Introduction:
Breast cancer is the most common cancer in women of the United States excluding skin cancers representing nearly 1 in 3 new female cancers each year. According to the American Cancer Society there will be nearly 300,000 new cases of invasive breast cancer and over 50,000 cases of ductal carcinoma in situ diagnosed in 2023 with over 43,000 deaths attributable to breast cancer in the United States alone [
1]. The high incidence and burden of breast cancer represents a tremendous challenge and opportunity to breast cancer screening programs. The purpose of any breast cancer screening program is to reduce the morbidity and mortality of breast cancer by identifying early small breast cancers to ensure accurate diagnosis and optimal treatment. Screening mammography is the only breast cancer screening modality with a proven mortality benefit, leading to the widespread adoption of mammography-based screening programs throughout the world.
Population based screening efforts have led to a large number of mammograms being performed annually, with nearly 40 million mammograms performed every year in the United State alone [
2]. The importance of screening mammography performance to breast cancer screening programs and the sheer volume of mammograms involved creates an imperative need to maximize performance and quality. In the United States, this is closely regulated by the Food & Drug Administration (FDA) via the Mammography Quality Standards Act (MQSA) including recent emphasis via the Enhancing Quality Using the Inspection Program (EQUIP) process initiated in 2017. These processes have helped ensure quality and uniformity for screening mammograms performed in the United States. However, even with these efforts there remain opportunities for improvement in performance metrics for screening mammography. As an illustration of this need, an evaluation of performance in the Breast Cancer Surveillance Consortium found a sensitivity of 86.9% and specificity of 88.9% for screening mammography with opportunities for improvement particularly noted in abnormal interpretation rates (false positives) in nearly half of the studied radiologists [
3].
Background:
The convergence in screening mammography as a widespread population health tool with opportunities to improve performance to reduce breast cancer mortality has generated significant interest and research. Using computers in an attempt to improve performance is not new and has a long history in breast imaging in the form of computer aided detection (CAD). The FDA first approved CAD for use in mammography was established in 1998 and by 2002, this technology was reimbursable by the Center for Medicare and Medicaid Services. This approval led to its rapid adoption in breast imaging, with 74% of mammograms in 2008 performed with CAD [
4].
Initial excitement and enthusiasm for the benefits of CAD in breast imaging has given way to the realization that traditional CAD may have limited or no increase in diagnostic performance [
4]. Multiple recent studies have raised concerns about the cost-effectiveness and clinical utility of CAD in breast imaging. An observational study of community-based mammography facilities from the Breast Cancer Surveillance Consortium performed by Fenton et al between 1998 and 2002 found that CAD use reduced overall radiologist reading accuracy as evaluated by receiver operating characteristic curve analysis [
5]. A subsequent study published in July 2011 found that CAD use reduced specificity by increasing recall rates, with no increase in sensitivity or invasive tumor characteristics (stage, size, or lymph node status) [
6]. Despite these concerns about its efficacy, CAD utilization for screening mammograms has become ubiquitous, with reimbursement bundled with screening mammography, and with utilization in 92% of all screening mammograms performed in the United States in 2016 [
7].The failure of conventional CAD to fulfill the need for improving and optimizing mammography performance creates a continued opportunity for artificial intelligence (AI) in breast imaging.
Artificial Intelligence:
AI is a large field that includes many diverse technologies and applications with the shared characteristics of using computer-based algorithms and data to solve problems or perform tasks that would typically require human intelligence. In the past 10-15 years there have been tremendous advances in the availability and accessibility of more powerful computational hardware for processing and storing data needed for AI applications. At the same time, and perhaps even more critically, there has been an exponential increase in the amount and availability of data for training AI algorithms. These changes have allowed for revolutionary developments in AI during the past 10 years with particular focus on machine learning (ML). ML is a subset of AI where computers are trained and perform functions without being explicitly programmed by humans on how to complete the tasks. ML commonly uses features and input from human programmers as the basis of learning. Further along the continuum of ML is representation learning which does not require human feature engineering, but rather learns the features itself. Deep learning (DL) is a step further where the features are extracted in a hierarchical fashion with many simple features making up more complex features [
8]. These changes and developments have allowed for DL applications that generate truly breakthrough performance enhancements in image analysis tasks [
8].
DL utilizing convolutional neural networks has seen an explosion of possibility and practical use for image analysis for non-medical images in the past 10 years. This includes many non-medical imaging related tasks such as image classification or detection which are already deeply ingrained in daily workflow. These successes led to interest for applications within radiology that could apply the success of AI algorithms in image analysis to perform clinically meaningful tasks such as classification (presence or absence of disease), segmentation (quantitative analysis of organs or lesions for surgical planning), and detection (determining the presence or absence of a lesion or nodule) amongst many other diverse applications for AI in radiology [
8].
Artificial Intelligence in Breast Imaging:
Opportunities in Breast Imaging for AI Applications
Breast imaging has many unique features and characteristics that create opportunities for meaningful AI applications (
Table 1). Specifically, the long standing and unique structured lexicon of breast imaging as defined by the Breast Imaging Reporting & Data System (BI-RADS®) from the American College of Radiology facilitates the development and implementation of AI. BI-RADS® provides a standardized and structured system of lexicon and terminology, reporting, classification, communication and medical auditing for mammography, breast ultrasound and breast MRI [
9]. This system supports the development and evaluation of AI applications in breast imaging in many ways, perhaps most importantly, by creating a predefined methodology and framework for the radiologist’s interpretation of breast imaging studies and mapping of results. When combined with medical outcomes, auditing and reporting there is a repository of data for breast imaging included in radiologist interpretations and clinical outcomes for mammography [
10]. Moreover, the standardized approach to screening mammography where 2 specific mammographic positions are imaged for each breast (craniocaudal and mediolateral oblique positions), improves the standardization of imaging data being utilized for training and validation.
This standardization and established methodology for determining and tracking results has facilitated the creation of multiple large data sets which are a prerequisite for the development of high performing AI algorithms. There are currently multiple large mammography data sets, some of which have greater than 1 million mammograms with associated patient factors and known clinical outcomes [
11,
12,
13,
14]. Many of the available data sets come from various sources including different practice locations, practice types and multiple mammography vendors. Some data sets are also focused on including a racially diverse case mix, critical to ensuring high levels of performance across the entire population [
11]. The availability of data sets is significantly more advanced for mammography, in particular screening mammography, when compared to other breast imaging modalities such as ultrasound or MRI.
Challenges of Breast Imaging for AI Applications
There are several unique aspects of breast imaging that make the development and implementation of high performing AI algorithms more challenging (
Table 1). For example, recent rapid adoption and widespread utilization of digital breast tomosynthesis (DBT) has created challenges on multiple fronts. The image data for DBT is unique and significantly different from standard full field digital mammography (FFDM) as many slices of images are created with each mammographic position versus the single FFDM image for each view. The appearance of the images including benign and malignant pathology differ significantly. Moreover, DBT file sizes are orders of magnitude greater than traditional FFDM images with files sizes for single exams approaching or exceeding 1 gigabyte. This creates significant challenges for storage, transfer, and consumption of this large volume of data, particularly in a busy clinical application.
There are also significant variations in the appearance of DBT images between various vendors with the differences being significantly greater than when comparing traditional FFDM mammographic images. Further compounding these challenges are the recent but variable use of synthetic mammographic images to replace traditional FFDM images. These synthetic images are generated from tomosynthesis imaging data which has the advantage of eliminating the need for standalone FFDM image, and thus significantly reducing the radiation dose for patients. The utilization of synthetic mammography is highly variable [
15]. Moreover, there are significant differences between the appearance of these synthetic images between vendors and between software upgrades of a vendor. This variability, and the recent heterogenous adoption of these technologies, has created a significant limitation and challenge for AI algorithms which may not have been developed for a certain image type or may not perform equally well across all vendors and systems.
An additional significant challenge for the development of high performing AI algorithms is the manner in which breast images are interpreted. Frequently, breast imaging studies are interpreted with the utilization of multiple prior comparison imaging exams, allowing radiologists to identify new, subtle, meaningful changes and dismiss stable benign variations. This is an additional process that must be either built into AI algorithms or otherwise accounted for in their application. Additionally, the interpretation of breast imaging is commonly a multimodality process, particularly outside of the screening mammography environment. Often mammography is used in conjunction with breast ultrasound, breast MRI, or other adjunctive imaging modalities to evaluate and workup breast problems. These tasks are also performed in a complex background in which the radiologist aggregates information in real-time about patients’ clinical and medical history, including risk scores amongst other factors, referring providers, and technologists, which can influence the most efficacious workup and diagnosis. These different disparate data sources and factors represent challenges for the development of high performing AI algorithms.
Applications for Artificial Intelligence in Breast Imaging:
Cancer Detection:
Much of the research, development, and excitement surrounding AI applications in breast imaging has been on cancer detection, notably cancer detection on screening mammography (
Table 2). Widespread adoption of breast cancer screening programs, the significant morbidity and mortality of breast cancer worldwide, and the efficacy of high performing screening mammography creates a unique and powerful opportunity for AI. This has led to a tremendous focus on AI based applications for mammography-based breast cancer detection.
There have been many published examples of AI algorithms which demonstrate excellent performance in cancer detection for screening mammography. This includes a number of algorithms trained and evaluated on single institution or homogenous internal data sets. However, there have also been multiple, more recent examples of AI algorithms trained on larger, more heterogenous or representative data sets. This includes an AI based cancer detection system trained on United Kingdom (UK) and United States (US) data comparing AI performance vs radiologist performance in a reader study finding an absolute reduction of 5.7% and 1.2% in false positives and 9.4% and 2.7% in false negatives (US and UK data sets) [
16]. The AI algorithm performed significantly better than all human readers in the reader study [
16]. Another seminal AI algorithm was developed as the result of an international crowdsourcing challenge which found that individual AI algorithms approached but did not exceed radiologist performance [
17]. Rather, the best performance was achieved when an ensemble of the best AI algorithms was combined with a radiologist [
17]. An additional published AI Model trained on greater than 1 million images achieved an excellent AUC for cancer detection of 0.895 when evaluated on a large data set. Further evaluation and comparison of this model's performance with a group of radiologists in a reader study found that the AI’s AUC exceeded that of all individual readers, however, importantly found that the performance of a radiologist-AI hybrid model was the highest performing in the reader study, exceeding both individual and AI alone performance [
18].
Such investigations have generated tremendous excitement for using AI applications for breast cancer detection with multiple commercially available products already available for use on the market, in addition to other investigational or open-source AI algorithms. However, there is currently a significant gap in the understanding of how these AI applications will perform in the real world when used in clinical practice by radiologists. A recent review article found no prospective studies testing accuracy of AI in screening practice [
19]. The review also noted significant issues with methodology and quality in published investigations, finding the majority of AI applications were less accurate than a single radiologist and all included algorithms were less accurate than the consensus of two or more radiologists. The authors also noted the pattern that small, more limited studies which found AI to be more accurate than radiologists demonstrated issues with bias and generalizability, and that their results were not yet replicated in larger studies [
19].
External attempts to evaluate the performance of AI algorithms have resulted in variable observed performance. For example, a high performing AI model demonstrated significantly inferior performance when used at an external site in its native form [
20]. This same AI model was then tested after training without transfer learning and after retraining with transfer learning (using a pretrained algorithm and then applying it to a new but related problem with some modification). Local retraining of that model with transfer learning allowed for improvement in performance that approached initial reported levels [
20]. The results suggest limitations and concerns for generalizability of performance in AI applications. Perhaps more importantly these results illustrate the possibilities for optimizing AI performance locally at sites using a generally available model. A study looking to externally evaluate and compare three different commercially available algorithms with human readers (single and double) found that performance for the single best AI algorithm was sufficiently high that it could be evaluated as an independent reader for screening mammography [
21]. Moreover, combining the first radiologist reader with the best AI algorithm found more cancers than using first and second radiologist readers. A systematic review of independent external validation of AI algorithms for cancer detection in mammography found only 13 data sets that met inclusion criteria with all being either retrospective reader or simulation studies. The review found mixed results with only some AI algorithms alone exceeding radiologist performance whereas in all reviewed instances radiologists combined with AI outperformed radiologists alone [
22]. An additional serious concern is that developed and available AI algorithms may not perform equally well in all subpopulations or patient groups. A study evaluating a well-known, previously externally validated, high performing AI algorithm on an independent, external, diverse population found that certain patient groups had much lower performance compared to other groups and previously published performances. These issues raise concerns about unintended secondary consequences for inadequate inclusion of all patient groups in testing and validation data sets [
23].
DBT represents an additional challenge when interpreting the available literature and evaluating AI performance for clinical use. Many of the large available data sets for training and previously published AI algorithms were created and validated entirely or predominantly using FFDM data sets. As DBT has gained widespread adoption and a high level of utilization, this creates more uncertainty when attempting to generalize expected performance from commercially available or investigational AI algorithms into clinical practice. A recent large study evaluating a well-known commercially available AI algorithm performance on FFDM versus DBT found significantly diminished levels of performance for the AI algorithm with the DBT data [
24]. The AI algorithm met or surpassed radiologist performance for FFDM but generated a markedly higher and undesirable false positive rate with DBT images illustrating both the challenges of this data and the difficulty in generalizing performance across settings [
24].
The high level of interest and focus on developing improved AI algorithms for screening mammography has led to the RSNA Screening Mammography Breast Cancer Detection Competition of 2023 [
25]. This competition will utilize data from the ADMANI data set and should further increase the attention and resources dedicated to breast cancer detection with AI applications [
14].
The development and evaluation of AI tools for cancer detection in breast imaging has overwhelmingly been focused on mammography. This is intuitive given the immense number of mammography exams that are performed worldwide and the relatively standardized nature of mammography. There have however been additional investigations regarding AI to increase cancer detection with breast ultrasound, breast MRI, and contrast enhanced mammography. A recent retrospective reader study evaluating hybrid AI and radiologists' performance in the interpretation of screening and diagnostic breast ultrasound found preserved sensitivity for breast cancer detection with the hybrid AI workflow but, with the advantage of reducing false positives by 37.3%, and decreasing benign biopsies by 27.8% for screening ultrasound [
26]. Screening breast ultrasound can be performed using a handheld technique or an automated technique. There are several benefits of automated breast ultrasound technology for screening however, the studies typically contain significantly greater than 1000 images which presents a significant challenge for radiologists’ efficiency and failing to detect a meaningful finding. A commercially available AI powered application is available for use as CAD in automated breast ultrasound studies which may be able to help address these challenges for automated breast ultrasound screening [
27]. AI applications for cancer detection with breast MRI are also in development including a recent study reporting non-inferiority between breast radiologists and an AI system for identifying malignancy in breast MRI [
28]. Contrast enhanced mammography is another important supplemental screening modality whose use is evolving rapidly. A DL model developed to evaluate contrast enhanced mammography images demonstrated excellent performance and radiologist use of the AI model led to significantly improved performance metrics for radiologists in the study [
29].
Decision Support:
Improving the efficacy of breast imaging interpretations is not restricted to cancer detection on screening exams. There is also a need to improve radiologist’s diagnostic performance when a lesion or area of interest is identified. Many opportunities for improvement are available in the realm of decision support, including limiting benign biopsy recommendations and minimizing false negative interpretations. Applications of decision support have been studied in several different scenarios across various breast imaging modalities. A study evaluating an AI based clinical decision support application for DBT found that radiologists using the decision support were able to increase sensitivity while preserving specificity, thus reducing the likelihood of false negative interpretations without increasing benign biopsy recommendations [
30]. A separate investigation evaluating AI decision support for mammography evaluated radiologist performance categorizing masses finding improved AUC when using AI decision support with both increased sensitivity and specificity [
31]. The authors also found that more junior radiologists made more interpretive adjustments for masses that were suspicious when using AI decision support, suggesting experience or confidence may be an important potential variable for the impact of decision support. Another study evaluating an AI based algorithm used images and clinical factors for predicting malignancy of suspicious microcalcifications seen on mammography, a common diagnostic problem encountered in breast imaging, demonstrating non-inferior diagnostic performance compared to a senior breast radiologist and outperforming junior radiologists [
32].
Decision support opportunities in breast imaging extend beyond mammography. A common diagnostic problem encountered is appropriately stratifying breast masses identified on ultrasound as either benign, needing short term follow up or requiring biopsy for tissue diagnosis. A multicenter retrospective review of a commercially available AI breast ultrasound decision support application found that radiologist reader performance increased significantly when using the AI decision support with an AUC increasing from 0.83 without decision support to 0.87 with decision support [
33]. Interestingly, the same study found that using decision support can reduce intrareader variability, providing an opportunity to standardize interpretive performance [
33]. In a recent retrospective study evaluating decision support for breast MRI, radiologist readers from academic and private practice centers compared radiologists reading with conventional MRI CAD software versus AI based MRI CAD software. The study found the AUC significantly improved with AI algorithm for all readers with an average improvement from 0.71 to 0.76 [
34]. These findings suggest a role for improving diagnostic performance within the context of complex breast MRI interpretations.
Breast Density:
Breast density reflects the mammographic amount of fibroglandular tissue in the breast, designated into four categories by BI-RADS: a) almost entirely fatty, b) scattered areas of fibroglandular density, c) heterogeneously dense, which may obscure small masses, and d) extremely dense which lowers the sensitivity of mammography. Approximately 40% of women in the United States have dense breasts, designated as category c and d [
35]. Breast density is an independent risk factor for breast cancer, with at least a moderate association with breast cancer risk [
36]. Due to this elevated risk, and decreased sensitivity of mammography, women with dense breasts may benefit from supplemental screening with modalities such as breast ultrasound, contrast-enhanced mammography, molecular breast imaging, or breast MRI. In most states across the United States, women are required by law to be notified of their breast density. Recently, the FDA issued a national requirement for breast density notification, which will go into effect in September 2024 [
37].
The accuracy of breast density reporting can be subject to interpersonal and intrapersonal variability amongst radiologists, highlighting the value of computer-based assessment in the standardization of breast density reporting. Early iterations required manual input to outline and define breast tissue density [
38]. Numerous fully automated DL algorithms are now available which use convolutional neural networks to define breast density, demonstrating high levels of accuracy in stratifying dense and non-dense breasts. For example, an externally validated algorithm demonstrated 89% accuracy in stratifying non-dense and dense breasts, with a 90% agreement between the algorithm and three independent readers [
39]. Other models have also demonstrated high levels of agreement in clinical use in binary categorization of dense and non-dense breasts, with 94% agreement amongst radiologists with the DL algorithm when evaluating greater than 10,000 mammography examinations [
40]. Diagnostic accuracy can be maintained in algorithms assessing breast density in synthetic mammograms, demonstrating an accuracy of 89.6% when differentiating dense and non-dense breasts [
41]. However, the possibility for altered performance of automated breast density assessments exists when moving from FFDM images to synthetic mammography including complex potential interactions with ethnicity and body mass index that require awareness and attention [
42]. Numerous FDA approved algorithms for quantification of breast density are currently available for use including some widely used in clinical practice (
Table 2). A study comparing mammographic density assessment in these models demonstrated the percentage density measured by some specific commercially available algorithms
also had a strong association with breast cancer risk, suggesting there may be utility in automated density assessment in cancer risk stratification [
43]
.
Cancer Risk Assessment:
Identifying women at increased risk of breast cancer is as an important assessment when determining the need for additional screening and preventative intervention. Current risk assessment models estimate the average risk of breast cancer for women with similar risk factors, as opposed to individual breast cancer risk. These models include the Gail Model (BCRAT), Tyrer-Cuzick model (IBIS),
Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm model (BOADICEA), BRCAPRO, and Breast Cancer Surveillance Consortium model (BCSC). Each of these models account for different factors such as age, age of menarche, obstetric history, first degree and multi-generational relatives with breast cancer, genetic information, number of previous biopsies, race and ethnicity, and body mass index amongst other factors. The models calculate 5-year, 10-year, or lifetime risk of breast cancer and are used to identify women who may benefit from supplemental high-risk screening for breast cancer, chemoprevention, or lifestyle modifications. As the different models rely on unique combinations of risk factors, including some factors while excluding others, there are several limitations to a sole model being used to independently predict cancer risk. For example, the Gail model can underestimate the risk of breast cancer in women with familial history of breast cancer, personal history of atypia, and in non-American and non-European populations [
44]. In a study evaluating the 10-year performance of the Gail, Tyrer-Cuzick, BOADICEA, BRCAPRO models, the authors identified that the integration of multigenerational family history, such as in the Tyrer-Cuzick and BOADICEA models, better demonstrates the ability to predict breast cancer risk [
45]. This analysis also suggested that a hybrid model incorporating various factors from each of these models may help improve accuracy in breast cancer detection risk. A separate cohort analysis comparing these five risk assessment models in 35,000 women over 6 years demonstrated similar, moderate predicative accuracy and good overall calibration amongst the models (AUC 0.61-0.64) [
46].
New developments in AI image-based risk models demonstrate promising results in cancer risk assessment, in some instances out-performing traditional cancer risk assessment models. A case-cohort study of an AI image-based mammography risk model assessed short-term and long-term performance of its model compared to Tyrer-Cuzick version 8 over a period of 10 years [
47]. The image based AI model outperformed Tyrer-Cuzick in both short-term and long-term assessment when evaluating approximately 8,600 women with age-adjusted AUC AI model performances ranging from 0.74 to 0.65 for breast cancers developed in 1 to 10 years, significantly exceeding the Tyrer-Cuzick age-adjusted AUCs of 0.62 to 0.60 in this time period [
47].
Mirai, a DL mammography-based risk model, incorporates digital mammographic features along with clinical factor inputs to provide breast cancer risk prediction within 5 years, and was recently validated across a broad, diverse international data set [
48]. Approximately 128,000 screening mammograms and pathologically confirmed breast cancers across 7 international sites in 5 countries including the United States, Israel, Sweden, Taiwan, and Brazil were evaluated [
48]. Of the 62,185 unique patients, 3,815 patients were diagnosed with breast cancer within 5 years of the index screening mammogram, with Mirai obtaining concordance indices of >/=0.75 and AUC performance of 0.75 for White women (0.71-0.78, 95% CI) and 0.78 for Black women (0.75-0.82, 95% CI), outperforming traditional cancer risk models. Such a model demonstrates the promise of an AI cancer risk assessment tool to significantly improve the accuracy of breast cancer risk assessments. Moreover, making personalized AI image-based assessments is an opportunity for improved performance for all ethnicities and groups, including those where previous risk assessment models did not perform as well.
Workflow Applications:
AI based triage tools can be used to prioritize patients and improve overall workflow for radiologists interpreting breast imaging studies. This has been most well-studied with screening mammography. Using AI based triage algorithms, a retrospective simulation study in which AI-based screening (normal – no radiologist, moderate risk – radiologist review, and suspicious – recalled) was compared to radiologist screening found non-inferior sensitivity and higher specificity (with 25.1% reduction in false positives) [
49]. The findings of this study were achieved while simultaneously achieving a workload reduction of 62.6%, with triaged normal studies read only by the AI system. Similarly, another retrospective simulation study showed that using AI to triage mammograms into no radiologist assessment and enhanced assessment categories could potentially reduce workload by more than 50% and preemptively detect a substantial proportion of cancers otherwise diagnosed later [
50]. These findings suggest a novel potential way of integrating AI based cancer detection into clinical workflows to preserve or improve clinical performance while reducing workloads. The implications for this type of workflow may differ between screening programs with a single versus a double reader paradigm.
Another study evaluating an AI system used in the detection of lesions on DBT found that when the algorithm was concurrently incorporated into the interpretation of the mammograms, it reduced reading times by approximately half while still improving accuracy with a statistically significant 0.057 average improvement in AUC [
51]. As reading times with DBT are significantly longer than with FFDM this provides an opportunity for increased efficiency which is particularly important given the current shortage of trained radiologists who can interpret mammograms. An alternative approach for improving interpretive efficiency for DBT is the replacement of traditional 1 mm thin tomosynthesis slices with 6 mm thick overlapping slices that has been implemented by a mainstream mammography modality manufacturer [
50]. These thick slices are created in part by using AI algorithms to make salient suspicious findings more conspicuous [
50]. This should allow for increased efficiency in interpretation of DBT by reducing the number of slices for review. By using these triage tools, radiology practices could prioritize examinations to be read immediately, categorize cases by complexity, and replace the second reader at sites offering double reading to enhance radiologist’s workflow [
53]. As of today, there are multiple commercially available algorithms that can assist in triage of mammographic interpretation (
Table 2).
Quality Assessment:
The importance of maintaining high quality positioning and technique has long been a focus for mammography. MQSA includes significant focus on ensuring standardization and quality for mammography in the United States. Poor positioning is often identified as a leading cause of clinical imaging deficiencies and misdiagnosis [
54]. This has led to the recently implemented FDA EQUIP initiative that began in 2017 to emphasize and focus on ensuring and improving quality for the effective performance of mammography. The need for uniform high quality mammographic technique and positioning creates an opportunity for AI algorithms to evaluate mammography exams and provide feedback and opportunities for improvement for performing technologists and interpreting physicians. A recent study found an AI algorithm could assess breast positioning on mammography to evaluate for common issues that can lead to inadequate positioning such as nipple in profile, breast rotation, visualization of the pectoral muscle, inframammary fold, and the pectoral nipple line with the algorithm being highly accurate in identifying these deficiencies [
55] Additional research studying the application of AI to breast positioning assessment has looked to replicate additional quality assessment tasks performed by radiologists when interpreting mammograms in hopes of standardizing the detection of these issues, finding some success as well [
56]. In fact, there is currently a commercially available application that utilizes AI to help evaluate, track and improve quality in mammographic positioning [
57].
Neoadjuvant Chemotherapy Response:
AI may also be used to assess treatment response to neoadjuvant chemotherapy for breast cancer. Neoadjuvant chemotherapy (chemotherapy given prior to surgery) can reduce tumor size, allowing for less invasive surgical procedures. It also enables in vivo evaluation of treatment response, allowing therapeutic treatment plans to be modified based on each patient’s individual response [
58]. Despite its relatively low sensitivity (63-88%) and specificity (54-91%), MRI is currently the most accurate imaging method for determining tumor response to neoadjuvant therapy [
59]. Recent research has demonstrated that AI has the potential to improve treatment response prediction. A meta-analysis by Liang et al. found that ML and MRI are highly accurate (AUC = 0.87, 95% CI = 0.84 to 0.91) in predicting response to neoadjuvant therapy [
60].
AI applied to imaging may predict tumor response to treatment prior to the
initiation of neoadjuvant chemotherapy. A proof-of-concept study by Skarping et al. demonstrated the effectiveness of a DL based model using baseline digital mammograms to predict patient’s response to neoadjuvant therapy, with an AUC of .71 [
61]. Their model predicted tumor response by deciphering breast parenchymal patterns and tumor appearances as reflected by different grey-level pixel presentations in digital mammography. This type of platform may help aid in clinical decision making prior to administering chemotherapy, significantly reducing patient morbidity. Likewise, a study evaluating ultrasound images of primary breast cancer in clinically node negative patients was able to predict the likelihood of having lymph node metastases at surgery with a high level of accuracy [
62]. These findings demonstrate the evolving possibilities for AI based applications to positively predict patient outcomes and may provide opportunities to individually tailor and improve patient care.
Image Enhancement:
There have been several recent novel investigations and developments using AI algorithms to enhance the appearance of images in breast imaging. One creative example is the use of an AI based process that first involved collapsing or merging suspicious regions of interest from DBT into ‘maximum suspicion projections’ that emphasizes the suspicious findings making them more conspicuous [
63]. These novel synthesized images are then used as an input for an AI cancer detection model to detect breast cancers reducing the burden for image and data preparation. Along the same lines of this approach, a major mammography modality vendor now has a commercially available AI based application that emphasizes features that are likely to be important for accurate imaging review such as bright foci which may represent calcifications, lines that can represent distortion, or rounded objects that may be masses [
52]. This information is derived from 1 mm slices from the DBT images but is then combined into overlapping thick 6 mm slabs that can significantly decrease the number of slices that need reviewed while still preserving the visibility of salient findings [
52].
Additional investigations have evaluated using AI applications to reduce the amount of intravenous contrast dose needed for breast MRI [
64]. This is especially important given the current recommendations for serial annual supplemental screening breast MRIs for patients at high risk for breast cancer and recent focus on the possibility of gadolinium retention. A recent study demonstrated an AI algorithm that was developed using a data set of breast MRI images with and without contrast. This AI model was then given inputs consisting only of non-contrast images from breast MRI studies and was able to generate simulated contrast enhanced breast MRI images [
65]. These simulated images were felt to be quantitatively similar and demonstrated high level of tumor overlap with the true contrast enhanced breast MRI images, with 95% of images found to be of diagnostic quality by the study radiologists. These developments demonstrate the power of AI applications to create clinical value and novel potential workflows using minimal or limited data sets.
Discussion and Future Directions:
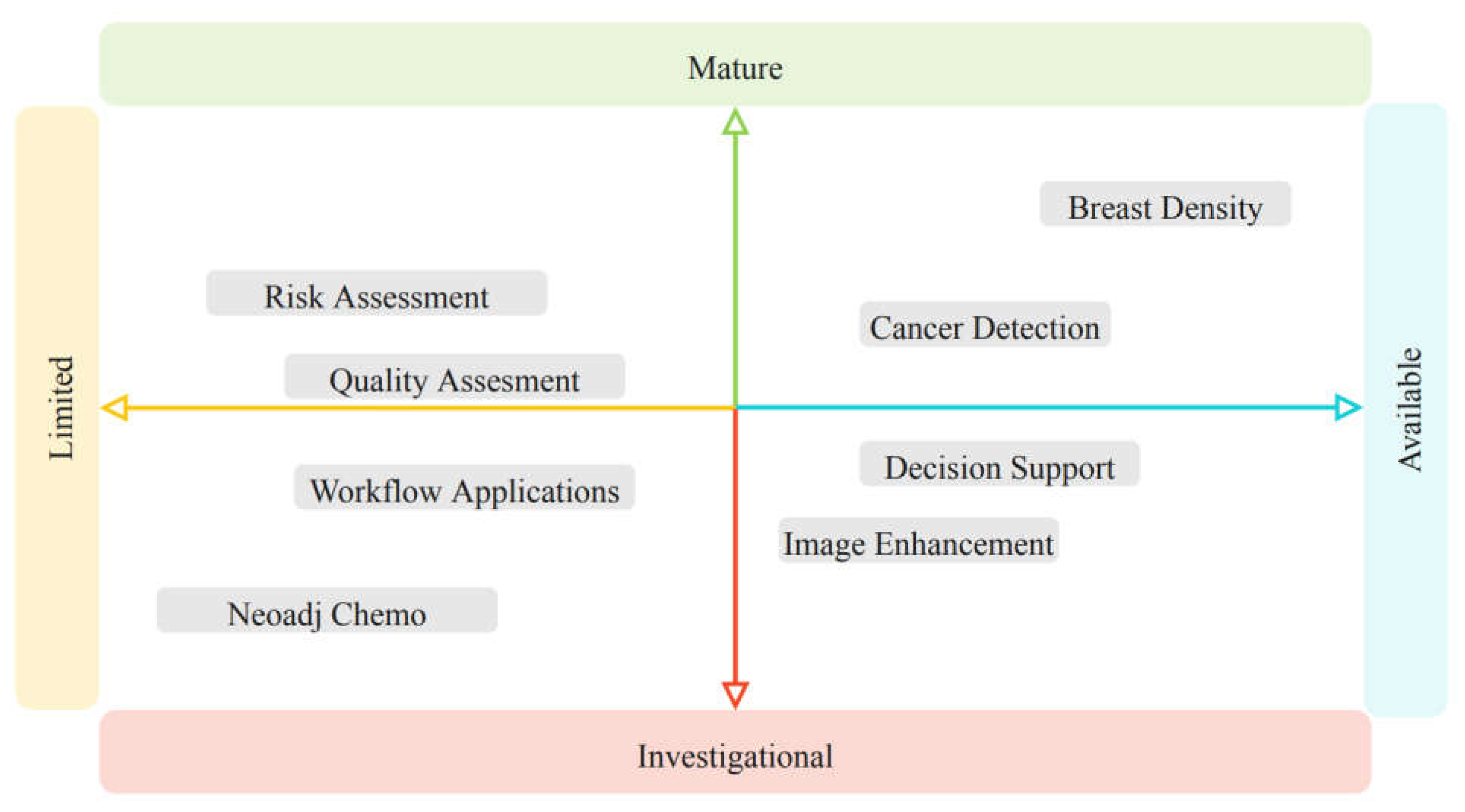
There are at least 20 available FDA AI based applications available today for breast imaging (
Table 2). Beyond currently commercially available applications, there are many more in development with potential applications for breast imaging at various degrees of maturity and availability (
Figure 1).
However, there are significant barriers to implementation of AI applications in breast imaging including inconsistent performance, significant cost, and IT requirements, along with the lack of radiologist, patient and referring provider familiarity and trust [
66]. Additionally, there are meaningful concerns for the generalizability of AI algorithms in breast imaging with a recent publication showing significant performance degradation of an AI algorithm that was trained using images from a specific manufacturer when tested using an updated system/software from that same manufacturer [
67]. This required site specific modification of the algorithm to improve performance. These issues demonstrate a more general concern for the ability of AI applications to generate consistent and uniform results between sites and clinical scenarios. This reinforces the need for careful evaluation of applications for each site and close monitoring of performance. Further complicating adoption is the lack of reimbursement for AI applications in breast imaging, which may drive focus and adoption towards applications that can provide convincing workflow or efficiency gains to counterbalance the costs of adoption and implementation.
During the first few months of 2023 there has been tremendous excitement and focus on a handful of AI natural language processing models, specifically large language models, that seem poised to generate evolutionary and disruptive change throughout many different fields and industries. ChatGPT, a conversation large language model, is perhaps the most well-known and discussed of these models that is extremely successful at automatically summarizing large inputs of information and answering questions in a conversational manner. Potential applications within breast imaging may include imaging appropriateness and clinical decision support, preauthorization needs, generating reports, summarizing information from the electronic medical record, and creating interactive computer aiding detection applications [
68]. Given the high degree of contact of breast imaging with patients and general population applications like ChatGPT, large language models may provide value in shaping and guiding patient interaction and education for breast imaging topics in the future.
It will be essential to build and shape the trust and perceptions of patients and referring providers towards AI in breast imaging. Patient attitudes and perceptions regarding AI in radiology are complex and include distrust and accountability, concerns about procedural knowledge, a preference for preserving personal interaction, efficiency, and remaining informed about use [
69]. More generally, approximately 50% of women of screening age (over 50) in England report positive feelings about the use of AI in reading mammograms, with the remainder being neutral or reporting negative feelings [
70]. This data suggests that there will be significant future work towards educating patients on how AI can be implemented in breast imaging and keeping patients aware of the benefits and limitations of its use.
References
- Breast Cancer Statistics | How Common Is Breast Cancer? Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 10 March 2023).
- Health C for D and, R.; MQSA National Statistics. FDA [Internet]. 2023. Available online: https://www.fda.gov/radiation-emitting-products/mqsa-insights/mqsa-national-statistics (accessed on 10 March 2023).
- Lehman, C.D.; Arao, R.F.; Sprague, B.L.; Lee, J.M.; Buist, D.S.M.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Tosteson, A.N.A.; Rauscher, G.H.; et al. National Performance Benchmarks for Modern Screening Digital Mammography: Update from the Breast Cancer Surveillance Consortium. Radiology 2017, 283, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Wellman, R.D.; Buist, D.S.M.; Kerlikowske, K.; Tosteson, A.N.A.; Miglioretti, D.L.; for the Breast Cancer Surveillance Consortium Diagnostic Accuracy of Digital Screening Mammography With and Without Computer-Aided Detection. JAMA Intern. Med. 2015, 175, 1828–1837. [CrossRef] [PubMed]
- Fenton, J.J.; Taplin, S.H.; Carney, P.A.; Abraham, L.; Sickles, E.A.; D’Orsi, C.; Berns, E.A.; Cutter, G.; Hendrick, R.E.; Barlow, W.E.; et al. Influence of Computer-Aided Detection on Performance of Screening Mammography. N. Engl. J. Med. 2007, 356, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.J.; Abraham, L.; Taplin, S.H.; Geller, B.M.; Carney, P.A.; D’Orsi, C.; Elmore, J.G.; Barlow, W.E.; for the Breast Cancer Surveillance Consortium Effectiveness of Computer-Aided Detection in Community Mammography Practice. JNCI J. Natl. Cancer Inst. 2011, 103, 1152–1161.
- Keen, J.D.; Keen, J.M.; Keen, J.E. Utilization of Computer-Aided Detection for Digital Screening Mammography in the United States, 2008 to 2016. J. Am. Coll. Radiol. 2018, 15, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep Learning: A Primer for Radiologists. RadioGraphics 2017, 37, 2113–2131. [Google Scholar] [CrossRef] [PubMed]
- Breast Imaging Reporting & Data System. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed on 30 March 2023).
- Mammography Quality Standards Act and Program | FDA. Available online: https://www.fda.gov/radiation-emitting-products/mammography-quality-standards-act-and-program (accessed on 30 March 2023).
- Jeong, J.J.; Vey, B.L.; Bhimireddy, A.; Kim, T.; Santos, T.; Correa, R.; Dutt, R.; Mosunjac, M.; Oprea-Ilies, G.; Smith, G.; et al. The EMory BrEast imaging Dataset (EMBED): A Racially Diverse, Granular Dataset of 3.4 Million Screening and Diagnostic Mammographic Images. Radiol. Artif. Intell. 2023, 5, e220047. [Google Scholar] [CrossRef] [PubMed]
- Halling-Brown, M.D.; Warren, L.M.; Ward, D.; Lewis, E.; Mackenzie, A.; Wallis, M.G.; Wilkinson, L.S.; Given-Wilson, R.M.; McAvinchey, R.; Young, K.C. OPTIMAM Mammography Image Database: A Large-Scale Resource of Mammography Images and Clinical Data. Radiol. Artif. Intell. 2021, 3, e200103. [Google Scholar] [CrossRef]
- Dembrower, K.; Lindholm, P.; Strand, F. A Multi-million Mammography Image Dataset and Population-Based Screening Cohort for the Training and Evaluation of Deep Neural Networks-the Cohort of Screen-Aged Women (CSAW). J. Digit. Imaging 2020, 33, 408–413. [Google Scholar] [CrossRef]
- Frazer, H.M.L.; Tang, J.S.N.; Elliott, M.S.; Kunicki, K.M.; Hill, B.; Karthik, R.; Kwok, C.F.; Peña-Solorzano, C.A.; Chen, Y.; Wang, C.; et al. ADMANI: Annotated Digital Mammograms and Associated Non-Image Datasets. Radiol. Artif. Intell. 2023, 5, e220072. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, S.P.; Sprague, B.L.; Weaver, D.L.; Herschorn, S.D.; Conant, E.F. Survey Results Regarding Uptake and Impact of Synthetic Digital Mammography With Tomosynthesis in the Screening Setting. J. Am. Coll. Radiol. JACR 2020, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H.; Back, T.; Chesus, M.; Corrado, G.S.; Darzi, A.; et al. International evaluation of an AI system for breast cancer screening. Nature 2020, 577, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Schaffter, T.; Buist, D.S.M.; Lee, C.I.; Nikulin, Y.; Ribli, D.; Guan, Y.; Lotter, W.; Jie, Z.; Du, H.; Wang, S.; et al. Evaluation of Combined Artificial Intelligence and Radiologist Assessment to Interpret Screening Mammograms. JAMA Netw. Open 2020, 3, e200265. [Google Scholar] [CrossRef]
- Wu, N.; Phang, J.; Park, J.; Shen, Y.; Huang, Z.; Zorin, M.; Jastrzebski, S.; Fevry, T.; Katsnelson, J.; Kim, E.; et al. Deep Neural Networks Improve Radiologists’ Performance in Breast Cancer Screening. IEEE Trans. Med. Imaging 2020, 39, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Geppert, J.; Stinton, C.; Todkill, D.; Johnson, S.; Clarke, A.; Taylor-Phillips, S. Use of artificial intelligence for image analysis in breast cancer screening programmes: systematic review of test accuracy. BMJ 2021, 374, n1872. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.J.J.; Oakden-Rayner, L.; Hall, K.A.; Reintals, M.; Holmes, A.; Carneiro, G.; Palmer, L.J. Replication of an open-access deep learning system for screening mammography: Reduced performance mitigated by retraining on local data [Internet]. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.05.28.21257892v1 (accessed on 30 March 2023). [CrossRef]
- External Evaluation of 3 Commercial Artificial Intelligence Algorithms for Independent Assessment of Screening Mammograms | Breast Cancer | JAMA Oncology | JAMA Network. Available online: https://jamanetwork.com/journals/jamaoncology/fullarticle/2769894 (accessed on 27 February 2023).
- Anderson, A.W.; Marinovich, M.L.; Houssami, N.; Lowry, K.P.; Elmore, J.G.; Buist, D.S.M.; Hofvind, S.; Lee, C.I. Independent External Validation of Artificial Intelligence Algorithms for Automated Interpretation of Screening Mammography: A Systematic Review. J. Am. Coll. Radiol. JACR 2022, 19, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.; Hippe, D.S.; Nakhaei, N.; Wang, P.-C.; Zhu, B.; Siu, N.; Ahsen, M.E.; Lotter, W.; Sorensen, A.G.; Naeim, A.; et al. External Validation of an Ensemble Model for Automated Mammography Interpretation by Artificial Intelligence. JAMA Netw. Open 2022, 5, e2242343. [Google Scholar] [CrossRef]
- Romero-Martín, S.; Elías-Cabot, E.; Raya-Povedano, J.L.; Gubern-Mérida, A.; Rodríguez-Ruiz, A.; Álvarez-Benito, M. Stand-Alone Use of Artificial Intelligence for Digital Mammography and Digital Breast Tomosynthesis Screening: A Retrospective Evaluation. Radiology 2022, 302, 535–542. [Google Scholar] [CrossRef]
- RSNA Screening Mammography Breast Cancer Detection. Available online: https://kaggle.com/competitions/rsna-breast-cancer-detection (accessed on 30 March 2023).
- Shen, Y.; Shamout, F.E.; Oliver, J.R.; Witowski, J.; Kannan, K.; Park, J.; Wu, N.; Huddleston, C.; Wolfson, S.; Millet, A.; et al. Artificial intelligence system reduces false-positive findings in the interpretation of breast ultrasound exams. Nat. Commun. 2021, 12, 5645. [Google Scholar] [CrossRef]
- QView Medical. Available online: https://www.qviewmedical.com (accessed on 17 April 2023).
- Witowski, J.; Heacock, L.; Reig, B.; Kang, S.K.; Lewin, A.; Pyrasenko, K.; Patel, S.; Samreen, N.; Rudnicki, W.; Łuczyńska, E.; et al. Improving breast cancer diagnostics with artificial intelligence for MRI [Internet]. medRxiv 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.02.07.22270518v1 (accessed on 12 January 2023). [CrossRef]
- Mao, N.; Zhang, H.; Dai, Y.; Li, Q.; Lin, F.; Gao, J.; Zheng, T.; Zhao, F.; Xie, H.; Xu, C.; et al. Attention-based deep learning for breast lesions classification on contrast enhanced spectral mammography: a multicentre study. Br. J. Cancer 2023, 128, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.; Rodriguez-Ruiz, A.; Pedersen, K.; Hofvind, S.; Wicklein, J.; Kappler, S.; Mann, R.M.; Sechopoulos, I. Impact of Artificial Intelligence Decision Support Using Deep Learning on Breast Cancer Screening Interpretation with Single-View Wide-Angle Digital Breast Tomosynthesis. Radiology 2021, 300, 529–536. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Y.; Zeng, W.; Xu, W.; Liu, J.; Ma, X.; Wei, J.; Zeng, H.; Xu, Z.; Wang, S.; et al. Can a Computer-Aided Mass Diagnosis Model Based on Perceptive Features Learned From Quantitative Mammography Radiology Reports Improve Junior Radiologists’ Diagnosis Performance? An Observer Study. Front. Oncol. 2021, 11, 773389. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.; Zhang, Y.; Wang, L.; Luo, R.; Wu, H.; Wu, C.; Zhang, H.; Tan, W.; Yin, H.; et al. A deep learning model integrating mammography and clinical factors facilitates the malignancy prediction of BI-RADS 4 microcalcifications in breast cancer screening. Eur. Radiol. 2021, 31, 5902–5912. [Google Scholar] [CrossRef] [PubMed]
- Mango, V.L.; Sun, M.; Wynn, R.T.; Ha, R. Should We Ignore, Follow, or Biopsy? Impact of Artificial Intelligence Decision Support on Breast Ultrasound Lesion Assessment. Am. J. Roentgenol. 2020, 214, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Edwards, A.V.; Newstead, G.M. Artificial Intelligence Applied to Breast MRI for Improved Diagnosis. Radiology 2021, 298, 38–46. [Google Scholar] [CrossRef]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of Mammographically Dense Breasts in the United States. JNCI J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Bovbjerg, V.E. Quantitative Assessment of Mammographic Breast Density: Relationship with Breast Cancer Risk. Radiology 2004, 230, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Commissioner, O. of the FDA Updates Mammography Regulations to Require Reporting of Breast Density Information and Enhance Facility Oversight. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-mammography-regulations-require-reporting-breast-density-information-and-enhance (accessed on 17 April 2023).
- Nguyen, T.L.; Aung, Y.K.; Evans, C.F.; Yoon-Ho, C.; Jenkins, M.A.; Sung, J.; Hopper, J.L.; Song, Y.-M. Mammographic density defined by higher than conventional brightness threshold better predicts breast cancer risk for full-field digital mammograms. Breast Cancer Res. 2015, 17, 142. [Google Scholar] [CrossRef]
- Magni, V.; Interlenghi, M.; Cozzi, A.; Alì, M.; Salvatore, C.; Azzena, A.A.; Capra, D.; Carriero, S.; Della Pepa, G.; Fazzini, D.; et al. Development and Validation of an AI-driven Mammographic Breast Density Classification Tool Based on Radiologist Consensus. Radiol. Artif. Intell. 2022, 4, e210199. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Yala, A.; Schuster, T.; Dontchos, B.; Bahl, M.; Swanson, K.; Barzilay, R. Mammographic Breast Density Assessment Using Deep Learning: Clinical Implementation. Radiology 2019, 290, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sexauer, R.; Hejduk, P.; Borkowski, K.; Ruppert, C.; Weikert, T.; Dellas, S.; Schmidt, N. Diagnostic accuracy of automated ACR BI-RADS breast density classification using deep convolutional neural networks. Eur. Radiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gastounioti, A.; McCarthy, A.M.; Pantalone, L.; Synnestvedt, M.; Kontos, D.; Conant, E.F. Effect of Mammographic Screening Modality on Breast Density Assessment: Digital Mammography versus Digital Breast Tomosynthesis. Radiology 2019, 291, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Astley, S.M.; Harkness, E.F.; Sergeant, J.C.; Warwick, J.; Stavrinos, P.; Warren, R.; Wilson, M.; Beetles, U.; Gadde, S.; Lim, Y.; et al. A comparison of five methods of measuring mammographic density: a case-control study. Breast Cancer Res. BCR 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Vianna, F.S.L.; Giacomazzi, J.; Oliveira Netto, C.B.; Nunes, L.N.; Caleffi, M.; Ashton-Prolla, P.; Camey, S.A. Performance of the Gail and Tyrer-Cuzick breast cancer risk assessment models in women screened in a primary care setting with the FHS-7 questionnaire. Genet. Mol. Biol. 2019, 42, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Liao, Y.; Whittemore, A.S.; Leoce, N.; Buchsbaum, R.; Zeinomar, N.; Dite, G.S.; Chung, W.K.; Knight, J.A.; Southey, M.C.; et al. 10-year performance of four models of breast cancer risk: a validation study. Lancet Oncol. 2019, 20, 504–517. [Google Scholar] [CrossRef]
- McCarthy, A.M.; Guan, Z.; Welch, M.; Griffin, M.E.; Sippo, D.A.; Deng, Z.; Coopey, S.B.; Acar, A.; Semine, A.; Parmigiani, G.; et al. Performance of Breast Cancer Risk-Assessment Models in a Large Mammography Cohort. JNCI J. Natl. Cancer Inst. 2020, 112, 489–497. [Google Scholar] [CrossRef]
- Eriksson, M.; Czene, K.; Vachon, C.; Conant, E.F.; Hall, P. Long-Term Performance of an Image-Based Short-Term Risk Model for Breast Cancer. J. Clin. Oncol. 2023, 22, 01564. [Google Scholar] [CrossRef]
- Yala, A.; Lehman, C.; Schuster, T.; Portnoi, T.; Barzilay, R. A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction. Radiology 2019, 292, 60–66. [Google Scholar] [CrossRef]
- Lauritzen, A.D.; Rodríguez-Ruiz, A.; von Euler-Chelpin, M.C.; Lynge, E.; Vejborg, I.; Nielsen, M.; Karssemeijer, N.; Lillholm, M. An Artificial Intelligence–based Mammography Screening Protocol for Breast Cancer: Outcome and Radiologist Workload. Radiology 2022, 304, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dembrower, K.; Wåhlin, E.; Liu, Y.; Salim, M.; Smith, K.; Lindholm, P.; Eklund, M.; Strand, F. Effect of artificial intelligence-based triaging of breast cancer screening mammograms on cancer detection and radiologist workload: a retrospective simulation study. Lancet Digit. Health 2020, 2, e468–e474. [Google Scholar] [CrossRef] [PubMed]
- Conant, E.F.; Toledano, A.Y.; Periaswamy, S.; Fotin, S.V.; Go, J.; Boatsman, J.E.; Hoffmeister, J.W. Improving Accuracy and Efficiency with Concurrent Use of Artificial Intelligence for Digital Breast Tomosynthesis. Radiol. Artif. Intell. 2019, 1, e180096. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Kshirsagar, A.; Smith, A. 3DQuorumTM Imaging Technology. Available online: https://www.hologic.com/sites/default/files/downloads/WP-00152_Rev001_3DQuorum_Imaging_Technology_Whitepaper%20%20(1).pdf.
- Lamb, L.R.; Lehman, C.D.; Gastounioti, A.; Conant, E.F.; Bahl, M. Artificial Intelligence (AI) for Screening Mammography, From the AJR Special Series on AI Applications. Am. J. Roentgenol. 2022, 219, 369–380. [Google Scholar] [CrossRef] [PubMed]
- 2022-12-27 08:32 | Archive of FDA. Available online: https://public4.pagefreezer.com/browse/FDA/27-12-2022T08:32/https://www.fda.gov/radiation-emitting-products/mqsa-insights/poor-positioning-responsible-most-clinical-image-deficiencies-failures (accessed on 17 April 2023).
- Brahim, M.; Westerkamp, K.; Hempel, L.; Lehmann, R.; Hempel, D.; Philipp, P. Automated Assessment of Breast Positioning Quality in Screening Mammography. Cancers 2022, 14, 4704. [Google Scholar] [CrossRef]
- Deep Learning Based Automatic Detection of Adequately Positioned Mammograms | SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-3-030-87722-4_22 (accessed on 10 April 2023).
- Optimized Program Performance – Volpara Health. Available online: https://www.volparahealth.com/breast-health-platform/optimized-program-performance/ (accessed on 17 April 2023).
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of Imaging in Neoadjuvant Therapy for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
- Imaging Neoadjuvant Therapy Response in Breast Cancer | Radiology. Available online: https://pubs.rsna.org/doi/full/10.1148/radiol.2017170180 (accessed on 18 April 2023).
- Liang, X.; Yu, X.; Gao, T. Machine learning with magnetic resonance imaging for prediction of response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2022, 150, 110247. [Google Scholar] [CrossRef]
- Skarping, I.; Larsson, M.; Förnvik, D. Analysis of mammograms using artificial intelligence to predict response to neoadjuvant chemotherapy in breast cancer patients: proof of concept. Eur. Radiol. 2022, 32, 3131–3141. [Google Scholar] [CrossRef]
- Zhou, L.-Q.; Wu, X.-L.; Huang, S.-Y.; Wu, G.-G.; Ye, H.-R.; Wei, Q.; Bao, L.-Y.; Deng, Y.-B.; Li, X.-R.; Cui, X.-W.; et al. Lymph Node Metastasis Prediction from Primary Breast Cancer US Images Using Deep Learning. Radiology 2020, 294, 19–28. [Google Scholar] [CrossRef]
- Lotter, W.; Diab, A.R.; Haslam, B.; Kim, J.G.; Grisot, G.; Wu, E.; Wu, K.; Onieva, J.O.; Boyer, Y.; Boxerman, J.L.; et al. Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach. Nat. Med. 2021, 27, 244–249. [Google Scholar] [CrossRef]
- Müller-Franzes, G.; Huck, L.; Tayebi Arasteh, S.; Khader, F.; Han, T.; Schulz, V.; Dethlefsen, E.; Kather, J.N.; Nebelung, S.; Nolte, T.; et al. Using Machine Learning to Reduce the Need for Contrast Agents in Breast MRI through Synthetic Images. Radiology 2023, 222211. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Calabrese, E.; Mongan, J.; Ray, K.M.; Hayward, J.H.; Kelil, T.; Sieberg, R.; Hylton, N.; Joe, B.N.; Lee, A.Y. Deep Learning to Simulate Contrast-enhanced Breast MRI of Invasive Breast Cancer. Radiology 2023, 306, e213199. [Google Scholar] [CrossRef] [PubMed]
- Bahl, M. Artificial Intelligence in Clinical Practice: Implementation Considerations and Barriers. J. Breast Imaging 2022, 4, 632–639. [Google Scholar] [CrossRef] [PubMed]
- de Vries, C.F.; Colosimo, S.J.; Staff, R.T.; Dymiter, J.A.; Yearsley, J.; Dinneen, D.; Boyle, M.; Harrison, D.J.; Anderson, L.A.; Lip, G.; et al. Impact of Different Mammography Systems on Artificial Intelligence Performance in Breast Cancer Screening. Radiol. Artif. Intell. 2023, e220146. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Heacock, L.; Elias, J.; Hentel, K.D.; Reig, B.; Shih, G.; Moy, L. ChatGPT and Other Large Language Models Are Double-edged Swords. Radiology 2023, 230163. [Google Scholar] [CrossRef]
- Ongena, Y.P.; Haan, M.; Yakar, D.; Kwee, T.C. Patients’ views on the implementation of artificial intelligence in radiology: development and validation of a standardized questionnaire. Eur. Radiol. 2020, 30, 1033–1040. [Google Scholar] [CrossRef]
- Lennox-Chhugani, N.; Chen, Y.; Pearson, V.; Trzcinski, B.; James, J. Women’s attitudes to the use of AI image readers: a case study from a national breast screening programme. BMJ Health Care Inform. 2021, 28, e100293. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).