Submitted:
22 April 2023
Posted:
23 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
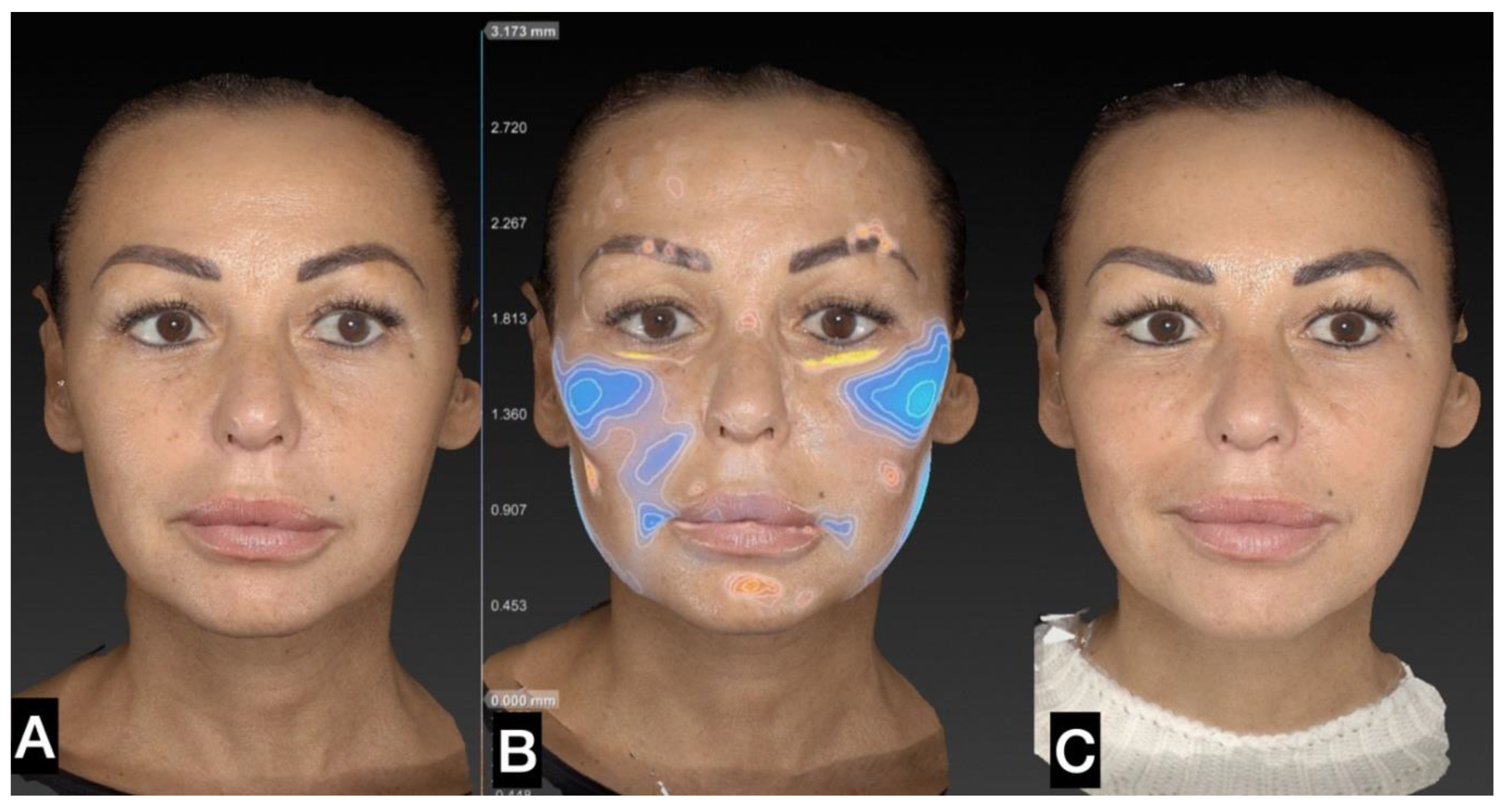
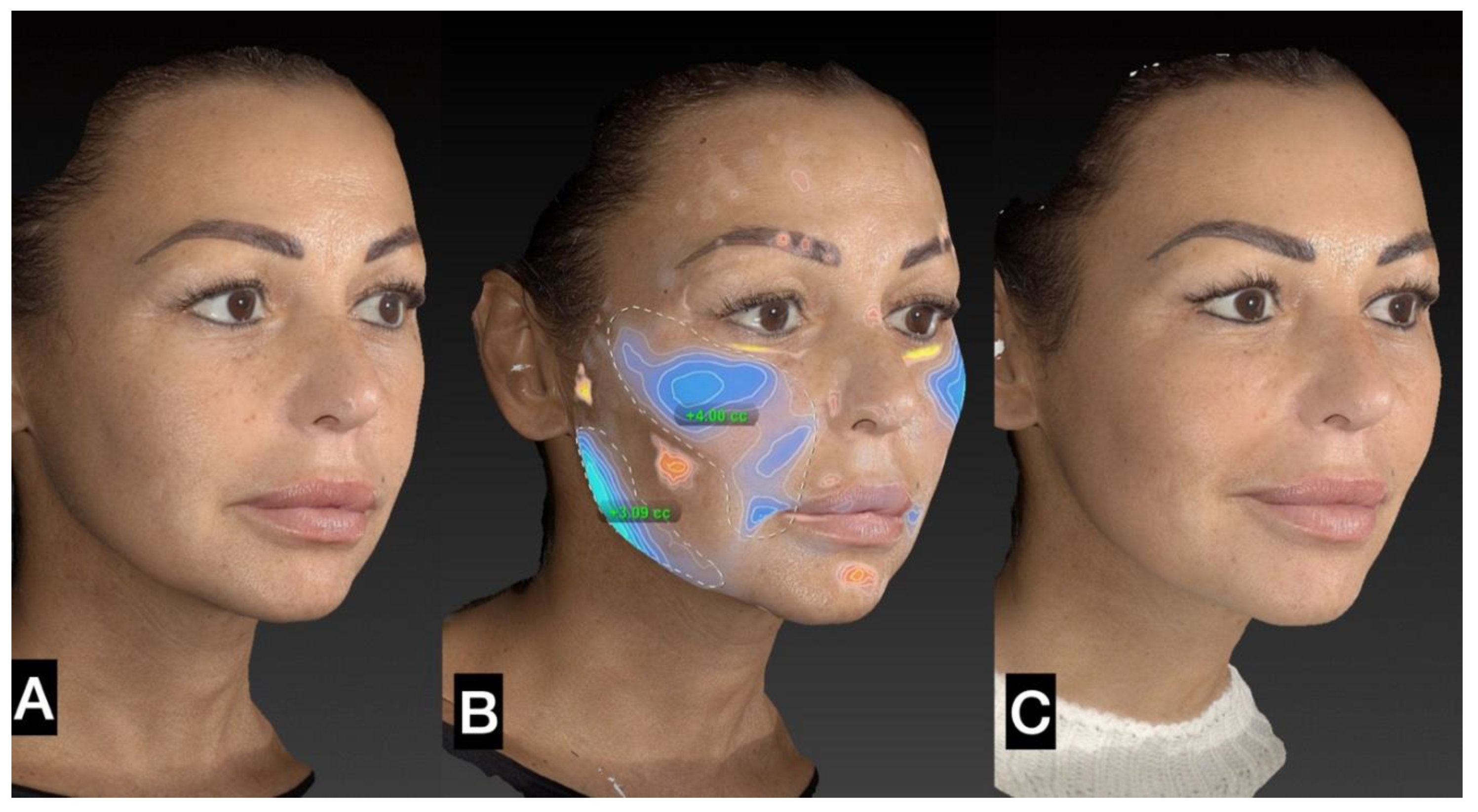
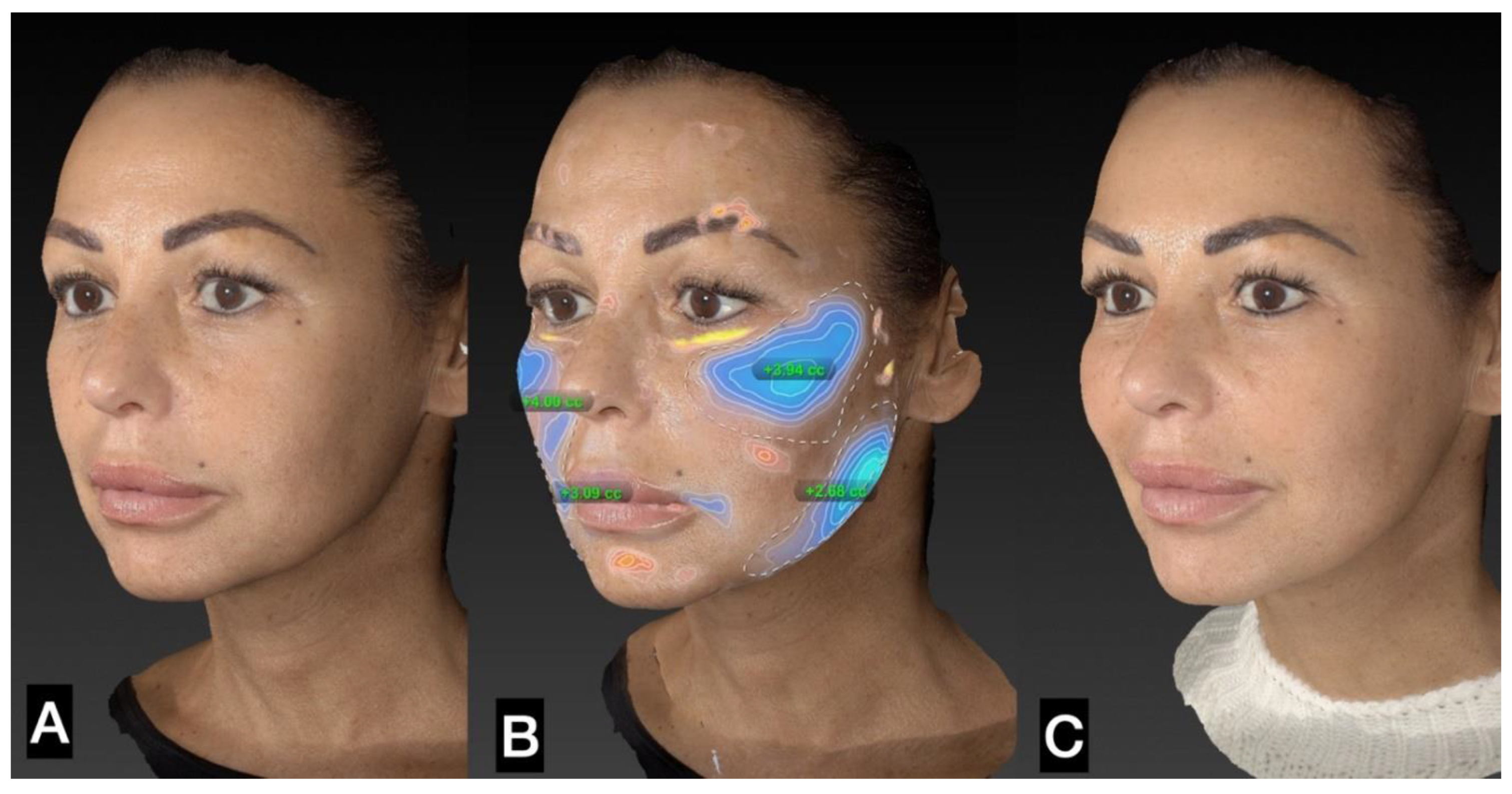
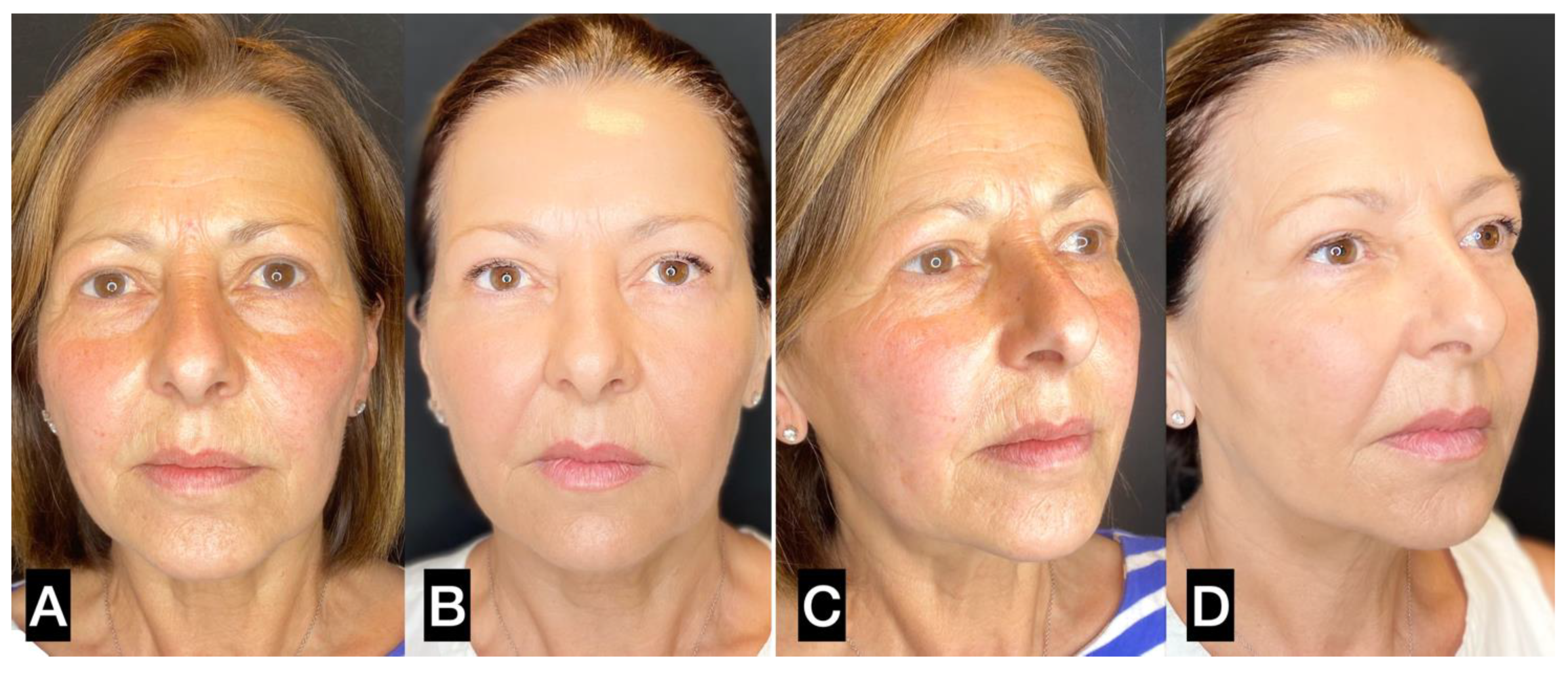
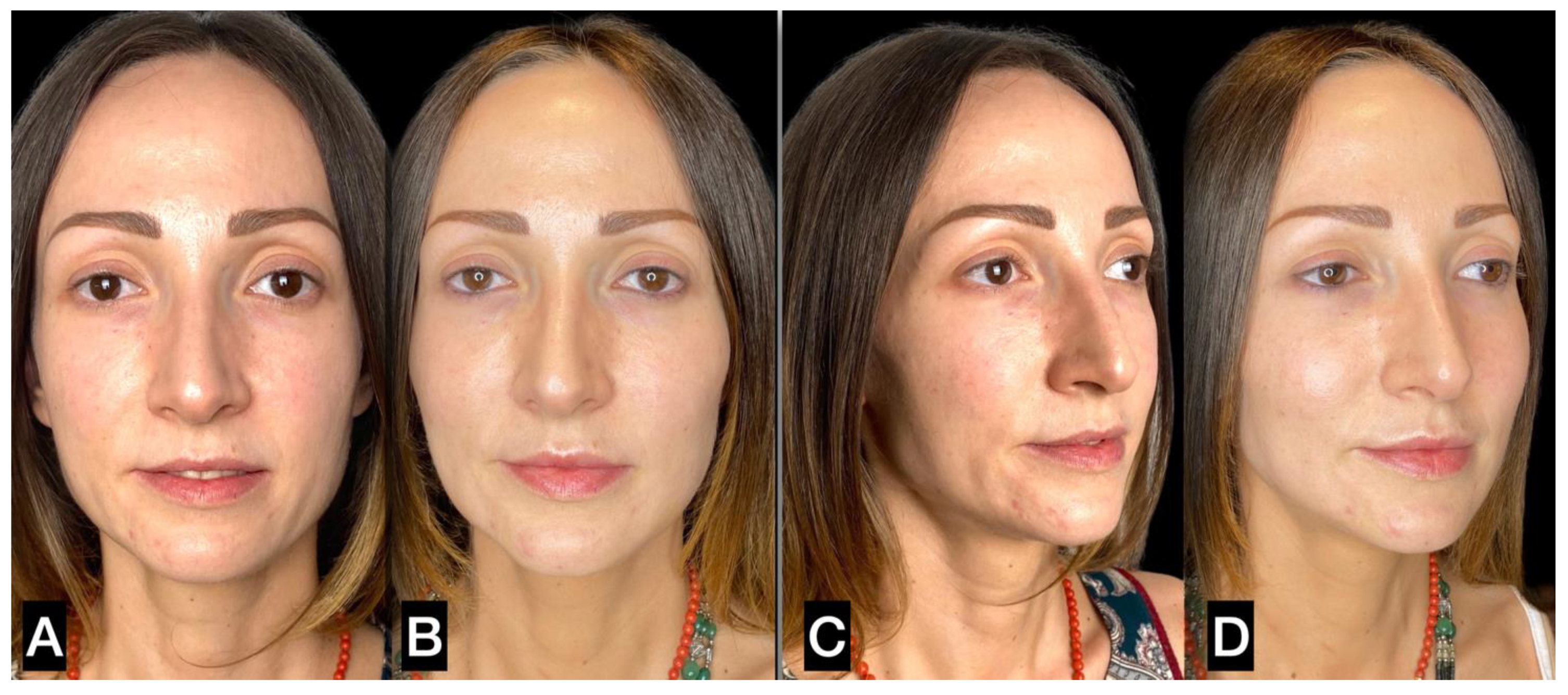
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fisher, G.J.; Kang, S.; Varani, J. , Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhess, J.J. Mechanisms of photoaging and chronological skin aging. Arch Dermatol 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Taub, A.F.; Pham, K. Stem cells in dermatology and anti-aging care of the skin. Facial Plast Surg Clin North Am 2018, 26, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduc. 2012, 2012, 64635. [Google Scholar] [CrossRef]
- Rohrich, R.J; Pessa, J.E. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg 2007, 119(7), 2219–2227. [Google Scholar] [CrossRef]
- Fitzgerald, R.; Graivier, M.H.; Kane, M.; Lorenc, Z.P.; Vleggaar, D.; Werschler, W.P.; Kenkel, J.M. Facial aesthetic analysis. Aesthet Surg J 2010, 30, S25–S27. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, L.R. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg 2008, 121(3), 1061. [Google Scholar] [CrossRef]
- Donofrio, L.M. Fat distribution: a morphologic study of the aging face. Dermatol Surg 2000, 26, 1107–1112. [Google Scholar] [CrossRef]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int J Mol Sci 2017, 18, 208. [Google Scholar] [CrossRef]
- Nailor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Cotta-Pereira, G.; Rodrigo, F.G.; Bittencourt-Sampaio, S. Oxytalan, elaulin and elastic fibers in the human skin. J Invest Dermatol 1976, 66, 143–148. [Google Scholar] [CrossRef]
- Gennai, A.; Tesauro, P.; Colli, M.; Roda, B.; Zattoni, A. Infranatant portion of microfragmented adipose tissue: a promising source of SVF for the management of androgenic alopecia. J Regen Med 2021, 1–7. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiado, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application Frontiers in Cell and Developmental. Biology 2020, 8, 236. [Google Scholar]
- Tallone T, Realini C, Bohmler A, Kornfeld C, Vassalli G et al. Adult human adipose tissue contains several types of multipotent cells. J Cardiovasc Transl Res 2011, 4, 200–210. [Google Scholar] [CrossRef]
- Del Papa, N.; Di Luca, G.; Sambataro, D.; Zaccara, E.; Maglione, W.; Gabrielli, A.; Fraticelli, P.; Moroncini, G.; Beretta, L.; Santianello, A.; et al. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant 2015, 24, 2297–2305. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007, 119, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Roda, B.; Zia, S.; Vigliotta, I.; Zannini, C.; Alviano, F.; Bonsi, L.; Zattoni, A.; Reschiglian, P.; Gennai, A. Characterization of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesthet Surg J 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ogura, F.; Wakao, S. Kuroda, Y.; Tsuchiyama, K.; Bagheri M., Heneidi, S.; Chazenbalk. G.; Aiba, S.; Dezawa, M. Human Adipose Tissue Possesses a Unique Population of Pluripotent Stem Cells with Nontumorigenic and Low Telomerase Activities: Potential Implications in Regenerative Medicine. Stem Cell Dev 2013, 2, 7. [Google Scholar]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Trivisonno, A.; Di Rocco, G.; Cannistra, C.; Finocchi, V.; Farr, S.T.; Monti, M.; Toietta, G. Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet Surg J. 2014, 34, 601–613. [Google Scholar] [CrossRef]
- Zeltzer, A.A.; Tonnard, P.L.; Verpaele, A.M. Sharp -needle intradermal fat grafting (SNIF). Aesthet Surg J 2012, 32, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalla, G.F.; Riccio, M.; Gigante, A. Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction From Adipose Tissue: Preliminary Results. Front Cell Dev Biol 2019, 7, 88. [Google Scholar] [CrossRef]
- Rubina, K.; Kalinina, N.; Efimenko, A.; Lopatina, T.; Melikhova, V.; Tsokolaeva, Z.; Sysoeva, V.; Tkachk, V.; Parfyonova, Y. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A 2009, 15, 2039–2050. [Google Scholar] [CrossRef]
- Cai, L.; Johnstone, B.H.; Cook, T.G.; Tan, J.; Fishbein, M.C.; Chen, PS.; Marcha, K.L. IFATS Collection: Human Adipose Tissue-Derived Stem Cells Induce Angiogenesis and Nerve Sprouting Following Myocardial Infarction, in Conjunction with Potent Preservation of Cardiac Function. Stem Cells 2009, 27, 230–237. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 2005, 332, 370–379. [Google Scholar] [CrossRef]
- Guo, J.; Dardik, A.; Fang, K.; Huang, R.; Gu, Y. Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Res Ther 2017, 8, 228. [Google Scholar] [CrossRef]
- Akita, S.; Akino, K.; Hirano, A.; Ohtsuru, A.; Yamashita, S. Noncultured Autologous Adipose-Derived Stem Cells Therapy for Chronic Radiation Injury. Stem Cells Int 2010, 1, 532704. [Google Scholar] [CrossRef]
- Gennai, A.; Bernardini, F.P. R3 facial rejuvenation through minimal incisions vertical endoscopic lifting (MIVEL) and superficial enhanced fluid fat injection (SEFFI): endoscopic repositioning, tissue regeneration volume restoration. Aesthetic Medicine 2015, 1. [Google Scholar]
- Bernardini, F.P.; Gennai, A.; Izzo, L.; Zambelli, A.; Repaci, E.; Baldelli, I.; Fraternali-Orcioni, G.; Hartstein, M.E.; Santi, P.L.; Quarto, R. Superficial Enhanced Fluid Fat Injection (SEFFI) to Correct Volume Defects and Skin Aging of the Face and Periocular Region. Aesthetic Surgery J 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.P.; Gennai, A. Superficial Enhanced Fluid Fat Injection for Volume Restoration and Skin Regeneration of the Periocular Aesthetic Unit. An Improved Fat Grafting Technique to enhance the beauty of the eye. JAMA Plastic Facial Surgery 2016, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Gennai, A.; Bernardini, F.P. Superficial enhanced fluid fat injection (SEFFI and MicroSEFFI) in facial rejuvenation. CellR4 2017, 5, e2239. [Google Scholar]
- Gennai, A.; Zambelli, A.; Repaci, E.; Quarto, R.; Baldelli, I.; Fraternali, G.; Bernardini, F.P. Skin Rejuvenation and Volume Enhancement with the Micro Superficial Enhanced Fluid Fat Injection (M-SEFFI) for Skin Aging of the Periocular and Perioral Regions. Aesthet Surg J 2017, 37, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Gennai, A.; Bernardini, F.P. Rejuvenation faciale par lifting endoscopique avec le petite incision incision vertical associée à une injection superficielle de graisse: un repositionement endoscopique, une regeneration tissulaire et une restauration volumetrique (La technique R3). J. Med. Esth. Et Chir. Derm. (article in French). 2017, 174, 87–95. [Google Scholar]
- Pignata, G. ; Gennai, A; Bernardini, F.P. Rejuvenation of the centre of the face: a new paradigm Endoscopic lifting with fat grafting. Plast Aesthet Res 2018, 5, 23. [Google Scholar] [CrossRef]
- Gennai, A.; Bovani, B.; Colli, M.; Melfa, F.; Piccolo, D.; Russo, R.; Roda, B.; Zattoni, A.; Reschiglian, P.; Zia, S. Comparison of Harvesting and Processing Technique for Adipose Tissue Graft: Evaluation of Cell Viability. Int J Regen Med 2021, 4, 2–5. [Google Scholar] [CrossRef]
- Gennai, A.; Bernardini, F.P.; Baldessin, M.; Bovani, B.; Camporese, A.; Colli, M.; Diaspro, A.; Melfa, F.; Piccolo, D.; Russo, R.; Tesauro, P.; Roda, B.; Zia, S. . Evaluation of The Viability and Phenotype of Adipose Derived Cells Harvested Using Different Harvesting and Processing Procedures: A Pilot Study. J Stem Cells Clin Pract 2022, 2, 101. [Google Scholar]
- Gennai, A.; Zia, S.; Roda, B.; Maggio, A.; Bonsi, L.; Alviano, F.; Zattoni, A.; Reschiglian, P.; Bernardini, F.P. SEFFI (Superficial Enhanced Fluid Fat Injection) for aesthetic and clinical regenerative treatments. Global Journal of Dermatology & Venereology 2020, 8, 32–40. [Google Scholar]
- Clauser, L.; Lucchi, A.; Tocco-Tussardi, I.; Gardin, C.; Zavan, B. Autologous Fat Transfer for Facial Augmentation and Regeneration: Role of Mesenchymal Stem Cells. Atlas Oral Maxillofac Surg Clin North Am 2018, 26, 25–32. [Google Scholar] [CrossRef]
- Charles de Sá, L.; Gontijo de Amorim, N.F; Takiya, C.M.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg 2015, 135, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Gennai, A.; Bovani, B.; Colli, M.; Melfa, F.; Piccolo, D.; Russo, R.; Tretti Clementoni, M.; Zia, S.; Roda, B.; Zattoni, A. Evaluation of the Number, Biophysical and Multipotent Characteristics of Adipose Derived Stem Cells Harvested by SEFFI Procedure and Interaction with Different Type of Hyaluronic Acids. Int J Regen Med 2021, 4, 2–10. [Google Scholar] [CrossRef]
- Molliard, S.G; Bétemps, J.B.; Hadjab, B.; Topchian, D.; Micheels, P.; Salomon, D. Key rheological properties of hyaluronic acid fillers: from tissue integration to product degradation. Plast Aesthet Res 2018, 5, 17. [Google Scholar] [CrossRef]
- Altman, A.M.; Khalek, F.J.A.; Seidensticker, M.; Minilla, S.; Yan, Y.; Coleman, M.; Song, Y.H.; Butler, C.E.; Alt, E.U. Human tissue-resident stem cells combined with hyaluronic acid gel provide fibrovascular-integrated soft-tissue augmentation in a murine photoaged skin model. Plast Reconstr Surg 2010, 125, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Colli, M.; Gennai, A.; Russo, R.; Tomeo, A; Bovani, B. ; Piccolo, D.; Melfa, F.; Tesauro, P.; Diaspro, A. Non-surgical Jowl and Jawline Rejuvenation with Resorbable Suspension Threads and Superficial Enhanced Fluid Fat Injection (SEFFI). J Reg Med Biol Res. 2022, 3, 1–19. [Google Scholar] [CrossRef]
- Zduńska, K.; Kołodziejczak, A.; Rotsztejn, H. Is skin microneedling a good alternative method of various skin defects removal. Dermatology Therapy 2018, 31, e12714. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) against Ultraviolet (UV) Radiation Effects and the Skin Photoaging. Biomedicines 2021, 9(5), 532. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Wu, H.; Xie, S.; Zhang, M.; Zhang, B.; Tang, S. Basic Fibroblast Growth Factor Influences Epidermal Homeostasis of Living Skin Equivalents through Affecting Fibroblast Phenotypes and Functions. Skin Pharmacol Physiol 2018, 31, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Carruthers, J. Botulinum toxin. In: Bolognia J.L., Jorizzo J.L., Rapini R.P., editors. Dermatology. 2nd ed. New York: Mosby Elsevier; 2008, 2381–2390.
- Gart, M.S.; Gutowski, K.A. Overview of botulinum toxins for aesthetic uses. Clin Plast Surg 2016, 43, 459–471. [Google Scholar] [CrossRef]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: a literature review of clinical use and pharmacological aspect. Clin Cosmet Investig Dermatol 2019, 12, 223–228. [Google Scholar] [CrossRef]
- Kroumpouzos, G.; Arora, G.; Kassir, M.; Galadari, H.; Wollina, U.; Lotti, T.; Grabbe, S.; Goldust, M. Carboxytherapy in dermatology. Clinics in Dermatology 2022, 40(3), 305–309. [Google Scholar] [CrossRef]
- El-Domyati, M.; El-Din, W.H.; Medhat, W.; Ibrahim, M.R.; Khaled, Y. The use of Carboxytherapy alone or in combination with fractional CO2 laser for facial rejuvenation: A split-face comparative study. J Cosmet Dermatol 2020, 19(7), 1648–1655. [Google Scholar] [CrossRef]
- Courderot-Masuyer, C.; Robin, S.; Tauzin, H.; Humbert, P. Evaluation of lifting and antiwrinkle effects of calcium hydroxylapatite filler. In vitro quantification of contractile forces of human wrinkle and normal aged fibroblasts treated with calcium hydroxylapatite. J Cosmet Dermatol 2016, 15, 260–268. [Google Scholar] [CrossRef]
- Lorenc, Z.P.; Bass, L.M.; Fitzgerald, R.; Goldberg, D.J; Graivier, M.H. Physiochemical Characteristics of Calcium Hydroxylapatite (CaHA). Aesthet Surg J 2018, 38(S1), S8–S12. [Google Scholar] [CrossRef]
- Rovatti, P.P.; Pellacani, G.; Guida, S. Hyperdiluted Calcium Hydroxylapatite 1:2 for Mid and Lower Facial Skin Rejuvenation: Efficacy and Safety. Dermatol Surg 2020, 46, e112–e117. [Google Scholar] [CrossRef]
- Bartoletti, E.; Melfa, F.; Renzi, M.; Rovatti, P. Systematic review of the literature on the properties, quality and reliability of calcium hydroxyapatite: results of an Italian experts’ meeting. Aesthetic Medicine 2022, 8. [Google Scholar]
- Coleman, S.R. Structural fat grafts: the ideal filler? Clin Plast Surg 2001, 28(1), 111e9. [Google Scholar] [CrossRef]
- Tonnard, P.L.; Verpaele, A.M.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting basic research and clinical applications. Plast Reconstr Surg J. 2013, 132, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.P.; Gennai, A.; Izzo, L.; Devoto, M.H. Minimal incisions vertical endoscopic lifting and fat grafting as a systematic approach to the rejuvenation of the periocular esthetic unit. Ophthalmic Plast Reconstr Surg 2013, 29, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.S.; Desouches, C.; Gay, A.M.; Hautier, A.; Magalon, G. Development of micro-injection as an innovative autologous fat graft technique: the use of adipose tissue as dermal filler. J Plast Reconstr Aesthet Surg 2012, 65, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- James, I.B.; Coleman, S.R.; Rubin, J.P. Fat, stem cells, and platelet-rich plasma. Clin Plast Surg 2016, 43(3), 473–488. [Google Scholar] [CrossRef]
- Mengzhu, L.V.; Zhang, S.; Jiang, B.; Cao, S.; Dong, Y.; Cao, L.; Guo, S. Adipose Adipose-derived stem cells regulate metabolic homeostasis and delay aging by promoting mitophagy. J FASEB 2021, 35, e21709. [Google Scholar]
- Kikap, K.; Fan, Y.; Lin, G.; Park, Y.K.; Pak, C.S.; Jeong, J.H.; Kim, S. Synergistic Effect of Adipose-Derived Stem Cells and Fat Graft on Wrinkles in Aged Mice. Plast Reconstr Surg 2019, 143(6), 1637–1616. [Google Scholar]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; Lattanzi, W. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy 2015, 17(8), 1076–1089. [Google Scholar] [CrossRef] [PubMed]
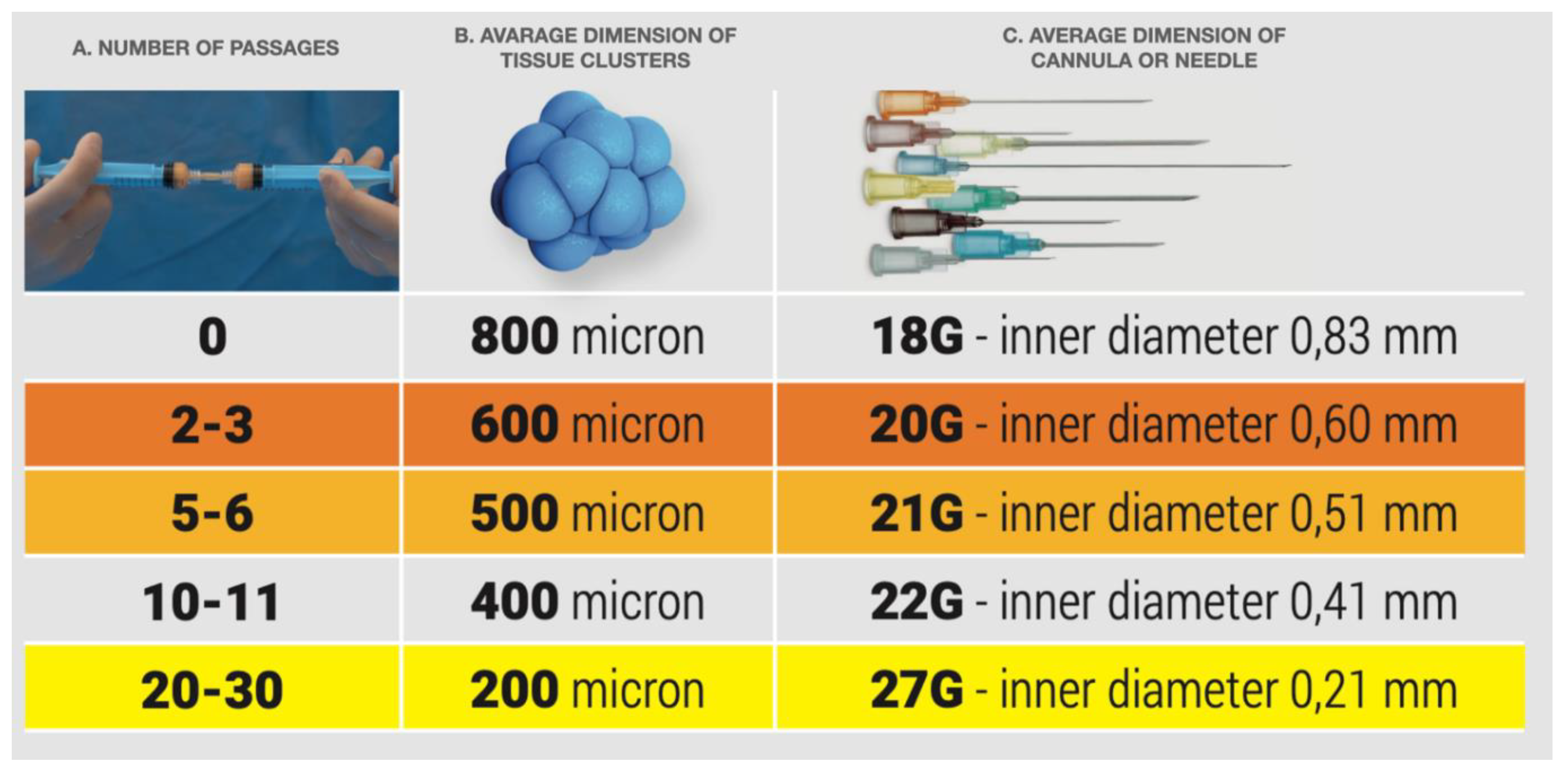
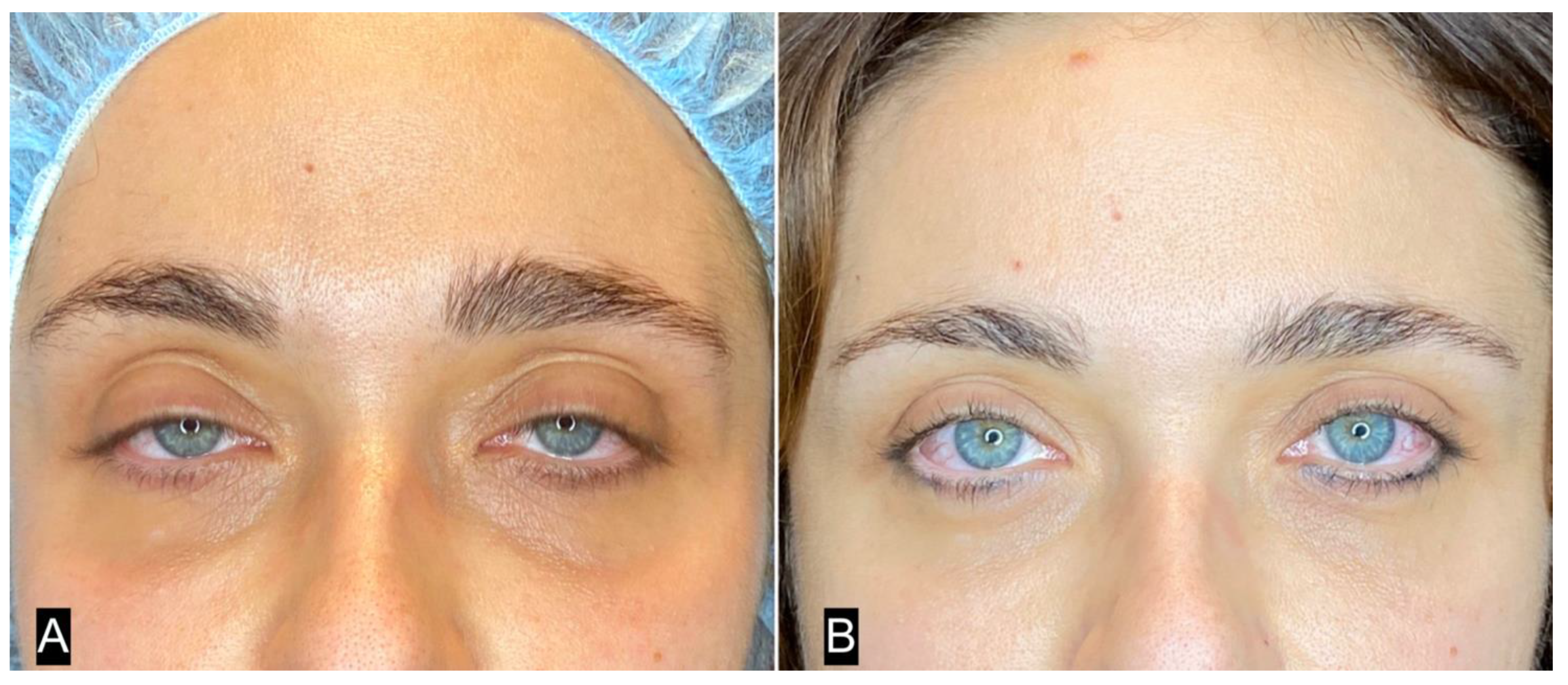
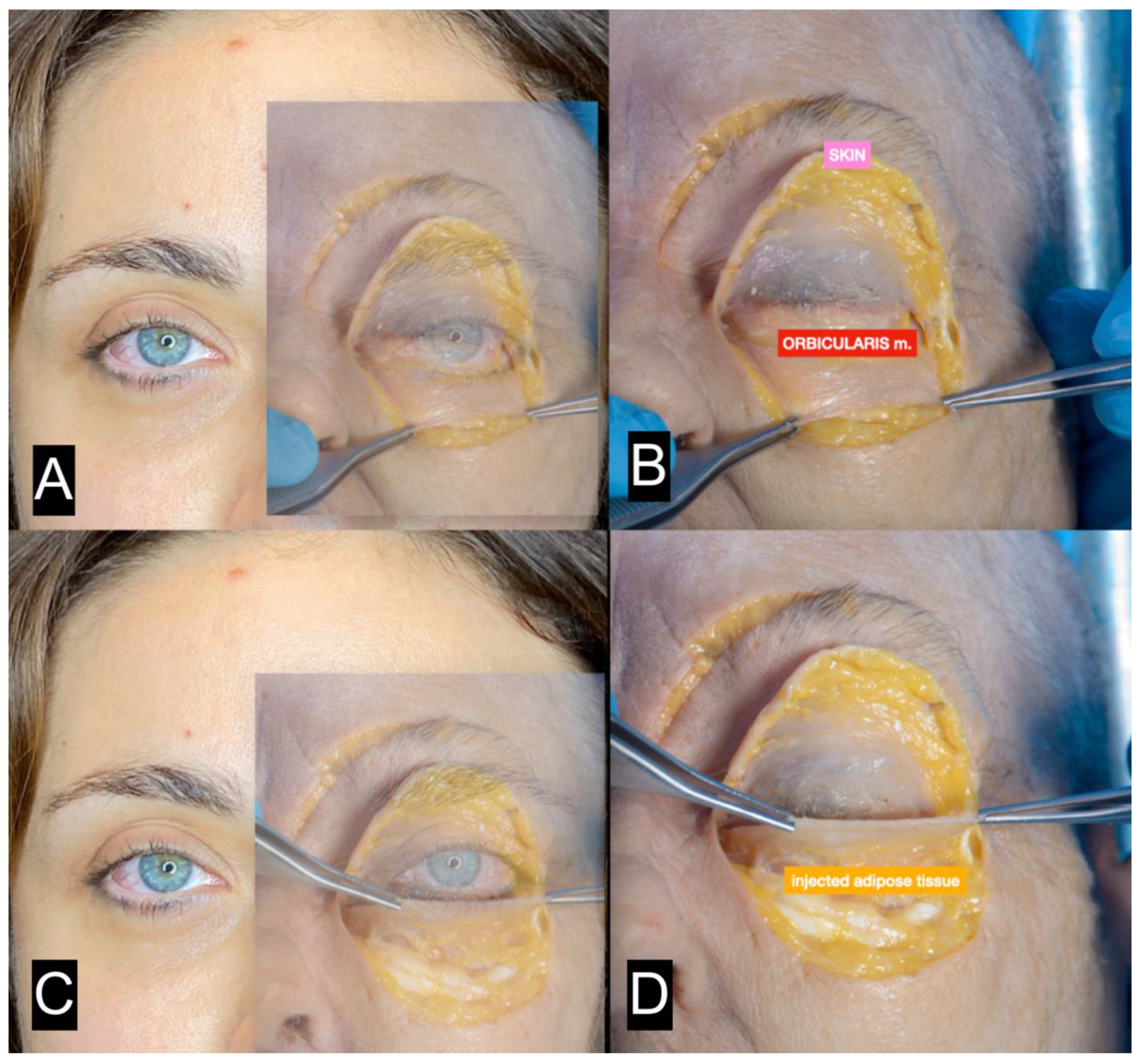
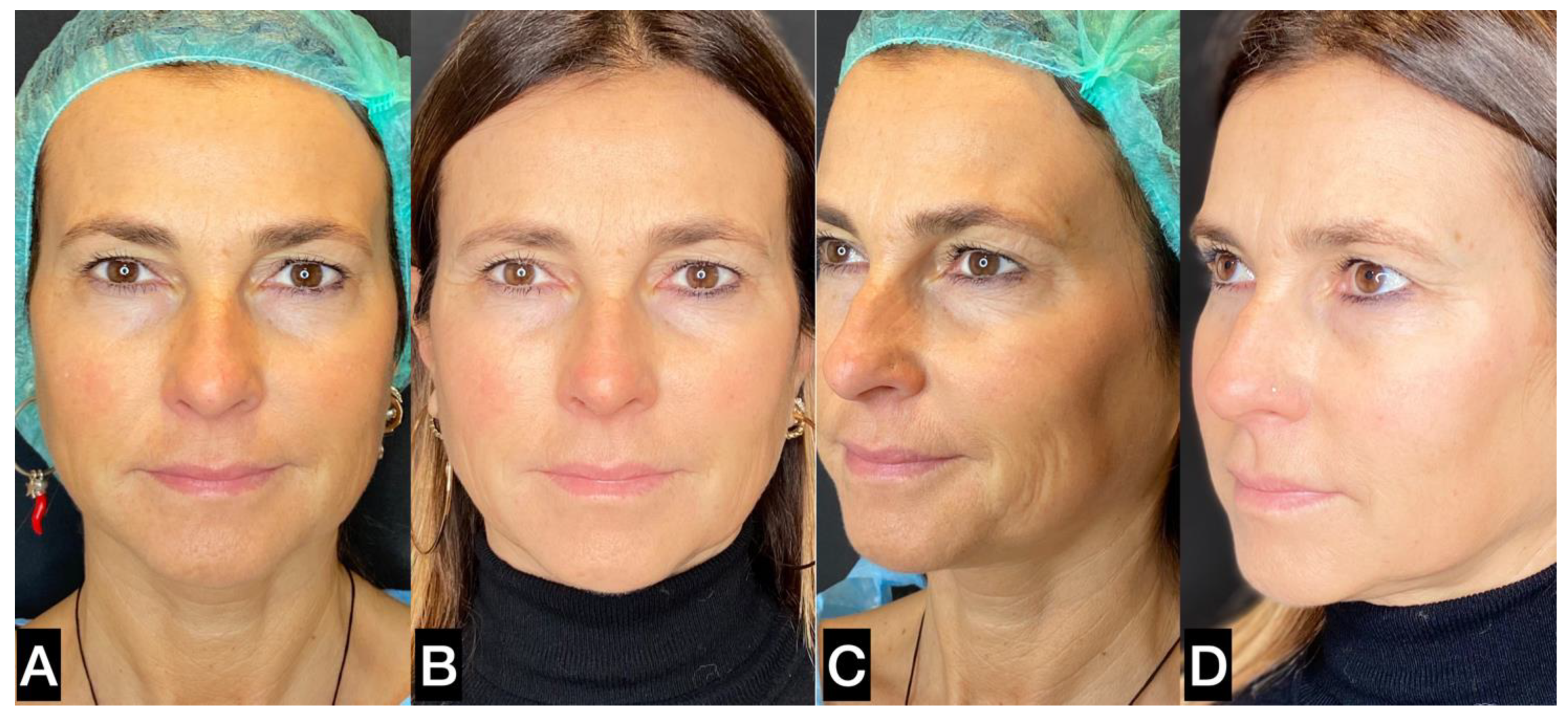
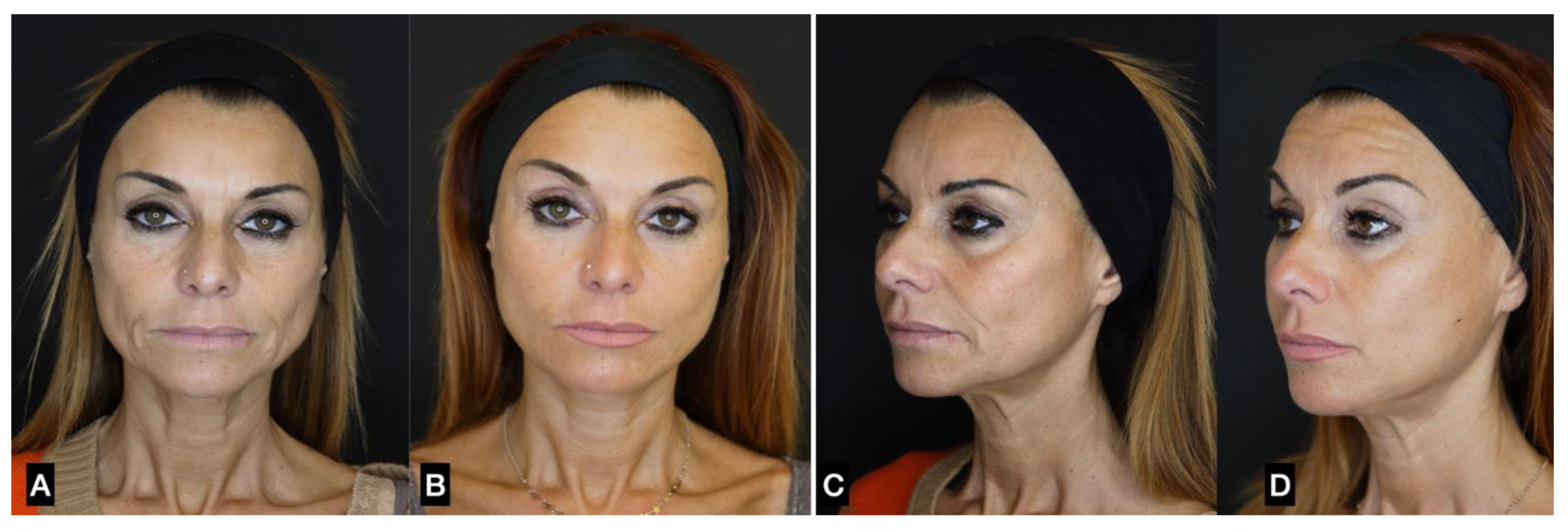
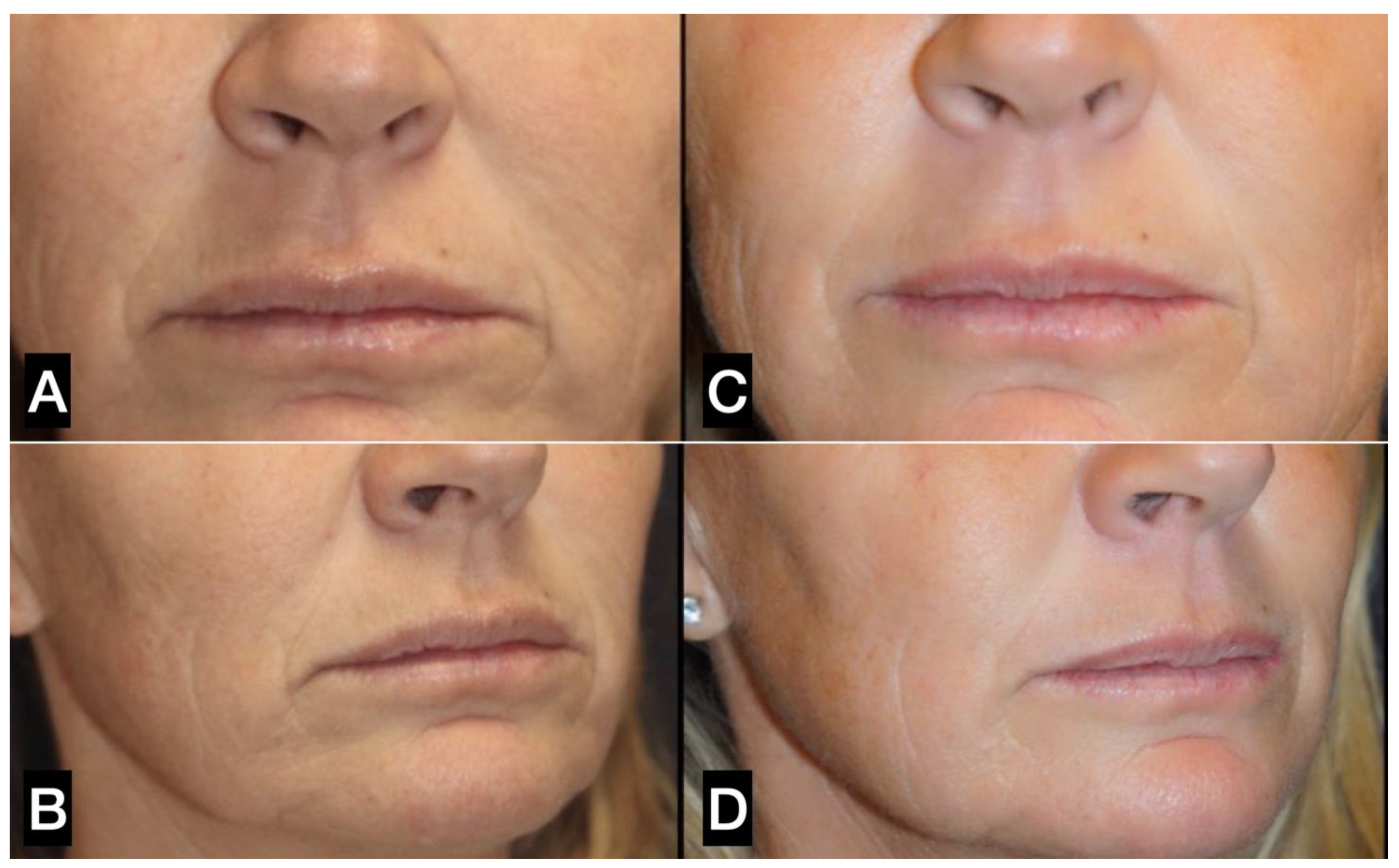
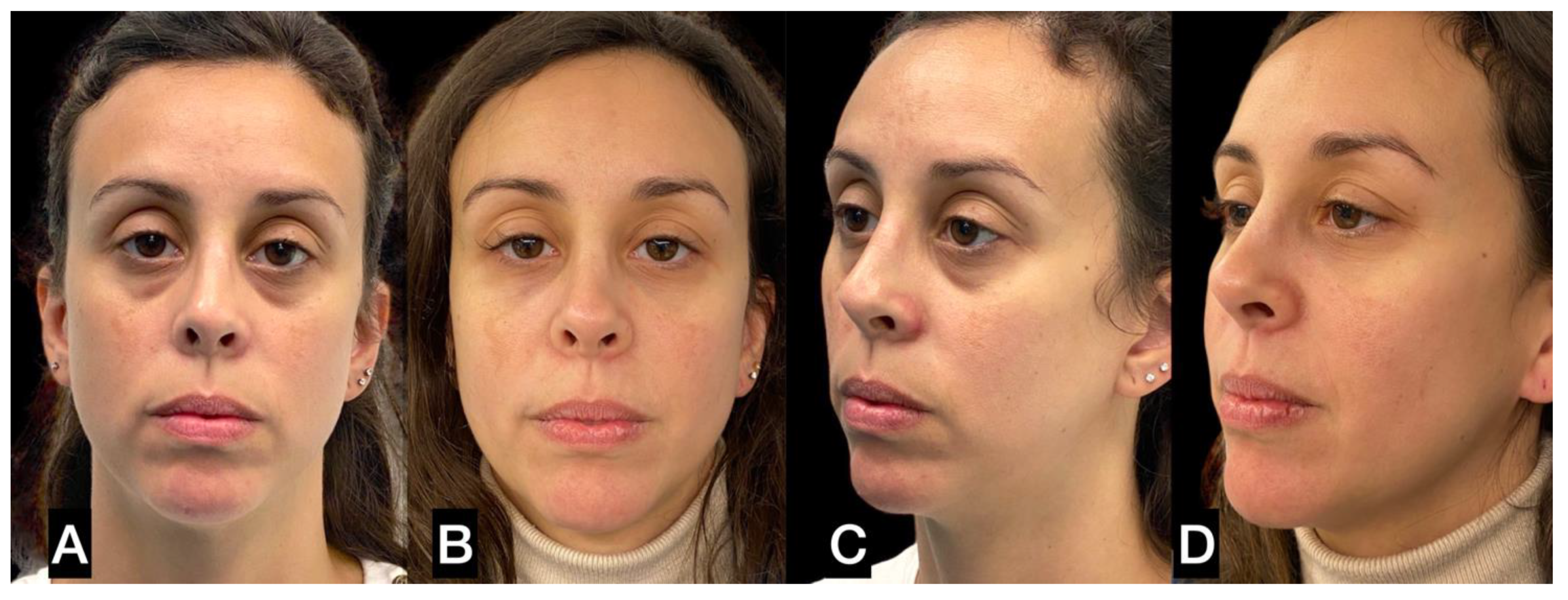
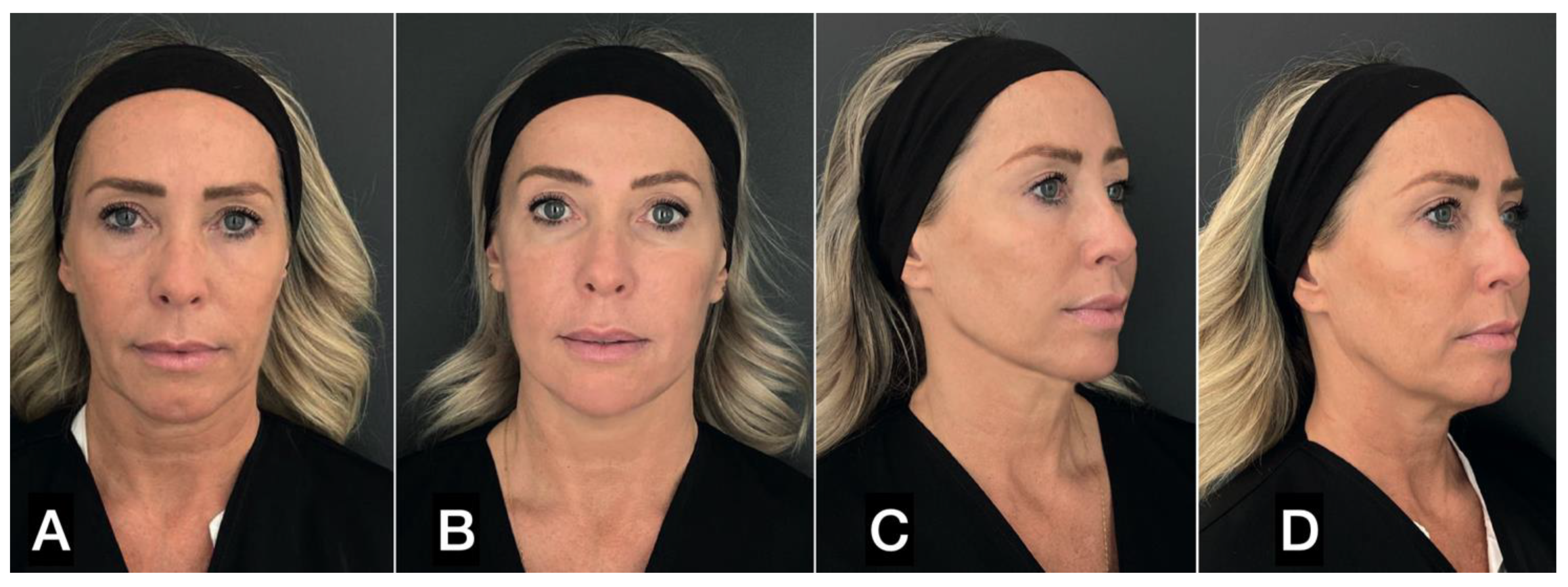
| FAT HARVESTING | Involved area | Number of patients (%) | Average harvested volume (ml) |
|---|---|---|---|
| Abdomen | 1231 (52.1) | 27.1 | |
| Trochanteric | 761 (32.4) | 27.1 | |
| Hip | 296 (12.6) | 22.6 | |
| Knee | 67 (2.8) | 6.8 | |
| Other | 3 (0.1) | 2.1 | |
| SEFFI INJECTION | Injection area | Number of patients (%) | Average injected volume (ml) |
| Single area | Periocular | 63 (2.7) | 1.4 |
| Temporal | 5 (0.2) | 1.1 | |
| Malar-zygomatic | 233 (9.9) | 4.4 | |
| Perioral/lip | 34 (1.4) | 1.3 | |
| Jawline | 91 (3.8) | 2.8 | |
| Other | 14 (0.6) | 5.2 | |
| Total | 440 (18.6) | ||
| Combined areas | Full face | 557 (23.6) | 14.8 |
| Two areas | 561 (23.7) | 5.1 | |
| Three areas | 639 (27.0) | 2.8 | |
| Four areas | 246 (10.4) | 8.3 | |
| Total | 1925 (81.4) |
| TAR PROCEDURES | Number of procedures | Percentage of patients | |
|---|---|---|---|
| Single procedure | 1,484 | 62.7 | |
| Combined procedures | Botulin toxin | 295 | 12.5 |
| Hyaluronic acids | 233 | 9.9 | |
| Threads | 122 | 5.2 | |
| Microneedling | 85 | 3.6 | |
| Calcium Hydroxylapatite | 65 | 2.7 | |
| Carboxytherapy | 51 | 2.6 | |
| Endolift | 24 | 1.0 | |
| Others | 0 | 0 | |
| Total | 875 | 37.0 |
| Site of complications | Type of complications | Number of events | Percentage of patients |
|---|---|---|---|
| DONOR SITE | Ecchymosis | 336 | 14.2 |
| Hematoma | 31 | 1.3 | |
| Prolonged erythema (> 48 hours) | 0 | 0 | |
| Skin necrosis | 0 | 0 | |
| Prolonged edema (> 20 days) | 0 | 0 | |
| Infection | 0 | 0 | |
| Fat necrosis | 0 | 0 | |
| Telangiectasias | 0 | 0 | |
| Skin irregularities (> 20 days) | 5 | 0.2 | |
| Embolism | 0 | 0 | |
| Other | 0 | 0 | |
| INJECTION SITE | Ecchymosis | 914 | 38.6 |
| Hematoma | 18 | 0.8 | |
| Prolonged erythema (> 48 hours) | 0 | 0 | |
| Skin necrosis | 0 | 0 | |
| Prolonged edema (> 5 days) | 14 | 0.6 | |
| Infection | 1 | <0.1 | |
| Fat necrosis | 0 | 0 | |
| Telangiectasias | 0 | 0 | |
| Activation of acne | 0 | 0 | |
| Visibility | 2 | 0.1 | |
| Skin irregularities (> 48 hours) | 1 | <0.1 | |
| Blindness | 0 | 0 | |
| Asymmetry | 3 | 0.1 | |
| Other | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





