Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. HT biomarkers and epidemiological data
1.2. Prevalence of HT diagnoses
1.3. Molecular biotechnologies (MB) and HT
2. Material and Methods
2.1. Data Sources
2.2. Study Selection
2.3. Inclusion criteria
2.4. Exclusion criteria
2.5. Data Extraction
2.6. Data Synthesis
3. Results
3.1. CTSs conducted on HT population.
3.2. Clinical application of MB in HT-CTSs
4. Discussion
5. Conclusion
References
- Yoo, W.S.; Chung, H.K. Recent Advances in Autoimmune Thyroid Diseases. Endocrinol Metab 2016, 31, 379. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, S.; Nielsen, C.H.; Feldt-Rasmussen, U. Recent Advances in Understanding Autoimmune Thyroid Disease: The Tallest Tree in the Forest of Polyautoimmunity. F1000Res 2017, 6, 1776. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune Thyroid Disorders. Autoimmunity Reviews 2015, 14, 174–180. [Google Scholar] [CrossRef]
- Trovato, M. A Historical Excursus of Diagnostic Methods for Hashimoto Thyroiditis and Graves’ Disease. Gazz Med Ital - Arch Sci Med 2020, 179. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-Thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and Molecular Basis of Thyroid Autoimmunity. European Thyroid Journal 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Sreedharan, S.; Khadilkar, U.N.; Deviprasad, D.; Kamath, M.P.; Bhojwani, K.M.; Alva, A. Clinical, Biochemical & Cytomorphologic Study on Hashimoto’s Thyroiditis. Indian J Med Res 2014, 140, 729–735. [Google Scholar]
- Kahaly, G.J.; Gottwald-Hostalek, U. Use of Levothyroxine in the Management of Hypothyroidism: A Historical Perspective. Front. Endocrinol. 2022, 13, 1054983. [Google Scholar] [CrossRef]
- Okosieme, O.; Gilbert, J.; Abraham, P.; Boelaert, K.; Dayan, C.; Gurnell, M.; Leese, G.; McCabe, C.; Perros, P.; Smith, V.; et al. Management of Primary Hypothyroidism: Statement by the British Thyroid Association Executive Committee. Clinical Endocrinology 2016, 84, 799–808. [Google Scholar] [CrossRef]
- Jordan, B.; Uer, O.; Buchholz, T.; Spens, A.; Zierz, S. Physical Fatigability and Muscle Pain in Patients with Hashimoto Thyroiditis. J Neurol 2021, 268, 2441–2449. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, J.; Li, H.; Zhong, H.; Wan, Q. Changes in Glucose-lipid Metabolism, Insulin Resistance, and Inflammatory Factors in Patients with Autoimmune Thyroid Disease. J Clin Lab Anal 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Bladowska, J.; Budrewicz, S.; Sąsiadek, M.; Dziadkowiak, E.; Ejma, M. The Evaluation of Hashimoto’s Thyroiditis with Event-Related Potentials and Magnetic Resonance Spectroscopy and Its Relation to Cognitive Function. Sci Rep 2021, 11, 2480. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. The Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat Rev Dis Primers 2022, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.; Korevaar, T.I.M.; Taylor, P. Trends, Determinants, and Associations of Treated Hypothyroidism in the United Kingdom, 2005–2014. Thyroid 2019, 29, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M.; Duntas, L.; Fadeyev, V.; Nygaard, B.; Vanderpump, M.P.J. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J 2012, 1, 55–71. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.C.; Cappola, A.R.; Celi, F.S.; Fliers, E.; Heuer, H.; McAninch, E.A.; Moeller, L.C.; Nygaard, B.; Sawka, A.M.; et al. Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: A Consensus Document. Thyroid 2021, 31, 156–182. [Google Scholar] [CrossRef]
- Lillevang-Johansen, M.; Abrahamsen, B.; Jørgensen, H.L.; Brix, T.H.; Hegedüs, L. Over- and Under-Treatment of Hypothyroidism Is Associated with Excess Mortality: A Register-Based Cohort Study. Thyroid 2018, 28, 566–574. [Google Scholar] [CrossRef]
- Thayakaran, R.; Adderley, N.J.; Sainsbury, C.; Torlinska, B.; Boelaert, K.; Šumilo, D.; Price, M.; Thomas, G.N.; Toulis, K.A.; Nirantharakumar, K. Thyroid Replacement Therapy, Thyroid Stimulating Hormone Concentrations, and Long Term Health Outcomes in Patients with Hypothyroidism: Longitudinal Study. BMJ 2019, l4892. [Google Scholar] [CrossRef]
- Davies, T.F.; Morshed, S.A.; Mezei, M.; Latif, R. Brief Report - Monoclonal Antibodies Illustrate the Difficulties in Measuring Blocking TSH Receptor Antibodies. Front. Endocrinol. 2022, 13, 943459. [Google Scholar] [CrossRef]
- Peterson, S.J.; Cappola, A.R.; Castro, M.R.; Dayan, C.M.; Farwell, A.P.; Hennessey, J.V.; Kopp, P.A.; Ross, D.S.; Samuels, M.H.; Sawka, A.M.; et al. An Online Survey of Hypothyroid Patients Demonstrates Prominent Dissatisfaction. Thyroid 2018, 28, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Bjerkreim, B.A.; Hammerstad, S.S.; Gulseth, H.L.; Berg, T.J.; Omdal, L.J.; Lee-Ødegård, S.; Eriksen, E.F. Effect of Liothyronine Treatment on Quality of Life in Female Hypothyroid Patients With Residual Symptoms on Levothyroxine Therapy: A Randomized Crossover Study. Front. Endocrinol. 2022, 13, 816566. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Nirantharakumar, K.; Hegedüs, L. Recent Evidence Sets Therapeutic Targets for Levothyroxine-Treated Patients with Primary Hypothyroidism Based on Risk of Death. European Journal of Endocrinology 2021, 184, C1–C3. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Hegedüs, L.; Nagy, E.V.; Papini, E.; Hay, H.A.; Abad-Madroñero, J.; Tallett, A.J.; Bilas, M.; Lakwijk, P.; Poots, A.J. The Impact of Hypothyroidism on Satisfaction with Care and Treatment and Everyday Living: Results from E-Mode Patient Self-Assessment of Thyroid Therapy, a Cross-Sectional, International Online Patient Survey. Thyroid 2022, thy.2022.0324. [Google Scholar] [CrossRef]
- Roberto Castello; Marco Caputo Thyroid Diseases and Gender. Italian Journal of Gender-Specific Medicine 2019. [CrossRef]
- Matana, A.; Popović, M.; Boutin, T.; Torlak, V.; Brdar, D.; Gunjača, I.; Kolčić, I.; Boraska Perica, V.; Punda, A.; Polašek, O.; et al. Genome-Wide Meta-Analysis Identifies Novel Gender Specific Loci Associated with Thyroid Antibodies Level in Croatians. Genomics 2019, 111, 737–743. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Practice & Research Clinical Endocrinology & Metabolism 2019, 33, 101367. [Google Scholar] [CrossRef]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune Disorders in Hashimoto’s Thyroiditis: What Do We Know So Far? Journal of Immunology Research 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global Prevalence and Epidemiological Trends of Hashimoto’s Thyroiditis in Adults: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. The Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Andersson, M.; Karumbunathan, V.; Zimmermann, M.B. Global Iodine Status in 2011 and Trends over the Past Decade. The Journal of Nutrition 2012, 142, 744–750. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine Deficiency. Endocrine Reviews 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed]
- Devdhar, M.; Drooger, R.; Pehlivanova, M.; Singh, G.; Jonklaas, J. Levothyroxine Replacement Doses Are Affected by Gender and Weight, But Not Age. Thyroid 2011, 21, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Poste, G.; Carbone, D.P.; Parkinson, D.R.; Verweij, J.; Hewitt, S.M.; Jessup, J.M. Leveling the Playing Field: Bringing Development of Biomarkers and Molecular Diagnostics up to the Standards for Drug Development. Clinical Cancer Research 2012, 18, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Troch, M.; Woehrer, S.; Streubel, B.; Weissel, M.; Hoffmann, M.; Müllauer, L.; Chott, A.; Raderer, M. Chronic Autoimmune Thyroiditis (Hashimoto’s Thyroiditis) in Patients with MALT Lymphoma. Annals of Oncology 2008, 19, 1336–1339. [Google Scholar] [CrossRef]
- Trovato, M.; Giuffrida, G.; Seminara, A.; Fogliani, S.; Cavallari, V.; Ruggeri, R.M.; Campennì, A. Coexistence of Diffuse Large B-Cell Lymphoma and Papillary Thyroid Carcinoma in a Patient Affected by Hashimoto’s Thyroiditis. Arch. Endocrinol. Metab. 2017, 61, 643–646. [Google Scholar] [CrossRef]
- Anil, C.; Goksel, S.; Gursoy, A. Hashimoto’s Thyroiditis Is Not Associated with Increased Risk of Thyroid Cancer in Patients with Thyroid Nodules: A Single-Center Prospective Study. Thyroid 2010, 20, 601–606. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Lin, C.-L.; Cheng, F.T.-F.; Sung, F.-C.; Kao, C.-H. Cancer Risk in Patients with Hashimoto’s Thyroiditis: A Nationwide Cohort Study. Br J Cancer 2013, 109, 2496–2501. [Google Scholar] [CrossRef]
- Resende De Paiva, C.; Grønhøj, C.; Feldt-Rasmussen, U.; Von Buchwald, C. Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, E.T.; Pavlidis, T.E. A Review of Primary Thyroid Lymphoma: Molecular Factors, Diagnosis and Management. Journal of Investigative Surgery 2019, 32, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sevilla, J.J.; Salar, A. Recent Advances in the Genetic of MALT Lymphomas. Cancers 2021, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Vielh, P. Thyroid and Molecular Testing. Advances in Thyroid Molecular Cytopathology. JMP 2021, 2, 77–92. [Google Scholar] [CrossRef]
- Trovato, M. Update on International Medical Taxonomies of Biomarkers and Their Applications in Management of Thyroid Cancers. Diagnostics 2022, 12, 662. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Available online: https://beta.clinicaltrials.gov/ (accessed on 16 April 2023).
- Strachan, D.P. Hay Fever, Hygiene, and Household Size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Tomer, Y.; Davies, T.F. Infection, Thyroid Disease, and Autoimmunity*. Endocrine Reviews 1993, 14, 107–120. [Google Scholar] [CrossRef]
- Desailloud, R.; Hober, D. Viruses and Thyroiditis: An Update. Virol J 2009, 6, 5. [Google Scholar] [CrossRef]
- Morohoshi, K.; Takahashi, Y.; Mori, K. Viral Infection and Innate Pattern Recognition Receptors in Induction of Hashimoto’s Thyroiditis. Discov Med 2011, 12, 505–511. [Google Scholar]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.-F. The ‘Hygiene Hypothesis’ for Autoimmune and Allergic Diseases: An Update. Clinical and Experimental Immunology 2010, 160, 1–9. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.-Y.; Bashi, T.; Bizzaro, G.; Blank, M.; Shoenfeld, Y. Unraveling the Hygiene Hypothesis of Helminthes and Autoimmunity: Origins, Pathophysiology, and Clinical Applications. BMC Med 2015, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Potaczek, D.P.; Pfefferle, P.I. The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. Front. Immunol. 2021, 12, 637087. [Google Scholar] [CrossRef] [PubMed]
- Kondrashova, A.; Seiskari, T.; Ilonen, J.; Knip, M.; Hyöty, H. The ‘Hygiene Hypothesis’ and the Sharp Gradient in the Incidence of Autoimmune and Allergic Diseases between Russian Karelia and Finland. APMIS 2013, 121, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Detels, R.; Brody, J.A.; Edgar, A.H. Multiple Sclerosis among American, Japanese and Chinese Migrants to California and Washington. Journal of Chronic Diseases 1972, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, U.; Kahana, E.; Alter, M. The Changing Frequency of Multiple Sclerosis in Israel. Archives of Neurology 1973, 29, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Bodansky, H.J.; Staines, A.; Stephenson, C.; Haigh, D.; Cartwright, R. Evidence for an Environmental Effect in the Aetiology of Insulin Dependent Diabetes in a Transmigratory Population. BMJ 1992, 304, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Staines, A.; Hanif, S.; Ahmed, S.; McKinney, P.A.; Shera, S.; Bodansky, H.J. Incidence of Insulin Dependent Diabetes Mellitus in Karachi, Pakistan. Archives of Disease in Childhood 1997, 76, 121–123. [Google Scholar] [CrossRef]
- Hammond, S.R. The Age-Range of Risk of Developing Multiple Sclerosis: Evidence from a Migrant Population in Australia. Brain 2000, 123, 968–974. [Google Scholar] [CrossRef]
- Bach, J.-F. Revisiting the Hygiene Hypothesis in the Context of Autoimmunity. Front. Immunol. 2021, 11, 615192. [Google Scholar] [CrossRef]
- Krassas, G.E.; Tziomalos, K.; Pontikides, N.; Lewy, H.; Laron, Z. Seasonality of Month of Birth of Patients with Graves’ and Hashimoto’s Diseases Differ from That in the General Population. eur j endocrinol 2007, 156, 631–636. [Google Scholar] [CrossRef]
- Attard, C.C.; Sze, W.C.C.; Vella, S. Predictors of Autoimmune Thyroid Disease. Baylor University Medical Center Proceedings 2022, 35, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Flicek, P.; Amode, M.R.; Barrell, D.; Beal, K.; Brent, S.; Chen, Y.; Clapham, P.; Coates, G.; Fairley, S.; Fitzgerald, S.; et al. Ensembl 2011. Nucleic Acids Research 2011, 39, D800–D806. [Google Scholar] [CrossRef] [PubMed]
- Borchers, C.H.; Kast, J.; Foster, L.J.; Siu, K.W.M.; Overall, C.M.; Binkowski, T.A.; Hildebrand, W.H.; Scherer, A.; Mansoor, M.; Keown, P.A. The Human Proteome Organization Chromosome 6 Consortium: Integrating Chromosome-Centric and Biology/Disease Driven Strategies. Journal of Proteomics 2014, 100, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kulski, J.K.; Suzuki, S.; Shiina, T. Human Leukocyte Antigen Super-Locus: Nexus of Genomic Supergenes, SNPs, Indels, Transcripts, and Haplotypes. Hum Genome Var 2022, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, K.; Gaberšček, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr Genomics 2011, 12, 576–588. [Google Scholar] [CrossRef]
- Douillard, V.; Castelli, E.C.; Mack, S.J.; Hollenbach, J.A.; Gourraud, P.-A.; Vince, N.; Limou, S. Approaching Genetics Through the MHC Lens: Tools and Methods for HLA Research. Front. Genet. 2021, 12, 774916. [Google Scholar] [CrossRef]
- De Santis, D.; Truong, L.; Martinez, P.; D’Orsogna, L. Rapid High-resolution HLA Genotyping by MinION Oxford Nanopore Sequencing for Deceased Donor Organ Allocation. HLA 2020, 96, 141–162. [Google Scholar] [CrossRef]
- Zawadzka-Starczewska, K.; Tymoniuk, B.; Stasiak, B.; Lewiński, A.; Stasiak, M. Actual Associations between HLA Haplotype and Graves’ Disease Development. JCM 2022, 11, 2492. [Google Scholar] [CrossRef]
- Stasiak, M.; Zawadzka-Starczewska, K.; Tymoniuk, B.; Stasiak, B.; Lewiński, A. Significance of HLA in the Development of Graves’ Orbitopathy. Genes Immun 2023, 24, 32–38. [Google Scholar] [CrossRef]
- Liao, W.-L.; Liu, T.-Y.; Cheng, C.-F.; Chou, Y.-P.; Wang, T.-Y.; Chang, Y.-W.; Chen, S.-Y.; Tsai, F.-J. Analysis of HLA Variants and Graves’ Disease and Its Comorbidities Using a High Resolution Imputation System to Examine Electronic Medical Health Records. Front. Endocrinol. 2022, 13, 842673. [Google Scholar] [CrossRef]
- Mori, K.; Yoshida, K. Viral Infection in Induction of Hashimotoʼs Thyroiditis: A Key Player or Just a Bystander? : Current Opinion in Endocrinology, Diabetes and Obesity 2010, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Weider, T.; Genoni, A.; Broccolo, F.; Paulsen, T.H.; Dahl-Jørgensen, K.; Toniolo, A.; Hammerstad, S.S. High Prevalence of Common Human Viruses in Thyroid Tissue. Front. Endocrinol. 2022, 13, 938633. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, W.P.; Liu, H.X.; Wang, D.; Li, Y.F.; Wang, W.Q.; Wang, L.; He, F.R.; Wang, Z.; Yan, Q.G.; et al. Detection of Human Parvovirus B19 in Papillary Thyroid Carcinoma. Br J Cancer 2008, 98, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Liu, H.; Wang, D.; Wang, W.; Li, Y.; Wang, Z.; Wang, L.; Zhang, W.; Huang, G. Parvovirus B19 Infection Associated with Hashimoto’s Thyroiditis in Adults. Journal of Infection 2010, 60, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Heidari, Z.; Jami, M. Parvovirus B19 Infection Is Associated with Autoimmune Thyroid Disease in Adults. Int J Endocrinol Metab 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Pastore, F. Hepatitis C Virus Infection and Thyroid Autoimmune Disorders: A Model of Interactions between the Host and the Environment. WJH 2016, 8, 83. [Google Scholar] [CrossRef]
- Caselli, E.; Zatelli, M.C.; Rizzo, R.; Benedetti, S.; Martorelli, D.; Trasforini, G.; Cassai, E.; Degli Uberti, E.C.; Di Luca, D.; Dolcetti, R. Virologic and Immunologic Evidence Supporting an Association between HHV-6 and Hashimoto’s Thyroiditis. PLoS Pathog 2012, 8, e1002951. [Google Scholar] [CrossRef]
- Seyyedi, N.; Dehbidi, G.R.; Karimi, M.; Asgari, A.; Esmaeili, B.; Zare, F.; Farhadi, A.; Dabbaghmanesh, M.H.; Saki, F.; Behzad-Behbahani, A. Human Herpesvirus 6A Active Infection in Patients with Autoimmune Hashimoto’s Thyroiditis. The Brazilian Journal of Infectious Diseases 2019, 23, 435–440. [Google Scholar] [CrossRef]
- Trovato, M.; Sciacchitano, S.; Facciolà, A.; Valenti, A.; Visalli, G.; Di Pietro, A. Interleukin-6 Signalling as a Valuable Cornerstone for Molecular Medicine (Review). Int J Mol Med 2021, 47, 107. [Google Scholar] [CrossRef]
- Trovato, M.; Ruggeri, R.M.; Sciacchitano, S.; Vicchio, T.M.; Picerno, I.; Pellicanò, G.; Valenti, A.; Visalli, G. Serum Interleukin-6 Levels Are Increased in HIV-Infected Patients That Develop Autoimmune Disease during Long-Term Follow-Up. Immunobiology 2018, 223, 264–268. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chung, Y.-H.; Shi, Y.-F.; Tzang, B.-S.; Hsu, T.-C. The VP1 Unique Region of Human Parvovirus B19 and Human Bocavirus Induce Lung Injury in Naïve Balb/c Mice. PLoS ONE 2018, 13, e0202667. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; Eis-Huebinger, A.M.; Deijs, M.; De Vries, M.; Drexler, J.F.; Oppong, S.K.; Müller, M.A.; Klose, S.M.; Wellinghausen, N.; Cottontail, V.M.; et al. Two Novel Parvoviruses in Frugivorous New and Old World Bats. PLoS ONE 2011, 6, e29140. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. Journal of General Virology 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Pénzes, J.J.; Söderlund-Venermo, M.; Canuti, M.; Eis-Hübinger, A.M.; Hughes, J.; Cotmore, S.F.; Harrach, B. Reorganizing the Family Parvoviridae: A Revised Taxonomy Independent of the Canonical Approach Based on Host Association. Arch Virol 2020, 165, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernandez, J.; Carton, R.; Tanner, A.R.; Puttick, M.N.; Blaxter, M.; Vinther, J.; Olesen, J.; Giribet, G.; Edgecombe, G.D.; Pisani, D. A Molecular Palaeobiological Exploration of Arthropod Terrestrialization. Phil. Trans. R. Soc. B 2016, 371, 20150133. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Fernandez, J.; Tanner, A.R.; Puttick, M.N.; Vinther, J.; Edgecombe, G.D.; Pisani, D. A Cambrian–Ordovician Terrestrialization of Arachnids. Front. Genet. 2020, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Pénzes, J.J.; De Souza, W.M.; Agbandje-McKenna, M.; Gifford, R.J. An Ancient Lineage of Highly Divergent Parvoviruses Infects Both Vertebrate and Invertebrate Hosts. Viruses 2019, 11, 525. [Google Scholar] [CrossRef]
- Cossart, Y.E.; Cant, B.; Field, A.M.; Widdows, D. PARVOVIRUS-LIKE PARTICLES IN HUMAN SERA. The Lancet 1975, 305, 72–73. [Google Scholar] [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a Human Parvovirus by Molecular Screening of Respiratory Tract Samples. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 12891–12896. [Google Scholar] [CrossRef]
- Lehmann, H.W.; Von Landenberg, P.; Modrow, S. Parvovirus B19 Infection and Autoimmune Disease. Autoimmunity Reviews 2003, 2, 218–223. [Google Scholar] [CrossRef]
- Adamson, L.A.; Fowler, L.J.; Clare-Salzler, M.J.; Hobbs, J.A. Parvovirus B19 Infection in Hashimoto’s Thyroiditis, Papillary Thyroid Carcinoma, and Anaplastic Thyroid Carcinoma. Thyroid 2011, 21, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gravelsina, S.; Nora-Krukle, Z.; Svirskis, S.; Cunskis, E.; Murovska, M. Presence of B19V in Patients with Thyroid Gland Disorders. Medicina 2019, 55, 774. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, P.; Cheng, F.; Li, Y.; Wang, Z.; Hao, S.; Wang, J.; Ning, K.; Ganaie, S.S.; Engelhardt, J.F.; et al. Cellular Cleavage and Polyadenylation Specificity Factor 6 (CPSF6) Mediates Nuclear Import of Human Bocavirus 1 NP1 Protein and Modulates Viral Capsid Protein Expression. J Virol 2020, 94, e01444–19. [Google Scholar] [CrossRef] [PubMed]
- Sowd, G.A.; Serrao, E.; Wang, H.; Wang, W.; Fadel, H.J.; Poeschla, E.M.; Engelman, A.N. A Critical Role for Alternative Polyadenylation Factor CPSF6 in Targeting HIV-1 Integration to Transcriptionally Active Chromatin. Proc. Natl. Acad. Sci. U.S.A. 2016, 113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Schubert, H.L.; Singh, P.K.; Martins, L.J.; Engelman, A.N.; D’Orso, I.; Hill, C.P.; Planelles, V. Cleavage and Polyadenylation Specificity Factor 6 Is Required for Efficient HIV-1 Latency Reversal. mBio 2021, 12, e01098-21. [Google Scholar] [CrossRef] [PubMed]
- Mattola, S.; Hakanen, S.; Salminen, S.; Aho, V.; Mäntylä, E.; Ihalainen, T.O.; Kann, M.; Vihinen-Ranta, M. Concepts to Reveal Parvovirus–Nucleus Interactions. Viruses 2021, 13, 1306. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Giovinazzo, S.; Certo, R.; Alibrandi, A.; Trimarchi, F.; Benvenga, S.; Trovato, M. TP53 Polymorphism May Contribute to Genetic Susceptibility to Develop Hashimoto’s Thyroiditis. J Endocrinol Invest 2015, 38, 1175–1182. [Google Scholar] [CrossRef]
- Li, Z.-H.; Han, J.; Wang, Y.-F.; Dai, J.; Zhang, H.; Li, C.-X.; Ma, Q. Association between Polymorphism of Interleukin-23 Receptor and Hashimoto’s Thyroiditis in Chinese Han Population of Shandong. Chinese Medical Journal 2015, 128, 2050–2053. [Google Scholar] [CrossRef]
- Chen, R.-H.; Chang, C.-T.; Chen, W.-C.; Tsai, C.-H.; Tsai, F.-J. Proinflammatory Cytokine Gene Polymorphisms among Hashimoto’s Thyroiditis Patients. J. Clin. Lab. Anal. 2006, 20, 260–265. [Google Scholar] [CrossRef]
- Durães, C.; Moreira, C.S.; Alvelos, I.; Mendes, A.; Santos, L.R.; Machado, J.C.; Melo, M.; Esteves, C.; Neves, C.; Sobrinho-Simões, M.; et al. Polymorphisms in the TNFA and IL6 Genes Represent Risk Factors for Autoimmune Thyroid Disease. PLoS ONE 2014, 9, e105492. [Google Scholar] [CrossRef]
- Jameson, J.L.; Longo, D.L. Precision Medicine — Personalized, Problematic, and Promising. N Engl J Med 2015, 372, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Zucca, E.; Arcaini, L.; Buske, C.; Johnson, P.W.; Ponzoni, M.; Raderer, M.; Ricardi, U.; Salar, A.; Stamatopoulos, K.; Thieblemont, C.; et al. Marginal Zone Lymphomas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Annals of Oncology 2020, 31, 17–29. [Google Scholar] [CrossRef] [PubMed]
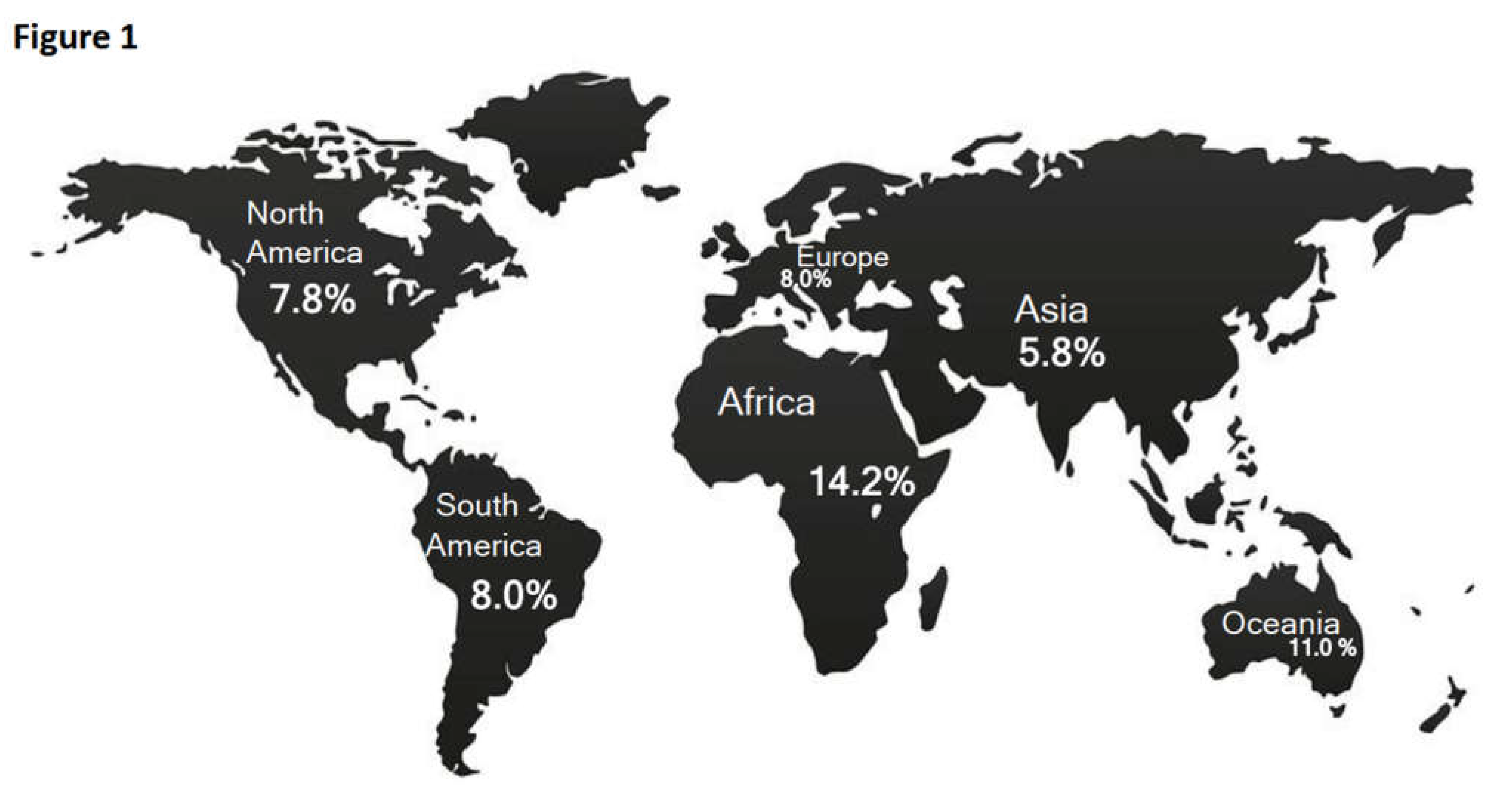
| Continent | Geolocation | ClinicalTrials.gov Identifier |
OFFICIAL TITLE | Start Date* |
Primary Completion Date* |
Study Completion Date* |
Last Verified |
Conclusion |
|---|---|---|---|---|---|---|---|---|
| Africa (n=2) 0.04% |
Egypt | NCT03289403 | The role of immunomodulatory treatment in success of ICSI in patients with autoimmune thyroiditis |
2018 | 2019 | 2019 | 2020 | Completed |
| Israel | NCT01270425 | Sonographic and laboratory evaluation of the thyroid gland in patients with systemic sclerosis |
2011 | 2011 | 2012 (anticipated) |
2013 | Completed | |
| American (n=7) 15.5% |
Brazil | NCT01129492 | Low-level laser therapy in chronic autoimmune thyroiditis |
2006 | 2009 | 2009 | 2010 | Completed |
| Brazil | NCT02240563 | Low level laser therapy for autoimmune thyroiditis |
2014 | 2016 | 2016 | 2017 | Completed | |
| Chile | NCT04778865 | Effect of treatment for vitamin D deficiency on thyroid function and autoimmunity in Hashimoto's thyroiditis |
2020 | 2021 (estimated) |
2021 (estimated) |
2021 | Recruiting | |
| USA | NCT00958113 | Autoimmune thyroid disease genetic study |
2009 | 2013 | 2015 | 2015 | Completed | |
| USA | NCT01428167 | Hashimotos thyroiditis and thyroid cancer (thyroid cancer) |
2011 | 2012 | 2012 | 2012 | Completed | |
| USA | NCT01551498 | Evaluating the dietary supplement anatabloc in thyroid health-ASAP (antabloc supplementation autoimmune prevention) (ASAP) |
2012 | 2013 | 2013 | 2015 | Completed | |
| USA | NCT04542278 | Preoperative steroids in autoimmune thyroid disease |
2020 | 2022 | 2022 | 2022 | Completed | |
| Asian (n= 6) 13.3% |
China | NCT03447093 | The oral microbiota is associated with autoimmune thyroiditis |
2017 | 2019 (estimated) |
2021 (estimated) |
2018 | Unknown |
| China | NCT04075851 | The prevalence of serum thyroid hormone autoantibodies in autoimmune thyroid diseases |
2019 | 2022 (estimated) |
2022 (estimated) |
2021 | Recruiting | |
| China | NCT04942769 | Study on the effect of selenium supplementation on the structure and function of autoimmune thyroiditis |
2019 | 2021 (estimated) |
2021 (estimated) |
2021 | Recruiting | |
| China | NCT03390582 | Gut microbiota is associated with autoimmune thyroid disease |
2017 | 2018 (estimated) |
2021 (estimated) |
2018 | Unknown | |
| Taiwan | NCT02126683 | The effect of Plaquenil on serum inflammatory markers and goiter in euthyroid young women with Hashimoto's thyroiditis |
2014 | 2016 (estimated) |
2016 (estimated) |
2014 | Unknown | |
| Taiwan | NCT01760421 | The effect of Hydroxychloroquine treatment in Hashimoto's thyroiditis |
2011 | 2012 | 2013 | 2014 | Completed | |
| Europe (n=22) 48.8% |
Denmark | NCT02013479 | Selenium supplementation in autoimmune thyroiditis (CATALYST) |
2014 | 2022 | 2022 (estimated) |
2022 | Active, not recruiting |
| France | NCT03114267 | Involvement of viral infections in the pathogenesis of chronic lymphocytic thyroiditis (Etude thyrovir) |
2012 | 2015 | 2015 | 2017 | Completed | |
| France | NCT03103776 | Involvement of polyomaviruses in the pathogenesis of autoimmune Thyroiditis and Goitrigenesis (IPoTAIG) |
2016 | 2018 (estimated) |
2018 (estimated) |
2018 | Unknown | |
| France | NCT04789993 | Additional autoimmune diseases with type 1 diabetes in pediatrics at diabetes diagnosis and during follow-up (AADT1D) |
2021 | 2021 (estimated) |
2021 (estimated) |
2021 | Enrolling by invitation |
|
| France | NCT05544448 | In vitro effect study of Interleukin-2 muteins on regulatory T cells of patients with different autoimmune, allo-immune or inflammatory diseases (MuTreg) |
2022 | 2023 (anticipated) |
2023 (anticipated) |
2022 | Not yet recruiting |
|
| Germany | NCT00552487 | Isolated ACTH deficiency in patients with Hashimoto thyroiditis |
2005 | NA | 2006 | 2007 | Completed | |
| Greece | NCT02491567 | DNA methylation and autoimmune thyroid diseases (THYRODNA) |
2014 | 2016 | 2018 | 2019 | Completed | |
| Greece | NCT02644707 | Selenium supplementation in youths with autoimmune thyroiditis (THYROSEL) |
2014 | 2016 | 2018 | 2020 | Completed | |
| Greece | NCT04693936 | Metabolic biomarkers in hashimoto's thyroiditis and psoriasis |
2021 | 2023 (estimated) |
2024 (estimated) |
2022 | Recruiting | |
| Greece | NCT02725879 | FGF-21 levels and RMR in children and adolescents with Hashimoto's thyroiditis (THYROMETABOL) (THYROMETABOL) |
2016 | 2020 (estimated) |
2020 (estimated) |
2020 | Unknown | |
| Italy | NCT03498417 | Anti-insulin-like growth factor- 1 receptor (IGF-1R) Antibodies in Graves' Disease and Graves' orbitopathy (IGF1RAbsGO) |
2018 | 2018 | 2018 | 2018 | Completed | |
| Italy | NCT01465867 | Selenium supplementation in pregnancy (Serena) |
2012 | 2017 | 2018 | 2018 | Completed | |
| Norway | NCT02319538 | Hashimoto - a surgical disease. total thyroidectomy makes antibodies disappear and ameliorates symptoms |
2012 | 2017 | 2017 | 2018 | Completed | |
| Poland | NCT04752202 | The influence of reducing diets on changes in thyroid parameters in obese women with Hashimoto's disease |
2019 | 2019 | 2019 | 2021 | Completed | |
| Poland | NCT04682340 | Analysis of BPA concentration in serum in women of reproductive age with autoimmune thyroid disease |
2020 | 2021 | 2022 | 2022 | Completed | |
| Romania | NCT04600349 | Identity oriented psychotrauma therapy on Hashimoto in adults |
2020 | 2020 | 2021 | 2021 | Completed | |
| Romania | NCT04472988 | Eye movement desensitization and reprocessing on autoimmune thyroiditis in adults |
2020 | 2020 | 2021 | 2021 | Completed | |
| Switzerland | NCT05017142 | Swiss pediatric inflammatory brain disease registry (Swiss Ped-IBrainD) |
2020 | 2071 (estimated) |
2071 (estimated) |
2021 | Recruiting | |
| Turkey | NCT01102205 | Evaluation of oxidative stress and effect of Levothyroxine treatment on oxidative stress in Hashimoto disease |
2010 | 2010 | 2010 | 2013 | Completed | |
| Turkey | NCT04754607 | Effects of low-level laser therapy on oxidative stress levels, fatigue and quality of life in patients with Hashimoto thyroiditis |
2021 | 2022 | 2022 | 2022 | Completed | |
| Turkey | NCT00271427 | Selenium treatment in autoimmune thyroiditis (AIT) |
2004 | NA | 2005 | 2006 | Completed | |
| Turkey | NCT01644318 | CXCL9 and CXCL11 levels in patients with autoimmune thyroiditis and habitual abortions |
NA | NA | NA | 2012 | Unknown | |
| Unknown (n=8) 17.7% |
Not provided |
NCT01884649 | Fetuin A as a new marker of inflammation in Hashimoto thyroiditis |
2012 | 2012 | 2012 | 2013 | Completed |
| Not provided |
NCT02318160 | Oxidative status in children with autoimmune thyroiditis |
2014 | 2014 | 2014 | 2014 | Completed | |
| Not provided |
NCT04613323 | Management of thyroid function in Hashimoto's thyroiditis during pregnancy |
2022 (estimated) |
2022 (estimated) |
2022 (estimated) |
2021 | Not yet recruiting |
|
| Not provided |
NCT02190214 | Thyroid disorders in Malaysia: a nationwide multicentre study (MyEndo-Thyroid) |
2014 | 2016 | 2016 | 2016 | Completed | |
| Not provided |
NCT03048708 | Thyroid in bariatric surgery (ThyrBar) |
2011 | 2013 | 2016 | 2018 | Completed | |
| Not provided |
NCT02302768 | Effect of Semet (80 and 160 mcg) versus placebo in euthyroid patients with AIT |
2012 | 2014 | 2015 (estimated) |
2014 | Unknown | |
| Not provided |
NCT05435547 | Preoperative corticosteroids in autoimmune thyroid disease |
2022 | 2025 (anticipated) |
2025 (anticipated) |
2022 | Not yet recruiting |
|
| Not provided |
NCT05276063 | A Phase 2b, study of Linsitinib in subjects with active, moderate to severe thyroid eye disease (TED) (LIDS) |
2022 | 2023 (estimated) |
2025 (estimated) |
2023 | Recruiting |
| ClinicalTrials.gov identifier | Target sequences | Analysis and methods | Biospecimen genetic retention and description | Type and model of study | Time perspective** | Enrollment of subjects | Responsible Party | Results Overview |
|---|---|---|---|---|---|---|---|---|
| NCT03114267 | Parvovirus | Analysis of the viral genome by PCR*, Analysis of the presence of capsid protein |
Not provided | Observational, cohort | Retrospective | 64 | Centre Hospitalier Universitaire, Amiens | No publications available |
| NCT03103776 | Polyoma Virus | Positive PCR* Frequencies for Polyomavir us | Blood, Urine and / or Thyroid Tissue | Interventional, parallel assignment | NA | 49 | Centre Hospitalier Universitaire, Amiens | No publications available |
| NCT03447093 | Oral microbiota | Measurement of microbiota by 16S rRNA gene. | Not provided | Observational, case control | Cross-Sectional | 120 | First Affiliated Hospital of Harbin Medical University | Publications available |
| NCT03390582 | Fecal microbiota | Measureme nt of microbiota by 16S rRNA gene. | Human feces | Observational, cohort | Cross-Sectional | 200 | First Affiliated Hospital of Harbin Medical University | No publications available |
| NCT00958113 | HLA, CTLA4, thyroglobulin, THSR, CD40, PTPN2 and PTPN22 | Map and identify genes that confer susceptibility to Autoimmune Thyroid Disease | Saliva | Observational, case control | Cross-Sectional | 199 | University of Colorado, Denver, USA | No publications available |
| NCT02491567 | CD40L, FOXP3, CTLA4, PTPN22, IL2RA, FCRL3 and HLADRB1 | DNA methylatio status of CpGs within gene promoters | Blood (leukocytes) | Observational, case control | Cross-Sectional | 110 | Medical School of Aristotle University of Thessaloniki | Publications available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





