Submitted:
21 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
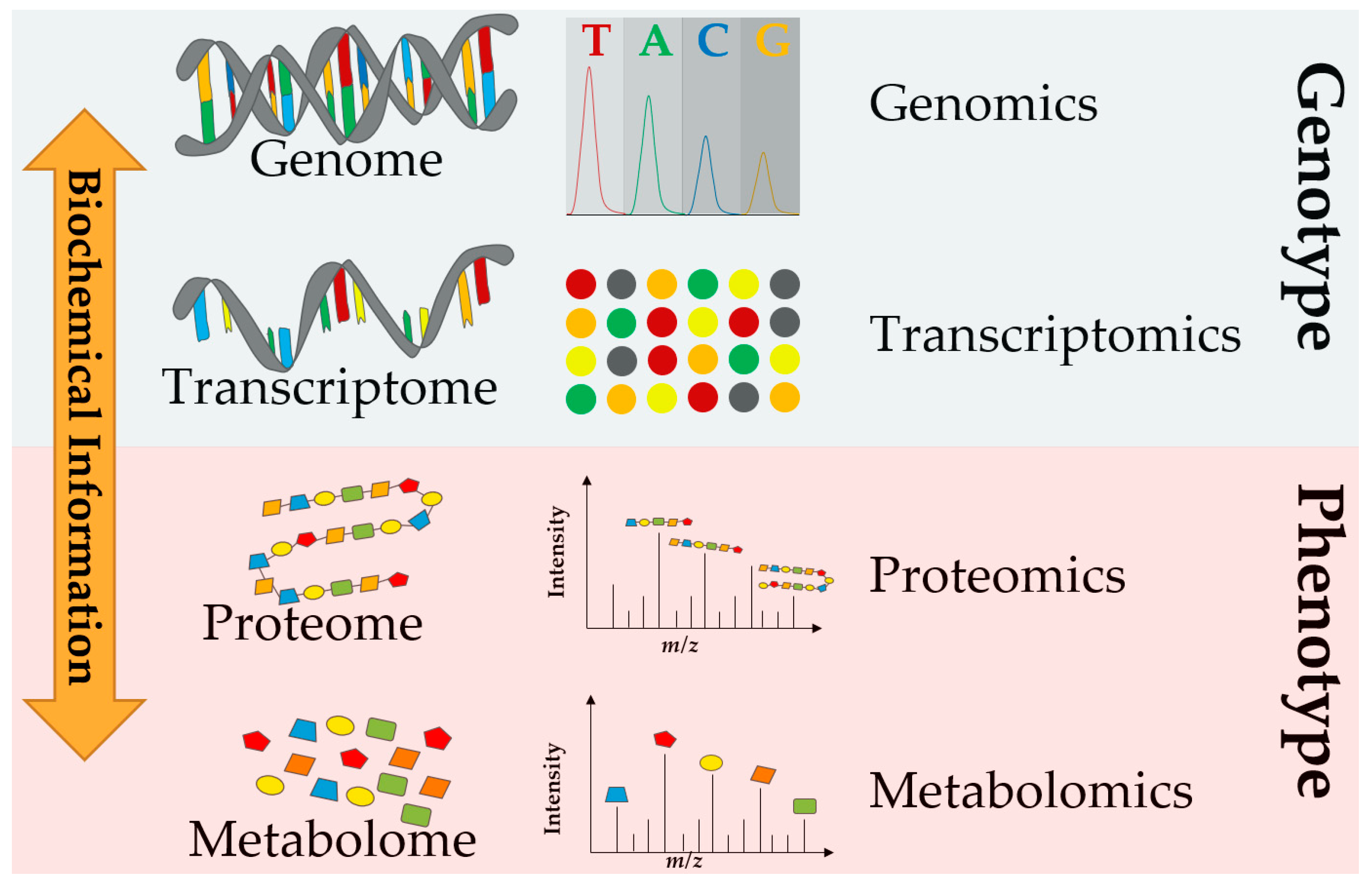
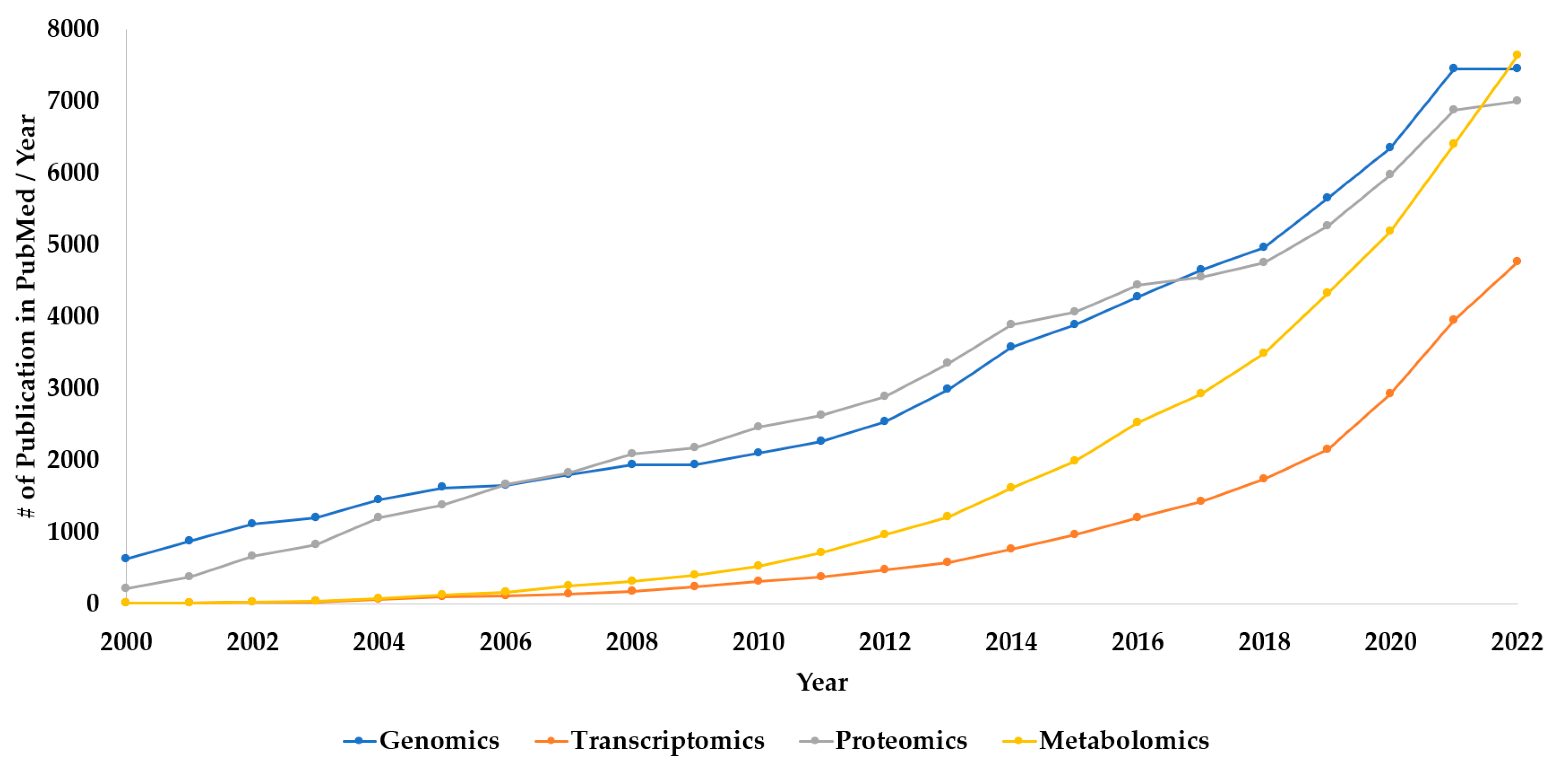
1. Introduction
2. Untargeted Metabolomics
2.1. Workflow
2.2. Applications and Opportunities
2.3. Limitations
3. Targeted Metabolomics
3.1. Workflow
3.2. Applications and Opportunities
3.3. Limitations
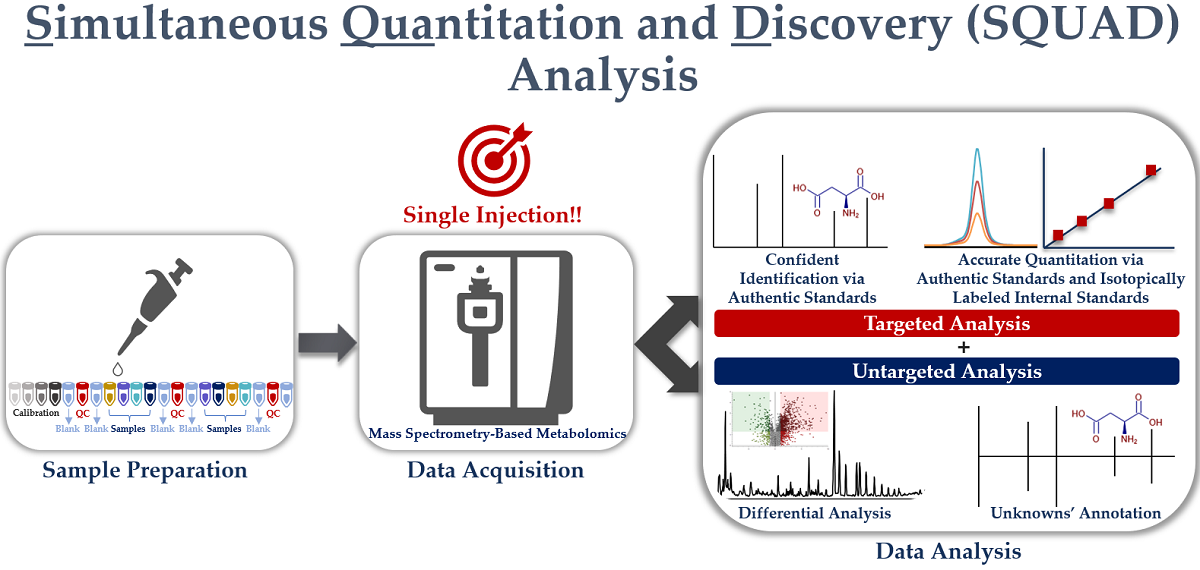
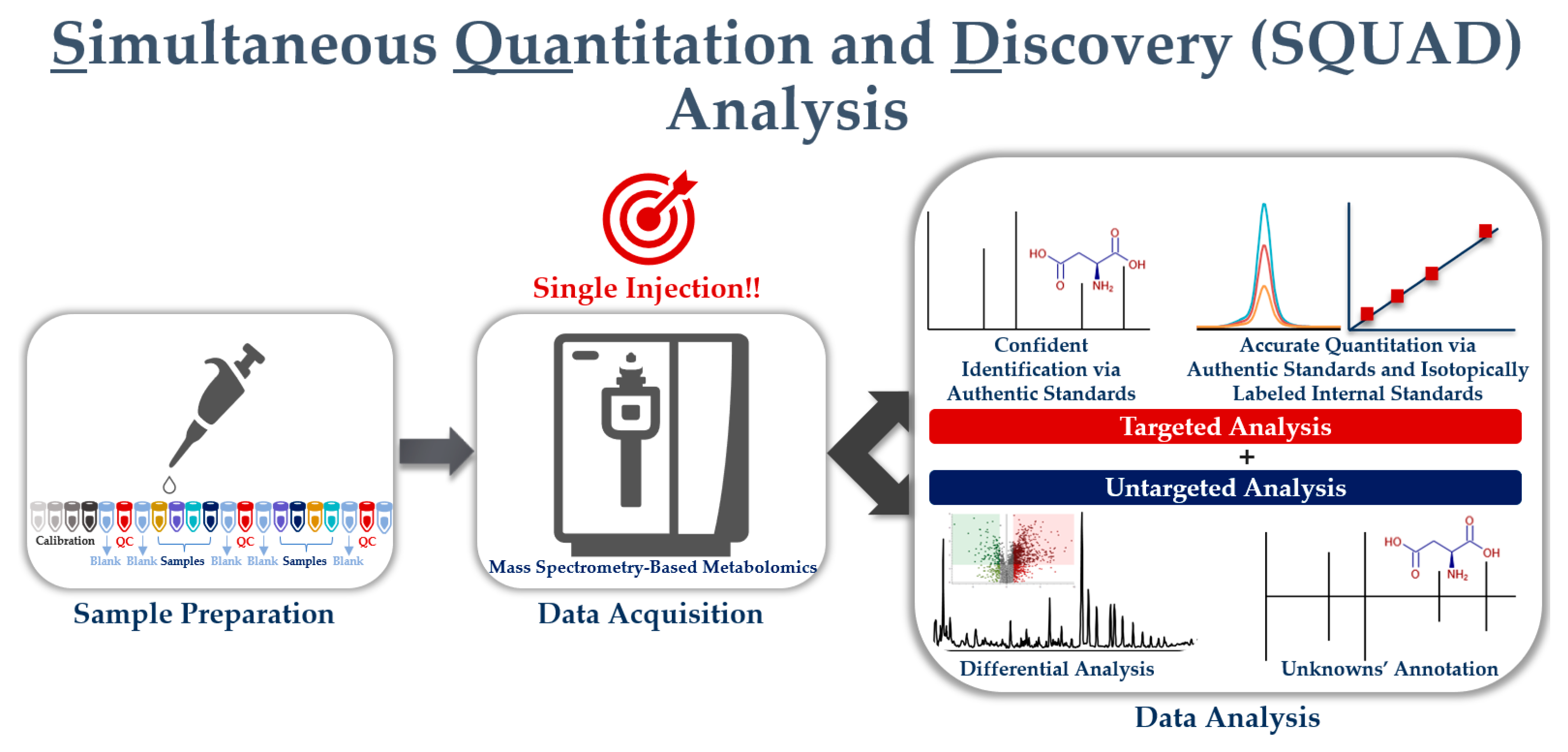
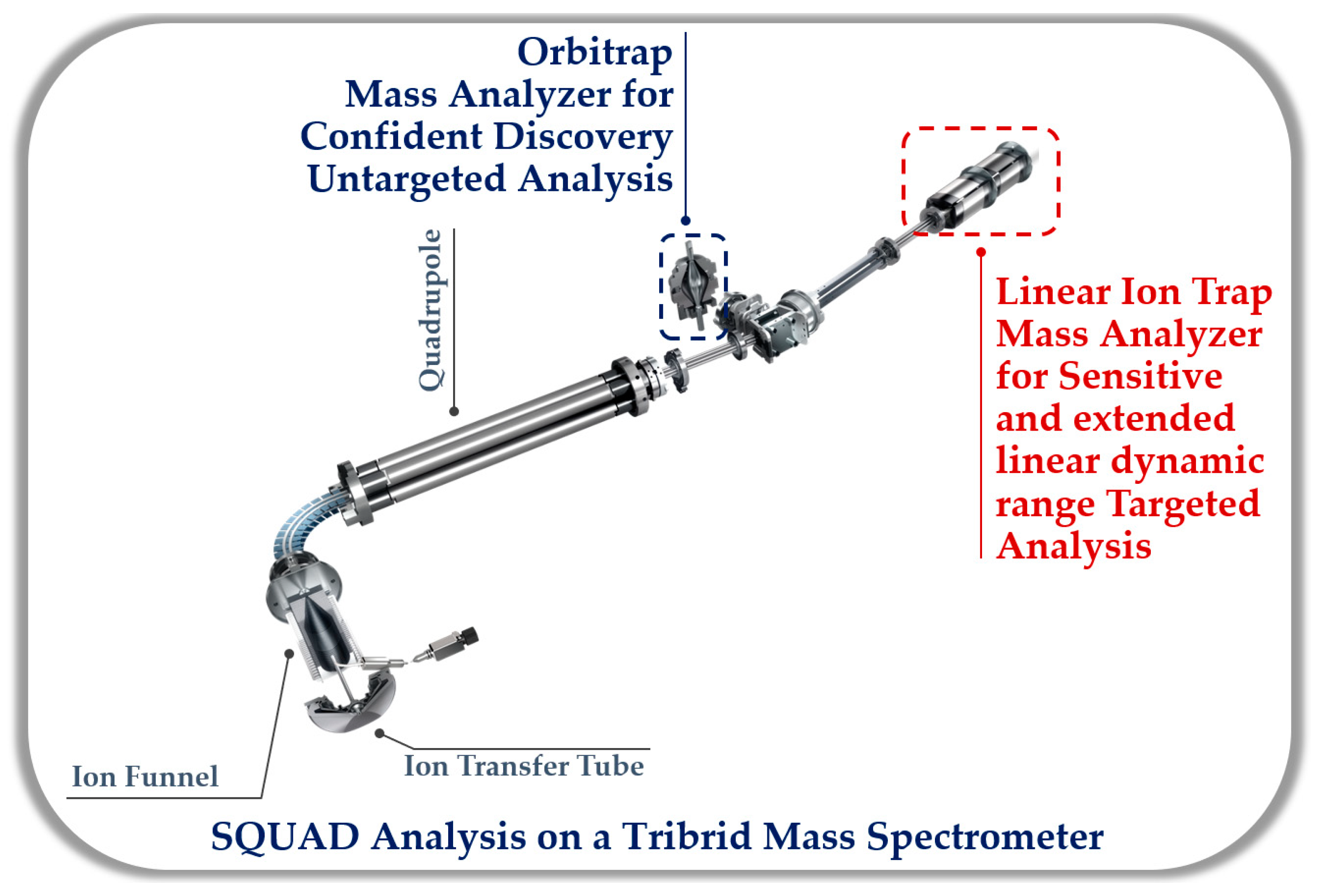
4. Simultaneous Quantitation and Discovery (SQUAD) Analysis
4.1. Nomenclature
4.2. Workflow Structures
4.2.1. Combined Targeted and Untargeted Metabolomics
4.2.2. Pseudo-targeted Metabolomics
4.2.3. Semi-targeted Metabolomics
4.2.4. Simultaneous Quantitation and Discovery (SQUAD) Analysis
Opportunities for SQUAD Analysis
Barriers to Adopting SQUAD
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baidoo, E.E.K.; Teixeira Benites, V. Mass Spectrometry-Based Microbial Metabolomics: Techniques, Analysis, and Applications. Methods in Molecular Biology 2019, 1859, 11–69. [Google Scholar] [CrossRef] [PubMed]
- Amer, B.; Baidoo, E.E.K. Omics-Driven Biotechnology for Industrial Applications. Front Bioeng Biotechnol 2021, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics - The Link between Genotypes and Phenotypes. Plant Mol Biol 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, P. NMR-Based Metabolomics and Fluxomics: Developments and Future Prospects. Analyst 2020, 145, 2457–2472. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhong, F.; Zhu, J. Bridging Targeted and Untargeted Mass Spectrometry-Based Metabolomics via Hybrid Approaches. Metabolites 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pagani, I.; Liolios, K.; Jansson, J.; Chen, I.M.A.; Smirnova, T.; Nosrat, B.; Markowitz, V.M.; Kyrpides, N.C. The Genomes OnLine Database (GOLD) v.4: Status of Genomic and Metagenomic Projects and Their Associated Metadata. Nucleic Acids Res 2012, 40, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; Mclean, J.A. Untargeted Metabolomics Strategies — Challenges and Emerging Directions. 2016, 1897–1905. [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr Protoc Mol Biol 2012, CHAPTER, Unit30.2. [CrossRef]
- Guo, J.; Huan, T. Comparison of Full-Scan, Data-Dependent, and Data-Independent Acquisition Modes in Liquid Chromatography − Mass Spectrometry Based Untargeted Metabolomics. 2020. [CrossRef]
- Nikolskiy, I.; Mahieu, N.G.; Chen, Y.; Tautenhahn, R.; Patti, G.J. An Untargeted Metabolomic Work Fl Ow to Improve Structural Characterization of Metabolites. 2013.
- Wishart, D.S. Emerging Applications of Metabolomics in Drug Discovery and Precision Medicine. Nature Publishing Group 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The Apogee of the Omics Trilogy. Nature Publishing Group. [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of Bioactive Metabolites Using Activity Metabolomics. Nat Rev Mol Cell Biol 2019, 20, 353. [Google Scholar] [CrossRef]
- Emwas, A.M.; Al-rifai, N.; Szczepski, K.; Alsuhaymi, S.; Rayyan, S.; Almahasheer, H.; Jaremko, M.; Brennan, L.; Lachowicz, J.I. You Are What You Eat : Application of Metabolomics Approaches to Advance Nutrition Research. 2021, 1–20.
- Brennan, L.; Hu, F.B.; Sun, Q. Metabolomics Meets Nutritional Epidemiology : Harnessing the Potential in Metabolomics Data. 2021.
- O’Gorman, A.; Brennan, L. Metabolomic Applications in Nutritional Research: A Perspective. J Sci Food Agric 2015, 95, 2567–2570. [Google Scholar] [CrossRef]
- Reisdorph, N.A.; Hendricks, A.E.; Tang, M.; Doenges, K.A.; Reisdorph, R.M.; Tooker, B.C.; Quinn, K.; Borengasser, S.J.; Nkrumah-Elie, Y.; Frank, D.N.; et al. Nutrimetabolomics Reveals Food-Specific Compounds in Urine of Adults Consuming a DASH-Style Diet. Sci Rep 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Bauermeister, A.; Mannochio-Russo, H.; Costa-Lotufo, L. V.; Jarmusch, A.K.; Dorrestein, P.C. Mass Spectrometry-Based Metabolomics in Microbiome Investigations. Nat Rev Microbiol 2022, 20, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Baidoo, E.E.K.; Teixeira Benites, V. Mass Spectrometry-Based Microbial Metabolomics: Techniques, Analysis, and Applications. Methods in Molecular Biology 2019, 1859, 11–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, A.; Miao, J.; Sun, H.; Han, Y.; Yan, G. Metabolomics Biotechnology, Applications, and Future Trends: A Systematic Review. 2019, 37245–37257. [CrossRef]
- Amer, B.; Juul, L.; Dalsgaard, T.K. Original Article Improved Solubility of Proteins from White and Red Clover – Inhibition of Redox Enzymes. 2021, 302–311. [CrossRef]
- Dudley, B.E.; Yousef, M.; Wang, Y.; Griffiths, W.J. TARGETED METABOLOMICS AND MASS SPECTROMETRY While a Great Emphasis Has Been Placed on Global Metabolomic Analysis I . Introduction Since the Success of Genome Analysis via Genomics , a Number of Related; 1st ed.; Elsevier Inc, 2010; Vol. 80.
- van der Lelie, D.; Oka, A.; Taghavi, S.; Umeno, J.; Fan, T.J.; Merrell, K.E.; Watson, S.D.; Ouellette, L.; Liu, B.; Awoniyi, M.; et al. Rationally Designed Bacterial Consortia to Treat Chronic Immune-Mediated Colitis and Restore Intestinal Homeostasis. Nat Commun 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Kobayashi, T.; Nishiumi, S.; Yamanaka, K.; Nakagawa, T.; Fujigaki, S.; Iemoto, T.; Kobayashi, M.; Okusaka, T.; Nakamori, S.; et al. Identification of Highly Sensitive Biomarkers That Can Aid the Early Detection of Pancreatic Cancer Using GC/MS/MS-Based Targeted Metabolomics. Clinica Chimica Acta 2017, 468, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kuhring, M.; Eisenberger, A.; Schmidt, V.; Kränkel, N.; Leistner, D.M.; Kirwan, J.; Beule, D. Concepts and Software Package for Efficient Quality Control in Targeted Metabolomics Studies: MeTaQuaC. Anal Chem 2020, 92, 10241–10245. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, F.N.; Cook, F.; Gounarides, J.; Marciano, S.; Haddad, L.; Tamaroff, A.J.; Casciato, P.; Narvaez, A.; Mascardi, M.F.; Anders, M.; et al. Plasma and Stool Metabolomic Biomarkers of Non-Alcoholic Fatty Liver Disease in Argentina. medRxiv 2020. [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal Chem 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Ruoppolo, M. Targeted Metabolomics; INC, 2022; ISBN 9780323850629.
- Analysis, S.L.; Reisz, J.A.; Zheng, C.; Alessandro, A.D.; Nemkov, T. Chapter 8. 1978, 121–135. [CrossRef]
- Mireault, M.; Prinville, V.; Ohlund, L.; Sleno, L. Semi-Targeted Profiling of Bile Acids by High-Resolution Mass Spectrometry in a Rat Model of Drug-Induced Liver Injury. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Rischke, S.; Hahnefeld, L.; Burla, B.; Behrens, F.; Gurke, R.; Garrett, T.J. Journal of Mass Spectrometry and Advances in the Clinical Lab Small Molecule Biomarker Discovery : Proposed Workflow for LC-MS-Based Clinical Research Projects. Journal of Mass Spectrometry and Advances in the Clinical Lab 2023, 28, 47–55. [Google Scholar] [CrossRef]
- Briscoe, C.J.; Stiles, M.R.; Hage, D.S. System Suitability in Bioanalytical LC/MS/MS. J Pharm Biomed Anal 2007, 44, 484–491. [Google Scholar] [CrossRef]
- Gonzalez-Covarrubias, V.; Martínez-Martínez, E.; Bosque-Plata, L. Del The Potential of Metabolomics in Biomedical Applications. Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Iwasa, M.; Ishihara, T.; Mifuji-Moroka, R.; Fujita, N.; Kobayashi, Y.; Hasegawa, H.; Iwata, K.; Kaito, M.; Takei, Y. Elevation of Branched-Chain Amino Acid Levels in Diabetes and NAFL and Changes with Antidiabetic Drug Treatment. Obes Res Clin Pract 2015, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature That Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a Peripheral Blood Metabolic Profile with Coronary Artery Disease and Risk of Subsequent Cardiovascular Events. Circ Cardiovasc Genet 2010, 3, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are We Close to Defining a Metabolomic Signature of Human Obesity? A Systematic Review of Metabolomics Studies; Springer US, 2019; Vol. 15; ISBN 0123456789.
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H. Targeted Metabolomics for Biomarker Discovery Angewandte. 2010, 5426–5445. [CrossRef]
- Altmaier, E.; Kastenmüller, G.; Römisch-Margl, W.; Thorand, B.; Weinberger, K.M.; Illig, T.; Adamski, J.; Döring, A.; Suhre, K. Questionnaire-Based Self-Reported Nutrition Habits Associate with Serum Metabolism as Revealed by Quantitative Targeted Metabolomics. Eur J Epidemiol 2011, 26, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Tebani, A.; Bekri, S. Paving the Way to Precision Nutrition through Metabolomics. Front Nutr 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Naureen, Z.; Cristoni, S.; Donato, K.; Medori, M.C.; Samaja, M.; Herbst, K.L.; Aquilanti, B.; Velluti, V.; Matera, G.; Fioretti, F.; et al. Metabolomics Application for the Design of an Optimal Diet. J Prev Med Hyg 2022, 63, E142–E149. [Google Scholar] [CrossRef]
- Brennan, L. Session 2: Personalised Nutrition Metabolomic Applications in Nutritional Research. Proceedings of the Nutrition Society 2008, 67, 404–408. [Google Scholar] [CrossRef]
- Szeremeta, M.; Pietrowska, K.; Niemcunowicz-Janica, A.; Kretowski, A.; Ciborowski, M. Applications of Metabolomics in Forensic Toxicology and Forensic Medicine. Int J Mol Sci 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Petersen, I.N.; Tortzen, C.; Kristensen, J.L.; Pedersen, D.S.; Breindahl, T. Identification of a New Metabolite of GHB: Gamma-Hydroxybutyric Acid Glucuronide. J Anal Toxicol 2013, 37, 291–297. [Google Scholar] [CrossRef]
- Narduzzi, L.; Dervilly, G.; Audran, M.; Le Bizec, B.; Buisson, C. A Role for Metabolomics in the Antidoping Toolbox? Drug Test Anal 2020, 12, 677–690. [Google Scholar] [CrossRef]
- Bedia, C. Metabolomics in Environmental Toxicology: Applications and Challenges. Trends in Environmental Analytical Chemistry 2022, 34, e00161. [Google Scholar] [CrossRef]
- Selamat, J.; Rozani, N.A.A.; Murugesu, S. Application of the Metabolomics Approach in Food Authentication. Molecules 2021, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass Appeal: Metabolite Identification in Mass Spectrometry-Focused Untargeted Metabolomics. Metabolomics 2013, 9, 44–66. [Google Scholar] [CrossRef]
- Chen, S.; Kong, H.; Lu, X.; Li, Y.; Yin, P.; Zeng, Z.; Xu, G. Pseudotargeted Metabolomics Method and Its Application in Serum Biomarker Discovery for Hepatocellular Carcinoma Based on Ultra High-Performance Liquid Chromatography/Triple Quadrupole Mass Spectrometry. Anal Chem 2013, 85, 8326–8333. [Google Scholar] [CrossRef]
- Pinel, G.; Weigel, S.; Antignac, J.P.; Mooney, M.H.; Elliott, C.; Nielen, M.W.F.; Le Bizec, B. Targeted and Untargeted Profiling of Biological Fluids to Screen for Anabolic Practices in Cattle. TrAC - Trends in Analytical Chemistry 2010, 29, 1269–1280. [Google Scholar] [CrossRef]
- Che, N.; Ma, Y.; Ruan, H.; Xu, L.; Wang, X.; Yang, X.; Liu, X. Integrated Semi-Targeted Metabolomics Analysis Reveals Distinct Metabolic Dysregulation in Pleural Effusion Caused by Tuberculosis and Malignancy. Clinica Chimica Acta 2018, 477, 81–88. [Google Scholar] [CrossRef]
- Turi, C.E.; Murch, S.J. Targeted and Untargeted Phytochemistry of Ligusticum Canbyi: Indoleamines, Phthalides, Antioxidant Potential, and Use of Metabolomics as a Hypothesis-Generating Technique for Compound Discovery. Planta Med 2013, 79, 1370–1379. [Google Scholar] [CrossRef]
- García-Villalba, R.; Tomás-Barberán, F.A.; Fança-Berthon, P.; Roller, M.; Zafrilla, P.; Issaly, N.; García-Conesa, M.T.; Combet, E. Targeted and Untargeted Metabolomics to Explore the Bioavailability of the Secoiridoids from a Seed/Fruit Extract (Fraxinus Angustifolia Vahl) in Human Healthy Volunteers: A Preliminary Study. Molecules 2015, 20, 22202–22219. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey Phenolic Compound Profiling and Authenticity Assessment Using Hrms Targeted and Untargeted Metabolomics. Molecules 2021, 26, 1–21. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Drakopoulou, S.K.; Aalizadeh, R.; Thomaidis, N.S. Targeted and Untargeted Metabolomics as an Enhanced Tool for the Detection of Pomegranate Juice Adulteration. Foods 2019, 8. [Google Scholar] [CrossRef]
- Drakopoulou, S.K.; Damalas, D.E.; Baessmann, C.; Thomaidis, N.S. Trapped Ion Mobility Incorporated in LC-HRMS Workflows as an Integral Analytical Platform of High Sensitivity: Targeted and Untargeted 4D-Metabolomics in Extra Virgin Olive Oil. J Agric Food Chem 2021, 69, 15728–15737. [Google Scholar] [CrossRef] [PubMed]
- Vorrink, S.U.; Ullah, S.; Schmidt, S.; Nandania, J.; Velagapudi, V.; Beck, O.; Ingelman-Sundberg, M.; Lauschke, V.M. Endogenous and Xenobiotic Metabolic Stability of Primary Human Hepatocytes in Long-Term 3D Spheroid Cultures Revealed by a Combination of Targeted and Untargeted Metabolomics. FASEB Journal 2017, 31, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Xu, J.; Zhou, W.W.; Brand, E.; Chen, H.B.; Zhao, Z.Z. Integrating Targeted and Untargeted Metabolomics to Investigate the Processing Chemistry of Polygoni Multiflori Radix. Front Pharmacol 2018, 9. [Google Scholar] [CrossRef]
- Eudy, B.J.; McDermott, C.E.; Liu, X.; da Silva, R.P. Targeted and Untargeted Metabolomics Provide Insight into the Consequences of Glycine-N-Methyltransferase Deficiency Including the Novel Finding of Defective Immune Function. Physiol Rep 2020, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jasbi, P.; Shi, X.; Turner, C.; Hrovat, J.; Liu, L.; Rabena, Y.; Porter, P.; Gu, H. Early Breast Cancer Detection Using Untargeted and Targeted Metabolomics. J Proteome Res 2021, 20, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Chaby, L.E.; Lasseter, H.C.; Contrepois, K.; Salek, R.M.; Turck, C.W.; Thompson, A.; Vaughan, T.; Haas, M.; Jeromin, A. Cross-Platform Evaluation of Commercially Targeted and Untargeted Metabolomics Approaches to Optimize the Investigation of Psychiatric Disease. Metabolites 2021, 11. [Google Scholar] [CrossRef]
- Taylor, A.L.; Davis, D.E.; Codreanu, S.G.; Harrison, F.E.; Sherrod, S.D.; McLean, J.A. Targeted and Untargeted Mass Spectrometry Reveals the Impact of High-Fat Diet on Peripheral Amino Acid Regulation in a Mouse Model of Alzheimer’s Disease. J Proteome Res 2021, 20, 4405–4414. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, S.; Han, C.; Lu, Y.; Ma, C.; Tian, J.; Bi, J.; Deng, Z.; Wang, Q.; Xu, Q. Targeted and Untargeted Metabolomics Profiling of Wheat Reveals Amino Acids Increase Resistance to Fusarium Head Blight. Front Plant Sci 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Zhang, C.; Li, C.; Fang, Z.; Zeng, Z.; Hu, B.; Chen, H.; Wu, W.; Wang, T.; et al. Targeted and Untargeted Metabolomic Analyses and Biological Activity of Tibetan Tea. Food Chem 2022, 384, 132517. [Google Scholar] [CrossRef]
- Qin, N.; Qin, M.; Shi, W.; Kong, L.; Wang, L.; Xu, G.; Guo, Y.; Zhang, J.; Ma, Q. Investigation of Pathogenesis of Hyperuricemia Based on Untargeted and Targeted Metabolomics. Sci Rep 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Zeng, L.; Jin, S.; Fu, Y.; Chen, L.; Yin, J.; Xu, Y. A Targeted and Untargeted Metabolomics Analysis of “Oriental Beauty” Oolong Tea during Processing. Beverage Plant Research 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Monti, M.C.; Frei, P.; Weber, S.; Scheurer, E.; Mercer-Chalmers-Bender, K. Beyond Δ9-Tetrahydrocannabinol and Cannabidiol: Chemical Differentiation of Cannabis Varieties Applying Targeted and Untargeted Analysis. Anal Bioanal Chem 2022, 414, 3847–3862. [Google Scholar] [CrossRef] [PubMed]
- Kioroglou, D.; Mas, A.; Portillo, M.C. Qualitative Factor-Based Comparison of NMR, Targeted and Untargeted GC-MS and LC-MS on the Metabolomic Profiles of Rioja and Priorat Red Wines. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Melnik, A. V.; Da Silva, R.R.; Hyde, E.R.; Aksenov, A.A.; Vargas, F.; Bouslimani, A.; Protsyuk, I.; Jarmusch, A.K.; Tripathi, A.; Alexandrov, T.; et al. Coupling Targeted and Untargeted Mass Spectrometry for Metabolome-Microbiome-Wide Association Studies of Human Fecal Samples. Anal Chem 2017, 89, 7549–7559. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling; 2016; Vol. 2016; ISBN 0471142727.
- Rochat, B. From Targeted Quantification to Untargeted Metabolomics: Why LC-High-Resolution-MS Will Become a Key Instrument in Clinical Labs. TrAC - Trends in Analytical Chemistry 2016, 84, 151–164. [Google Scholar] [CrossRef]
- Gonzalez-Riano, C.; Sanz-Rodríguez, M.; Escudero-Ramirez, J.; Lorenzo, M.P.; Barbas, C.; Cubelos, B.; Garcia, A. Target and Untargeted GC–MS Based Metabolomic Study of Mouse Optic Nerve and Its Potential in the Study of Neurological Visual Diseases. J Pharm Biomed Anal 2018, 153, 44–56. [Google Scholar] [CrossRef]
- Coene, K.L.M.; Kluijtmans, L.A.J.; van der Heeft, E.; Engelke, U.F.H.; de Boer, S.; Hoegen, B.; Kwast, H.J.T.; van de Vorst, M.; Huigen, M.C.D.G.; Keularts, I.M.L.W.; et al. Next-Generation Metabolic Screening: Targeted and Untargeted Metabolomics for the Diagnosis of Inborn Errors of Metabolism in Individual Patients. J Inherit Metab Dis 2018, 41, 337–353. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Zhang, R.; He, J.; Chen, Y.; Bi, N.; Song, Y.; Wang, L.; Zhan, Q.; Abliz, Z. Development of a Metabolic Pathway-Based Pseudo-Targeted Metabolomics Method Using Liquid Chromatography Coupled with Mass Spectrometry. Talanta 2019, 192, 160–168. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, M.; Wei, X.; Zhu, H.; Zhang, J.; Zhang, Y.; Xue, L.; Huang, L.; Chen, G.; Chen, M.; et al. Pseudotargeted Metabolomic Fingerprinting and Deep Learning for Identification and Visualization of Common Pathogens. Front Microbiol 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Deng, H.; He, R.; Xia, H.; Xu, N.; Deng, Q.; Liang, D.; Lin, L.; Liao, L.; Xiong, B.; Xie, X.; et al. Ultra-HPLC-MS Pseudo-Targeted Metabolomic Profiling Reveals Metabolites and Associated Metabolic Pathway Alterations in Asian Plum (Prunus Salicina) Fruits in Response to Gummosis Disease. Functional Plant Biology 2022, 49, 936–945. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Li, P.; He, C.; Wang, R.; Su, H.; Wan, J.B. An Improved Pseudotargeted Metabolomics Approach Using Multiple Ion Monitoring with Time-Staggered Ion Lists Based on Ultra-High Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. Anal Chim Acta 2016, 927, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Jia, W.; Du, A.; Shi, L. Pseudo-Targeted Metabolomics Analysis of the Therapeutic Effect of Phenolics-Rich Extract from Se-Enriched Green Tea (Camellia Sinensis) on LPS-Stimulated Murine Macrophage (RAW264.7). Food Research International 2022, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Xiang, Q.; Kim, J.; Dufresne, C.; Liu, Y.; Li, T.; Chen, S. Creation of a Plant Metabolite Spectral Library for Untargeted and Targeted Metabolomics. Int J Mol Sci 2023, 24, 2249. [Google Scholar] [CrossRef] [PubMed]
- Genta-Jouve, G.; Croué, J.; Weinberg, L.; Cocandeau, V.; Holderith, S.; Bontemps, N.; Suzuki, M.; Thomas, O.P. Two-Dimensional Ultra High Pressure Liquid Chromatography Quadrupole/Time-of-Flight Mass Spectrometry for Semi-Targeted Natural Compounds Identification. Phytochem Lett 2014, 10, 318–323. [Google Scholar] [CrossRef]
- Salem, M.A.; Giavalisco, P. Semi-Targeted Lipidomics of Plant Acyl Lipids Using UPLC-HR-MS in Combination with a Data-Independent Acquisition Mode. Methods in Molecular Biology 2018, 1778, 137–155. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Singh, A.; Vishnu, K. V.; Ajeeshkumar, K.K.; Anandan, R.; Ashok Kumar, K.; Mathew, S. Authentication of Two Bio-Active Fish Oils by Qualitative Lipid Profiling Using Semi-Targeted Approach: An Exploratory Study. J AOAC Int 2021, 103, 78–82. [Google Scholar] [CrossRef]
- Castañé, H.; Iftimie, S.; Baiges-Gaya, G.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Garrido, P.; Castro, A.; Camps, J.; Joven, J. Machine Learning and Semi-Targeted Lipidomics Identify Distinct Serum Lipid Signatures in Hospitalized COVID-19-Positive and COVID-19-Negative Patients. Metabolism 2022, 131. [Google Scholar] [CrossRef]
- Baiges-Gaya, G.; Iftimie, S.; Castañé, H.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Castro, A.; Camps, J.; Joven, J. Combining Semi-Targeted Metabolomics and Machine Learning to Identify Metabolic Alterations in the Serum and Urine of Hospitalized Patients with COVID-19. Biomolecules 2023, 13, 163. [Google Scholar] [CrossRef]
- Jensen-Kroll, J.; Demetrowitsch, T.; Clawin-Rädecker, I.; Klempt, M.; Waschina, S.; Schwarz, K. Microbiota Independent Effects of Oligosaccharides on Caco-2 Cells -A Semi-Targeted Metabolomics Approach Using DI-FT-ICR-MS Coupled with Pathway Enrichment Analysis. Front Mol Biosci 2022, 9, 1–17. [Google Scholar] [CrossRef]
- Ma, Y.J.; Yuan, L.H.; Xiao, J.M.; Jiang, H.Y.; Sa, Y.H.; Sun, H.Q.; Song, J.Y.; Sun, Z.G. The Mechanism of Traditional Chinese Medicine Based on Semi-Targeted Metabolomics to Improve IVF Outcomes in Senile Patients. Evidence-based Complementary and Alternative Medicine 2021, 2021. [Google Scholar] [CrossRef]
- Bayle, M.L.; Wopereis, S.; Bouwman, J.; van Ommen, B.; Scalbert, A.; Pujos-Guillot, E. Semi-Targeted Metabolomic Approaches to Validate Potential Markers of Health for Micronutrients: Analytical Perspectives. Metabolomics 2012, 8, 1114–1129. [Google Scholar] [CrossRef]
- Burgess, K.; Creek, D.; Dewsbury, P.; Cook, K.; Barrett, M.P. Semi-Targeted Analysis of Metabolites Using Capillary-Flow Ion Chromatography Coupled to High-Resolution Mass Spectrometry. Rapid Communications in Mass Spectrometry 2011, 25, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Kotamreddy, J.N.R.; Hansda, C.; Mitra, A. Semi-Targeted Metabolomic Analysis Provides the Basis for Enhanced Antioxidant Capacities in Pigmented Rice Grains. Journal of Food Measurement and Characterization 2020, 14, 1183–1191. [Google Scholar] [CrossRef]
- Billet, K.; Malinowska, M.A.; Munsch, T.; Unlubayir, M.; Adler, S.; Delanoue, G.; Lanoue, A. Semi-Targeted Metabolomics to Validate Biomarkers of Grape Downy Mildew Infection under Field Conditions. Plants 2020, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Lu, W.; Su, X.; Klein, M.S.; Lewis, I.A.; Fiehn, O.; Rabinowitz, J.D. Metabolite Measurement: Pitfalls to Avoid and Practices to Follow. Annu Rev Biochem 2017, 86, 277–304. [Google Scholar] [CrossRef]
- Jourdan, F.; Breitling, R.; Barrett, M.P.; Gilbert, D. MetaNetter: Inference and Visualization of High-Resolution Metabolomic Networks. Bioinformatics 2008, 24, 143–145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



