Submitted:
23 April 2023
Posted:
24 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Participants
2.2. Defnition of EO and non-EO patients
2.3. Assessment of Psychiatric Symptoms and Cognitive Functions
2.4. Blood Sampling and TAOC Measurement
2.5. Statistical Analysis
3. Results
3.1. Demographic, Clinical and Cognitive Parameters in EO and non-EO Patients
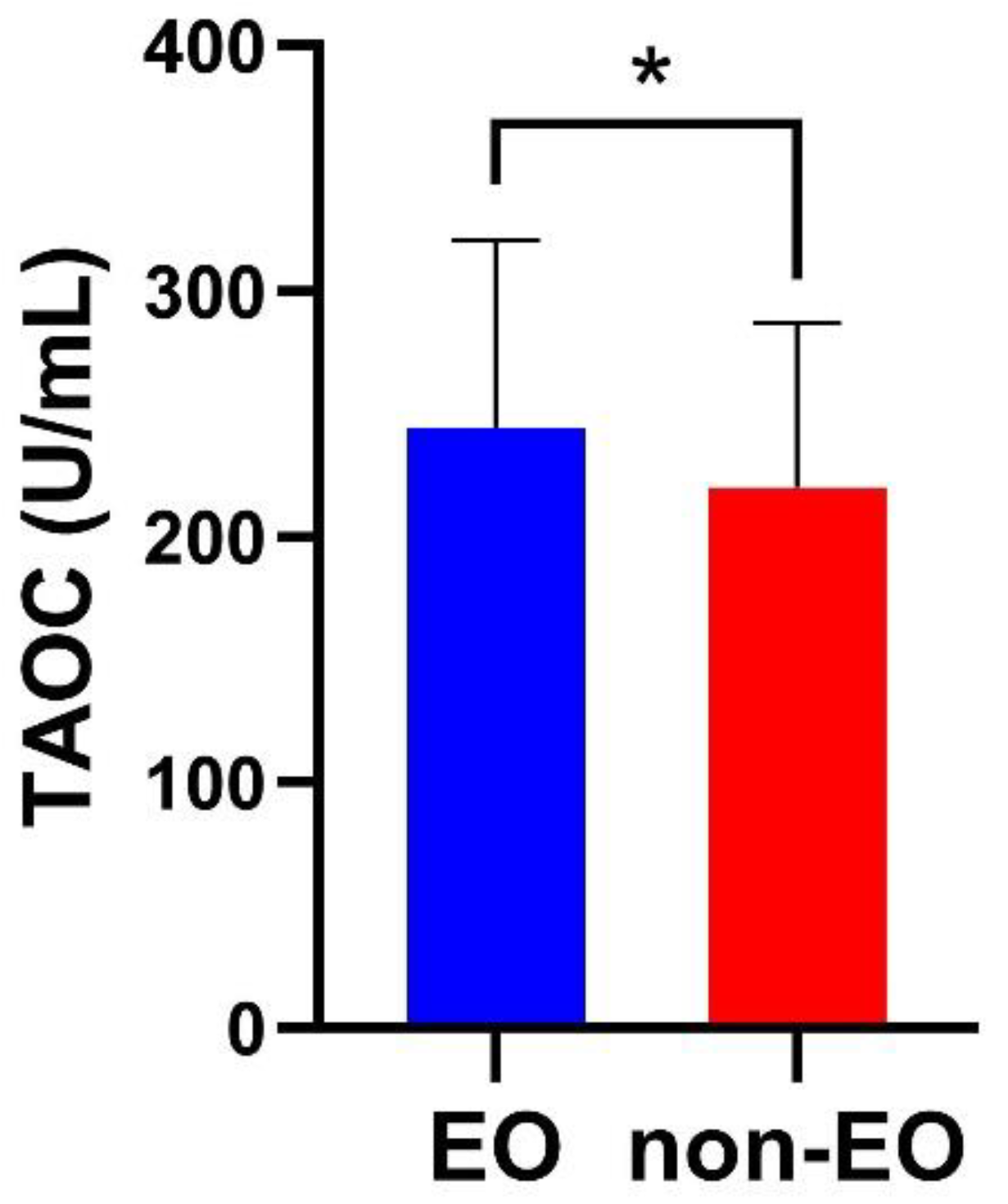
3.2. TAOC Levels in EO and non-EO Schizophrenia Patients
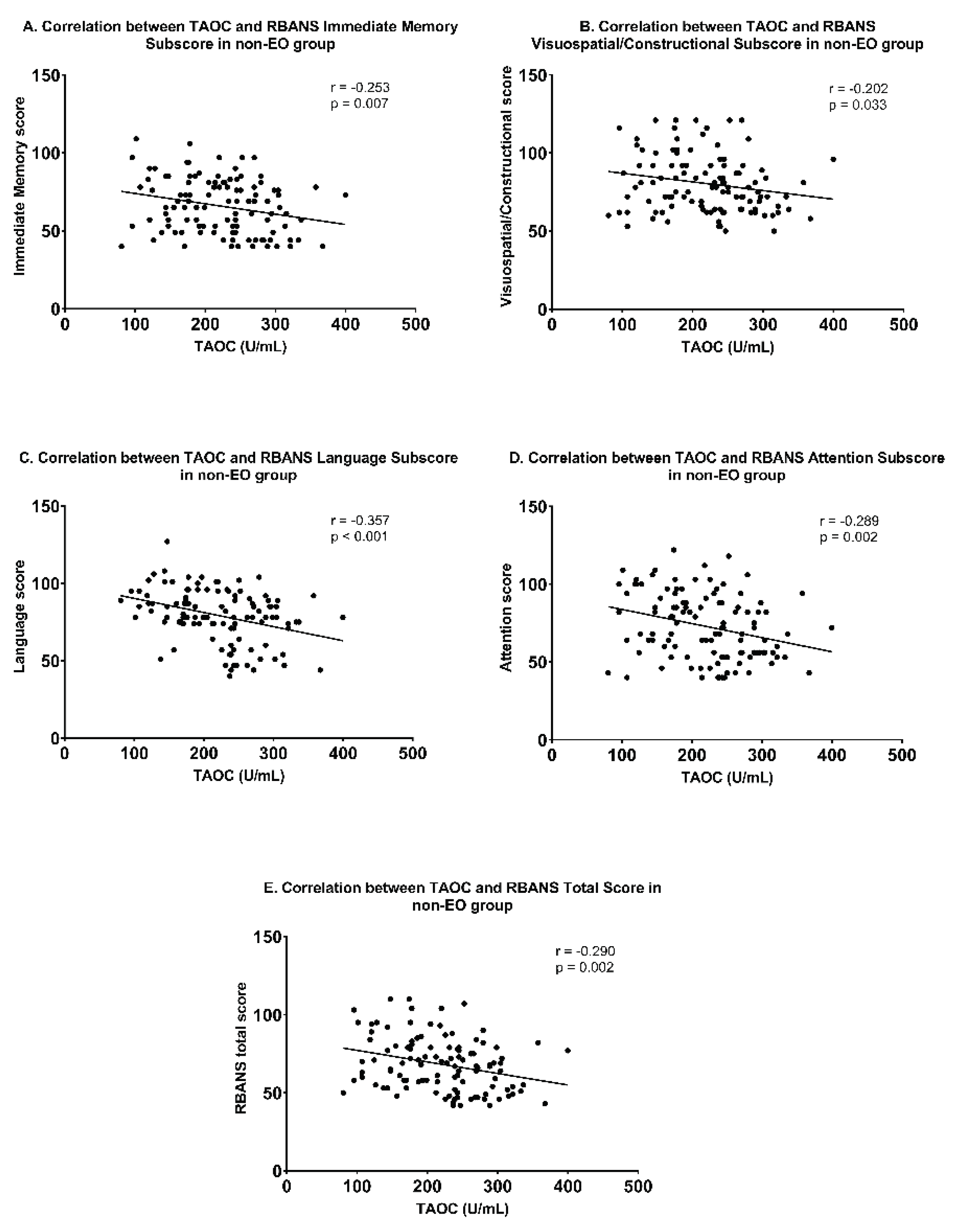
3.3. Association between TAOC Levels and Cognitive Function in EO and non-EO Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H.A. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Delborde, Y.; Ahmadpanah, M.; Seifrabiee, M.A.; Jahangard, L.; Bazzazi, N.; Brand, S. Non-linear associations between retinal nerve fibre layer (RNFL) and positive and negative symptoms among men with acute and chronic schizophrenia spectrum disorder. J. Psychiatr. Res. 2021, 141, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Immonen, J.; Jääskeläinen, E.; Korpela, H.; Miettunen, J. Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv. Psychiatry 2017, 11, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hafner, H.; Nowotny, B. Epidemiology of early-onset schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 1995, 245, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Linke, M.; Jankowski, K.S.; Ciołkiewicz, A.; Jędrasik-Styła, M.; Parnowska, D.; Gruszka, A.; Denisiuk, M.; Jarema, M.; Wichniak, A. Age or age at onset? Which of them really matters for neuro and social cognition in schizophrenia? Psychiatry Res. 2015, 225, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, J.S.; Zhou, Y.J.; Chen, D.C.; Xiu, M.H.; Wang, L.; Zhang, X.Y. BDNF affects the mediating effect of negative symptoms on the relationship between age of onset and cognition in patients with chronic schizophrenia. Psychoneuroendocrinology 2021, 125, 105121. [Google Scholar] [CrossRef]
- Fraguas, D.; Díaz-Caneja, C.M.; Rodríguez-Quiroga, A.; Arango, C. Oxidative Stress and Inflammation in Early Onset First Episode Psychosis: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2017, 20, 435–444. [Google Scholar] [CrossRef]
- Kostyak, J.C.; Kris-Etherton, P.; Bagshaw, D.; DeLany, J.P.; Farrell, P.A. Relative fat oxidation is higher in children than adults. Nutr. J. 2007, 6, 19. [Google Scholar] [CrossRef]
- Kyriakopoulos, M.; Dima, D.; Roiser, J.P.; Corrigall, R.; Barker, G.J.; Frangou, S. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 911–920.e912. [Google Scholar] [CrossRef]
- Vancampfort, D.; Stubbs, B.; Mitchell, A.J.; De Hert, M.; Wampers, M.; Ward, P.B.; Rosenbaum, S.; Correll, C.U. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015, 14, 339–347. [Google Scholar] [CrossRef]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Mihaljević-Peleš, A.; Bajs Janović, M.; Šagud, M.; Živković, M.; Janović, Š.; Jevtović, S. Cognitive deficit in schizophrenia: an overview. Psychiatr. Danub. 2019, 31, 139–142. [Google Scholar] [PubMed]
- Boudriot, E.; Schworm, B.; Slapakova, L.; Hanken, K.; Jager, I.; Stephan, M.; Gabriel, V.; Ioannou, G.; Melcher, J.; Hasanaj, G.; et al. Optical coherence tomography reveals retinal thinning in schizophrenia spectrum disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bozikas, V.P.; Kosmidis, M.H.; Kioperlidou, K.; Karavatos, A. Relationship between psychopathology and cognitive functioning in schizophrenia. Compr Psychiatry 2004, 45, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Rajji, T.K.; Ismail, Z.; Mulsant, B.H. Age at onset and cognition in schizophrenia: meta-analysis. Br. J. Psychiatry 2009, 195, 286–293. [Google Scholar] [CrossRef]
- Tuulio-Henriksson, A.; Partonen, T.; Suviusaari, J.; Haukka, J.; Lonnqvist, J. Age at onset and cognitive functioning in schizophrenia. Br. J. Psychiatry 2004, 185, 215–219. [Google Scholar] [CrossRef]
- van der Werf, M.; Kohler, S.; Verkaaik, M.; Verhey, F.; van Os, J.; Investigators, G. Cognitive functioning and age at onset in non-affective psychotic disorder. Acta Psychiatr. Scand. 2012, 126, 274–281. [Google Scholar] [CrossRef]
- Wang, D.M.; Du, Y.X.; Zhu, R.R.; Tian, Y.; Chen, J.J.; Chen, D.C.; Wang, L.; Zhang, X.Y. The relationship between cognitive impairment and superoxide dismutase activity in untreated first-episode patients with schizophrenia. World J. Biol. Psychiatry 2022, 23, 517–524. [Google Scholar] [CrossRef]
- Mico, J.A.; Rojas-Corrales, M.O.; Gibert-Rahola, J.; Parellada, M.; Moreno, D.; Fraguas, D.; Graell, M.; Gil, J.; Irazusta, J.; Castro-Fornieles, J.; et al. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry 2011, 11, 26. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Gabr, S.A.; Anwer, S.; Li, H. Associations between vitamin E, oxidative stress markers, total homocysteine levels, and physical activity or cognitive capacity in older adults. Sci. Rep. 2021, 11, 12867. [Google Scholar] [CrossRef]
- Martinez-Cengotitabengoa, M.; Mico, J.A.; Arango, C.; Castro-Fornieles, J.; Graell, M.; Paya, B.; Leza, J.C.; Zorrilla, I.; Parellada, M.; Lopez, M.P.; et al. Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr. Res. 2014, 156, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Palomar-Bonet, M.; Atienza, M.; Hernandez-Ledesma, B.; Cantero, J.L. Associations of Salivary Total Antioxidant Capacity With Cortical Amyloid-Beta Burden, Cortical Glucose Uptake, and Cognitive Function in Normal Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1839–1845. [Google Scholar] [CrossRef]
- Morera-Fumero, A.L.; Diaz-Mesa, E.; Abreu-Gonzalez, P.; Fernandez-Lopez, L.; Cejas-Mendez, M.D. Low levels of serum total antioxidant capacity and presence at admission and absence at discharge of a day/night change as a marker of acute paranoid schizophrenia relapse. Psychiatry Res. 2017, 249, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, Q.W.; Luo, X.G.; Tian, L.; Wang, Z.R.; Tan, S.P.; Chen, S.; Yang, G.G.; An, H.M.; Yang, F.D.; et al. Plasma total antioxidant status and cognitive impairments in first-episode drug-naive patients with schizophrenia. Cogn. Neurodyn. 2019, 13, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Bošković, M.; Vovk, T.; Kores Plesničar, B.; Grabnar, I. Oxidative stress in schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar]
- Wang, K.Q.; Xiu, M.H.; Su, X.R.; Wu, F.C.; Zhang, X.Y. Association between Changes in Total Antioxidant Levels and Clinical Symptom Improvement in Patients with Antipsychotic-Naive First-Episode Schizophrenia after 3 Months of Risperidone Monotherapy. Antioxidants 2022, 11, 646. [Google Scholar] [CrossRef]
- Al-Chalabi, B.M.; Thanoon, I.A.J.; Ahmed, F.A. Potential effect of Olanzapine on total antioxidant status and lipid peroxidation in sSchizophrenic patients. Neuropsychobiology 2009, 59, 8–11. [Google Scholar] [CrossRef]
- Basso, M.R.; Nasrallah, H.A.; Olson, S.C.; Bornstein, R.A. Cognitive deficits distinguish patients with adolescent- and adult-onset schizophrenia. Neuropsychiatry Neuropsychol. Behav. Neurol. 1997, 10, 107–112. [Google Scholar]
- Greig, T.C.; Bell, M.D.; Kaplan, E.; Bryson, G. Object relations and reality testing in early- and late-onset schizophrenia. J. Clin. Psychol. 2000, 56, 505–517. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zheng, Y.L.; Xiu, M.H.; Chen, D.C.; Kosten, T.R.; Zhang, X.Y. Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Golshani, S.; Najafpour, A.; Hashemian, S.S.; Goudarzi, N.; Shahmari, F.; Golshani, S.; Babaei, M.; Firoozabadi, K.; Duersteler, K.M.; Bruehl, A.B.; et al. When Much Is Too Much-Compared to Light Exercisers, Heavy Exercisers Report More Mental Health Issues and Stress, but Less Sleep Complaints. Healthcare 2021, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Brosius, F. SPSS: umfassendes Handbuch zu Statistik und Datenanalyse; MITP-Verlags GmbH & Co. KG: 2018.
- Bellino, S.; Rocca, P.; Patria, L.; Marchiaro, L.; Rasetti, R.; Di Lorenzo, R.; Paradiso, E.; Bogetto, F. Relationships of age at onset with clinical features and cognitive functions in a sample of schizophrenia patients. J. Clin. Psychiatry 2004, 65, 908–914. [Google Scholar] [CrossRef]
- Jeste, D.V.; Harris, M.J.; Krull, A.; Kuck, J.; McAdams, L.A.; Heaton, R. Clinical and neuropsychological characteristics of patients with late-onset schizophrenia. Am. J. Psychiatry 1995, 152, 722–730. [Google Scholar]
- Sachdev, P.; Brodaty, H.; Rose, N.; Cathcart, S. Schizophrenia with onset after age 50 years. 2: Neurological, neuropsychological and MRI investigation. Br. J. Psychiatry 1999, 175, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, X.; Fatjo-Vilas, M.; Munoz, M.J.; Campanera, S.; Miret, S.; Minano, M.J.; Aguilera, M.; Miralles, M.L.; Navarro, M.E.; Lazaro, L.; et al. Increased familiarity of intellectual deficits in early-onset schizophrenia spectrum disorders. World J. Biol. Psychiatry 2012, 13, 493–500. [Google Scholar] [CrossRef]
- Crow, T.J.; Colter, N.; Frith, C.D.; Johnstone, E.C.; Owens, D.G. Developmental arrest of cerebral asymmetries in early onset schizophrenia. Psychiatry Res. 1989, 29, 247–253. [Google Scholar] [CrossRef]
- Raz, S.; Raz, N. Structural brain abnormalities in the major psychoses: a quantitative review of the evidence from computerized imaging. Psychol. Bull. 1990, 108, 93–108. [Google Scholar] [CrossRef]
- Caldiroli, A.; Serati, M.; Orsenigo, G.; Caletti, E.; Buoli, M. Age at onset and social cognitive impairment in clinically stabilized patients with schizophrenia: An ecological cross-sectional study. Iran. J. Psychiatry 2018, 13, 84–93. [Google Scholar] [PubMed]
- Vidal, C.N.; Rapoport, J.L.; Hayashi, K.M.; Geaga, J.A.; Sui, Y.; McLemore, L.E.; Alaghband, Y.; Giedd, J.N.; Gochman, P.; Blumenthal, J.; et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch. Gen. Psychiatry 2006, 63, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.L.; Giedd, J.N.; Gogtay, N. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Notaras, M.; Du, X.; Gogos, J.; van den Buuse, M.; Hill, R.A. The BDNF Val66Met polymorphism regulates glucocorticoid-induced corticohippocampal remodeling and behavioral despair. Transl. Psychiatry 2017, 7, e1233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Yan, Q.Z.; Yan, X.M.; Li, C.B.; Fang, H.; Zheng, Y.L.; Zhang, C.X.; Yao, H.J.; Chen, D.C.; Xiu, M.H.; et al. The study of BDNF Val66Met polymorphism in Chinese schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.R.; Kumar, S.S.; Brown, A.S. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol. Psychiatry 2014, 75, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Berretta, S.; Pantazopoulos, H.; Markota, M.; Brown, C.; Batzianouli, E.T. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr. Res. 2015, 167, 18–27. [Google Scholar] [CrossRef]
- Solana, C.; Pereira, D.; Tarazona, R. Early senescence and leukocyte telomere shortening in SCHIZOPHRENIA: A role for cytomegalovirus infection? Brain. Sci. 2018, 8, 188. [Google Scholar] [CrossRef]
- Copoglu, U.S.; Virit, O.; Kokacya, M.H.; Orkmez, M.; Bulbul, F.; Erbagci, A.B.; Semiz, M.; Alpak, G.; Unal, A.; Ari, M.; et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 2015, 229, 200–205. [Google Scholar] [CrossRef]
| EO (n=90) | Non-EO (n=111) | F or X2 | df | P value | Partial η² | ||
|---|---|---|---|---|---|---|---|
| Age (y) | 18.56±1.69 | 33.01±8.44 | 255.711 | 200 | <0.001 | 0.56 | |
| Sex | |||||||
| Male (%) | 47/90 (52.22%) | 60/111 (54.05%) | 0.067 | 1 | 0.887 | / | |
| Female (%) | 43/90 (47.78%) | 51/111 (45.95%) | |||||
| Education (y) | 8.37±2.33 | 9.78±4.40 | 7.524 | 197 | 0.007 | 0.04 | |
| BMI (kg/m2) | 20.31±3.52 | 21.79±3.25 | 8.990 | 186 | 0.003 | 0.05 | |
| Smoker (%) | 10/90 (11.11%) | 39/111 (35.14%) | 13.992 | 1 | <0.001 | / | |
| Age of onset (y) | 17.79±1.53 | 32.26±8.48 | 255.259 | 200 | <0.001 | 0.56 | |
| PANSS | |||||||
| Positive symptoms | 20.68±5.66 | 21.40±6.11 | 0.748 | 200 | 0.388 | 0.004 | |
| Negative symptoms | 19.79±6.94 | 17.44±6.38 | 6.272 | 200 | 0.013 | 0.01 | |
| General psychopathology | 34.48±8.38 | 35.08±8.75 | 0.246 | 200 | 0.621 | 0.001 | |
| Total Score | 74.84±14.66 | 73.64±15.72 | 0.310 | 200 | 0.579 | 0.002 | |
| RBANS | |||||||
| Immediate Memory | 64.12±14.99 | 66.00±17.59 | 0.647 | 200 | 0.422 | 0.003 | |
| Visuospatial/Constructional | 73.38±13.81 | 80.39±18.38 | 9.015 | 200 | 0.003 | 0.04 | |
| Language | 67.91±18.21 | 79.33±17.15 | 20.927 | 200 | <0.001 | 0.095 | |
| Attention | 76.34±18.30 | 72.86±21.03 | 1.538 | 200 | 0.216 | 0.01 | |
| Delayed Memory | 66.41±19.94 | 70.86±20.14 | 2.453 | 200 | 0.119 | 0.01 | |
| Total Score | 63.31±13.34 | 68.29±17.05 | 5.134 | 200 | 0.025 | 0.03 | |
| TAOC (U/mL) | 244.48±76.06 | 220.08±67.05 | 5.861 | 200 | 0.016 | 0.03 | |
| EO (n=90) | Non-EO (n=111) | All participants (n=201) | |
|---|---|---|---|
| Education (y) | -0.363 (0.000) | -0.382 (0.000) | -0.374 (0.000) |
| Age of onset | 0.121 (0.256) | -0.039 (0.685) | -0.135 (0.056) |
| BMI (kg/m2) | -0.176 (0.109) | -0.180 (0.068) | -0.212 (0.003) |
| PANSS | |||
| Positive symptoms | -0.180 (0.089) | -0.342 (0.000) | -0.273 (0.000) |
| Negative symptoms | 0.038 (0.719) | -0.158 (0.096) | -0.029 (0.681) |
| General psychopathology | 0.016 (0.883) | -0.265 (0.005) | -0.138 (0.050) |
| Total Score | -0.042 (0.693) | -0.346 (0.000) | -0.196 (0.005) |
| RBANS | |||
| Immediate Memory | -0.112 (0.295) | -0.253 (0.007) | -0.196 (0.005) |
| Visuospatial/Constructional | -0.064 (0.548) | -0.202 (0.033) | -0.173 (0.014) |
| Language | -0.134 (0.208) | -0.357 (0.000) | -0.284 (0.000) |
| Attention | -0.217 (0.040) | -0.289 (0.002) | -0.236 (0.001) |
| Delayed Memory | -0.105 (0.323) | -0.163 (0.086) | -0.151 (0.032) |
| total Score | -0.172 (0.104) | -0.290 (0.002) | -0.258 (0.000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





