1. Introduction
Limonene is a typical monoterpene that with diverse functions in food, medicine, pesticide, solvent, fuel, etc [
1,
2,
3,
4,
5,
6,
7]. Due to its wide range of applications, the global demand for limonene continues to grow in the past decade and is expected to USD 248 million by 2028 [
8]. The traditional plant-based extraction is hindered by the limited acreage and vulnerable to seasonal and climatic diversions. The chemical synthesis is unfavored due to high energy consumption, low efficiency, environmental concerns, and toxic impurities, which hinder their largescale applications in certain areas like food and medicine [
1]. With the rapid development of synthetic biology and metabolic engineering, microbial cell factory is becoming a sustainable alternative platform for the production of limonene. Importantly, limonene produced via the microbial technology is generally regarded as safe (GRAS) [
9].
To date, various microbes include
Escherichia coli [
10],
Saccharomyces cerevisiae [
11,
12,
13],
Yarrowia lipolytica[
14,
15] and
Rhodosporidium toruloides [
16] have been employed for limonene production. However, the maximum titer was only 3.63 g/L with engineered
E. coli BL21 (DE3) in a bioreactor [
10]. Therefore, the current maximum productivity and carbon yield are economically infeasible for largescale production [
1]. Generally, there are several aspects that influence the bioproduction of limonene: the choice of efficient microbial host, the enable genetic toolbox for the specific host, the available genetic elements for constructing an efficient heterologous synthetic route and the agreeable cultivation conditions for the recombinant strains. Among which the optimization of fermentation conditions is the last but critical procedure for establishing an industrial profitable process, which is featured with easy operation, low input and high efficiency (titer, yield, and productivity) [
17].
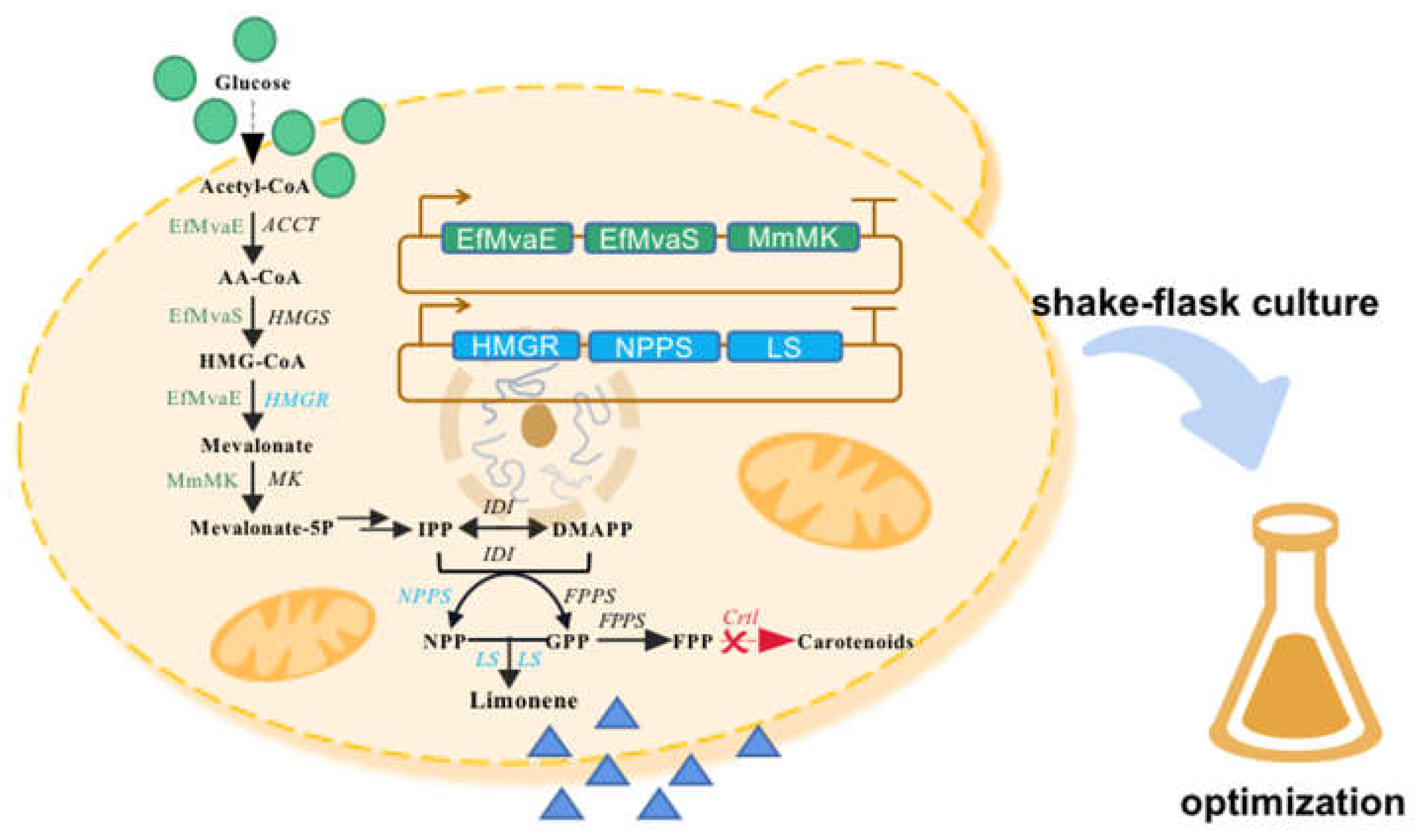
As the unconventional yeast,
R. toruloides can metalize a wide range of low-cost carbon sources to support its cell growth and lipid accumulation. Most interestingly, it is endowed with a naturally robust mevalonate pathway (MVA), which has been rewired for manufacturing various terpenoids including limonene, ent-kaurene bisabolene, and carotenoids [
16,
18,
19,
20,
21]. As in the case of our previous study, the biosynthesis of limonene was achieved by introducing the neryl pyrophosphate synthase (
SlNPPS) from
Solanum lycopersicum, limonene synthase (
CltLS1) from
Citrus limon, acetoacetyl-CoA thiolase (
EfMvaE) and mevalonate synthase (
EfMvaS) from
Enterococcus faecalis, the mevalonate kinase (MmMK) from
Methanosarcina mazei and overexpressing the truncated endogenous hydroxy-methyl-glutaryl-CoA reductase (HMGR). The average titer of only 54.4 mg/L limonene was generated in 250-mL flask [
16]. The undesired results might be caused by inappropriate strain and cultivation conditions.
To tap the potential of the engineered strains and improve the production efficiency, the cultivation conditions were investigated in the present study through single-factor and othogonal design expriments (
Figure 1). First, the recombinant
R. toruloides strains were compared for the stability of limonene production; Second, the effects of carbon source, inoculation, working volume, temperature, pH, dodecane coverage, and media on limonene production were investigated; Finally, the cultivation conditions were assessed via orthogonal experiments. The final limonene titer was improved from 52.5 to 358.1 mg/Lon 250-mL flask, a 586% net increase. Our results here indicated the possibility of elevating limonene production efficiency via operating at fermentation level, and the performance of limonene producing strains should be further enhanced at genetic level.
2. Materials and Methods
2.1. Material and Reagents
Limonene standard was purchased from Sigma-Aldrich (Shanghai, China), and 3, 5-dinitrosalicylic acid, hexane, and dodecane were purchased from Shanghai Kefeng Industry Co., Ltd. (Shanghai, China). All the other reagents were obtained locally. All the genes used in the present study were synthesized in our previous study [
16]. the plasmids: P1, PGK-Ble-Tnos-Pxyl-CltLS1-NPPS-tHMGR-Thsp and P2, PGK-Ntc- Tnos-Pxyl-MmMK-EfMvaS-EfMvaE-Thsp were constructed in our previous study [
16]. SlNPPS: neryl pyrophosphate synthase from
Solanum lycopersicum, CltLS1: limonene synthase from
Citrus limon, the EfMvaE: acetoacetyl-CoA thiolase from
Enterococcus faecalis, EfMvaS: mevalonate synthase from
Enterococcus faecalis, MmMK: mevalonate kinase from
Methanosarcina mazei, tHMGR: truncated endogenous hydroxymethyl glutaryl-CoA reductase [
16]. All the genes were under the control of the xylose reductase promoter, and the strains obtained were constructed in our previous study and preserved in the College of Enology, Northwest A&F University (Shanxi, China) [
16].
2.2. Microbial Strain, Media, and Fermentations
The carotenogenesis-defcient
R. toruloides NP11 was kindly provided by Prof. Zongbao kent Zhao from Dalian Institute of Chemical Physics, CAS, and used as the parental strain. The
R. toruloides engineered strain was constructed through the
Agrobacterium-mediated transformation (ATMT) [
16]. The ATMT experiment was conducted as follows [
16]. First, the vector carrying
Agrobacterium tumefaciens cells were cultivated in LB medium supplemented with 50 μg/mL kanamycin at 28 ℃ for 15 h, and
R. toruloides NP11 cells in YPD medium at 30 ℃for 15 h. Second, cells were collected by centrifugation at 8,000 g for 30 s, then washed twice and diluted with sterilized water to an optical density 0.6 (OD600). For transformation, 100 μL of each cell suspension was mixed, and spread onto the IM agar plates (MM medium with 200 μM aceto- syringone and 20 g/L agar) , and incubated at 25 ℃ for 2 days. Then, the transformant was transferred onto the selection plate to incubate until colony generated. The obtained transformants were streaked on selection YPD for five successive generations to verify their phenotype stability. Finally, the resultant tranformants were subjected for testing limonene production efficiency and stability.
The medium were as follows. YPD liquid medium (g/L): glucose 20, peptone 20, yeast extract 10, (solid YPD was supplied with Agar powder 20). SD medium (g/L): glucose 20, YNB 6.7 (yeast unit without amino acids). MM (Minimal medium) (g/L): glucose 20, ammonium sulfate 5, potassium dihydrogen phosphate 3, magnesium sulfate heptahydrate 0.5, 1 mL/L trace element solution, and 1 mL/L vitamin solution. NL (nitrogen limiting) medium (g/L): glucose 20, yeast powder 0.5, ammonium chloride 0.33, magnesium sulfate heptahydrate 1.5, potassium dihydrogen phosphate 1, disodium hydrogen phosphate 1, pH = 6.0. Trace elements (g/L): Calcium chloride dihydrate 4, ferrous sulfate heptahydrate 0.55, hydrated citric acid 0.52, zinc sulfate heptahydrate 0.1, manganese sulfate 0.076, 18 mol/L sulfuric acid 100 µL. Vitamin solution (g/L): Biotin 0.05, 4-aminobenzoic acid 0.2, nicotinic acid 1, D-Calcium pantothenate 1, pyridoxamine dihydrochloride 1.
For 250-mL flask experiments, the seed culture was prepared by inoculating the single colony into 5 mL YPD and cultured at 28 °C and 180 r/min for 24 h, and were then inoculated into 50 mL YPD with a ratio of 2% (v/v) and cultured at 28 °C and 180 r/min for 24 h. The seed culture was subsequently inoculated into YPD with an initial OD600 of 0.6. If not specified, 20% (v/v) dodecane was used as the overlay before culturing at 28 °C, 180 rpm/min for a certain time (usually 120 h), and glucose was used as the carbon source. The initial pH of the YPD liquid medium in its natural state was 5.3. and other medium were ajusted to pH 5.3 with 0.5 M NaOH. The cultures were sampled at intervals of 24 h to determine sugar, OD600 and limonene.
2.3. Single-Factor Effects on Limonene Production
For the Single-Factor test, the influence of the following factors including intial cell optic density, working volume, temperature, intial pH, carbon source, medium type, organic solvent overly ratio were investigated with limoene titer as the index. The initial cell density (OD600) was set as 0.2, 0.4, 0.6 and 0.8; working volume (mL): 25, 50, 75, 100, and 125; temperature (°C): 22, 25, 28 and 31; , initial pH: 4, 5, 6, 7, 8;, medium: YPD medium, MM medium, SD medium and NL medium; carbon source: glucose, xylose, fructose and sucrose, and dodecane coverage ratio(v/v,% ) : 10, 20, 30 and 40.
2.4. Orthogonal Experiments For Elevating Limonene Production
Based on the single-factor experiments above, an orthogonal array of L
9 (3
4) was conducted considering four most important factors (working volume, temperature, pH and dodecane coverage) (
Table 1). The limonene titer was used as the evaluation criterion. All experiments were performed in triplicates, and the data were presented as mean and standard deviations. Statistical significance was determined using one-way ANOVA followed by Tukey multiple ranged tests (SPSS22.0 software, Chicago, IL, USA), and statistical differences between the means were evaluated using least significant difference (LSD) analysis at
p = 0.05. Histograms were drawn using Datagraph 4.3.0 (USA).
2.5. Analytical Methods
The pH was determined using a pH meter (FiveEasy Plus, METTLER-TOLEDO), and biomass was measured using an ultraviolet spectrophotometer (EVOLUTION 220, Beijing). The glucose was estimated by a 3,5-dinitryl-salicylic acid (DNS) colorimetry assay [
22]. Limonene was quantified using gas chromatography (GC-2014C, Shimadzu) as previously described [
16]. Briefly, the KB-1 column (60 m × 0.25 mm × 0.25 µm, Kormat Corporation, USA) with a column temperature of 145 °C, loading temperature of 240 °C, and detector temperature of 260 °C. Nitrogen was used as a carrier gas, the mobile phase was n-hexane, the flow rate was a constant 1.0 mL/min, and the sample size was 1 µL.
3. Results
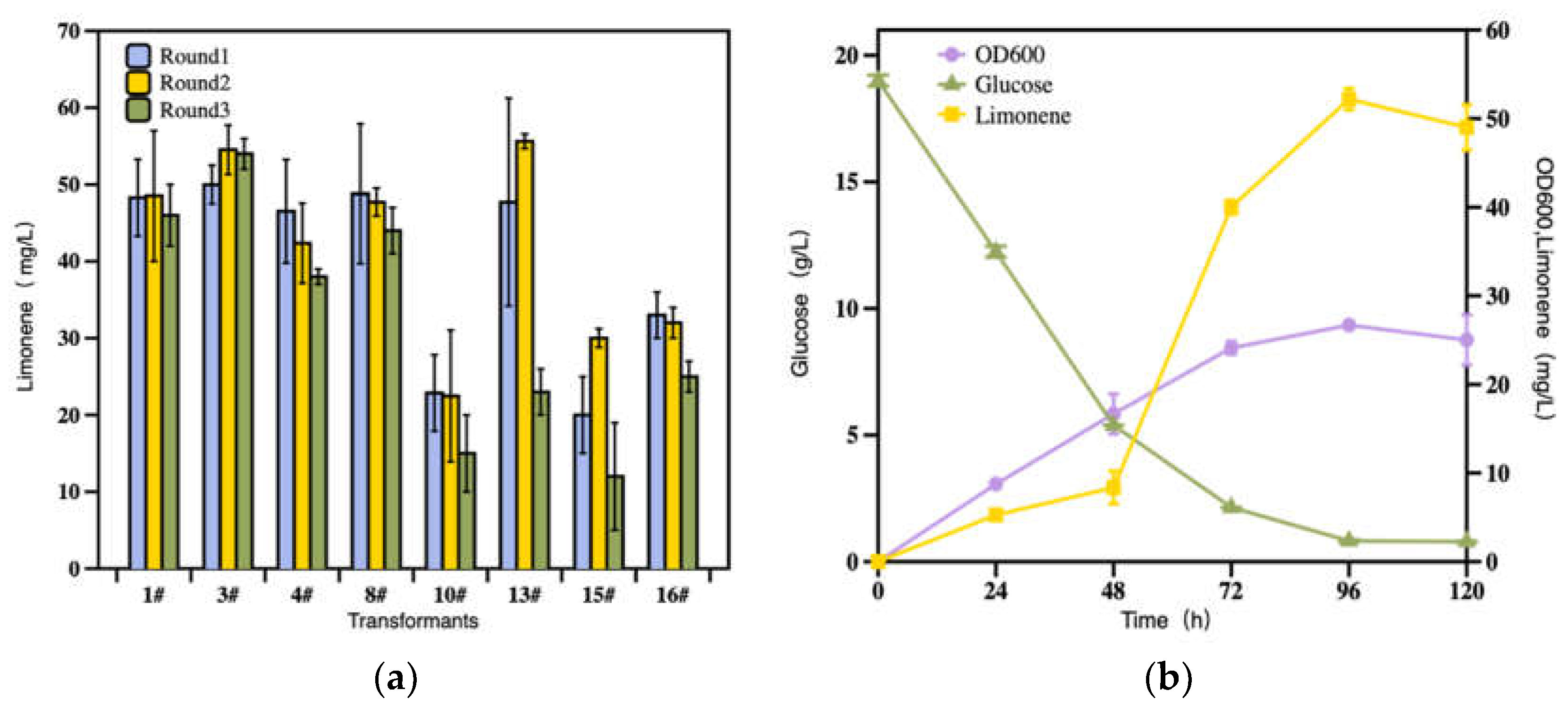
3.1. Limonene Production Stability of Engineered R. toruloides Strains
The production stability of engineered
R. toruloides strains is vital for establishing an efficient bioprocess. As such, eight of the previously constructed transformants were compared for their limonene production stability in the YPD medium for three rounds as described by Liu et al [
16]. The limonene titers were determined at 96 h. As shown in
Figure 2a, the strain 3# exhibited better observable stability than others and was employed for the following experiments.
The dynamic evolution of 3# in OD
600, glucose consumption, limonene production was monitored for 120 h. The fermentation was started with an initial OD
600 of 0.6 and maintained at 28 °C, 180 r/min for 120 h. As shown in
Figure 2b,
R. toruloides grew to OD
600 of 26.7 within 96 h, but decreased to OD
600 of 25.3 at 120 h; The limonene titer evolved with a similar pattern as the cell density. The limonene biosynthesis was started at the beginning of the fermentation, accelerated from 48 h, and reached the top at 96 h (52.2 mg/L, 2.0 mg/OD
600). The glucose was mostly consumed within 72 h and depleted at 96 h. Notably, the limonene biosynthesis and glucose depletion were coupled in an opposite direction, and limonene was produced only when glucose was present. According to the results above, the fermentation should be ended when the carbon source was exhausted.
3.2. The Impacts of Fermentation Parameters on Limonene Production
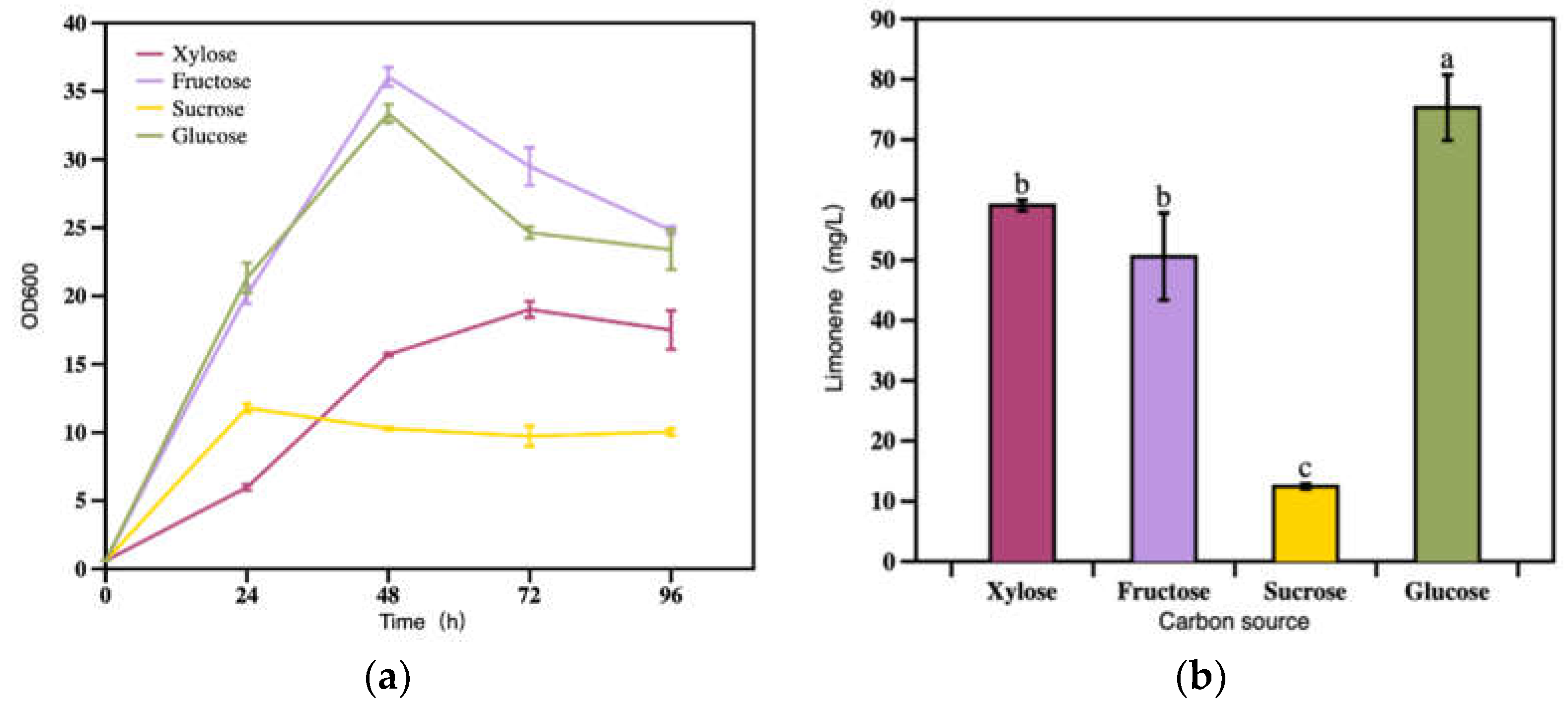
3.2.1. The Effect of Carbon Source on Limonene Production
Carbon source is essential to microorganisms in supporting the biosynthesis of structural macromolecules, the energy generation for cell proliferation and the production of desired products [
23,
24,
25]. Besides, microbes, especially the engineered ones always prefer varied carbon sources for producing specific products [
26]. Thus, it is necessary to optimize the carbon sources when studying the bioproduction of limonene with engineered
R. toruloides strains. In the present study, xylose, glucose, fructose and sucrose were used as the candidate carbon sources, and evaluated for their effects on limonene production. As shown in
Figure 3a, fructose and glucose were more suitable as carbon sources for supporting the growth of engineered
R. toruloides than xylose and sucrose. Fructose was the most suitable carbon source, which resulted in the highest cell density of OD
600 36.0. and sucrose was not desirable. In terms of limonene titer, the maximium was (75.3 mg/L) achieved with glucose, and the lowest was 10 mg/L with sucrose. When it comes to the limonene production efficiency, the results 3.4 mg/OD
600, 2.0 mg/OD
600, 1.3 mg/OD
600 and 3.3 mg/OD
600 for xylose, fructose, sucrose and glucose, respectively. In summary, glucose was the most viable carbon source , followed by xylose and fructose, for sustaining cell growth and limonene production. while sucrose was most unsuitable (
Figure 3b). Thus, glucose was used as the carbon source in the following experiments.
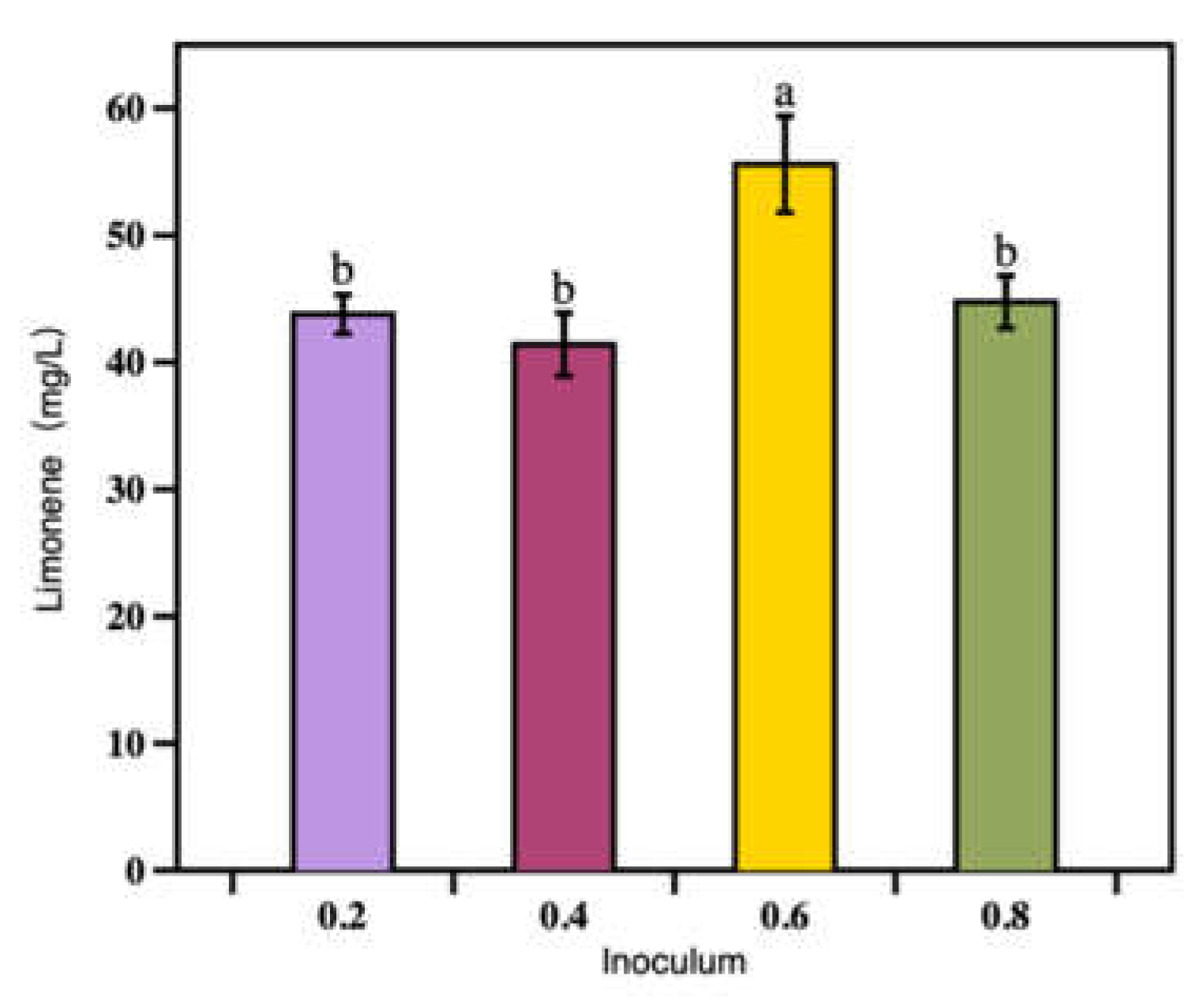
3.2.2. The Effect of Initial Cell Density on Limonene Production
The concentration of the inoculum affects the start of the fermentation process and product production [
27]. Usually, higher inoculum would shorten the lag-phase, allow the product to form earlier, and reduce the chance of microbiological contamination. The optimal inoculum usually depends on the concentration of dissolved oxygen, nutrients in the medium, and the chosen microbial strains [
27]. To determine the optimal intial cell density for limonene production, the initial optic densities were set as 0.2, 0.4, 0.6 and 0.8 in 250 mL shake flasks with working volume of 50 mL and cultivated at 28 ℃ for 96 h. As can be seen from
Figure 4, the highest titer was 55.6 mg/L when the initial was set at OD
600 = 0.6 (
p = 0.001 < 0.05, significant difference) (
Figure 4). When the initial OD
600 was 0.2, 0.4, and 0.8, the titers of limonene were significantly lower than that of the initial OD
600 of 0.6, and there was no significant difference among the three groups (
p = 0.246, 0.657 > 0.05). Therefore, the optimal initial OD
600 was set to 0.6 in the following experiments.
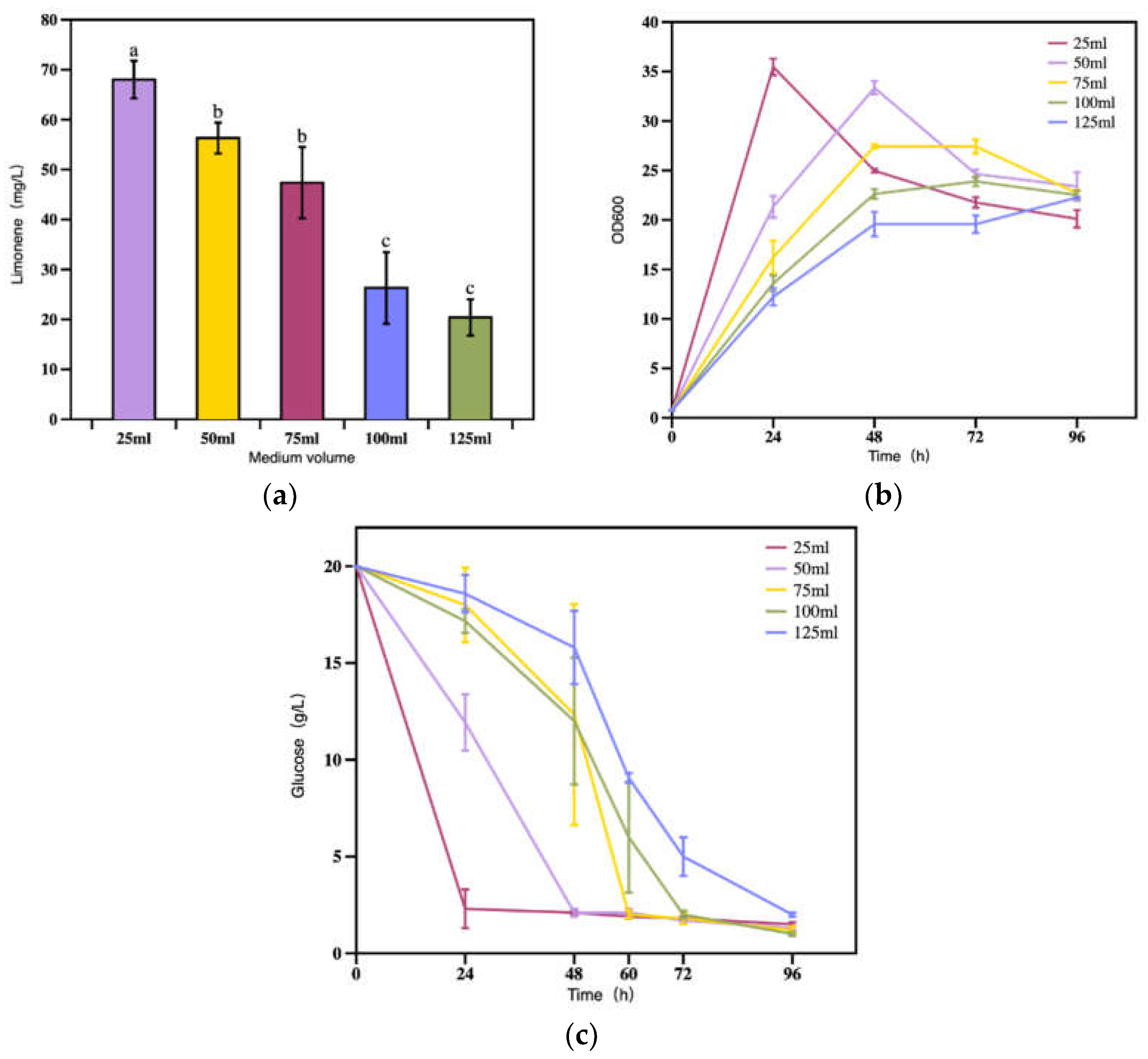
3.2.3. The Effect of Working Volume on Limonene Production
As a restrict aerobic yeast, the cell growth and metabolism of
R. toruloides are highly oxygen demanding. Further, the terpenoids biosynthesis is also an energy consuming process. Sufficient dissolved oxygen supply is critical to maintain the cell viability and limonene biosynthesis. To investigate the effect of aeration on limonene production, the medium loading volume were set within a range of 25 to 125 mL on 250-mL flasks. It was obvious that the limonene titer decreased when the working volume increased from 25 to 125 mL (
Figure 5a). Moreover the time to reach the maximum cell density increased as the media volume elevated (
Figure 5b). When the working volume was 25 mL, the biomass of
R. toruloides reached the maximum and all glucose was depleted within 24 h (
Figure 5c), and the limonene reached its maximum of 68.2 mg/L at 96 h. For the group with a loading medium of 50 mL, the titer of limonene reached 56.3 mg/L at 96 h. When the volume was further increased to 75 mL, 100 mL, and 125 mL, the resulted limonene titers were 48.3 mg/L, 26.3 mg/L, 20.4 mg/L at 96 h, respectively. The results indicated that an excessively high medium loading can lead to insufficient dissolved oxygen, which in turn will affect cell growth and the limonene titer [
27,
28]. Additionally, too high media volume might also influence the mass transfer efficiency, which was also an important factor for efficient bioproduction.
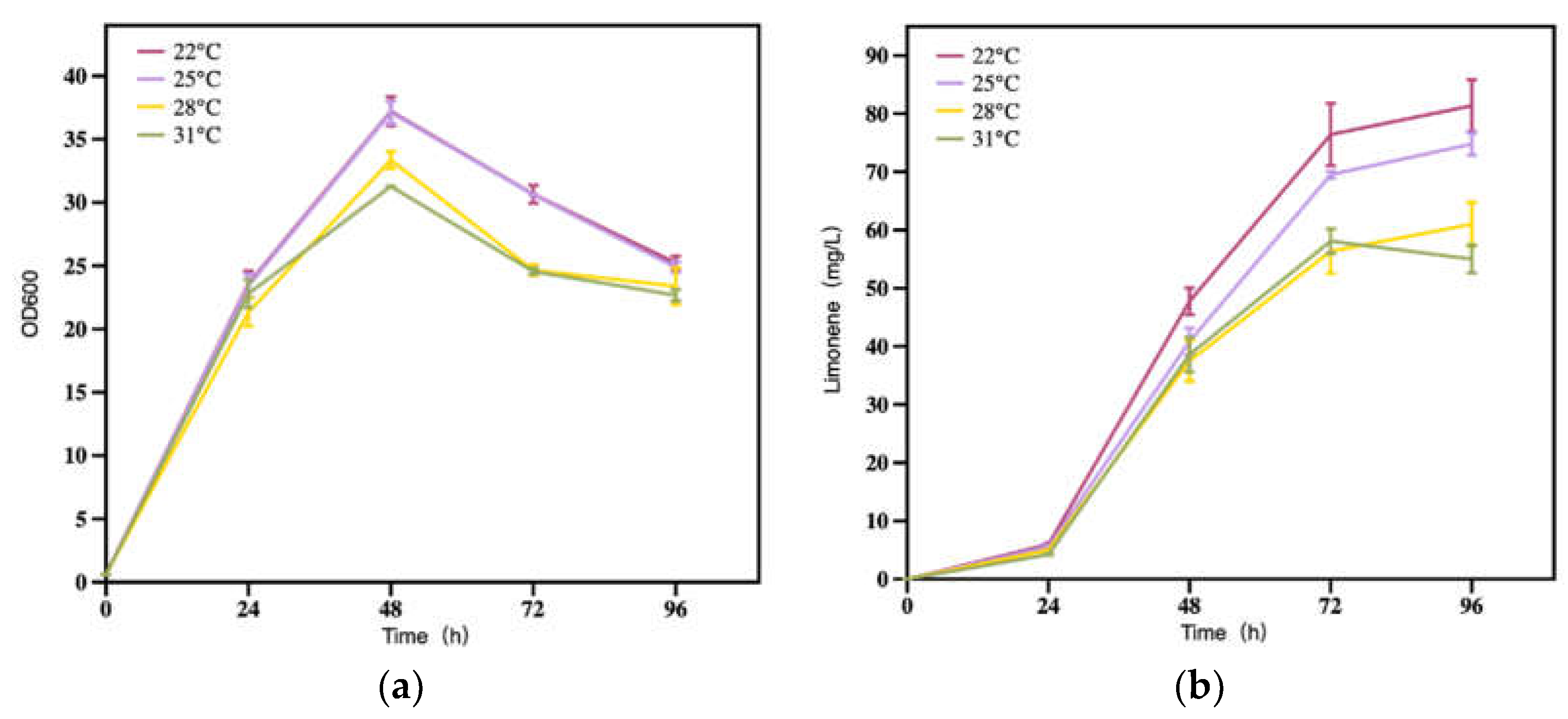
3.2.4. The Effect of Temperature on Limonene Production
The operation temperature could affect the cell growth via modulating the substrate viscosity, mass transfer, decomposition, and absorption, the dissolved oxygen content, the enzymatic activity in the biochemical process, and finally influence the product synthesis efficiency [
17]. Although the optimal fermentation temperature of
R. toruloides was reported as 28 °C [
21], it was probable not such a case for limonene production with the engineered strains. Thus, the effects of temperature on limonene production were explored. The results of the present study showed that the cell grew best at 22 °C and 25 °C (
Figure 6a). The highest titers were 81.4 mg/L, 78.0 mg/L and 75.3 mg/L at 22 °C, 25 °C and 28 °C, respectively (
Figure 6b). It was worth noting that the limonene was only 18.1 mg/L at 120 h under 31 °C, which was 70.5% lower than that at 96 h (not shown in figure). Considering the volatility of limonene and the temperature 31 °C, the significant lower limonene titer might be attributed to the accelerated evaporation of limonene itself as well as the inadequate enzymatic activity of the yeast cells.
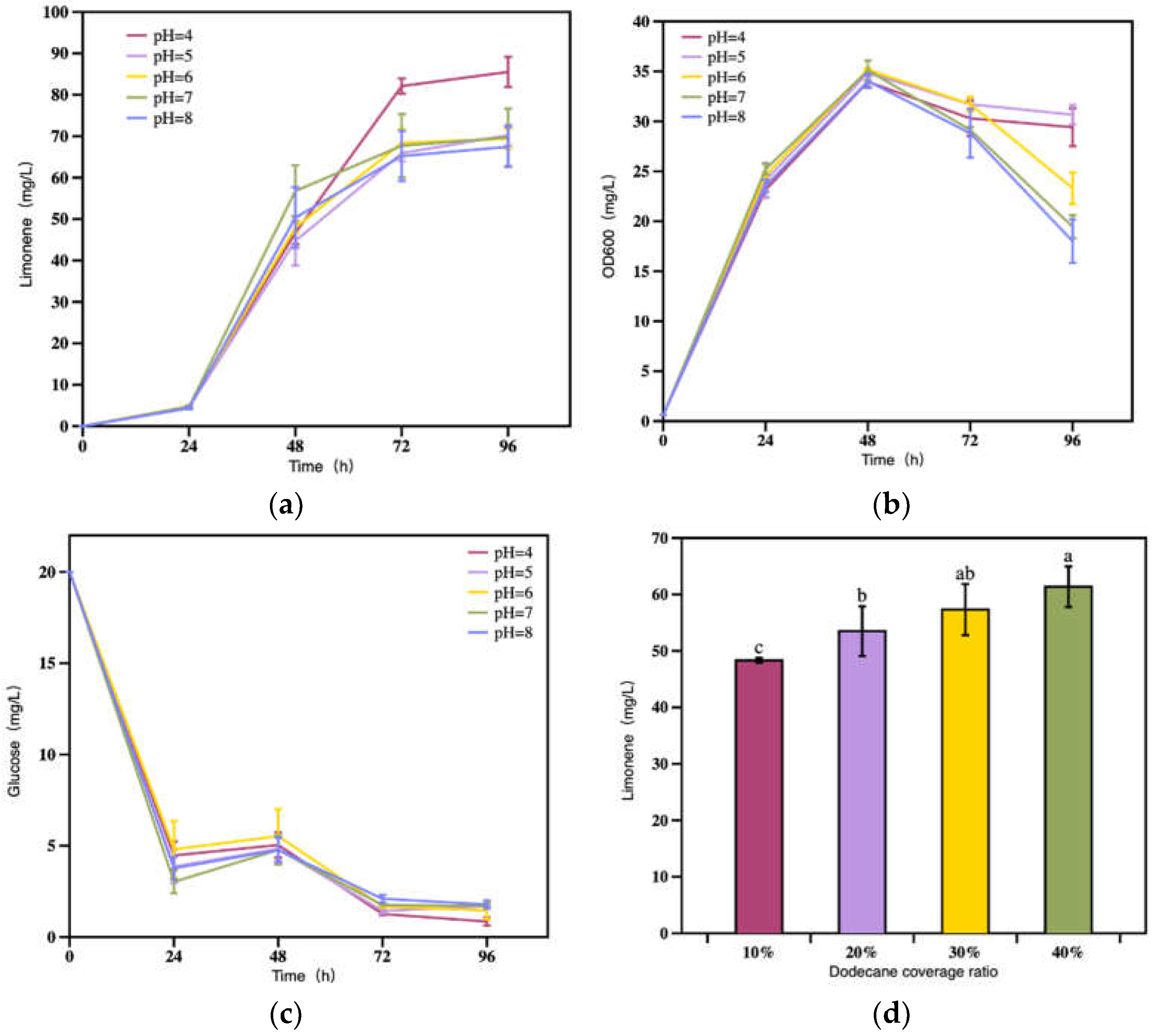
3.2.5. The Effect of Initial pH on Limonene Production
The pH in the cultivation medium impacts cell growth and bioproduction efficiency by affecting the activity of enzymes and changing the cell membrane permeability by altering its charge state, thereby affecting the absorption of nutrients and the efflux of metabolites [
29,
30,
31,
32]. To investigate its effect on limonene production, the initial pH in the medium was set from 4 to 7 (
Figure 7a,b). The results demonstrated that the initial pH of the YPD medium had no obvious effect on cell growth and glucose consumption of
R. toruloides in the first 48 h (
Figure 7b,c), but had a significant effect on the production of limonene from 48 h to 72 h (
Figure 7a). The highest titer of limonene (85.5 mg/L) was obtained at the initial pH 4, significantly higher than others (
p = 0.002, 0.001, 0.001, 0.001 < 0.05). Possibly, a lower initial pH facilitated the maintenance of cell viability when the fermentation entered the stationary phase, where the yeast tend to autophagy and autolysis. The release of intracellular material, especially the bioamine, usually causes the increase in the alkalinity of the media, thus affects the yeast viability.
3.2.6. The Effect of Dodecane Coverage Ratio on Limonene Production
Monoterpenes are usually volatile and can pose certain cytotoxicity on microbial host. To facilitate the instant extraction of monoterpenes and eliminate its cytotoxicity, the bi-phasic fermentation system is common employed with biocompatible organic solvent such as diisononyl phthalate, dibutyl phthalate and dodecane as an overlay [
1]. Dodecane stands has been extensively used for
in situ production of monoterpenes, including limonene [
10,
11,
12,
13,
14,
15,
16]. Moreover, the addition of dodecane is also beneficial to increase the dissolved oxygen in the fermentation system [
1]. In the present study, the dodecane to water ratios (v/v) were explored for their effects on limonene production. As is shown in
Figure 7d, the limonene production increased with the raise of dodecane to water ratio. However, only 14% increase was observed in terms of limonene titer when the dodecane ratio was elevated from 20% (53.5 mg/L) to 40% (61.4 mg/L) (
Figure 7d). The results here indicated that less dodecane coverage would result in the evaporation of limonene during fermentation. In light of the solvent cost in large scale application, the organic orverlay maybe combined with a cooling reflux system to achieve a high product recovery efficiency.
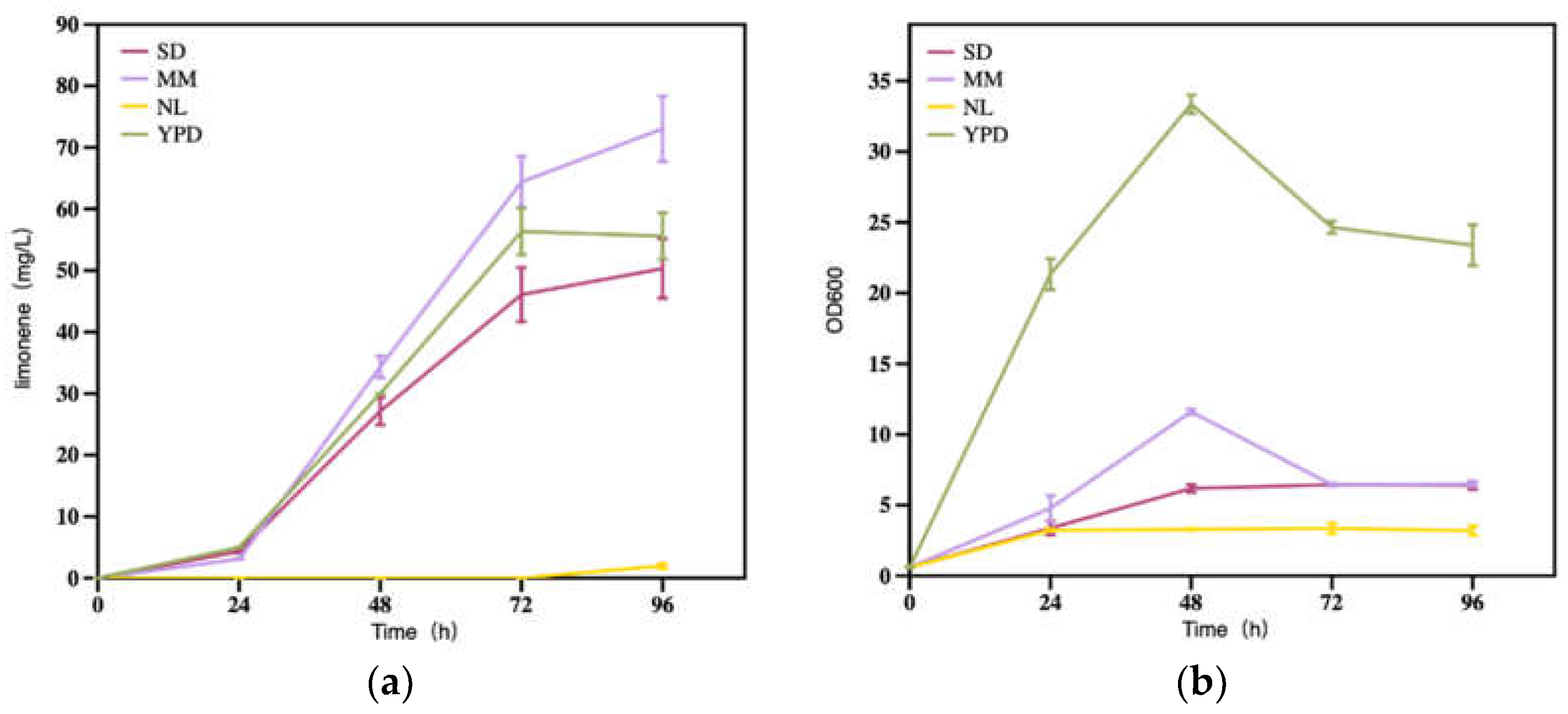
3.2.6. The Effect of the Medium on Limonene Production
The composition of the medium employed during fermentation could influence the viability of the yeast inoculated. While rich medium is favored by microbes for cell mass accumulation, the synthetic ones are preferred for assessing the capacity of microbial host toward a specific product. To facilitate the biosynthesis of limonene with engineered
R. toruloides, the medium of YPD, SD, MM, and NL were examined. The results showed that
R. toruloides had the best biomass performance in YPD (
p ≈ 0 < 0.05), and the result was constant with the previous study [
33]. However, the MM medium was the best for limonene production (73.0 mg/L), 31.3% higher than that obtained in YPD (55.6 mg/L). Notably, the limonene production efficiency (11.4 mg/OD
600) in the MM medium was significantly higher than those of other medium at 96 h, which represented a much higher conversion from glucose (3.65 mg/g with the YPD medium). In the SD medium, the cell growth was relatively weaker than in MM. This result might be attributed to the absence of amino acids in SD medium, which are essential components for synthesizing proteins within the cell, and thus maintaining the state of cells. The maximum limonene titer was 50.3 mg/L at 96 h with the SD medium (
Figure 8a). In the NL medium, the maximum OD
600 was 3.4 and the limonene was only 2.0 mg/L (
Figure 8b). Taken together, the MM medium was the best choice for limonene production while the NL medium could not be considered further. Thus, the MM medium was used for subsequent experimental operations.
3.3. Opitimization of Limonene Production through Orthogonal Experiments
Based on single-factor experiment results above, four main influential factors were obtained: working volume, temperature, pH, and the dodecane coverage ratio. To optimize the fermentation system, a L
9 (3
4) orthogonal design was employed. From the range analysis in
Table 2, it can be seen that the effect of various factors on limonene titers ranked as A > D > C > B (medium volume > dodecane coverage ratio > pH > temperature). The optimal combinations were A
3D
3C
3B
3 and A
2D
3C
3B
1 according to the K value and the orthogonal results, respectively (
Table 2). By referring to the single-factor experimental results, the working volume of 75 mL was better than 50 mL. Needless to say, a pH of 6 is optimal, as is a dodecane coverage ratio of 40%. From the point of applicability, a 40% dodecane coverage will encounter serious economic infeasibility. It can be seen from the experimental data of No. 9 that the culture conditions of a 20% (v/v) dodecane covering ratio, pH = 6, 28 °C, and 75 mL allowed an acceptable titer. Therefore, the optimal fermentation conditions were as follows: A (working volume) = 50 mL, B (temperature) = 22 °C, C (pH) = 6, and D (dodecane coverage ratio) = 20%, at the 250-mL flask level. Finally, the limonene titer was elevated from 52.5 mg/L to 358.1 mg/L, which was 586% net increase in titer and 34.8 fold improvement in production efficiency (mg/ OD600). Still, it must be noticed that the yield of limonene from glucose was only 1.79% (w/w). The low conversion yield might be attributed to intrinsic inefficiency of the engineered yeast, which should only be overcome by indepth metabolic rewiring rather than the fermentation optimization [
34].
4. Conclusions
The red yeast R. toruloides is a versatile platform for producing various products from low-cost feedstock. Our experiments demonstrated that R. toruloides can be potentially used as an excellent host for limonene production, and the fermentation conditions pose greatly impact on limoene production. The results of single-factor and the L9 (34) orthogonal experiments showed that the optimal fermentation conditinons was the MM medium for limonene production and the optimal parameters were: initial OD600 of 0.6, temperature of 22 °C, intial of pH 6, dodecane coverage ratio of 20% and working volume of 50 mL on the 250-mL flask. Under the optimized conditions, the limonene titer of engineered R. toruloides was improved from 52.5 to 358.1 mg/L, a 586% net increase. Collectively, our results lay a good foundation for improving the production of monoterpenes with engineered R. toruloides and the performance of limonene producing strains should be further enhanced at genetic level.
Author Contributions
D.Z. Conceptualization, methodology, formal analysis, investigation; Q.D.G. Methodology, formal analysis, and fata curation; X.C.Z. Writing review & editing; S.S.L. investigation; Q.S.Q. Project administration and funding acquisition; X.W. Formal analysis, resources and data curation; X.B.Y. Writing review & editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was Supported by State Key Laboratory of Microbial Technology Open Projects Fund (M2022-13), Chinese Universities Scientifc Fund (2452018314), and Natural Science Foundation of Shaanxi Province (2020JM-177).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Ren, Y.; Liu, S.; Jin, G.; Yang, X.; Zhou, Y.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 107628. [Google Scholar] [CrossRef] [PubMed]
- Wl, A.; Sw, A.; Mc, B.; Des, A.; Kjlc, D.; Ylac, D. Developing poly(vinyl alcohol)/chitosan films incorporate with d-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar]
- d'Alessio, P.A.; Mirshahi, M.; Bisson, J.F.; Bene, M.C. Skin repair properties of d-Limonene and perillyl alcohol in murine models. Anti Inflamm. Anti Allergy Agents Med. Chem. 2014, 13, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Jaradat, N.; Maqboul, L. The Formation of self-assembled nanoparticles loaded with doxorubicin and D-Limonene for cancer therapy. ACS Omega. 2022, 7, 42096–42104. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Jaradat, N.; Maqboul, L.; Battista, F.; Remelli, G.; Zanzoni, S.; Bolzonella, D. Valorization of residual orange peels: limonene recovery, volatile fatty acids, and biogas production. ACS Sustain. Chem. Eng. 2020, 8, 6834–6843. [Google Scholar]
- Chubukov, V.; Mingardon, F.; Schackwitz, W.; Baidoo, E.; Mukhopadhyay, A. Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase AhpC. Appl. Environ. Microbiol. 2015, 81, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, X.; Jiang, G.; Wu, J.; Zhang, J.; Lei, D.; Yuan, Y. Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Theodoropoulos, C.; Scrutton, N.S. Techno-economic assessment of microbial limonene production. Bioresour. Technol. 2019, 300, 122666. [Google Scholar] [CrossRef]
- Jongedijk, E.; Cankar, K.; Buchhaupt, M. Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biot. 2016, 100, 2927–2938. [Google Scholar] [CrossRef]
- Rolf, J.; Julsing, M.K.; Rosenthal, K. A gram-scale limonene production process with engineered Escherichia coli. Molecules. 2020, 25, 1881. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, S.; Cao, J.; Qao, J.; Zhao, G.R. Systematic optimization of limonene production in engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Meng, Y.; Zhang, L.; Qiao, J.; Zhao, G.R. Combinatorial engineering of Saccharomyces cerevisiae for improving limonene production. Biochem. Eng. J. 2021, 176, 108155. [Google Scholar] [CrossRef]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. P. Natl. Acad. Sci. USA. 2020, 117, 31789–31799. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wei, L.; Lv, Y.; Chen, J.; Hua, Q. Elevating limonene production in oleaginous yeast Yarrowia lipolytica via genetic engineering of limonene biosynthesis pathway and optimization of medium composition. Biotechnol. Bioprocess Eng. 2019, 24, 500–506. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, Y.; Li, S.; Zhao, Y.; Yu, A. Engineering the oleaginous yeast Yarrowia lipolytica to produce limonene from waste cooking oil. Biotechnol. Biofuels. 2019, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, M.; Ren, Y.; Jin, G.; Tao, Y.; Lyu, L.; Zhao, Z.K.; Yang, X. Engineering Rhodosporidium toruloides for limonene production. Biotechnol. Biofuels. 2021, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Doriano, C. Optimizing scale-up fermentation processes. Trends Biotechnol. 2002, 20, 103–105. [Google Scholar]
- Zhao, Y.; Song, B.; Li, J.; Zhang, J. Rhodotorula toruloides: an ideal microbial cell factory to produce oleochemicals, carotenoids, and other products. World J. Microb. Bio. 2022, 38, 1–19. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, Z.K. Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. J. Biotechnol. 2008, 136, S363–S364. [Google Scholar] [CrossRef]
- Geiselman, G.M.; Zhuang, X.; Kirby, J.; Tran-Gyamfi, M.B.; Gladden, J.M. Production of ent-kaurene from lignocellulosic hydrolysate in Rhodosporidium toruloides. Microb. Cell Factories. 2020, 19, 23. [Google Scholar] [CrossRef]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Gladden, J.M. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels. 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lopes, H.; Bonturi, N.; Kerkhoven, E.; Miranda, E.; Lahtvee, P. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biot. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, J.; Zhao, H.; Shi, S. Engineering oleaginous yeast Rhodotorula toruloides for overproduction of fatty acid ethyl esters. Biotechnol. Biofuels. 2021, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, T.; Li, X.; Chen, Y.; Campbell, K.; Nielsen, J.; Chen, Y. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 2019, 10, 4976. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Cucchetti, D.; Pizza, F.; Franzosi, G.; Bianchi, D.; Compagno, C. Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour. Technol. 2012, 111, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Hafiza, Z.; Maskat, M.; Liew, S.L.; Mamot, S. Fermentation of morinda citrifolia extract by Saccharomyces cerevisiae as affected by substrate concentration, inoculum size, temperature and fermentation time. Int. Food Res. J. 2013, 20, 1889–1894. [Google Scholar]
- Pursell, M.R.; Mendes-Tatsis, M.A.; Stuckey, D.C. Effect of fermentation broth and biosurfactants on mass transfer During liquid–liquid extraction. Biotech. Bioeng. 2004, 80, 155–165. [Google Scholar] [CrossRef]
- Arino, J. Integrative responses to high pH stress in Saccharomyces cerevisiae. Omics. 2010, 14, 517–523. [Google Scholar] [CrossRef]
- Serra-Cardona, A.; Canadell, D.; Arino, J. Coordinate responses to alkaline pH stress in budding yeast. Microb. Cell. 2015, 2, 182–196. [Google Scholar] [CrossRef]
- Pereira, A.S.; Miranda, S.M.; Lopes, M.; Belo, I. Factors affecting microbial lipids production by Yarrowia lipolytica strains from volatile fatty acids: Effect of co-substrates, operation mode and oxygen. J. Biotechnol. 2021, 331, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Diakov, T.; Kane, P.M. Regulation of vacuolar proton-translocating ATPase activity and assembly by extracellular pH. J. Biol. Chem. 2010, 285, 23771–23778. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Bedekar, A.; Liu, J.; Jin, S.; Rao, C.V. Production of galactitol from galactose by the oleaginous yeast Rhodosporidium toruloides IFO0880. Biotechnol. Biofuels. 2019, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, T.; Li, X.; Chen, Y.; Campbell, K.; Nielsen, J.; Chen, Y. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 2019, 10, 4976. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).