1. Introduction
Raccoons belong to the family
Procyonidae, which includes 18 species in total. The genus
Procyon has 7 species.
Procyon lotor is located in a large geographic area of North America. It extends from southern Canada through most of the continental United States and Central America [
1]. In the United States, regular outbreaks occur in free-ranging raccoons (
Procyon lotor), a species that might have a role in the epidemiology of CDV within domestic dogs in that region [
2]. In Europe, particularly in Romania, the racoons are wild animals kept in zoos, but the utilization as pets may be a new trend.
The disease is caused by a virus from the family of
Paramyxoviridae, genus
Morbillivirus. It is mortal in most cases and affects both wild and domestic animals, with dogs being the most common victims [
3,
4,
5,
6,
7].
The sources of infection can be primary and secondary. The primary sources are ill and recovered animals, which eliminate large amounts of the virus through all their secretions and excretions. Secondary sources include all elements of the environment that have come into contact with pathological products, such as active animated vectors (humans during viremia) or passive vectors such as water, food, means of transport and care, etc..Infection occurs directly through the contact with ill or recovered animals and indirectly through secondary sources. The entrance pathways of the virus into the animal body are respiratory, digestive and conjunctival [
3,
4,
5,
6,
8].
The virus is transmitted extremely easily by air through the infected animal’s secretions. The virus is easily transferred from domestic to wild animals and vice versa. Another way of transmission is the contact with bedding or other objects touched by infected animals. Following the infection, the virus first settles in the tonsils and then reaches the internal organs and blood. Respiratory tract, digestive tract and nervous system are most sensitive to the virus. In domestic animals, any unvaccinated dog is prone to CDV disease, but the most susceptible are dogs that live in poor conditions and are not properly fed. The similar situation is expected to occur in wildlife. [
9,
10]. From a clinical point a view, about 6 days after exposure, fever (around 40°C), ocular and nasal secretions occur. These symptoms may be confused with these of a cold, but they worsen after an animal loses its appetite and becomes less active. Following the initial disease period, diarrhea and vomiting may also occur, leading to dehydration of the affected animal. In dogs, after two weeks, neurological disorders manifested by vertigo appear and dogs may end up not recognizing their owner [
3].
The only way to prevent the disease in dogs is to vaccinate at the age of eight weeks with booster after two weeks. The chances of survival increase depending on how quickly the disease is diagnosed and what the body response to the virus is. A strong immune system gives an animal a high chance of recovery from the disease, and the immunity to the virus can be developed [
11,
12,
13].
The study was conducted to establish the cause of disease of seven raccoons from Timisoara Zoo, and the cause of death of two, through a series of laboratory tests and the necropsy, in support of the hypothesis that the clinical signs and death of two raccoons were caused by the interspecific infection with Canine Distemper Virus.
2. Materials and Methods
The methods included identification techniques of CDV disorders in racoons to compare those with well-known lesions caused by CDV in dogs. This study was performed after receiving the approval from the Ethical Committee of the Faculty of Veterinary Medicine in Timisoara.
2.1. Case history, examination and preliminary treatment.
The veterinarian from Timisoara Zoo reported that two out of seven raccoons (Procyon lotor), aged 7 months, showed clinical signs of impaired digestive system, characterized by severe diarrhea, up to hematemesis, which are common aspects of CDV disease. In five animals, the clinical signs were not observed. For all racoons, the blood samples were collected in EDTA Vacuum Blood Collection Tubes at the beginning of antibiotic treatment, and transported on ice to the infectious diseases laboratory for screening. Although the veterinarian attempted treatment with Enrofloxacin, 5mg/kg body weight, twice a day, and rehydration, two raccoons died after 2 and 3 days, respectively.
2.2. Detection of canine distemper virus by qRT-PCR.
Detection of canine distemper virus in raccoons was performed using the qRT-PCR technique. Blood plasma was obtained after centrifugation of samples. Viral genome extraction was performed from plasma samples using the QIAamp Viral RNA Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The detection of viral particles was performed using the in vitro diagnostic One step Distemper virus detection kit (Bioingentech Biotechnologies, Concepción, Chile) in a qualitative assay using an Mx3005P Real-Time PCR System (Agilent Technologies, Santa Clara, California, USA) according to manufacturer's instructions. Each sample was analyzed in triplicate; a negative control, a positive control and an internal reaction control were used for each run. Results were interpreted according to Ct values as follows: a value lower than 11 and higher than 40 was considered negative, Ct values between 12 and 35 were considered positive, and values higher than 35 and lower than 40 were considered inconclusive.
2.3. Necropsy.
Raccoon cadavers were transported in biosecurity conditions in sealed plastic bags from Timisoara Zoo to the Laboratory of Pathological Anatomy of the Faculty of Veterinary Medicine in Timisoara to establish the cause of death. The necropsy was performed according to the mammalian autopsy technique after skinning the carcasses, opening the thoracic and abdominal cavities, and examining the tissues and organs, with increased attention to those with lesions visible macroscopically.
2.4. Toxicological screening.
Since toxicological examination required a short turnaround time, it preceded the PCR examination. The purpose of this rapid intervention was to save the remaining raccoons, in case of intoxication being the cause of clinical manifestations and death. In order to exclude any possible intoxication, prior to obtaining the results from qRT-PCR, and after the necropsy of both dead raccoons, the liver samples were analyzed to identify the main anticoagulant raticides present on the Romanian market (difenacoum, difetialone, difacinonone, brodifacoum, bromodiolone, chlorofacinone).The technique used here involved high performance liquid chromatography.
2.5. Microbiological and mycosis screening.
To exclude any possible bacterial infections, the inoculations from the small intestine (duodenal and jejunal fragments), liver and lymph nodes, were performedtoisolate the bacterial strains. The primary culture was performed on various media, depending on the species of microorganisms that were suspectedto be involved.For the isolation of Escherichia coli, the primary cultivation was performed using nutrient broth and agar media, acknowledging that these microorganisms are not demanding during cultivation due to the rich enzymatic content. All incubations were performed in aerobic conditions for 24 hours at 37°C. Sabouraud-glucose-agar gel (Beckton Dickinson GmbH, Heidelberg, Germany) with chloramphenicol and gentamicin (50 mg of each antibiotic / 1000 ml) were used for mycological examination. Inoculation was performed in aerobic conditions for 48 hoursat 37°C.
2.6. Immunohistochemical / histopathological examination.
For the histopathological examination, samples were collected from the lung and intestine to highlight the inclusions produced by the virus. Techniques applied in staining intestinal and lung samples included: fixation (formalin), embedding (dehydration, clarification, fixing in the paraffin and inclusion), sectioning (6 µm with the Slee Mainz microtome) and staining (Hematoxylin – Eosin).
A kit containing a specific immunoglobulin conjugate coupled with peroxidase was used to detect CDV (nucleocapsid) antigens present in infected cells. For this purpose, portions of intestine with macroscopic pathological lesions were collected from the carcasses. From each intestinal portion, sufficient tissue amounts were collected to be included into a 3-part working protocol.
In part I, each sample was fixed in 4% paraformaldehyde for 24 hours, after which the samples were washed in tap water and kept in 50% alcohol (1 hour), 70% alcohol (1 hour), 95% alcohol (1 hour), 100% alcohol (1 hour) andalcohol:toluene1: 1 mixture (1 hour). The samples were then placed in paraffin I enclosures and kept inthermostat at 60°C for two hours and in paraffin II inthermostat at 60°C for one hour. The paraffin used had the following composition: 100 g paraffin + 5 g wax.
In part II, the blocks were sectioned using a microtome ( 4 µm thickness), after which they were placed on glass slides. Then, the following steps were taken: paraffin removal with toluene (2 baths for 15 minutes each), rehydration with ethanol (100% 5 min, 96% -5min, 70% - 5 minutes), washing the slides with distilled water and removing excess water, neutralizing endogenous peroxidase with peroxidase block for 10 minutes, washing with TBS 1 (2 baths for 5 minutes), incubating with protein block for 10 minutes and washing with TBS 1 (2 baths for 5 minutes).
The next step performed in the sectioned slides was to add a conjugate consisting of the primary antibody coupled with peroxidase in a dilution of 1:100. Then, the slides were kept in the refrigerator in trays with water until the next day.
In part III, the slides removed from the refrigerator were subjected to the following steps: washing with TBS 1 (2 baths for 5 minutes), incubation with post primary (30 minutes), washing with TBS 1 (2 baths for 5 minutes) and incubation with novolink polymer containing the primary antibody for 30 minutes, rinsing with TBS 1 (2 baths for 5 minutes), treating the slide with 3,3` diamino-benzidine (DAB) for 5 minutes, washing with distilled water. Subsequently, hematoxylin to stain the nuclei, was added to the slides for 40 seconds. Finally, the slides were washed with distilled water (2 baths for 5 minutes), followed by the final washing of the slides with: unyhol, plus and bioclear.
2.7. The electron-microscopic examination.
The electron microscopy examination was performed by the Pasteur National Institute in Bucharest, a partner of the Faculty of Veterinary Medicine in Timisoara. Viral particles were visualized in a suspension (sample) by their specific adsorption on the surface of a double membrane electrolytic network (formvar and carbon), followed by fixation, washing, negative staining (deposition of electro dense substances around viral particles) and examination of transmission under the electron microscope. The smaller the electron-dense substance used, the deeper it penetrates the surface structures of the viruses, obtaining more details. For the direct negative staining method by electron microscopy (EM-DNSM), the fragments of lung and intestine were placed in a suspension consisting of quartz sand (Merck, KGaA, Darmstadt, Germany) and phosphate buffered saline (PBS, 5 ml at pH 7.2 -7.4) in sterile conditions. The obtained suspension was harvested and subjected to clarification centrifugation at 400 xg for 20 min at + 400C. From the resulting supernatant, 50 µl suspension was taken, over which electrolytic grids of 150 msh (copper mesh grids) were covered with double membrane (formvar and carbon) for 1-2 min. The grids were contrasted with 2% uranyl acetate in distilled water, followed by the examination under the electron microscope. The lung and intestine fragments taken were fixed for 30 minutes in cold water in PBS, with 2.5% glutaraldehyde. Subsequently, the parts were post fixed in osmium tetraoxide (OsO4) solution, dehydrated by successive passages in ethyl alcohol baths in increasing concentrations, followed by propylene oxide clarification and included in Epon 812. Ultrafine preparations sectioned at the LKB III ultramicrotome were deposited on electrolytic grids and double contrast with the Reynolds solution.
3. Results
3.1. Clinical outcome.
The five raccoons did not show any clinical signs, which meant that the disease did not affect all the animals. Both dead raccoons with clinical signs had good body condition scores before their death, but had a lower body growth status, lagging behind in development compared to other siblings. Perhaps, due to anorexia, they were deprived of the proteins needed for antibody synthesis and this could have affected their immune system. None of the raccoons, healthy or ill, have been vaccinated against CDV, as the disease had not been reported in zoos in raccoons or other disease-susceptible species in this habitat. Possibly, the infection and disease were caused by secondary sources of infection through the active animated vectors (animal caregivers during the possible period of viremia) or the passive abiotic vectors (water, food, means of transport and care, etc.).This can be explained by the fact that the zoo is located on the outskirts of the city of Timisoara in a wooded area and many stray dogs can reachit (unfortunately, the problem of stray dogs in Timisoara is currently still not fully resolved). Unvaccinated dogs have a high prevalence of this disease and can be sources of infection. Moreover, they could have come into contact with animal lovers (visitors, caretakers, etc.) who later entered the zoo and came into contact with raccoons.
3.2. Detection of canine distemper virus by qRT-PCR.
Following the analysis by qRT-PCR, viral genome was detected in all seven whole blood samples collected. The results are presented in
Table 1.1and dead animals are highlighted.
Detecting CDV by qRT-PCR method is a common technique in CDV diagnosis in dogs [
22,
23] and it can be used in raccoons as well.
3.3. Necropsy exam.
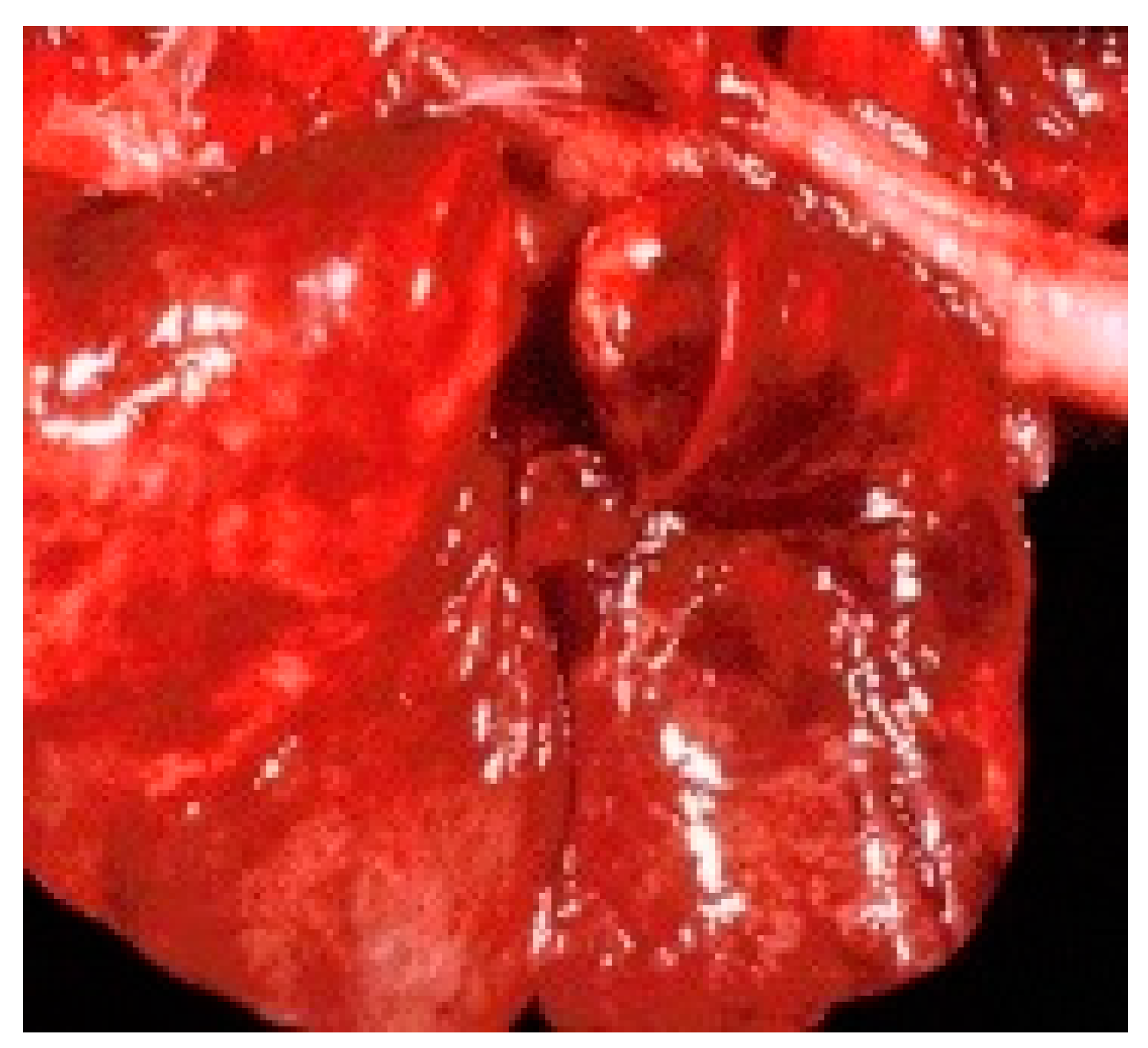
Following the necropsy, several lesions were noted, such as: hemorrhagic gastroenteritis (
Figure 1), pulmonary congestion (
Figure 2), pharyngeal ulcers, foci of necrosis on the surface of the liver and pancreas. The severity of the clinical signs and the observed anatomo-pathological lesions proved the septicemic evolution of the disease.
3.4. Toxicological screening.
Due to the presence of hemorrhagic lesions, toxicological examinations were performed using the classical method of determining anticoagulants to rule out possible poisoning with raticides. The results were negative.
3.5. Microbiological and mycosis exam.
Based on the biochemical characteristics of the xylose lysine deoxycholate agar subculture (XLD) (Microbiology Labor-Technik, Arad, Romania), it has been concluded that the isolated bacterial cultures were Escherichia coli.Escherichia coli and Candida spp. were identified bybacteriological examination of samples taken from the intestine, liver and lymph nodes. The morphological characteristics of the isolated strains, observed on smears stained with methylene blue, revealed the presence of Candida spp. In the case of Candida spp.,the samples for the histopathological examination were collected from the brain, lungs and intestines.
3.6. Immunohistochemical / histopathological examination
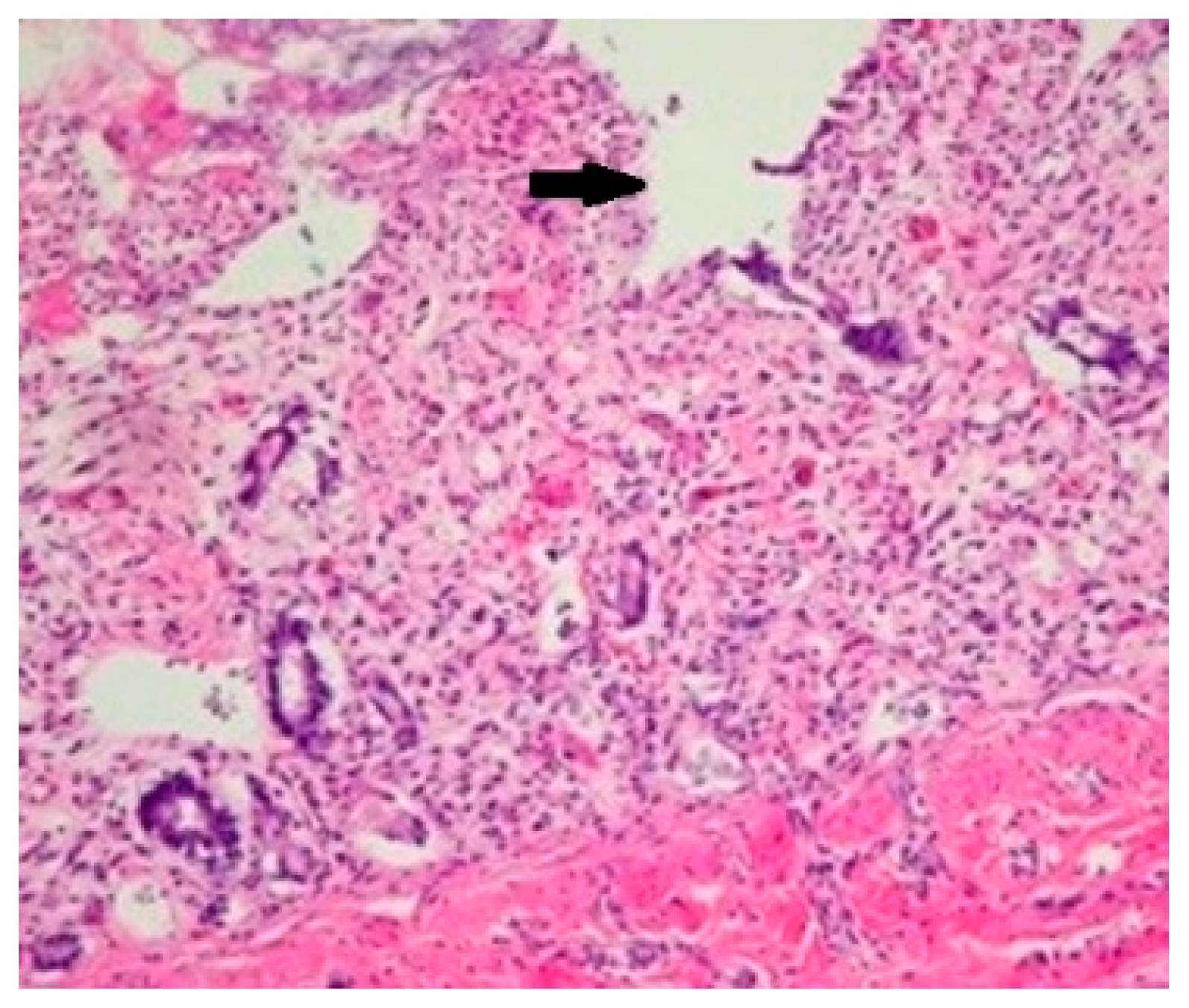
Due to the fact that the histopathological lesions of the digestive tract resemble the lesions of parvovirus, namely the necrosis of the glandular crypts (
Figure 3), the immunohistochemical examination was performed to exclude it. However, the results were negative, as they lacked brown areas characteristic to the antigen-antibody reaction in the case of positive samples.
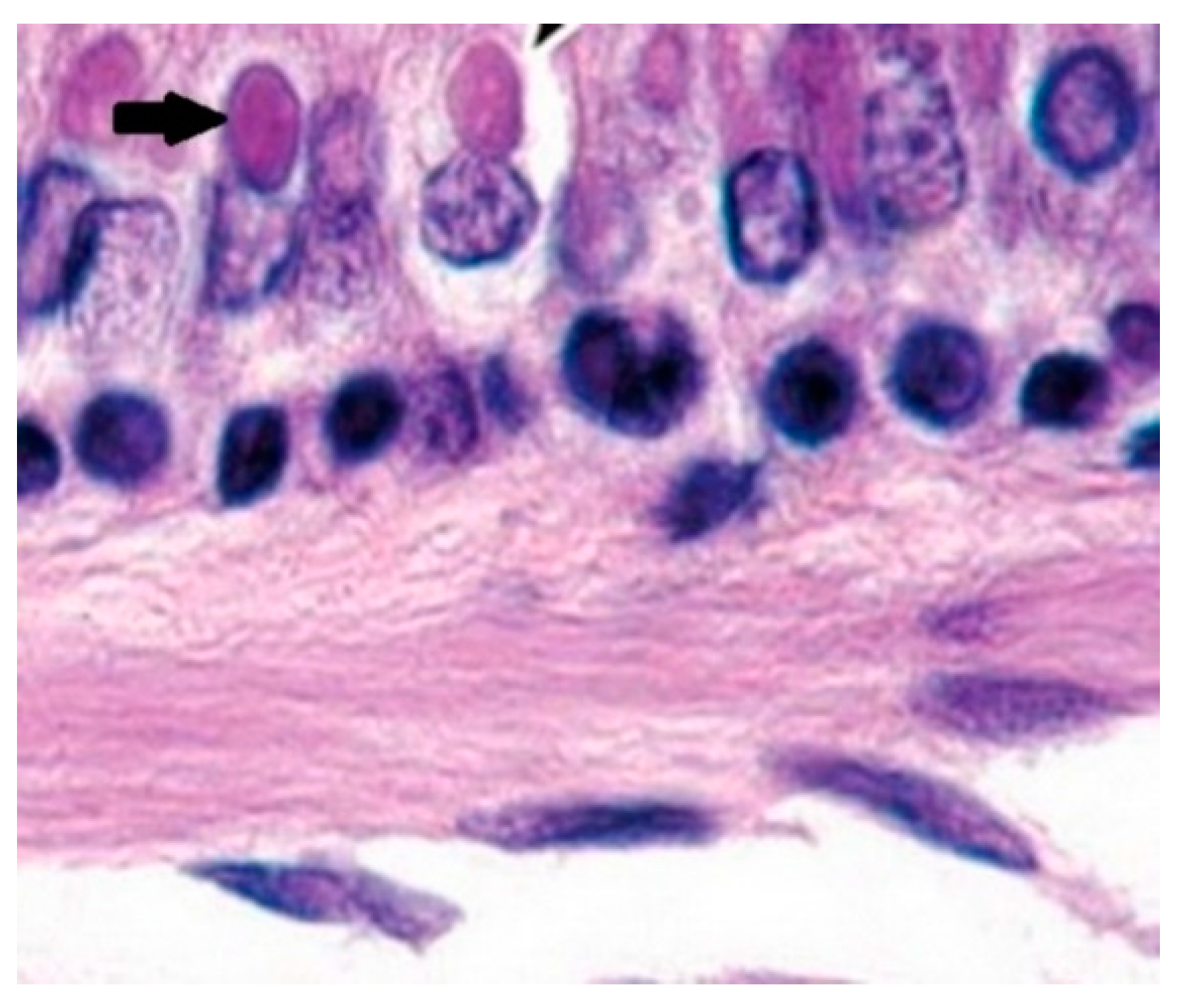
In the lung samples, after performing the histopathological examination, inclusions induced by both intracytoplasmic viruses (eosinophils) and intranuclearviruses were observed (
Figure 4 and
Figure 5). Examination of the small intestine revealed nematodes of the genus
Ascaris spp. Their presence explains the evidence of eosinophils during the histopathological examination. Additionally, even if no signs were reported, histopathological examinations of the brain were performed but the results were negative. These characteristic lesions occur very rarely, but some adenoviruses may cause inclusions.
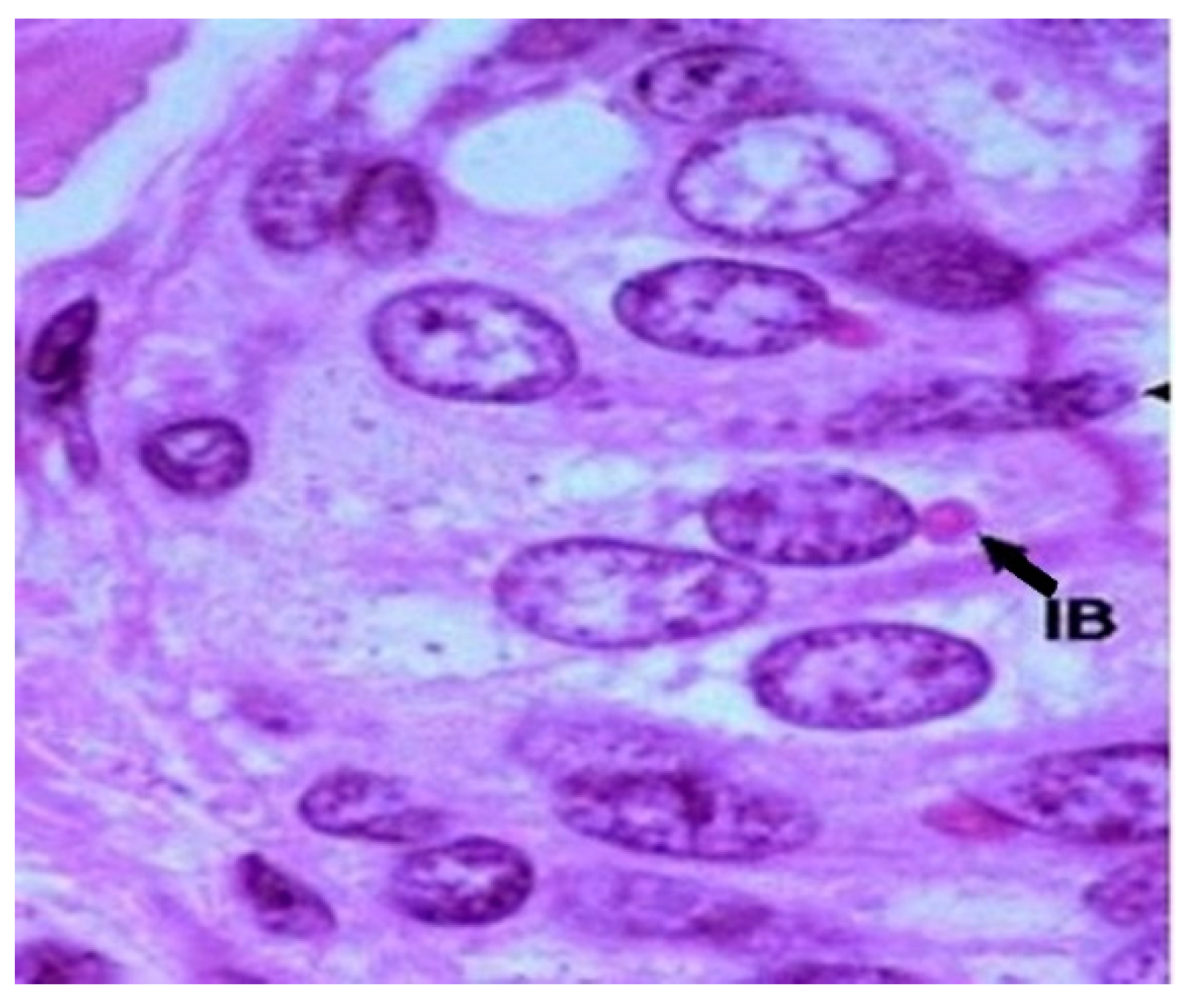
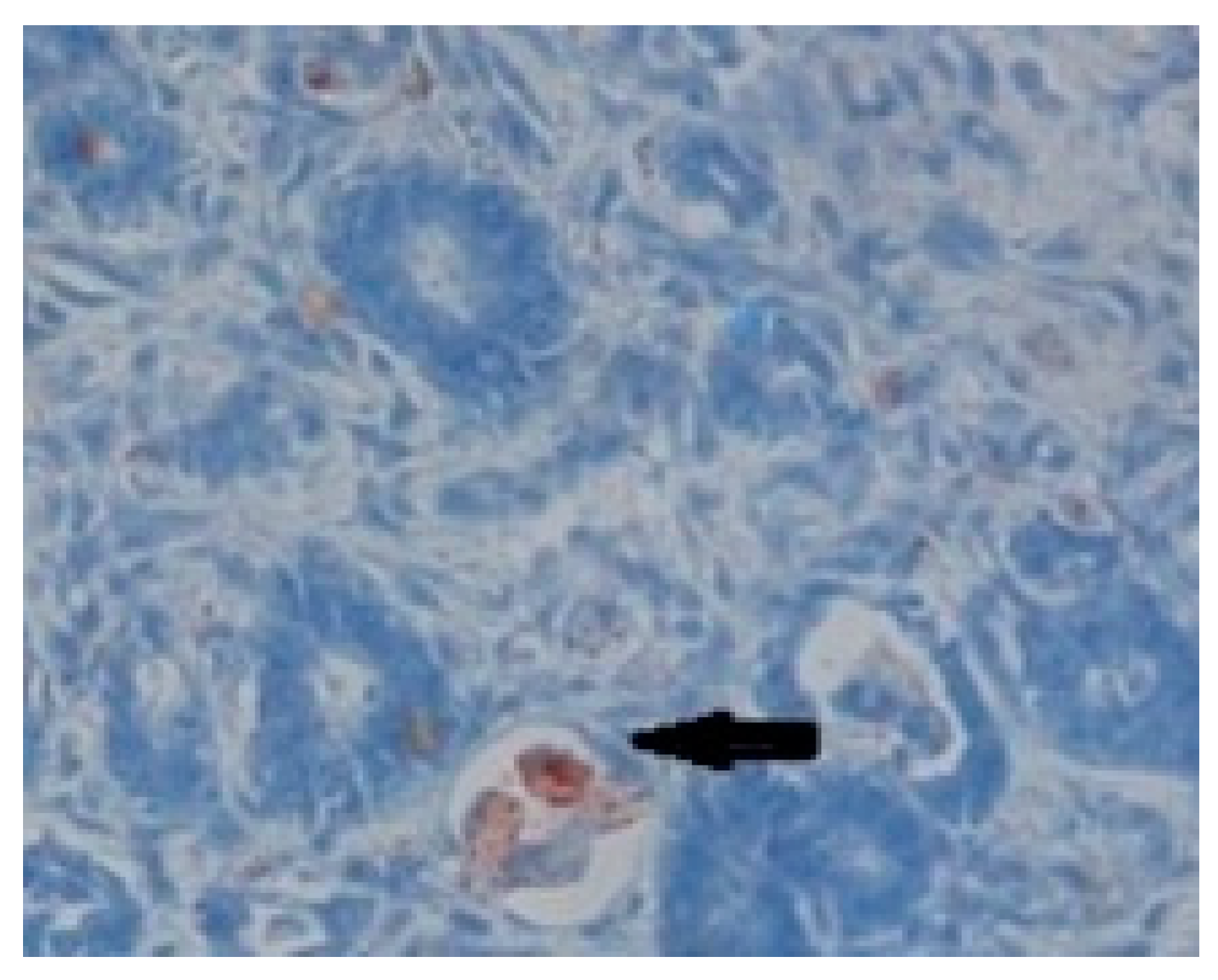
Immunohistochemical analysis allowed the fixation of the primary antibodies coupled with peroxidaseby the viral nucleocapsid. Subsequently, the antigen-antibody-peroxidase complexes were visualized with the help of secondary antibodies and by adding 3,3`-diaminobenzidine, which reacts with peroxidase and results in a dark brown granular color indicating the presence of viral antigens in the cytoplasm of the cells. In the examined sections, browncolorwas observed and it was present in the cytoplasm of the cells from the germinal centers of the medullary area (
Figure 6). This aspect is considered a confirmation of the CDV, and it represents a positive for the presence of viral antigens in the cytoplasm of enterocytes.
The inclusions produced by morbilliviruses have been reported by some researchers in cats [
3], while in raccoons they have not been shown. Thus, to confirm the etiological diagnosis with certainty, we utilized electron microscopy.
3.7. The electron-microscopic examination
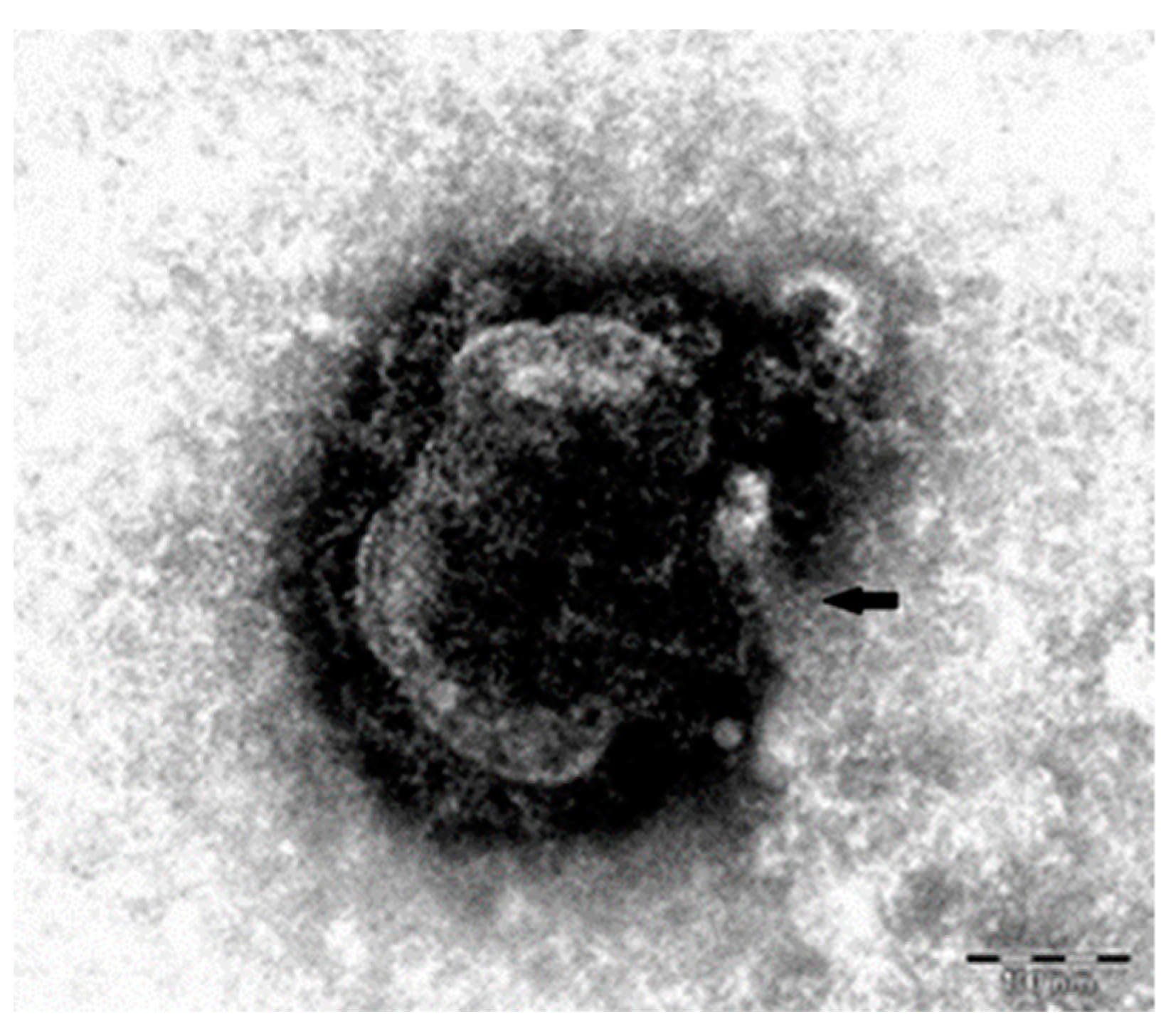
Following this examination, ultrastructural aspects of enterocytes were highlighted: electron-dense, enveloped agglomerations with spherical geometry, diameter of about 150 nm, characteristic of morbilliviruses that replicate in the cytoplasm by attaching the virus to the host cell (
Figure 7), aspect also reported by Habermann et al. [
14].
Electron microscopic examination revealed viral particles characteristic of morbilliviruses in lung samples.
4. Discussion
There is no special treatment to cure this disease, but there are ways to treat the symptoms. Depending on the severity of the condition and how advanced the disease is, fluid infusions are recommended if the animal is dehydrated and a drug treatment is administered to control episodes of vomiting and encephalitis. The response to the maintenance treatment and the treatment of CDV disease differ, depending on the sizes of the animals, their health, their age and how advanced the disease is. The best way to prevent the disease is to get them vaccinated with CDV vaccines at the age of two months with a booster administered two weeks later. [
14,
20,
21]. Although general immunity to this disease is not described in the literature, it has played an important role in the onset and evolution of the CDV infestation.
The clinical and lesion manifestations in CDV vary from respiratory to digestive, cutaneous and nervous. The clinical signs found in raccoons were similar to those found in dogs [
25], the animal species that is most commonly affected by this disease, and included: digestion, vomiting, diarrhea, hematemesis. Due to the fact that the death occurred after 2 and 3 days respectively, no clinical signs of nervousness were reported, as these usually set in after approximately 14 days from the onset of the disease [
15].As othersreport[
22], the disease due to CDV may range from subclinical to severe and is sometimes fatal. Clinical signs may include fever, inappetence, coughing, ocular or nasal discharge, difficult respiration, vomiting, diarrhea, lethargy, and/or neurologic abnormalities. Neurologic disease may occur during the acute infection or may manifest in the weeks following exposure. Clinical signs may also include muscle twitching, weakness, blindness or even seizures. Infection of the tissues of the nasal planum and footpads may result in excessive or thickened tissue. Puppies surviving CDV may have enamel defects of their permanent teeth.
Diagnostic testing for CDV in racoons can be performed by analyzing nasal swabs as well as tissue samples by PCR, like in dogs. From our experience with the PCR analysis, an ideal sample type varies based on clinical signs. The recommendation for PCR exam is made after observing the following clinical signs: gastrointestinal signs (whole blood and/or feces), respiratory signs (nasal, pharyngeal, or ocular swabs) or neurologic signs (whole blood, urine, and/or a conjunctival SWAB).
Lesions reported during the gross necropsy examination of raccoons included those observed by other researchers in dogs with this disease, e.g. severe dehydration due to severe enteric phenomena, pulmonary congestion, hemorrhagic enteritis [
12].
Like in dogs, the presence of bacteria and fungi did not cause the death of the raccoons, because the lesions present were not characteristic of such. The presence of bacteria and fungi was also reported by Konjević et al. [
16].The infection of lymphoid cells by CDV leads to immunosuppression, the severity of which can lead to variability in the clinical disease with the potential of secondary bacterial infection (for this reason the antibiotic treatment is used), up to and including the development of neurological signs in its later stage [
23]. Additionally, Ascaris nematodes have been identified, an observationalso made by Kazacos et al. [
17].
The histopathological lesions characteristic of CDV infection, namely intracytoplasmic and intranuclear inclusions, corresponded to those mentioned by other researchers in racoons [
13] as well as in dogs [
26].
The results obtained by using the IHC technique are similar to the results found in the literature on the use of this method in the diagnosis of CDV [
18,
19], such as in dogs [
26].
Clinical signs (characterized by severe diarrhea which led to severe dehydration, and eventual death by hypovolemic shock), histopathological results (including immunohistochemical observations) and electron microscopy images are comparable between a dog and a racoon. The implications of the CD viruses in clinical signs, immunological response, body organs damage (followed by death) are also comparable between dogs and racoons.
As stated in the literature, during the CDV evolution, immunity has played an important role in triggering the disease and maintaining its severe evolution. Due to the high morbidity and mortality rates and broad host range (domestic dogs and wildlife, in at least six orders and over 20 families of mammals), understanding the epidemiology of CDV is important for its control in both, domestic animals and wildlife [
27]. From the epidemiological point a view, canine distemper cases in Timisoara Zoo raccoons increase the risk of transmission to pets in the Timisoara area. Unvaccinated or improperly vaccinated dogs between 3 and 6 months of age are at greatest risk of infection [
28]. Through the reports in literature [
27,
28,
29,
30] and through the obtained results, the interspecific infection is clearly possible and the epidemiological risk of infection transmitted by racoons to domestic animals, such as dogs, and cats is present.
5. Conclusions
Canine distemper virus generally affects dogs (Family: Canidae, Subspecies: Canis lupus familiaris), but it can also be identified in other species such as raccoons(Family: Procyonidae,Genus Procyon loto). Clinical signs (characterized by severe diarrhea which leads to severe dehydration and eventual death caused by hypovolemic shock), histopathological results (including immunohistochemical observations) and electron microscopy images are comparable between dogs and racoons. The easiest and the highest accuracy method to diagnose CDV involves performing a PCR assay on the harvested blood, nasal swabs or tissue samples.
Author Contributions
Conceptualization, S.A and H.I.; methodology, S.A.; validation, H.I.; formal analysis, P.S.A and L.I ; investigation, H.A.S and B.O; resources L,I.; data curation, A.S and L.B.; writing—original draft preparation, V.O.S.; writing—review and editing, I.O.R.; visualization, P.S.A.; supervision, H.I.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Experimental Units of Horia Cernescu Research Unit–Contract no. 4833 of 04.09.2014 and the APC was funded by Life Science University Timisoara.
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Committee of Experimental Units of Life Science University - Faculty of Veterinary Medicine in Timisoara.
Data Availability Statement
The data presented was obtained from all subjects involved in this study. Data Availability Statements are available.
Acknowledgments
The authors acknowledges to the Experimental Units and University of Life Science for financial support. Also the authors acknowledges for the technical support, materials and sample processing to Laboratories from Horia Cernescu Research Unit Timisoara and to the Pasteur National Institute in Bucharest.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamir, A.N. Pathology of neurologic disorders of raccoons (Procyon lotor). J. Veter-Diagn. Investig. 2011, 23, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R.J. Canine distemper in terrestrial carnivores: A review. J. Zoo Wildl. Med. 2000, 31, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, B.; Knotek, Z.; Farkas, J.; Ádám, É.; Dobay, O.; Nász, I. Adenovirus Infection in Cats. An Epidemiological Survey in the Czech Republic. Acta Veter-Brno 1999, 68, 275–280. [Google Scholar] [CrossRef]
- Frölich, K.; Czupalla, O.; Haas, L.; Hentschke, J.; Dedek, J.; Fickel, J. Epizootiological investigations of canine distemper virus in free-ranging carnivores from Germany. Veter-Microbiol. 2000, 74, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Munson, L. Feline Morbillivirus infection. In Infectious Diseases of Wild Mammals; Williams, E.S., Barker, I.K., Eds.; Blackwell Publishing: London, UK, 2001; pp. 59–62. [Google Scholar]
- Creevy, K.E.; Evans, J.B. Canine Distemper in MDS Manual–Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA.
- Shi, N.; Le, Z.; Xiuhua, Y.; Xiangyu, Z.; Shu, Z.; Daining, Z.; Ming, D. Insight into an Outbreak of Canine Distemper Virus Infection in Masked Palm Civets in China. Front Vet Sci. 2021, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.M.; Saenz, J.R. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet Res. 2016, 12, 1–11. [Google Scholar]
- Headley, S.A.; Oliveira, T.E.S.; Pereira, A.H.T.; Moreira, J.R.; Michelazzo, M.M.Z.; Pires, B.G.; Marutani, V.H.B.; Xavier, A.A.C.; Di Santis, G.W.; Garcia, J.L.; et al. Canine morbillivirus (canine distemper virus) with concomitant canine adenovirus, canine parvovirus-2, and Neospora caninum in puppies: A retrospective immunohistochemical study. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Lempp, C.; Spitzbarth, I.; Puff, C.; Cana, A.; Kegler, K.; Techangamsuwan, S.; Baumgärtner, W.; Seehusen, F. New Aspects of the Pathogenesis of Canine Distemper Leukoencephalitis. Viruses 2014, 6, 2571–2601. [Google Scholar] [CrossRef]
- Jamison, R.K.; Lazar, E.C.; Binn, L.N.; Alexander, A.D. Survey for antibodies to canine viruses in selected wild mammals. J. Wildl. Dis. 1973, 9, 2–3. [Google Scholar] [CrossRef]
- Williams, E.S.; Barker, I.K. Infectious Diseases of Wild Mammals, 3rd ed.; Iowa State University Press: Ames, IA, USA, 2001; p. 43. [Google Scholar]
- Cranfield, M.R.; Barker, I.K.; Mehren, K.G.; Rapley, W.A. Canine Distemper in Wild Raccoons (Procyon lotor) at the Metropolitan Toronto Zoo. Can Vet J. 1984, 25, 63–66. [Google Scholar]
- Habermann, R.T.; Herman, C.M.; Williams, F.P. Distemper in raccoons and foxes suspected of having rabies. J Am Vet Med Assoc. 1958, 132, 31–35. [Google Scholar] [PubMed]
- Shabbir, M.Z.; Rabbani, M.; Ahmad, A.; Ahmed, A.; Muhammad, K.; Anwar, I. Comparative evaluation of clinical samples from naturally infected dogs for early detection of canine distemper virus. Turk. J. Veter- Anim. Sci. 2010, 34, 547–552. [Google Scholar] [CrossRef]
- Konjević, D.; Sabočanec, R.; Grabarević, Ž.; Zurbriggen, A.; Bata, I.; Beck, A.; Kurilj, A.G.; Cvitković, D. Canine distemper in Siberian tiger cubs from Zagreb ZOO: Case report. Acta Veter-Brno 2011, 80, 47–50. [Google Scholar] [CrossRef]
- Kazacos, K.R.; Wiriz, W.L.; Burger, P.P.; Chrisimas, C.S. Raccoon ascarid larvae as a cause of fatal central nervous system disease in subhuman primates. J Am Vet Med Assoc. 1981, 179, 1089–1094. [Google Scholar]
- Haines, D.M.; Martin, K.M.; Chelack, B.J.; Sargent, R.A.; Outerbridge, C.A.; Clark, E.G. Immunohistochemical detection of canine distemper virus in haired skin, nasal mucosa, and footpad epithelium: A method for antemortem diagnosis of infection. J. Veter-Diagn. Investig. 1999, 11, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.G.; Karikalan, M.; Siddappa, C.M.; Mahendran, K.; Srivastava, G.; Rajak, K.K.; Bhardwaj, Y.; Varshney, R.; War, Z.A.; Singh, R.; et al. Molecular and pathological screening of canine distemper virus in Asiatic lions, tigers, leopards, snow leopards, clouded leopards, leopard cats, jungle cats, civet cats, fishing cat, and jaguar of different states, India. Infect. Genet. Evol. 2022, 98, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Robinson, V.B.; Newberne, J.W.; Brooks, D.M. Distemper in the American raccoon (Procyon lotor). J Am Vet Med Assoc. 1957, 131, 276–278. [Google Scholar] [PubMed]
- Maurer, K.E.; Nielsen, S.W. Neurologic disorders in the raccoon in northeastern United States. J Am Vet Med Assoc. 1981, 179, 1095–1098. [Google Scholar]
- Wang, J.; Wang, J.; Li, R.; Liu, L.; Yuan, W. Rapid and sensitive detection of canine distemper virus by real-time reverse transcription recombinase polymerase amplification. BMC Veter-Res. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Di Trani, L.; Buonavoglia, C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 2006, 136, 171–176. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Khosravi, M.; Alves, L.; Ader-Ebert, N.; Bringolf, F.; Zurbriggen, A.; Plemper, R.K.; Plattet, P. Canine Distemper Virus Envelope Protein Interactions Modulated by Hydrophobic Residues in the Fusion Protein Globular Head. J. Virol. 2015, 89, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.F.; Baumgärtner, W.; Tontis, D.; Polizopoulou, Z.; Saridomichelakis, M.N.; Lekkas, S. Histopathology and Immunohistochemistry of Canine Distemper Virus-induced Footpad Hyperkeratosis (Hard Pad Disease) in Dogs with Natural Canine Distemper. Veter-Pathol. 2004, 41, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruíz-Sáenz, J. Evolution and Interspecies Transmission of Canine Distemper Virus—An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef]
- Niederwerder, M., Boyer, N., Canine Distemper: A Recent Uptick in the Number of Positive Raccoon Diagnostic Samples Underscores the Importance of Vaccination in Dogs. https://ksvdl.org/resources/news/diagnostic_insights _for_technicians/october2017/raccoons_CDV.html.
- Beineke, A.; Baumgärtner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus—An update. One Heal. 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Kapil, S.; Yeary, T.J. Canine Distemper Spillover in Domestic Dogs from Urban Wildlife. Veter- Clin. North Am. Small Anim. Pr. 2011, 41, 1069–1086. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).