1. Introduction
Bovine mastitis, caused by complex interactions between the host, environment, and infectious organisms, is one of the most common diseases of dairy cattle. It significantly impacts global dairy production by reducing milk quantity and quality [
1]. Aside from its direct effects on dairy production,
Staphylococcus (S.) aureus, a major infectious agent in bovine mastitis worldwide [
2], is also a human health concern.
S. aureus strains with high pathogenic potential (e.g., harboring virulence factors and antimicrobial resistance genes) can infect humans through a variety of channels, including food. As a result, food-producing animals, particularly cattle and pigs, are significant producers of
S. aureus in the food chain [
3]. Mastitis can be characterized as infectious or environmental, depending on the micro-organisms that cause infection. During milking, contagious bacteria spread across infected quarters [
5].
S. aureus is a facultative anaerobe with capsule, nonmotile, and non-spore-forming cocci that are Gram-positive, catalase- and coagulase-positive. This bacterium is regarded as one of the primary pathogens involved in bovine mastitis worldwide because it is highly contagious and causes long-term chronic infections [
6]. Although several mastitis prevention initiatives have been adopted in various countries,
S. aureus prevalence in cows remains high [
7]. This inflammatory reaction caused by mastitis increases the somatic cell count, which lowers synthetic activity in the mammary tissue and alters milk composition. Furthermore, increased vascular permeability can result in ions, protein, and enzyme leakage. It also lowers milk quality by decreasing the production of various milk components, such as lactose, fat, non-fat solids, and casein. Mastitis costs include milk production losses, medicine and veterinary service expenditures, and the death or early euthanasia of affected animals [
8]. Aside from its value in animal production,
S. aureus in milk and other dairy products can cause human infections, which is important to public health. These infections can be more severe if the microbe manufactures toxins [
9]. Effective diagnostic approaches can facilitate mastitis control and encourage antimicrobial stewardship [91]. Clinical mastitis must be objectively scored to predict therapy outcomes [92] and adjust treatment procedures accordingly [
10].
In the dairy sector, automated monitoring devices (AMDs) have become more widely available [
11]. Adoption of AMD is likely to increase as dairy herd size and consolidation continue [
12,
13,
14]. Since clinical mastitis (CM) is a severe concern for dairy farmers, many AMDs have been developed to aid in early diagnosis [
15,
16]. Given the difficulties in visually analyzing milk quality in herds with automatic milking systems, applying AMDs to detect intramammary infection (IMI) can be critical [
1]. Milk yield, composition, somatic cell count (SCC), electrical conductivity, and flow rate are commonly used to determine IMI [
17]. Furthermore, activity-recording devices that count steps or time spent lying, standing, walking, or ruminating have been used with different degrees of efficacy [
18,
19]. Continuous observation of reticuloruminal pH data has revealed a robust and predictable short-term pattern that can be characterized by a simple sine wave and frequency of one cycle per day [
20]. Thus, evaluating reticuloruminal pH is of interest to most doctors, who define the state based on single observations. Continuous monitoring of pH values using remote sensing data is now possible; however, this generates a massive volume of data that can be difficult to comprehend. As a result, researchers who use continuous pH monitoring techniques to study food and acidosis frequently use average values or threshold procedures to evaluate reticuloruminal pH [
20].
Previous studies showed that body site temperature is an effective technique for determining IMI due to the feverish state caused by the immune response to infections [
21,
22]. However, most studies have concentrated on the udder skin surface or rectal temperature, with the latter being the most common approach on dairy farms [
18,
23]. Intra-reticuloruminal AMD provides an alternative to physically monitoring rectal temperature, allowing for automatic and regular monitoring without holding the cows [
24]. The period when rumen pH is below a given threshold is traditionally used to explain variation in rumen pH [
25], albeit threshold levels vary between research and monitoring methods [
26]. A bolus placed into the rumen can measure a cow’s temperature in real time (reticulorumen). Temperature and pH can be measured using boluses. Wireless boluses send data every ten minutes. The information can be saved in the cloud or on a computer. Measurements can be recorded for up to a year, depending on the battery life of the various bolus variants [
27]. More research is needed to assess mastitis status, including spontaneous infections with various pathogens and other variations in reticulorumen temperature [
28]. In our past studies, we determined that veterinarians and farmers should examine the likelihood of stillbirth during late gestation based on clinical mastitis. This practice may help develop methods for enhancing reproductive performance in dairy cows [
29]. The findings show clinical mastitis impacts the time and rumination chews registered by sensor systems [
30].
We hypothesized that reticuloruminal temperature, reticuloruminal pH, and cow activity could be used as biomarkers for the early diagnosis of clinical mastitis in dairy cows. In this study, we aimed to determine the relationship between reticuloruminal temperature, reticuloruminal pH, cow activity, and clinical mastitis in dairy cows.
2. Materials and Methods
2.1. Ethical Approval
The Lithuanian Law on Animal Welfare and Protection were followed in conducting this investigation. The approval number of this study is G2-227.
2.2. Animals farm and feeding
From July to December 2022, the experiment was conducted at the Lithuanian University of Health Sciences and a dairy farm (55.792368° N, 24.017499° E) with 600 milking Holstein cows. The study was conducted with cows at a second lactation, with an average daily milk yield of 35 kg per cow, an average feed intake of 19 kg DM/day, milk fat of 4.3, milk protein of 3.55, an average of milk somatic cell count of 190,000/mL, and an average of milk urea nitrogen of 22%. Cows were milked using a DeLaval milking parlor twice daily and kept in a stall-free barn.
Individual attribute data (lactation number, breed, latest calving date, and milk yield) were acquired from the farm’s computer system and documented on a spreadsheet (Delpro DeLaval Inc, Tumba, Sweden). The number of days in milk (DIM) for each cow was obtained for each data collection period by calculating the number of days between the last calving date and the first day of the data collecting period. All cows were fed a total mixed ration (TMR) consisting primarily of maize and alfalfa silage. TMR comprised 38% grass silage, 38% corn silage, and 24% flaked grain concentrate with a mineral mixture. Depending on the circumstances, the ration was designed to meet or exceed the needs of a 550 kg Holstein cow producing 35 kg of milk daily. Cows were fed daily at 8:00 a.m. and 4:00 p.m.
2.3. Research design
From 01 July to 31 December 2022, 28 clinical mastitis cases were diagnosed among the herd (n = 600) by clinical symptoms, such as abnormal milk appearance (watery, flakes, fibrin clots, etc.; mild), abnormal milk appearance with a swollen or painful quarter, abnormal milk appearance with a swollen or painful quarter, and systemic signs of illness (fever, decreased appetite, dehydration, etc.; severe). A general clinical examination revealed that no cows had clinical indications compatible with disease or other variables such as heat stress or estrus. Cows with these characteristics (n = 3) were excluded from the research. This group’s total number of cows was 25 (195.65 ± 2.5 days in milk).
All cows’ (n=25) milk samples were collected for microbiological investigation. The microbology test showed Streptococcus spp. and S. aureus, as well as amoxicillin and clavulanic acid sensitivity. Cows with CM were treated with intramammary antibiotics and anti-inflammatory drugs (Synulox LC + NSAID). The latter included Melovem® 20 mg/ml. Cows were treated with a single subcutaneous injection at a dosage of 2.5 ml/100 kg of body weight. The antimicrobial used was Synulox LC (for lactating cows). Each 3 g syringe contained 200 mg of amoxicillin (as amoxicillin trihydrate), 50 mg of clavulanic acid (as potassium clavulanate), and 10 mg of prednisolone that was administered by intramammary infusion soon after milking and at 12-hour intervals for three consecutive milkings. Following the final milking, antibiotic infusions were administered as follows: Trained staff wearing clean disposable gloves cleansed the teat ends for at least 5 seconds with 70% isopropyl alcohol-soaked cotton swabs before the antibiotic treatment was infused into the mammary gland [
31].
Twenty-five cows (second and more lactation numbers and 193.65 ± 2.4 days in milk) were categorized as clinically healthy with an SCC of <200,000 cells/mL and no clinical signs of disease.
2.4. Measurements.
2.4.1. Measurement Equipment
We recorded the following parameters during the experiment: reticulorumen pH (pH), reticulorumen temperature (RR temp.), and cow activity. Using smaXtec boluses (smaXtec animal care technology®, Graz, Austria), we could monitor real-time data such as pH, reticulorumen content (TRR) temperature, and cow activity [
33,
34]. The data was collected using antennas (smaXtec animal care technology®). An indwelling and wireless data-transmitting device was used to monitor RT, pH, TRR, and cow activity (smaXtec animal care GmbH, Graz, Austria). A microprocessor controlled the system. Data on pH and TRR were acquired using an A/D converter and stored on an external memory chip for further analysis and interpretation. To begin the experiment, the pH probes were calibrated with buffer solutions of pH 4 and 7 to ensure correct operation. The smaXtec messenger® computer software collected all data.
2.4.2. Duration of measurements
In this investigation, we compared reticulorumen data from a week before diagnosis with HG data from the same time period. CM cows were observed on the same days as healthy cows.
2.4.3. Statistical analysis
The experimental animals were divided into four classes based on the reticulorumen pH assay: first class <6.22; second class 6.22–6.42; third class 6.42–6.62; fourth class >6.62. Classes were assigned according to our previous publication [
35]. SPSS 25.0 (SPSS Inc., Chicago, IL, USA) was used for statistical data analysis. Using descriptive statistics, the Kolmogorov–Smirnov test assessed the normal distribution of variables. A linear regression equation was calculated to determine the statistical relationship between the study’s recorded parameters— reticulorumen pH (pH), reticulorumen temperature (RR temp.), and cow activity (dependent variables)— in the days before diagnosis or between reticulorumen pH classes (independent indicators). A backward stepwise logistic model was applied to exclude all non-essential explanatory variables (according to the Wald test’s significance). The estimates and 95% Wald limits were used to calculate the odds ratio (OR) for the probability of success to failure and the 95% confidence interval (CI). The Pearson correlation was used to detect the linear relationship between the investigated traits.
Repeated measures analysis of variance (ANOVA) was used for comparing means across the investigated variables based on observations from -7 to 0 days before diagnosis.
3. Results
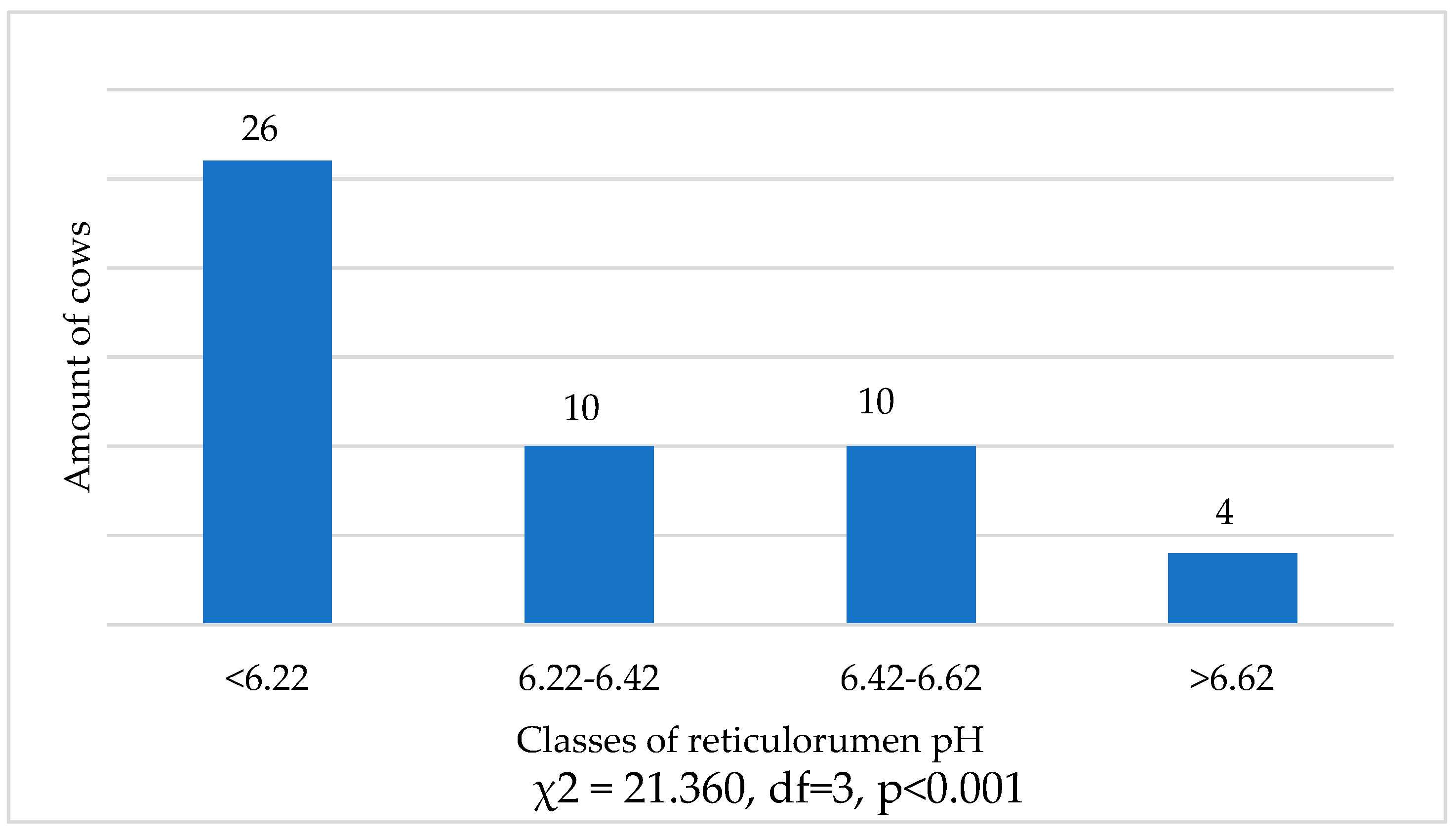
The distribution of cows according to reticulorumen pH classes showed 93.22% more cows in the first class of reticulorumen pH than in the fourth class (χ2 = 48.016, df=1,
p=0.001). There were 55.93% more cows in the first class than in the second (χ2 = 12.812 df=1,
p = 0.001) and third classes at 81.36% (χ2 = 32.914 df=1,
p = 0.001) (
Figure 1).
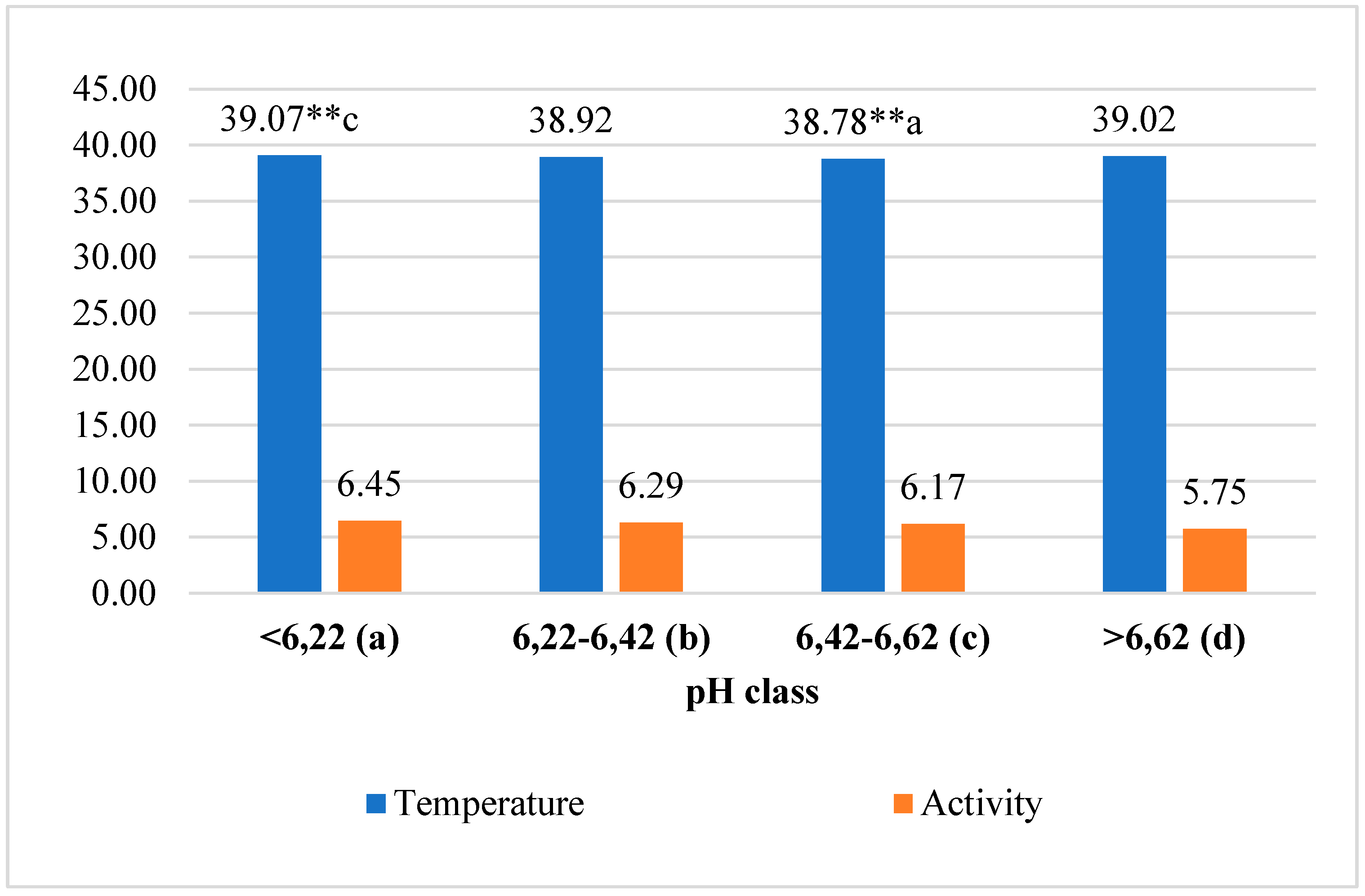
The average reticulorumen temperature of the first-class cows was 0.74% higher than in the third class (p<0.01) and 0.13–0.38% higher than in the second and fourth classes (p>0.05). Data analysis of reticulorumen temperature revealed a statistically significant relationship between classes of reticulorumen pH. The reticulorumen temperature decreased by about 0.029 (y = -0.029x + 39.02; R² = 0.0857). The average walking activity of first-class cows was 10.85% higher than in the fourth class and 2.48–4.34% higher than in the second and third classes (p>0.05). Data analysis of walking activity showed a statistically significant relationship between reticulorumen pH classes. Walking activity decreased by about 0.222 steps/min (y = -0.222x + 6.72; R² = 0,9157) (
Figure 2).
Different letters a, b, c, and d indicate statistically significant differences between means of different pH classes ** p<0.01.
Analysis of the investigated traits in a group of cows showed that the highest average pH value was estimated in the control group, 7.32% higher than in the investigated group of cows (p<0.001). Reticulorumen temperature was 1.25% higher in the investigated group than in the control group (p<0.05). For walking activity, the higher average value was determined in the control group, which was 17.37% higher than in the investigated group of cows (p<0.001) (
Table 1).
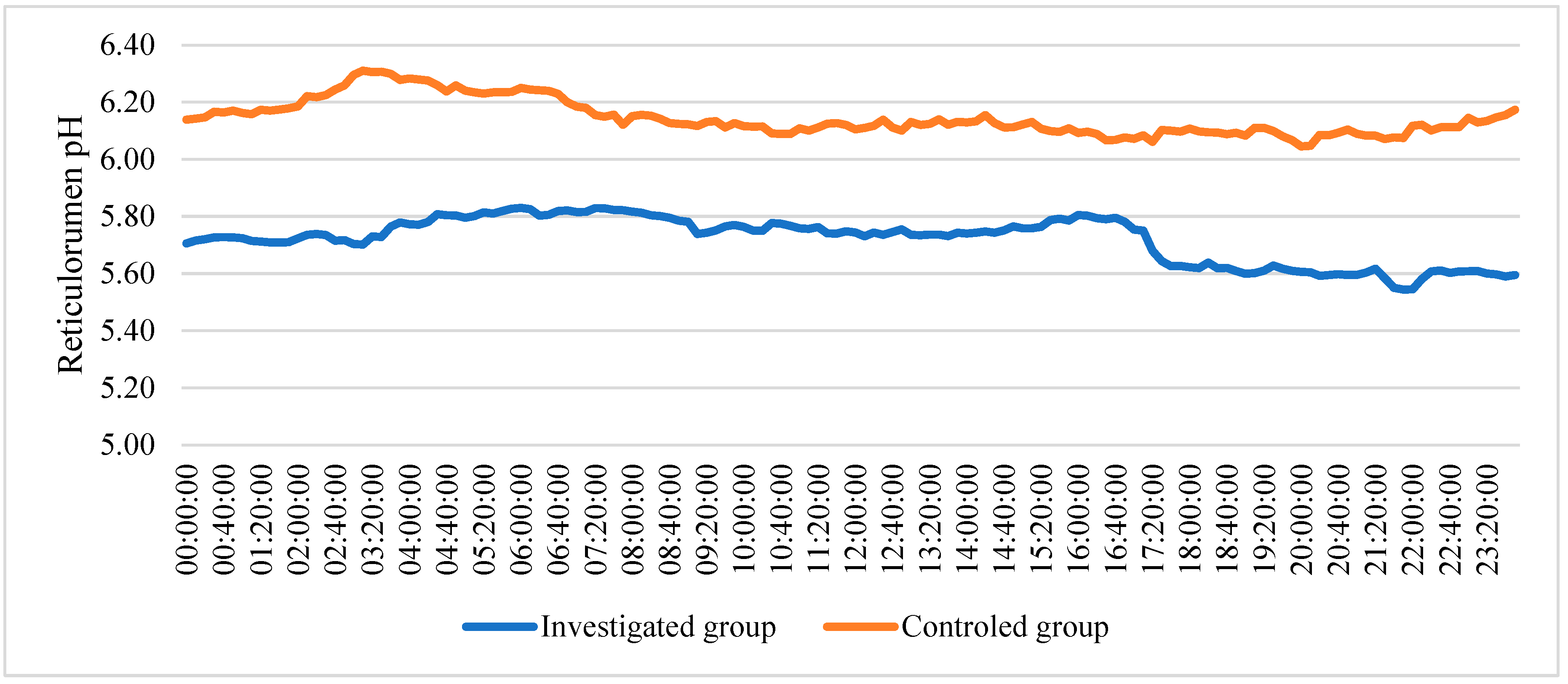
The data of reticulorumen pH changes over 24 h showed that pH changed from 5.53 to 5.83 in investigated group. In the control group, pH changed from 6.09 to 6.35 (
Figure 3).
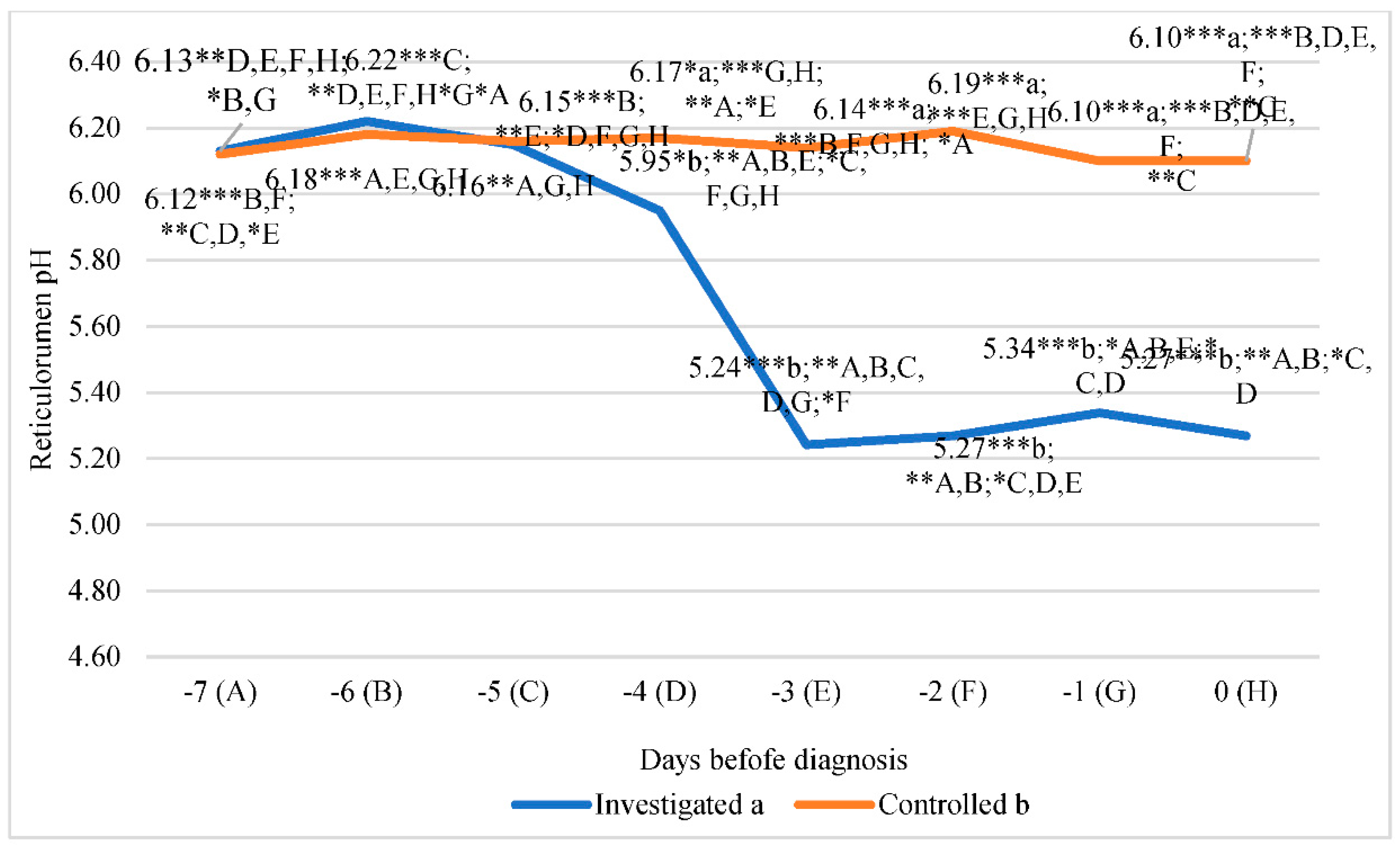
The lowest reticulorumen pH in the investigated group was detected on the third day before diagnosis. It was 15.76% lower than the highest reticulorumen pH detected on the sixth day before diagnosis in the investigated group (p<0.001). The lowest reticulorumen pH in the control group was detected at 0 and 1 days before diagnosis, it was 1.45% lower compared to the highest reticulorumen pH detected on the second day before diagnosis in the control group (p<0.001).
Analysis of the reticulorumen pH in a group of cows showed that the highest average pH value was estimated in cows on the sixth day before diagnosis. According to comparisons of pH means between groups, we recorded statistically significant differences on the third day before diagnosis (14.63% lower in the investigated group than in the control p<0.001), the second day before diagnosis (14.89% lower in the investigated group than in the control group, p<0.001), first day before diagnosis (12.49% lower in the investigated group than in the control group, p<0.001) and diagnosis day (13.63% lower in the investigated group than in the control group, p<0.001).
Data analysis of reticulorumen pH in the investigated group of cows revealed a significant relationship between days before diagnosis. Reticulorumen pH decreased by approx. 0.164/ day in the investigated groups of cows (y = -0,1642x + 6,4346; R² = 0,790). The control group remained almost the same ( y = -0,0057x + 6,1707; R² = 0,1558) (
Figure 4).
Letters a and b indicate statistically significant mean differences between the two groups * p<0.05, ** p<0.01, *** p<0.001. A, B, C, D, E, F, G, and H indicate statistically significant differences between days in the same group.
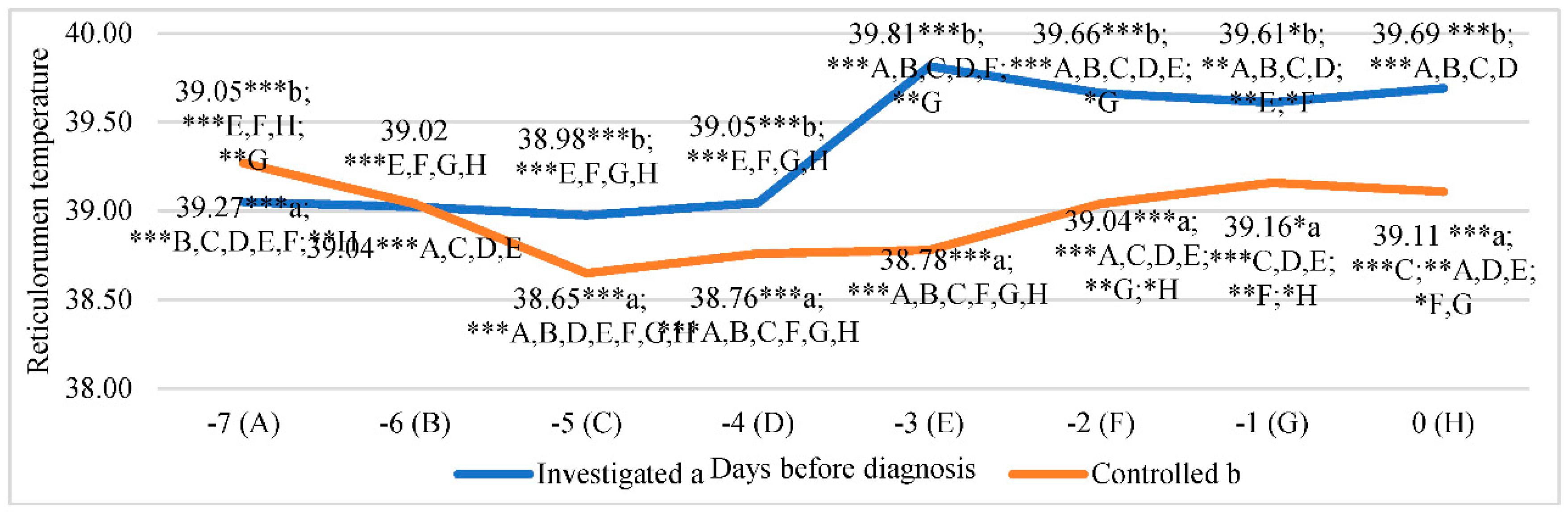
The lowest reticulorumen temperature in the investigated group was detected on the fifth day before diagnosis. It was 2.08% lower than in the highest reticulorumen temperature detected on the third day before diagnosis in the investigated group (p<0.001).
The lowest reticulorumen temperature in the control group was detected on the fifth day before diagnosis. It was 1.58% lower than the highest reticulorumen temperature detected on the seventh day before diagnosis in the control group (p<0.001).
According to reticulorumen temperature means between groups, we estimated statistically significant differences between all days except the sixth day before diagnosis.
The reticulorumen temperature mean difference ranged from 0.56% on the seventh day before diagnosis, higher in the control group (p<0.001), to 2.60% on the third day before diagnosis (p<0.001).
Data analysis of reticulorumen temperature showed a significant relationship between days before diagnosis. The reticulorumen temperature increased by approx. 0.12°C/ day (y = 0.1221x + 38.811; R² = 0.6748), whereas in the control group, reticulorumen temperature remained almost the same (y = 0,008x + 38,94; R² = 0,0079) (
Figure 5).
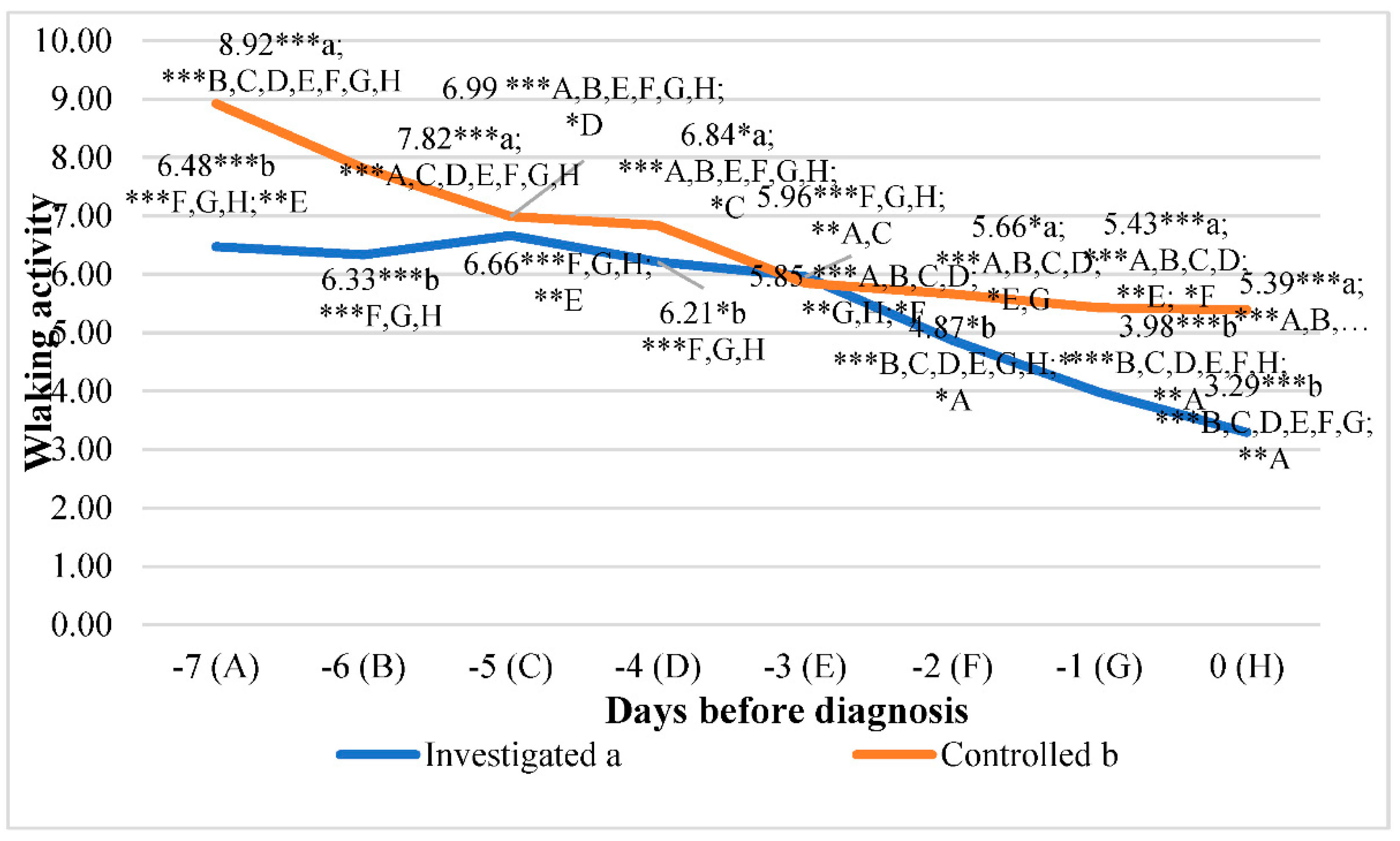
The lowest walking activity in the investigated group was detected at 0 days before diagnosis, 50.60% lower than the fifth day before diagnosis (p<0.001). In the control group of cows, the lowest walking activity was detected 0 days before diagnosis at -39.57% than on the seventh day before diagnosis (p<0.001).
Regarding walking activity means between groups, the highest statistically significant differences were estimated seven days before diagnosis (27.41% lower in the investigated group than in the control group, p<0.0001), the first day before diagnosis (26.67% lower in the investigated group than in the control group, p<0.001), and the sixth day before diagnosis (19.02% lower in the investigated group than in the control group, p<0.001).
Data analysis of walking activity showed a significant relationship between days before diagnosis. Walking activity decreased by approx. 0.472 steps/min/day (y = -0.472x + 7.5974; R² = 0.8302) in the investigated group. In the control group, we detected a similar relationship. Walking activity decreased by approx. 0.4957 steps/min/day (y = -0.4957x + 8.8432; R² = 0.9087) (
Figure 6).
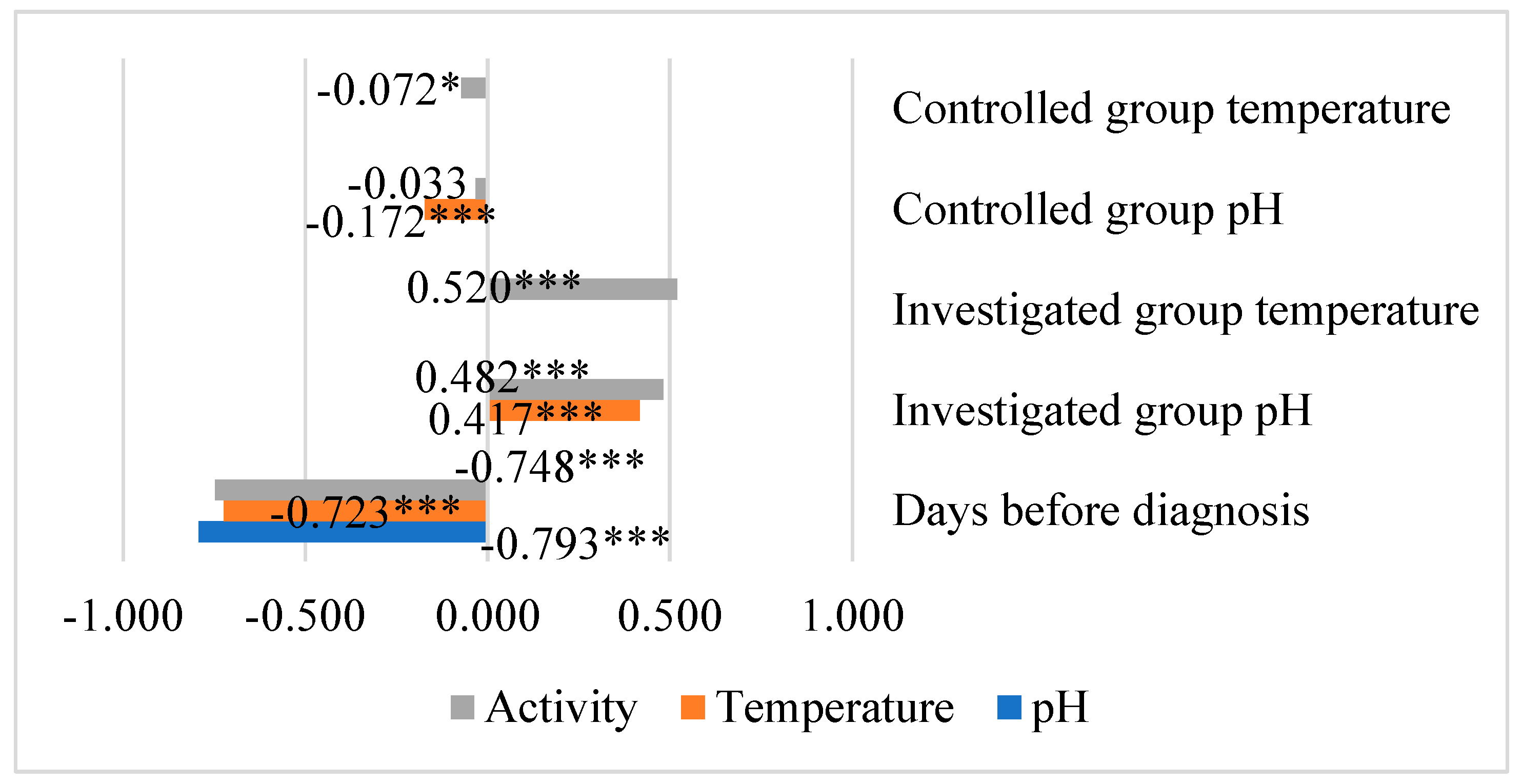
The days before diagnosis showed a highly significant negative relationship with walking activity, reticulorumen temperature, and reticulorumen pH (p<0.001). The investigated group showed a moderately significant positive relationship between reticulorumen temperature and walking activity and reticulorumen pH with temperature and activity (p<0.001). A non-significant negative relationship was detected in the control group of reticulorumen pH with temperature and activity (p<0.05) (
Figure 7).
The data analysis revealed that mastitis was associated with rumination time, rumination chews, and boluses (p < 0.001) (
Table 2).
We used a logistic regression model to determine associations. One of the dimensions had an outcome of interest with two categories (pH value: first group <6.22 and second group >6.22), indicating that subclinical mastitis among the cows had a higher possibility with temperature (OR = 1.211 times, p<0.05) and activity (OR = 1.954 times, p<0.001) (
Table 2).
4. Discussion
Due to increased interest and acceptance of automatic (robotic) milking systems (AMS), reliable automatic diagnosis of mastitis is needed due to the reduced inspection time required to identify cows with mastitis that require veterinary care [
19]. Efficient mastitis identification allows farmers to implement early and sufficient treatment procedures, reduce antibiotic overuse, conserve animal health and welfare by minimizing pain and suffering, increase recovery rates, and maximize economic return [
36]. Accurate diagnosis methods can facilitate mastitis control and promote wise antimicrobial use [
37]. We hypothesized that reticuloruminal temperature, reticuloruminal pH, and cow activity could be indicators for early detection of clinical mastitis in dairy cows. We aimed to find a link between reticuloruminal temperature, pH, cow activity, and clinical mastitis in dairy cows. CM prediction using milk-related variables (e.g., conductivity, SCS, lactate dehydrogenase, and milk yield) alone or in combination was widely tested with varying results [
17]. Reliable mastitis diagnosis by automated technologies allows us to implement early treatment programs, avoid antibiotic misuse, conserve cow health and welfare, avoid discomfort and pain, and boost recovery rate and farm economic viability [
38].
RR pH in the healthy group was 7.32% higher than in CM cows. Reticulorumen temperature was 1.25% higher in the CM group than in the control group. The healthy group had a higher average value for walking activity and was 17.37 % higher than the CM group. The data of reticulorumen pH changes over 24 h showed that pH changed from 5.53 to 5.83 in the CM group. In the control group, pH changed from 6.05 to 6.31. According to the existing literature, ruminal pH is the best indicator for subacute ruminal acidosis (SARA) risk due to rumen pH variations in dairy cows [
39]. As a result, cows’ vulnerability to SARA varies [
40]. Rumination and fermentation processes are inextricably linked. Thus, decreased rumination activity leads to decreased saliva buffering, raising the risk of SARA [
41]. Clinically sick cows generally exhibited a low rumen pH and reduced feed intake. It is unknown if low pH is a cause or results from the condition [
42]. Decreased feed intake is a common symptom of sickness. For example, Van Winden et al. [
43] discovered a decreased total DMI prior to diagnosing displaced abomasum. In the investigation by Huzzey et al. [
44], cows at risk of metritis could also be identified by total DMI. Lukas et al. [
45] used DMI changes as an indication of health status
. Denwood et al. [
20] proposed forecasting illness episodes by monitoring deviations from an expected pattern (of feed intake or rumen pH) as a future study emphasis. In our previous study, we found that higher reticulorumen pH and temperature, higher milk lactose levels, and lower concentrate consumption are all associated with cow reproduction success. On the other hand, changes in cows’ productivity and activity have also been reported as dependable predictors of cow reproduction success [
46].
In our study, the lowest reticulorumen pH in the CM group was detected on the third day before diagnosis. It was 15.76% lower than the highest reticulorumen pH detected on the sixth day before diagnosis. Although the lowest reticulorumen pH in CM cows was detected at 0 and 1 days before diagnosis, it was 1.45% lower than the highest reticulorumen pH detected on the second day before diagnosis.
The use of RRT to predict CM has received little attention [
28]. In this study, we found that the lowest reticulorumen temperature in the CM group was detected on the fifth day before diagnosis. It was 2.08% lower than the highest reticulorumen temperature detected on the third day before CM diagnosis. Adams et al. [
24] evaluated the association between RRT and naturally occurring mastitis using a novel intra-reticuloruminal device that monitored the reticular temperature at each milking. The RRT was within the normal temperature range of 38.0-39.4°C before the challenge [
47] and increased following the intramammary challenge, remaining constant until the end of the study period [
28]. RRT increased by 2.4°C in quarters experimentally infected with
Escherichia coli, according to AlZahal et al. [
39]. We found a lower increase in RRT, likely due to pathogenicity differences between
Strep. uberis and
E. coli, highlighting pathogen-specific aspects of temperature fluctuations after IMI [
28]. Increased RRT may be related to the number of bacteria in the infected quarters since an association between bacterial concentration and body temperature was previously reported [
21,
22]. Several parameters, including ambient temperature, drinking water temperature, feed intake, and diet composition, have been shown to alter the strength of the connections [
28]. Research differences may be attributed to the algorithms and temperature limits used to create alarms. In our investigation, we set the alarm threshold to one standard deviation from the baseline RRT temperature [
28]. The increase in RRT following an intramammary challenge with
Strep. uberis triggered warnings based on one standard deviation of the baseline temperature [
28]. According to the literature, the reticulorumen temperature during the week preceding the challenge was within the normal temperature range of 38.0-39.4°C. It increased after the intramammary challenge, remaining steady until the end of the study period [
47].
The lowest walking activity in the CM group was detected 0 days before diagnosis, which was 50.60% lower than the fifth day before diagnosis. The lowest walking activity was detected 0 days before diagnosis, which was -39.57% lower than the seventh day before diagnosis. Mastitis, a cause of pain in afflicted animals, is one of the most important diseases in dairy cows. It has widely known negative impacts on dairy farms’ welfare and profitability [
48]. Increased animal activity prior to disease discovery may be related to increased stress [
49]. Cows spent less time sleeping, ruminating, and drinking when their udders were highly swollen and fever-ridden [
50]. Our previous research has shown that circadian rhythm and seasonal reproductive cycles alter nursing cows’ reticulorumen temperature and pH [
51]. Changes in activity and knowledge about temporal effects at least 10 days following a mastitis diagnosis contribute to our understanding of naturally occurring cases [
52]. During illness, laying increases across animal species. Reduced activity is useful for energy conservation and a febrile response [
53]. As a result, decreased laying behavior may be perceived as a response to an udder infection in both experimentally produced and natural cases of acute clinical mastitis [
52].
5. Conclusions
In this study, we found that reticuloruminal temperature, reticuloruminal pH, and cow activity could be used as biomarkers for the early diagnosis of clinical mastitis in dairy cows. The RR pH of healthy cows was 7.32% higher than CM cows. Data of reticulorumen pH changes over 24 h showed that CM cows’ pH changed from 5.53 to 5.83, whereas in the healthy group, the pH changed from 6.05 to 6.31. Regarding walking activity, a higher average value was determined in the healthy group, 17.37% higher than the CM group.
CM occurrences must be tracked over shorter time periods so that farmers can receive information that facilitates wise management decisions. In future CM research, more cows should be studied, and this dataset should be used to create prediction models through machine learning approaches. Retrospective clinical mastitis prediction systems that use machine learning could benefit from these data.
Author Contributions
R.A.: supervision of the whole study; L.A.: software and algorithm development; G.P. and A.R.: data collection and investigation; W.B.: writing—review and editing.
Funding
Research Council of Lithuania. Project number: S-MIP-22-137
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee (study approval number: PK016965. 2017.06.06).
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hogeveen, H.; Klaas, I.C.; Dalen, G.; Honig, H.; Zecconi, A.; Kelton, D.F.; Sánchez Mainar, M. Novel Ways to Use Sensor Data to Improve Mastitis Management. J. Dairy Sci. 2021, 104, 11317–11332. [CrossRef]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The Cost of Clinical Mastitis in the First 30 Days of Lactation: An Economic Modeling Tool. Prev. Vet. Med. 2015, 122, 257–264. [CrossRef]
- van Loo, I.; Huijsdens, X.; Tiemersma, E.; de Neeling, A.; van de Sande-Bruinsma, N.; Beaujean, D.; Voss, A.; Kluytmans, J. Emergence of Methicillin-Resistant Staphylococcus Aureus of Animal Origin in Humans. Emerg. Infect. Dis. 2007, 13, 1834–1839. [CrossRef]
- Blowey, R.W.; Edmondson, P. Mastitis Control in Dairy Herds; CABI, 2010; ISBN 978-1-84593-751-5.
- Bradley, A.J. Bovine Mastitis: An Evolving Disease. Vet. J. 2002, 164, 116–128. [CrossRef]
- Levison, L.J.; Miller-Cushon, E.K.; Tucker, A.L.; Bergeron, R.; Leslie, K.E.; Barkema, H.W.; DeVries, T.J. Incidence Rate of Pathogen-Specific Clinical Mastitis on Conventional and Organic Canadian Dairy Farms. J. Dairy Sci. 2016, 99, 1341–1350. [CrossRef]
- Dore, S.; Liciardi, M.; Amatiste, S.; Bergagna, S.; Bolzoni, G.; Caligiuri, V.; Cerrone, A.; Farina, G.; Montagna, C.O.; Saletti, M.A.; et al. Survey on Small Ruminant Bacterial Mastitis in Italy, 2013–2014. Small Rumin. Res. 2016, 141, 91–93. [CrossRef]
- Guimarães, J.L.B.; Brito, M.A.V.P.; Lange, C.C.; Silva, M.R.; Ribeiro, J.B.; Mendonça, L.C.; Mendonça, J.F.M.; Souza, G.N. Estimate of the Economic Impact of Mastitis: A Case Study in a Holstein Dairy Herd under Tropical Conditions. Prev. Vet. Med. 2017, 142, 46–50. [CrossRef]
- Hennekinne, J.-A.; Ostyn, A.; Guillier, F.; Herbin, S.; Prufer, A.-L.; Dragacci, S. How Should Staphylococcal Food Poisoning Outbreaks Be Characterized? Toxins 2010, 2, 2106–2116. [CrossRef]
- Krömker, V.; Leimbach, S. Mastitis Treatment—Reduction in Antibiotic Usage in Dairy Cows. Reprod. Domest. Anim. 2017, 52, 21–29. [CrossRef]
- Bell, M.J.; Tzimiropoulos, G. Novel Monitoring Systems to Obtain Dairy Cattle Phenotypes Associated With Sustainable Production. Front. Sustain. Food Syst. 2018, 2.
- Barkema, H.W.; von Keyserlingk, M.A.G.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.-P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited Review: Changes in the Dairy Industry Affecting Dairy Cattle Health and Welfare. J. Dairy Sci. 2015, 98, 7426–7445. [CrossRef]
- González, L.A.; Tolkamp, B.J.; Coffey, M.P.; Ferret, A.; Kyriazakis, I. Changes in Feeding Behavior as Possible Indicators for the Automatic Monitoring of Health Disorders in Dairy Cows. J. Dairy Sci. 2008, 91, 1017–1028. [CrossRef]
- Luby, C.D.; Waldner, C.; Jelinski, M.D. Update on Demographics of the Canadian Dairy Industry for the Period 2011 to 2016. Can. Vet. J. 2020, 61, 75–78.
- Delgado, H.A.; Cue, R.I.; Haine, D.; Sewalem, A.; Lacroix, R.; Lefebvre, D.; Dubuc, J.; Bouchard, E.; Wade, K.M. Profitability Measures as Decision-Making Tools for Québec Dairy Herds. Can. J. Anim. Sci. 2018, 98, 18–31. [CrossRef]
- Liang, D.; Arnold, L.M.; Stowe, C.J.; Harmon, R.J.; Bewley, J.M. Estimating US Dairy Clinical Disease Costs with a Stochastic Simulation Model. J. Dairy Sci. 2017, 100, 1472–1486. [CrossRef]
- Fadul-Pacheco, L.; Delgado, H.; Cabrera, V.E. Exploring Machine Learning Algorithms for Early Prediction of Clinical Mastitis. Int. Dairy J. 2021, 119, 105051. [CrossRef]
- Stangaferro, M.L.; Wijma, R.; Caixeta, L.S.; Al-Abri, M.A.; Giordano, J.O. Use of Rumination and Activity Monitoring for the Identification of Dairy Cows with Health Disorders: Part III. Metritis. J. Dairy Sci. 2016, 99, 7422–7433. [CrossRef]
- Khatun, M.; Clark, C.E.F.; Lyons, N.A.; Thomson, P.C.; Kerrisk, K.L.; García, S.C. Early Detection of Clinical Mastitis from Electrical Conductivity Data in an Automatic Milking System. Anim. Prod. Sci. 2017, 57, 1226–1232. [CrossRef]
- Denwood, M.J.; Kleen, J.L.; Jensen, D.B.; Jonsson, N.N. Describing Temporal Variation in Reticuloruminal PH Using Continuous Monitoring Data. J. Dairy Sci. 2018, 101, 233–245. [CrossRef]
- Rambeaud, M.; Almeida, R.A.; Pighetti, G.M.; Oliver, S.P. Dynamics of Leukocytes and Cytokines during Experimentally Induced Streptococcus Uberis Mastitis. Vet. Immunol. Immunopathol. 2003, 96, 193–205. [CrossRef]
- Tassi, R.; McNeilly, T.N.; Fitzpatrick, J.L.; Fontaine, M.C.; Reddick, D.; Ramage, C.; Lutton, M.; Schukken, Y.H.; Zadoks, R.N. Strain-Specific Pathogenicity of Putative Host-Adapted and Nonadapted Strains of Streptococcus Uberis in Dairy Cattle. J. Dairy Sci. 2013, 96, 5129–5145. [CrossRef]
- Machado, N.A.F.; Da Costa, L.B.S.; Barbosa-Filho, J.A.D.; De Oliveira, K.P.L.; De Sampaio, L.C.; Peixoto, M.S.M.; Damasceno, F.A. Using Infrared Thermography to Detect Subclinical Mastitis in Dairy Cows in Compost Barn Systems. J. Therm. Biol. 2021, 97, 102881. [CrossRef]
- Adams, A.E.; Olea-Popelka, F.J.; Roman-Muniz, I.N. Using Temperature-Sensing Reticular Boluses to Aid in the Detection of Production Diseases in Dairy Cows. J. Dairy Sci. 2013, 96, 1549–1555. [CrossRef]
- Abdela, N. Sub-Acute Ruminal Acidosis (SARA) and Its Consequence in Dairy Cattle: A Review of Past and Recent Research at Global Prospective. Achiev. Life Sci. 2016, 10, 187–196. [CrossRef]
- Kleen, J.L.; Cannizzo, C. Incidence, Prevalence and Impact of SARA in Dairy Herds. Anim. Feed Sci. Technol. 2012, 172, 4–8. [CrossRef]
- Levit, H.; Pinto, S.; Amon, T.; Gershon, E.; Kleinjan-Elazary, A.; Bloch, V.; Ben Meir, Y.A.; Portnik, Y.; Jacoby, S.; Arnin, A.; et al. Dynamic Cooling Strategy Based on Individual Animal Response Mitigated Heat Stress in Dairy Cows. Animal 2021, 15, 100093. [CrossRef]
- Rodriguez, Z.; Kolar, Q.K.; Krogstad, K.C.; Swartz, T.H.; Yoon, I.; Bradford, B.J.; Ruegg, P.L. Evaluation of Reticuloruminal Temperature for the Prediction of Clinical Mastitis in Dairy Cows Challenged with Streptococcus Uberis. J. Dairy Sci. 2022. [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Jonike, V.; Baumgartner, W.; Paulauskas, A. Subclinical Mastitis Detected during the Last Gestation Period Can Increase the Risk of Stillbirth in Dairy Calves. Animals 2022, 12, 1394. [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M.; Urbutis, M.; Rutkaukas, A.; Šertvytytė, G.; Baumgartner, W. Identification of Changes in Rumination Behavior Registered with an Online Sensor System in Cows with Subclinical Mastitis. Vet. Sci. 2022, 9, 454. [CrossRef]
- Castro-Costa, A.; Salama, A.A.K.; Moll, X.; Aguiló, J.; Caja, G. Using Wireless Rumen Sensors for Evaluating the Effects of Diet and Ambient Temperature in Nonlactating Dairy Goats. J. Dairy Sci. 2015, 98, 4646–4658. [CrossRef]
- Nielen, M.; Schukken, Y.H.; Brand, A.; Deluyker, H.A.; Maatje, K. Detection of Subclinical Mastitis from On-Line Milking Parlor Data. J. Dairy Sci. 1995, 78, 1039–1049. [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Jonike, V.; Baumgartner, W.; Paulauskas, A. Milk Lactose as a Biomarker of Subclinical Mastitis in Dairy Cows. Animals 2021, 11, 1736. [CrossRef]
- Yang, F.L.; Li, X.S.; He, B.X.; Yang, Z.L.; Li, G.H.; Liu, P.; Huang, Q.H.; Pan, X.M.; Li, J. Malondialdehyde Level and Some Enzymatic Activities in Subclinical Mastitis Milk. Afr. J. Biotechnol. 2011, 10, 5534–5538. [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Inline Reticulorumen PH as an Indicator of Cows Reproduction and Health Status. Sensors 2020, 20, 1022. [CrossRef]
- Kamphuis, C.; Mollenhorst, H.; Heesterbeek, J. a. P.; Hogeveen, H. Data Mining to Detect Clinical Mastitis with Automatic Milking.; Wellington, New Zealand, 2010; pp. 568–572.
- Ashraf, A.; Imran, M. Causes, Types, Etiological Agents, Prevalence, Diagnosis, Treatment, Prevention, Effects on Human Health and Future Aspects of Bovine Mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [CrossRef]
- Kuipers, A.; Koops, W.J.; Wemmenhove, H. Antibiotic Use in Dairy Herds in the Netherlands from 2005 to 2012. J. Dairy Sci. 2016, 99, 1632–1648. [CrossRef]
- AlZahal, O.; Kebreab, E.; France, J.; McBride, B.W. A Mathematical Approach to Predicting Biological Values from Ruminal PH Measurements. J. Dairy Sci. 2007, 90, 3777–3785. [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute Ruminal Acidosis in Dairy Cows: The Physiological Causes, Incidence and Consequences. Vet. J. 2008, 176, 21–31. [CrossRef]
- Schmitz, R.; Schnabel, K.; von Soosten, D.; Meyer, U.; Hüther, L.; Spiekers, H.; Rehage, J.; Dänicke, S. Changes of Ruminal PH, Rumination Activity and Feeding Behaviour during Early Lactation as Affected by Different Energy and Fibre Concentrations of Roughage in Pluriparous Dairy Cows. Arch. Anim. Nutr. 2018, 72, 458–477. [CrossRef]
- Heirbaut, S.; Børge Jensen, D.; Jing, X.P.; Stefańska, B.; Lutakome, P.; Vandaele, L.; Fievez, V. Different Reticuloruminal PH Metrics of High-Yielding Dairy Cattle during the Transition Period in Relation to Metabolic Health, Activity, and Feed Intake. J. Dairy Sci. 2022, 105, 6880–6894. [CrossRef]
- Van Winden, S.C.L.; Jorritsma, R.; Müller, K.E.; Noordhuizen, J.P.T.M. Feed Intake, Milk Yield, and Metabolic Parameters Prior to Left Displaced Abomasum in Dairy Cows. J. Dairy Sci. 2003, 86, 1465–1471. [CrossRef]
- Huzzey, J.M.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Prepartum Behavior and Dry Matter Intake Identify Dairy Cows at Risk for Metritis. J. Dairy Sci. 2007, 90, 3220–3233. [CrossRef]
- Lukas, J.M.; Reneau, J.K.; Wallace, R.L.; De Vries, A. A Study of Methods for Evaluating the Success of the Transition Period in Early-Lactation Dairy Cows. J. Dairy Sci. 2015, 98, 250–262. [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Televičius, M.; Malašauskienė, D.; Urbutis, M.; Baumgartner, W. Relation of Subclinical Ketosis of Dairy Cows with Locomotion Behaviour and Ambient Temperature. Animals 2020, 10, 2311. [CrossRef]
- Bewley, J.M.; Grott, M.W.; Einstein, M.E.; Schutz, M.M. Impact of Intake Water Temperatures on Reticular Temperatures of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3880–3887. [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The Hidden Cost of Disease: I. Impact of the First Incidence of Mastitis on Production and Economic Indicators of Primiparous Dairy Cows. J. Dairy Sci. 2021, 104, 7932–7943. [CrossRef]
- Antanaitis, R.; Žilaitis, V.; Kucinskas, A.; Juozaitienė, V.; Leonauskaite, K. Changes in Cow Activity, Milk Yield, and Milk Conductivity before Clinical Diagnosis of Ketosis, and Acidosis. Vet. Ir Zootech. 2015, 70, 3–9.
- Siivonen, J.; Taponen, S.; Hovinen, M.; Pastell, M.; Lensink, B.J.; Pyörälä, S.; Hänninen, L. Impact of Acute Clinical Mastitis on Cow Behaviour. Appl. Anim. Behav. Sci. 2011, 132, 101–106. [CrossRef]
- Antanaitis, R.; Zilaitis, V.; Juozaitiene, V.; Stoskus, R.; Televicius, M. Changes in Reticulorumen Content Temperature and PH According to Time of Day and Yearly Seasons. Pol. J. Vet. Sci. 2016, 19. [CrossRef]
- Fogsgaard, K.K.; Bennedsgaard, T.W.; Herskin, M.S. Behavioral Changes in Freestall-Housed Dairy Cows with Naturally Occurring Clinical Mastitis. J. Dairy Sci. 2015, 98, 1730–1738. [CrossRef]
- Johnson, R.W. The Concept of Sickness Behavior: A Brief Chronological Account of Four Key Discoveries. Vet. Immunol. Immunopathol. 2002, 87, 443–450. [CrossRef]
Figure 1.
Distribution of cows (average pH during the research) according to reticulorumen class.
Figure 1.
Distribution of cows (average pH during the research) according to reticulorumen class.
Figure 2.
Analysis of investigated traits by reticulorumen pH classes.
Figure 2.
Analysis of investigated traits by reticulorumen pH classes.
Figure 3.
Reticulorumen pH changes over 24 h in a group of cows.
Figure 3.
Reticulorumen pH changes over 24 h in a group of cows.
Figure 4.
Comparison of reticulorumen pH between two groups of cows days before diagnosis: investigated group (a), control group (b).
Figure 4.
Comparison of reticulorumen pH between two groups of cows days before diagnosis: investigated group (a), control group (b).
Figure 5.
Comparison of reticulorumen temperature between days before diagnosis in two groups of cows (n=293): investigated group (a), control group (b). Letters a and b indicate statistically significant mean differences between the two groups * p <0.05, ** p<0.01, *** p<0.001. A, B, C, D, E, F, G, and H indicate statistically significant differences between days in the same group.
Figure 5.
Comparison of reticulorumen temperature between days before diagnosis in two groups of cows (n=293): investigated group (a), control group (b). Letters a and b indicate statistically significant mean differences between the two groups * p <0.05, ** p<0.01, *** p<0.001. A, B, C, D, E, F, G, and H indicate statistically significant differences between days in the same group.
Figure 6.
Walking activity comparison between the control and investigated groups days before diagnosis. Letters a and b indicate statistically significant mean differences between the two groups: investigated group (a), control group (b). * p<0.05, ** p<0.01, *** p<0.001. A, B, C, D, E, F, G, and H indicate statistically significant differences between days in the same group.
Figure 6.
Walking activity comparison between the control and investigated groups days before diagnosis. Letters a and b indicate statistically significant mean differences between the two groups: investigated group (a), control group (b). * p<0.05, ** p<0.01, *** p<0.001. A, B, C, D, E, F, G, and H indicate statistically significant differences between days in the same group.
Figure 7.
Correlations between investigated indicators.* p<0.05, ** p<0.01, *** p<0.001.
Figure 7.
Correlations between investigated indicators.* p<0.05, ** p<0.01, *** p<0.001.
Table 1.
Investigated traits means (M) and standard errors (SE) in a group of cows.
Table 1.
Investigated traits means (M) and standard errors (SE) in a group of cows.
| Group |
pH |
Temperature |
Walking activity |
Investigated a
(n=25) |
5.70±0.009*** b
|
39.36±0.011* b
|
5.47±0.027*** b
|
Controlled b
(n=75) |
6.15±0,038*** a
|
38.87±0,020 * a
|
6.62±0.112*** a
|
Table 2.
Analysis of mastitis risk indicators using a logistic regression model. Cows of the investigated group were grouped according to pH value: first group <6.22 and second group >6.22.
Table 2.
Analysis of mastitis risk indicators using a logistic regression model. Cows of the investigated group were grouped according to pH value: first group <6.22 and second group >6.22.
| Indicators |
Groups of investigation |
B |
S.E. |
Wald |
df |
p-value |
OR: odds ratio
(95% CI for OR) |
| Temperature |
pH value:
first group <6.22
second group >6.22 |
0.191 |
0.093 |
4.281 |
1 |
0.039 |
1.211
(1.010-1.452) |
| Activity |
pH value:
first group <6.22
second group >6.22 |
0.670
|
0.073
|
84.609
|
1
|
p<0.001
|
1.954
(1.694-2.253
|
| Constant |
|
-13.391
|
3.465
|
14.935
|
1
|
p<0.001
|
0.000
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).