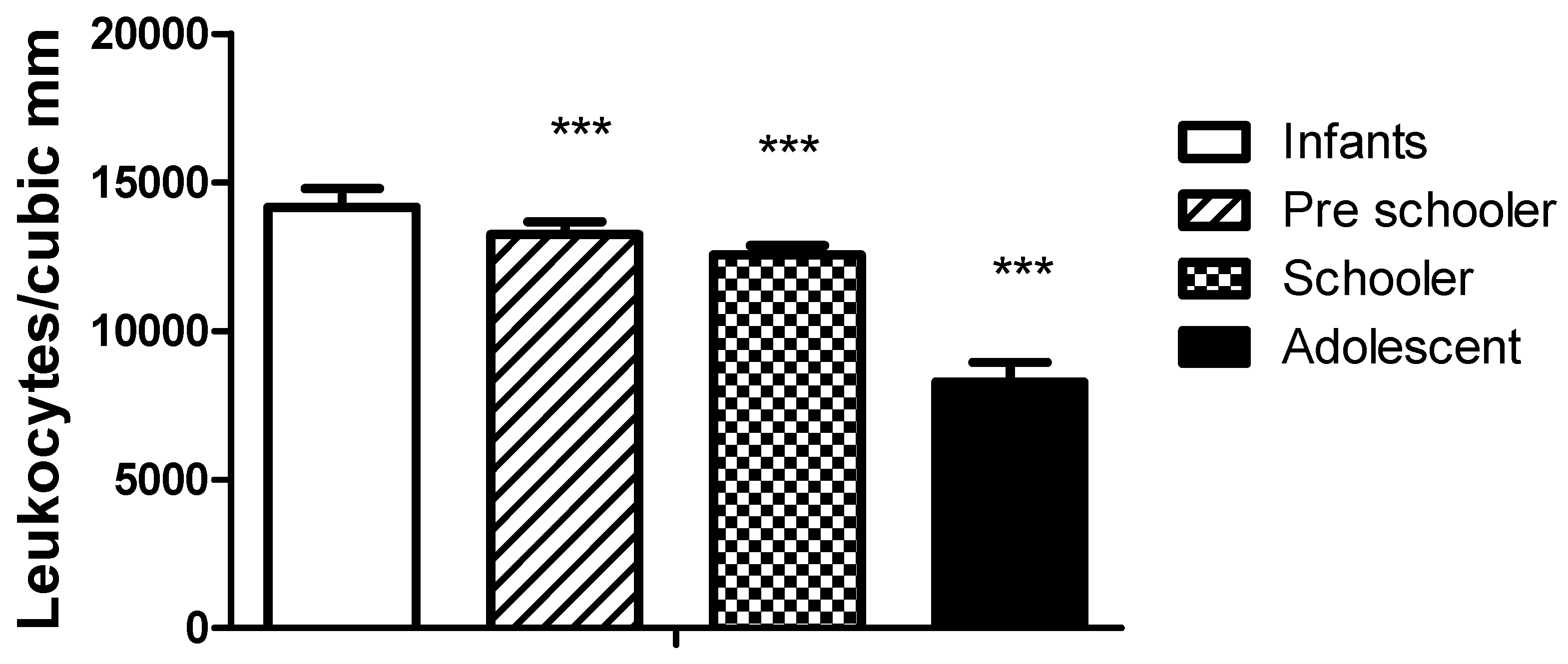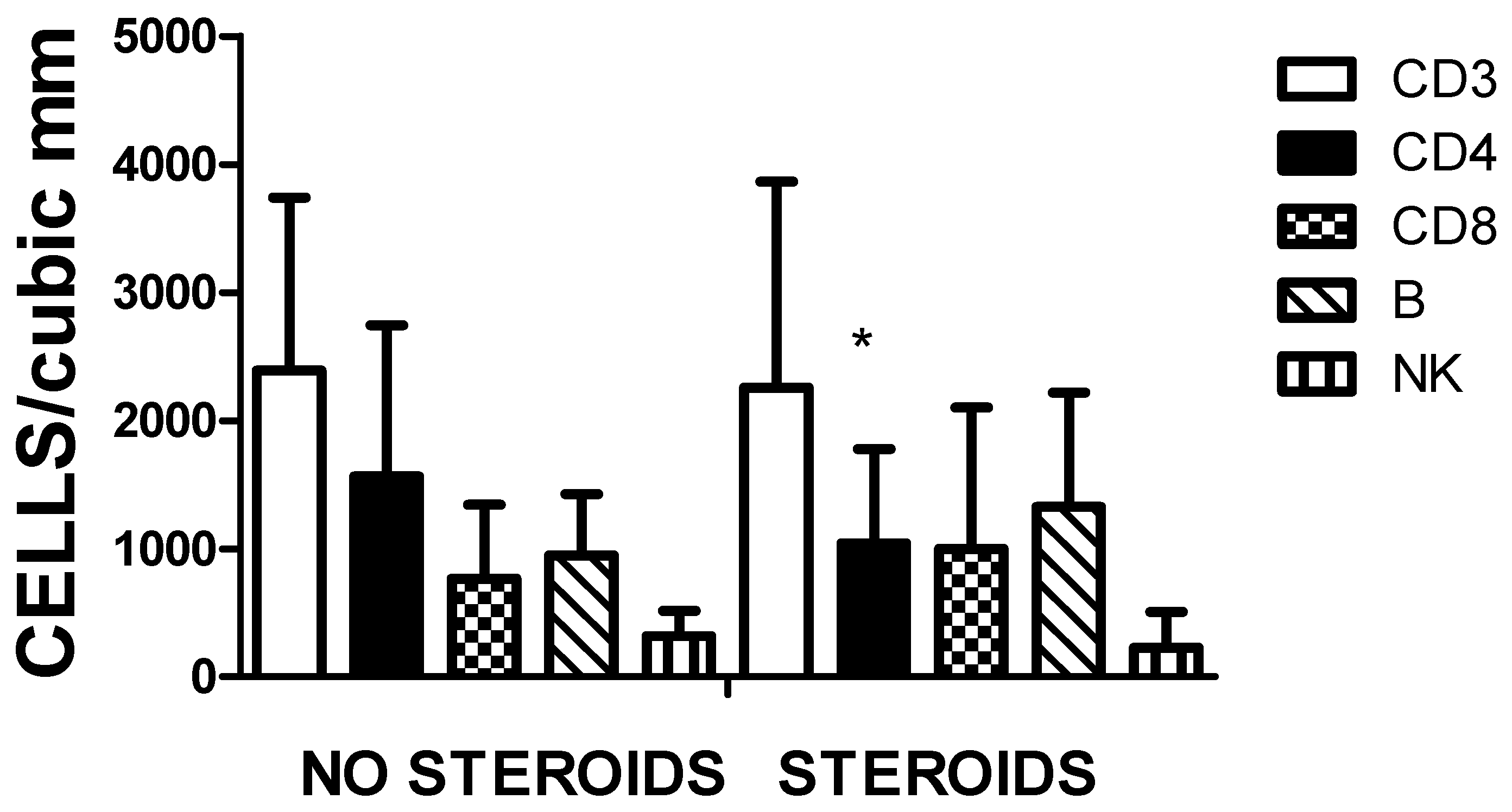1. Introduction
Coronaviruses (CoVs) are enveloped viruses with non-segmented, positive-sense single-stranded RNA genomes and are characterised by distinctive crown-like spikes that protrude from the capsid [
1]. Circulating human coronaviruses can be isolated in only 4-8% of all children with acute respiratory tract infections. The disease symptoms are mild unless the child is immunocompromised [
2]. SARS-CoV-2 virus shares similar characteristics and immune responses to other coronaviruses [
2]; children are less frequently affected by SARS-CoV-2 [
2,
3,
4,
5].
According to statistics published by the Chinese Centres for Disease Control and Prevention, only 1% of the cases reported at the pandemic’s start were in children under 10 years of age, reaching up to 12.3% in February 2020 [
2]. Several hypotheses emerged about the low incidence of COVID-19 in children: they were less exposed to the virus, were asymptomatic or had mild symptoms similar to those of a common cold; fever was the common indication for PCR test. Recent studies have shown that in the pediatric population, COVID-19 is often asymptomatic or less severe than in adults [
2,
3,
4,
5]. The contagion occurs through respiratory droplets, mainly in contact with an adult. Prolonged exposure to high concentrations of aerosolised virus can facilitate transmission. Gastrointestinal symptoms are the primary physical disturbance recorded [
3,
4,
5].
The pediatric population usually is coinfected with other viruses such as Adenovirus, Bocavirus, Rhinovirus, Respiratory Syncytial Virus, Influenza or Parainfluenza [
3,
4]. There seems to be a cyclical pattern with seasonal outbreaks between December and May or March to November in the southern hemisphere, which could condition the immune response and COVID-19 symptoms [
3,
4]. SARS-CoV-2 primarily uses the complex angiotensin-converting enzyme-2 receptor (ACE2), a glycosylated transmembrane protein, to infect and invade the target cell [
6,
7]. The maximum expression of ACE2 is observed in the respiratory epithelium, lungs, kidneys, intestines, testis (Sertoli and Leydig cells), uterus, vagina, endothelium and the heart, and its expression is dependent on age [
6,
7,
8,
9]. A lower ACE2 transcript and expression in the child’s organs may explain why SARS Cov-2 infection is less prevalent in children [
6,
7,
8,
9].
In a group of patients, a rare but severe disorder associated with COVID-19 termed multisystem inflammatory syndrome (MIS) can affect children (MIS-C) and adults (MIS-A) [
10]. It is characterised by dysregulated immune responses leading to endothelial dysfunction and a hyperinflammatory state. Hyperinflammation causes a capillary leak, followed by multiorgan failure. MIS-C is characterised by inflammation in multiple organs such as the brain, kidneys, heart, eyes, lungs, skin, or gastrointestinal system [
10]. The most common symptoms of MIS-C are fever, gastrointestinal (abdominal pain, vomiting, and diarrhoea), and mucocutaneous (conjunctivitis, strawberry tongue, skin rash, and dried-cracked lips) [
11]. Nutritional status or other medical conditions are usually not considered in pediatric COVID-19 or MIS-C [
10,
11,
12,
13,
14,
15]. Patients with MIS-A present comorbidities such as obesity and hypertension [
10,
11,
12,
13,
14]. The male gender, in MIS-a and MIS-c, is the most affected since circulating inflammatory markers, such as IL-6 and C-reactive protein, are higher than in females [
10,
11,
12,
13,
14]. Fever and rash are the most common presenting symptoms in patients with MIS-A. Since the initial clinical presentation can be non-specific, mimicking acute infection, a 4-6 weeks follow-up after COVID-19 recovery is recommended for patients with the aforementioned clinical complaints [
10,
11,
12,
13,
14].
The epidemiologic dynamic of SARS-CoV-2 infection in the pediatric population in Venezuela changed dramatically, according to official statistics (
https://covid19.patria.org.ve/estadisticas-venezuela/). The percentage of positive cases reported in patients under 19 years of age changed from 2.5% (third week of September 2020) to 11% in January 2021, 15% by January 2022, and 18% in October 2022 [
16]. The increase in pediatric cases may be due to different viral variants and the possible reluctance of parents to vaccinate their children against the virus.
Since the pediatric hospital, Dr. José Manuel de los Ríos is a national centre of reference for pediatric diseases, a particular war was created for patients with SARS-CoV-2 infection and specific attention was given to their nutritional status other medical conditions. The study aimed to analyse the immune response of patients admitted to the hospital and the possible influence of different parameters, age, gender, nutritional status, treatment and medical conditions.
2. Materials and Methods
The Dr José Manuel de los Ríos Children’s Hospital is a national centre for pediatric patients for children with complex medical conditions and chronic pathologies (cardiopathies, nephropathies, metabolic diseases, autoimmunity). Patients included in the study were in a special hospital ward for medical follow-up.
The study was approved by the bioethics committee of the Dr José Manuel de los Ríos Children’s Hospital (HJMR-25/11/2020) and of the Dr Nicolás Bianco Colmenares Institute of Immunology, Faculty of Medicine of the Central University of Venezuela, Caracas (IDI-30/11/2020). Written informed consent from parents or guardians was taken. Informed assent from adolescents was taken along with their parents or guardians.
A descriptive cross-sectional prospective case series observational field study was conducted. The study comprises two periods in which the ward was most active, March to May 2021 and January to March 2022. A total of 72 pediatric patients with SARS-CoV-2 infection were included. The patient’s ages were between 29 days old and 18 years old. All cases met clinical/epidemiological criteria defined by the WHO, ad admission 30 (41,7%) had a positive PCR for the virus and 42 (58,3%) had a positive Antigenic rapid diagnostic test (RDT). A structured interview was conducted with parents and representatives to obtain the information in the medical records to verify the required data and new information. The clinical history included symptoms, comorbidities, nutritional diagnosis according to WHO growth patterns, use of steroids and MIS-C diagnosis. Patients whose parents did not sign the informed consent were excluded from the study. No patient required oxygen, and there were fatal cases
A venipuncture was performed on all patients to obtain two tubes of 2-3 ml of peripheral blood, one with EDTA as an anticoagulant and one for a serum sample. A haematological control was performed. Flow cytometry was used to quantify T cell subsets, B cells and NK lymphocytes. The following monoclonal antibodies from BioLegend were used anti-CD3/Pecy5, anti-CD4/Pe, anti-CD8/FITC; anti-CD19/PeCy5, anti-CD20/Pe anti-CD3/FITC-CD16-CD56/Pe. The commercial ELISA kits from Legend Max™ (BioLengnd) IFN-γ, IL-6, and IL-10 levels were used for cytokine quantification. The ELISA tests were performed as suggested by the manufacturer. According to the manufacturer, the minimum detectable concentrations are IFN-γ 5.6 pg/mL, IL-6 1.6 pg/mL, and IL-10 2 pg/mL. In the youngest group, cytokine detection was difficult to ascertain; therefore, to facilitate the analysis, the patients were organised by age group according to age, establishing four groups: infants: 29 days old to 23 months with 29 days old; preschoolers: 2 years old to 6 years 11 months and 29 days old; school-age children: 7 years old to 9 years 11 months and 29 days old; adolescents: 10 years old to 18 years 11 months and 29 days old.
Statistical Analysis
The techniques of descriptive statistics were applied for the first analysis. Tables and figures of the absolute and relative frequencies were represented. This organisation, comparisons, and necessary calculations were performed with Microsoft Excel program version 16.70. The arithmetic mean and standard deviation were calculated for the continuous variables; in the case of nominal variables, their frequencies and percentages were calculated
The correlations between the immunological markers white blood cell count, CD4+ T cell percentage, CD8+ T cell percentage, CD4+ T cell/CD8+ T cell ratio, NK cell percentage, NKT cell percentage, B cell percentage, IL levels -6, IL-10 and IFN-γ were evaluated with the Pearson correlation coefficient. The principal component factorial analysis model was used to determine the relationships among variables using the Kaiser-Meyer-Olking measures and the Bartlett sphericity test to validate the results. From the data matrix, explanatory variances were calculated from the principal component model and varimax rotation was used to determine the final correlations. A multivariate general linear model analysis (MLGM or MANOVA) was used for the relationships between immunological markers and epidemiological indicators. All immunological features were included as predictor variables, and age, use of steroids, nutritional diagnosis and severity of SARS-CoV-2 infection were included as independent variables. The effect size measures were also calculated as a direct indicator of the impact of the epidemiological variables using the partial eta statistic (h2). Also, the following values were used for interpretation: h2 < 0.01 as a small or unimportant outcome, h2 between 0.02 and 0.14 as a medium or relevant result, and h2 greater than 0.14 as an important and significant effect. To validate the consistency of the MANOVA models, the Pillai Trace statistic was used to indicate feasibility for calculation. Since this statistic varies between 0 and 1 for values close to unity, it is considered that the MANOVA model can be carried out without problems. A value was considered statistically significant if p < 0.05. Data were tabulated with STATA version 17.0 developer StataCorp [
17].
3. Results
3.1. Characteristic of the Cohort
The sample consisted of 72 pediatric patients whose general characteristics are illustrated in
Table 1. Remarkably, 34.7% of the patients were undernourished, and 4.2% were obese-overweight. Around 28% of the individuals had comorbidities: 7 (35%) were asthmatic, 5 (25%) had cancer, 5 (25%) had nephropathy, 2 (10%) had hemoglobinopathy, and 1 (5%) had type 1 diabetes mellitus. Most patients did not have significant medical issues (59.7%). However, 22 patients had MIS-C 22 and 6 bacterial and one viral coinfection (
Table 1). Also,
Table 1 illustrates the treatment used; 30 patients did not receive steroid treatment since they did not have critical medical issues during the infection and dexamethasone was often used in the patients that required steroid therapy (30). The prevalent symptom was fever, cough, and diarrhoea (
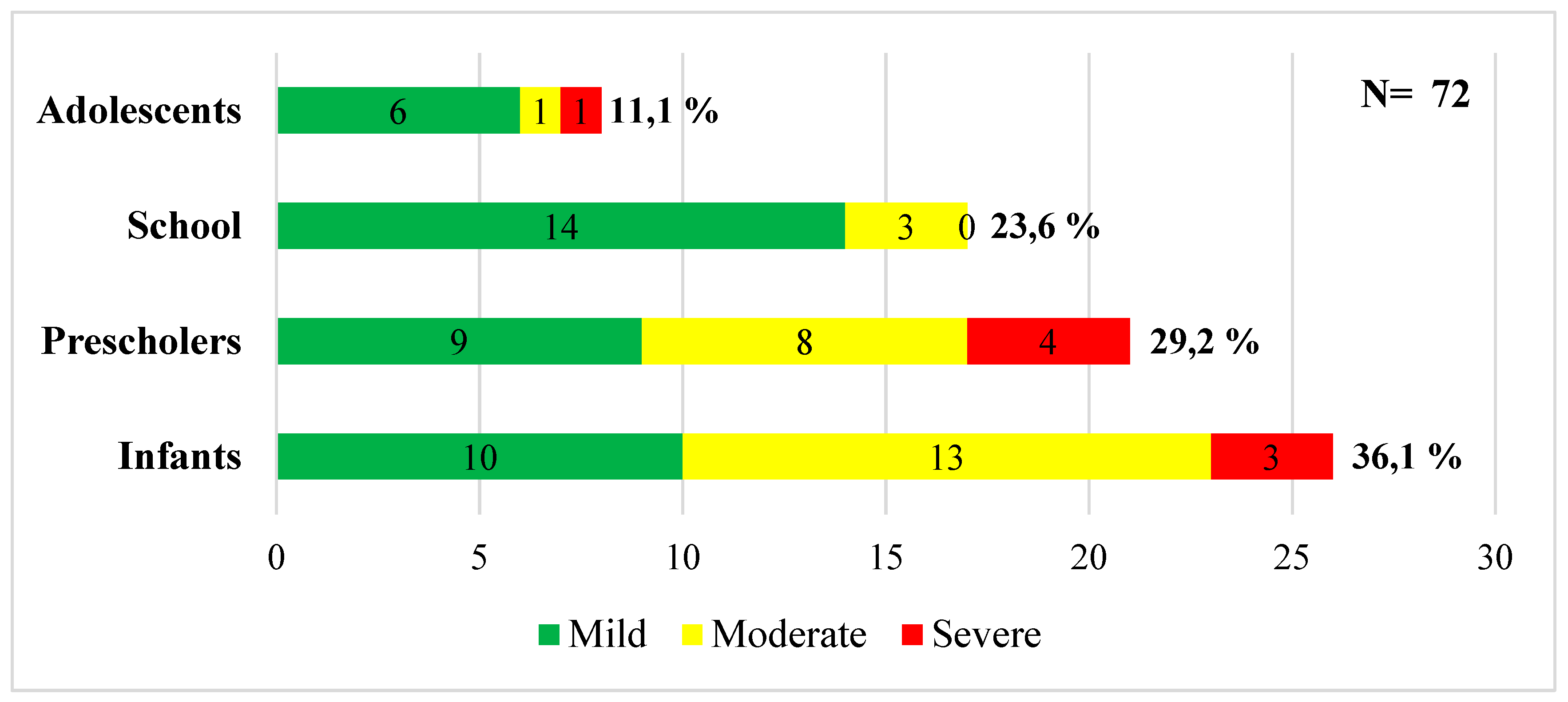
Table 1). The distribution of patients by age and clinical severity is depicted in
Figure 1.
3.2. The Influence Of Age On Lymphocyte Populations and Cytokine Values
Age is an important influence on the haematological and cytokine parameters observed in the group.
Figure 2 illustrates the difference in leukocyte values obtained in haematological counts. As expected, the values for the lower age group were higher and decreased with age. The values did not differ between genders.
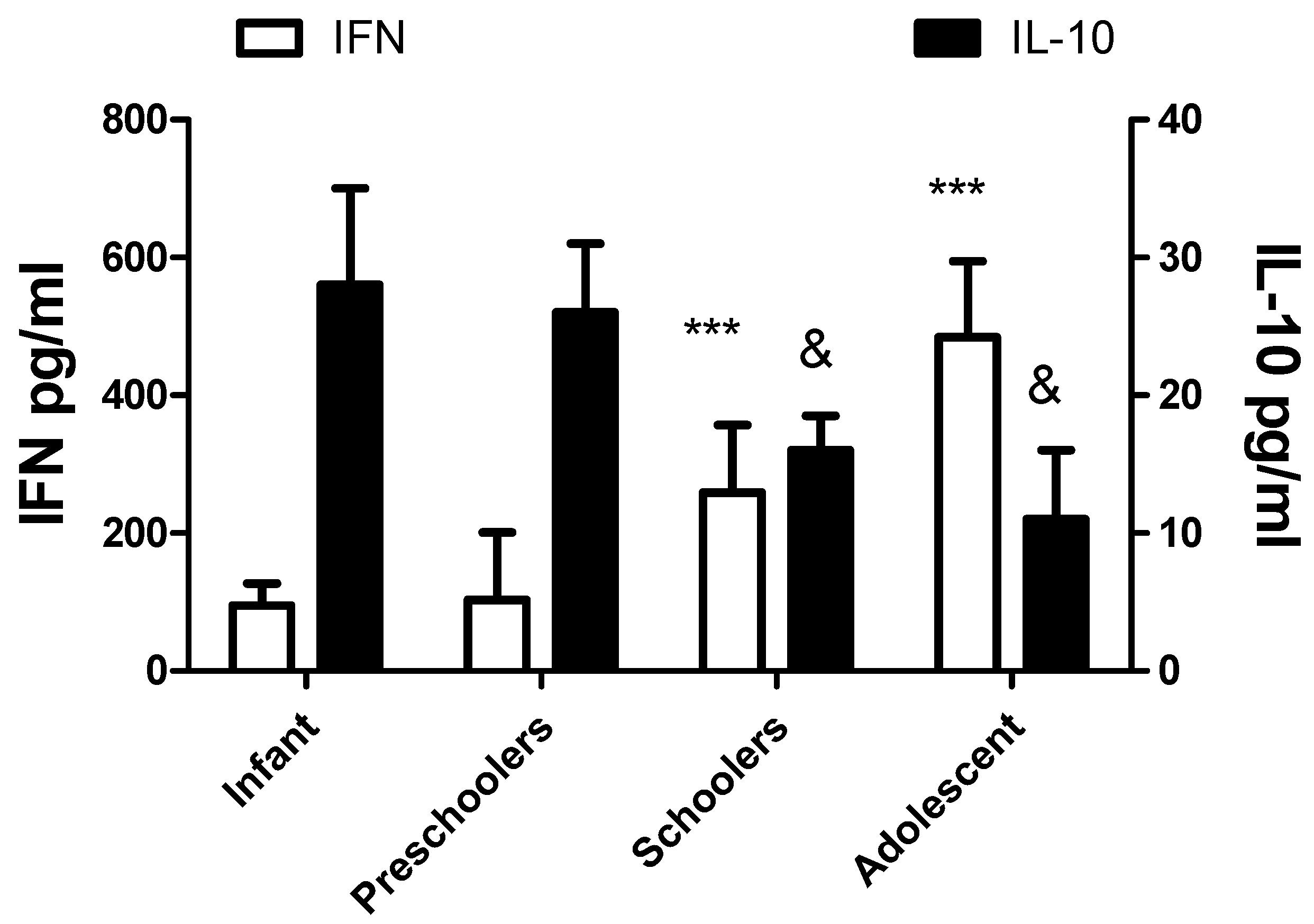
The levels of cytokines IFNγ and IL-10 are also different with age, as depicted in
Figure 3. No significant differences in gender were recorded. The values of IFN γ increase with age, and the contrary occurs with IL-10. No significant effects with age or gender were recorded with IL-6 levels.
3.3. Nutritional Status and Lymphocyte Populations.
As recorded in
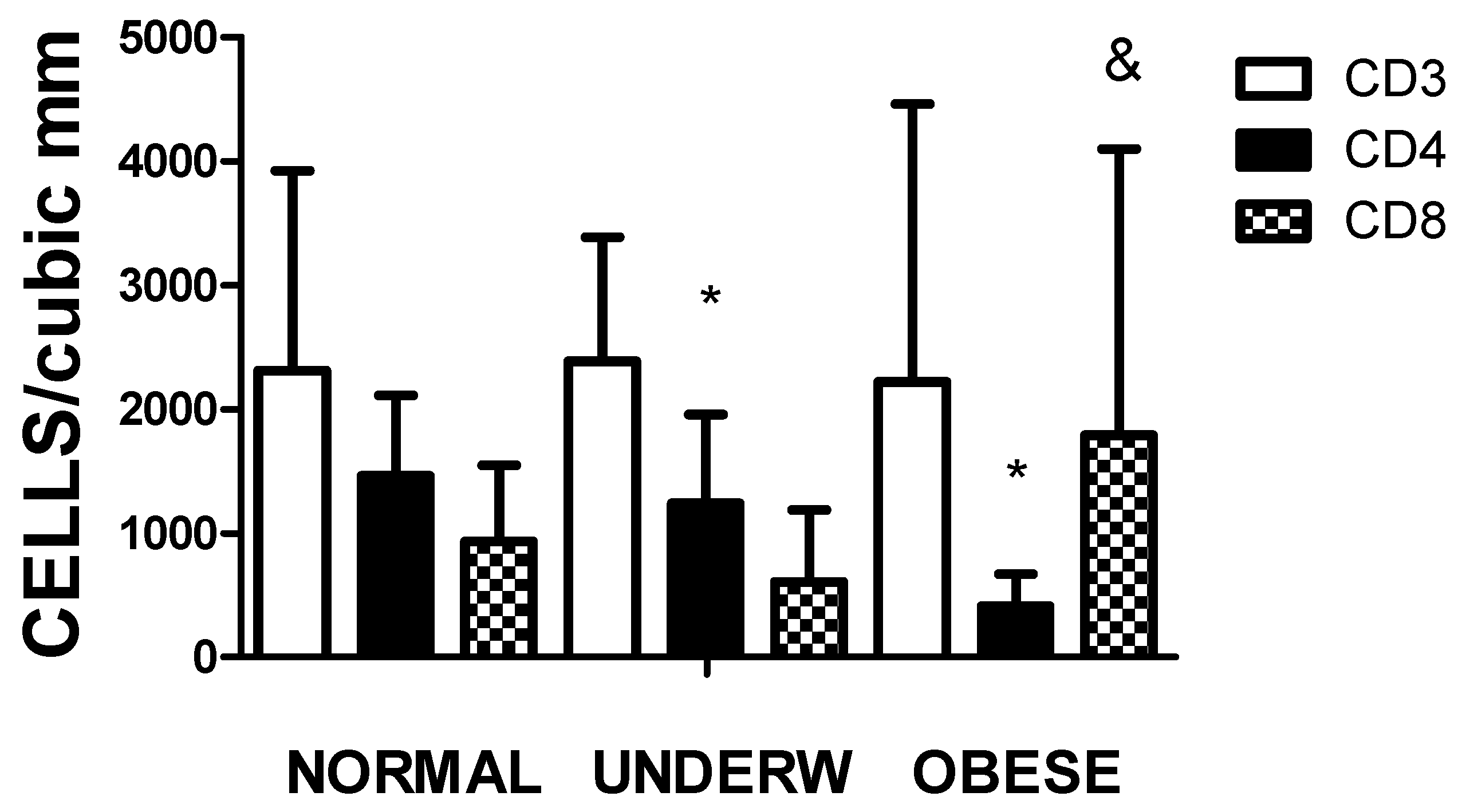
Table 1, the nutritional status of the children admitted to the hospital ward differed independently of the severity or treatment for SARS-CoV-2 infection. In
Figure 4, the values of CD4 significantly decreased in the underweight and overweight individuals (p<0.01). In malnourished individuals compared to overweight-obese patients, the values of CD8 do not compensate for the decrease in CD4 levels. As expected, the CD4/CD8 ratio is higher in the normal and underweight groups and drops dramatically in the overweight-obese group.
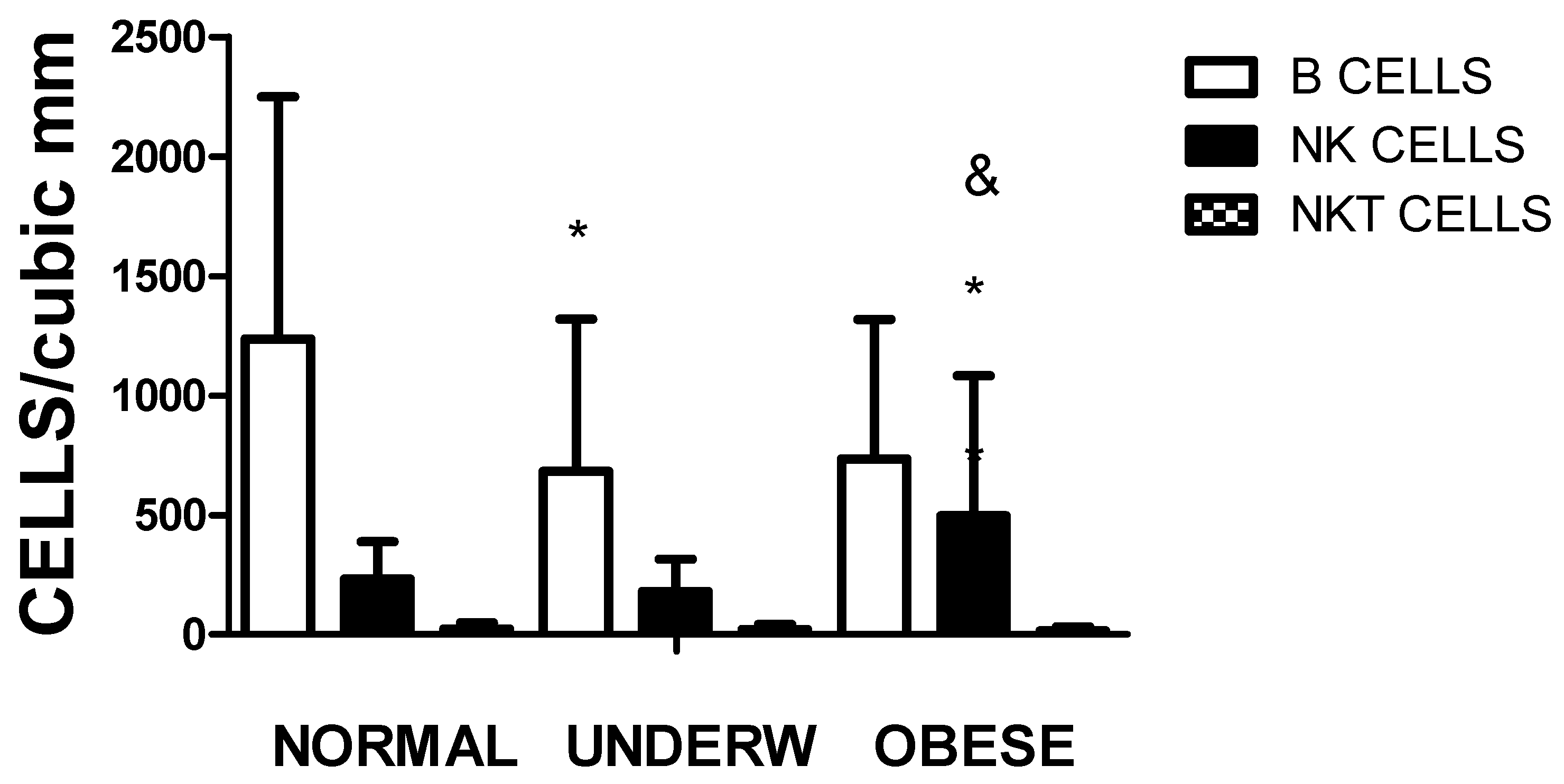
Figure 5 depicts that not only the amount of CD3CD4 subpopulation differs according to the nutritional status, but also the number of circulating B cells in underweight individuals and NK cells in the obese group as compared to the normal individuals.
3.4. MIS-C, COVID-19 Severity and IL-6 Concentrations
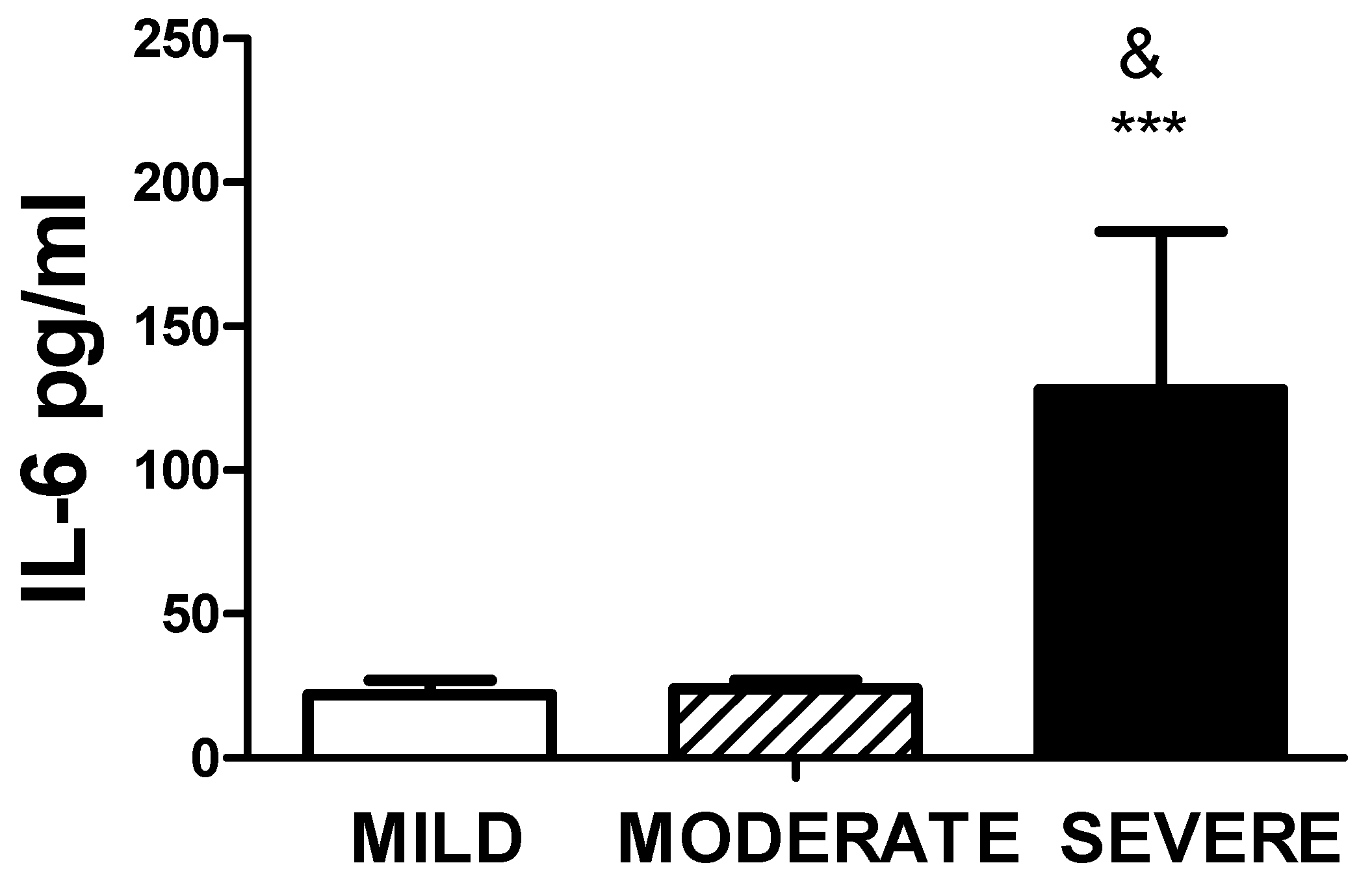
Higher amounts of serum Il-6 levels were observed in patients with severe disease as compared to moderate or mild disease, as shown in
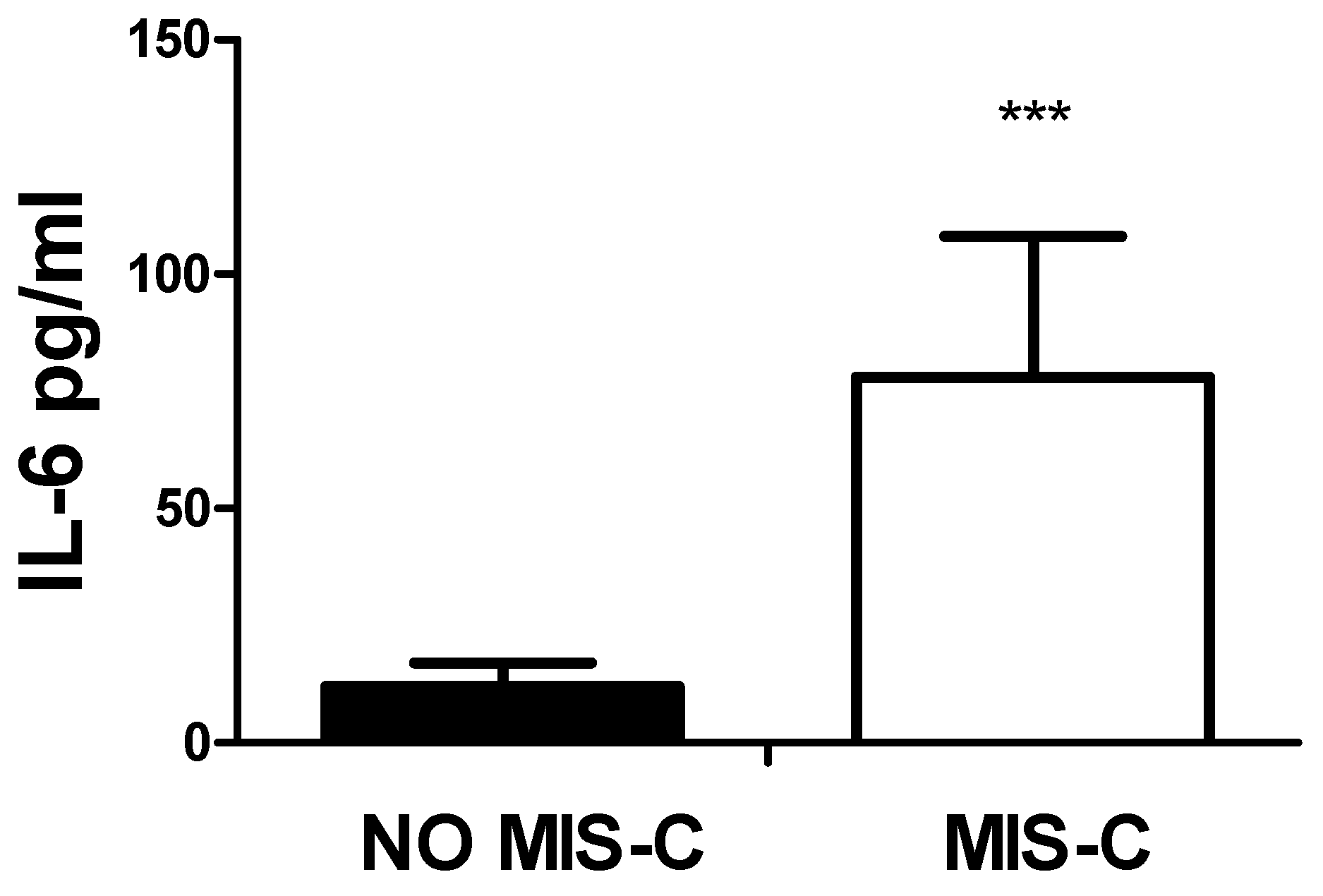
Figure 6. As expected, the values of IL-6 were also significantly higher in patients with MIS-C,
Figure 7.
3.5. Steroid Use, Cytokine Concentration and Lymphocyte Population
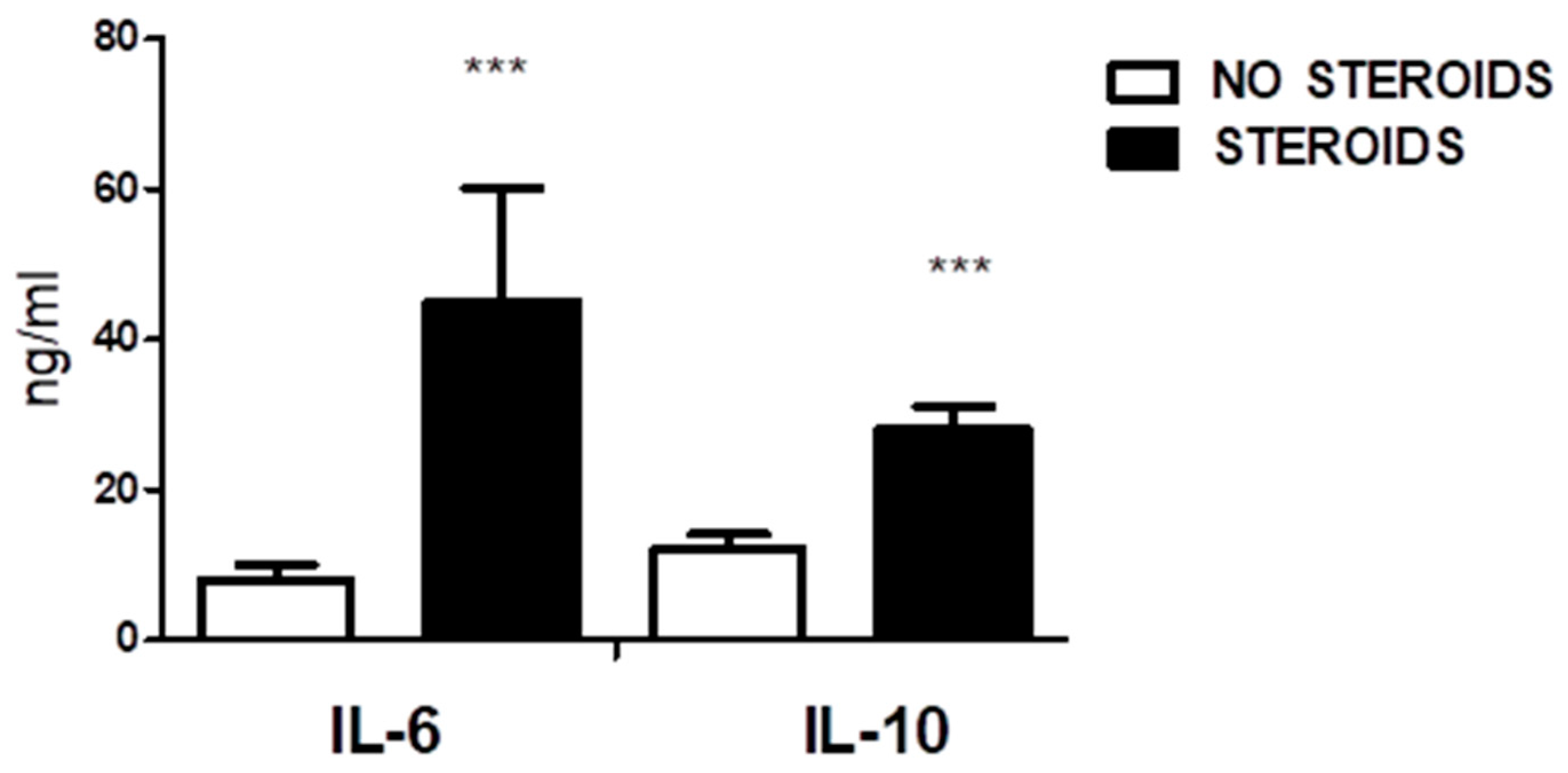
The effect of steroids on the immune response of pediatric cases was analysed. Statistical differences were recorded in IL-6 and IL-10 serum levels (
Figure 8). The increase in IL-6 in the steroid group refers to the group of MIS-C under treatment. The group of no steroids corresponds with patients with mild and some with moderate disease.
In
Figure 9, the cell populations in the two groups were represented. A significant decrease in the CD3CD4 cell subpopulation was observed in the treated group. No other significant differences were encountered.
4. Discussion
The immune response against SARS-CoV-2 in pediatric patients differs from adults [
2,
3,
4,
6,
7,
8]. Leukopenia and lymphopenia are frequently reported in adults but are less frequent in the pediatric population [
2,
3,
4,
6,
7,
8]. Also, MIS and cytokine storm are generally observed in adult individuals with comorbidities infected with the virus [
2,
3,
4,
6,
7,
8]. There are few studies on pediatric COVID-19, probably because most infected patients have a mild disease course [
2,
3,
4,
5,
8].
The main target of SARS-CoV-2 is the respiratory tract, and respiratory infections are frequent in the first years of life. These infections lead to memory T and B cell subpopulations that prevent reinfection or severe disease [
6,
7]. Several factors have been proposed to explain the immune response against pathogens in children [
2,
3,
4,
8]. 1) A low expression of ACE2 decreases SARS CoV-2 infection efficiency in target cells and organs [
2,
3,
4,
8,
9]. 2) In the early stages of infection, the production of IgM, which has broad reactivity and variable affinity, facilitates the decrease of pathogenic burden [
18,
19]. 3) In children, fetal-derived B1 lymphocytes contribute to such natural antibody production without antigenic stimulation during the first days of viral infection [
18,
19]. These B1 lymphocytes and memory B cells are abundant and adaptable to new antigens. Early polyclonal B cell response constitutes an advantage that reduces tissue damage and favours the child’s survival to eliminate unknown or known pathogens [
18,
19]. In adults, this response is not observed [
6]. 4) An efficient innate immune response protects pediatric patients; the cytokine storm is usually observed in adults, not children [
2,
3,
4,
6]. 5) A cross-reactive immune response from other coronavirus infections may be protective [
4]. Also, other common viral respiratory coinfections in children may limit SARS-CoV-2 replication [
4,
11].
The most common clinical manifestations in pediatric patients with COVID-19 were fever, cough, and diarrhoea which have also been reported [
2,
3,
4,
20,
21,
22]. The comorbidities observed in the cohort were asthma (7: 35%) was the most frequent, followed by cancer (5: 25%), nephropathies (5: 25%), hemoglobinopathies (sickle cell anaemia) (2: 10%) and type 1 diabetes mellitus (1: 5%). Most patients with comorbidities (67%) had mild disease, suggesting the medical follow-up was successful. Other groups reported no increased risk in asthmatic patients [
23,
24]. Moderate or severe disease was observed in six (30%) of the patients; patients with chronic kidney disease on renal replacement therapy (4: 20%) and cancer patients (2: 10%).
Several factors were encountered in the cohort analysed in this report that may affect the response and outcome to the viral infection. The first point is the difference in circulating leukocyte levels and the levels of cytokines present in the serum. The second issue is that a substantial number of patients under five years old were undernourished, suggesting that the course of the disease may be affected [
15,
25]. It has been described that underweight children have impaired immune response; there is an increase in IL-10 and a decrease in serum IFNγ levels with a reduction in circulating B cells and an inadequate response to polyclonal antigens [
25]. We observed a reduction in circulating CD3CD4 and B lymphocytes in underweight patients, which agrees with the general description reported without SARS-CoV-2 infection [
25]. It is not easy to define the clinical impact of COVID-19 on these patients. To our knowledge, there are no reliable clinical trials to ascertain the role of undernourishment in pediatric patients infected with COVID-19, especially under five years old.
The prevalence of moderate or severe COVID-19 was higher in children under two years old, suggesting the need to evaluate and provide exhaustive follow-up for SARS-CoV-2 infections in infants. The levels of IL-6 can be an excellent prognostic factor; however, C-reactive protein, the acute phase reactant induced by IL-6, may provide helpful guidelines at a lower cost [
26,
27]. Among the main differential findings of cytokine levels and cellularity in relation to the age group, the highest levels of IL-10 and leukocyte count in infants stand out regarding preschoolers, schoolchildren and adolescents, which is considered part of the expected physiological behavior due to the maturity of the immune system, where the number of leukocytes is high at birth, with an increase in the first 12 weeks of life and then followed by a decrease and stability during the first 2 years of age, to then continue a progressive decrease in the ages of preschool, school and adolescence until reaching values similar to those of adults. Breastfeeding in this age group may confer a protective role;breast milk contains a multitude of immunologically active components, including interleukin (IL)-10, a major regulatory cytokine of inflammatory responses, which, helps infants in the essential immune adaptations [
28,
29]. The high IL-10 levels may play a role in T reg response in the pediatric population [
21,
26,
27]. The levels of IFNγ only were correlated with age and contrasted those of IL-10, suggesting that at a younger age, the Th1 response is less prominent. However, in this group, the amount of undernourished individuals is higher and possibly nutrition may increase IL-10 and decrease IFNγ production [
15,
25].
The high prevalence of MIS-C is 30.6% [
10,
11,
12,
13,
14] of the patients, primarily infants and preschoolers (18: 81.8%), who initially presented mild disease. The possibility of overdiagnosis of MIS-C is suggested in this cohort, although IL-6 levels were higher than in the other group [
30,
31]. In addition, a meta-analysis performed with more than 2000 studies refers to the complexity of the syndrome [
32].
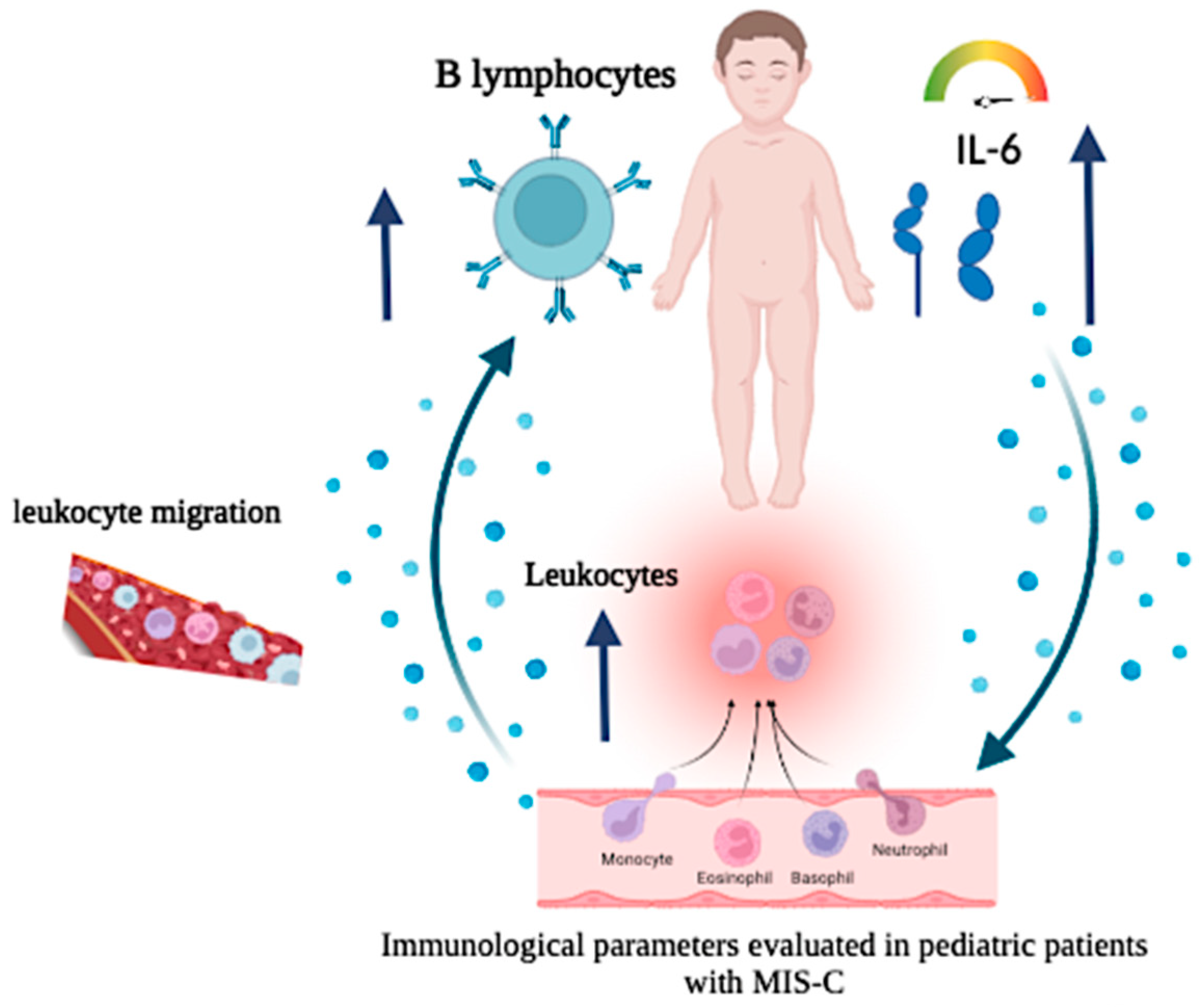
Figure 10 represents a mechanism relating to circulating IL-6 levels in MIS-C.
Differences in circulating lymphocyte populations and cytokine levels were observed in patients similarly treated with steroids as reported [
33,
34]. Increased levels of IL-10 and a decreased amount of CD4 were recorded. The levels of IL-6 were also increased in the treated group, but some patients were diagnosed with MIS-C, which would probably affect the cytokine levels. The analysis did not include the dose or time of steroids received.
In a previous report [
35], the role of memory T cells and age were analysed in adult patients infected with COVID. Younger individuals responded and maintained the amount of memory T cells compared to older individuals whose memory T cells decreased. It would be interesting to assess the memory response of children of this cohort as well as others that have been vaccinated.
The immune response to SARS-CoV-2 infection appears to be a critical factor in the development and prognosis of patients with COVID-19. That is why, by the data presented in this report, it is possible to clarify some hypotheses and open new research topics on the immune response of the pediatric population against SARS-CoV-2 infection. Fortunately, pediatric patients have a lower tendency to develop severe forms and lower mortality.
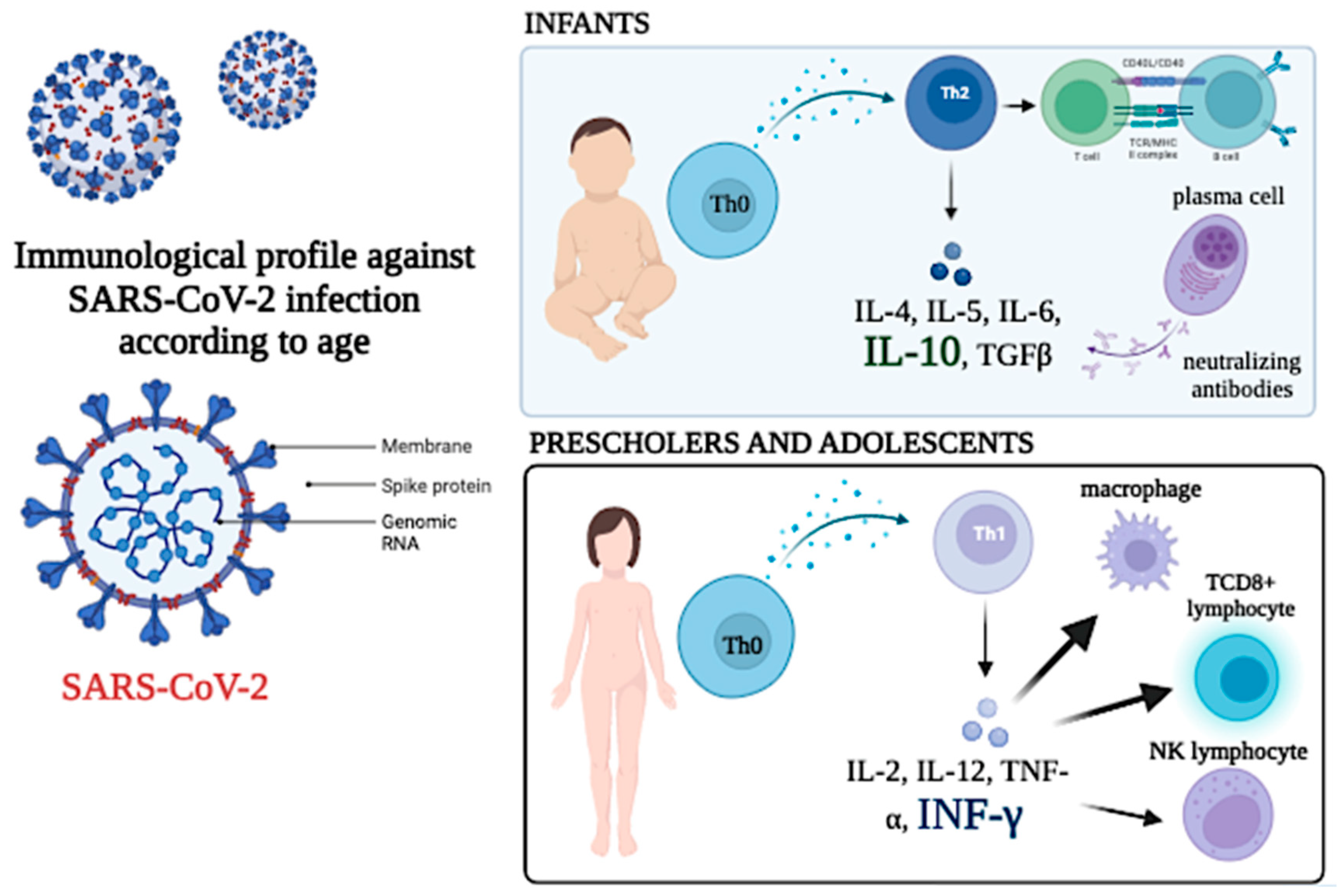
Figure 11 represents a scheme of the findings reported. The difference between infants and preschoolers and teenagers is described.
5. Conclusions
No significant alterations in the leukocyte count or percentage of lymphocytes were found with the symptoms. Higher levels of IL-10 were observed in infants. In contrast, higher IFN- γ levels were found in preschoolers, schoolchildren, and adolescents, showing a more dominant antiviral and Th1-type response. The levels of IL-6 are related to the severity of the disease, and it is higher in patients with diagnosed MIS-C. The nutritional status and the administration of systemic steroids, to a greater extent, the use of dexamethasone, influenced levels of cytokines and percentages of T lymphocytes, B lymphocytes and NK lymphocytes.
The presence of asthma as a comorbidity was not associated with greater severity. Like recent evidence, it is concluded that it does not constitute a risk factor for contagion or severity of COVID-19 in the Venezuelan pediatric population.
It is essential to highlight the differences between eutrophic patients and patients with normal weight and malnutrition in cell populations and subpopulations. These results suggest that nutritional status may be critical in the immune response against SARS-CoV-2.
6. Limitations
This study had several restrictions. The limited availability of COVID-19 pediatric cohort studies that evaluate the patient’s immunophenotype, cytokines, and biosocial aspects at international and regional levels. The small number of patients with severe disease could partly induce bias in the analysis. The impact of nutrition may also be a bias since it may affect the response to treatment and the progression of the disease. However, the medical intervention at the ward decreased the effect of malnutrition. Since most of the patients were discharged in a short period due to mild symptoms, patient follow-up was not possible,
Author Contributions
Conceptualisation, FIC and AHG; methodology, JBDS; validation, LHD and FIT; formal analysis, JBD, FIC and AHG.; investigation, FIC, SJM, WYM and AHG; resources, MEZ; data curation, JBD; writing original draft preparation, FIC, SJM, AHG.; writing—review and editing, FIC, AHG and JBDS; visualisation, AHG, JBDS.; supervision, AHG; project administration, FIC.; funding acquisition, AHG. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the National Fund for Science, Technology and Innovation (FONACIT), an entity attached to the Ministry of Popular Power for Science and Technology of the Bolivarian Republic of Venezuela (MINCYT), Project number: 202000PGP3. It was coordinated by M. Sc. Soriuska Mayora, Institute of Immunology Dr. Nicolás E. Bianco C. UCV.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Institute of Immunology Dr. Nicolás E. Bianco C. UCV (IDI 30/11/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.”
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J. , Wong, G.W.K. SARS-CoV-2 Infection in Children. N Engl J Med, 2020, 382, 1663–1665. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020, S0092867420311570. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020, 39, 355–68. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020, 20, 689–96. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, J.B.; García, A.H.; Moreno, D.; Hajduch, M. Coronavirus infection: An immunologists’ perspective. Scand J Immunol 2021, 93, e130432021. [Google Scholar] [CrossRef]
- Garmendia, J.V.; García, A.H.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Autoimmunity and Immunodeficiency in Severe SARS-CoV-2 Infection and Prolonged COVID-19. Curr Issues Mol Biol. 2022, 45, 33–50. [Google Scholar] [CrossRef]
- Carsetti, R.; Quintarelli, C.; Quinti, I.; Piano Mortari, E.; Zumla, A.; Ippolito, G.; Locatelli, F. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc Health. 2020, 4, 414–416. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Hosseini, P.; Fallahi, M.S.; Erabi, G.; Pakdin, M.; Zarezadeh, S.M.; Faridzadeh, A.; Entezari, S.; Ansari, A.; Poudineh, M.; Deravi, N. Multisystem Inflammatory Syndrome and Autoimmune Diseases Following COVID-19: Molecular Mechanisms and Therapeutic Opportunities. Front. Mol. Biosci. 2022, 9, 804109. [Google Scholar] [CrossRef]
- Kunal, S.; Ish, P.; Sakthivel, P.; Malhotra, N.; Gupta, K. The emerging threat of multisystem inflammatory syndrome in adults (MIS-A) in COVID-19: A systematic review. Heart Lung. 2022, 54, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Panaro, S.; Cattalini, M. The Spectrum of Manifestations of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV2) Infection in Children: What We Can Learn From Multisystem Inflammatory Syndrome in Children (MIS-C). Front. Med. 2021, 8, 747190. [Google Scholar] [CrossRef]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; et al. Rate of thrombosis in children and adolescents hospitalised with COVID-19 or MIS-C. Blood. 2021, 138, 190–198. [Google Scholar] [CrossRef]
- James, P.T.; Ali, Z.; Armitage, A.E.; Bonell, A.; Cerami, C.; Drakesmith, H.; Prentice, A.M. The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review. J Nutrit, 2021, 151, 1854–1878. [Google Scholar] [CrossRef]
- Venezuelan Ministry of Health Epidemiological Bulletin April 8 2021-December 8 2022 (Ministerio del Poder Popular para la Salud. COVID-19 Boletín Nacional. 8 de abril 2021 y 8 de diciembre 2022). Available online: https://covid19.patria.org.ve/estadisticas-venezuela/.
- Hair Jr., J. F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis (5th ed.). 1998. Upper Saddle River, NJ: Prentice Hall.
- Holodick, N.E.; Rodríguez-Zhurbenko, N.; Hernández, A.M. Defining Natural Antibodies. Front Immunol 2017, 8, 872. [Google Scholar] [CrossRef] [PubMed]
- Capolunghi, F.; Rosado, M.M.; Sinibaldi, M.; Aranburu, A.; Carsetti, R. Why do we need IgM memory B cells? Immunol Lett 2013, 152, 114–20. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhao, Z.; Zhang, T. , Guo, W., Guo, W., Zheng, J., Cai, C. A systematic review and meta-analysis of children with Coronavirus Disease 2019 (COVID-19). J Med Virol. 2021, 93, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Herold, B.C. Immune responses to SARS-CoV-2 infection in hospitalised pediatric and adult patients. Sci Transl Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Majeed, T.; Azam Ansari, M.; Ali, U. : Sabit, H., Al-Suhaimi, E.A. Current Scenario of COVID-19 in Pediatric Age Group and Physiology of Immune and Thymus response. Saudi J Biol Sci. 2020, 27, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Sinha, I.; Fernandes, R.M. : Hawcutt, D.B. Pediatric asthma and COVID-19: The known, the unknown, and the controversial. Pediatr Pulmonol. 2020, 55, 3573–3578. [Google Scholar] [CrossRef]
- Adir. Y.; Saliba, W.; Beurnier, A.; Humbert, M. Asthma and COVID-19: an update. Eur Respir Rev. 2021, 30, 210152. [Google Scholar] [CrossRef]
- Rytter, M.J.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The immune system in children with malnutrition--a systematic review. PLoS One. 2014, 9, e105017. [Google Scholar] [CrossRef]
- Trofin, F.; Nastase, E.V.; Vâță, A. , Iancu, L.S.; Luncă, C.; Buzilă, E.R.; Vlad, M.A.; Dorneanu, O.S. The Immune, Inflammatory and Hematological Response in COVID-19 Patients, According to the Severity of the Disease. Microorganisms. 2023, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Morhart, P.; Kehl, S.; Schuh, W.; Hermes, K.; Meltendorf, S.; et al. Age-related Differences in Immune Reactions to SARS-CoV-2 Spike and Nucleocapsid Antigens. In Vivo. 2023, 37, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.J. , & Sánchez, G. Cytokine modulation (IL-6, IL-8, IL-10) by human breast milk lipids on intestinal epithelial cells (Caco-2). The Journal of Maternal-Fetal & Neonatal Medicine, 2015, 1–8. [CrossRef]
- Fiocchi, A. , Schünemann, H. J., Brozek, J., Restani, et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA): A summary report. Journal of Allergy and Clinical Immunology, 2010, 126, 11191128e12. [Google Scholar] [CrossRef]
- Patel, V.K.; Shirbhate, E.; Patel, P.; Veerasamy, R.; Sharma, P.C.; Rajak, H. Corticosteroids for treatment of COVID-19: effect, evidence, expectation and extent. Beni Suef Univ J Basic Appl Sci. 2021, 10, 78. [Google Scholar] [CrossRef]
- Avcu, G.; Arslan, A.; Arslan, S.Y.; Sahbudak Bal, Z.; Turan, C.; Ersayoglu, I.; Cebeci, K.; Kurugol, Z.; Ozkinay, F. Misdiagnosis of the multisystemic inflammatory syndrome in children: A diagnostic challenge. J Paediatr Child Health. 2023. [CrossRef]
- Sachdeva, M.; Agarwal, A.; Sra, H.K.; Rana, M.; Pradhan, P.; Singh, M.; Saini, S.; Singh, M. Multisystem Inflammatory Syndrome Associated With COVID-19 in Children (MIS-C): A Systematic Review of Studies From India. Indian Pediatr. 2022, 59, 563–569. [Google Scholar] [CrossRef]
- Vasichkina, E.; Alekseeva, D.; Kudryavtsev, I.; Glushkova, A.; Starshinova,A. Y.; Malkova, A.; Kudlay, D.; Starshinova, A. COVID-19 Heart Lesions in Children: Clinical, Diagnostic and Immunological Changes. Int J Mol Sci. 2023, 24, 1147. [Google Scholar] [CrossRef]
- Khoo, W.H.; Jackson, K.; Phetsouphanh, C.; Zaunders, J.J.; Alquicira-Hernandez, J.; Yazar, S.; et al. Tracking the clonal dynamics of SARS-CoV-2-specific T cells in children and adults with mild/asymptomatic COVID-19. Clin Immunol. 2023, 246, 109209. [Google Scholar] [CrossRef] [PubMed]
- Mayora, S.; Zabaleta-Lanz, M.; Martínez, W.; Toro, F.; De Sanctis, J.B; García, A. Lymphocyte subpopulations in Venezuelan patients infected with SARS CoV-2. Gac Méd Caracas 2020, 128 (Supl 1), S74–S78. [Google Scholar] [CrossRef]
Figure 1.
Distribution of patients by age and clinical severity. The figure shows the distribution of patients according to age group and clinical severity. The majority of patients with severe disease were under 5 years of age. Source: calculations made in Microsoft Excel version 16.70.
Figure 1.
Distribution of patients by age and clinical severity. The figure shows the distribution of patients according to age group and clinical severity. The majority of patients with severe disease were under 5 years of age. Source: calculations made in Microsoft Excel version 16.70.
Figure 2.
Amount of circulating leukocytes according to age classification. The figure represents the number of circulating leukocytes in different age groups. The significant differences correspond to comparing the infant and the rest of the groups, * p<0.05, *** p< 0.0001. The values recorded in adolescents are similar to the normal range in healthy adults.
Figure 2.
Amount of circulating leukocytes according to age classification. The figure represents the number of circulating leukocytes in different age groups. The significant differences correspond to comparing the infant and the rest of the groups, * p<0.05, *** p< 0.0001. The values recorded in adolescents are similar to the normal range in healthy adults.
Figure 3.
Serum cytokine concentration depends on the age group. The left Y axis and the white bars represent the values of IFN γ. The right axis and the black bars show the levels of IL10, The concentration of IFNγ and IL-10 significantly differ with age when the values recorded in the toddler group were compared to schoolers and adolescents (***p<0.0001 for IFNγ and & p<0.01 for IL-10).
Figure 3.
Serum cytokine concentration depends on the age group. The left Y axis and the white bars represent the values of IFN γ. The right axis and the black bars show the levels of IL10, The concentration of IFNγ and IL-10 significantly differ with age when the values recorded in the toddler group were compared to schoolers and adolescents (***p<0.0001 for IFNγ and & p<0.01 for IL-10).
Figure 4.
The figure illustrates the total T-cell amount and subpopulations according to their nutritional status (Underw refers to underweight and Obese to obese and overweight). The differences among the groups were significant by one-way ANOVA for CD4 and CD8, not for CD3; * representing p<0.01 CD4 values when the normal group are compared to underweight and obese-overweight individuals and & representing p<0.01 CD8 values of the undernourished patients compared with the overweight-obese group.
Figure 4.
The figure illustrates the total T-cell amount and subpopulations according to their nutritional status (Underw refers to underweight and Obese to obese and overweight). The differences among the groups were significant by one-way ANOVA for CD4 and CD8, not for CD3; * representing p<0.01 CD4 values when the normal group are compared to underweight and obese-overweight individuals and & representing p<0.01 CD8 values of the undernourished patients compared with the overweight-obese group.
Figure 5.
The figure illustrates the circulating B, NK and NKT cell populations (Underw refers to underweight and Obese to obese and overweight). The differences among the groups were significant by one-way ANOVA for B cells and NK cells, not for NKT cells. * representing p<0.01 B cell values when the normal group are compared to underweight, and when NK values of normal individuals were compared with obese-overweight individuals and & representing p<0.01 NK values of the undernourished patients compared with the overweight-obese group.
Figure 5.
The figure illustrates the circulating B, NK and NKT cell populations (Underw refers to underweight and Obese to obese and overweight). The differences among the groups were significant by one-way ANOVA for B cells and NK cells, not for NKT cells. * representing p<0.01 B cell values when the normal group are compared to underweight, and when NK values of normal individuals were compared with obese-overweight individuals and & representing p<0.01 NK values of the undernourished patients compared with the overweight-obese group.
Figure 6.
Levels of IL-6 and COVID-19 status. The figure represents the values of serum IL-6 assessed by ELISA of the three groups. Significant differences were observed between mild and moderate (*** p<0.0001) and moderate and severe (& p< 0.0001).
Figure 6.
Levels of IL-6 and COVID-19 status. The figure represents the values of serum IL-6 assessed by ELISA of the three groups. Significant differences were observed between mild and moderate (*** p<0.0001) and moderate and severe (& p< 0.0001).
Figure 7.
Serum levels of IL-6 and MIS-C. A significant (***p<0.0001) higher amount of IL-6 was observed in patients with MIS-C as compared to patients without it.
Figure 7.
Serum levels of IL-6 and MIS-C. A significant (***p<0.0001) higher amount of IL-6 was observed in patients with MIS-C as compared to patients without it.
Figure 8.
Effect of steroids on serum IL-6 and IL-10. The figure illustrates the values of cytokines detected by ELISA in the serum of individuals treated with steroids. The significant differences were recorded using an unpaired Student’s t-test (*** represents p<0.0001).
Figure 8.
Effect of steroids on serum IL-6 and IL-10. The figure illustrates the values of cytokines detected by ELISA in the serum of individuals treated with steroids. The significant differences were recorded using an unpaired Student’s t-test (*** represents p<0.0001).
Figure 9.
Effect of steroids on different lymphocyte populations. The only significant (* p < 0.01) change in cell number is the amount of CD3CD4 cell subpopulation. There are no other important changes in the other cell types.
Figure 9.
Effect of steroids on different lymphocyte populations. The only significant (* p < 0.01) change in cell number is the amount of CD3CD4 cell subpopulation. There are no other important changes in the other cell types.
Figure 10.
Description: Cellular and cytokine alterations were observed in COVID-19 patients with MIS-C diagnosis.
Figure 10.
Description: Cellular and cytokine alterations were observed in COVID-19 patients with MIS-C diagnosis.
Figure 11.
Profile of cytokines and circulating lymphocytes in two different age groups. A Th2 profile in infants and a Th1 profile in schoolchildren and adolescents is suggested.
Figure 11.
Profile of cytokines and circulating lymphocytes in two different age groups. A Th2 profile in infants and a Th1 profile in schoolchildren and adolescents is suggested.
Table 1.
Characteristic of pediatric patients with SARS-CoV-2 infection.
Table 1.
Characteristic of pediatric patients with SARS-CoV-2 infection.
| Variables |
n |
% |
| Sex |
|
|
| Male |
34 |
47,2 |
| Female |
38 |
52,8 |
| Age group |
|
|
| Infant |
26 |
36.1 |
| Preschooler |
21 |
29,2 |
| School-age |
17 |
23,6 |
| Adolescent |
8 |
11,1 |
| COVID-19 severeness |
|
|
| Mild |
38 |
52,8 |
| Moderate |
26 |
36,1 |
| Severe |
8 |
11,1 |
| Nutrition Diagnosis |
|
|
| Malnutrition |
25 |
34,7 |
| Normal |
44 |
61,1 |
| Overweight-obese |
3 |
4,2 |
| Comorbidities |
|
|
| Yes |
20 |
27,8 |
| No |
52 |
72,2 |
| Complications |
|
|
| No |
43 |
59,7 |
| Bacterial coinfection |
6 |
8,3 |
| Viral coinfection |
1 |
1,4 |
| MIS-C |
22 |
30,6 |
| Treatment received |
|
|
| No steroids |
30 |
41,7 |
| Dexamethasone |
30 |
41,7 |
| Hydrocortisone |
5 |
6,9 |
| Methylprednisolone |
4 |
5,6 |
| Dexamethasone +immunoglobulin |
3 |
4,2 |
| Symptoms |
|
|
| Fever |
66 |
91,6 |
| Cough |
56 |
77,7 |
| Diarrhoea |
38 |
52,7 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.c |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).