1. Introduction
Pollutants deposited in the ecological systems especially heavy metals are a major concern due to their toxicity. Heavy metals are of great interest due to the role they play in environmental and biological systems. Compared to other organic pollutants, heavy metals are very stable substrates at ambient conditions so they accumulate in soil and water bodies and thus are transported to humans and mammals through the food chain [
1,
2,
3,
4]. Most of the reported heavy metals receive great attention from environmental sectors and health agencies due to their harmful effect on humans and living environments even in very small concentrations. In specific, studies showed that the presence of lead, cadmium, and mercury contaminants may damage many organs and tissues including the brain, bone, liver, and calcium metabolism disorders, leading to certain types of cancers [
5,
6,
7,
8,
9,
10]. Despite the harm they cause to the environment and human health, they are widely used in our daily life since they play a key role in many industries. For instance, mercury is deposited into the environment through various industrial actions including mining, coal combustion, metal smelter exhaust, and paper mills [
11,
12,
13]. Combustion and industrial sources are significant for mercury emission into the environment where most of the anthropogenic mercury emissions is associated with the burning of fossil fuel, coal and oil. In addition, being part of major industrial products of daily use including batteries, paper, latex paints, and electronics, mercury deposition to the environment is significant especially when no effective and direct recycling processes are applied. Mercury accumulated in the environment in three forms with Hg(II) is the most widely spread in the environment.
In contrast to mercury, iron is one of the major elements that is widely used in various industrial applications [
14,
15]. As an essential element, iron is a very important element to humans and other living organisms. Despite its importance in human health, the iron content should be optimized where cases with deficient or saturated levels of iron are reported to cause function disorders, weak immunity, and lack of sleep [
16,
17,
18]. As a result, high levels of iron contamination in drinking water poses health concerns, thus the World Health Organization recommended that the iron (III) levels in drinking water to be very low [
19].
Given the high stability of heavy metal along with the direct and indirect effect they pose to human health, there is a demand to selectively and sensitively detect their presence in both environmental and biological samples. Several instruments and methods were modified to detect and quantify mercury and other toxic metal ions in water bodies. The common techniques in use include atomic absorption spectrometry (AAS) [
20,
21], inductively coupled plasma techniques [
22,
23,
24], and electrochemistry [
25]. In some cases, these methods were modified and used along with various separation chromatographic methods including ion chromatography, gas chromatography, and high-performance liquid chromatography [
26,
27]. While these methods are very sensitive with a wide range of linearity, still the high cost and long operation, and complicated sampling processes of such techniques increase the need of finding simpler and faster methods that provide similar results in a shorter time. Recently, chemiluminescent sensors were modified to investigate organic and inorganic analytes in biological and environmental systems [
28,
29,
30]. This provides a simple method to detect target molecules/ions with low detection limits over a short time. Importantly, luminescence-based sensors can be further designed to attain a wide range of signaling “antenna” when the analyte covalently binds to the luminescent probe providing opportunities to monitor the concentrations of the target within a short time. This antenna can be observed through changes in the signal’s intensities, wavelength, lifetime, and chirality [
28,
29,
30,
31,
32].
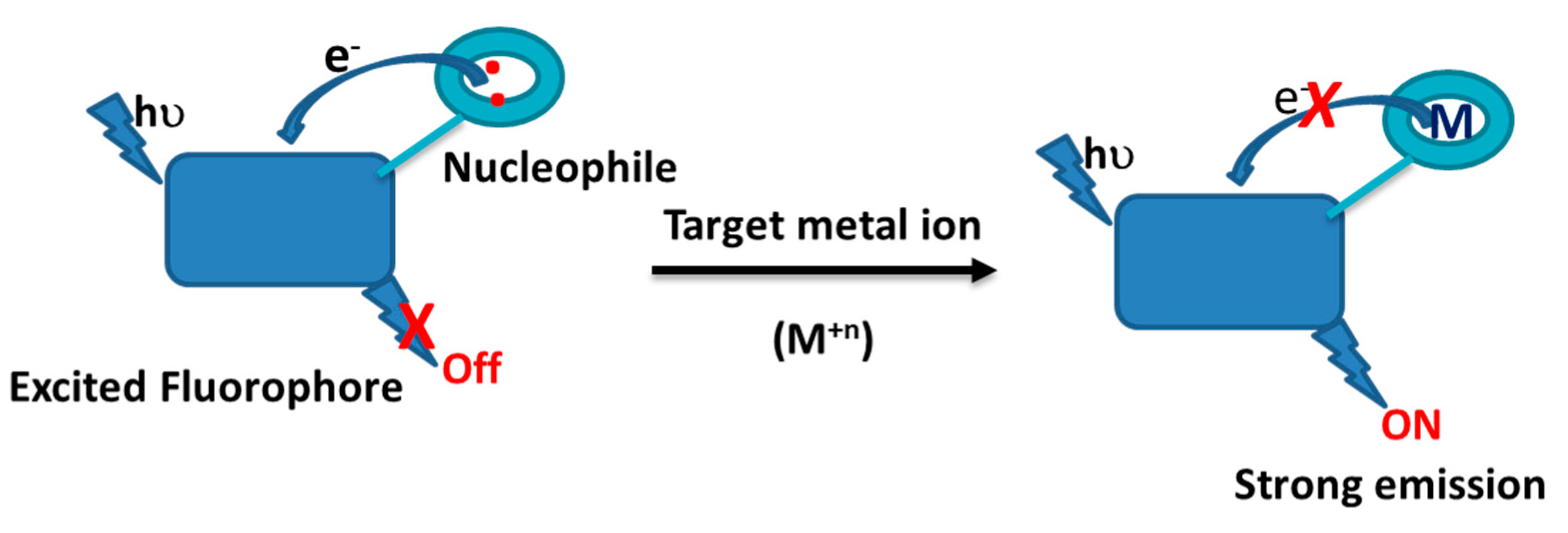
The photophysical properties of a fluorophore can be designed by establishing proton-, energy-, or electron-transfer processes that are sensitive toward the target molecules including heavy metal ions. One of the most common strategies used to build luminescent devices is to facilitate the photoinduced electron transfer (PET) process that turns the emission
on or off upon target analyte binding/interaction (see
Scheme 1). Three major features must be present for designing a luminescent sensor: the luminophore, spacer, and receptor. When the target analyte binds to the receptor, an immediate change to the emission occur where emission intensity may be enhanced or quenched making
‘off-on’ or
‘on-off’ sensors as illustrated in
Scheme 1. Variations to the emission spectra may occurs through various mechanisms including PET, Ligand-to-metal charge transfer (LMCT), or metal-to-ligand charge transfer (MLCT) [
30,
31,
32,
33,
34]. Several studies reported that the LMCT involves electronic transitions from the localized orbitals in the conjugated linker to a metal-centered orbital and is highly sensitive to the ionic size and the coordination geometry of the linker [
30,
31,
32,
33,
34,
35,
36,
37].
While several conjugated organic molecules were reported for metal ion detection at low concentration levels [
30,
31,
32,
33,
34,
35,
36,
37], the available fluorescent sensors that selectively detect mercury at ambient environmental conditions are limited. Much attention has been focused on porphyrin derivatives with strong fluorescent properties that are quenched upon the addition of mercury ions as
on-off type sensors [
38,
39,
40]. Additionally, most of the reported luminescent chemo sensors lack selectivity and water solubility which mimic their use in real environmental samples. In the present investigation we synthesized N
1, N
3, N
5-tris(2-hydroxyphenyl)benzene-1,3,5-tricarboxamide (
Sensor A) that is tailored to three different suitable sites to capture metal ions forming five-membered ring associated between the linker and the target metal ion of use. Both the orientation of the donor sites and the associated space are designed to covalently bind to Hg (II). For the potential use of
Sensor A for real water samples, we tested several water samples of environmental, tap, and deionized water. Since our goal is to find a selective and sensitive sensor for the detection of specific metal ions in water bodies, the photophysical properties of
Sensor A toward various concentrations of metal ions including Hg(II), Mg
2+, Na
+, Zn
2+, Ni
2+, Pb
2+, Fe
3+, and Ca
2+ ions were investigated at various aqueous solutions adjusted at three different pH values (pH 5, 7, and 10).
2. Materials and Methods
All chemicals and reagents including 1,3,5-benzenetricarboxylic acid (trimesic acid), o-aminophenol, iron (III) nitrate, mercury (II) nitrate, zinc nitrate, magnesium nitrate, sodium nitrate, nickel (II) nitrate, ethanol, and phosphate buffers were purchased from Sigma-Aldrich and used without further purifications. Also, 1000 ppm standard solutions were purchased from Sigma-Aldrich and used for quality control in this study. All solvents used are of analytical reagent grade and the water was doubly distilled. The metal ion solutions were prepared from their nitrate salts with specific metal concentrations calculated to represent the quencher concentrations.
UV-visible measurements were recorded on a single-beam UV-visible spectrophotometer (carry 50 conc) equipped with a xenon arc lamp. Fluorescence spectra were recorded from a carry eclipse Varian spectrophotometer equipped with 150 W continuous xenon lamp along with a sensitive grating and PMT detector. The machine has a feature to survey the excitation and emission profile of the fluorophore. Excitation and emission curves were recorded upon exposure to the selected emission and excitation wavelengths, respectively. Infrared spectra were recorded using the ABB Bomem MB3000 series combined with the intuitive Horizon MBTM FTIR software. NMR spectra were recorded on Bruker 400 MHz.
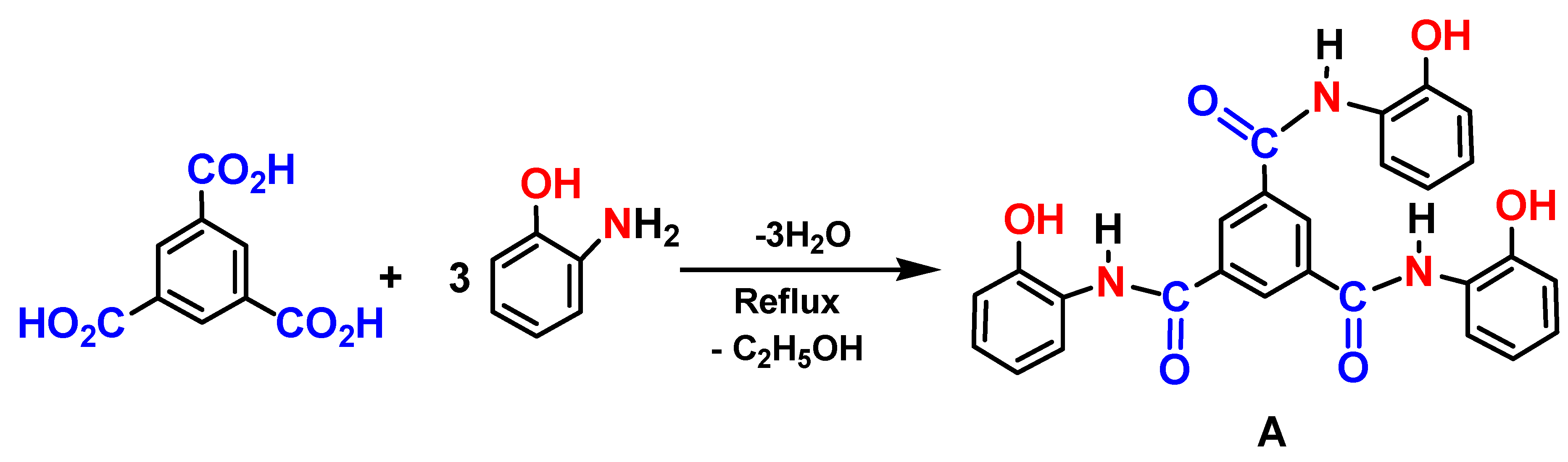
2.1. Synthesis of Sensor A.
Compound A was synthesized by a solvothermal process where trimesic acid (0.693 g, 3.3 mmol) and o-aminophenol (1.06 g, 9.9 mmol) were dissolved in ethanol (20 mL each). The trimesic acid solution was connected to reflux at 80°C. When all the solids dissolved, the o-aminophenol solution was gradually added and continued under reflux for 6 h with continuous stirring where no starting materials remains as depicted from thin layer chromatography. The red precipitate formed after slow cooling to room temperature and was filtered and crystallized from ethanol. The collected precipitate was dried in an oven at 80°C for 2 h and then air-dried for 1 h before further use in this study. The material was characterized using UV-visible, Luminescence, FTIR, and NMR spectroscopic techniques. The reaction yield obtained was 93% with a high melting point of m.p. > 300℃. FTIR (KBr): ν (cm−1) 3373, 1691, 1604, 1495, 1460, 1381, 1280, 1110, 928 cm-1. Proton NMR (1H NMR, 400 MHz, d6-DMSO) showed δ (ppm) 8.90 (s, 3H), 8.0 (s, 3H), 7.47 (d, 3H), 6.7-6.84 (d, 9H). 5.0 (s, 3H).
2.2. Binding Studies
We rely on the SSLS to monitor the changes associated to the fluorimetric and colorimetric features for detecting different metal ionswater samples. 30-ppm solutions of Sensor A were prepared in methanol: water, 10:90 v:v buffered solutions at pH 5, 7, and 10 using phosphate buffers. Several concentrations of the tested analytes including Zn (II), Hg (II), Mg2+, Na+, Ca2+, Ni (II), and Fe (III) ions were prepared from their nitrate salts with concentrations ranging from 0.5 to 1000 ppm. The initial luminescence intensity was recorded for a solution containing 2.75 mL of Sensor A diluted with water to a 3.0 mL total volume. The fluorescence intensity was monitored to reach stable emission thus maximum binding and quenching affinity was reached. Using 100 ppm solution of the metal ion of interest, it was noticed that immediate responses occurred with iron and mercury ions with no change to the emission profile occurred when other metal ions are used even after being monitored for 30 min. Even though the binding affinity occurred immediately, we followed the binding affinity for all tested various metal ion concentrations after 2-min intervals for a complete binding to be established. To ensure that the observed inhibition to the luminescence intensity is directed to the binding affinity between the sensor and the target metal ions, equal volumes of water (0.25 mL) were added to the organic sensor for an initial emission intensity that will be used as F0.
2.3. Equilibration adsorption measurements.
To evaluate the adsorption of mercury ions on
Sensor A, a 2 mL solution containing 6.0 mg of
Sensor A was monitored upon adding various volumes of 50 ppm mercury ions solution at pH 7 for 10 min. The initial and the final equilibrium concentrations were determined from the emission intensity at 515 nm. The equilibrium adsorption capacity of mercury ion on
Sensor A was then determined using equation 1.
where Q
eqm is the equilibrium adsorption capacity,
Co is the initial concentration of the sensor and
Ceqm is the equilibrium concentration of the adsorbent with V and W represent the solution's volume and the mass of adsorbent, respectively. Finally, the the removal efficiency from the initial concentrations, η %, is determined using equation 2:
3. Results and Discussion
The luminescent
Sensor A was prepared in a one-pot reaction of trimesic acid and
o-aminophenol in a 1:3 mole ratio,
Scheme 2. The product was crystallized from ethanol and dried at 100℃ for 1 h.
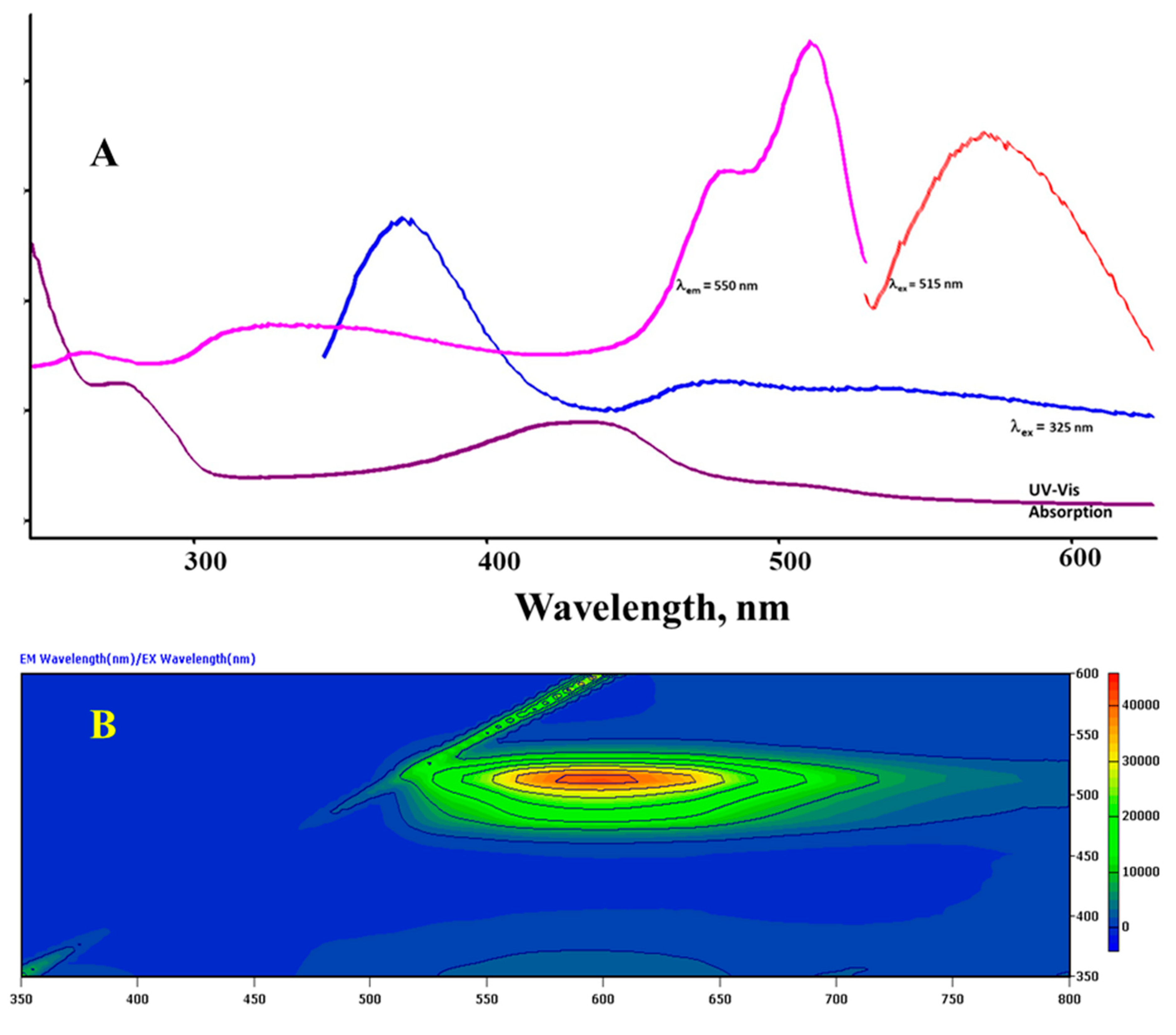
Figure 1 shows the UV-Vis absorption spectrum along with the excitation and emission spectra of
Sensor A depicted at the indicated wavelengths. The UV-Vis spectrum of
A showed absorption peaks with maximum absorbance appearing at 275 and 436 nm along with a shoulder peak at 515 nm. The compound also possesses strong excitation and emission profiles in the UV and visible regions. The emission profile depends on the selected excitation wavelength. For instance, a strong emission appears at 571 nm upon excitation at 515 nm, whereas strong emissions were observed at 375 and 515 nm upon excitation at 325 nm. The excitation profile monitored at 550 nm also indicates bands at 512 and 325 nm aligned to the absorption bands depicted from the UV-Vis absorption spectrum. The obtained absorption and emission profiles are due to the high conjugation chain along with plausible n-π* transitions.
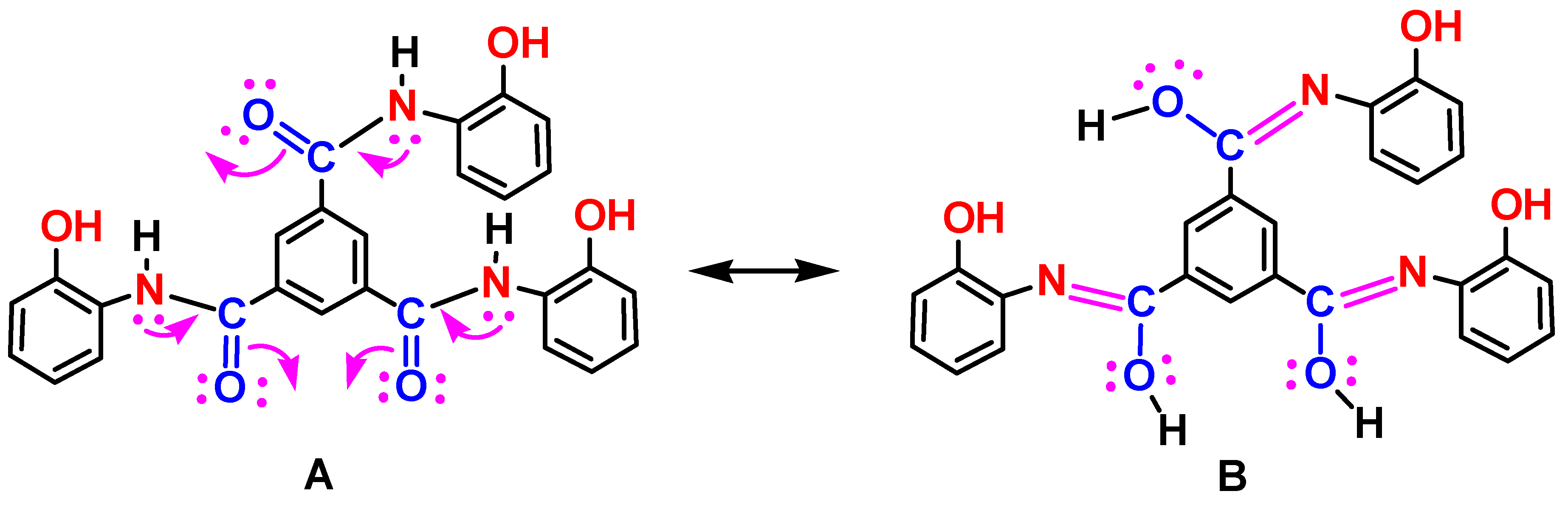
Figure 1B shows a full-range 2D excitation-emission profile where the maximum luminescence features appear in the visible range from 500-700 nm. The appearance of the emission bands in the visible region corresponds to the presence of the extended conjugation of the designed fluorophore with the two aminophenol substrates
via the possible formation of amide-imido tautomers in the solution that extends the conjugation to all fused rings,
Scheme 3.
The selection of
Sensor A for metal ion detection stems from the strong chain of conjugation that provides reasonable clear and strong fluorescence and absorption properties with a special molecular skeleton, with efficient binding sites for metal ions that causes a direct and sudden change to its luminescence features. Finally, the material is highly soluble in polar solvents thus the testing protocols can be applied to real environmental samples as illustrated therein. Given the broad features of the UV-visible absorption bands and the lack of well-defined emission bands due to the selected excitation wavelengths,
Figure 1A, we rely on the Synchronous Scan Luminescence Spectroscopy (SSLS) rather than ordinary emission or excitation spectra for monitoring the binding interaction with the target metal ions. This method involves recording the luminescence intensity while both the excitation and emission monochromators are varied at a constant wavelength difference (Δλ) [
41,
42]. Using this technique, one can monitor luminescence profiles for materials with weak luminophores or low concentrations with well-defined bands especially when both excitation and emission intensities will be gathered as previously reported [
41,
42,
43].
The degree of covalency between the metal ions and the organic sensor depends on the pH and the linkage sites require tailoring the sensor under optimized conditions. For example, a study illustrated the solvent effect on the structural relaxation that follows the displacement of the electron cloud in the dipolar merocyanine dyes where variations were observed in the fluorescence quantum yield that is associated with the dielectric constant of the solvent. In specific, using a series of solvents ordered by increasing dielectric constant the optical gap was found to decrease as the polarity of the solvent increases which results in the positive solvatochromic shift observed in the absorption and emission spectrum [
44]. Therefore, all the SSLS recorded in this study represent analysis for solutions using similar aqueous compositions. Besides the high sensitivity and the low detection limit, SSLS also helps to study changes that may occur during the process when new luminophores are formed during the binding processes under various pH buffer solutions and regular ambient water samples.
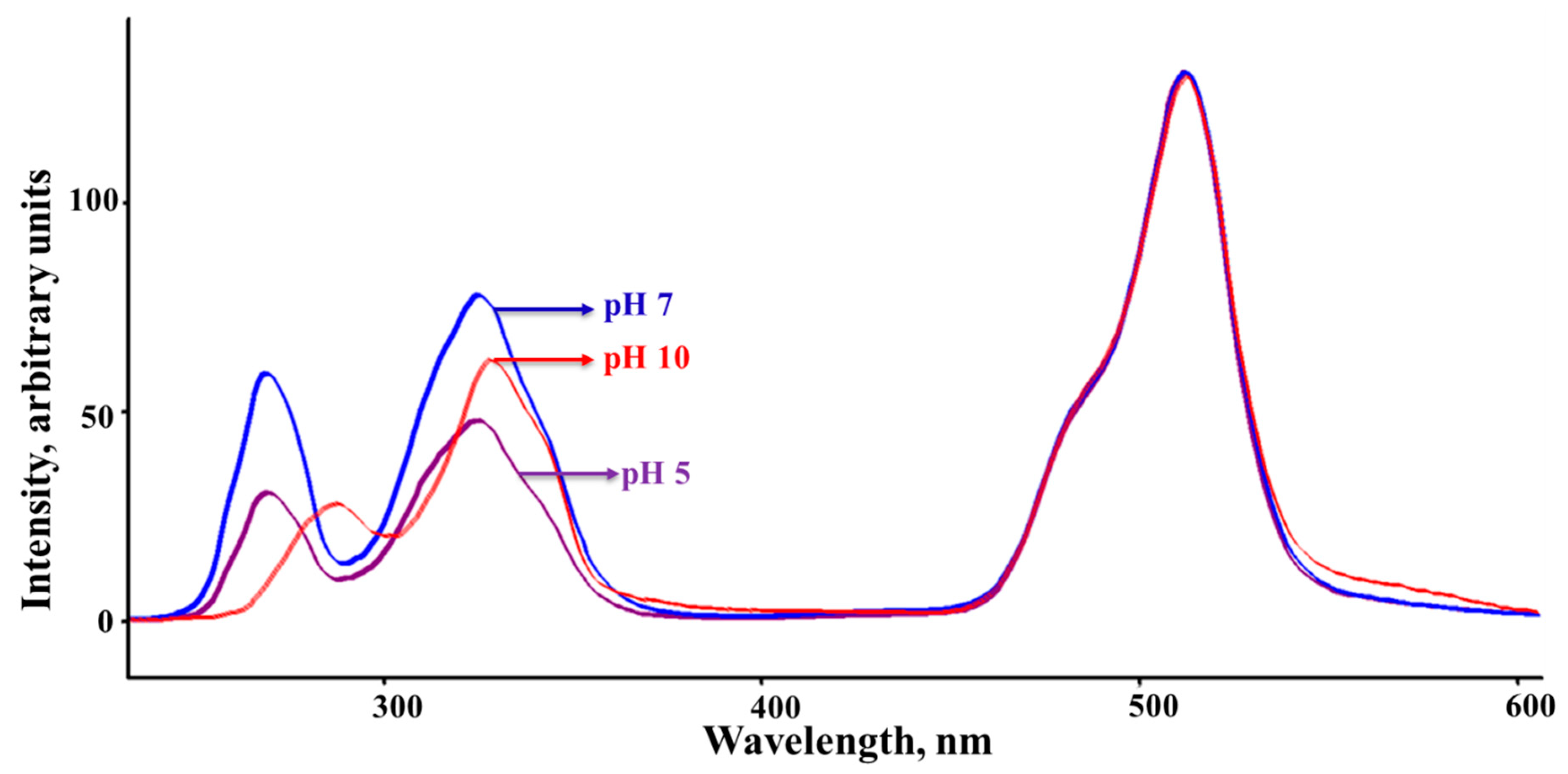
Figure 2 shows the SSLS recorded at Δλ = 50 nm for
Sensor A prepared in water solution as well as adjusted pH buffer solutions. As shown in
Figure 2,
Sensor A has a strong luminescence band in the visible range along with two bands in the UV region at 275 and 325 nm indicating the presence of several luminophores. As shown in
Figure 2, the band that appears in the visible range is solvent and pH-independent while the high energy bands are pH as well as solvent dependent. This supports the fact that the low energy band (visible region) is associated with the long chain of conjugation, while the high-energy modes are related to n-π* transitions that may be affected by varying solutions pH in polar solvents. This provides both luminescent and colorimetric detection of the metal ions. The SSLS recorded under basic conditions, showed a redshift in the high energy bands compared to the analogue spectra recorded at neutral and acidic conditions. This shift occurs because of the formation of a highly conjugated system obtained via the phenolate ions formation.
Since our goal is to find a selective and sensitive sensor for the detection of specific metal ions in water bodies, the photophysical properties of Sensor A toward various concentrations of metal ions including Hg(II), Mg2+, Na+, Zn2+, Ni2+, Pb2+, Fe3+, and Ca2+ ions were investigated at various aqueous solutions adjusted at three different pH values (pH 5, 7, and 10). To test the ability of Sensor A as a luminescent sensor for the given target metal ion, its SSLS was monitored as the metal ion concentration increased in the solution. In brief, SSLS were first recorded for 2.75 mL of 30 ppm solution of Sensor A mixed with 0.25 mL of water and the obtained intensity is taken as a reference (F0). Then, 2.75 mL of the sensor solution was mixed with 0.25 mL of various concentrations of the target metal ion and the SSLS were recorded (referred to as F). To test the time required for complete binding (if any) between Sensor A and the target metal ion, we monitored the emission intensity of Sensor A combined with each metal ion for 15 min. Interestingly, an immediate response has occurred with mercury and iron ions (within 30 seconds) with no response observed for the other metal ions even at higher exposure time. Repeating the measurements for various concentrations, it was noticed that the sensor provides responses in a short time where the luminescence intensity is stabilized immediately upon mixing. However, in the testing protocol followed herein, all solutions were mixed for 2 min after which the SSLS were recorded to get stable and consistent results as presented thereafter.
Sensor A shows an immediate and strong response to both mercury and iron ions with no changes occurring for all other tested metal ions including Na
+, Mg
2+, Ca
2+, Ni
2+, Pb
2+, and Zn (II). This is evidenced by the clear significant changes to the luminescence bands of
Sensor A before and after the addition of various concentrations of mercury and iron ions.
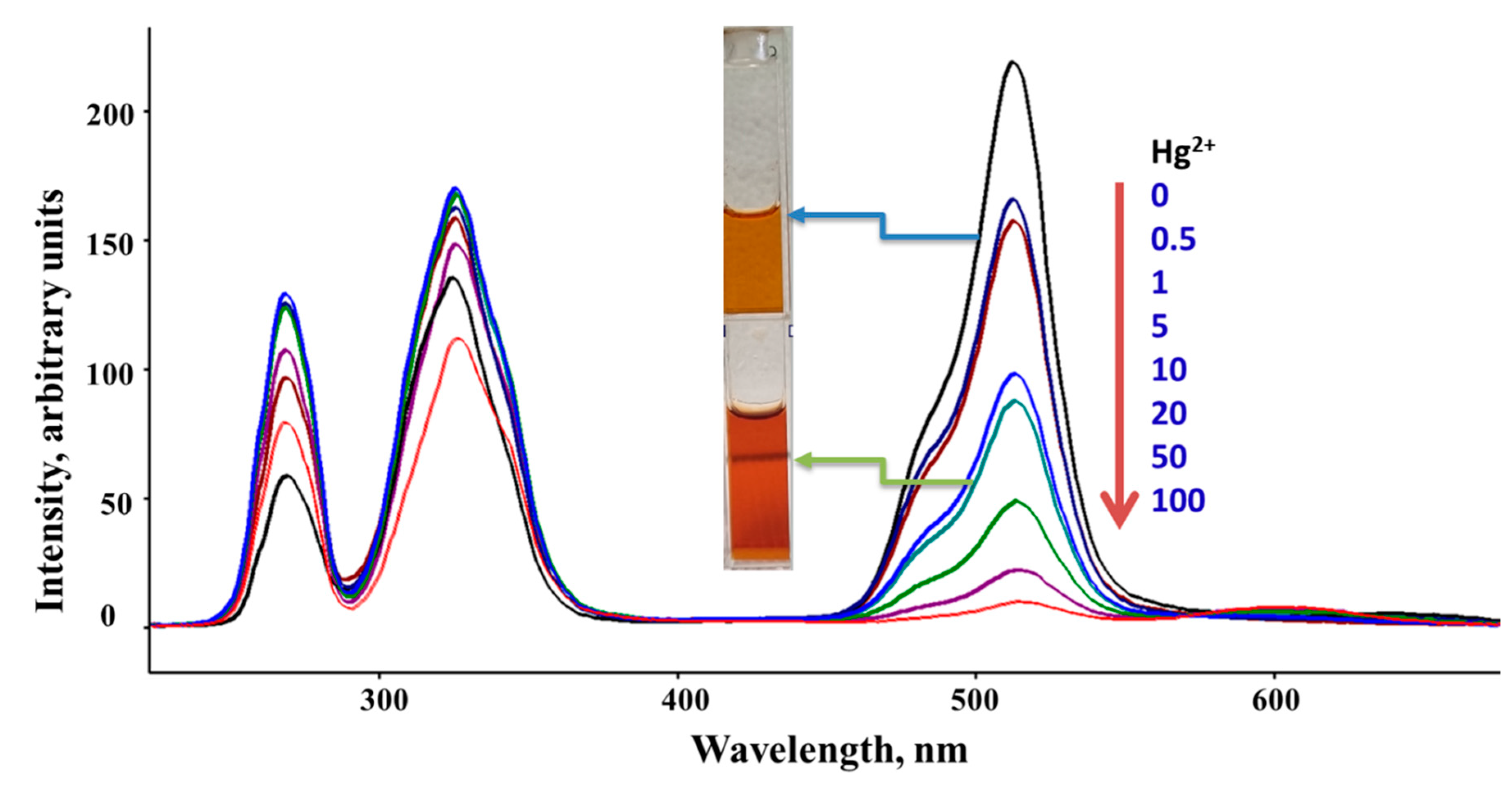
Figure 3 shows the SSLS of
Sensor A before and after adding various concentrations of mercury ions as monitored at pH 5.0. As shown in
Figure 3, the luminescence band intensities are gradually reduced when higher concentrations of mercury ions are added with the quenching magnitude found to be directly proportional to the concentration of the quencher Hg (II). As presented in
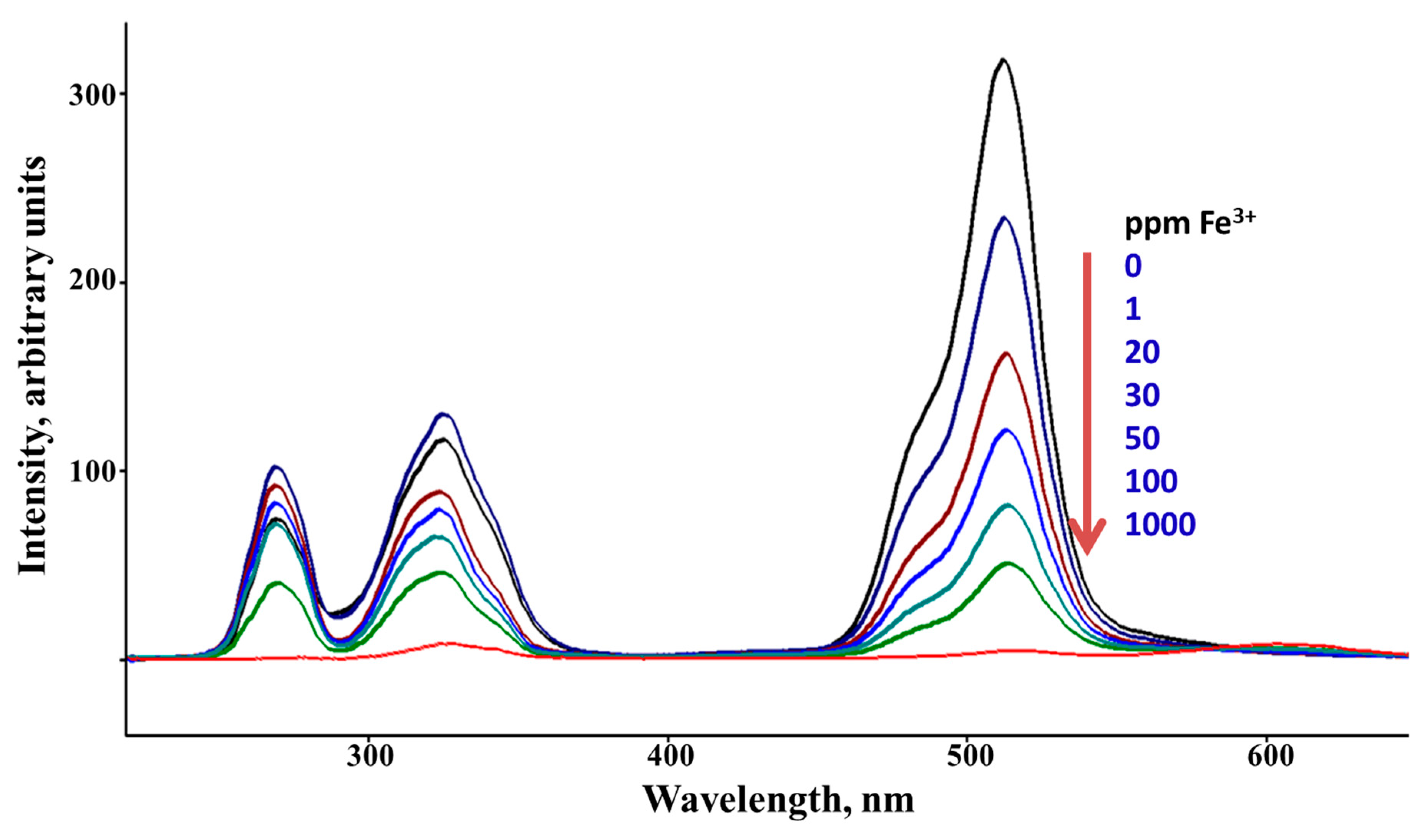
Figure 3, a complete quenching for the band at 515 nm occurred at a 100 ppm Hg (II) concentration level. Similarly, the emission intensity is quenched when different concentrations of Fe(III) were adequately mixed with sensor A. As presented in
Figure 4, the emission intensity observed at 510 nm tends to decline when various levels of ferric ions were added with complete quenching occurred at 1000ppm metal ion concentration. The conjugated probe tend to react with the target metal ion through the suitable donor sites affecting the electron cloud distribution of the sensor, thereby changing the output of the emission intensity of the starting material. Therefore, both results, presented in
Figure 3 and
Figure 4, indicate the high affinity for
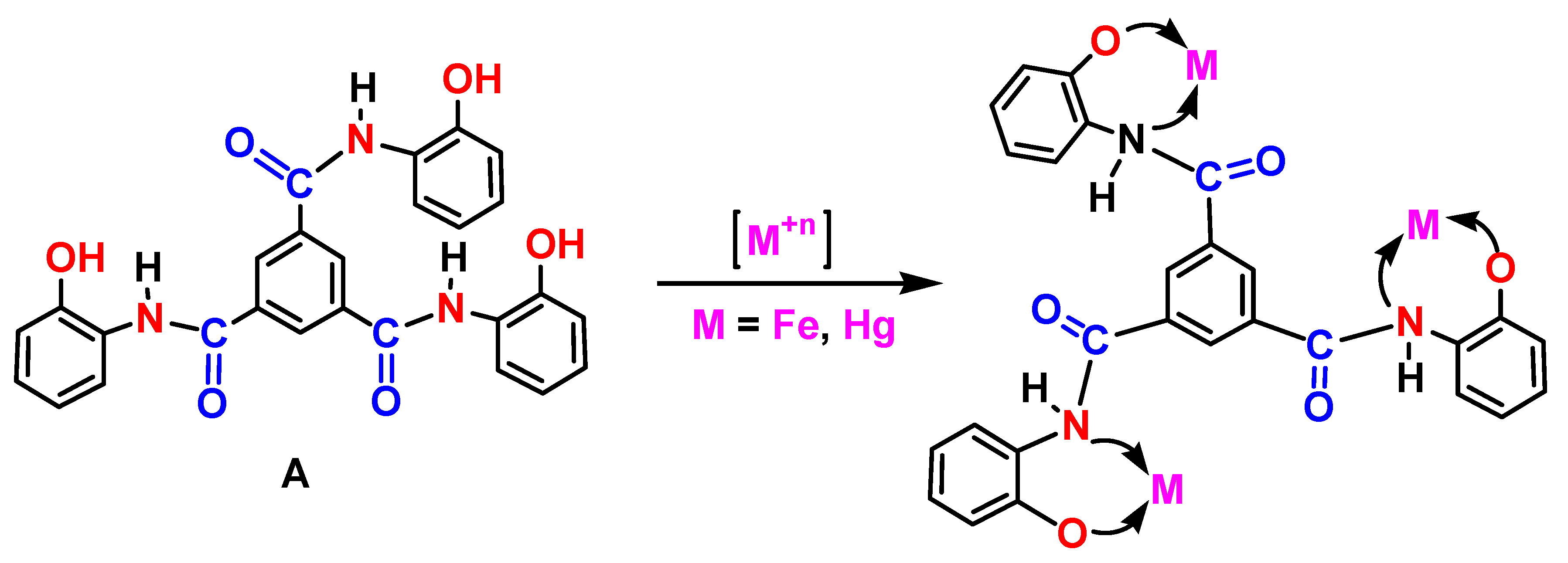
Sensor A to capture mercury and iron ions from solutions forming five-membered chelating rings through complexation with the probe via the imidazole nitrogen atom, pyridine nitrogen atom, and phenolic oxygen atom,
Scheme 4.
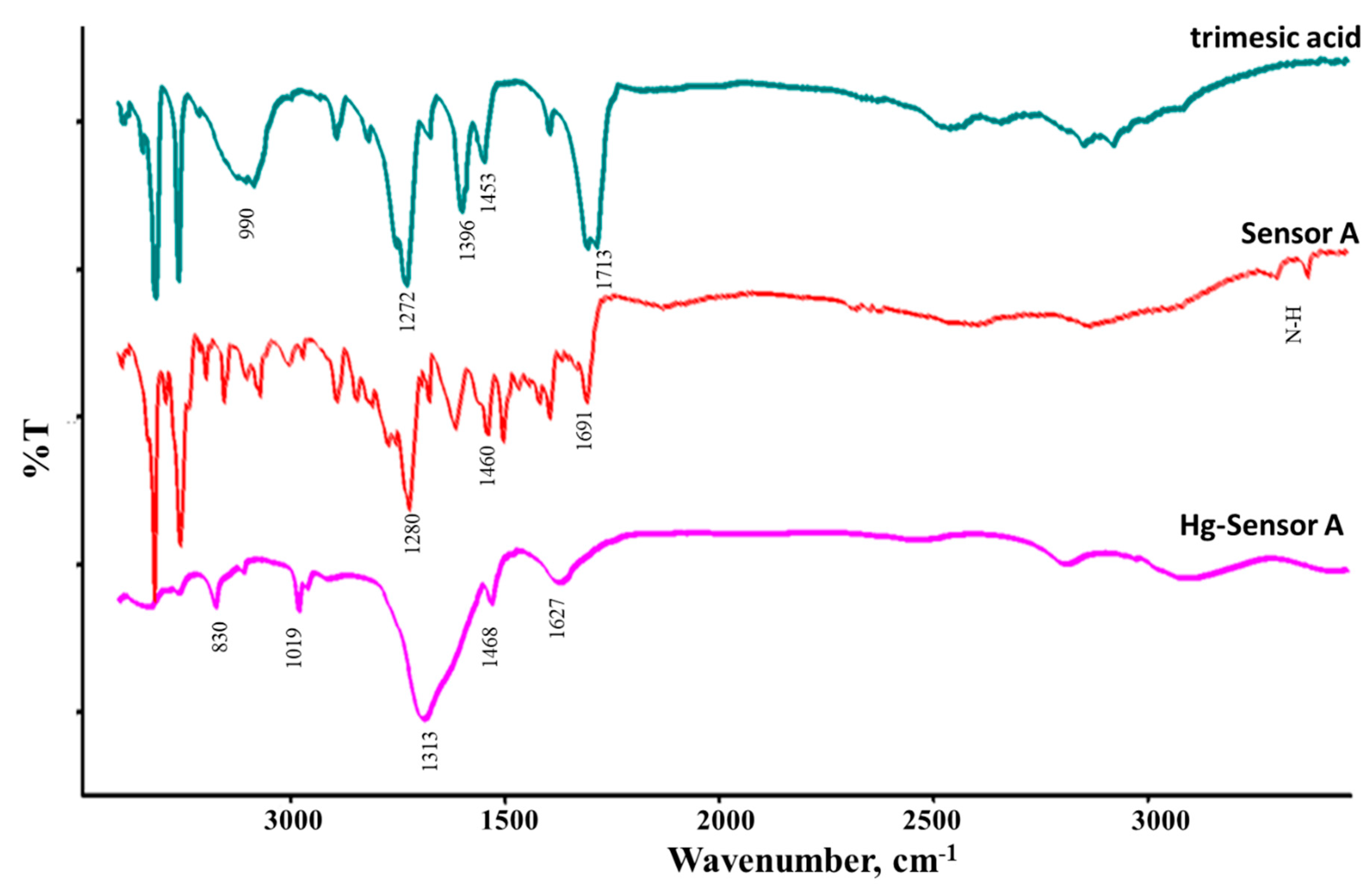
We used FT-ATR spectroscopy to monitor the changes that occurred between the starting material, sensor, and the sensor binding to metal ions.
Figure 5 shows the FT-ATR spectra for trimesic acid, and
Sensor A before and after binding to 50 ppm mercury ions solution (after removing the solvent). Trimeric acid showed bands in the range of 1105, 1250, and 1272 cm
-1 assigned for the aromatic C-H in-plane bending vibrations. The bands that appear at 690, 740, and 990 cm
-1 are assigned to the C-H out-of-plane bending modes. The characteristic infrared absorption bands of C=O appear at 1691 and 1713 cm
-1. The ring C=C stretching modes are observed at 1453 and 1604 cm
−1. Compared to the bands observed for trimesic acid,
Sensor A shows stretching bands at 3303 and 3373 cm
−1 assigned to the N-H stretching mode. The presence of the C=O mode at 1690 cm
−1 along with the C=C and C=N modes appear at 1460 and 1498 cm
-1 supporting the formation of the amide moieties. Upon binding to mercury ions, bands were observed at 1627, and 1468, along with a broad band centered at 1313 cm
-1. The C=N and C=O modes were shifted to appear at 1627 cm
−1 supporting their binding to mercury ions that weakened the C=O and C-N modes,
Scheme 4.
To find the affinity of the binding association (Ksv) between
Sensor A (fluorophore F) and the metal ion of interest (quencher) we use the Stern-Volmer equation shown below providing that static quenching is the dominant quenching process, equation 4 [
42,
43].
Where [Q] is the metal ion concentration (quencher), F
0 and F represent the fluorescence intensity of the sensor before and after adding the quencher.
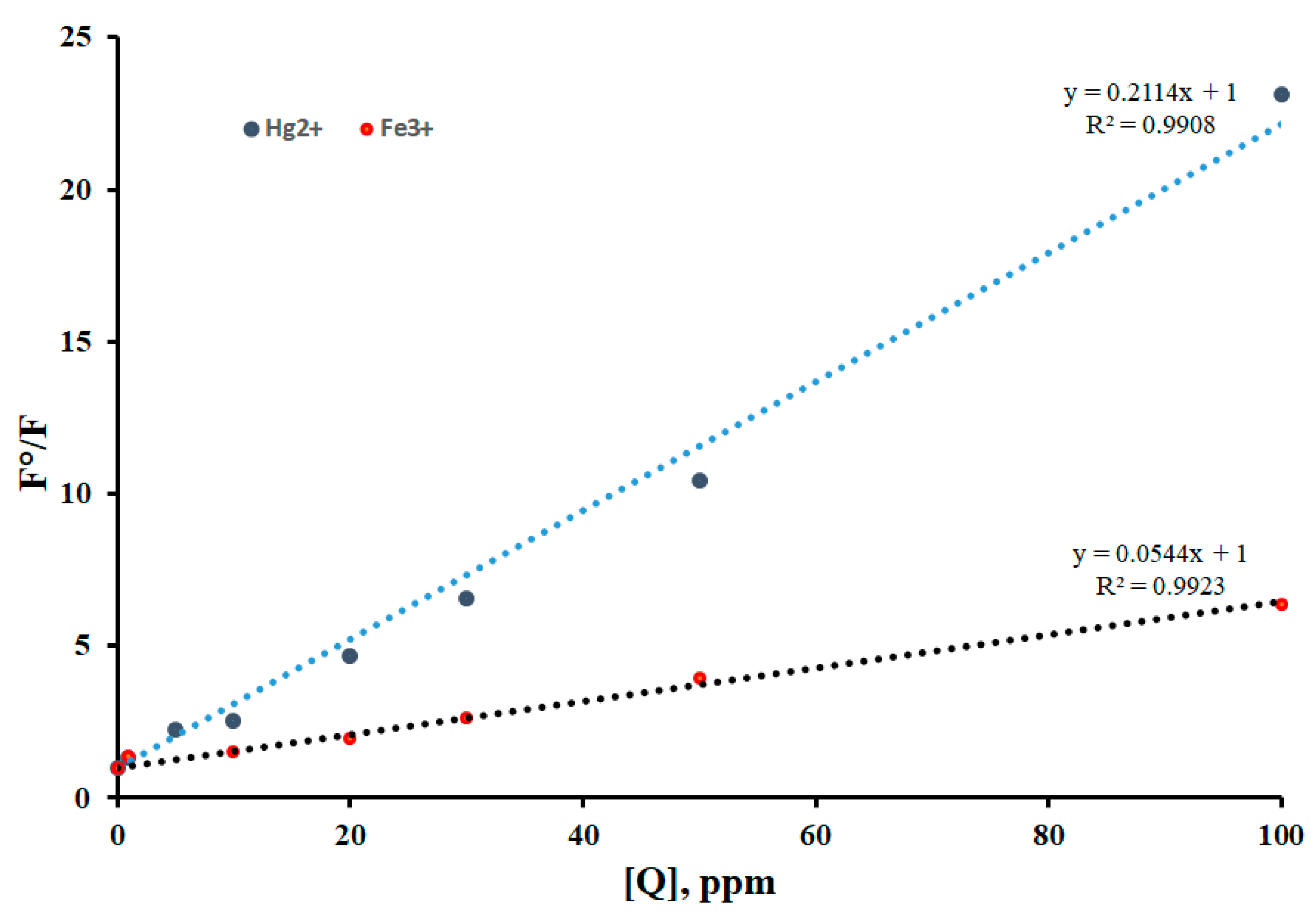
Figure 6 shows the Stern-Volmer plot that describes the affinity of the quencher to reduce the initial emission intensity of the sensor upon mixing reported at pH = 5. As shown in
Figure 6, the plot provides a good correlation between the normalized emission intensity before and after adding various concentrations of Hg(II) and Fe(III) where the R
2=0.9908 and 0.9923, respectively. This is expected given that more covalency occured between the iron or mercury ions that are classified as soft acids with the intermediate basic sites represented via oxygen and imine nitrogen linkage . As presented in
Figure 6, the binding affinity of
Sensor A to Hg (II) is four times higher than that observed for Fe (III),
Table 1. The binding affinity also tends to vary when the pH of the solution is varied using buffers at pH 7 and 10. The binding constants obtained for
Sensor A at various pH conditions are summarized in
Table 1. As depicted in
Table 1,
Sensor A has a strong binding affinity for both metal ions detected in acidic and neutral conditions.
The response toward Hg (II) was linear in the concentration range of 4.2 x 10
-5 to 2.0 x 10
-8 M with a limit of detection of 1.0 x 10
-8 M. In addition,
Sensor A showed a wide linear response in the range of 1.5 x 10
-3 to 1.5 x 10
-8 M toward iron ions. This is very important to detect low levels of Fe (III) in an aqueous medium at the nM scale since Fe (III) is considered among the most important metal ions in biological systems and exerts an incomparable role in many biological processes including RNA and DNA synthesis, and metabolism. While the sensor showed a good response toward both mercury and iron ions, the binding affinity was found to vary at a given pH condition. For example, the relative binding affinity of
Sensor A with mercury ions to that of iron ions is 1.5 at pH 10. This affinity enhanced to around 4 and 9 folds under acidic and neutral conditions, respectively. The stong binding with mercury is expected due to its preference to bind to N-based donor ligands especially the one associated to aromatic and conjugated systems as presented herein. The sensor also provides special skeletal arrangement that allows good size fitting to capture large metal ions under all pH conditions employed,
Scheme 4.
For the potential use of
Sensor A for real water samples, we tested several environmental, tap, and deionized water samples. Further, tap water samples contaminated with 50 ppm iron ions and mercury ions in isolated and mixture forms were also evaluated. The SSLS of
Sensor A was not affected by adding deionized water. The tap water showed a slight reduction in the synchronous scan luminescence bands estimating the iron level to a few ppm levels which were confirmed by ICP analysis. The spiked water samples were added in a different sequence. When 0.25 mL of the spiked sample with 50 ppm iron ions was mixed with
Sensor A, the emission intensity was reduced by 65% with this reduction reaching 97% when the same volume of 50 ppm of mercury ions spiked water sample was added. Similarly, when the spiked sample with mixed ions or with mercury ions alone was used, a direct quenching occurred with above 95% reduction observed to the initial emission intensity. This supports the results obtained in
Table 1 where the sensor provides a high binding affinity toward mercury ions compared to iron ions.
In summary, a highly conjugated organic molecule was synthesized in one step reaction using low cost and safe starting materials. Besides its high solubility in polar solvents, the unique electronic and skeletal structures depicted by the orientation of the N-, and O-, provides multiple active sites that are suitable to attract mercuric and ferric ions (scehme 4) with low detection limits and wide linear detection range (mM to nM) thus providing a wide range of applications to biological and environmental samples. Further, sensor A, is a highly conjugated organic framework that provides both colorimetric and fluorometric signatures when it is covalently binds to the target metal ions. It is shown that both the emission intensity and the color of the dye have been immediately changed upon mixing with mercuric ions (less than 30 seconds). Finally, the material was tested for detection of mercuric and ferric ions in real water samples with high affinity to adsorb mercuric ions from water bodies with the measured adsorption capacity of 1.2-2.0 mg/g and the removal efficiency from water samples reached 98.8% at pH 7.0.