1. Introduction
The apple leaf-curling midge,
Dasineura mali Kieffer (Diptera: Cecidomyiidae), native to Europe, is a monophagous herbivore that attacks apple trees in Europe, South and North America, and New Zealand [
1,
2,
3,
4,
5]
. Its larvae feed on young leaves and shoots, reducing photosynthesis, resulting in poor shoot development, and potentially affecting the long-term yield [
6]. Although the midge does not feed on fruit, its cocooned larvae can contaminate fresh fruit, causing quarantine problems [
7,
8].
Dasineura mali infestations in commercial orchards have raised concerns in the apple industry in New Zealand and some regions that trade with New Zealand, such as Australia, California, China, India, Japan, and Taiwan [
9,
10]
.
Dasineura mali adults emerge from the soil in the early morning and swarm around the emergence sites for mating, after which time females lay their eggs on growing apple shoots [
11]. Eggs hatch in 3–5 days depending on the temperature and larval feeding on young leaves causes them to roll or produce galls alongside their outer edges; mature larvae (3
rd instar) leave the curled leaves and burrow in the soil (≤ 10 mm in depth) where they spin cocoons and pupate [
12] but some can spin cocoons on fruit on their way to the ground [
9,
13]. The number of generations of
D. mali per year varies depending on climate conditions and availability of food, for example, there are three generations in Canada [
5,
14], three to six generations in Europe [
15] and four to five generations in New Zealand [
12,
16,
17,
18].
Knowledge on the development, survival, and emergence of an insect species under different environmental conditions is vital to its pest risk analysis, forecast and management. Temperature and daylength are two main environmental factors that influence these biological parameters [
19]. The low temperature threshold (temperature below which no measurable development takes place) and thermal constant (the number of degree-days above the lower threshold temperature for completion of development) are the two most important thermal parameters that determine how the temperature affects the developmental rate of ectotherms [
5,
20] and help predict pest phenology [
21,
22]. Many studies show that both temperature and daylength affect survival and development of cecidomyiid species [
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35] although daylength does not appear to have any effect on these parameters in several cecidomyiid species [
26,
29]. To date, little is known about how temperature and daylength influence
D. mali, making its risk analysis, forecast and control difficult.
To generate critical information for development of measures for effective pest risk analysis, forecast and control of D. mali, we carried out experiments under a series of temperature and daylength conditions. We tested how temperature and day length affected the development and survival of this pest. Based on our findings, we then calculated developmental thresholds and thermal requirements and developed a model for prediction of the number of D. mali generations per year under different climate conditions in New Zealand. Our findings would help countries that trade with D. mali-infested regions perform pest risk analysis and develop forecast and management measures.
2. Materials and Methods
2.1. Insects
We established a breeding colony in the laboratory from the field-collected mature larvae (3rd instar) in a mature organic apple orchard in Plant Growth Unit, Massey University, Palmerston North, New Zealand. The larvae were transferred to the rearing medium (vermiculite) in Petri dishes (5.5 cm in diameter × 1.3 cm in height) and maintained at 20 ± 1ºC, 65 ± 5% RH and 15:9 h (light:dark) for pupation. Newly emerged adults were released into an aluminium-framed experimental cage (43 × 42 × 40 cm) in which 10 apple seedlings (≈ 20 cm height, bred from rootstock MM 106 FSV) were maintained for oviposition. The cage had a fine metal mesh (aperture size = 0.25 mm) on the back and both sides and Perspex on the top and front and aluminium alloy on the bottom.
2.2. Effect of Temperature on Development and Survival
We investigated the effect of temperature on the development, survival and emergence of D. mali at five constant temperatures: 5, 10, 15, 20 and 25°C with a day length of 15 hrs in the laboratory. For each test temperature, at the onset of photophase we released 30 newly emerged D. mali adults from the above colony with a sex ratio of about 1:1 into an above-mentioned experimental cage for ovipositon, where 10 apple seedlings were maintained as above. We removed seedlings 12 hrs after oviposition, caged them individually using transparent permeable plastic bags (42 cm in length × 23 cm in width with aperture = 0.25 mm) and maintained them at the test temperature. Because midge adults laid few eggs at 5 and 10°C, we allowed adults to lay eggs on seedlings at 20°C for 12 hrs and then individually caged the oviposited seedlings as above and transferred them to these two temperature conditions.
To determine the egg incubation duration and hatch rate, we made daily examination of two infested shoots at each temperature under a stereomicroscope (Olympus, Japan). The seedlings were maintained at the same temperature until larvae became mature (3rd instar, orange in colour). We started examining larval maturation by gently opening curled leaves when they turned brown. We counted and removed mature larvae from leaves once a day until all larvae became mature. Egg hatch rate (no. of newly hatched larvae/no. of eggs) and larval survival rate (no. of mature larvae/no. of newly hatched larvae) were calculated. Developmental duration of eggs and immature larvae were recorded.
Mature larvae collected from 10 seedlings under each test temperature were transferred into Petri dishes with the rearing medium as mentioned above and maintained at the same temperature until emergence. There were 18, 20 and 20 dishes with 20, 30 and 25 larvae per dish established at 15, 20 and 25°C, respectively. Because no eggs hatched at 5°C and no larvae completed development at 10°C, we collected mature larvae from 20ºC, and immediately transferred them to 10°C in 12 dishes with 25 mature larvae each and maintained them at this temperature until adults emerged. Newly emerged adults were counted and sexed daily according to Gagné and Harris [
36]. After all adults had emerged, midge cocoons from each dish were examined under the microscope to determine the pupation rate of larvae (no. of pupae/no. of mature larvae) and emergence rate of adults from the pupae (no. of emerged adults/no. of pupae).
2.3. Effect of Daylength on Development and Survival
In this experiment we tested the effect of day length on the development, survival and emergence of D. mali under six daylength conditions: 10, 11, 12, 13, 14 and 15 h light based on a 24-h cycle at 20ºC in the laboratory. This experiment was carried out and data were recorded as in the temperature experiment. There were 13, 17, 15, 25, 25 and 20 dishes with 25 mature larvae per dish at above day lengths, respectively.
2.4. Low Temperature Thresholds and Thermal Constants
Based on the developmental time at test temperatures, we used the method of Campbell et al. [
37] to estimate the low temperature threshold (
t) and thermal constant (degree-days,
K) for
D. mali:
r =
a +
bT, where
r is the developmental rate [1/developmental duration (days)],
T is the temperature, and
a and
b are estimates of the
r intercept and slope, respectively. The low temperature threshold and thermal constant were calculated as
t = -
a/
b and
K = 1/
b, respectively. The standard errors of
t and
K were calculated as
and
, respectively, where
SE(
b) is the
SE
of
b,
s2 is the residual mean square of
, is the sample mean, and
N is the sample size. At a given constant temperature, thermal requirement for
D. mali to complete development from eggs to adults was calculated as:
K =
n×(
T-
t), where
n is the number of days required to complete development.
2.5. Estimation of the Number of Generations in the Field
Based on the low temperature threshold and degree-days (see Results), we estimated the number of generations ALCM might have in four regions in New Zealand, Hawke’s Bay (latitude 39.6°S), Palmerston North (latitude 40.4°S), Nelson (latitude 41.3°S) and Central Otago (latitude 45.3°S), by developing a degree-day model [
21]:
, where
Ti is the mean daily air temperature (1981–2010) [
38] at day
i after the midge emergence from the overwintered generation, and
n is the number of days during the growing season. To avoid overestimation or underestimation, we calculated the degree-days since the mean date of the first adult emergence peak, i.e., 30 September (2004–2017) in Hawker’s Bay, 02 October (2004–2017) in Nelson and 11 October (2001–2017) in Central Otago [
18] and 10 October (2005–2007) in Palmerston North [
39]. Hawke’s Bay, Nelson and Central Otago are the three main apple growing regions for export in New Zealand [
40].
2.6. Statistical Analysis
We performed analyses using SAS v. 9.4 software (SAS Institute Inc., NC, USA). Data on the development of different stages at different temperatures and daylengths and the thermal requirement (degree-days) were not normally distributed (Shapiro-Wilk test, UNIVARIATE Procedure) and thus analyzed using a generalized linear model (GLMMIX Procedure) followed by a Tukey-Kramer test for multiple comparisons between temperature. The relationship between the developmental rate and temperature was estimated using a general linear model (GLM Procedure). The survival data were analyzed using a generalized linear model (GENMOD Procedure) with a Binomial distribution and logit function. Multiple comparisons between temperatures or between day lengths were performed using the Contrast statement.
3. Results
3.1. Effect of Temperature on Development and Survival
Eggs failed to hatch at 5°C. At 10°C eggs hatched but larvae failed to complete their development. Mature larvae transferred from 20°C to 10°C successfully pupated and developed to adults in about 60 days (
Table 1). The midge developed significantly faster with the increase of temperature (
F3,127 = 70.72 for egg,
F2,993 = 379.87 for larva,
F3,1171 = 3135.28 for larva-adult,
F2,993 = 1250.65 for total,
F2,470 = 586.28 for male and
F2,523 = 664.01 for female;
P < 0.0001) (
Table 1).
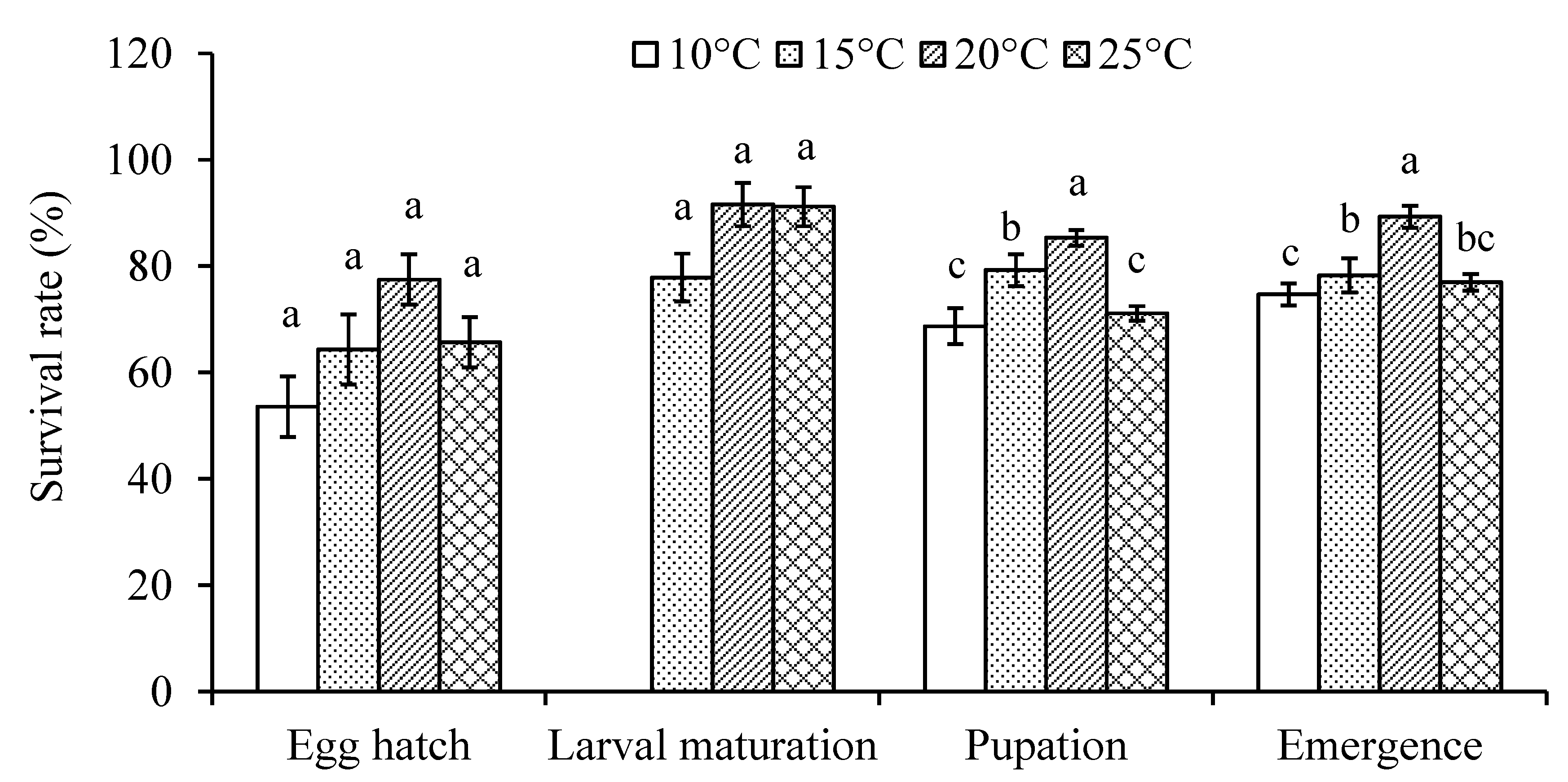
Egg hatch and larval survival rates were higher at 20°C but there was no significant difference between temperatures (
= 6.59,
P = 0.0863 for egg hatch rate;
= 3.49,
P = 0.1747 for larval survival rates) (
Figure 1). Significantly more mature larvae pupated and adults emerged at 20°C than at 10 and 25°C (
= 47.65 and 33.20 for pupation and emergence rates, respectively;
P < 0.0001) (
Figure 1).
3.2. Effect of Daylength on Development and Survival
Our results show that daylength has no significant effect on the development of
D. mali (
F5,231 = 0.53 for egg,
F5,207 = 0.18 for larva,
F5,1743 = 1.55 for larva-adult,
F5,1743 = 0.77 for complete lifecycle,
F5,806 = 0.42 for male and
F5,937 = 0.59 for female;
P > 0.05) (
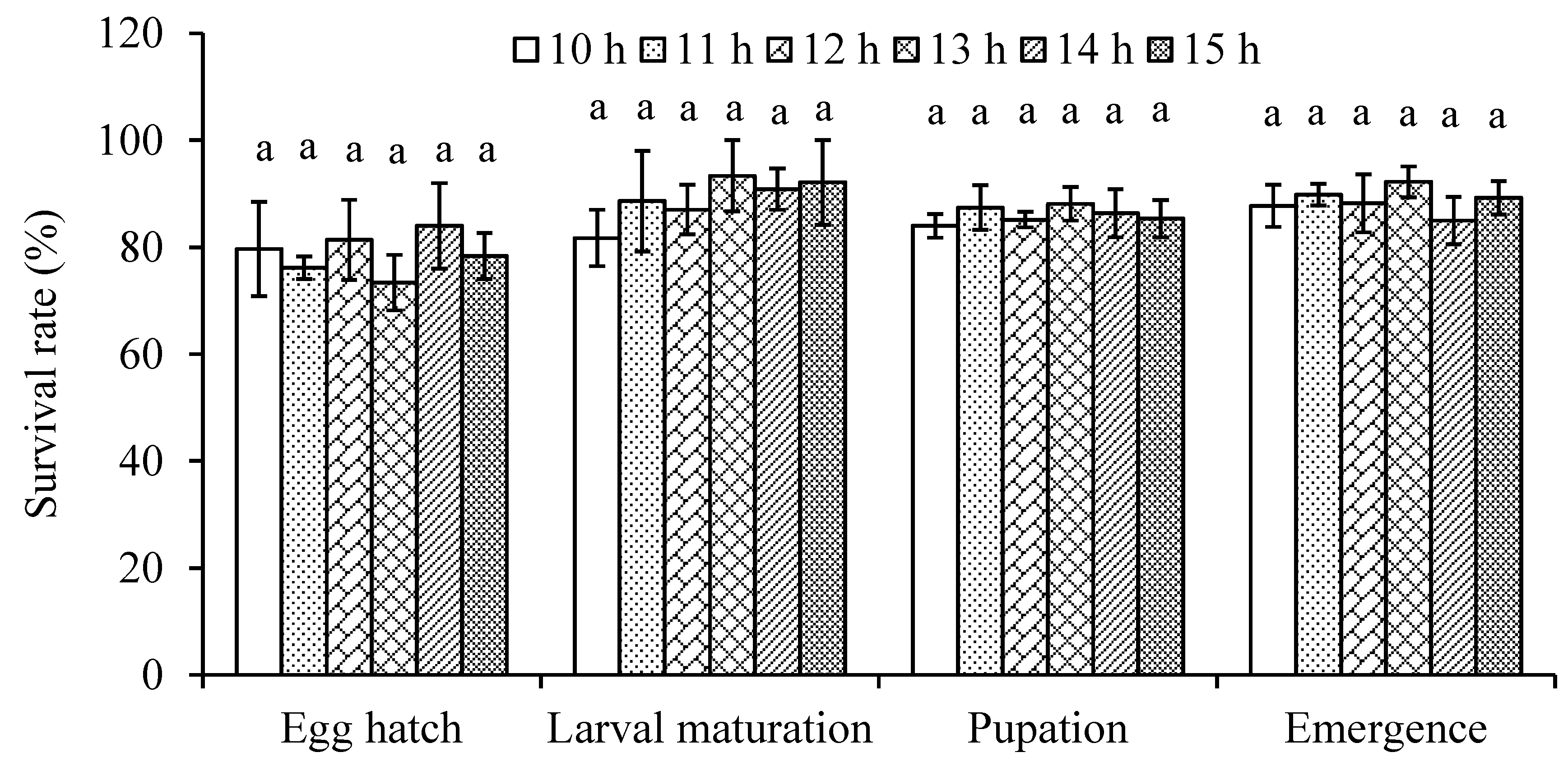
Table 2). Similarly, daylength had no effect on the midge survival (
= 2.12, 7.18, 4.66 and 5.98 for egg hatch, larval survival, pupation and emergence rates, respectively,
P > 0.05) (
Figure 2).
3.3. Low Temperature Thresholds and Thermal Constants
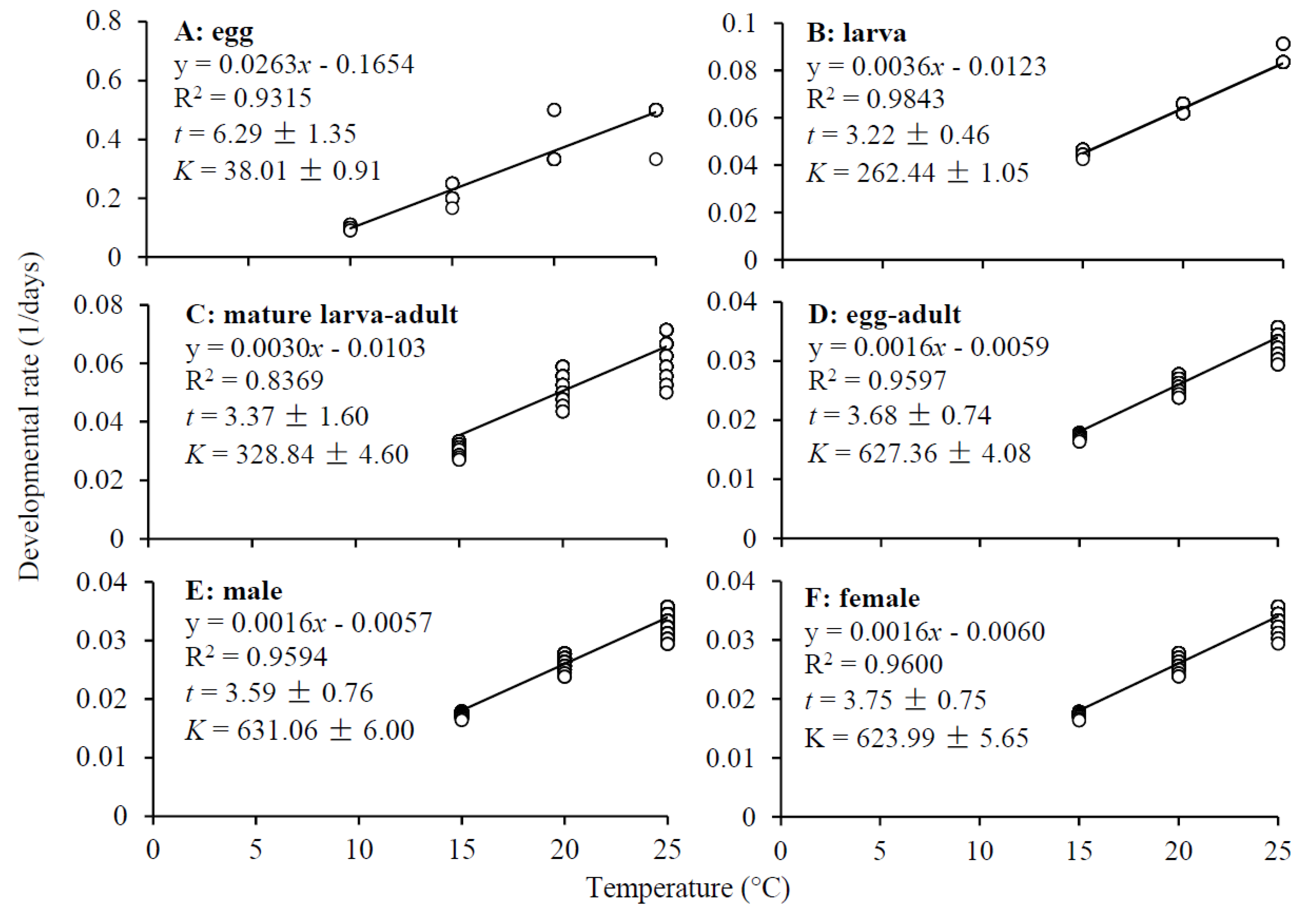
The low temperature threshold was 6.7°C for egg development, which was twice as high as that for larval (3.2°C) and pupal (3.4°C) development (
Figure 3A–C). Thermal requirement for
D. mali developing from eggs to adults was about 630 degree-days with a low temperature threshold of about 3.7°C (
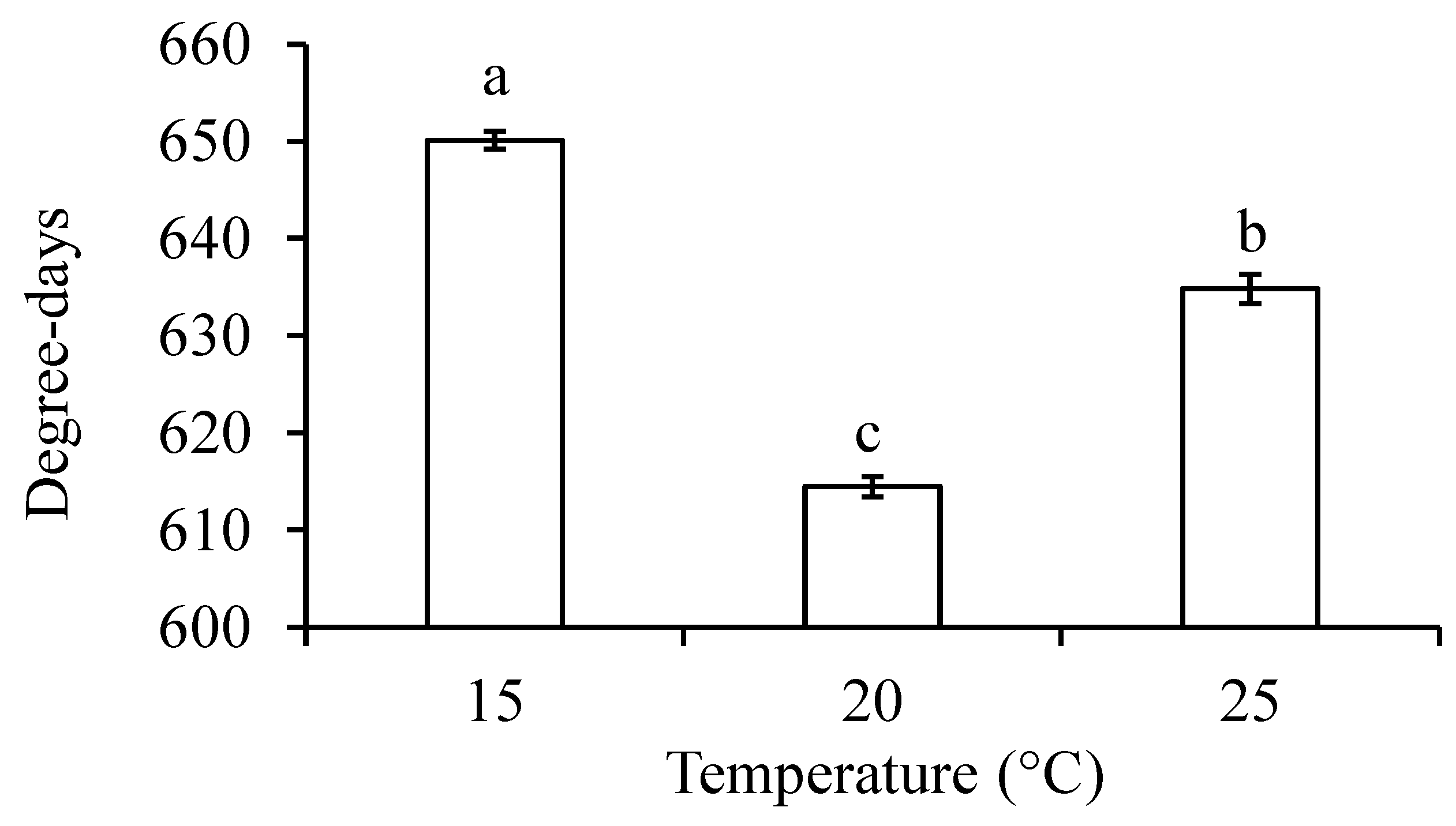
Figure 3D–F). Thermal requirement for the midge to complete development from eggs to adults was significantly different between three test temperatures with the lowest thermal constant being at 20°C (ANOVA:
F2,993 = 151.09,
P < 0.0001) (
Figure 4).
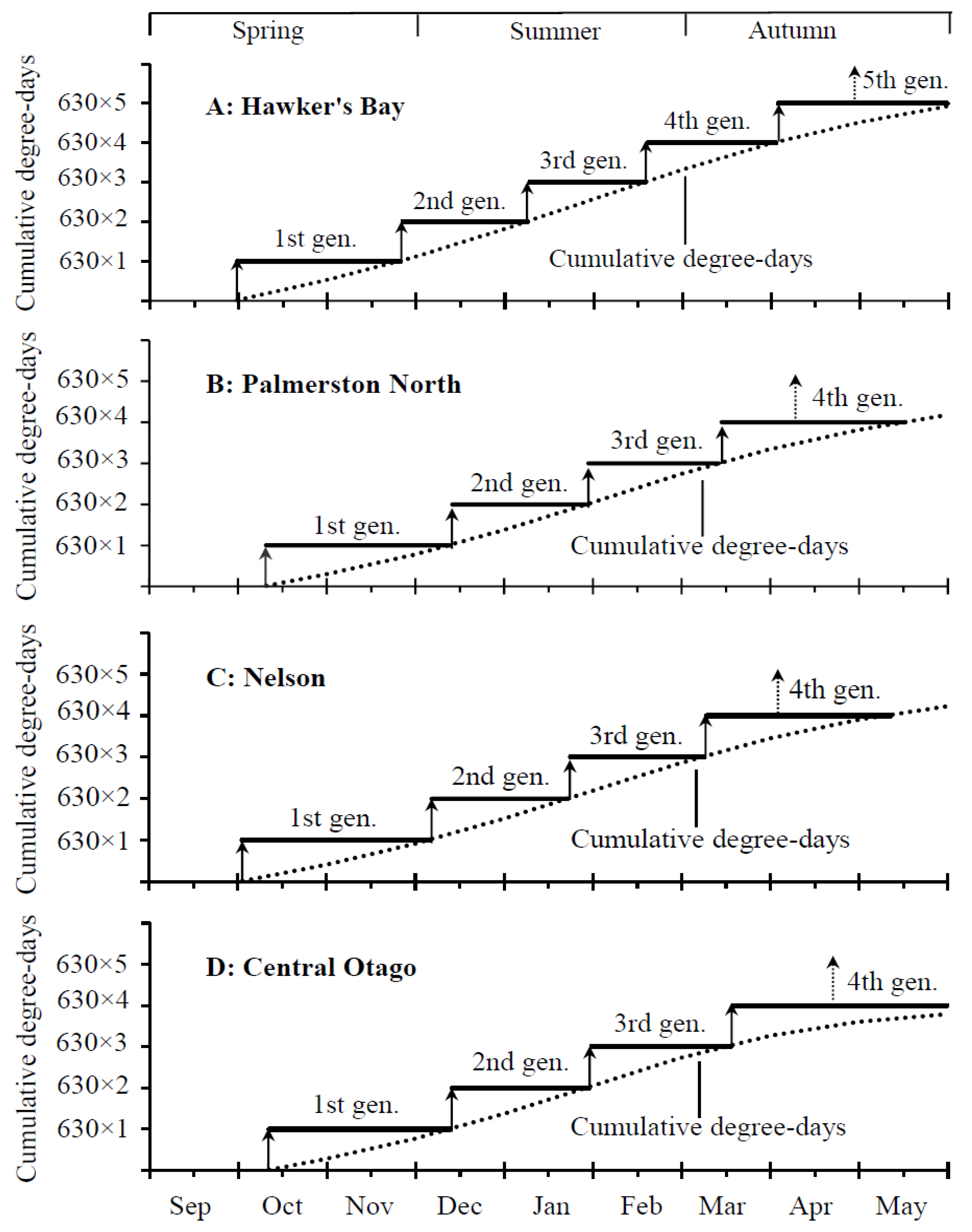
3.4. Estimation of the Number of Generations in the Field
Our model predicts that
D. mali should have five generations a year in Hawker’s Bay with four completed generations between October and late March/early May, and mature larvae produced by the 4
th generation would enter overwintering during late April (
Figure 5A). However, four generations should occur in Palmerston North, Nelson and Central Otago, with three completed generations during the growing seasons (October–mid-March) and mature larvae made by adults of the 3
rd generation entering overwintering during mid-April (
Figure 5B–D).
4. Discussion
Our results show that
D. mali could not complete its lifecycle at 10°C but the high tolerance of its mature larvae and pupae to this temperature (
Table 1) may allow it to survive lengthy shipment in cold containers and reach the countries that trade with midge-infested regions. The midge had significantly higher thermal requirement for completion of its lifecycle at 25°C than at 20°C (
Figure 4), suggesting that the temperature ≥ 25°C may be less suitable for its development and explaining its summer outbreaks in New Zealand where average maximum temperature rarely exceeds 25°C [
41]. Furthermore, the significantly lower pupation and emergence rates at 25°C (
Figure 1) or higher [
42] may lower the probability of its population build-up in regions where the average maximum temperature during the summer is over 25°C. The susceptibility to higher temperatures has also been reported in other cecidomyiid species such as the sorghum midge,
Contarinia sorghicola (Coquillett) [
24] and blackcurrant leaf midge,
D. tetensi (Rübsaamen) [
43].
The low temperature threshold of 6.7°C for egg development in
D. mali may explain why its eggs failed to hatch at 5°C. The low temperature thresholds for the development of other
D. mali stages were about 3.5°C (
Figure 3B–D), lower than the mean air temperatures during the winter in New Zealand [
38], probably because this midge is native to colder Europe. Using thermal parameters of several midge pests, various authors have developed models for prediction of their population dynamics in the field, providing powerful tools for decision makings in pest management [
23,
28,
43,
44,
45,
46]. For example, the accurate prediction of adult emergence time is critical to the applications of pheromone traps and insecticides [
47,
48,
49,
50].
Based on our findings in the present study (
Figure 3D) and long-history climate data [
38], we have developed a thermal model to predict the number of generations and adult emergence time of each generation in four locations in New Zealand. We show that the model provides an accurate prediction of five generations in Hawker’s Bay and four generations in Palmerston North, Nelson, and Central Otago (
Figure 5), agreeing to the field data reported [
16,
18,
39]. Like other gall-forming insects [
51,
52,
53], the availability of host plants for
D. mali is also a crucial factor for the occurrence of the number of generations per year. For example, the lack of food in the autumn [
16] may prevent it from having the fifth generation in Palmerston North, Nelson, and Central Otago.
Like some midge species [
26,
29], our results demonstrate that day length had no effect on
D. mali development (
Table 2) and survival (
Figure 2). Previous studies indicate that the effect of short-day length, if any, on development and survival of midge species is usually associated with low temperatures [
29,
30,
33] or lack of food [
16]. Therefore, day length is unlikely to affect these life parameters of
D. mali during the apple growing season in New Zealand when the temperature is suitable. However, it is still unknown whether combinations of darkness and low temperature during fruit storage and shipment affect development of its mature larvae, which warrants further investigations.
In conclusion, we show that temperature rather than day length significantly affects D. mali development and survival. We suggest that countries or regions with temperatures of ≥ 25°C during the growing seasons may be less suitable for D. mali population build-up in apple orchards. The thermal model developed in this study can accurately predict the number of generations a year and the time of adult emergence in each generation in different locations of New Zealand. We recommend that this model be used to predict population dynamics of the pest in other parts of the world. Information generated in the present study can be used for pest risk analysis, forecast and control.
Author Contributions
Conceptualization, XZH and Qiao; experimental design, XZH and Qiao; data collection, XZH; data analysis, XZH and Qiao; manuscript preparation and revision, XZH and Qiao.
Funding
The work reported here was funded by a Massey University Research Fund to QW.
Data Availability Statement
All data are included in the manuscript.
Acknowledgments
We thank Plant Growth Unit, Massey University for allowing us to collect insects from the apple orchards and providing apple seedlings for experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marrison, L.G. Apple leaf-curling midge in New Zealand. New Zeal. J. Agri. 1953, 86, 565–566. [Google Scholar]
- Carl, K.P. Observations on the apple leaf-curling midge, Dasineura mali Kieffer and a new species from apple leaf-galls, Macrolabis sp. (Dipt. Cecidomyiidae). Anz. Schädlingskund. Pfl. Umweltschutz 1980, 53, 99–102. [Google Scholar] [CrossRef]
- Gagné, R.J. The plant-feeding gall midges of North America. Cornell University Press, Ithaca, New York, 1989, 356 p.
- CABI/EPPO. Dasineura mali. Distribution Maps of Plant Pests No. 711, 2008. [CrossRef]
- Cossentine, J.; Brauner, A.; Franklin, J.; Robertson, M.; Buhl, P.; Blatt, S.; Gariepy, T.D.; Fraser, H.; Appleby, M.; Grigg-Mcguffin, K.; Mason, P. Parasitism and phenology of Dasineura mali (Diptera: Cecidomyiidae) in Canadian apple (Rosaceae) orchards. Can. Entomol. 2020, 152, 355–373. [Google Scholar] [CrossRef]
- Allison, P.A.; Meekings, J.A.; Tomkins, R.; Wilson, D.J. Effects of leaf damage by apple leafcurling midge (Dasyneura mali) on photosynthesis of apple leaves. Proceedings of the 48th New Zealand Plant Protection, Hastings, New Zealand, 8–10 August 1995, pp. 121–124. [CrossRef]
- Tomkins, A.R.; Wilson, D.J.; Hutchings, S.O.; June, S. A survey of apple leafcurling midge (Dasyneura mali) management in Waikato orchards. Proceedings of the 47th New Zealand Plant Protection, Waitangi, New Zealand, 9–11 August 1994, pp. 346–349. [CrossRef]
- Bayer CropScience. Apple leaf curling midge Dasineura mali. 2019. https://www.cropscience.bayer.co.nz/pests/insects/apple-leaf-curling-midge (accessed on 25 February 2023).
- Lowe, S. Apple leafcurling midge. Pipmark Technical Bulletin, New Zealand apple and pear marketing board, 1993, 5 p.
- New Zealand Government. Australia - measures affecting the importation of apples from New Zealand (WT/DS367). First written submission of New Zealand to WTO, 20 June 2008, Geneva.
- Heath, J.J.; Zhang, A.; Roelofs, W.L.; Smith, R.F. Flight activity and further evidence for a female-produced sex pheromone of the apple leaf midge, Dasineura mali, in Nova Scotia. Northeast. Nat. 2005, 12, 93–102. [Google Scholar] [CrossRef]
- Todd, D.H. A preliminary account of Dasyneura mali Kieffer (Cecidomyiidae: Dipt.) and an associated hymenopterous parasite in New Zealand. New Zeal. J. Sci. Tech. A 1956, 37, 462–464. [Google Scholar]
- Smith, J.T. Aspects of the ecology and management of apple leafcurling midge (Dasineura mali) (Diptera: Cecidomyiidae) on the Waimea Plains, Nelson, New Zealand. PhD thesis, Lincoln University, New Zealand, 2000, 192 p.
- Laroche, M.; Provost, C. Suivi des populations de cécidomyie du pommier et méthodes de captures. Rapport final, MAPAQ, Prime-vert, 2015, 13 p.
- Cross, J.V.; Solomon, M.G.; Brabandreier, D.; Blommers, L.; Easterbrook, M.A.; Jay, C.N.; Jenser, G.; Jolly, R.L.; Kuhlmann, U.; Lilley, R.; Olivella, E.; Toepfer, S.; Vidal, S. Biocontrol of pests of apples and pears in Northern and Central Europe: 2. Parasitoids. Biocont. Sci. Tech. 1999, 9, 277–314. [Google Scholar] [CrossRef]
- Shaw, P.W.; Wallis, D.R.; Alspach, P.A.; Sandanayaka, W.R.M. Phenology of apple leafcurling midge (Dasineura mali) in relation to parasitism by Platygaster demades. New Zeal. Plant Prot. 2005, 58, 306–310. [Google Scholar] [CrossRef]
- Wearing, C.H.; Marshall, R.R.; Attfield, B.; Colhoun, C. Phenology and distribution of the apple leafcurling midge (Dasineura mali (Kieffer)) (Diptera: Cecidomyiidae) and its natural enemies on apples under biological and integrated pest management in Central Otago, New Zealand. New Zeal. Entomol. 2013, 36, 87–106. [Google Scholar] [CrossRef]
- Lo, P.L.; Walker, J.T.S. Annual and regional variability in adult Dasineura mali (apple leafcurling midge) emergence in New Zealand. New Zeal. Plant Prot. 2017, 70, 131–136. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal adaptations of insects. Oxford University Press, Oxford, 1986, 411 p.
- Higley, L.G.; Pedigo, L.P.; Ostile, K.R. DEGDAY: a program for calculating degree-days, and assumptions behind the degree-day approach. Environ. Entomol. 1986, 15, 999–1016. [Google Scholar] [CrossRef]
- Muñiz, M.; Zalom, F.G. Developmental rate and number of generation estimates for Ceratitis capitata (Weiedemann) in fruit growing regions of California. IOBC/wprs Bull. 1997, 20, 55–66. [Google Scholar]
- Rowley, C.; Cherrill, A.; Leather, S.R.; Pope, T.W. Degree-day based phenological forecasting model of saddle gall midge (Haplodiplosis marginata) (Diptera: Cecidomyiidae) emergence. Crop Prot. 2017, 102, 154–160. [Google Scholar] [CrossRef]
- Baxendale, F.P.; Teetes, G.L.; Sharpe, P.J.H. Temperature-dependent model for sorghum midges (Diptera, Cecidomyiidae) spring emergence. Environ. Entomol. 1984, 13, 1566–1571. [Google Scholar] [CrossRef]
- Baxendale, F.P.; Teetes, G.L.; Sharpe, P.J.H.; Wu, H. Temperature-dependent model for development of nondiapausing sorghum midges (Diptera, Cecidomyiidae). Environ. Entomol. 1984, 13, 1572–1576. [Google Scholar] [CrossRef]
- Moore, A.D. Effects of temperature and length of photophase on development and diapause in Cystiphora schmidti (Rübsaamen) (Diptera: Cecidomyiidae). J. Aust. Entomol. Soc. 1987, 26, 349–354. [Google Scholar] [CrossRef]
- Wellso, S.G. Aestivation and phenology of the Hessian fly (Diptera: Cecidomyiidae) in Indiana. Environ. Entomol. 1991, 20, 795–801. [Google Scholar] [CrossRef]
- Gillespie, D.R.; Opit, G.; Roitberg, B. Effects of temperature and relative humidity on development, reproduction, and predation in Feltiella acarisuga (Vallot) (Diptera: Cecidomyiidae). Biol. Cont. 2000, 17, 132–138. [Google Scholar] [CrossRef]
- Hellqvist, S. Phenology of the blackcurrant leaf midge (Dasineura tetensi) in northern Sweden. Acta Agri. Scand., Section B Soil Plant Sci. 2001, 51, 84–90. [Google Scholar] [CrossRef]
- Gillespie, D.R.; Quiring, D.M.J. Effects of photoperiod on induction of diapause in Feltiella acarisuga (Diptera: Cecidomyiidae). Can. Entomol. 2002, 134, 69–75. [Google Scholar] [CrossRef]
- Wise, I.L.; Lamb, R.J. Diapause and emergence of Sitodiplosis mosellana (Diptera: Cecidomyiidae) and its parasitoid Macroglenes penetrans (Hymenoptera: Pteromalidae). Can. Entomol. 2004, 136, 77–90. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.H.; Chung, Y.J. Temperature-dependent post-diapause development and prediction of spring emergence of the pine needle gall midge (Dipt., Cecidomyiidae). J. Appl. Entomol. 2007, 131, 674–683. [Google Scholar] [CrossRef]
- Des Marteaux, L.E.; Habash, M.B.; Schmidt, J.M.; Hallett, R.H. A method for induction and quantification of diapause entry in the swede midge (Diptera: Cecidomyiidae). Can. Entomol. 2012, 144, 792–800. [Google Scholar] [CrossRef]
- Yamane, M.; Yano, E.; Matsumoto, Y.; Yoshioka, S.; Kawai, T.; Toyonishi, H.; Nakamura, T. Effect of photoperiod and temperature on the induction of diapause in a Japanese strain of Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Appl. Entomol. Zool. 2012, 47, 17–26. [Google Scholar] [CrossRef]
- Yukawa, J.; Ichinose, M.; Kim, W.; Uechi, N.; Gyoutoku, N.; Fujii, T. Lower development threshold temperatures and thermal constants for four species of Asphondylia (Diptera: Cecidomyiidae) in Japan and their larval developmental delay caused by heat stress. Appl. Entomol. Zool. 2016, 51, 71–80. [Google Scholar] [CrossRef]
- Cheng, W.; Long, Z.; Zhang, Y.; Liang, T.; Zhu-Salzman, K. Effects of temperature, soil moisture and photoperiod on diapause termination and post-diapause development of the wheat blossom midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae). J. Insect Physiol. 2017, 103, 78–85. [Google Scholar] [CrossRef]
- Gagné, R.J.; Harris, M.O. The distinction between dasineura spp. (Diptera: Cecidomyiidae) from apple and pear. Proc. Entomol. Soc. Wash. 1998, 100, 445–448. [Google Scholar]
- Campbell, A.; Frazier, B.D.; Gilbert, N.; Guitierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- NIWA. Mean monthly temperatures (°C) 1981-2010. https://niwa.co.nz/education-and-training/schools/resources/climate/meanairtemp (accessed on 28 February 2023).
- He, X.Z.; Wang, Q. Phenological dynamics of Dasineura mali Kieffer (Diptera: Cecidomyiidae) and its parasitoid Platygaster demades Walker (Hymenoptera: Platygasteridae) in apple orchards. J. Econ. Entomol. 2011, 104, 1640–1646. [Google Scholar] [CrossRef]
- Anon. Statistical annual 2016. Pipfruit New Zealand Incorporated. 2017, 27 p.
- New Zealand Meteorological Service. Daily climatological observations: monthly reports. Massey University, New Zealand, 2005–2007.
- Sandanayaka, W.R.M.; Ramankutty, P. Temperature dependent emergence and survival of Platygaster demades (Hymenoptera: Platygastridae), parasitoid of apple leaf curling midge. Biol. Cont. 2007, 42, 41–47. [Google Scholar] [CrossRef]
- Cross, J.V.; Crook, D.J. Predicting spring emergence of blackcurrant leaf midge (Dasineura tetensi) from air temperatures. Entomol. Exp. Appl. 1999, 91, 421–430. [Google Scholar] [CrossRef]
- Gordon, S.C.; Barrie, I.A.; Woodford, J.A.T. Predicting spring oviposition by raspberry cane midge from accumulated derived soil temperatures. Ann. Appl. Biol. 1989, 114, 419–427. [Google Scholar] [CrossRef]
- Oakley, J.N.; Cumbleton, P.C.; Corbett, S.J.; Saunders, P.; Green, D.I.; Young, J.E.B.; Rodgers, R. Prediction of orange wheat blossom midge activity and risk of damage. Crop Prot. 1998, 17, 145–149. [Google Scholar] [CrossRef]
- Hallett, R.H.; Goodfellow, S.A.; Weiss, R.M.; Olfert, O. MidgEmerge, a new predictive tool, indicates the presence of multiple emergence phenotypes of the overwintered generation of swede midge. Entomol. Exp. Appl. 2009, 130, 81–97. [Google Scholar] [CrossRef]
- Burnip, G.M.; Gibb, A.R.; Suckling, D.M. Target and non-target impacts from diazinon applied against Dasineura mali in a Canterbury apple orchard. Proceedings of the 51st New Zealand Plant Protection, Hamilton, New Zealand, 11–13 August 1998, pp. 143–147. [CrossRef]
- Walker, J.T.S.; Wearing, C.H.; Bradley, S.J.; Shaw, P.W.; Burnip, G.M.; Tokins, A.R.; Richardson, C.A.; Hodson, A.J. Integrated fruit production (IFP) for New Zealand pipfruit: pest management recommendations. Proceedings of the 51st New Zealand Plant Protection, Hamilton, New Zealand, 11–13 August 1998, pp. 166–172. [CrossRef]
- Cross, J.V.; Hall, D.R.; Shaw, P.; Anfora, G. Exploitation of the sex pheromone of apple leaf midge Dasineura mali Kieffer (Diptera: Cecidomyiidae): Part 2. Use of sex pheromone traps for pest monitoring. Crop Prot. 2009, 28, 128–133. [Google Scholar] [CrossRef]
- Jacquemin, G.; Chavalle, S.; De Proft, M. Forecasting the emergence of the adult orange wheat blossom midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae) in Belgium. Crop Prot. 2014, 58, 6–13. [Google Scholar] [CrossRef]
- Price, P.W.; Roininen, H.; Tahvanainen, J. Plant age and attack by the bud galler, Euura mucronata. Oecologia. 1987, 73, 334–337. [Google Scholar] [CrossRef]
- Yukawa, J. Synchronization of gallers with host plant phenology. Popul. Ecol. 2000, 42, 105–113. [Google Scholar] [CrossRef]
- Yukawa, J.; Akimoto, K. Influence of synchronization between adult emergence and host plant phenology on the population density of Pseudasphondylia neolitseae (Diptera: Cecidomyiidae) inducing leaf galls on Neolitsea sericea (Lauraceae). Popul. Ecol. 2006, 48, 13–21. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).