1. Introduction
Although much progress has been made in the field of SARS CoV-2 diagnostics, to date the dynamics of SARS-CoV-2 replication, shedding and infectivity in humans remain incompletely understood (1). Infectivity by any viral pathogen is a very complex process that involves multiple host and viral factors. Different studies on SARS CoV-2 infection have suggested that the magnitude and duration of viral shedding correlates with biological characteristics of the virus, disease severity, patient age and sex, viral load, stage of the infection and immune status of the infected individual (1-5).
SARS-CoV-2 RNA has been detected in different body fluids in addition to nasal swabs during acute infection, including saliva, peripheral blood, ocular secretions, anal swabs, and urine [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. A recent meta-analysis found that the mean shedding time of SARS-CoV-2 in the upper respiratory tract, lower respiratory tract, stools, and serum was 17.0, 14.6, 17.2, and 16.6 days, respectively [
5]. However, results from multiple investigations have been very heterogeneous [8). Most of these studies used only a single time point to collect samples, included limited sites of biologic specimen collection, and only utilized patients with severe forms of COVID-19[
5,
9,
10]. As a result, variations in time between symptom onset and sample testing can be a confounding factor when analyzing data from these studies on viral shedding and infectivity. In addition, virus isolation from non-respiratory tract specimens has been unsuccessful in most cases[
1,
9,
10,
11,
12,
13,
14,
15].
To investigate SARS-CoV-2 persistence and replication competence in individuals with mild or severe symptoms we carried out the present study on 125 COVID-19 cases. The dynamics of infectious virus shedding from multiple biologic sites during acute infection were determined through weekly longitudinal sampling in 123 individuals. We estimated viral clearance rates and potential infectivity at multiple sites, among immunocompetent and immunosuppressed individuals with mild or severe COVID-19 disease. In addition, the shedding dynamics of the B.1.1.28 lineage (ancestral strain) and the Gamma strains of SARS-CoV-2 were compared.
The objective of the study was to investigate the presence and potential infectivity of SARS-CoV-2 in nasopharyngeal swabs, saliva, urine, blood, and feces (anal swab), among immunocompetent and immunocompromised patients, during the acute and convalescence phases of COVID-19, in patients infected with two different virus strains.
2. Materials and Methods
2.1. Study Design and Population
This was an observational, prospective study, developed in patients with SARS-CoV-2 infection, using a convenience sample. Patients were followed-up for 5 weeks after their initial COVID-19 diagnosis, or until SARS-CoV-2 testing was negative in the collected samples.
Two groups of patients were included: 1-Immunocompetent patients with mild disease who were infected with either the ancestral SARS-CoV-2 (B.1.1.28 lineage) or the Gamma variant; 2- Immunocompromised patients, hospitalized with severe disease infected with either the ancestral SARS-CoV-2 (B.1.1.28 lineage) or the Gamma variant.Hospitalized patients were seen either at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (USP) (a public hospital) or at Hospital 9 de Julho (a private hospital) in São Paulo, Brazil, between May 2020 and March 2021.
All immunocompromised patients included in the study were individuals with a previous diagnosis of different types of neoplasms and were under immunosuppressive therapy at the time they were infected with SARS CoV-2. The outpatient subjects were seen between March and May 2020. They were participants in
The Corona São Caetano Program, a primary care initiative offering COVID-19 care to all residents of São Caetano do Sul, Brazil [
16]. None of the participants received any COVID-19–specific anti-viral therapy or vaccines for SARS-CoV-2, before inclusion in the study. Age, sex, disease severity, clinical variables, and date of the onset of symptoms were retrieved from medical records, from the laboratory information system of the two hospitals and from
The Corona São Caetano Program.
2.2. Laboratory Tests and Sample Collection
Sample Collection
All recruited patients, aged > 18 years, had a confirmed diagnosis of COVID- 19, through the identification of SARS-CoV-2 by RT-PCR from a nasopharyngeal swab.
After their initial COVID-19 diagnosis, during a 5-week period, with a 7-day interval, each included patient was invited to collect different samples, as follows: anal and nasal swab, saliva, urine, and blood. All samples were then subjected to SARS- CoV-2 testing by RT-PCR.
Real-time PCR for SARS-CoV-2
Quantitative assays (SARS-CoV-2 N or E gene) for SARS-CoV-2 were performed according to protocols adapted with primers and probes for the RT PCR assay [
17,
18,
19]. All samples were deemed suitable for amplification by RT-PCR based on analysis of the internal control consisting of primers and probe for the human Ribonuclease P gene [
20]. Sequences of oligonucleotides were resuspended in known concentrations (serial dilution to base 10) for use as a positive control and for the construction of viral load quantification curves. The synthetic oligo sequences designed for the current study were based on a protocol previously described for other pathogens [
21]. The sequences of specific oligos are described in
Table 1.
The RT PCR data was expressed as the value of the Cycle threshold (Ct), corresponding to the initial amplification cycle, which is inversely proportional to the number of copies of the target sequence of interest, given by the measurement of the number of copies per reaction.
Viral culture
Viral culture for SARS-CoV-2, conducted in a biosafety level-3 facility, utilized Vero CCL81 cells (ATCC® CCL-81™) in Dulbecco minimal essential medium supplemented with 5% heat-inactivated fetal bovine serum and anti biotics/antimycotics. SARS-CoV-2 PCR-positive samples were inoculated into a Vero cell culture in plastic bottles (Jet biofilm, 12,5 cm2 area, 25 mL capacity) and incubated in a 37°C incubator in an atmosphere of 5% CO2. Cultures were maintained for at least 2 weeks and observed daily for evidence of cytopathic effects (CPEs). At least two subcultures were performed on each sample. The detection of CPEs was investigated using an inverted microscope (Nikon, Japan) and the presence of virus in supernatants from cultures showing CPEs was determined by specific RT-PCR, as described above. Viral isolation (culture) was performed on all RT-PCR positive samples 10 or more days after onset of symptoms. The Cts of supernatant and the original clinical sample (Ct sample) were compared, and positive cultures were defined where Ct sample - Ct culture was ≥3. Culture positivity was utilized as a proxy for infectivity.
SARS-CoV-2 Whole-Genome Sequencing
The viral RNA, extracted as described above, was also used for whole-genome sequencing (WGS) analysis. In brief, SARS-CoV-2 complementary DNA and multiplex PCR steps were performed, and the amplicons were sequenced using the MinION platform (Oxford Nanopore Technologies, United Kingdom) and Miseq (Illumina, San Diego, USA) for lineage characterization [
22]. Variant calling and consensus sequences were performed using artic minion with Nanopolish version from the ARTIC bioinformatics pipeline (
https://github.com/artic-network/fieldbioinformatics). Genome regions with a depth of 50 times genome coverage were used to lineage classification by Pangolin version 3.1.5 (
http://pangolin.cog-uk.io/) [
23] and Nextclade version 1.4.0 (
https://clades.nextstrain.org) and confirmed by manual genotyping.
Co-infection with Influenza A and B virus and syncytial respiratory virus (SRV)
All individuals from Group 1 and Group 2 were tested (nasal swab samples collected at week 1) for Influenza A and B virus and syncytial respiratory virus (SRV) at our laboratory, by using the Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay.
2.3. Definitions
The persistence of SARS-CoV-2 was defined based on the time spam (days) from the onset of symptoms to the last positive results that samples remained RT-PCR positive. Individuals with RT-PCR positive samples in any biologic specimen 10 or more days after onset of symptoms were deemed to have a persistent infection. The ability to propagate virus in an
in vitro culture was used as a proxy for infectivity, as proposed by Wolfel et al. [
9].
2.4. Statistics
Differences between immunocompetent and immunosuppressed subjects, infection with the B1 or Gamma SARS-CoV-2 strains and viral detection between biological sites were analyzed by Fisher’s exact test or the Mann-Whitney test, as appropriate. The quantitative parameters of viral detection were described for each infecting SARS-CoV-2 strain and subjects’ immune status by week of collection using absolute and relative frequencies. Persistence of viral detection for more than 10 days in any sample was described according to clinical and demographic characteristics and the association of persistence with qualitative characteristics was verified using the chi-square test or Fisher's exact test. Quantitative characteristics were compared according to persistence using the student t test or Mann-Whitney test. The analyses were performed using the IBM-SPSS for Windows version 20.0 software and tabulated using the Microsoft-Excel 2003 software. All tests were performed with a significance level of 5%.
3. Results
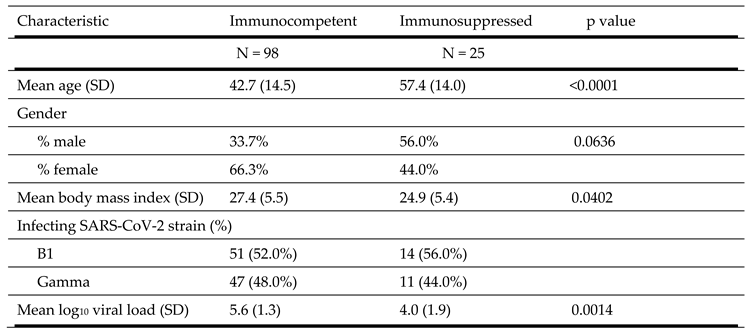
Ninety-eight immunocompetent patients and 25 immunosuppressed individuals were included in the study. Characteristics of the study population is shown in
Table 2. The immunocompetent group were younger (42.7 vs. 57.4 years old, p<0.0001), had a higher mean body mass index (27.4 vs. 24.9 kg/m
2, p = 0.0402) and a higher mean log
10 SARS-CoV-2 viral load (5.6 vs. 4.0, p = 0.0014) than the immunosuppressed group. The viral genome sequences obtained from all clinical specimens from immunocompetent patients with mild forms of disease belonged to either the B.1.128 lineage (51 cases) or the Gamma lineage (47 cases). The Gamma strain emerged in Brazil in 2020 [
24,
25,
26]. Similarly, among immunosuppressed individuals the viral genome sequences also belonged to either the B.1.128 lineage (14 patients) or to the Gamma variant (11 patients).
None of the included patients were positive forInfluenza A and B virus and syncytial respiratory virus (SRV), when we tested nasal swab samples collected at week 1.
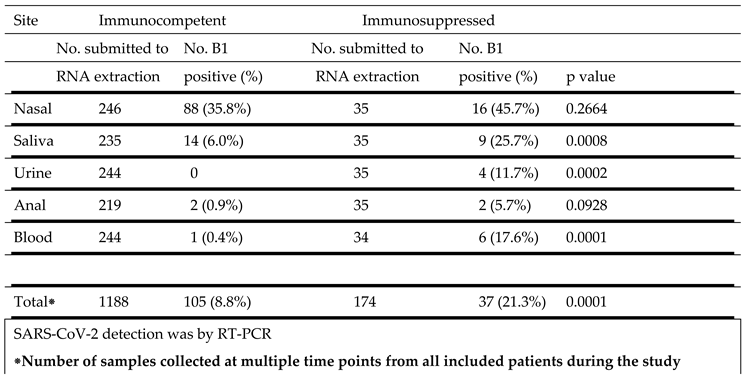
SARS-CoV-2 B1 strain detection at different sites in immunocompetent and immunosuppressed patients is show in
Table 3. The highest percentage of virus detection was in nasal samples, 35.8% of samples from immunocompetent subjects and 45.7% from immunosuppressed individuals. Saliva was the second most frequently positive site, 6.0% in immunocompetent subjects and 25.7% in the immunocompromised group . This difference was highly significant (p = 0.0008). Similarly, urine (p = 0.0002) and blood (p = 0.0001) samples from immunosuppressed patients were more frequently positive than from immunocompetent individuals. A small and comparable percentage of anal swabs from both groups were also positive.
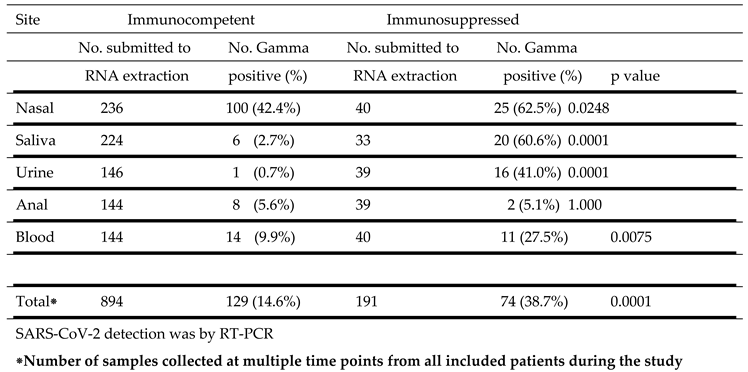
SARS-CoV-2 Gamma strain detection at different sites in immunocompetent and immunosuppressed patients is detailed in
Table 4. Similar to the results with the B1 strain, the highest percentage of positive samples were from the nasal cavity in both immunocompetent (42.4%) and immunosuppressed (62.5%) subjects. This difference was significant (p = 0.0248). Virus was also detected in a higher percentage of samples from saliva, (p = 0.0001), urine (p = 0.0001) and blood (p = 0.0075) from immunosuppressed than from immunocompetent individuals. A small and comparable percentage of anal swabs from both groups were also positive.
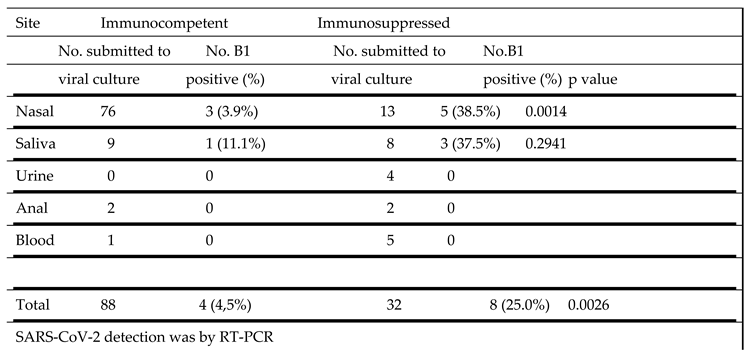
The ability to propagate the B1 strain of SARS-CoV-2 in culture from different sites in immunocompetent and immunosuppressed patients is shown in
Table 5. In samples from immunocompetent individuals virus could be cultured from only 3 of 76 nasal samples (3.9%) and 1 of 9 saliva samples (11.1%). In marked contrast, virus was cultured from 5 of 13 nasal samples (38.5%) and 3 of 8 saliva samples (37.5%) from the immunosuppressed group. This difference in vírus cultivation from nasal samples was significant (p = 0.0014).
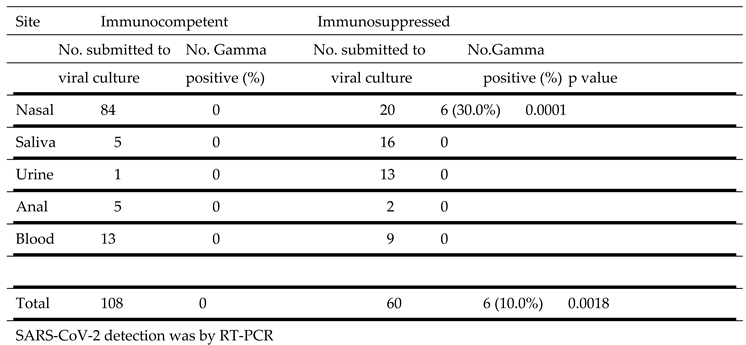
SARS-CoV-2 Gamma strain propagation in culture from samples obtained from different sites is described in
Table 6. Virus was cultured from samples obtained from the nasal cavity of immunosuppressed (6 of 20, 30.0%) but not from immunocompetent (0 of 84) individuals. No virus was obtained from cultures of saliva, urine, anal and blood from all patients.
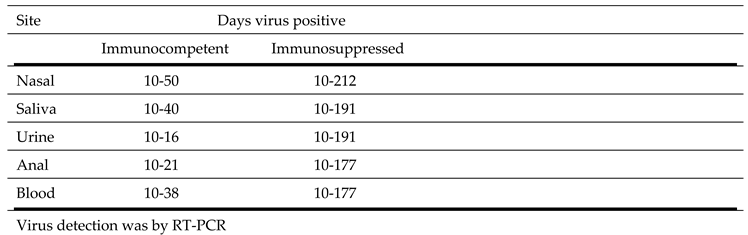
The duration of viral shedding at different sites in immunocompetent and immunosuppressed individuals over time in individuals with prolonged viral shedding of at least 10 days is shown in
Table 7. Among the immunocompetent patients viral shedding from different sites varied from 16 days to 50 days and was longest (50 days) in nasal specimens. For immunosuppressed individuals viral shedding varied from 177 days to 212 days and was also longest for nasal specimens (212 days).
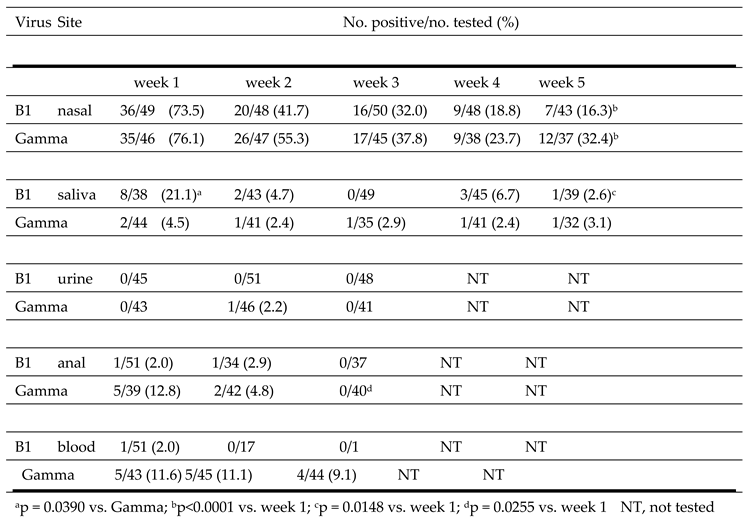
The detection of the B1 and Gamma SARS-C0V-2 strains at different body sites over time is shown in
Table 8. The highest number of positive samples was observed in nasal samples collected at week 1. The percent positive was similar for B1 (73.5%) and Gamma (76.1%) isolates. The second highest percent positive was in week 1 saliva for B1 (21.1%) and in week 1 anal swabs for Gamma (12.8%). The percent virus positive in saliva was significantly higher for individuals infected with B1 (21.1%) than in those infected with the Gamma strain (4.5%) (p = 0.0390). The B1 strain was only detected in 1/51 blood samples collected at week 1 and in none of the samples collected at weeks 2 and 3. In contrast, the Gamma strain was detected in 11.6%, 11.1% and 9.1% of blood samples collected at week 1, 2 or 3, respectively. B1 and Gamma detection in nasal samples (p<0.0001), and B1 detection in saliva (p=0.0148), progressively decreased over the 5-week testing period.
Analysis of all viral genome sequences obtained from clinical specimens of all patients did not reveal anymutations which would be associated with prolonged viral shedding , viral replication or pathogenicity.
4. Discussion
Among immunocompetent and immunosuppressed individuals with COVID, a greater number were positive for SARS-CoV-2 in nasopharyngeal swabs than in any other sites tested regardless of the infecting viral strain or the time after infection initiation that the sample was collected. At sites other than the nasopharynx -- saliva, urine and blood – virus was detected more frequently in immunosuppressed individuals than in those who were immunocompetent. The few positive anal samples were not significantly different between the immunocompetent and immunosuppressed populations. Virus detection from all sites declined over time but persisted longer in immunosuppressed individuals. Viral propagation in culture was also achieved more frequently in samples from the immunosuppressed group. All these findings were consistent with earlier reports [
1,
27,
28,
29]. In immunocompetent patients, considering only samples collected ten or more days after onset of disease, 3.6%, 1.9% and 0.25% of blood, anal and urine samples were positive for SARS-CoV-2. In contrast, among immunosuppressed individuals, considering only samples collected ten or more days after onset of disease, SARS CoV-2 was identified in 41.17%, 5.4% and 22.9% of blood, urine and anal samples. Our data also confirmed that in immunocompetent individuals with mild COVID-19, successful virus cultivation from clinical samples obtained ten days after the onset of symptoms is an uncommon event, regardless of the viral strain involved [
1,
9,
10,
30] and that immunocompromised patients with COVID-19 are at elevated risk for prolonged viral shedding and persistent replicating capacity [
30,
31,
32]. In our study, despite the frequent finding of SARS-CoV-2 in different biological materials and in some cases for very prolonged periods, viral replication
in vitro was identified only in nasopharyngeal and saliva samples. SARS-CoV-2 has been described in different body fluids, by many authors, from different parts of the world. Most of these reports, however, included only a small number of prospectively collected samples from only the nasopharynx and saliva. The present study reports finding from multiple sites in a large series of immunocompromised and immunocompetent individuals.
One of our patients, a 40-year-old male who had undergone a prior autologous hematopoietic stem cell transplant due to a diffuse large B-cell lymphoma, was found to persistently shed SARS-CoV-2 that could be propagated in culture from nasal swab and saliva samples for more than 196 days. Other studies have identified atypical cases with prolonged shedding of infectious virus for up to 200 days [
30,
31,
32,
33,
34].
Several prior studies have proposed that the persistence of SARS-CoV-2 capable of replication was related to immunosuppression [
35,
36,
37]. As extensively described for numerous viral infections, both innate immunity and T cell-mediated adaptive immune response are essential for the clearance and long-term inhibition of viral infections [
35,
36]. It is also important to acknowledge that studies have associated the persistence of SARS-CoV-2 with severity of disease, the presence of co-morbidities and use of glucocorticoids [
38,
39,
40,
41]. In our series of immunosuppressed patients, all were hospitalized with severe forms of COVID 19.
Viral evolution of SARS-CoV-2 over time has led to the emergence of numerous variants. Differences in immune evasion, viral loads, and duration of shedding between variants have been described [
1,
5]. It was not surprising, therefore, that in our study differences in viral detection, persistence, and proliferation in culture between the B1 and Gamma strains were observed.
Only a few studies have described temporal changes in SARS-CoV-2 detection [
42,
43,
44]. The virus detection rate in blood in different studies was between 28% and 32% for hospitalized patients. However, patients in intensive care had rates up to 78% [
3,
42,
43,
44]. Our study is unique in that it describes the prospective presence of SARS CoV-2 in non-hospitalized individuals with only mild forms of COVID-19. In addition, to our knowledge no prior investigation has reported
in vitro viral propagation from SARS-CoV-2-positive blood samples.
Review studies have found overall rates of SARS-CoV-2-positive urine samples to be low and levels correlated with severe disease status [
45]. The present study is in agreement with these prior reports. SARS CoV-2- positive urine samples were identified in 0.25% of immunocompetent COVID-19 patients with mild disease, but in 22.9% of immunosuppressed patients with severe disease. None of the virus-positive samples yielded virus upon cultivation. Similarly, SARS-CoV-2 has been identified in anal swabs [
6,
10,
11,
12]. The dynamics of SARS-CoV-2 shedding at this site has been described as erratic, with the highest viral loads reported during the first weeks after symptom onset and described in severe cases [
5,
6,
10,
11,
12,
38]. In our study, SARS CoV-2 detection in anal swabs was observed in 10 samples (1.9%) from immunocompetent individuals and in 5.4% of swabs from immunosuppressed individuals. Replicative virus was not obtained from any anal sample. This was in accord with findings from prior studies [
5,
38,
46].
Much progress has been made in understanding the transmission dynamics of SARS-CoV-2 and duration of infectivity. The World Health Organization (WHO) and the Center for Disease Control (CDC) have modified their recommendations in response to data indicating that infectivity decreases to essentially zero after about 10 days from symptom onset in mild to moderately ill patients and after about 15 days in critically ill and immunocompromised patients, with a maximum reported interval thus far of 20 days [
47]. To our knowledge, the present study is unique in comparing both SARS-CoV-2 detection by RT-PCR and by the presence of culturable virus in serially collected samples from different clinical sites, in both immunocompetent and immunosuppressed individuals. We also directly compared the shedding dynamics of two different SARS-CoV-2 strains - B and Gamma - in these individuals. This enabled us to estimate the time period of virus detection and its relationship to capacity for replication in different biological specimens.
It is necessary to acknowledge the limitations of our study. All of our cases occurred during the initial two years of the pandemic, before the widespread circulation of additional viral variants. Therefore, our findings might not be generalizable to current and future SARS-CoV-2 variants. Additionally, with the continuous circulation of SARS-CoV-2 in the community, previous or recurrent infection, vaccination, or a combination of both could alter viral shedding patterns differently from those observed in our study. In addition, while study staff were trained in sample collections it is possible that sample variation in quality could have occurred. However, the consistency in findings from longitudinal sampling suggest that this variation was likely minimal.
5. Conclusions
The present study describes the longitudinal dynamics of SARS-CoV-2 infection in immunocompetent individuals with mild disease as well as in immunocompromised individuals with severe disease. Delineation of the duration of virus detection and propagation capability in diverse biological specimens from these two different populations is fundamental to an improved understanding of contagion and development of more effective, evidence-based intervention policies. Additional studies are needed to further clarify the risk factors and features associated with persistent shedding of potentially infectious SARS-CoV-2 among other groups of individuals infected with various viral strains.
Author Contributions
Conceptualization: Maria Cássia Mendes-Corrêa, Steven S. Witkin, Matias Chiarastelli Salomão, Fábio Ghilardi , Fabio E Leal; Methodology: Tania Regina Tozetto-Mendoza, Lucy S Villas-Boas, Anderson Vicente de Paula, Jaqueline G de Jesus, Erika R Manuli ,Flavia C. Sales, Ingra M. Claro, Antonio Charlys da Costa,Camila Romano, Ester C. Sabino; Data curation: Heuder Gustavo Oliveira Paiao, Geovana M. Pereira ,Evelyn Patricia Sánchez Espinoza , Wilton Freire, Noely E Ferreira, Fábio Ghilardi ,Andrea de Barros Coscelli Ferraz, Evelyn Patricia Sánchez Espinoza; Formal analysis: Maria Cássia Mendes-Corrêa, Steven S. Witkin; Writing and review and supervision: Maria Cássia Mendes-Corrêa, Steven S. Witkin, Matias Chiarastelli Salomão. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant/Award Number: FAPESP (2020/05623-0) and Merck Investigator Studies Program – MISP Protocol No: IISP # 60384.
Institutional Review Board Statement
The study was approved by the Research Ethics Committee of Clinical Hospital of the University of Sao Paulo School of Medicine, or HC-FMUSP (CAAE Registry No.: 30419320.7.0000.0068 dated April 18, 2020).
Informed Consent Statement
Informed consent was obtained from all the participants.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023 Mar;21(3):147-161. [CrossRef]
- Néant N, Lingas G, Le Hingrat Q, Ghosn J, Engelmann I, Lepiller Q et al; French COVID Cohort Investigators and French Cohort Study groups. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci U S A. 2021 Feb 23;118(8):e2017962118. [CrossRef]
- Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A et al.; Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020 Oct 30;11(1):5493. PMID: 33127906; PMCID: PMC7603483. [CrossRef]
- Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020 Apr 21;369:m1443. PMID: 32317267; PMCID: PMC7190077. [CrossRef]
- Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021 Jan;2(1):e13-e22. Epub 2020 Nov 19. PMID: 33521734; PMCID: PMC7837230. [CrossRef]
- Peng L, Liu J, Xu W, Luo Q, Chen D, Lei Z et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J Med Virol. 2020 Sep;92(9):1676-1680. [CrossRef]
- Pérez-Bartolomé F, Sánchez-Quirós J. Ocular manifestations of SARS-CoV-2: Literature review. Arch Soc Esp Oftalmol (Engl Ed). 2021 Jan;96(1):32-40. English, Spanish. [CrossRef]
- Yan D, Zhang X, Chen C, Jiang D, Liu X, Zhou Y et al. Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis. Front Public Health. 2021 Mar 19; 9:652842. PMID: 33816427; PMCID: PMC8017277. [CrossRef]
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA et al.. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 May;581(7809):465-469. [CrossRef]
- Jeong HW, Kim SM, Kim HS, Kim YI, Kim JH, Cho JY et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin Microbiol Infect. 2020 Nov;26(11):1520-1524. [CrossRef]
- Cerrada-Romero C, Berastegui-Cabrera J, Camacho-Martínez P, Goikoetxea-Aguirre J, Pérez-Palacios P, Santibáñez S et al. Excretion and viability of SARS-CoV-2 in feces and its association with the clinical outcome of COVID-19. Sci Rep. 2022 May 5;12(1):7397. PMID: 35513481; PMCID: PMC9070969. [CrossRef]
- Dergham J, Delerce J, Bedotto M, La Scola B, Moal V. Isolation of Viable SARS-CoV-2 Virus from Feces of an Immunocompromised Patient Suggesting a Possible Fecal Mode of Transmission. J Clin Med. 2021 Jun 18;10(12):2696. PMID: 34207314; PMCID: PMC8235306. [CrossRef]
- Xiao F, Sun J, Xu Y, Li F, Huang X, Li H et al. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg Infect Dis. 2020 Aug;26(8):1920-1922. [CrossRef]
- Sun J, Zhu A, Li H, Zheng K, Zhuang Z, Chen Z et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect. 2020 Dec;9(1):991-993. PMID: 32342724; PMCID: PMC7301718. [CrossRef]
- Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P et al. SARS-CoV-2 Isolation from Ocular Secretions of a Patient With COVID-19 in Italy with Prolonged Viral RNA Detection. Ann Intern Med. 2020 Aug 4;173(3):242-243. [CrossRef]
- Leal FE, Mendes-Correa MC, Buss LF, Costa SF, Bizario JCS, de Souza SRP et al. Clinical features and natural history of the first 2073 suspected COVID-19 cases in the Corona São Caetano primary care programme: a prospective cohort study. BMJ Open. 2021 Jan 12;11(1):e042745. PMID: 33436471; PMCID: PMC7805372. [CrossRef]
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020 Jan;25(3):2000045. Erratum in: Euro Surveill. 2020 Apr;25(14): Erratum in: Euro Surveill. 2020 Jul;25(30). [CrossRef]
- Chu DKW, Pan Y, Cheng SMS, Hui KP, Krishnan P, Liu Y, et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem 2020; 66(4):549–5. PMID: 32031583. [CrossRef]
- Mendes-Correa MC, Tozetto-Mendoza TR, Freire WS, Paiao HGO, Ferraz ABC, Mamana AC et al. Torquetenovirus in saliva: A potential biomarker for SARS-CoV-2 infection? PLoS One. 2021 Aug 24;16(8):e0256357. PMID: 34428230; PMCID: PMC8384193. [CrossRef]
- Emery SL, Erdman DD, Bowen MD, Newton BR, Winchell JM, Meyer RF et al. Real-time reverse transcription-polymerase chain reaction assay for SARS-associated coronavirus. Emerg Infect Dis. 2004 Feb;10(2):311-6. PMID: 15030703; PMCID: PMC3322901. [CrossRef]
- Lima LR, Silva AP, Schmidt-Chanasit J, Paula VS. Diagnosis of human herpes virus 1 and 2 (HHV-1 and HHV-2): use of a synthetic standard curve for absolute quantification by real time polymerase chain reaction. Mem Inst Oswaldo Cruz. 2017 Mar;112(3):220-223. Epub 2017 Feb 16. PMID: 28225902; PMCID: PMC5319368. [CrossRef]
- Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021 May 21;372(6544):815-821. [CrossRef]
- Rambaut A, Holmes EC, O'Toole Á, Hill V, McCrone JT, Ruis C et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020 Nov;5(11):1403-1407. [CrossRef]
- Candido DS, Claro IM, de Jesus JG, Souza WM, Moreira FRR, Dellicour S et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020 Sep 4;369(6508):1255-1260. [CrossRef]
- Nicolete VC, Rodrigues PT, Fernandes ARJ, Corder RM, Tonini J, Buss LF et al. Epidemiology of COVID-19 after Emergence of SARS-CoV-2 Gamma Variant, Brazilian Amazon, 2020-2021. Emerg Infect Dis. 2022 Mar;28(3):709-712. [CrossRef]
- Sabino EC, Buss LF, Carvalho MPS, Prete CA Jr, Crispim MAE, Fraiji NA et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021 Feb 6;397(10273):452-455. [CrossRef]
- Tallmadge RL, Laverack M, Cronk B, Venugopalan R, Martins M, Zhang X et al. Viral RNA Load and Infectivity of SARS-CoV-2 in Paired Respiratory and Oral Specimens from Symptomatic, Asymptomatic, or Postsymptomatic Individuals. Microbiol Spectr. 2022 Jun 29;10(3): e0226421. [CrossRef]
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 May 12;323(18):1843-1844. PMID: 32159775; PMCID: PMC7066521. [CrossRef]
- Ke R, Martinez PP, Smith RL, Gibson LL, Mirza A, Conte M et al. Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. Nat Microbiol. 2022 May;7(5):640-652. [CrossRef]
- Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. (2020) 383:2291–3. [CrossRef]
- Sung A, Bailey AL, Stewart HB, McDonald D, Wallace MA, Peacock K et al. Isolation of SARS-CoV-2 in Viral Cell Culture in Immunocompromised Patients with Persistently Positive RT-PCR Results. Front Cell Infect Microbiol. 2022 Feb 2; 12:804175. PMID: 35186791; PMCID: PMC8847756. [CrossRef]
- Kim, M. C., Cui, C., Shin, K. R., Bae, J. Y., Kweon, O. J., Lee, M. K., et al. (2021). Duration of Culturable SARS-CoV-2 in Hospitalized Patients with Covid-19. N Engl. J. Med. 384 (7), 671–673. [CrossRef]
- Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020 Dec 23;183(7):1901-1912.e9. [CrossRef]
- Cunha MDP, Vilela APP, Molina CV, Acuña SM, Muxel SM, Barroso VM et al. Atypical Prolonged Viral Shedding with Intra-Host SARS-CoV-2 Evolution in a Mildly Affected Symptomatic Patient. Front Med (Lausanne). 2021 Nov 26; 8:760170. PMID: 34901074; PMCID: PMC8661089. [CrossRef]
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020 May 28;181(5):1036-1045.e9. [CrossRef]
- O'Connell P, Aldhamen YA. Systemic innate and adaptive immune responses to SARS-CoV-2 as it relates to other coronaviruses. Hum Vaccin Immunother. 2020 Dec 1;16(12):2980-2991. [CrossRef]
- Yang B, Fan J, Huang J, Guo E, Fu Y, Liu S et al. Clinical and molecular characteristics of COVID-19 patients with persistent SARS-CoV-2 infection. Nat Commun. 2021 Jun 9;12(1):3501. [CrossRef]
- Okita Y, Morita T, Kumanogoh A. Duration of SARS-CoV-2 RNA positivity from various specimens and clinical characteristics in patients with COVID-19: a systematic review and meta-analysis. Inflamm Regen. 2022 Jun 1;42(1):16. PMID: 35642011; PMCID: PMC9156361. [CrossRef]
- Folgueira MD, Luczkowiak J, Lasala F, Pérez-Rivilla A, Delgado R. Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19. Clin Microbiol Infect. 2021 Jun;27(6):886-891. [CrossRef]
- Dadras O, Afsahi AM, Pashaei Z, Mojdeganlou H, Karimi A, Habibi P et al. The relationship between COVID-19 viral load and disease severity: A systematic review. Immun Inflamm Dis. 2022 Mar;10(3):e580. [CrossRef]
- Kawasuji H, Morinaga Y, Tani H, Yoshida Y, Takegoshi Y, Kaneda M et al. SARS-CoV-2 RNAemia with a higher nasopharyngeal viral load is strongly associated with disease severity and mortality in patients with COVID-19. J Med Virol. 2022 Jan;94(1):147-153. [CrossRef]
- Leblanc JF, Germain M, Delage G, O’Brien S, Drews SJ, Lewin A. Risk of transmission of severe acute respiratory syndrome coronavirus 2 by transfusion: a literature review. Transfusion. 2020; 60:3046–54.
- Chen L, Wang G, Long X, Hou H, Wei J, Cao Y et al. Dynamics of Blood Viral Load Is Strongly Associated with Clinical Outcomes in Coronavirus Disease 2019 (COVID-19) Patients: A Prospective Cohort Study. J Mol Diagn. 2021 Jan;23(1):10-18. [CrossRef]
- Christensen J, Kumar D, Moinuddin I, Bryson A, Kashi Z, Kimball P, et al. Coronavirus disease 2019 viremia, serologies, and clinical course in a case series of transplant recipients. Transplant Proc. 2020; 52:2637–41.
- Roshandel MR, Nateqi M, Lak R, Aavani P, Sari Motlagh R, F Shariat S, et al. Diagnostic and methodological evaluation of studies on the urinary shedding of SARS-CoV-2, compared to stool and serum: a systematic review and meta-analysis. Cell Mol Biol (Noisyle-Grand). 2020; 66:148–56. 180.
- Morone G, Palomba A, Iosa M, Caporaso T, De Angelis D, Venturiero V et al. Incidence and Persistence of Viral Shedding in COVID-19 Post-acute Patients with Negativized Pharyngeal Swab: A Systematic Review. Front Med (Lausanne). 2020 Aug 28; 7:562. PMID: 32984389; PMCID: PMC7483760. [CrossRef]
- United States Centers for Disease Control and Prevention. Ending Isolation and Precautions for People with COVID-19: Interim Guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. (Accessed on March 03, 2023).
Table 1.
Description of the oligo sequences for the detection of CoV-2 by RT-qPCR.
Table 1.
Description of the oligo sequences for the detection of CoV-2 by RT-qPCR.
| Oligo |
Description |
Sequence 5'-3' |
| Forward |
HKU-NF |
5’-TAA TCA GAC AAG GAA CTG ATT A-3’ |
| Reverse |
HKU-NR |
5’-CGA AGG TGT GAC TTC CAT G-3’ |
| Probe |
HKU-NP |
format 5’-Cy5/TAO/3’-IABkRQ): 5’-GC AAA TTG TGC AAT TTG CGG-3’ |
| Curve |
Synthetic |
5’-ttcgtCGAAGGTGTGACTTCCATGcgtatCCGCAAATTGCACAATTTGCatgcgtAATCAGTTCCTTGTCTGATTActgata-3’ |
| Forward |
E-Sarberco F1 |
5’- ACA GGT ACG TTA ATA GTT AAT AGC GT-3’ |
| Reverse |
E-sarberco-R2 |
5’-ATA TTG CAG CAG TAC GCA CAC A-3’ |
| Probe |
E_sarberco P1 |
format 5’-VIC/ZEN/3’IABkFQ: 5’- ACA CTA GCC ATC CTT ACT GCG CTT CG - 3’ |
| Curve |
Synthetic |
5´-ttcgtATATTGCAGCAGTACGCACACcgtatCGAAGCGCAGTAAGGATGGCTAGTGTatgcgtACGCTATTAACTATTAACGTACCTGTctgata -3’ |
Table 2.
Clinical characteristics of study population.
Table 2.
Clinical characteristics of study population.
Table 3.
SARS-CoV-2 B1 strain detection at different sites in immunocompetent and immunosuppressed patients.
Table 3.
SARS-CoV-2 B1 strain detection at different sites in immunocompetent and immunosuppressed patients.
Table 4.
SARS-CoV-2 Gamma strain detection at different sites in immunocompetent and immunosuppressed patients.
Table 4.
SARS-CoV-2 Gamma strain detection at different sites in immunocompetent and immunosuppressed patients.
Table 5.
SARS-CoV-2 B1 strain propagation in culture from samples obtained from different sites.
Table 5.
SARS-CoV-2 B1 strain propagation in culture from samples obtained from different sites.
Table 6.
SARS-CoV-2 Gamma strain propagation in culture from samples obtained from different sites.
Table 6.
SARS-CoV-2 Gamma strain propagation in culture from samples obtained from different sites.
Table 7.
Duration of SARS-CoV-2 detection at different sites in immunocompetent and immunosuppressed individuals.
Table 7.
Duration of SARS-CoV-2 detection at different sites in immunocompetent and immunosuppressed individuals.
Table 8.
Detection of SARS-CoV-2 B1 and Gamma strains over time from different sites.
Table 8.
Detection of SARS-CoV-2 B1 and Gamma strains over time from different sites.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).