1. Introduction
Over 700 microbial species, including bacteria and fungi, inhabit the human oral cavity [
1]. Colonization of the mouth by yeasts was recognized over 2000 years ago, when Hippocrates reported the presence of oral thrush caused by
Candida species in infants and people with weakened immunity [
2,
3].
Candida albicans and several related
Candida species are opportunistic pathogens that live as benign commensal organisms in the oral cavities of healthy individuals, especially young children [
4]. One reason that
C. albicans is so successful is its ability to adapt and proliferate in a broad range of host environments, such as in acidic conditions [
5]. An acidic environment is detrimental to oral health because it promotes tooth cavitation.
C. albicans can co-adhere with other commensal species, helping in biofilm formation when suitable sugar resources are available in the diet [
6]. Common superficial
Candida infections include vaginal and oral candidiasis (thrush) and chronic mucocutaneous candidiasis [
7].
The literature reports that in children, the prevalence of oral thrush varies between 4% to 15% [
8,
9], with up to 40% in children with human immunodeficiency virus [
10]. Although most cases of oral thrush are mild, the disease can spread to the lungs, pleura, or diaphragm, or even to the gastrointestinal tract or kidneys, by dissemination through the bloodstream [
11]. In the past decade, cross-sectional clinical studies have observed that
C. albicans, along with
Streptococcus mutans, is highly abundant in the oral cavity of children with early childhood caries (ECC) [
12,
13]. Moreover,
C. albicans is twice as prevalent in the biofilm of children with ECC than in caries-free children [
14]. A recent systematic review and meta-analysis confirmed that the prevalence of oral
C. albicans in children with ECC is significantly higher than in caries-free children (p-value < 0.01), and that children with oral
C. albicans have 6.5 higher odds of experiencing ECC compared to those without this yeast [
15]. In keeping with these data,
C. albicans exhibits cariogenic traits: it is acidogenic, aciduric, and
is able to secrete aspartyl protease, which degrades dentinal collagen [
16,
17]
. Given the cariogenic potential of oral
Candida, it is crucial to evaluate its profile of susceptibility to commonly available antifungal drugs, so we can develop better ways to control this yeast in the oral cavity, especially in children at high risk for ECC.
The three major classes of antifungal drugs are polyene, azole, and echinocandin [
2]. Nystatin is a polyene drug that binds to the ergosterol in the cytoplasmic membrane of fungi, resulting in leakage of cell components [
18]. Nystatin is not absorbed by the gastrointestinal tract; however, it has topical effects with low hepatotoxicity, no reported drug interference, and minimal adverse effects [
19]. Because of the fungicidal and fungistatic properties of nystatin, it is commonly used topically for the treatment of oral candidiasis [
20] and is available in suspensions, oral rinses, gels, creams, tablets, and pastilles [
21]. A nystatin suspension of 100,000 IU (international units) is the treatment of choice for infants with oral thrush [
22]. In fungi, the development of nystatin resistance is frequently associated with a decrease in the ergosterol content of the resistant cells [
23].
Fluconazole is a systemic antifungal agent widely used in treating moderate to severe oral candidiasis [
24,
25]. It is fungistatic; it disrupts the synthesis of the fungal cytoplasmic membrane by inhibiting the lanosterol 14-α demethylase enzyme [
26]. Fluconazole is well tolerated by patients, has mild side effects and low toxicity (although higher than nystatin), and is relatively inexpensive [
25,
27]. For children with mucosal candidiasis, the recommendation is a single daily dose of 3mg/kg fluconazole suspension [
24,
28].
With the emergence of azole resistance in
Candida species [
19,
29], particularly
non-albicans [
30], drugs from the new echinocandin class, such as caspofungin, are currently the first-line choice in the management of invasive candidiasis [
31]. These drugs are used to treat serious fungal infections, including candidemia, esophageal candidiasis, and aspergillosis [
32]. Caspofungin requires IV administration via a single daily infusion over approximately 1 hour. [
33]. It is fungicidal, disrupting cell wall synthesis by inhibiting the enzyme 1,3-β-D glucan synthase. Echinocandins generally display superior activity against
Candida species than azoles [
33,
34].
It is unknown whether children display a similar antifungal response to oral Candida as their mothers, or whether, in children with recurrent colonization of Candida, their susceptibility profile changes over time. It is also unknown whether Candida becomes resistant to the drug over time. To our knowledge, no studies have compared the intra- and inter-antifungal drug susceptibility between children and their mothers. Answering these questions could provide us with information about predicting the susceptibility profile of both mothers and children. Potentially, treatment with antifungal drugs could prevent the transmission of Candida from mothers to their children. The present study aimed to evaluate and compare the in vitro susceptibility to nystatin, fluconazole, and caspofungin of oral Candida isolates collected from mothers and their children.
2. Materials and Methods
2.1. Study Design
This prospective study addressed 4 objectives in a cohort of mother/child dyads from birth to 2 years of age. We aimed to 1) Assess in vitro the antifungal susceptibility of oral Candida isolates from the mother-child cohort, 2) Compare Candida susceptibility between isolates from dyads of mothers and children; and 3) Assess longitudinal changes in the susceptibility of the isolates from children collected between 0-2 years. 4) In a subset of 5 children who provided data through 24 months, we also evaluated the clinical effectiveness of nystatin and fluconazole in reducing infection with oral C. albicans. Of these five, 4 were treated with nystatin and one with fluconazole; none was treated with caspofungin. 5) Further explore the mutations conserved among the group of C. albicans isolates with nystatin resistance, non-wild type fluconazole, and borderline high MIC values to caspofungin.
2.2. Study Sample
Data from pregnant women and their children were obtained from a prior birth cohort study that assessed the association between oral
Candida in early life and the onset of dental caries in children [
35]. The study protocol was approved by the University of Rochester Research Subject Review Board (#1248). Mother-child dyads carrying the same
Candida species in their oral cavity were included in the antifungal susceptibility testing study (Objectives 1-3). Medical and medication information, including prior receipt of antifungal and antibiotic medications, was obtained from mothers by self-report questionnaire and was further confirmed from participants’ medical records. For Objective 4, only children with 24 months of data and antifungal treatment were included.
2.3. Clinical Isolate Verification
Clinical isolates of Candida species were obtained from saliva and dental plaque of pregnant women and their children. Isolate types were identified by specific color on tests using BBL™ CHROMagar™ Candida (BD, Sparks, MD, USA) and further verified with whole genome sequencing (WGS). Cells from -80°C stock cultures were streaked on Yeast Peptones Dextrose agar (YPD) and incubated at 37°C for 20-24 hours.
2.4. DNA Extraction and Library Preparation for Whole Genome Sequencing
As a second and more reliable step in the verification of the Candida isolates, whole genome DNA (gDNA) was extracted from the clinical isolates using the MasterPure™ kit (Lucigen Corp, WI, USA) following the manufacturer’s instructions. Libraries were constructed using the Nextera XT kit (Illumina, Inc., USA) with 3 ng of gDNA as input. Fragment size profiles and quantification of the libraries were measured using the Fragment Analyzer and Qubit, respectively. The libraries were normalized to 1.75 nM and sequenced on NovaSeq 6000 using a SP flow cell with 150-bp paired-end reads. Sequence analysis was performed for the whole gene sequences of the genes associated with antifungal resistance, ERG3, ERG11, CDR1, CDR2, MDR1, and GSC1 (also known as FKS1).
2.5. Laboratory Preparation of Antifungal Drugs
Candida isolates were tested against nystatin (VWR International, LLC, NY, USA), fluconazole, and caspofungin (APExBIO, Houston, TX, USA). The drugs were dissolved in water or dimethyl sulfoxide (DMSO, Alfa Aesar, Ward Hill, MA, USA) according to the manufacturer's instructions. A stock drug solution was prepared which was 100 times higher than the desired testing concentration or at least 1.28 mg/mL. For each drug, two-fold serial dilutions were prepared, yielding final concentrations ranging from 16 to 0.03 μg/mL for nystatin, 64 to 0.125 μg/mL for fluconazole, and 8 to 0.015 μg/mL for caspofungin.
2.6. In Vitro Antifungal Drug Susceptibility Testing
Antifungal susceptibility testing was performed using the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 [
36] with modification, using RPMI 1640 growth medium (0.2% glucose with glutamine and without bicarbonate) (Gibco™, Thermo Fisher Scientific, MA, USA). The medium was buffered with 3-(N-morpholino) propanesulfonic acid (MOPS, Sigma-Aldrich, St. Louis, MO, USA) at a pH of 7. Yeast inoculums were prepared in 5 mL of RPMI 1640 medium, and the cell density in the resulting suspension was adjusted to 0.5 McFarland standard, corresponding to a stock inoculum of 1 × 10
6 - 5
× 10
6 CFU/mL (colony forming units). The suspension was diluted to obtain a final working cell density of 1 x 10
3 - 5 x 10
3 CFU/mL. Subsequently, 100 µL of the working inoculum solution and 100 µL of antifungal solution were seeded into a sterile, disposable, flat-bottomed 96-well plate (Greiner Bio-One, North Carolina, USA). In each well, the final inoculum density was 0.5 x 10
3 - 2.5 x 10
3 CFU/mL. The plates were incubated in ambient air without agitation at 35° C for 24 to 48 h, and the cell densities were noted visually.
C. albicans SC5314 or
C. krusei ATCC 6258 were used as a reference for quality control in all the experiments. To ensure rigorous and reproducible data, each experiment was performed in technical triplicates.
2.7. Spectrophotometric Measurement and Determination of Minimum Inhibitory Concentration (MIC) of Antifungal Drugs
To eliminate subjective determination of MIC values by visual inspection, spectrophotometer readings were carried out according to EUCAST guidelines. The MIC for fluconazole and caspofungin was defined as the lowest drug concentration that resulted in 50% growth inhibition compared to that of the growth control wells. For nystatin, the MIC was defined as the lowest drug concentration that resulted in 90% growth inhibition compared to that of the growth control wells. [
37]. The plates were read spectrophotometrically at 600 nm absorbance after 24 and 48 hours, using a microtiter plate reader, Tecan Infinite M200 PRO (Tecan, Männedorf, Switzerland). The MIC values and range were calculated. The isolates were classified as susceptible (S), intermediate (I), susceptible dose-dependent (SDD), and resistant (R), based on clinical breakpoints (CBP) recommended in the documents M27-A3 and M27-S4 [
36] and expressed visually as heatmaps (
Figure S4). However, polyenes, including nystatin, lack defined CBPs. To facilitate comparisons with other antifungal drugs, epidemiological cutoff values (ECVs) of amphotericin B (AMB, 2 μg/mL) were selected for all isolates [
38]. Moreover, because no defined CBPs were available for
C. dubliniensis and
C. lusitaniae, ECVs were used to separate susceptible and resistant isolates.
The antifungal susceptibility of isolates from mothers, compared to those of isolates from their respective children, was determined, as well as MIC change over time for children.
2.8. Bioinformatic Analysis of Antifungal Medication Resistance Genes
Pre-processed reads (adapters and low-quality bases removed) were mapped to the reference genome of
C. albicans SC5314 with the assembly accession reference sequence GCA_000784635.1 using BWA MEM (v 0.7.17) [
39]. The output SAM alignment file was converted to a sorted BAM file using samtools (v1.9) [
40]. Variant call files (VCFs) were generated from the sorted BAMs using bcftools mpileup (v1.4) [
40] with a minimum mapping quality score of 5. The VCFs were further filtered to exclude variants with quality scores of < 20 and coverage depth < 10. Variants were annotated with snpEff [
41] (v4.3t) using the pre-compiled Candida_albicans_sc5314_gca_000784635 database. Annotated VCF files were further filtered using bcftools filter (v1.14; [
40]) with the option –regions-file to include only genomic regions pertaining to 6 antifungal genes of interest. Furthermore, the bcftools isec (v1.14 [
40] was used to identify variants common or unique to different groups of
C. albicans strains. For each drug, two groups were made for the comparison as follows nystatin resistant vs. susceptible,
fluconazole wild type (WT; MIC ≤ 0.5 μg/mL)
vs. non-wild type (NWT; MIC > 0.5 μg/mL)
, and caspofungin normal MIC values (MIC < 0.25 μg/mL)
. vs. borderline high MIC values (MIC = 0.25 μg/mL)
. A set of conserved variants across all isolates of a given group were constructed using bcftools isec with the --nfiles option set to the number of isolates (individual VCF files) being compared. The resulting VCF file containing the conserved variants across the resistance group was then used as input to bcftools isec to identify a unique variant signature for each group.
2.9. Data and Statistical Analysis
Data were analyzed using SAS (SAS Institute) and displayed using GraphPad Prism version 9.4.1. Categorical variables were expressed as frequencies (%) and analyzed using Pearson’s chi-square test or Fisher’s exact test. The unpaired t-test and Mann–Whitney U test were used to compare differences in MICs among the mother/child isolates. Multiple logistic regression was used to assess factors associated with nystatin resistance (Y/N) of the C. albicans isolated from mothers and children. Factors included in the regression were race, ethnicity, gender, and prior clinical antifungal medication consumption. Differences were considered significant at a p-value <0.05.
3. Results
A total of 41 mother-child dyads in whom the same type of oral
Candida was detected were included in the study. The demographic, socioeconomic, medical, and oral characteristics of mothers and their children are shown in
Table 1 and
Table S1. The participating mothers were 26.4 ± 5.5 years old. Among the young children, 53.7% were females. With respect to race, about 67% of the dyads were Black, and 20% were White. Approximately 10% of mothers and 5% of children were of other or mixed races. The majority of the dyads were non-Hispanic. Approximately one-fifth of the children were delivered by c-section 9 (22%). Regarding oral health conditions, most mothers had dental caries (78%), whereas 10 children had ECC by two years of age (33.3%). Diaper rash in children was more prevalent than oral thrush (39% versus 19.5%). Children’s daycare attendance was low and remained below 20% throughout the period from birth to two years. Nearly all mothers were care providers for their children, whereas a range of 39% - 62% of fathers were involved in caring for their child between birth and 2 years.
The feeding patterns, including breastfeeding (exclusively), bottle feeding (exclusively), combined breast and bottle feeding, night feeding, and consumption of solid food, are illustrated in
Figure S1. Exclusive breastfeeding gradually decreased from 32% at one month to 0% at 18 months. On the other hand, exclusive bottle feeding nearly doubled from 36% at one month to 61% at six months. It remained stable between 6 and 12 months, and sharply decreased at 18 months. The number of children who had night bottle feeding was high (72%) during the first six months, with a sharp drop after six months, reaching 3% at the age of two years. Consumption of solid food started as early as two months and reached 100% at 12 months.
3.1. MIC of Clinical Candida Isolates
A total of 126 clinical Candida isolates from saliva (123) and plaque (3) were examined; these included 114 C. albicans, 6 Candida parapsilosis, 4 Candida dubliniensis, and 2 Candida lusitaniae. All isolates were confirmed by WGS; sequence reads were deposited in the NCBI Sequence Read Archive (SRA: Bioproject number PRJNA926612). The in vitro susceptibility of clinically isolated Candida species to nystatin, fluconazole, and caspofungin is shown in
Table 2. Each species of Candida demonstrated a somewhat different range of susceptibility to the three drugs tested.
3.2. Isolate susceptibilities to antifungal drugs
Classification of isolate susceptibilities to the tested antifungal drugs was based on clinical breakpoints (CBP) recommended in CLSI documents [
42,
43], as shown in
Table 3,
Table 4 and
Table 5, and
Table S2-S6. CBP is the MIC value that is used to categorize the susceptibility of an isolate (S, SDD, I, or R). To date, no CBPs or ECVs for nystatin have been defined. For this study, to allow comparison with other antifungal drugs, ECVs of amphotericin B (AMB, 2 μg/mL) were selected for all species [
38].
Most drugs were active against oral Candida isolates. Caspofungin was the most active drug; all isolates were susceptible to this drug. C. parapsilosis isolates (33.3%) fell in the SDD category to fluconazole. The overall resistance rate to nystatin was 5.6% (7 out of 126 isolates). Prenatal and child isolate resistance to nystatin was associated with maternal smoking (Fisher exact test; p-value=0.027). Moreover, child isolate resistance to nystatin was associated with 6 months of solid food intake (Fisher exact test; p-value=0.012), 18 month carriage of C. krusei in plaque (Fisher exact test; p-value=0.005), and 24 month carriage of S. mutans in saliva (Fisher exact test; p-value=0.05). In addition, isolate resistance to nystatin was related to the child’s experience of 2 prior episodes of otitis media and 2 periods of treatment with oral amoxicillin (Fisher exact test; p-value=0.027). Resistance to nystatin was not associated with a history of yeast infection or antifungal treatment (Fisher exact test; p-value=0.57).
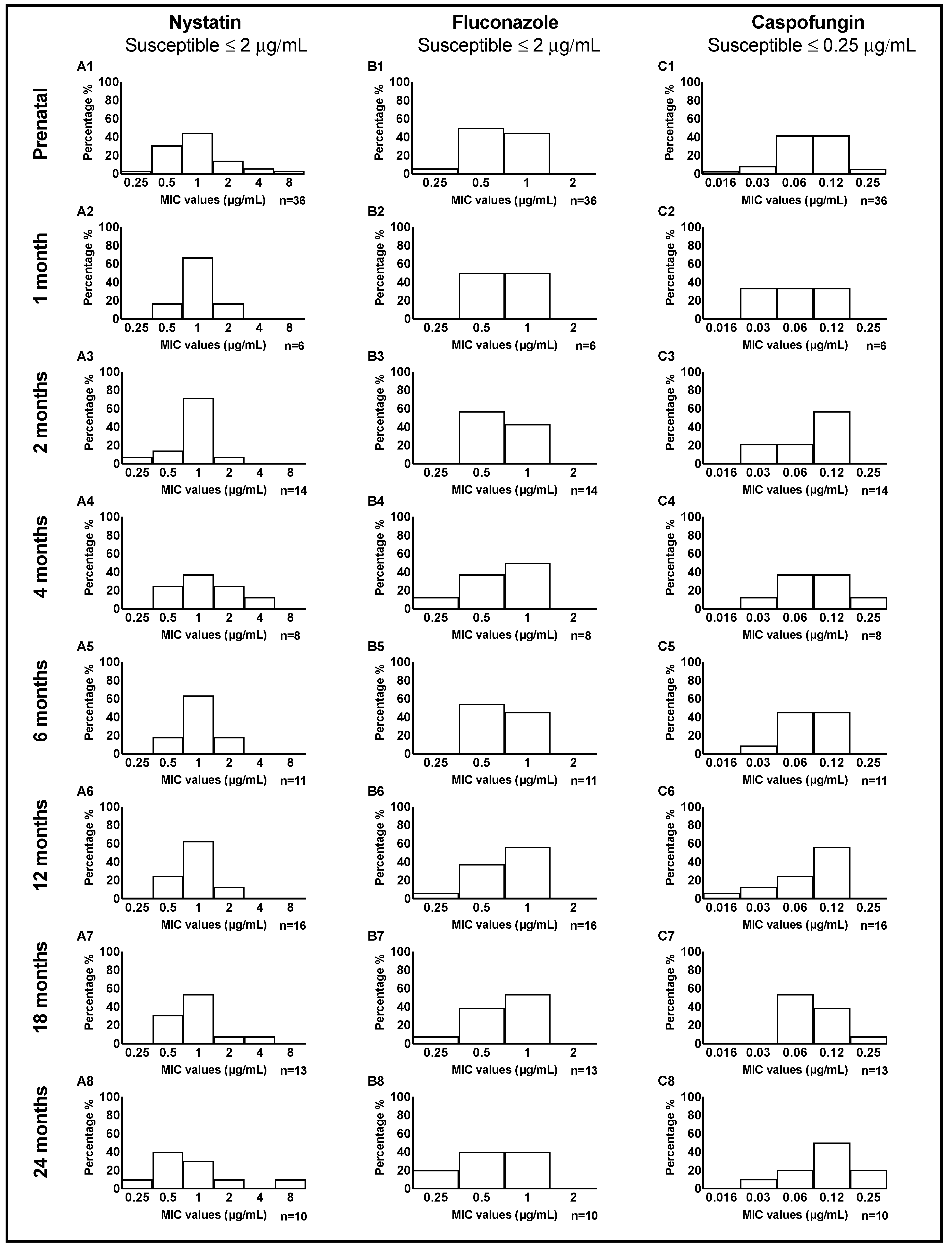
3.3. Candida MIC distribution among mother-child dyads
The distribution of MIC values for nystatin, fluconazole, and caspofungin in a total of 114
C. albicans clinical isolates from mother-child dyads are illustrated in
Figure 1. Overall, for nystatin and fluconazole, we observed a somewhat similar distribution of MIC values between mother and children. For caspofungin, at 24 months, we noticed more isolates with higher MIC values of 0.12 and 0.25 μg/mL. The distribution of MIC values tested in four
C. dubliniensis and six
C. parapsilosis isolates from mother-child dyads is illustrated in
Figure S2 and Figure S3, respectively.
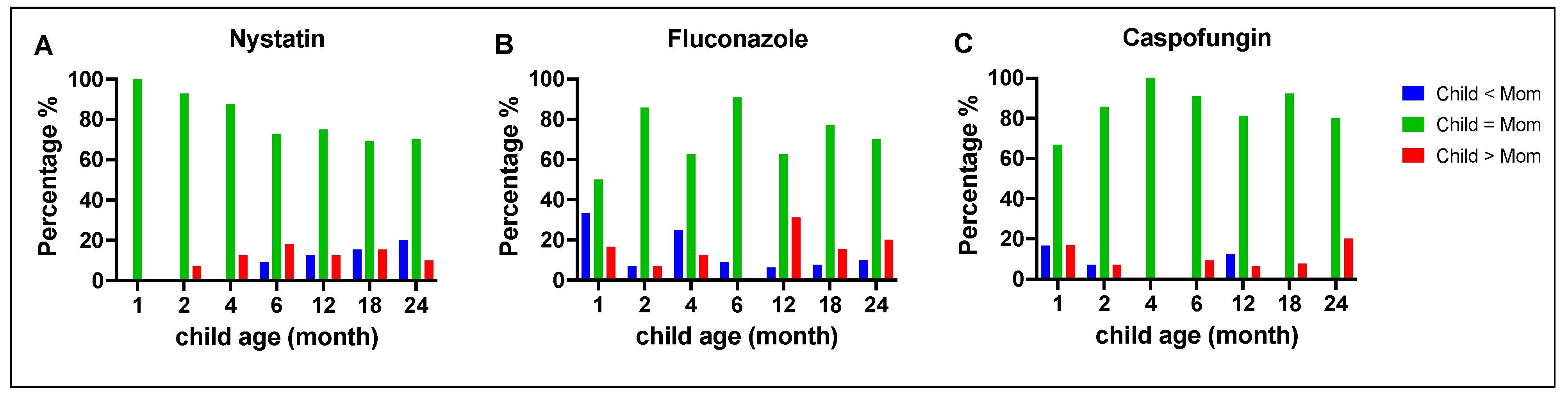
3.4. Comparison of antifungal susceptibility between mothers and children
C. albicans MIC values between mothers and children were compared at different time points, as illustrated in
Figure 2. Overall, the majority of the children's
C. albicans isolates had MIC values that were similar to strains isolated from their mothers (green bars). Notably, for fluconazole, at 12 months, 31% of the children's
C. albicans isolates had MIC values that were higher than the isolates from their mothers (red bars). For caspofungin, at 24 months, 20% of the children's
C. albicans isolates had MIC values that were higher than the isolates from their mothers (red bars).
When we compared the MIC values of the three C. parapsilosis dyads for nystatin and fluconazole, we found a different pattern: an equal proportion of those with the same, high, or low MIC values (each 33.33%). For caspofungin, two dyads had the same MIC values, and in the third dyad, the child isolate had a lower MIC value than the mother.
When we compared the two C. dubliniensis dyads, we found that the MIC values were the same for mothers and children for nystatin and fluconazole. For caspofungin, one dyad had the same MIC values; in the second dyad, the mother's strain had a higher MIC value than the child's. Finally, for C. lusitaniae, the single dyad had the same MIC values for nystatin and fluconazole, but for caspofungin, the child's isolate had a higher MIC value than the mother's.
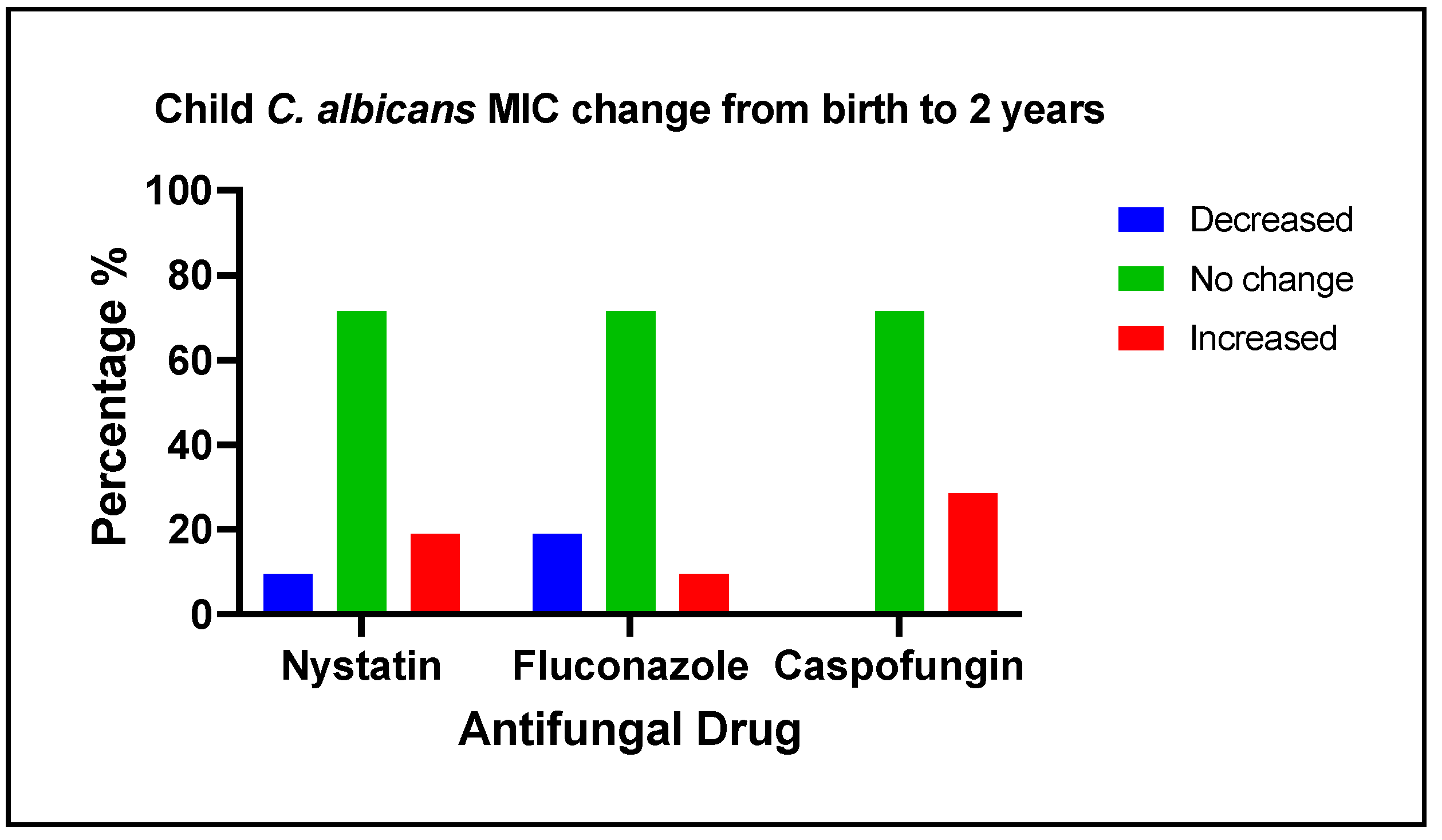
3.5. Changes of antifungal medication susceptibility in early life
The changes in
C. albicans MIC values to antifungal medications among 21 children from birth to 2 years of age are categorized as decreased, no change, or increased, as shown in
Figure 3. Nystatin MIC value increase was associated with mother hypertension (Fisher exact test; p-value=0.029). Fluconazole MIC value increase was associated with 6 months solid food intake (Fisher exact test; p-value=0.029), and 18 months sweet index (Fisher exact test; p-value=0.019). Caspofungin MIC value increase was associated with mother hypertension (Fisher exact test; p-value=0.048), mother plaque
C. albicans detection (Fisher exact test; p-value=0.012), 4- and 6-months breastfeeding, and 6 months night bottle feeding (Fisher exact test; p-value=0.048), 18 months
C. krusei carriage and having multiple vomiting diarrhea and constipation (Fisher exact test; p-value=0.035). No factors were found to be associated with a decrease in the isolates’ MIC values.
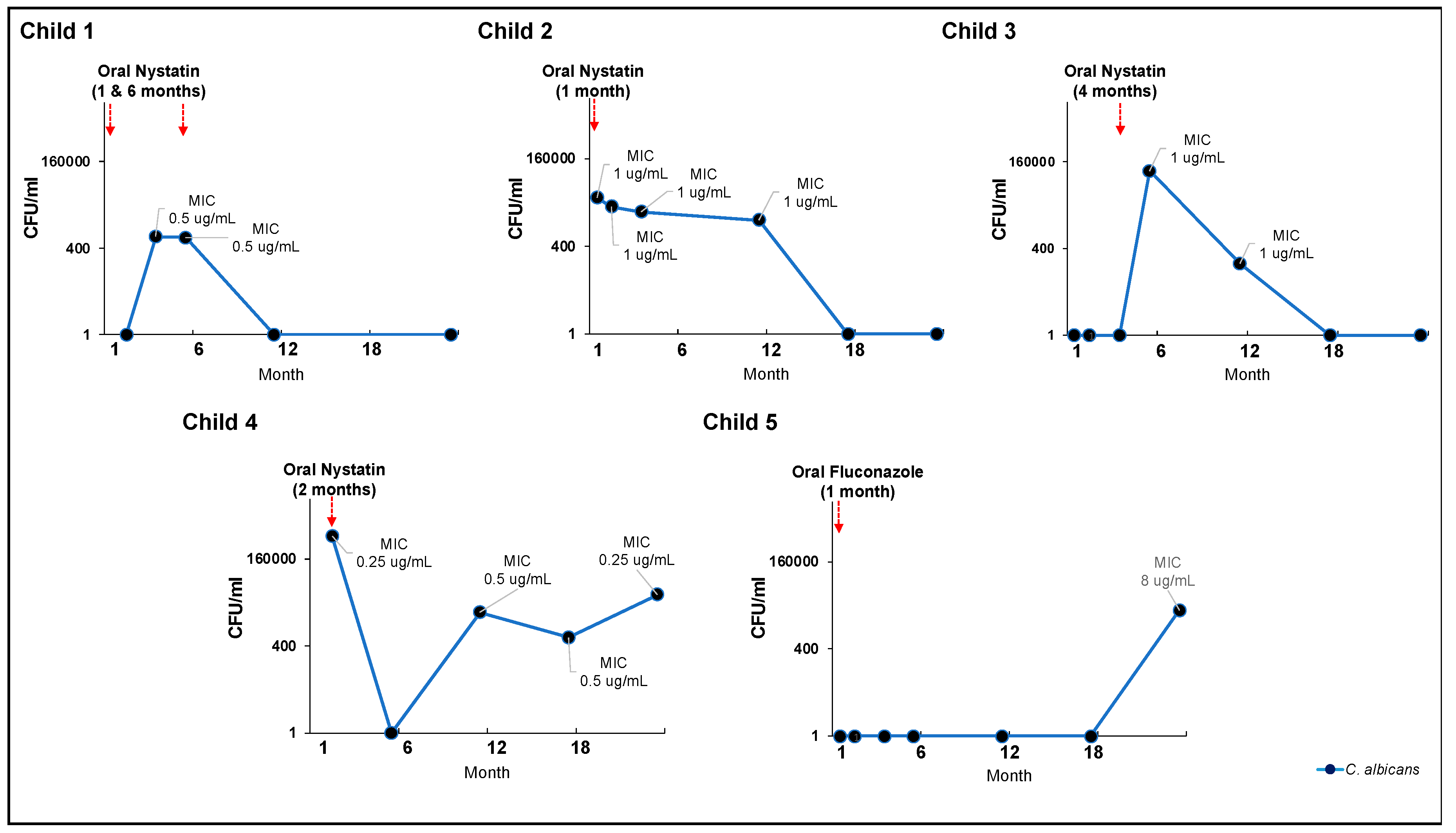
3.6. Effectiveness of clinical oral consumption of antifungal medication in early life
Five children had oral antifungal treatment (either nystatin or fluconazole) and completed the 24-month visit. Most of the antifungal medications were taken in early life, between one and six months. At 24 months, three children no longer had detectable salivary
C. albicans, while two were still positive. Their
C. albicans salivary carriage was still >400 CFU/ml, the cut-off value for the diagnosis of oral candidiasis [
45], despite treatment with oral antifungal medication. Notably, early fluconazole treatment in child 5 resulted in a high
C. albicans’ nystatin MIC value at 24 months.
Five children completed the 24-month visit and received oral antifungal treatment (nystatin or fluconazole). The majority of antifungal drugs were taken between the ages of one and six months. At 24 months, three children were negative for
C. albicans in their saliva, while two remained positive. Despite treatment with oral antifungal medication, their
C. albicans salivary carriage was still >400 CFU/ml, the cut-off value for diagnosing oral candidiasis [[
45]. Notably, child 5 was given fluconazole at the age of 1 month, which resulted in a high nystatin MIC value for
C. albicans by the time he was 24 months old.
3.7. Potentially Causal Resistance Mutations
Two missense mutations in the
CDR2 gene and 28 synonymous mutations compared to the
SC5314 reference sequences were found in the six
C. albicans strains resistant to nystatin (
Table 6,
Table S7). The nystatin-susceptible isolates did not share a common mutation. For fluconazole, no mutations were conserved among NWT isolates, and 5 synonymous mutations were shared among the WT isolates (
Table S8). Four missense mutations in three genes and 29 synonymous mutations were identified in the seven isolates with a caspofungin borderline high MIC value of 0.25 μg/mL (
Table 6,
Table S9).
4. Discussion
Our in vitro study showed that caspofungin was the most effective antifungal drug against all the tested Candida isolates, followed by fluconazole and nystatin. Resistance to nystatin was observed in 5.6% of the isolates. Although most of the children’s C. albicans isolates had MIC values similar to the strains isolated from their mothers, children also carried strains that were less susceptible to antifungal drugs than the maternal isolates. In nystatin-resistant isolates, two missense mutations in the CDR2 gene were identified.
Our finding that
Candida was resistant to nystatin in 5.6% of the samples requires interpretation. Since CBP and ECV cut-offs have not been defined for nystatin, we used ECV of amphotericin B instead, because both drugs have the same mechanism of action. Sutton et al. also used a value > 2 μg/mL as an endpoint to determine resistance to nystatin, and their finding of
C. albicans with the highest level of resistance compared to other strains agrees with our results. Moreover, De-Ia-Torre et al. and Zida et al. reported nystatin resistance in 5% and 6.3%, of the isolates tested, respectively [
27,
46]. Our findings further confirm that some
Candida strains can display resistance to nystatin (although at a relatively low percentage).
Results from our longitudinal cohort (Figure 4) indicated that clinical use of oral nystatin was ineffective in eliminating or reducing the carriage of C. albicans in children's oral cavities. Although oral nystatin was prescribed for 7 children,
C. albicans salivary carriage remained higher than 400 CFU/mL after treatment, a value consistent with a laboratory diagnosis of oral candidiasis [
45].
Previous research has demonstrated that nystatin is less effective than oral fluconazole or miconazole gel in treating oral thrush in immunocompetent infants [
28,
47]. This finding is supported by our
in vitro results. However, in our study, only one child received oral fluconazole treatment while seven received oral nystatin treatment, making it difficult to compare the clinical efficacy of those two medications.
Moreover, although we found that children’s isolates of C. albicans were not more susceptible to antifungals than adult isolates, pediatric antifungal medication contains only one-sixth of the dose used for adult antifungal medication. This difference could potentially explain the frequent failure of antifungal therapy in the children studied.
Our
in vitro data from Objectives 1, 2 and 3 reveal that none of the tested isolates were resistant to fluconazole, a finding similar to that of Brito et al. [
45], who examined
Candida species oral isolates from HIV and control groups, and Jewtuchowicz et al.[
48,
49], who studied
Candida species in healthy subjects with periodontal disease. In contrast, Zida et al. reported the highest resistance to azole, particularly fluconazole (66.5%), with a drug resistance rate of 72.3% to
Candida isolates from a vulvovaginal source and 59.0% of isolates from an oral source [
46].
C. krusei is inherently resistant to fluconazole [
42,
50]. These differences from our results could be attributed to the difference in the
Candida species studied. In our mother-child pairs, we did not detect
C. krusei.
In our study, although none of the subjects were exposed to caspofungin, we saw an increase in the MIC value of caspofungin in 29% of the children’s isolates. This increase in the MIC value may suggest the development of a more drug-tolerant strain in children who harbor
C. albicans for extended periods. According to recent reports on mucosal candidiasis, caspofungin treatment was not superior to fluconazole, and there was concern about relapse and reinfection after caspofungin treatment [
33]. These findings emphasize the importance of developing a new antifungal medication with superior activity.
In our study, two missense mutations in the
CDR2 gene, which codes for efflux pumps, were identified in all strains with MIC values above the ECV. However, those mutations were also found in some susceptible isolates, and casual inferences could not be made. Polyene resistance has been linked to a reduction in the ergosterol content of the plasma membrane [
51]. More research is needed to quantify membrane ergosterol levels and confirm the link between ergosterol content and resistance level. Furthermore, our findings failed to identify mutations specific to fluconazole NWT
C. albicans strains. Mechanisms of fluconazole resistance have been attributed to an alteration in
ERG3/ERG11 and/or
CDR/
MDR genes [
33]. Moreover, several point mutations in the
FKS1 subunit of glucan synthase have been identified and linked to the echinocandin resistance [
52]. Caspofungin resistance was not observed in our study; however, isolates with borderline high MIC values were grouped together, and four missense mutations were identified, one of which was in the target gene
FKS1.
Our finding of no cross-resistance between fluconazole, nystatin, and caspofungin confirms previous studies, and supports the view that no cross-resistance occurs between the classes of azoles, polyenes, and echinocandins [
29,
46,
53]. This finding suggests that if an isolate is resistant to one drug, it may be vulnerable to another. However, De-Ia-Torre et al. reported that multiple isolates exhibited cross-resistance or a combination of resistance in non-wild type (NWT) strains to different drugs (fluconazole, nystatin, itraconazole, posaconazole, amphotericin B and voriconazole) [
27]. Further study is needed to resolve this discrepancy.
We found that the susceptibility of
Candida isolates in children who received oral antifungals for all tested drugs did not differ from isolates taken from those who received no oral antifungals. In this small sample, logistic regression did not identify significant factors associated with
C. albicans resistance to nystatin or child MIC increase for nystatin, fluconazole, and caspofungin. Some studies have reported a correlation between antifungal use and resistance, but isolates in those studies were obtained from subjects with repeated or long-term antifungal use, or children given antifungal prophylaxis due to immunosuppression [
54,
55]. Our subjects, in contrast, were healthy with no immunosuppression or long-term antifungal use.
Even though our laboratory findings showed
C. albicans' susceptibility to antifungal drugs, we observed treatment failure. A lack of treatment compliance, incorrect dosage, or frequency could all be possible explanations. Nystatin should be given four times per day for 10-14 days for infants and children with oral candidiasis [
56]. Our findings are in line with previous clinical studies indicating that the mycological cure rates are low after nystatin treatment ranging between 5-11% [
10,
28,
57] . Although the
in vitro antifungal susceptibility testing could be a useful tool for predicting therapeutic outcomes, it does not always accurately reflect what occurs
in vivo due to individual natural variability, drug properties, and the differential characteristics of microorganisms in each individual [
18,
58].
Limitations and alternative approach
Our study is limited by a relatively small sample size, and a population from a single site in one US city, so the results may not generalize to other populations. In addition, only oral samples were collected and assessed, not samples from other body parts that can harbor Candida. Objective 4 was a preliminary investigation with only 5 children, without a systematic treatment protocol.
In addition, our in vitro broth microdilution method was performed on planktonic Candida, which does not mimic the biofilm nature of Candida in the oral cavity. The experiment could have been performed after Candida biofilm formation in the 96-well microtiter plates. Alternatively, we could have tested the effect of the drugs on the duo species biofilm of S. mutans and C. albicans, using the biofilm model. The latter method is helpful in allowing direct visualization and quantification of the extrapolysaccharide (EPS) and bacterial cells within intact biofilms, and it allows measurement of dry weight, total protein, and polysaccharide composition. We chose our method because it is the gold standard for antifungal susceptibility testing and thus could provide a foundation for future experiments.
5. Conclusions
Despite the fact that nystatin is currently the recommended drug for the treatment of infants with oral candidiasis, its in vitro efficacy is inferior to fluconazole and caspofungin. Our findings highlight the existence of Candida strains that are not susceptible to nystatin, confirmed by laboratory Candida detection after nystatin treatment. The implications of the preliminary results highlight the need for a drug other than nystatin for the treatment of infants with oral candidiasis. To determine the burden of infections caused by antifungal-resistant Candida strains, we suggest using proactive surveillance methods to evaluate resistance development in Candida infections. The high activity of fluconazole and lack of in vitro resistance of isolates to this drug may suggest the value of continuing to use this medication for the treatment of oral candidiasis and, potentially, dental caries.
The majority of the children’s C. albicans isolates from birth to two years had similar MIC values to the strains isolated from their mothers, although children also carry strains that are less susceptible to antifungal drugs than those from their mothers. This could explain treatment failure and suggests that a different dosage is required to more effectively treat children's Candida infections. The increase in caspofungin MIC values in children's C. albicans may indicate decreased activity of this antifungal medication in individuals who carry C. albicans for a long time. Given that caspofungin is not appropriate for mild disease, because it requires intravenous administration, it would be advisable to identify some alternative antifungal agents as an adjunct for the therapeutic prevention of oral diseases.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org, Table S1. Medical conditions and medication use characteristics of 41 mother-child dyads; Table S2. Classification of clinical oral
C. dubliniensis isolates susceptibility to fluconazole according to CLSI guidelines using epidemiological cutoff values (ECV); Table S3. Classification of clinical oral
C. lusitaniae isolates susceptibility to fluconazole according to CLSI guidelines using epidemiological cutoff values (ECV); Table S4. Classification of clinical oral
C. parapsilosis susceptibility to caspofungin according to CLSI guidelines using clinical breakpoints (CBP); Table S5. Classification of clinical oral
C. dubliniensis isolates susceptibility to caspofungin according to CLSI guidelines using epidemiological cutoff values (ECV); Table S6. Classification of clinical oral
C. lusitaniae isolates susceptibility to caspofungin according to CLSI guidelines using epidemiological cutoff values (ECV); Table S7. List of mutations conserved among nystatin-resistant
C. albicans strains (MIC> 2 μg/mL; n=6); Table S8. List of mutations conserved among fluconazole wild-type
C. albicans strains (MIC≤ 0.5 μg/mL; n=60); Table S9. List of mutations conserved among caspofungin borderline high MIC values (MIC= 0.25 μg/mL)
C. albicans strains (n=7); Figure S1. Proportions of child feeding (breast, bottle, and both breast and bottle feeding), night breast and bottle feeding, solid food intake, daycare attendance, mother and father as care provider measured at 1-, 2-, 4-, 6-, 12-, 18-, and 24-months; Figure S2. Distribution of antifungal medication minimal inhibitory concentration (MIC) values of 4
C. dubliniensis clinical isolates from mother-child dyads; Figure S3. Distribution of antifungal medication minimal inhibitory concentration (MIC) values of 6
C. parapsilosis clinical isolates from mother-child dyads; Figure S4. Heatmaps representing the relative growth of
Candida isolates in the presence of different drug concentrations.
Author Contributions
N. Alkhars, contributed to the conception, design, data acquisition, analysis, and interpretation, drafted, and critically revised the manuscript; A. Gaca, Y. Zeng and N. Al Jallad, contributed to data acquisition and analysis, critically revised the manuscript; E. Rustchenko, E. Eliav, contributed to data acquisition, and critically revised the manuscript; T.T. Wu, contributed to data analysis and interpretation, drafted and critically revised the manuscript; J. Xiao, contributed to the conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agreed to the published version of the manuscript.
Funding
This study was supported financially by grants K23DE027412 and R01DE031025 from the National Institute of Dental and Craniofacial Research (J. Xiao).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the University of Rochester Research Subject Review Board (#1248).
Informed Consent Statement
Informed consent was obtained from all subjects or their legal guardians for the study.
Data Availability Statement
Data may be accessed by contacting the corresponding author.
Acknowledgments
The authors gratefully acknowledge First Tooth Study members for their assistance in collecting saliva and plaque samples from the study subjects. We also thank the physicians, staff, and clinical administration at the University of Rochester Highland Family Medicine and Perinatal Dental Clinic for their generous support in conducting study visits. We are also thankful to the University of Rochester Genomic Research Center for conducting the WGS data.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Slavkin, H.C. Streptococcus mutans, early childhood caries and new opportunities. J Am Dent Assoc 1999, 130, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front Microbiol 2015, 6, 1391. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.A. A history of research on yeasts 12: medical yeasts part 1, Candida albicans. Yeast 2008, 25, 385–417. [Google Scholar] [CrossRef] [PubMed]
- Kadir, T.; Uygun, B.; Akyuz, S. Prevalence of Candida species in Turkish children: relationship between dietary intake and carriage. Arch Oral Biol 2005, 50, 33–37. [Google Scholar] [CrossRef]
- Cannon, R.D.; Holmes, A.R.; Mason, A.B.; Monk, B.C. Oral Candida: clearance, colonization, or candidiasis? Journal of dental research 1995, 74, 1152–1161. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, L.; Lei, Y.L.; Ren, B.; Zhou, X. The Interactions Between Candida albicans and Mucosal Immunity. Front Microbiol 2021, 12, 652725. [Google Scholar] [CrossRef]
- Molero, G.; Díez-Orejas, R.; Navarro-García, F.; Monteoliva, L.; Pla, J.; Gil, C.; Sánchez-Pérez, M.; Nombela, C. Candida albicans: genetics, dimorphism and pathogenicity. Interantl Microbiol 1998, 1(2), 95–106. [Google Scholar]
- Stecksen-Blicks, C.; Granstrom, E.; Silfverdal, S.A.; West, C.E. Prevalence of oral Candida in the first year of life. Mycoses 2015, 58, 550–556. [Google Scholar] [CrossRef]
- Vainionpaa, A.; Tuomi, J.; Kantola, S.; Anttonen, V. Neonatal thrush of newborns: Oral candidiasis? Clin Exp Dent Res 2019, 5, 580–582. [Google Scholar] [CrossRef]
- Flynn, P.M.; Cunningham, C.K.; Kerkering, T.; San Jorge, A.R.; Peters, V.B.; Pitel, P.A.; Harris, J.; Gilbert, G.; Castagnaro, L.; Robinson, P. Oropharyngeal candidiasis in immunocompromised children: A randomized, multicenter study of orally administered fiuconazole suspension versus nystatin. The Journal of Pediatrics 1995, 127, 322–328. [Google Scholar] [CrossRef]
- Harris, L.J.; Pritzker, H.G.; Eisen, A.; Steiner, J.W.; Shack, L. The effect of nystatin (mycostatin) on neonatal candidiasis (thrush): A method of eradicating thrush from hospital nurseries. Canadian Medical Association Journal 1958, 79, 891–896. [Google Scholar] [PubMed]
- Klinke, T.; Urban, M.; Luck, C.; Hannig, C.; Kuhn, M.; Kramer, N. Changes in Candida spp., mutans streptococci and lactobacilli following treatment of early childhood caries: a 1-year follow-up. Caries Res 2014, 48, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Li, W.; Lin, Y.; Yu, D.; Zhao, W. Genotypic diversity and cariogenicity of Candida albicans from children with early childhood caries and caries-free children. BMC Oral Health 2015, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Hodson, J.J.; Craig, G.T. The incidence of Candida albicans in the plaques of teeth of children. Dent Pract Dent Rec 1972, 22, 296–301. [Google Scholar] [PubMed]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res 2018, 52, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; Hughes, A.; Weetman, D.A.; MacFarlane, T.W. Growth and acid production of Candida species in human saliva supplemented with glucose. J Oral Pathol 1986, 15, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Klinke, T.; Kneist, S.; de Soet, J.J.; Kuhlisch, E.; Mauersberger, S.; Forster, A.; Klimm, W. Acid production by oral strains of Candida albicans and lactobacilli. Caries Res 2009, 43, 83–91. [Google Scholar] [CrossRef]
- Choukri, F.; Benderdouche, M.; Sednaoui, P. In vitro susceptibility profile of 200 recent clinical isolates of Candida spp. to topical antifungal treatments of vulvovaginal candidiasis, the imidazoles and nystatin agents. J Mycol Med 2014, 24, 303–307. [Google Scholar] [CrossRef]
- Khalandi, H.; Masoori, L.; Farahyar, S.; Delbandi, A.A.; Raiesi, O.; Farzanegan, A.; Khalandi, G.; Mahmoudi, S.; Erfanirad, T.; Falahati, M. Antifungal Activity of Capric Acid, Nystatin, and Fluconazole and Their In Vitro Interactions Against Candida Isolates from Neonatal Oral Thrush. Assay Drug Dev Technol 2020, 18, 195–201. [Google Scholar] [CrossRef]
- Tonon, C.C.; Francisconi, R.S.; Bordini, E.A.F.; Huacho, P.M.M.; Sardi, J.C.O.; Spolidorio, D.M.P. Interactions between Terpinen-4-ol and Nystatin on biofilm of Candida albicans and Candida tropicalis. Braz Dent J 2018, 29, 359–367. [Google Scholar] [CrossRef]
- Ellepola, A.N.; Samaranayake, L.P. Oral candidal infections and antimycotics. Crit Rev Oral Biol Med 2000, 11, 172–198. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Krüger, C.; Neumeister, C.; Schwantes, U.; Koch, D. In vitro susceptibility testing of yeasts to nystatin – low minimum inhibitory concentrations suggest no indication of in vitro resistance of Candida albicans, Candida species or non-Candida yeast species to nystatin. Clinical and Medical Investigations 2016, 1. [Google Scholar] [CrossRef]
- Mast, J.; Pina, E. Disappearance of Nystatin Resistance in Candida Mediated by Ergosterol. Journal of General Microbiology 1980, 117, 249–252. [Google Scholar]
- Martin, M.V. The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a review. Journal of Antimicrobial Chemotherapy 1999, 44, 429–437. [Google Scholar] [CrossRef]
- Quindos, G.; Gil-Alonso, S.; Marcos-Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med Oral Patol Oral Cir Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef]
- Boros-Majewska, J.; Salewska, N.; Borowski, E.; Milewski, S.; Malic, S.; Wei, X.Q.; Hayes, A.J.; Wilson, M.J.; Williams, D.W. Novel Nystatin A(1) derivatives exhibiting low host cell toxicity and antifungal activity in an in vitro model of oral candidosis. Med Microbiol Immunol 2014, 203, 341–355. [Google Scholar] [CrossRef]
- De-la-Torre, J.; Ortiz-Samperio, M.E.; Marcos-Arias, C.; Marichalar-Mendia, X.; Eraso, E.; Echebarria-Goicouria, M.A.; Aguirre-Urizar, J.M.; Quindos, G. In Vitro Antifungal Susceptibility of Oral Candida Isolates from Patients Suffering from Caries and Chronic Periodontitis. Mycopathologia 2017, 182, 471–485. [Google Scholar] [CrossRef]
- Goins, R.A.; Ascher, D.; Waecker, N.; Arnold, J.; Moorefield, E. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. The Pediatric Infectious Disease Journal 2002, 21, 1165–1167. [Google Scholar] [CrossRef]
- Kalkanci, A.; Berk, E.; Aykan, B.; Caglar, K.; Hizel, K.; Arman, D.; Kustimur, S. Epidemiology and antifungal susceptibility of Candida species isolated from hospitalized patients. J. Mycol. Med. 2007, 17, 16–20. [Google Scholar] [CrossRef]
- Dismukes, W.E. Introduction to Antifungal Drugs. Clinical Infectious Diseases 2000, 30, 653–657. [Google Scholar] [CrossRef]
- Wanjare, S.; Gupta, R.; Mehta, P. Caspofungin MIC Distribution amongst Commonly Isolated Candida Species in a Tertiary Care Centre - An Indian Experience. Journal of clinical and diagnostic research : JCDR 2016, 10, DC11–DC13. [Google Scholar] [CrossRef]
- Chryssanthou, E.; Cuenca-Estrella, M. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J Clin Microbiol 2002, 40, 3841–3844. [Google Scholar] [CrossRef] [PubMed]
- Eschenauer, G.; DePestel, D.D.; Carver, P.L. Comparison of echinocandin antifungals. Therapeutics and Clinical Risk Management 2007. [Google Scholar] [CrossRef] [PubMed]
- Serefko, A.; Los, R.; Biernasiuk, A.; Malm, A. Comparison of microdilution method and E-test procedure in susceptibility testing of caspofungin against Candida non-albicans species. New Microbiol 2008, 31, 257–262. [Google Scholar] [PubMed]
- Alkhars, N.; Zeng, Y.; Alomeir, N.; Al Jallad, N.; Wu, T.T.; Aboelmagd, S.; Youssef, M.; Jang, H.; Fogarty, C.; Xiao, J. Oral Candida Predicts Streptococcus mutans Emergence in Underserved US Infants. Journal of dental research 2022, 101, 54–62. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of, Yeasts; Third Informational Supplement; CLSI document M27-S3; Clinical and Laboratory Standards Institute: Wayne PA, 2008; 28. [Google Scholar]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. EUCAST E.DEF 7.3.2 2020. [Google Scholar]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin Microbiol Rev 2020, 33. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 2013. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 2014, 6, 80–92. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI supplement M60. Clinical and Laboratory Standards Institute 2022. [Google Scholar]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 2012, 50, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Pearsall, N.N.; Truelove, E.L. Quantitative relationships between Candida albicans in saliva and the clinical status of human subjects. J Clin Microbiol 1980, 12, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Zida, A.; Yacouba, A.; Bamba, S.; Sangare, I.; Sawadogo, M.; Guiguemde, T.; Kone, S.; Traore, L.K.; Ouedraogo-Traore, R.; Guiguemde, R.T. In vitro susceptibility of Candida albicans clinical isolates to eight antifungal agents in Ouagadougou (Burkina Faso). J Mycol Med 2017, 27, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Su, C.W.; Gaskie, S.; Jamieson, B.; Triezenberg, D. Clinical inquiries. What is the best treatment for oral thrush in healthy infants? J Fam Pract 2008, 57, 484–485. [Google Scholar] [PubMed]
- Jewtuchowicz, V.M.; Brusca, M.I.; Mujica, M.T.; Gliosca, L.A.; Finquelievich, J.L.; Lovannitti, C.A.; Rosa, A.C. Subgingival distribution of yeast and their antifungal susceptibility in immunocompetent subjects with and without dental devices. Acta Odontol Latinoam 2007, 20, 17–22. [Google Scholar] [PubMed]
- Brito, G.N.B.; Inocêncio, A.C.; Querido, S.M.R.; Jorge, A.O.C.; Koga-Ito, C.Y. In vitro antifungal susceptibility of Candida spp. oral isolates from HIV positive patients and control individuals. Braz Oral Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Fluconazole: Rationale for the EUCAST clinical breakpoints, version 3.0, European Committee on Antimicrobial Susceptibility Testing, 2020.
- Khalaf, R.A.; Fattouh, N.; Medvecky, M.; Hrabak, J. Whole genome sequencing of a clinical drug resistant Candida albicans isolate reveals known and novel mutations in genes involved in resistance acquisition mechanisms. J Med Microbiol 2021, 70. [Google Scholar] [CrossRef]
- Spettel, K.; Barousch, W.; Makristathis, A.; Zeller, I.; Nehr, M.; Selitsch, B.; Lackner, M.; Rath, P.M.; Steinmann, J.; Willinger, B. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PLoS One 2019, 14, e0210397. [Google Scholar] [CrossRef]
- Kuriyama, T.; Williams, D.W; Bagg, J.; Coulter, W.A.; Ready, D.; Lewis, M.A.O. In vitro susceptibility of oral Candida to seven antifungal agents. Oral Microbiology Immunology 2005. [Google Scholar] [CrossRef]
- Redding, S.; Smith, J.; Farinacci, G.; Rinaldi, M.; Fothergill, A.; Rhine-Chalberg, J.; Pfaller, M. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation by in vitro susceptibility testing and DNA subtype analysis. Clin Infect Dis 1994, 18, 240–242. [Google Scholar] [CrossRef]
- Heald, A.E.; Cox, G.M.; Schell, W.A.; Bartlett, J.A.; Perfect, J.R. Oropharyngeal yeast flora and fluconazole resistance in HIV-infected patients receiving long-term continuous versus intermittent fluconazole therapy. Aids 1996, 10, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Zhao, C.; Yan, Z.M.; Hua, H. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther 2016, 10, 1161–1171. [Google Scholar] [CrossRef]
- Hoppe, J.E. Treatment of oropharyngeal candidiasis and candidal diaper dermatitis in neonates and infants: review and reappraisal. Pediatr Infect Dis J 1997, 16, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Pfaller, M.A.; Walsh, T.J.; Chaturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rinaldi, M.G.; Sheehan, D.J.; et al. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev 2001, 14, 643–658. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Distribution of MIC values of 3 antifungal medications, tested in 114 C. albicans clinical isolates from mother-child dyads. MIC values (averaged for triplicate measurements) are illustrated separately for nystatin, fluconazole, and caspofungin. For nystatin (A1-8), the MIC of most of the clinical isolates clustered around 1 μg/mL; a higher MIC value (4-8 μg/mL) was seen in 8% of isolates from mothers and 4% of isolates from children. Regarding fluconazole (B1-8), mothers and children had the same frequency distribution of MIC values, centered around 0.5-1 μg/mL at almost all child ages. For caspofungin (C1-8), 42% of the mothers had a MIC value of 0.06 and the same percentage had MIC value of 0.12 μg/mL. In early infancy, most children had a low MIC value with a shift towards higher values of 0.12 and 0.25 μg/mL at 24 months.
Figure 1.
Distribution of MIC values of 3 antifungal medications, tested in 114 C. albicans clinical isolates from mother-child dyads. MIC values (averaged for triplicate measurements) are illustrated separately for nystatin, fluconazole, and caspofungin. For nystatin (A1-8), the MIC of most of the clinical isolates clustered around 1 μg/mL; a higher MIC value (4-8 μg/mL) was seen in 8% of isolates from mothers and 4% of isolates from children. Regarding fluconazole (B1-8), mothers and children had the same frequency distribution of MIC values, centered around 0.5-1 μg/mL at almost all child ages. For caspofungin (C1-8), 42% of the mothers had a MIC value of 0.06 and the same percentage had MIC value of 0.12 μg/mL. In early infancy, most children had a low MIC value with a shift towards higher values of 0.12 and 0.25 μg/mL at 24 months.
Figure 2.
Comparison between clinical isolates of mothers and children of C. albicans susceptibility to antifungal medications. The MIC values (averaged for triplicate measurements) of C. albicans for nystatin (A), fluconazole (B), and caspofungin (C) were compared between mothers and children at different time points. For nystatin, a high similarity percentage was observed, ranging between 70-100% (A). For fluconazole, the similar percentage ranged from 50-90%, with a higher proportion of children having high MIC values at 12 and 24 months (B). For caspofungin, at 1 month of age, 17% of the children’s isolates had lower MIC values than the isolates from their mothers, which reduced to 0% at 24 months. In contrast, the proportion of children with higher MIC values increased from 17% at one month to 20% at 24 months (C).
Figure 2.
Comparison between clinical isolates of mothers and children of C. albicans susceptibility to antifungal medications. The MIC values (averaged for triplicate measurements) of C. albicans for nystatin (A), fluconazole (B), and caspofungin (C) were compared between mothers and children at different time points. For nystatin, a high similarity percentage was observed, ranging between 70-100% (A). For fluconazole, the similar percentage ranged from 50-90%, with a higher proportion of children having high MIC values at 12 and 24 months (B). For caspofungin, at 1 month of age, 17% of the children’s isolates had lower MIC values than the isolates from their mothers, which reduced to 0% at 24 months. In contrast, the proportion of children with higher MIC values increased from 17% at one month to 20% at 24 months (C).
Figure 3.
Changes in C. albicans MIC to antifungal medications among 21 children in early life. The changes in C. albicans MIC values (averaged for triplicate measurements) to antifungal medications among children from birth to two years of age are categorized as decreased, no change, or increased. Approximately, 70% of the clinical isolates from children did not have changed MIC values for nystatin, fluconazole, and caspofungin. For nystatin, 19% of the clinical isolates had an increased MIC, and 14% had decreased MIC values over time. For fluconazole, 10% of the clinical isolates had an increase, whereas 19% had a decrease in their MIC values over time. Notably, for caspofungin, the MIC values increased in 29% of the isolates.
Figure 3.
Changes in C. albicans MIC to antifungal medications among 21 children in early life. The changes in C. albicans MIC values (averaged for triplicate measurements) to antifungal medications among children from birth to two years of age are categorized as decreased, no change, or increased. Approximately, 70% of the clinical isolates from children did not have changed MIC values for nystatin, fluconazole, and caspofungin. For nystatin, 19% of the clinical isolates had an increased MIC, and 14% had decreased MIC values over time. For fluconazole, 10% of the clinical isolates had an increase, whereas 19% had a decrease in their MIC values over time. Notably, for caspofungin, the MIC values increased in 29% of the isolates.
Figure 4.
C. albicans carriage level and oral antifungal treatment (nystatin or fluconazole). CFU: colony forming unit. MIC: minimum inhibitory concentration. Values averaged for triplicate measurements.
Figure 4.
C. albicans carriage level and oral antifungal treatment (nystatin or fluconazole). CFU: colony forming unit. MIC: minimum inhibitory concentration. Values averaged for triplicate measurements.
Table 1.
Demographic, socioeconomic, medical, and oral characteristics of mother-child dyads (n=41).
Table 1.
Demographic, socioeconomic, medical, and oral characteristics of mother-child dyads (n=41).
| Categories |
|
Mother n (%) |
Child n (%) |
| Demographic |
| Gender |
Female |
41 (100) |
22 (53.7) |
| Race |
Black |
28 (68.3) |
27 (65.9) |
| White |
9 (22.0) |
8 (19.5) |
| Ethnicity |
Non-Hispanic |
36 (87.8) |
36 (87.8) |
| |
|
|
|
| Socioeconomic-behavior factors |
| Marital status |
Married |
2 (4.9) |
na |
| Employment |
Employed |
24 (58.5) |
na |
| Education |
Middle school |
2 (4.9) |
na |
| |
High school |
25 (61.0) |
na |
| |
Associate |
4 (9.8) |
na |
| |
≥College |
10 (24.4) |
na |
| |
|
|
|
| Medical and oral conditions (Y) |
| Smoking |
|
4 (9.8) |
na |
| Emotional condition |
|
21 (51.2) |
0 (0) |
| Diabetes |
|
0 (0) |
0 (0) |
| Hypertension |
|
5 (12.2) |
0 (0) |
| Asthma |
|
2 (4.9) |
0 (0) |
| History of yeast infection |
12 (29.3) |
16 (39.0) |
| Diaper rash (Child) |
|
na |
16 (39.0) |
| Oral thrush (Child) |
|
na |
8 (19.5) |
| Caries |
|
32 (78.0) |
10 (33.3)#
|
| |
|
|
|
| Medication use (Y) |
| Antifungal* (Prenatal) |
9 (21.9) |
na |
| Antibiotic* (Prenatal) |
18 (43.9) |
na |
| Antifungal* (6 months postpartum or child by 24m) |
5 (12.2) |
16 (39.0) |
| Antibiotic* (6 months postpartum or child by 24m) |
11 (26.8) |
10 (24.4) |
| Inhaler |
2 (4.9) |
1 (2.4) |
| |
|
|
| Oral hygiene practice |
Brushing twice daily |
28 (68.3) |
16 (53.3) |
| |
|
|
|
| Birth route |
Vaginal |
na |
32 (78.0) |
Table 2.
Minimal Inhibition Concentration (μg/mL) of nystatin, fluconazole, and caspofungin against oral Candida isolates, determined by CLSI broth microdilution method.
Table 2.
Minimal Inhibition Concentration (μg/mL) of nystatin, fluconazole, and caspofungin against oral Candida isolates, determined by CLSI broth microdilution method.
| Species (n) |
Antifungal agent |
MIC (μg/mL) |
| |
|
Range |
|
Candida albicans (n=114) |
Nystatin |
0.25-8 |
| Fluconazole |
0.25-1 |
| Caspofungin |
0.016-0.25 |
|
Candida parapsilosis (n=6) |
Nystatin |
0.5-4 |
| Fluconazole |
2-4 |
| Caspofungin |
0.25-0.5 |
|
Candida dubliniensis (n=4) |
Nystatin |
0.5 |
| Fluconazole |
0.5 |
| Caspofungin |
0.06-0.12 |
|
Candida lusitaniae (n=2) |
Nystatin |
2 |
| Fluconazole |
1 |
| Caspofungin |
0.03-0.12 |
Table 3.
Susceptibility of clinical isolates to nystatin, interpreted according to CLSI guidelines.
Table 3.
Susceptibility of clinical isolates to nystatin, interpreted according to CLSI guidelines.
Species and Child Age at Isolation
(number of isolates) |
Susceptible (S)
MIC ≤2 μg/mL*
n (%) |
Resistant (R)
MIC >2 μg/mL*
n (%) |
| Candida albicans |
|
|
| Prenatal (36) |
33 (91.7) |
3 (8.3) |
| 1 month (6) |
6 (100) |
0 (0) |
| 2 months (14) |
14 (100) |
0 (0) |
| 4 months (8) |
7 (87.5) |
1 (12.5) |
| 6 months (11) |
11 (100) |
0 (0) |
| 12 months (16) |
16 (100) |
0 (0) |
| 18 months (13) |
12 (92.3) |
1 (7.7) |
| 24 months (10) |
9 (90) |
1 (10) |
| Candida parapsilosis |
|
|
| Prenatal (3) |
2 (66.7) |
1 (33.3) |
| 4 months (1) |
1 (100) |
0 (0) |
| 12 months (2) |
2 (100) |
0 (0) |
| Candida dubliniensis |
|
|
| Prenatal (2) |
2 (100) |
0 (0) |
| 24 months (2) |
2 (100) |
0 (0) |
| Candida lusitaniae |
|
|
| Prenatal (1) |
1 (100) |
0 (0) |
| 24 months (1) |
1 (100) |
0 (0) |
Table 4.
Susceptibility of clinical C. albicans and C. parapsilosis to fluconazole, according to CLSI guidelines.
Table 4.
Susceptibility of clinical C. albicans and C. parapsilosis to fluconazole, according to CLSI guidelines.
| Child age (Number of isolates) |
Susceptible (S)
MIC ≤2 μg/mL*
n (%) |
Susceptible Dose-Dependent (SDD)
MIC= 4 μg/mL*
n (%) |
Resistant (R)MIC ≥8 μg/mL*
n (%) |
| Candida albicans |
|
|
|
| Prenatal (36) |
36 (100) |
0 (0) |
0 (0) |
| 1 month (6) |
6 (100) |
0 (0) |
0 (0) |
| 2 months (14) |
14 (100) |
0 (0) |
0 (0) |
| 4 months (8) |
8 (100) |
0 (0) |
0 (0) |
| 6 months (11) |
11 (100) |
0 (0) |
0 (0) |
| 12 months (16) |
16 (100) |
0 (0) |
0 (0) |
| 18 months (13) |
13 (100) |
0 (0) |
0 (0) |
| 24 months (10) |
10 (100) |
0 (0) |
0 (0) |
| Candida parapsilosis |
|
|
|
| Prenatal (3) |
2 (66.7) |
1 (33.3) |
0 (0) |
| 4 months (1) |
1 (100) |
0 (0) |
0 (0) |
| 12 months (2) |
1 (50) |
1 (50) |
0 (0) |
Table 5.
Susceptibility of clinical C. albicans to caspofungin according to CLSI guidelines.
Table 5.
Susceptibility of clinical C. albicans to caspofungin according to CLSI guidelines.
| Species and Isolation timepoint (Number of Candida albicans isolates) |
Susceptible (S)
MIC ≤0.25 μg/mL*
n (%) |
Intermediate (I)
MIC =0.5 μg/mL*
n (%) |
Resistant
MIC ≥1 μg/mL*
n (%) |
| Prenatal (36) |
36 (100) |
0 (0) |
0 (0) |
| 1 month (6) |
6 (100) |
0 (0) |
0 (0) |
| 2 months (14) |
14 (100) |
0 (0) |
0 (0) |
| 4 months (8) |
8 (100) |
0 (0) |
0 (0) |
| 6 months (11) |
11 (100) |
0 (0) |
0 (0) |
| 12 months (16) |
16 (100) |
0 (0) |
0 (0) |
| 18 months (13) |
13 (100) |
0 (0) |
0 (0) |
| 24 months (10) |
10 (100) |
0 (0) |
0 (0) |
Table 6.
List of missense mutations conserved among different C. albicans clinical isolates.
Table 6.
List of missense mutations conserved among different C. albicans clinical isolates.
| Groups based on Susceptibility |
Gene |
Gene length |
Gene position |
Nucleotide substitution |
Aminoacid substituition |
Allele |
| Nystatin resistance (MIC >2 μg/mL) |
CDR2 |
4500 |
chr 3 |
2048G>A |
Arg683Lys |
homo/hetero |
| CDR2 |
4500 |
chr 3 |
4011G>C |
Leu1337Phe |
homo/hetero |
| Caspofungin reduced susceptibility (MIC= 0.25 μg/mL) |
FKS1 |
5694 |
chr 1 |
5657C>G |
Ser1886Thr |
homo/hetero |
| CDR2 |
4500 |
chr 3 |
2048G>A |
Arg683Lys |
homo/hetero |
| CDR2 |
4500 |
chr 3 |
4011G>C |
Leu1337Phe |
homo/hetero |
| ERG11 |
1587 |
chr 5 |
348T>A |
Glu116Asp |
homo/hetero |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).