Submitted:
24 April 2023
Posted:
24 April 2023
You are already at the latest version
Abstract
Keywords:
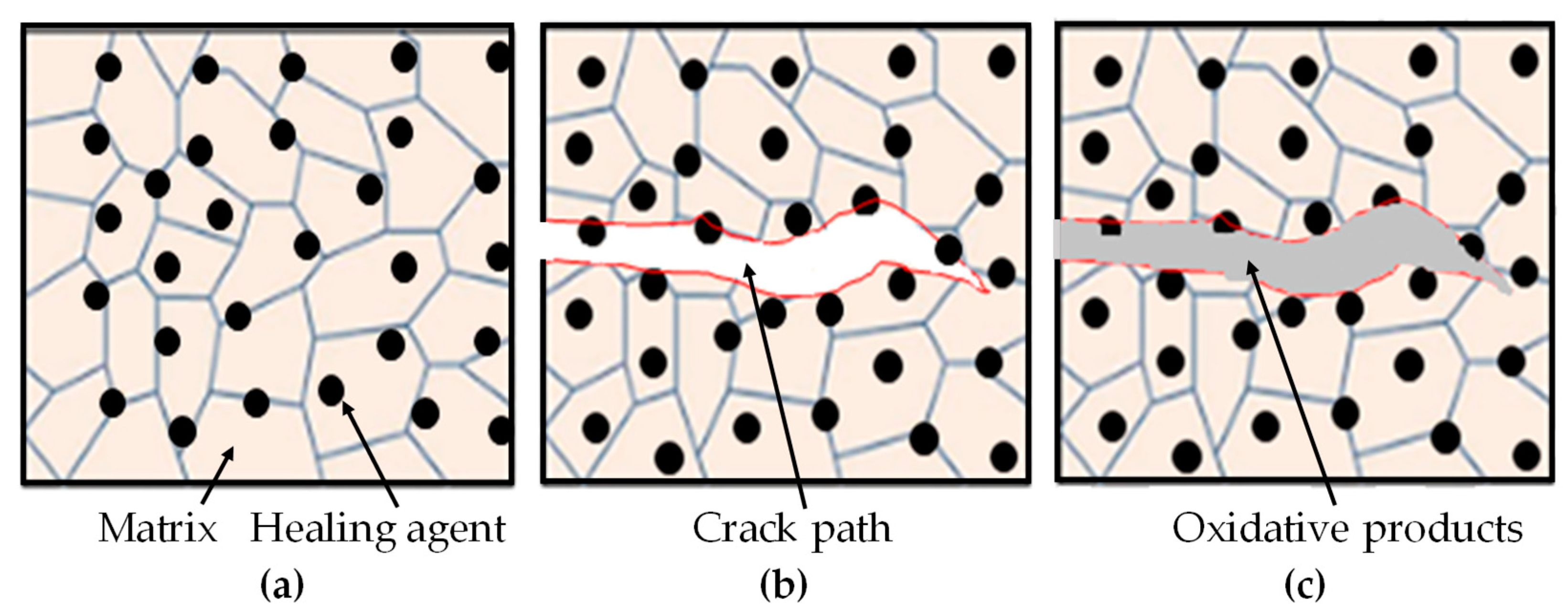
1. Introduction
2. Universal kinetic model of strength recovery
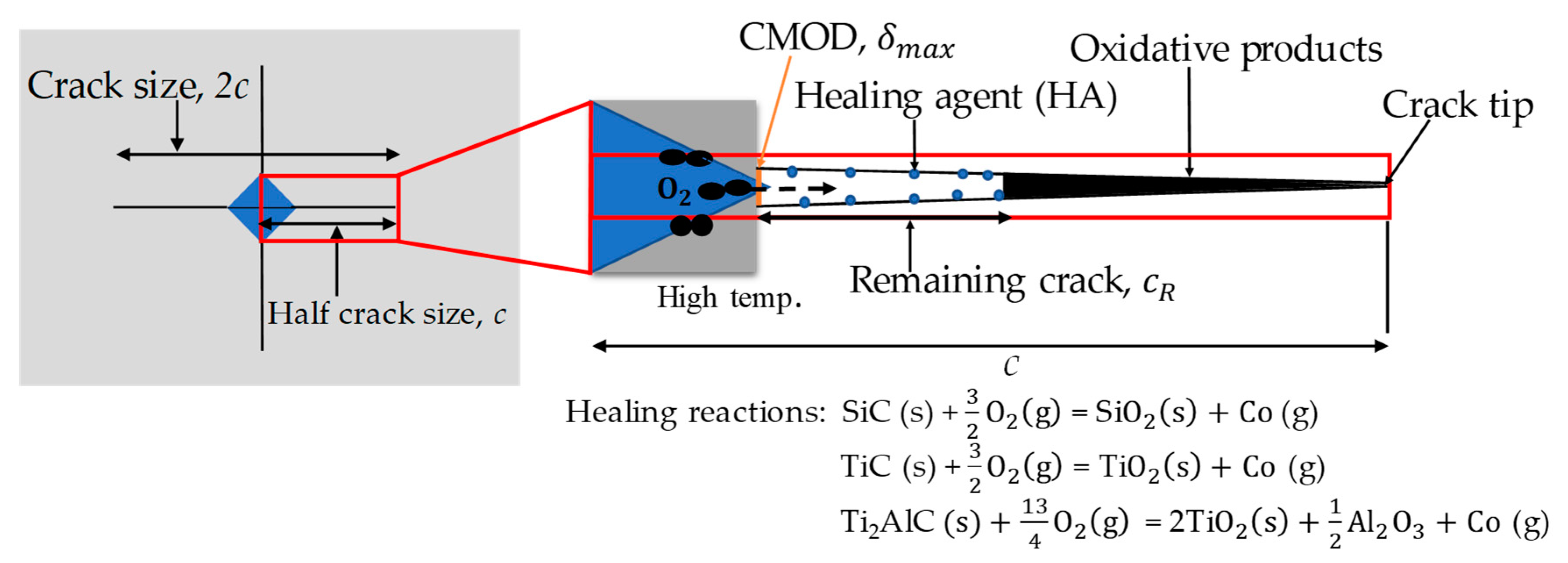
2.1. Volume-gain kinetics model
2.2. Tip-to-mouth crack-gap filling model
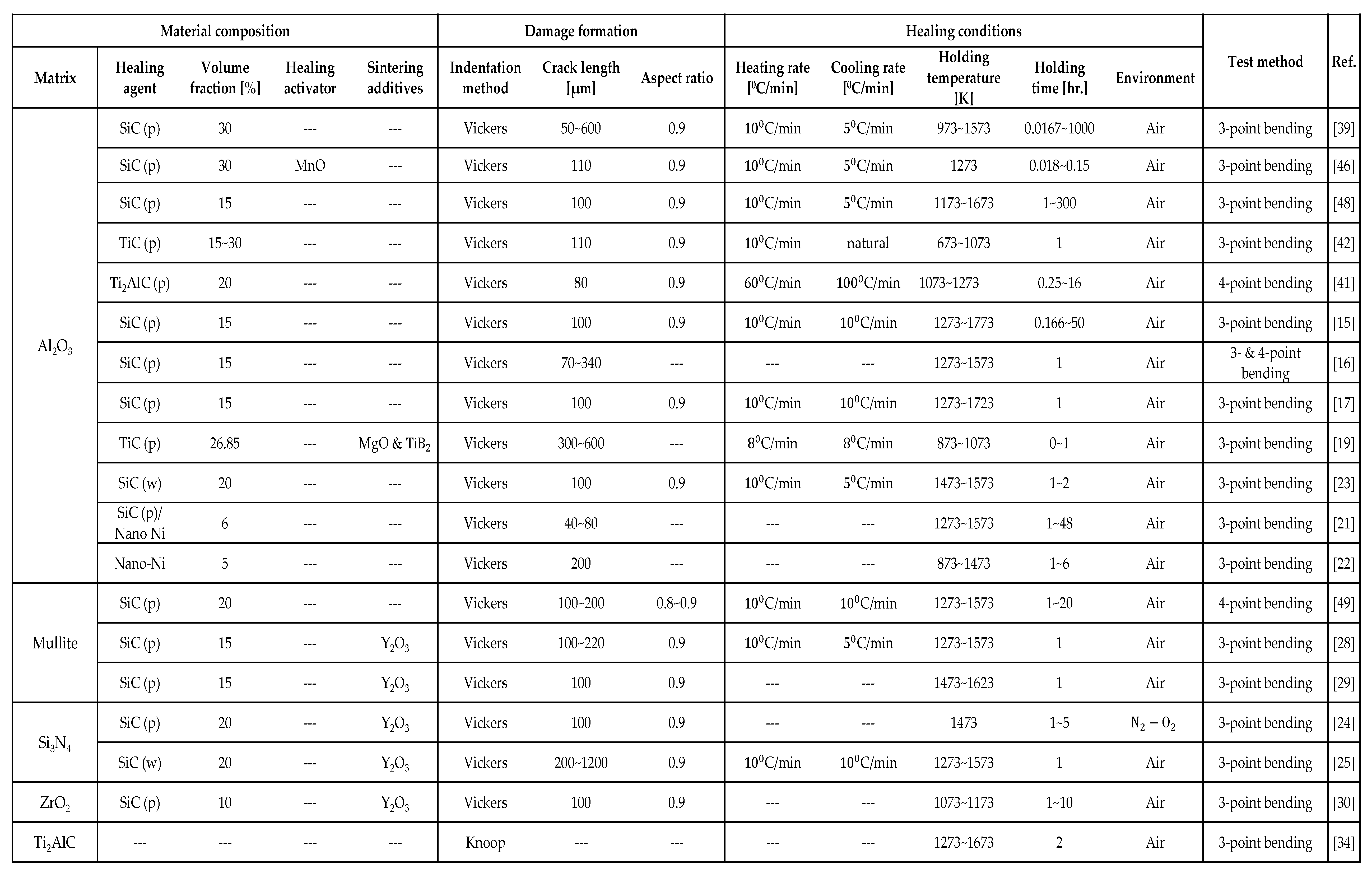
3. Parameter determination method
3.1. Target composites
3.2. Parameter optimization
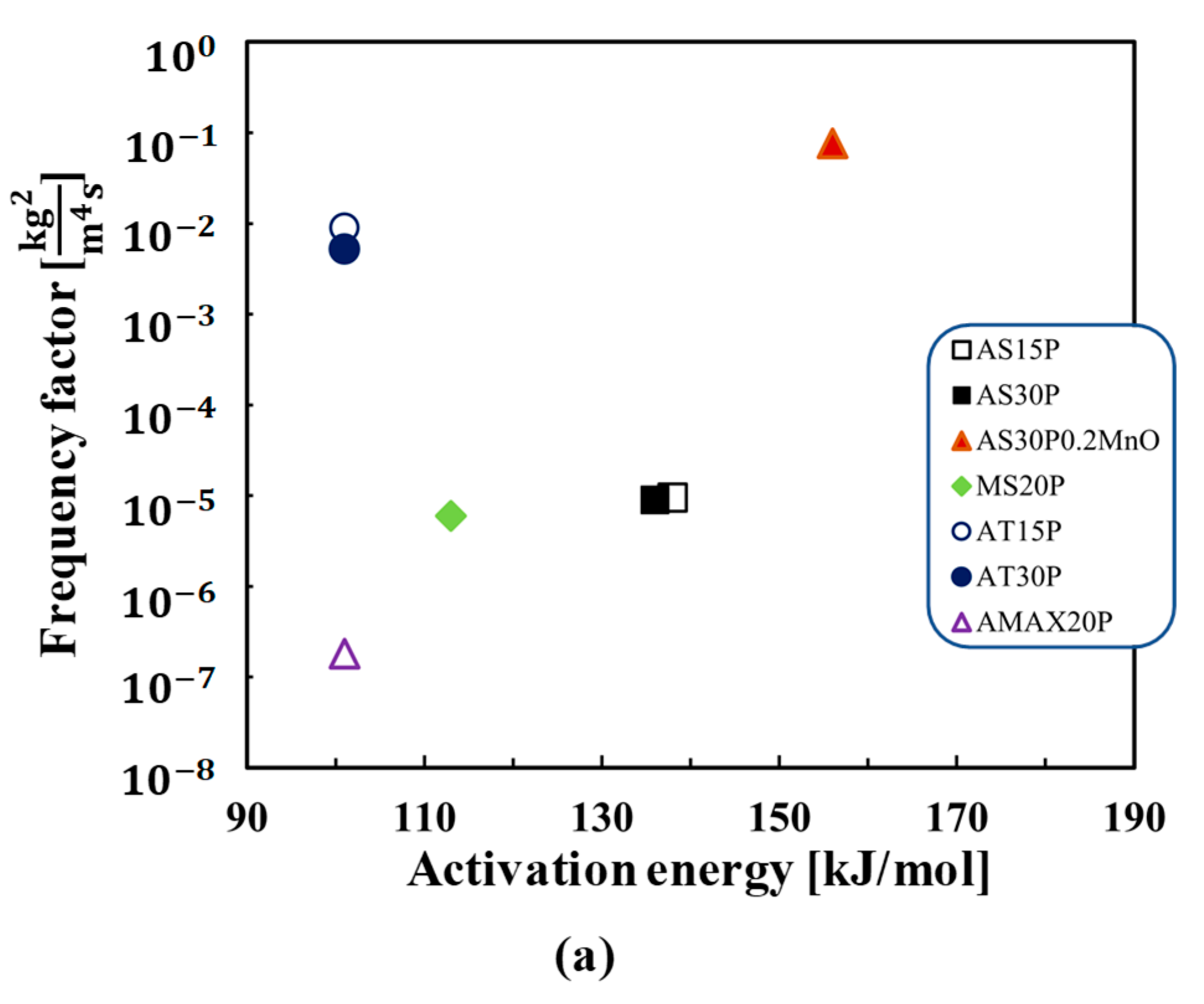
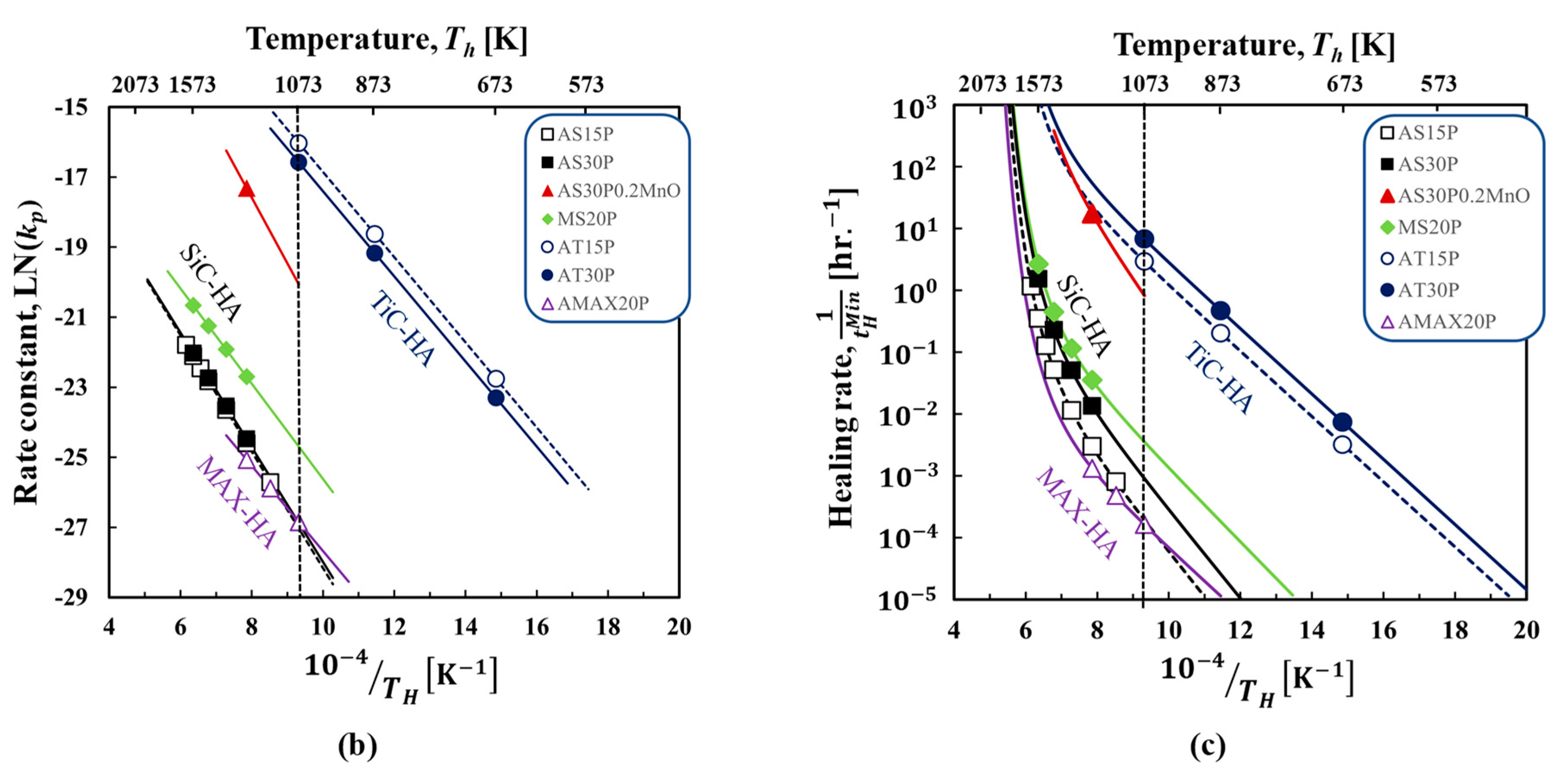
4. Results
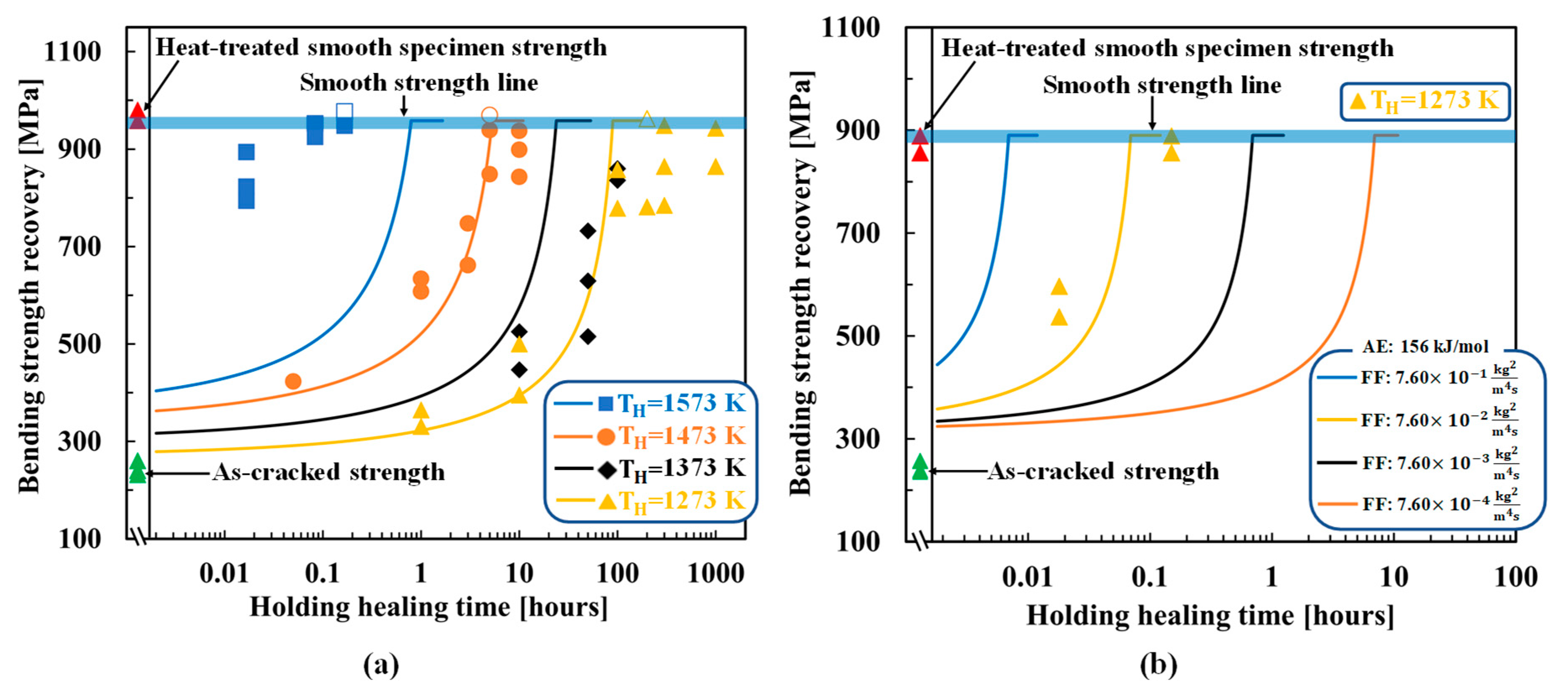
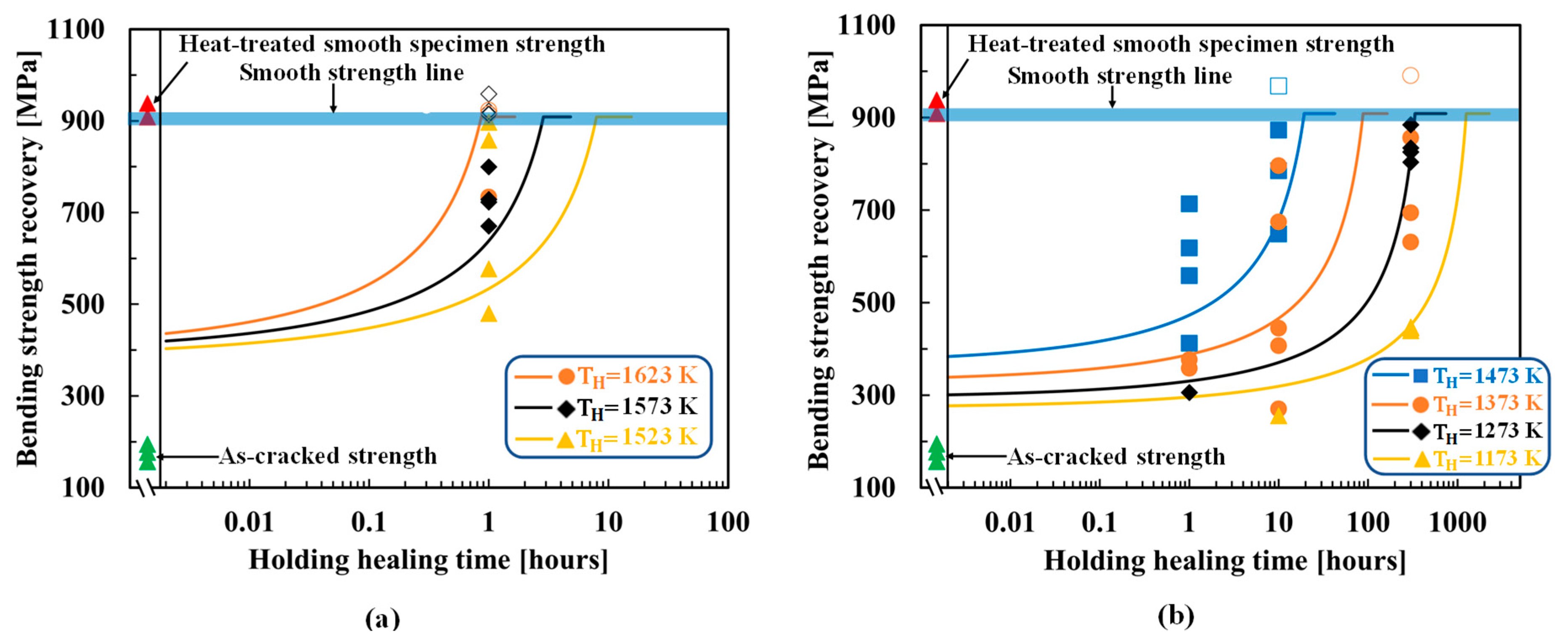
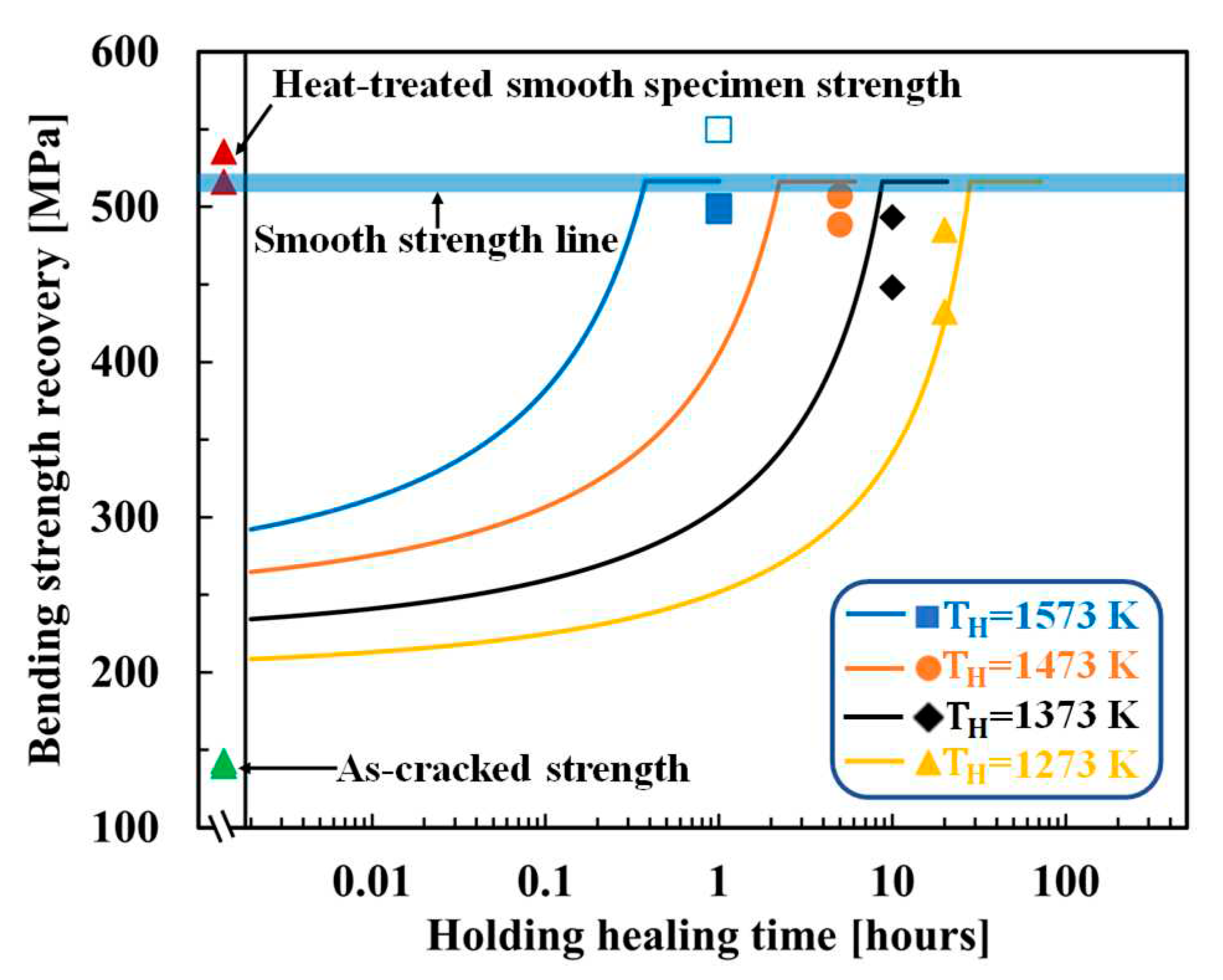
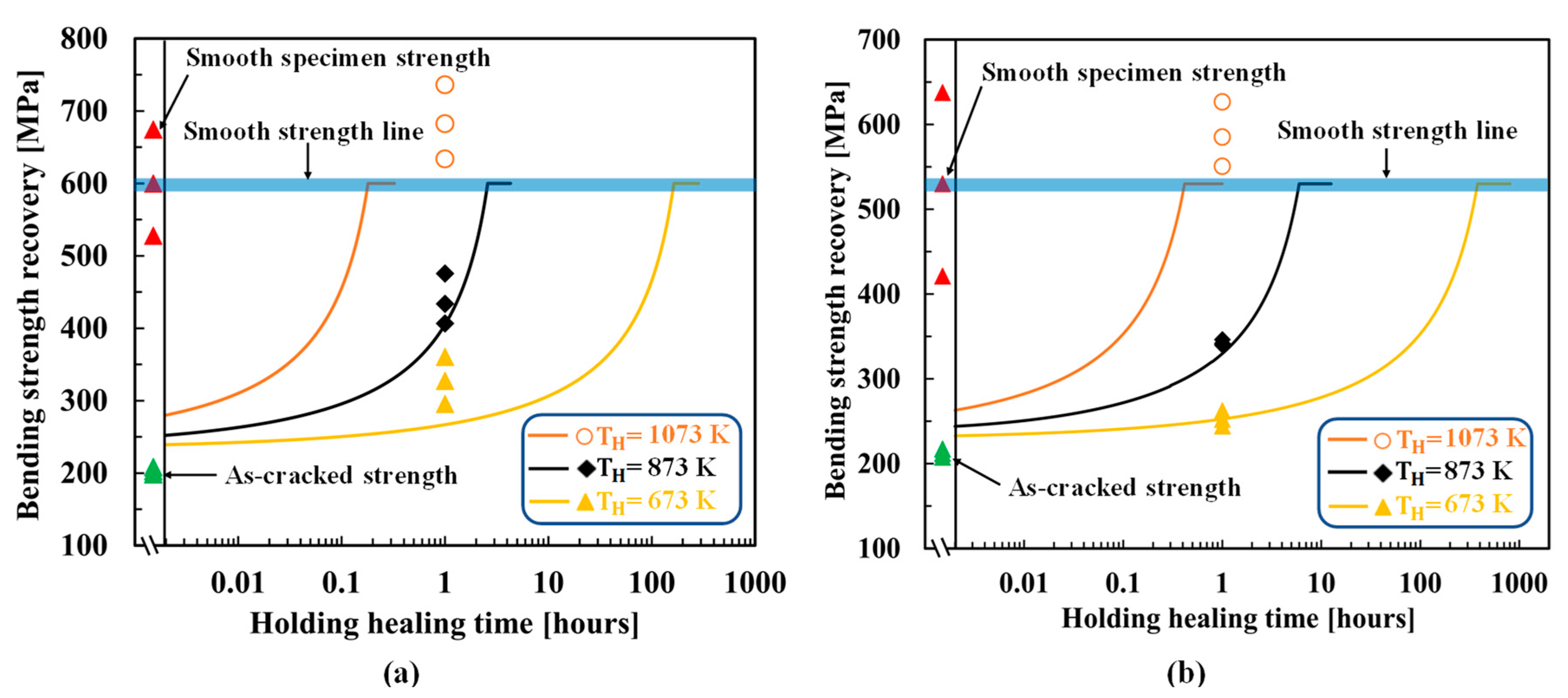
4.1. SiC–HA-based alumina and mullite composites
4.2. TiC–HA-based alumina composite
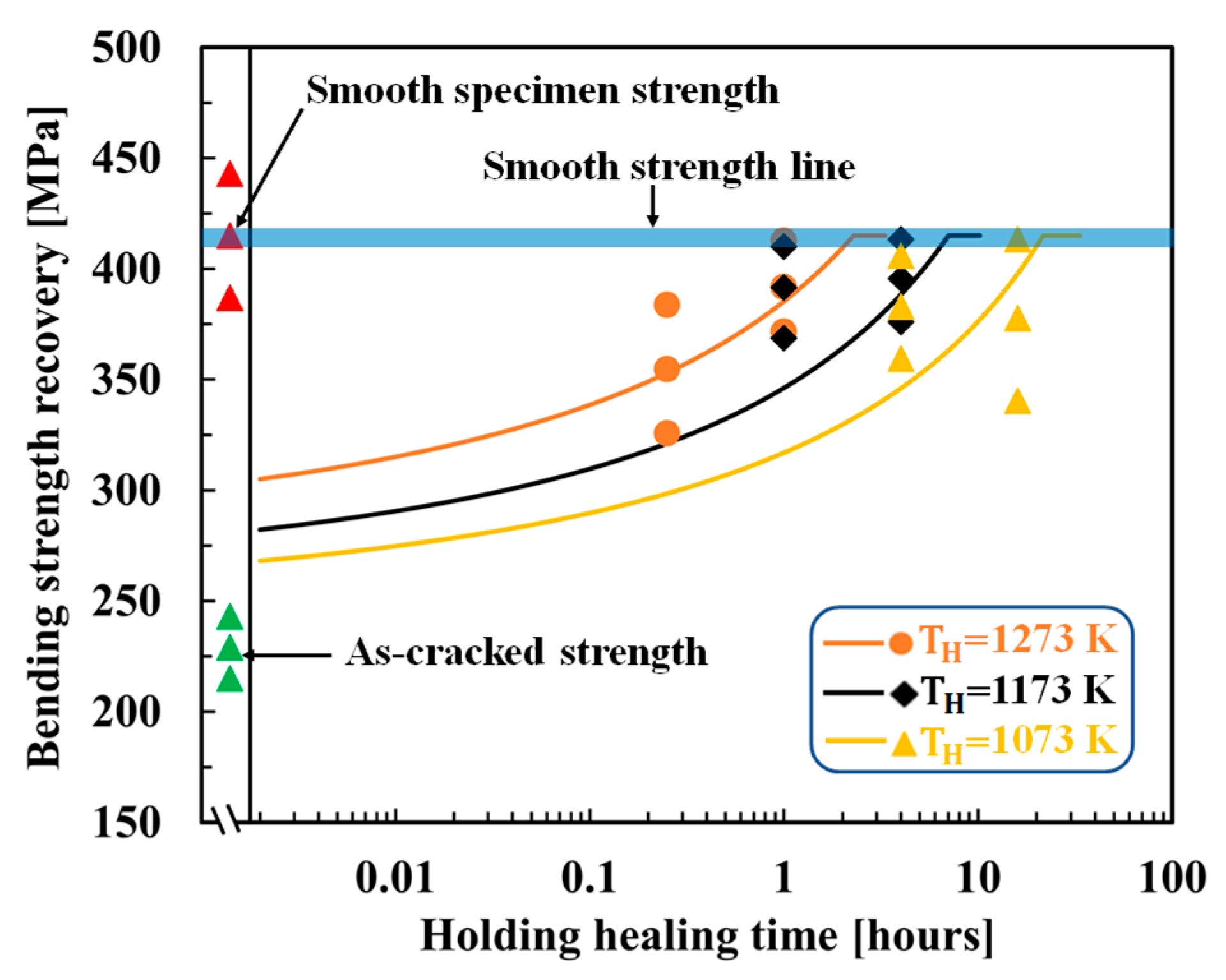
4.3. MAX-phase HA-based alumina composite
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greil, P. Self-Healing Engineering Ceramics with Oxidation-Induced Crack Repair. Adv. Eng. Mater. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Levine, S.R.; Opila, E.J.; Halbig, M.C.; Kiser, J.D.; Singh, M.; Salem, J.A. Evaluation of Ultra-High Temperature Ceramics for Aeropropulsion Use. J. Eur. Ceram. Soc. 2002, 22, 2757–2767. [Google Scholar] [CrossRef]
- Padture, N.P. Advanced Structural Ceramics in Aerospace Propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef]
- Quinn, G.D.; Morrell, R. Design Data for Engineering Ceramics: A Review of the Flexure Test. J. Am. Ceram. Soc. 1991, 74, 2037–2066. [Google Scholar] [CrossRef]
- Purohit, R.; Gupta, N.K.; Purohit, M.R.; Patil, A.; Bharilya, R.K.; Singh, S.K. An Investigation on Manufacturing of Self-Healing Materials. Mater. Today Proc. 2015, 2, 3371–3377. [Google Scholar] [CrossRef]
- van der Zwaag, S. An Introduction to Material Design Principles: Damage Prevention versus Damage Management. Springer Ser. Mater. Sci. 2007, 100, 1–18. [Google Scholar]
- Huet, R.; Sakona, A.; Kurtz, S.M. Strength and Reliability of Alumina Ceramic Femoral Heads: Review of Design, Testing, and Retrieval Analysis. J. Mech. Behav. Biomed. Mater. 2011, 4, 476–483. [Google Scholar] [CrossRef]
- Sitnikov, N.N.; Khabibullina, I.A.; Mashchenko, V.I.; Rizakhanov, R.N. Prospects of Application of Self-Healing Materials and Technologies Based on Them. Inorg. Mater. Appl. Res. 2018, 9, 785–793. [Google Scholar] [CrossRef]
- H. Heuer and J. P. Roberts. The Influence of Annealing on the Strength of Corundum Crystals. Proc. Br. Ceram. Soc. 1966, 6, 17–27.
- LANGE, F.F.; GUPTA, T.K. Crack Healing by Heat Treatment. J. Am. Ceram. Soc. 1970, 53, 54–55. [Google Scholar] [CrossRef]
- Chu, M.C.; Sato, S.; Kobayashi, Y.; Ando, K. DAMAGE HEALING AND STRENGTHENING BEHAVIOUR IN INTELLIGENT MULLITE/SiC CERAMICS. Fatigue Fract. Eng. Mater. Struct. 1995, 18, 1019–1029. [Google Scholar] [CrossRef]
- Ando, K.; Ikeda, T.; Sato, S.; Yao, F.; Kobayasi, Y. A Preliminary Study on Crack Healing Behaviour of Si3N4/SiC Composite Ceramics. Fatigue Fract. Eng. Mater. Struct. 1998, 21, 119–122. [Google Scholar] [CrossRef]
- K. ANDO, M.C. CHU, F.Y. and S. S. Fatigue Strength of Crack-Healed Si3N4/SiC Composite Ceramics. Fatigue Fract. Eng. Mater. Struct. 1999, 22, 897–903.
- Osada, T.; Nakao, W.; Takahashi, K.; Ando, K.; Saito, S. Strength Recovery Behavior of Machined Al2O3/SiC Nano-Composite Ceramics by Crack-Healing. J. Eur. Ceram. Soc. 2007, 27, 3261–3267. [Google Scholar] [CrossRef]
- Osada, T.; Nakao, W.; Takahashi, K.; Ando, K. Kinetics of Self-Crack-Healing of Alumina/Silicon Carbide Composite Including Oxygen Partial Pressure Effect. J. Am. Ceram. Soc. 2009, 92, 864–869. [Google Scholar] [CrossRef]
- Chlup, Z.; Flasar, P.; Kotoji, A.; Dlouhy, I. Fracture Behaviour of Al2O3/SiC Nanocomposite Ceramics after Crack Healing Treatment. J. Eur. Ceram. Soc. 2008, 28, 1073–1077. [Google Scholar] [CrossRef]
- Liu, S.P.; Ando, K. Fatigue Strength Characteristics of Crack-Healing Materials - Al2O3/SiC Composite Ceramics and Monolithic Al2O3. J. Chinese Inst. Eng. Trans. Chinese Inst. Eng. A/Chung-kuo K. Ch’eng Hsuch K’an 2004, 27, 395–404. [Google Scholar] [CrossRef]
- Boatemaa, L.; Brouwer, J.C.; van der Zwaag, S.; Sloof, W.G. The Effect of the TiC Particle Size on the Preferred Oxidation Temperature for Self-Healing of Oxide Ceramic Matrix Materials. J. Mater. Sci. 2018, 53, 5973–5986. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, L.; Guo, N.; Xu, C.; Zhang, S. Crack Healing and Strength Recovery of Al2O3/TiC/TiB2 Ceramic Tool Materials. Int. J. Refract. Met. Hard Mater. 2020, 87, 105167. [Google Scholar] [CrossRef]
- Maruoka, D.; Nanko, M. Recovery of Mechanical Strength by Surface Crack Disappearance via Thermal Oxidation for Nano-Ni/Al2O3 Hybrid Materials. Ceram. Int. 2013, 39, 3221–3229. [Google Scholar] [CrossRef]
- Maruoka, D.; Sato, Y.; Nanko, M. Crack-Healing Effectiveness of Nano Ni + SiC Co-Dispersed Alumina Hybrid Materials. Adv. Mater. Res. 2010, 89–91, 365–370. [Google Scholar] [CrossRef]
- Salas-Villaseñor, A.L.; Lemus-Ruiz, J.; Nanko, M.; Maruoka, D. Crack Disappearance by High-Temperature Oxidation of Alumina Toughened by Ni Nano-Particles. Adv. Mater. Res. 2009, 68, 34–43. [Google Scholar] [CrossRef]
- Nakao, W.; Ono, M.; Lee, S.K.; Takahashi, K.; Ando, K. Critical Crack-Healing Condition for SiC Whisker Reinforced Alumina under Stress. J. Eur. Ceram. Soc. 2005, 25, 3649–3655. [Google Scholar] [CrossRef]
- Takahashi, K.; Jung, Y.S.; Nagoshi, Y.; Ando, K. Crack-Healing Behavior of Si3N4/SiC Composite under Stress and Low Oxygen Pressure. Mater. Sci. Eng. A 2010, 527, 3343–3348. [Google Scholar] [CrossRef]
- Hu, J.F.; Deng, X.; Xu, T.Z.; Chen, Z. Experimental and Theoretical Investigation on the Effect of Crack Dimension on the Crack-Healing Performance of Si3N4/SiCw Composite Ceramic. Results Phys. 2019, 14, 102411. [Google Scholar] [CrossRef]
- Saucedo-Mora, L.; Mostafavi, M.; Khoshkhou, D.; Reinhard, C.; Atwood, R.; Zhao, S.; Connolly, B.; Marrow, T.J. Observation and Simulation of Indentation Damage in a SiC-SiCfibre Ceramic Matrix Composite. Finite Elem. Anal. Des. 2016, 110, 11–19. [Google Scholar] [CrossRef]
- Nakatani, M.; Nishimura, J.; Hanaki, S.; Uchida, H. Crack-Healing Behavior Induced by Oxidation in SiN/SiC Nanolaminated Films. Thin Solid Films 2014, 556, 68–73. [Google Scholar] [CrossRef]
- Ando, K.; Furusawa, K.; Chu, M.C.; Hanagata, T.; Tuji, K.; Sato, S. Crack-Healing Behavior under Stress of Mullite/Silicon Carbide Ceramics and the Resultant Fatigue Strength. J. Am. Ceram. Soc. 2001, 84, 2073–2078. [Google Scholar] [CrossRef]
- Lee, S.K.; Ono, M.; Nakao, W.; Takahashi, K.; Ando, K. Crack-Healing Behaviour of Mullite/SiC/Y2O3 Composites and Its Application to the Structural Integrity of Machined Components. J. Eur. Ceram. Soc. 2005, 25, 3495–3502. [Google Scholar] [CrossRef]
- Nam, K.W.; Hwang, J.R. The Crack Healing Behavior of ZrO 2/SiC Composite Ceramics with TiO 2 Additive. J. Mech. Sci. Technol. 2012, 26, 2093–2096. [Google Scholar] [CrossRef]
- Song, G.M.; Pei, Y.T.; Sloof, W.G.; Li, S.B.; De Hosson, J.T.M.; van der Zwaag, S. Oxidation-Induced Crack Healing in Ti3AlC2 Ceramics. Scr. Mater. 2008, 58, 13–16. [Google Scholar] [CrossRef]
- Sloof, W.G.; Pei, R.; McDonald, S.A.; Fife, J.L.; Shen, L.; Boatemaa, L.; Farle, A.S.; Yan, K.; Zhang, X.; Van Der Zwaag, S.; Lee, P.D.; Withers, P.J. Repeated Crack Healing in MAX-Phase Ceramics Revealed by 4D in Situ Synchrotron X-Ray Tomographic Microscopy. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Pei, Y.T.; Rao, J.C.; De Hosson, J.T.M. Self-Healing Performance of Ti 2AlC Ceramic. J. Mater. Chem. 2012, 22, 8304–8313. [Google Scholar] [CrossRef]
- Li, S.; Song, G.; Kwakernaak, K.; van der Zwaag, S.; Sloof, W.G. Multiple Crack Healing of a Ti 2AlC Ceramic. J. Eur. Ceram. Soc. 2012, 32, 1813–1820. [Google Scholar] [CrossRef]
- Song, G.M.; Schnabel, V.; Kwakernaak, C.; Van Der Zwaag, S.; Schneider, J.M.; Sloof, W.G. High Temperature Oxidation Behaviour of Ti2AlC Ceramic at 1200°C. Mater. High Temp. 2012, 29, 205–209. [Google Scholar] [CrossRef]
- Ozaki, S.; Osada, T.; Nakao, W. Finite Element Analysis of the Damage and Healing Behavior of Self-Healing Ceramic Materials. Int. J. Solids Struct. 2016, 100–101, 307–318. [Google Scholar] [CrossRef]
- Kurumatani, M.; Terada, K.; Kato, J.; Kyoya, T.; Kashiyama, K. An Isotropic Damage Model Based on Fracture Mechanics for Concrete. Eng. Fract. Mech. 2016, 155, 49–66. [Google Scholar] [CrossRef]
- Ozaki, S.; Yamamoto, J.; Kanda, N.; Osada, T. Kinetics-Based Constitutive Model for Self-Healing Ceramics and Its Application to Finite Element Analysis of Alumina/SiC Composites. Open Ceram. 2021, 6, 100135. [Google Scholar] [CrossRef]
- Osada, T.; Hara, T.; Mitome, M.; Ozaki, S.; Abe, T.; Kamoda, K.; Ohmura, T. Self-Healing by Design: Universal Kinetic Model of Strength Recovery in Self-Healing Ceramics. Sci. Technol. Adv. Mater. 2020, 21, 593–608. [Google Scholar] [CrossRef]
- Nakao, W.; Abe, S. Enhancement of the Self-Healing Ability in Oxidation Induced Self-Healing Ceramic by Modifying the Healing Agent. Smart Mater. Struct. 2012, 21. [Google Scholar] [CrossRef]
- Boatemaa, L.; Bosch, M.; Farle, A.S.; Bei, G.P.; van der Zwaag, S.; Sloof, W.G. Autonomous High-Temperature Healing of Surface Cracks in Al2O3 Containing Ti2AlC Particles. J. Am. Ceram. Soc. 2018, 101, 5684–5693. [Google Scholar] [CrossRef]
- Yoshioka, S.; Boatemaa, L.; Zwaag, S. van der; Nakao, W.; Sloof, W.G. On the Use of TiC as High-Temperature Healing Particles in Alumina Based Composites. J. Eur. Ceram. Soc. 2016, 36, 4155–4162. [Google Scholar] [CrossRef]
- Ando, K.; Shirai, Y.; Nakatani, M.; Kobayashi, Y.; Sato, S. (Crack-Healing + Proof Test): A New Methodology to Guarantee the Structural Integrity of a Ceramics Component. J. Eur. Ceram. Soc. 2002, 22, 121–128. [Google Scholar] [CrossRef]
- Ando, K.; Furusawa, K.; Takahashi, K.; Sato, S. Crack-Healing Ability of Structural Ceramics and a New Methodology to Guarantee the Structural Integrity Using the Ability and Proof-Test. J. Eur. Ceram. Soc. 2005, 25, 549–558. [Google Scholar] [CrossRef]
- Newman, J.C.; Raju, I.S. An Empirical Stress-Intensity Factor Equation for the Surface Crack. Eng. Fract. Mech. 1981, 15, 185–192. [Google Scholar] [CrossRef]
- Osada, T.; Kamoda, K.; Mitome, M.; Hara, T.; Abe, T.; Tamagawa, Y.; Nakao, W.; Ohmura, T. A Novel Design Approach for Self-Crack-Healing Structural Ceramics with 3D Networks of Healing Activator. Sci. Rep. 2017, 7, 1–2. [Google Scholar] [CrossRef]
- COSTELLO, J.A.; TRESSLER, R.E. Oxidation Kinetics of Hot-Pressed and Sintered A-SiC. J. Am. Ceram. Soc. 1981, 64, 327–331. [Google Scholar] [CrossRef]
- Ando, K.; Kim, B.S.; Chu, M.C.; Saito, S.; Takahashi, K. Crack-Healing and Mechanical Behaviour of Al2O3/SiC Composites at Elevated Temperature. Fatigue Fract. Eng. Mater. Struct. 2004, 27, 533–541. [Google Scholar] [CrossRef]
- Ando, K. ;, Min-Cheol Chu, Kiichi Tsuji, T. H.; Yasuyoshi Kobayashi, S.S. Crack Healing Behaviour and High-Temperature Strength of Mullite/SiC Composite Ceramics. J. Eur. Ceram. Soc. 2002, 22, 1313–1319. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





