1. Introduction
Carbapenems effectively treat serious infections because of their broad spectrum covering multidrug-resistant (MDR) gram-negative bacteria, such as extended-spectrum β-lactamase (ESBL)-producing or AmpC-hyperproducer Enterobacteriaceae, and nosocomial non-fermenters, such as
Pseudomonas aeruginosa and
Acinetobacter baumannii [
1]. Over the past decade, the prevalence of MDR gram-negative pathogens has considerably risen, contributing to the global escalation in carbapenem usage [
2,
3]. Carbapenems are the third most used antibiotic for community-acquired infections in the intensive care unit (ICU) (10.7%) and the first for hospital-acquired infections (21.5%) [
3]. Excessive carbapenem use leads to an increased cost burden, adverse effects, and patient mortality [
4]. Excessive carbapenem consumption is an important predisposing factor for worsening infections rates caused by multidrug-resistant multidrug-resistant
P. aeruginosa (MRPA),
A. baumannii (MRAB), and carbapenem-resistant Enterobacteriaceae (CRE) [
5,
6,
7]. Recently, the growing incidence of carbapenem-resistant gram-negative bacilli has become an urgent global healthcare challenge [
8]. Therefore, the appropriate carbapenem use is an important patient safety, public health, and a national priority.
Approximately 66% of patients in a French survey were prescribed carbapenems on an empirical basis for a median duration of 8 days in the ICU [
9]. In a previous study, we reported that carbapenems are prescribed to approximately half the patients (44.4%) in the highest amount (301.2 days of therapy (DOT) per 1000 patient-days) within the last two weeks of life [
10]. A better understanding of carbapenem prescribing habits will help develop comprehensive recommendations for carbapenem use in patients in their last days of life.
We conducted a post-hoc analysis of a previous nationwide study to investigate the current carbapenem prescribing status to patients within their last days of life to guide the judicious use of carbapenems.
2. Results
2.1. Characteristics of carbapenem use in patients within the last 2 days of their life
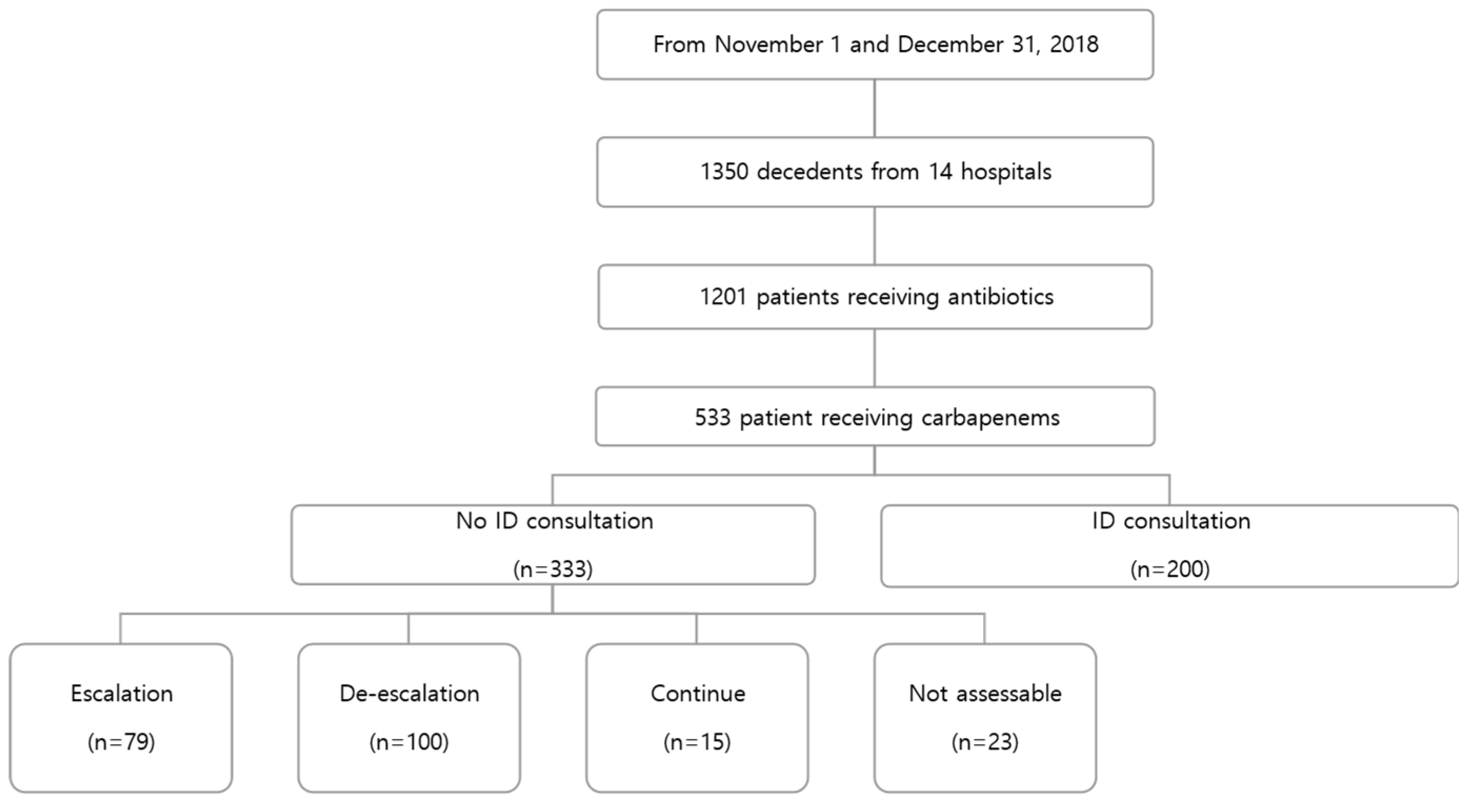
A total of 1,350 patients died at 14 hospitals during the study period. A total of 1201 patients received an antimicrobial agent during the last two weeks of their lives, of whom 533 (44.4%) received at least one carbapenem (
Figure 1). The median carbapenem treatment duration was 7 (3-12) days. The most prescribed carbapenem was meropenem (n=444, 83.3%).
The median patient age was 71.0 years. A total of 224 (42.0%) patients had an underlying cancer. Nearly two-thirds (n=341, 64.0%) of the patients died due to an infectious disease, Cancer was the second most common cause of death (n=110, 20.6%). At the time of death, 407 patients (76.0%) had LST documents. A total of 510 (95.7%) patients had microbiological samples drawn and 196 (36.8%) yielded carbapenem-resistant pathogens. Of the 533 patients receiving carbapenems, only 200 (37.5%) were referred to ID specialists; formal ID consultations were not requested for 333 (62.5%) patients. Among these 333 patients, 79 (23.7%) were assessed as requiring escalation, 100 (30.3) as de-escalation, 116 (34.8%) as continuation, 15 (4.5%) as discontinuation, and 23 (6.9%) were not assessable (
Table 1).
2.2. Comparison of characteristics between “optimal” and “not optimal” carbapenem prescriptions in patients without an ID specialist consultation
After an ID specialist review, 194 (62.6%) prescriptions were assessed as “not optimal,” and 116 (37.4%) were assessed as “optimal.”
Table 2 shows the characteristics of patients prescribed either “not optimal” or “optimal” antibiotics. The carbapenem treatment duration, sex, age, underlying comorbidities, cause of death, LST form completion, MDR pathogens isolated, and antibiotic class used showed no differences between the two groups. The number of antibiotic changes during the last 14 days of their life was significantly less in the “not optimal” group (odds ratio [OR] 0.83, 95% confidence interval (CI): 0.71-0.97, P = 0.023) than in the “optimal” group.
3. Discussion
This nationwide cohort study provided insights into the current practice of carbapenem use within the last few days of life. We demonstrated that carbapenems were frequently administered to patients within the last few days of life, and 36.8% of patients with carbapenem treatment had carbapenem-resistant pathogens. Although carbapenem prescription was universally restricted by a computerised antibiotic control program, a considerable amount (62.5%) was prescribed without an ID specialist consultation, of whom only a small portion of carbapenem use (34.8%) was “optimal”.
Carbapenems are the drugs of choice for infections caused by MDR bacteria, such as ESBL-producers and several non-fermenters. However, studies have shown that there is a link between carbapenem use and resistance at both the individual and unit levels of infecting flora and gut microbiota [
11,
12,
13,
14]. Additionally, carbapenem use has been associated with an increased risk of colonization of carbapenem-resistant
K. pneumoniae in the ICU [
15]. Our study showed that 533 (39.5%) patients received at least one carbapenem among the 1,350 decedents during the last two weeks of their lives; microbiologic analysis of these patients yielded carbapenem-resistant pathogens in 36.8%. However, we did not investigate the causal relationship between carbapenem use and resistance. Numerous uncontrolled factors, such as infection control and the impact of non-carbapenem antibiotics, could have affected the emergence of carbapenem-resistant organisms [
12].
Carbapenem use was controlled using a computerised antibiotic restriction program at all centres during the study period. The ID specialists at most centres provided recommendations on whether carbapenem should be continued for a specific period or discontinued after a chart review. Notwithstanding the existing antibiotic restriction program system and the high microbiological study performance (95.7%), only 116 (34.8%) prescriptions were assessed as “optimal” among the 333 carbapenem prescriptions without a formal ID specialist consultation. The duration of carbapenem use was similar among patients with and without microbiological results in a French survey [
9]. Hence, the authors suggested that the antibiotic stewardship program (ASP) of carbapenem prescriptions may not be efficient in controlling its prescription, and antibiotic consultations may help in achieving de-escalation [
9]. In our study, 194 (58.2%) of the 333 patients without ID consults were assessed as “not optimal”: 79 (23.7%) required escalation, 100 (30.0%) required de-escalation, and 15 (4.5%) discontinued. In addition, the number of antibiotic changes was significantly less in the “not optimal” group. Therefore, we assumed that many physicians continue carbapenems without ID consults although antibiotic change is needed. A previous study showed that antimicrobial use is influenced not only by ASP but also ID specialist consultation [
6]. ID consultation lead the reduction of carbapenem use, resulting favorable outcomes like shorter hospital stay and reduced mortality [
6]. A study conducted in Germany demonstrated that prospective audits and feedback from ID specialists lead to the reduction of both the overall use of antimicrobial agents and the proportion of broad-spectrum antibacterial use [
16]. A Swedish study established that an ID specialist-guided antimicrobial stewardship program consisting of prospective audits twice weekly profoundly reduced antibiotic use with no negative effect on patient outcomes [
17]. ID-guided consultations for
Staphylococcus aureus bacteraemia demonstrated better survival benefits and improved use of guideline-recommended strategies compared with patients without consultations [
18]. Although comprehensive ASP with additional ID specialist consultations may be burdensome, it might be necessary for the optimal antibiotic use in patients within the last 2 days of their life.
The combination of ID consultation and ASP also appears to enhance appropriate therapy in terminally ill patients [
19,
20]. In a study involving 459 patients in their terminal stages of illness, it was found that cessation of antibiotics after interventions by ASP did not result in higher mortality rates [
21]. The ASP team, which included clinical pharmacists and ID physicians, conducted audits of patients who were prescribed intravenous antibiotics, including carbapenem, fluoroquinolones, and piperacillin-tazobactam [
21]. Frequent antibiotic consultant interventions may help decrease carbapenem use even in terminally ill patients.
Our study had a few limitations. First, the computerised antibiotic control programme adherence to treatment recommendations was not objectively investigated. The effect of ASP on the behaviour of professionals varied considerably across studies [
22]. Second, physicians at each hospital participating in the evaluation assessed the optimality of antibiotics, which could lead to variations in their evaluations Nevertheless, we attempted to address this limitation by enlisting the expertise of an ID specialist. Third, this research was unable to establish causality definitively as a retrospective and epidemiological study. Last, detailed information regarding patient comorbidities was not available, and no assessment of the stage of illness was conducted. The definition of the last days of life was not clear due to patient characteristics; however, all deceased patients were included because the goal of the study was to examine the last antibiotic administered regardless of the patient’s disease status.
4. Materials and Methods
4.1. Study setting and population
The multicentre retrospective cohort study was conducted in 14 South Korean teaching hospitals between 1 November 2018 and 31 December 2018. All centers had appointed infectious disease (ID) specialists. During the study period, carbapenem use was universally restricted by a computerised antibiotic control program. The ID specialists provided recommendations on carbapenem use or discontinuation within 72 h, based on the clinical status and microbiological results through a chart review. A follow-up evaluation was performed after the approved period of carbapenem use, which was set by the ID specialist. The study included all patients above 18 years of age who passes away during the study period at each hospital, identified through a review of their electronic medical records. The Institutional Review Board of Kyungpook National University Chilgok Hospital (KNUCH 2019-09-008) and all the participating hospitals granted approval for the study, and the requirement for informed consent was waived given the retrospective observational nature of the study involving deceased patients.
4.2. Data collection and Definitions
The following data were obtained from the patient medical records using a standardised case report form: demographics, date of completion of life-sustaining treatment (LST) documents, class and number of antimicrobial agents used during the last 2 weeks of life, use of antimicrobial agent on the day of death, ID specialist consultation, microbiological testing, and isolation of MDR organisms. The amount of antimicrobial agent administered was determined by calculating the DOT per 1,000 patient-days. ID specialists retrospectively assessed carbapenem prescriptions without formal ID consultations as needing escalation, de-escalation, continuation, discontinuation, or not assessable. Cases assessed as “needing continuation” were considered “optimal” antibiotic prescription; others were classified as “not optimal” antibiotic prescriptions. MDR organisms included were methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), MRPA, MRAB, and CRE.
4.3. Statistical analysis
Frequencies and percentages were used to present discrete data, while continuous variables were expressed as mean ± standard deviation or as median and interquartile range following the Shapiro–Wilk normality test. The χ2, Fisher’s exact, two-sample t-, or Mann–Whitney U-tests were used, as appropriate to compare characteristics between subgroups of optimal and not optimal carbapenem prescribing practice. Factors associated with not optimal carbapenem administration were analysed using univariate and multivariate logistic regression analyses. When the distribution of continuous data was skewed, log transformations were applied for univariate analyses. Variables with a P value of <0.10 in the univariate analysis were considered for the multivariate analysis. IBM SPSS Statistics for Windows (version 23.0; IBM Corp., Armonk, NY, USA) was used to perform all analyses.
5. Conclusions
In conclusion, carbapenems are widely administered to patients in their last days of life, and a considerable proportion of them are administered inappropriately. Frequent consultant with antibiotic specialists in addition to antimicrobial control programs may be necessary to ensure appropriate carbapenem use.
Author Contributions
Conceptualization, K.K.T., C.H.H., K.S.W.; methodology, K.K.T., C.H.H., K.S.W.; software, W.Y.M., H.S., B.S., K.Y.; validation, W.Y.M., H.S., B.S., K.Y.; formal analysis, W.Y.M., H.S., B.S., K.Y.; investigation, W.Y.M., H.S., B.S., K.Y., C.H.S., L.S., J.D.S., S.K.M., M.C., H.S.T., K.B., L.M.S., H.J., K.J., Y.Y.K..; resources, K.K.T.; data curation, W.Y.M., H.S., B.S., K.Y., C.H.S., L.S., J.D.S., S.K.M., M.C., H.S.T., K.B., L.M.S., H.J., K.J., Y.Y.K.; writing—original draft preparation, W.Y.M, K.K.T.; writing—review and editing, W.Y.M., J.C.H., K.S.H., H.S., B.S., K.Y., C.H.S., L.S., J.D.S., S.K.M., M.C., H.S.T., K.B., L.M.S., H.J., K.J., Y.Y.K.; visualization, W.Y.M., H.S., B.S., K.Y.; supervision, K.K.T.; project administration, K.K.T.; funding acquisition, K.K.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Biomedical Research Institute grant from Kyungpook National University Hospital (2019-09-008).
Institutional Review Board Statement
The Institutional Review Board of Kyungpook National University Chilgok Hospital (KNUCH 2019-09-008) and all the participating hospitals granted approval for the study, and the requirement for informed consent was waived given the retrospective observational nature of the study involving deceased patients.
Data Availability Statement
Data available on request due to restrictions
Acknowledgments
Part of these data was presented at ECCMID 2023.
Conflicts of Interest
All authors report no conflicts of interest relevant to this article.
References
- Breilh D, Texier-Maugein J, Allaouchiche B, Saux MC, Boselli E. Carbapenems. J Chemother 2013;25:1-17.
- Armand-Lefèvre L, Angebault C, Barbier F, et al. Emergence of Imipenem resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 2013;57:1488–1495. [CrossRef]
- Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health 2018;6:e619–629. [CrossRef]
- Kwak YG, Moon C, Kim ES, Kim BN. Frequent Prescription of Antibiotics and High Burden of Antibiotic Resistance among Deceased Patients in General Medical Wards of Acute Care Hospitals in Korea. PLoS One 2016;11:e0146852. [CrossRef]
- Yoon YK, Yang KS, Lee SE, Kim HJ, Sohn JW, Kim MJ. Effects of Group 1 Versus Group 2 Carbapenems on the Susceptibility of Acinetobacter baumannii to Carbapenems: A Before and After Intervention Study of Carbapenem-Use Stewardship. PLoS One 2014;9:e99101. [CrossRef]
- Horikoshi Y, Suwa J, Higuchi H, et al. Sustained pediatric antimicrobial stewardship program with consultation to infectious diseases reduced carbapenem resistance and infection-related mortality. Int J Infect Dis 2017;64:69-73. [CrossRef]
- McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob Agents Chemother 2013;57:5131-5133. [CrossRef]
- O’Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations 2014 Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
- Gauzit R, Pean Y, Alfandari S, et al. Carbapenem use in French hospitals: a nationwide survey at the patient level. Int J Antimicrob Agents 2015;46:707–712. [CrossRef]
- Wi YM, Kwon KT, Hwang S, et al. Use of Antibiotics Within the Last 14 Days of Life in Korean Patients: A Nationwide Study. J Korean Med Sci 2023;38:e66. [CrossRef]
- Coppry M, Jeanne-Leroyer C, Noize P, et al. Antibiotics associated with acquisition of carbapenem-resistant Pseudomonas aeruginosa in ICUs: a multicentre nested case-case-control study. J Antimicrob Chemother 2019;74:503–510. [CrossRef]
- Woerther PL, Lepeule R, Burdet C, Decousser JW, Ruppé É, Barbier F. Carbapenems and alternative β-lactams for the treatment of infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae: What impact on intestinal colonisation resistance? Int J Antimicrob Agents 2018;52:762–770. [CrossRef]
- Raman G, Avendano EE, Chan J, Merchant S, Puzniak L. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control 2018;7:79. [CrossRef]
- Holt AFV, Severin JA, Lesaffre EMEH, Vos MC. A systematic review and metaanalyses show that carbapenem use and medical devices are the leading risk factors for carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2014;58:2626–2637. [CrossRef]
- Ruiz J, Gordon M, Villarreal E, et al. Influence of antibiotic pressure on multidrug resistant Klebsiella pneumoniae colonisation in critically ill patients. Antimicrob Resist Infect Control 2019; 8:1–7. [CrossRef]
- Stocker H, Mehlhorn C, Jordan K, Eckholt L, Jefferys L, Arastéh K. Clinical and economic effects of an antimicrobial stewardship intervention in a surgical intensive care unit. Infection 2020;48:509-519. [CrossRef]
- Nilholm H, Holmstrand L, Ahl J, et al. An Audit-Based, Infectious Disease Specialist-Guided Antimicrobial Stewardship Program Profoundly Reduced Antibiotic Use Without Negatively Affecting Patient Outcomes. Open Forum Infect Dis 2015;2:ofv042. [CrossRef]
- Paulsen J, Solligard E, Damas JK, DeWan A, Asvold BO, Bracken MB. The impact of infectious disease specialist consultation for Staphylococcus aureus bloodstream infections: a systematic review. Open Forum Infect Dis 2016;3:ofw048. [CrossRef]
- Butt AA, Al Kaabi N, Saifuddin M, et al. Impact of infectious diseases team consultation on antimicrobial use, length of stay and mortality. Am J Med Sci 2015;350:191–194. [CrossRef]
- Messacar K, Campbell K, Pearce K, et al. A handshake from antimicrobial stewardship opens doors for infectious disease consultations. Clin Infect Dis 2017;64:1449–1452. [CrossRef]
- Hung KC, Lee LW, Liew YX, et al. Antibiotic stewardship program (ASP) in palliative care: antibiotics, to give or not to give. Eur J Clin Microbiol Infect Dis 2022;41:29-36. [CrossRef]
- Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012;6:CD000259. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).