Submitted:
21 April 2023
Posted:
23 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
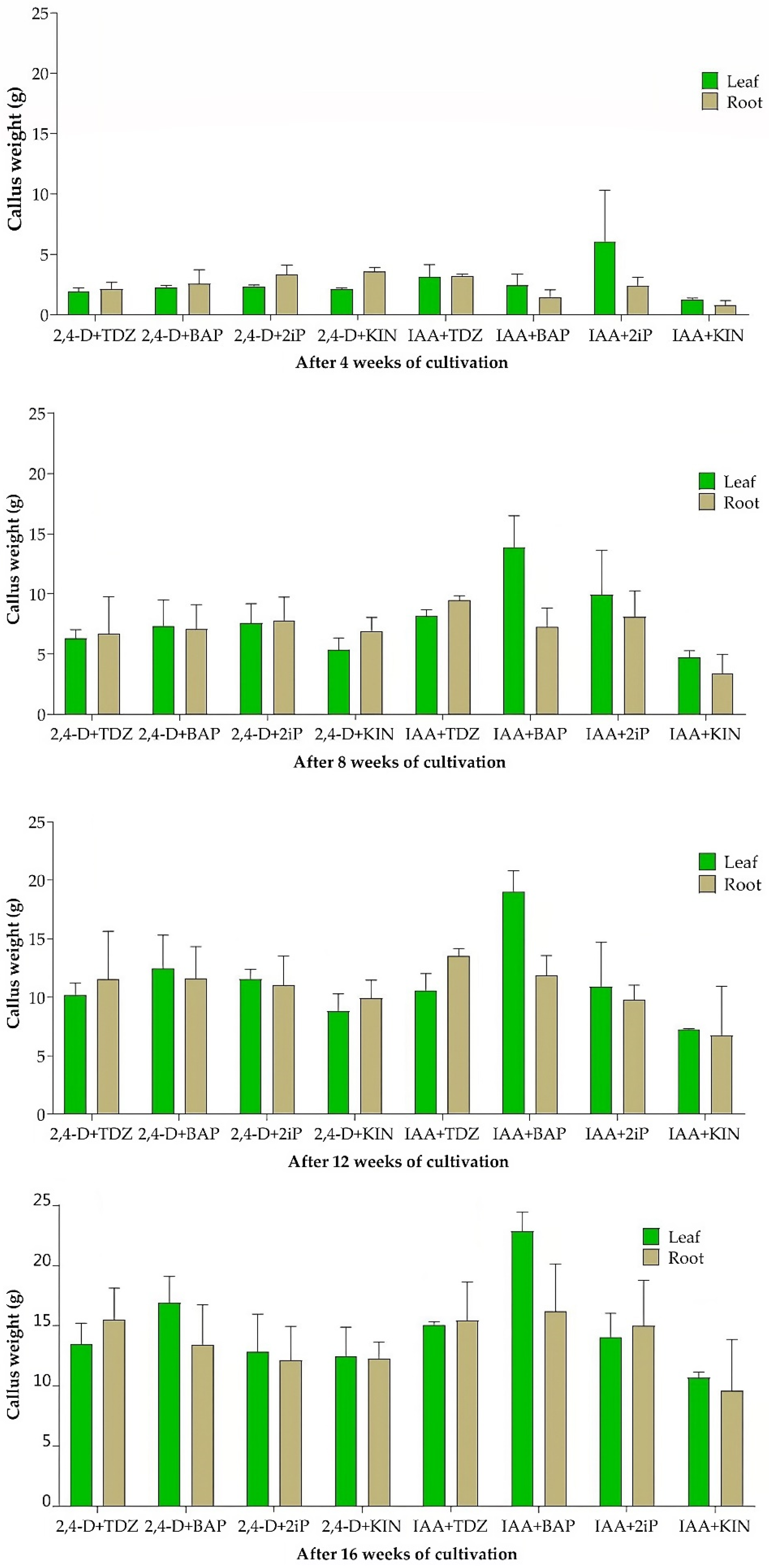
2.1. Callus Formation
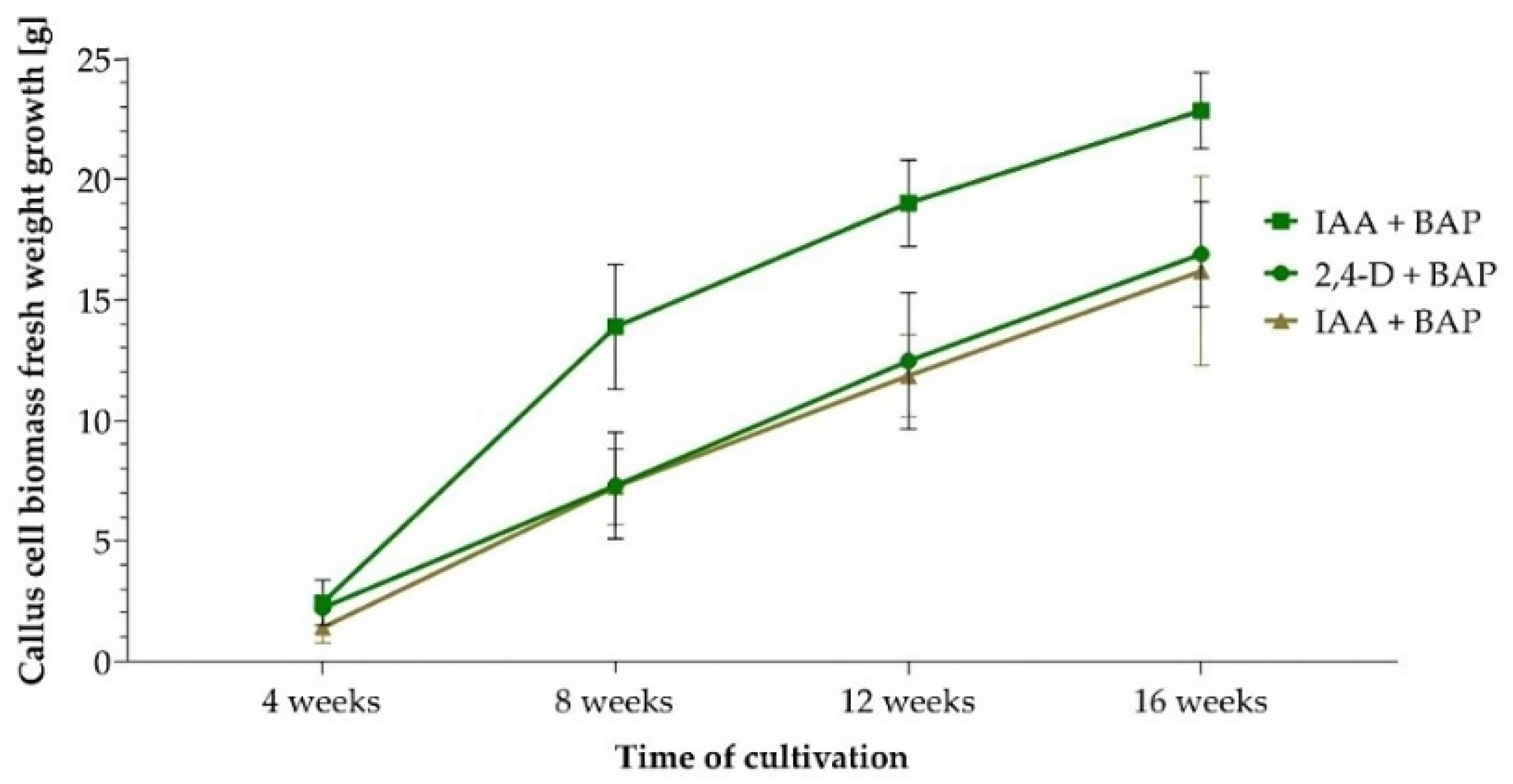
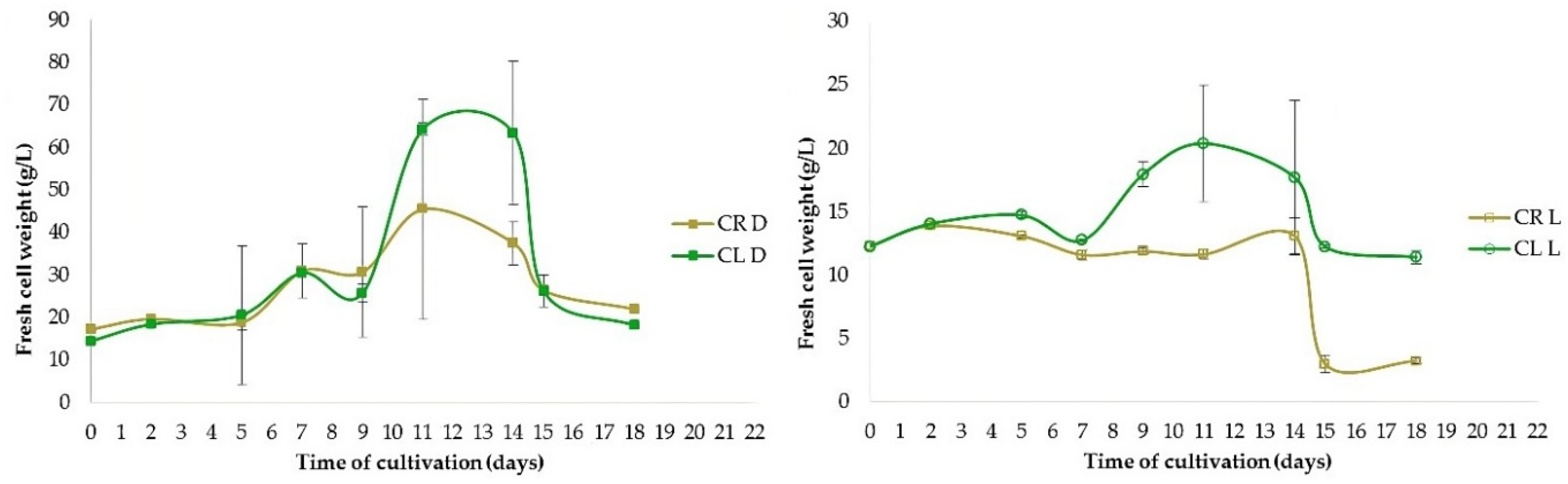
2.2. Callus Growth
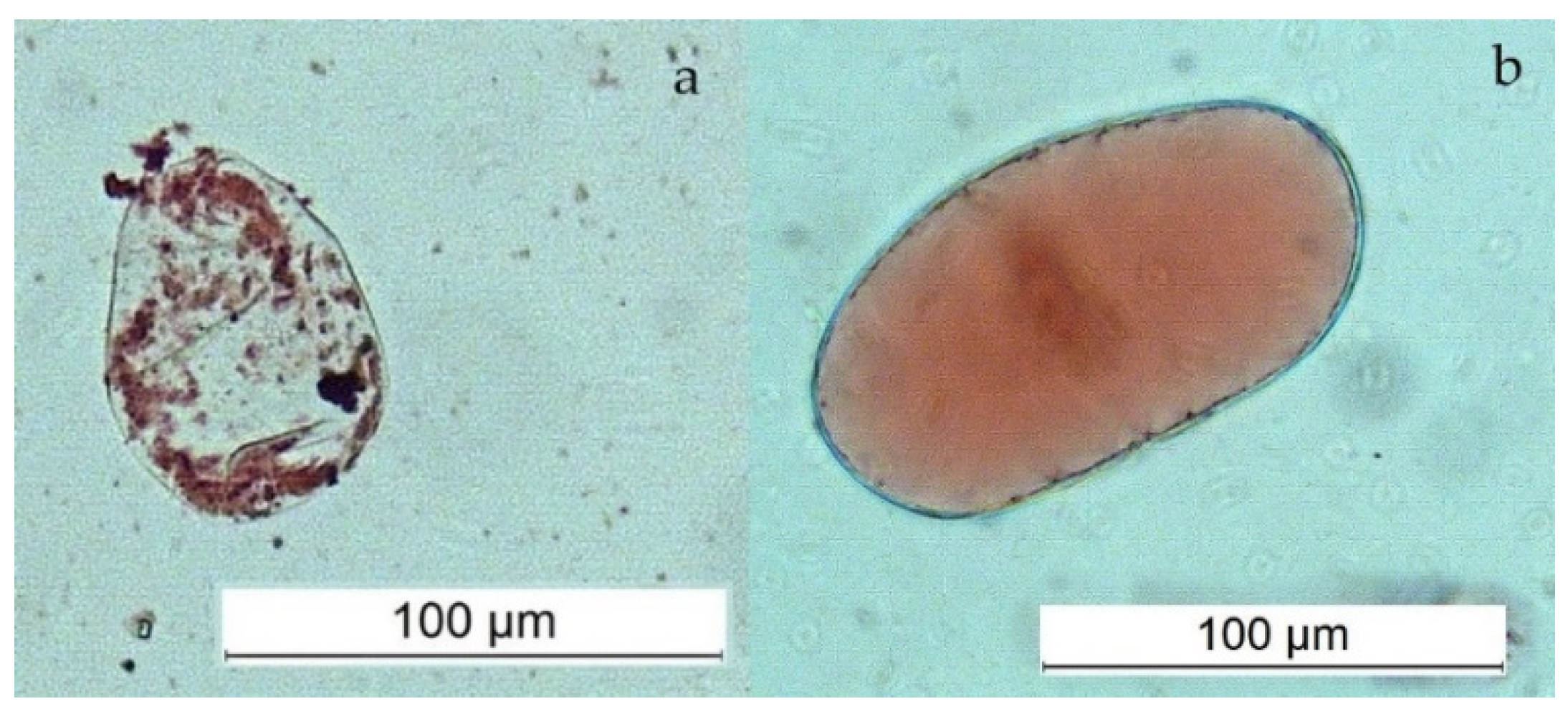
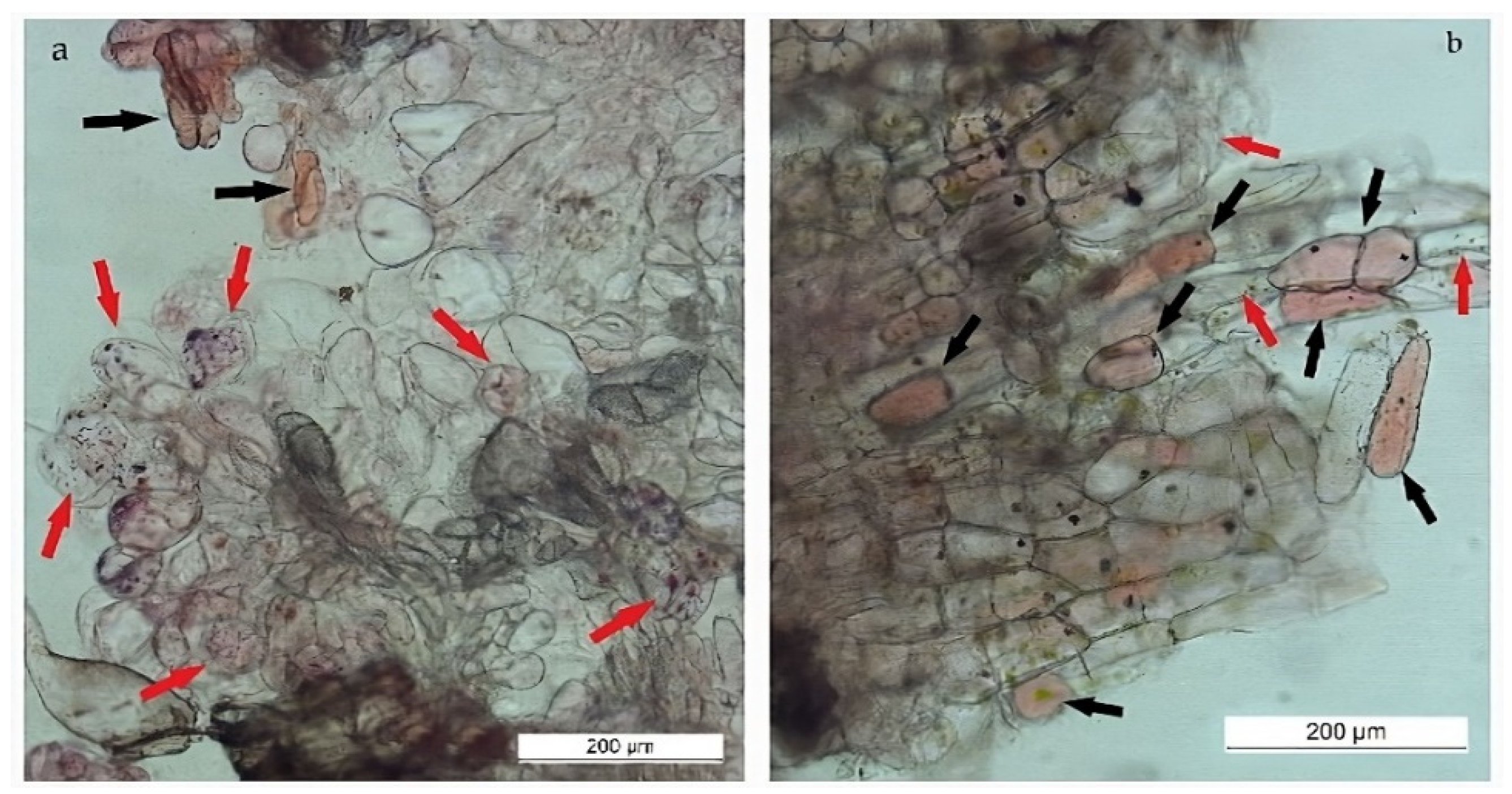
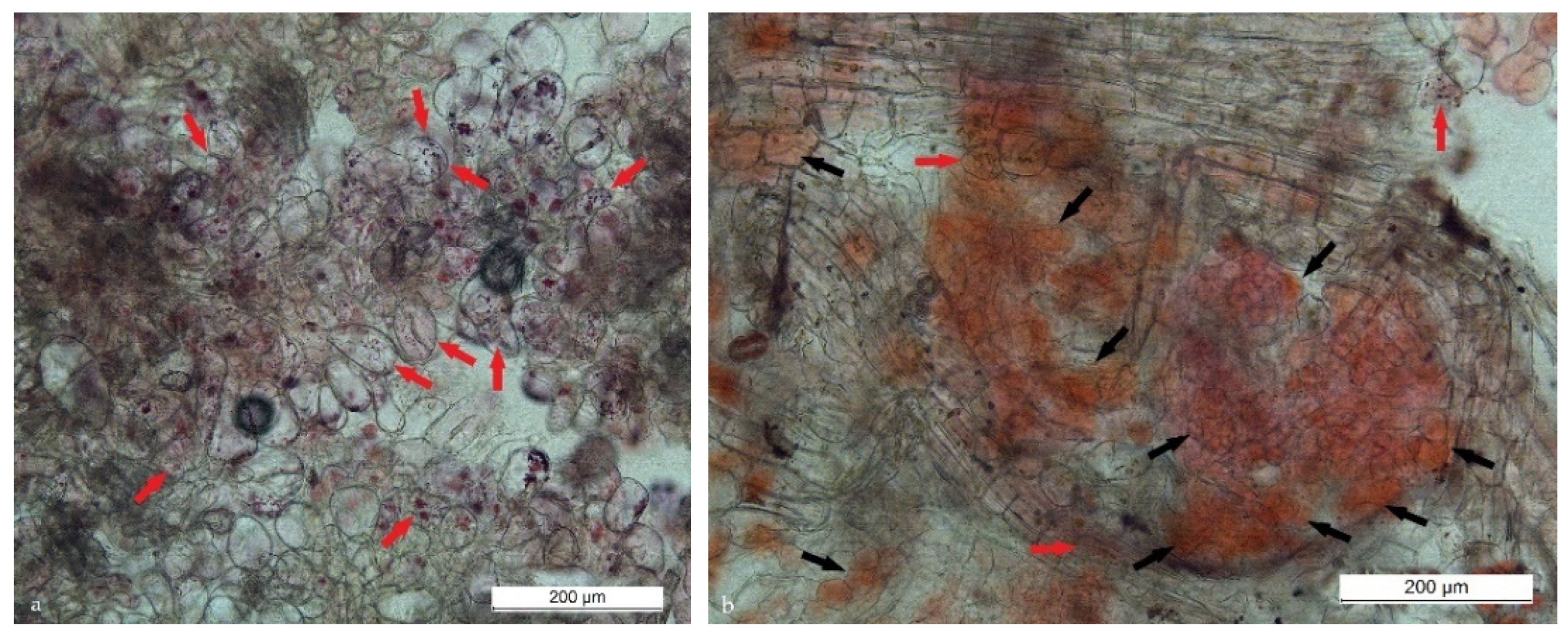
2.3. Stem Cells in Callus
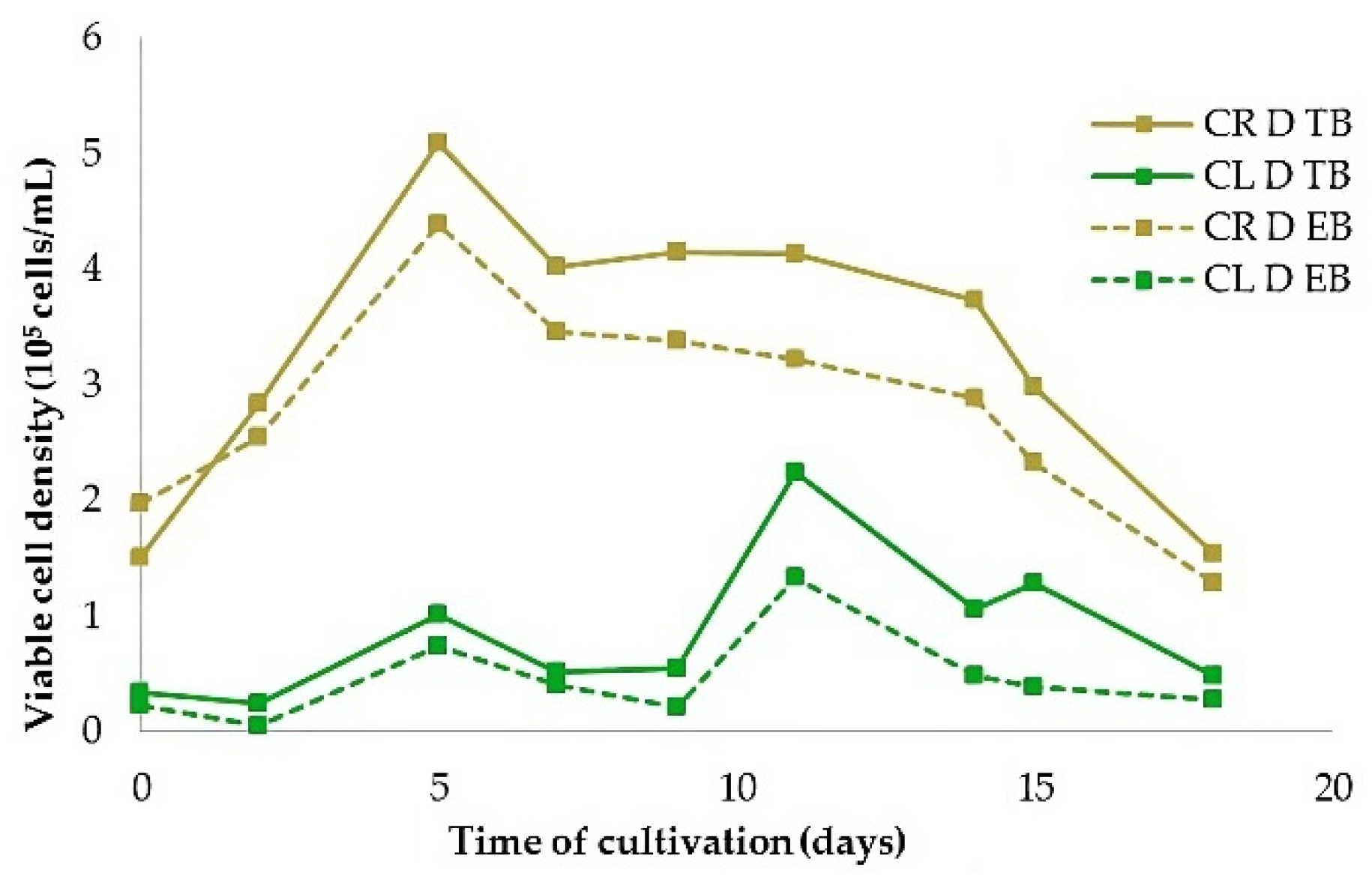
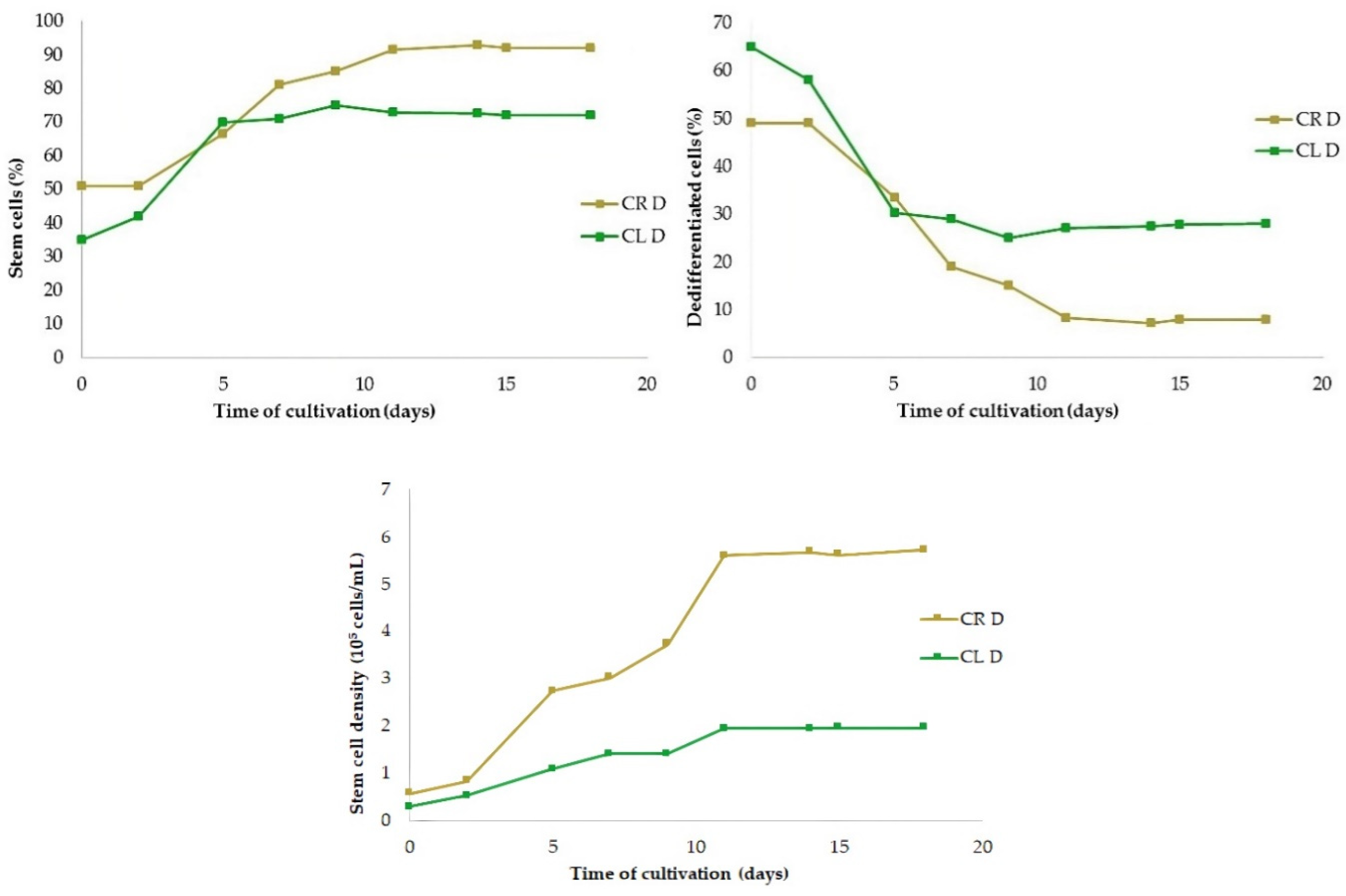
2.4. Cell Suspension Cultures
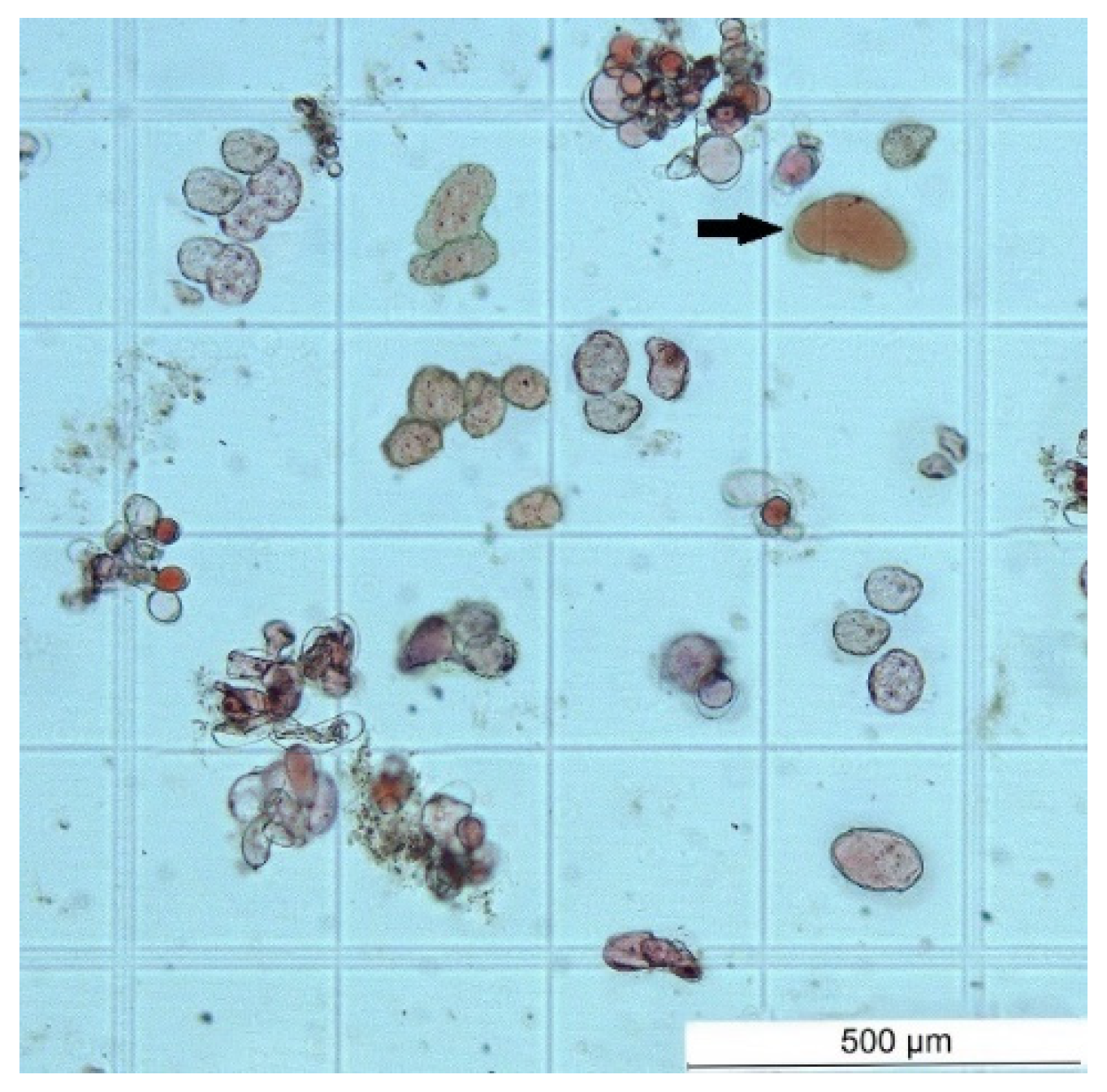
2.5. Stem Cell Suspension Cultures
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Callus Cultures
4.3. Cell Suspension Cultures
4.4. Stem Cell Cultures
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Ashwlayan, V.D.; Kumar, A.; Verma, M.; Garg, V.K.; Gupta, S.K. Therapeutic potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar] [CrossRef]
- Jan, N.; Andrabi, K.I.; John, R. Calenula officinalis—An important medicinal plant with potential biological properties. Proc. Indian Natl. Sci. Acad. 2017, 4, 769–787. [Google Scholar]
- Xuan, S.H.; Kim, G.Y.; Yu, J.Y.; Kim, J.W.; Yang, Y.R.; Jeon, Y.H; Jeong, Y.J.; Kim, A.R.; Park, S.N. Antioxidant and cellular protective effects against oxidative stress of Calendula officinalis flowers extracts in human skin cells. Appl. Chem. Eng. 2016, 27, 620–626. [Google Scholar] [CrossRef]
- Akhtar, N.; Zaman, S.U.; Khan, B.A.; Amir, M.N.; Ebrahimzadeh, M.A. Calendula extract: Effects on mechanical parameters of human skin. Acta Pol. Pharm. 2011, 68, 603–701. [Google Scholar]
- Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Koc, K.; Ekiert, H. Pot marigold (Calendula officinalis L.)—A position in classical phytotherapy and newly documented activities. Acta Sci. Pol. Hortorum Cultus 2020, 19, 47–61. [Google Scholar] [CrossRef]
- Andersen, F.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks Jr, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Final Report of the Cosmetic Ingredient Review Expert Panel amended safety assessment of Calendula officinalis–derived cosmetic ingredients. Int. J. Toxicol. 2010, 29S4, 221S–243S. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef]
- Çöçü, S.; Uranbey, S.; Ipek, A.; Khawar, K.M.; Sarihan, E.O.; Kaya, M.D.; Parmaksız, I.; Özcan, S. Adventitious shoot regeneration and micropropagation in Calendula officinalis L. Biol. Plant. 2004, 48, 449–451. [Google Scholar] [CrossRef]
- Wiktorowska, E.; Dlugosz, M.; Janiszowska, W. Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzyme Microb. Technol. 2010, 46, 14–20. [Google Scholar] [CrossRef]
- Długosz, M.; Wiktorowska, E.; Wiśniewska, A.; Pączkowski, C. Production of oleanolic acid glycosides by hairy root established cultures of Calendula officinalis L. Acta Biochim. Pol. 2013, 60, 467–473. [Google Scholar] [CrossRef]
- Çetin, B.; Kalyoncu, F.; Kurtuluş, B. Antibacterial activities of Calendula officinalis callus extract. Int. J. Sec. Metabolite 2017, 4, 257–263. [Google Scholar] [CrossRef]
- Grzelak, A.; Janiszowska, W. Initiation and growth characteristics of suspension cultures of Calendula officinalis cells. Plant Cell, Tissue Organ Cult. 2002, 71, 29–40. [Google Scholar] [CrossRef]
- Auguścińska, E.; Kasprzyk, Z. Studies on the labelling of terpenoids in shoots and cells or protoplasts from Calendula officinalis leaves. Acta Biochim. Pol. 1982, 29, 7–13. [Google Scholar] [PubMed]
- Długosz, M.; Markowski, M.; Pączkowski, C. Source of nitrogen as a factor limiting saponin production by hairy root and suspension cultures of Calendula officinalis L. Acta Physiol. Plant. 2018, 40, 35. [Google Scholar] [CrossRef]
- Alsoufi, A.S.M.; Pączkowski, C.; Szakiel, A.; Długosz, M. Effect of jasmonic acid and chitosan on triterpenoid production in Calendula officinalis hairy root cultures. Phytochem. Lett. 2019, 31, 5–11. [Google Scholar] [CrossRef]
- Rogowska, A.; Paczkowski, C.; Szakiel, A. Modulation of steroid and triterpenoid metabolism in Calendula officinalis plants and hairy root cultures exposed to cadmium stress. Int. J. Mol. Sci. 2022, 23, 5640. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, A.A.; Khodadadi, E.; Sadeghi, Z.; Lia shooshtari. An investigation of tissue culture and co-cultures of different explants in Calendula officinalis. Int. J. Biosci. 2013, 12, 201–205. [Google Scholar] [CrossRef]
- Kaya, N.; Aki, C. In vitro effects of plant growth regulators on callus formation in Calendula officinalis L. and Calendula arvensis L. species. Ann. Biol. Res. 2017, 8, 1–7. [Google Scholar]
- Al-Abasi, I.N.; Bashi, B.Z.K.; Al-Mallah, M.K. Design of culture medium and leaf clones are determinant factors in callus induction of Calendula officinalis L. Eur. Acad. Res. 2018, 6, 1901–1913. [Google Scholar]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Jin, Y.W.; Park, J.H.; Yoo, Y.M.; Hong, S.M.; Amir, R.; Yan, Z.; Kwon, E.; Elfick, A.; Tomlinson, S.; Halbritter, F.; Waibel, T.; Yun, B.-W.; Loake, G.J. Cultured cambial meristematic cells as a source of plant natural products. Nat. Biotechnol. 2010, 28, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Howat, S.; Jang, M.O.; Kim, I.S.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Cambial meristematic cells: a platform for the production of plant natural products. New Biotechnol., 2015, 32, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H. Vascular tissue differentiation and pattern formation in plants. Annu. Rev. Plant Biol. 2002, 53, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Laux, T. The stem cell concept in plants: a matter of debate. Cell 2003, 113, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Yan, Z.; Amir, R.; Hong, S.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant natural products: history, limitations and the potential of cambial meristematic cells. Biotechnol. Genet. Eng. Rev. 2012, 28, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.B.; Elias, C.B.; Patole, M.S. Role of hydrodynamic shear in the cultivation of animal, plant and microbial cells. Chem. Eng. J., 1996, 62, 121–141. [Google Scholar] [CrossRef]
- Lee, S.B.; Cho, H.I.; Jin, Y.W.; Lee, E.K.; Ahn, J.Y.; Lee, S.M. Wild ginseng cambial meristematic cells ameliorate hepatic steatosis and mitochondrial dysfunction in high-fat diet-fed mice. J. Pharm. Pharmacol. 2016, 68, 119–127. [Google Scholar] [CrossRef]
- Moon, S.H.; Venkatesh, J.; Yu, J.W.; Park, S.W. Diferential induction of meristematic stem cells of Catharanthus roseus and their characterization. C. R. Biol. 2015, 338, 745–756. [Google Scholar] [CrossRef]
- Song, Y.; Chen, S.; Wang, X.; Zhang, R.; Tu, L.; Hu, T.; Liu, X.; Zhang, Y.; Huang, L.; Gao, W. A novel strategy to enhance terpenoids production using cambial meristematic cells of Tripterygium wilfordii Hook. f. Plant Methods 2019, 15, 129. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Qin, N.; Zhang, Q.; Yan, C. Biotransformation of 4-methylcoumarins by cambial meristematic cells of Camptotheca acuminate. RSC Adv. 2019, 9, 9449. [Google Scholar] [CrossRef] [PubMed]
- Mehring, A.; Haffelder, J.; Chodorski, J.; Stiefelmaier, J.; Strieth, D.; Ulber, R. Establishment and triterpenoid production of Ocimum basilicum cambial meristematic cells. Plant Cell Tissue Organ Cult. 2020, 143, 573–581. [Google Scholar] [CrossRef]
- He, L.; Zhang, J.; Guo, D.; Tian, H.; Cao, Y.; Ji, X.; Zhan, Y. Establishment of the technology of cambial meristematic cells (CMCs) culture from shoots and high expression of FmPHV (PHAVOLUTA) functions in identification and differentiation of CMCs and promoting the shoot regeneration by hypocotyl in Fraxinus mandshurica. Plant Physiol. Biochem. 2021, 160, 352–364. [Google Scholar] [CrossRef]
- Legha, M.R.; Prasad, K.V.; Singh, S.K.; Kaur, C.; Arora, A.; Kumar, S. Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell. Dev. Biol.—Plant 2012, 48, 99–106. [Google Scholar] [CrossRef]
- Leal, F.; Rodrigues, A.; Fernandes, D.; Nunes, F.M.; Cipriano, J.; Ramos, J.; Teixeira, S.; Vieira, S.; Carvalho, L.M.; Pinto-Carnide, O. In vitro multiplication of Calendula arvensis for secondary metabolites extraction. In Proceedings of the IIIrd International Symposium on Acclimatization and Establishment of Micropropagated Plants, Faro, Portugal, 28 February 2009. [Google Scholar]
- Ibrahim, M.M.; Abed, R.M.; Ali, F.Q. Influence of biotic elicitor Aspergillus niger on salicylic acid products in callus cultures of Calendula officinalis L. plant. J. Phys.: Conf. Ser. 2019, 1294, 062016. [Google Scholar] [CrossRef]
- Sugimoto, K.; Gordon, S.P.; Meyerowitz, E.M. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–218. [Google Scholar] [CrossRef]
- Grafi, G. How cells dedifferentiate: a lesson from plants. Dev. Biol. 2004, 268, 1–6. [Google Scholar] [CrossRef]
- Verdeil, J.-L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: dependance versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef]
- Parizot, B.; Laplaze, L.; Ricaud, L.; Boucheron-Dubuisson, E.; Bayle, V.; Bonke, M.; De Smet, I.; Poethig, S.R.; Helariutta, Y.; Haseloff, J.; Chriqui, D.; Beeckman, T.; Nussaume, L. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation1[W]. Plant Physiol. 2008, 146, 140–148. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Tsukaya, H. Behavior of leaf meristems and their modification. Front. Plant Sci. 2015, 6, 1060. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.M.; Bonetta, D.; Tsukaya, H.; Dengler, R.E.; Dengler, N.G. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 1999, 215, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, R.; Erickson, R.O. Development of the lamina in Xanthium italicum represented by the plastochron index. Am. J. Bot. 1960, 47, 451–459. [Google Scholar] [CrossRef]
- Alvarez, J.P.; Furumizu.; Efroni.; Eshed Y.; Bowman J.L. Active suppression of a leaf meristem orchestrates determinate leaf growth. eLife 2016, 5, e15023. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Guan, C.; Jiao, Y. Molecular mechanisms of leaf morphogenesis. Mol. Plant 2018, 11, 1117–1134. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J Exp. Bot. 2020, 71, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Qi, M.; Xu, L.; Zhang, G.; Li, J.; Qin, P.; Ruan, Y.; Huang, H.; Zhang, Y.; Xu, L. Divergent regeneration-competent cells adopt a common mechanism for callus initiation in angiosperms. Regeneration 2017, 3, 132–139. [Google Scholar] [CrossRef]
- Müller-Xing, R.; Xing, Q. The plant stem-cell niche and pluripotency: 15 years of an epigenetic perspective. Front. Plant Sci. 2022, 13, 1018559. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Seth Favero, D.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 3.1–3.30. [Google Scholar] [CrossRef]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




