological, and cardiac functions |
|
NCOR2 (Nuclear receptor corepressor 2) |
Transcriptional regulator of the retinoic acid signaling | B and T lymphocytes, regulation of the immune response |
|
EP300 (E1A binding protein P300) |
Transcriptional regulator of the retinoic acid signaling | B and T lymphocytes, regulation of the immune response |
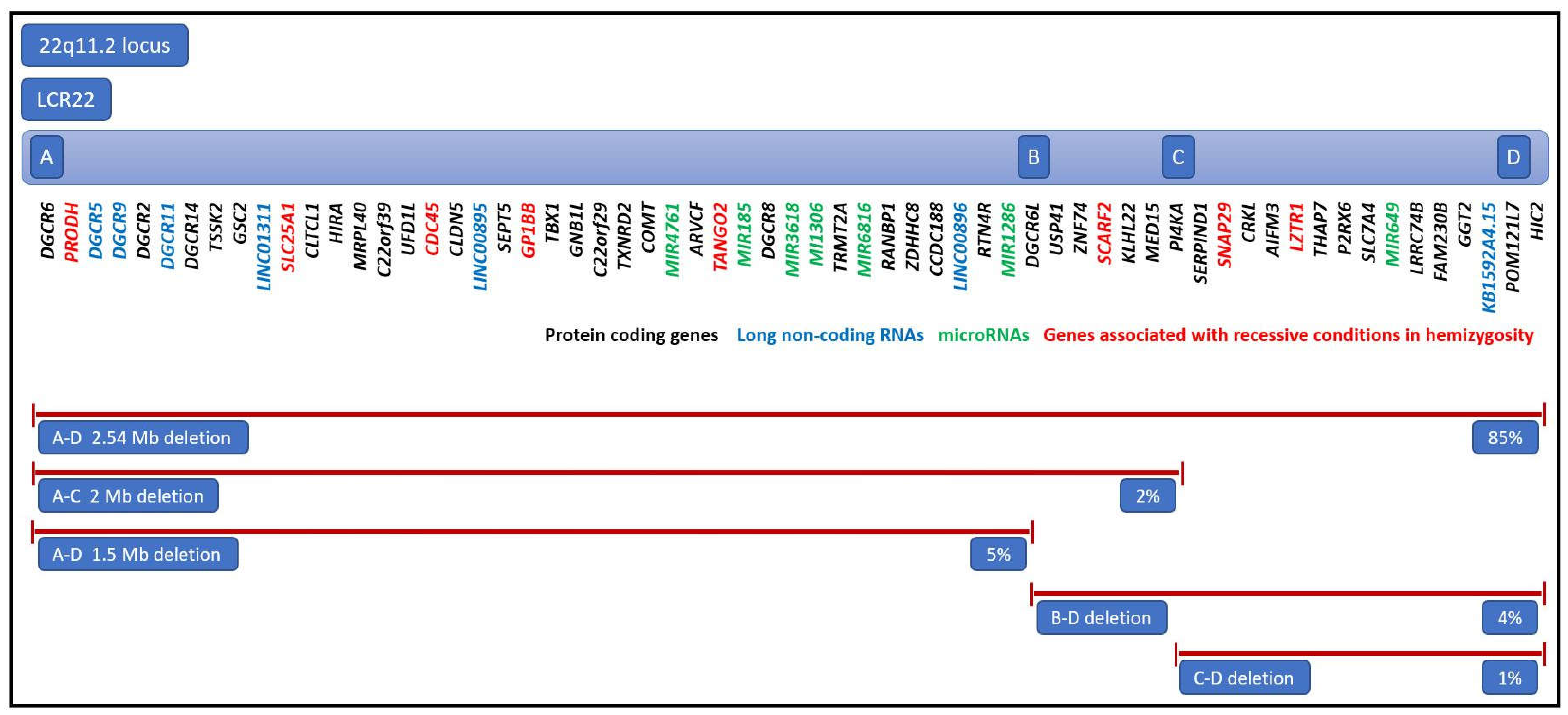
| Gene | Disease | OMIM# | Phenotype |
|---|---|---|---|
| PRODH | Hyperprolinemia type 1 | 239500 | General: neurological deficits Specific: psychomotor delay, hypotonia, and seizures |
| SLC25A1 | D2A2AD syndrome | 615182 | General: severe muscular weakness, respiratory distress, failed psychomotor development, early death Specific: encephalopathy, seizures |
| GP1BB | Bernard-Soulier syndrome | 231200 | Specific: hematologic disease, thrombocytopenia, increased megakaryocytes |
| SCARF2 | Van den Ende-Gupta syndrome | 600920 | General: joint dislocations Specific: contractual arachnodactyly, hooked clavicles, and blepharophimosis |
| SNAP29 | CEDNIK syndrome | 609528 | General: neuropathy Specific: cerebral dysgenesis, ichtyosis, and keratoderma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




