1. Introduction
Since December 2019, the deadly severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 762 million people, led to approximately seven million deaths, and caused unprecedented disruptions to public health and the economy [
1,
2,
3]. Real-time polymerase chain reaction (RT-PCR) for the detection of viral nucleic acids is currently the gold standard for the diagnosis of coronavirus disease 2019 (COVID -19) [
4,
5]. However, practical limitations prevent it from keeping pace with the rapid growth of the virus. For example, collecting samples from the nasopharynx is a painful process that can take a long time and requires licensed laboratories and specialized skills [
6]. Therefore, RT-PCR is not suitable for rapid and straightforward patient screening. Rapid screening to identify SARS-CoV-2-infected patients is essential to prevent transmission of the virus, ensure timely treatment, and support mass testing. Considering these factors, the focus has shifted to point-of-care testing (POCT) because it is easy and quick to perform and can screen large numbers of patients [
7,
8,
9,
10,
11,
12,
13]. In addition, experts agree that mortality rates can be reduced if more people are vaccinated and that complete elimination of COVID19 may not be possible until all people have access to vaccination. Despite the availability of 13.33 billion vaccine doses worldwide over the past two years, by April 2023, less than half of World Health Organization member countries (WHO) have reached the organization's goal of 70%, and only 37% of health professionals in low-income countries have received primary vaccination [
14,
15]. Booster vaccinations and additional doses account for approximately 20% of all administered vaccinations today [
16]. According to the director general of WHO, Tedros, "Blanket booster programs are likely to prolong the pandemic, rather than ending it, by diverting supply to countries that already have high levels of vaccination coverage, giving the virus more opportunity to spread and mutate" [
17]. In addition, there is the question of vaccination parity—who should receive booster vaccinations and when.
During the pandemic COVID -19, there has been an urgent need for rapid and accurate testing methods to detect SARS-CoV-2 infection. Immunodiagnostic kits that detect IgM/IgG antibodies formed by vaccination or infection have been developed to qualitatively identify the presence of antibodies in patient samples [
13,
18,
19]. Although the concentrations of IgG and IgM antibodies can provide information about a person's immune response to infection, they do not necessarily reflect the person's immunity to the pathogen or ability to prevent or control infection. Nevertheless, detection of IgG and IgM antibodies to SARS-CoV-2 has been shown to be a reliable indicator of infection. IgM antibodies can be detected in the blood of patients at 3 to 6 days and IgG at 8 days after COVID-19 infection [
10]. Detection of both IgM and IgG antibodies could provide valuable information about the time course of viral infection, and rapid detection of these antibodies could improve the diagnosis and treatment of COVID -19.
One of the promising methods for the detection of IgM/IgG antibodies in patient samples is the lateral flow assay (LFA), which is based on the light scattering phenomenon of nanoparticles. In this method, antibodies bind to gold nanoparticles (GNP) conjugated to a non-pathogenic antigen and move in a specific direction [
20,
21]. LFA tests are commonly used at the point-of-care (POC) because they are user-friendly, allow on-site testing, and provide results within minutes, making them cost-effective [
22]. However, rapid LFA tests can only provide qualitative information about the presence or absence of antibodies, rather than quantification of specific antibodies.
The conventional method for measuring an LFA signal uses clinical or research laboratory equipment, such as a microplate reader, which offers exceptional performance. However, their high cost and size make them unsuitable for many potential applications. [
23,
24,
25,
26,
27]. To overcome this limitation, dedicated analytical systems are currently being developed to provide quantitative information on antibodies by monitoring light scattering from GNP [
28,
29,
30]. Additionally, smartphone-based systems are also being developed to provide a rapid, user-friendly, and cost-effective method for antibody quantification [
31,
32].
Several researchers have developed smartphone platforms to quantify various analytes, including concentrations of vitamin D, iron, and vitamin B12. Lee et al. and Srinivasan et al. developed a smartphone platform to quantify vitamin D and iron [
33,
34]. However, these systems usually require an external device connected to the smartphone, which limits the functionality and increases the cost. For example, Lee et al. developed a low-cost POC quantification system for vitamin B12 concentrations using silver amplification to increase the initial signal and detection limit within the required range [
31]. However, they used a specially designed LFA kit with a "spacer pad" to extend the duration of the critical competitive binding reaction. Similarly, Foysal et al. developed a smartphone-based analyte detection method to measure albumin in human serum on an LFA strip [
30]. However, this method cannot provide accurate data for slight misalignment under the camera. The LFA kits must be accurately placed in the field of view to avoid errors in the calculation of the number of pixels. Tong et al. developed an AI-assisted colorimetric LFA platform based on polydopamine nanoparticles using the receptor-binding domain of viral spike protein and mouse lgG for the quantification of neutralizing antibodies from vaccines [
13]. They used a portable smartphone-based reader to image the LFIA results and output them to the AI algorithm to analyze the concentration of neutralizing antibodies. This approach is a promising solution for accurate quantification of LFAs using smartphones.
Here, we present a smartphone- and cloud-based artificial intelligence analysis system (SCAISY) for SARS-CoV-2-specific IgG antibodies detected on LFA strips. SCAISY allows users to quickly and easily measure antibody production rates and quantify antigen and SARS-CoV-2 antibody retention rates for various purposes based on the rapid LFA-type immunodiagnostic kit. This system provides statistical data in addition to antibody retention values without the need for an external device. In addition, SCAISY ensures robust performance under a wide range of illumination conditions and camera orientations without the need for an external illumination device. As demand for vaccination and immunity testing rapidly increases, SCAISY is a simple and powerful method to track vaccination effectiveness in large populations, especially in resource-limited countries. This technique can be used as a real-time public health tool to monitor immunity levels and determine the need for additional vaccinations and immunity to specific antigens based on vaccination status, vaccine type, and duration after vaccination or infection.
1. Materials and Methods
2.1. LFA Kit and Human Blood Sampling
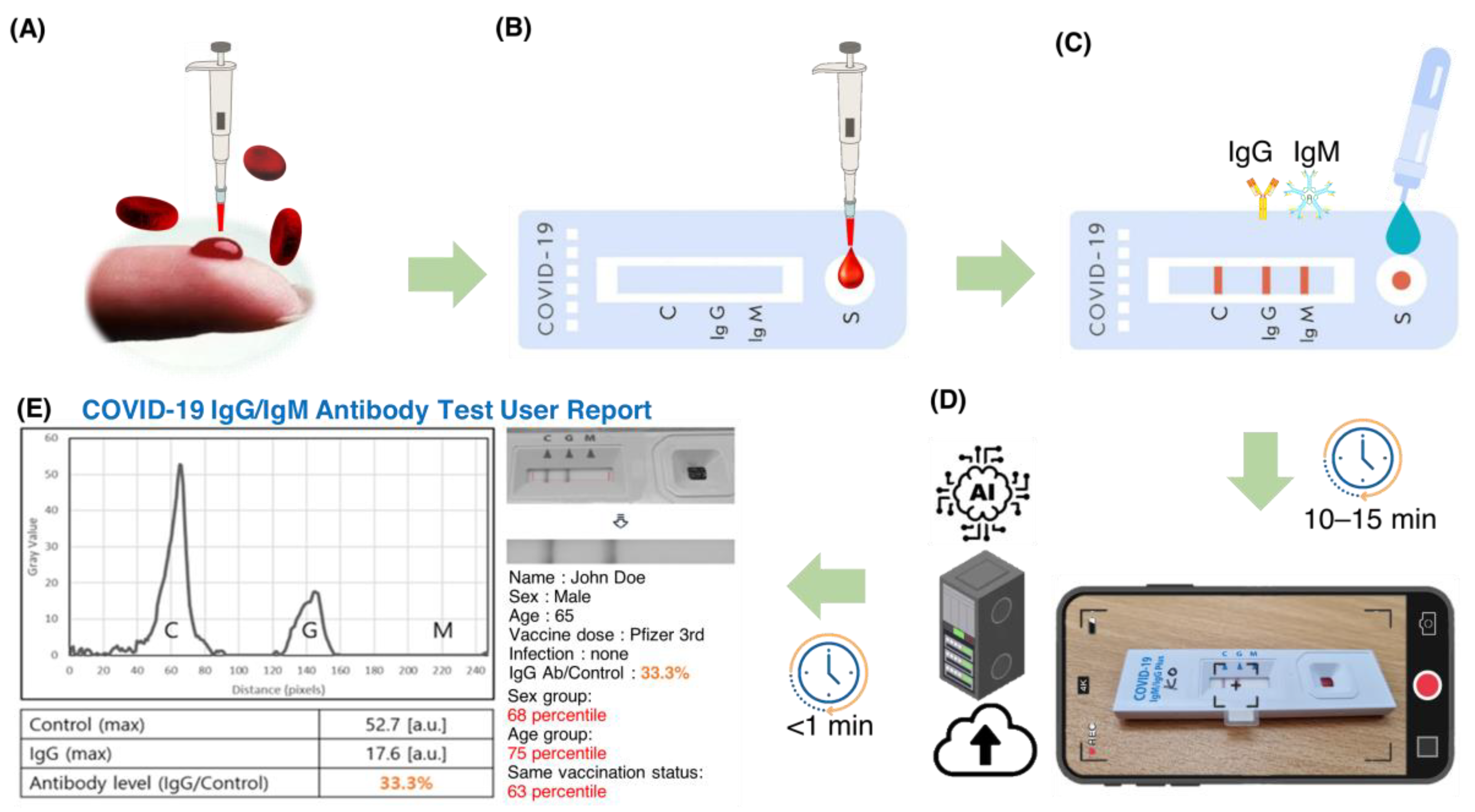
Figure 1 shows a flowchart of the steps involved in using an AI-based system for quantitative evaluation of LFA kits. Blood samples were collected from the subjects by pricking the finger and directly entered into the sample pad of a COVID -19 IgM/IgG Plus test kit (SD Biosensor, Suwon, Korea). Whole blood samples were collected with approval from the Institutional Review Board (#2021AN0040) of Anam Hospital of Korea University. A buffer solution (3–4 drops) provided by the kit manufacturer was added to the sample pad. The sample was then passed through a conjugation pad where antibody and conjugated markers were stored. When the target molecule was present in the sample, it bound to the immobilized conjugated antibodies and markers and continued to move through the LFA kit. As the sample moved through the LFA, the reagents on the nitrocellulose membrane bound to the target molecule on the test line. The presence of the target analyte in the sample was indicated by a colored line formed by light scattering from the GNP, the density of which varied depending on the amount of target analyte present.
2.2. Display of Results
The LFA strip used in this study has three detection lines: a control line (C) and two test lines (M and G) for detecting IgM and IgG antibodies, respectively. The control line serves as a safety measure to indicate that the test was performed correctly. A positive result for IgM or IgG antibodies is indicated by the appearance of a bright red line on the corresponding M or G test line. The intensity of the color on these test lines reflects the concentration of the analyte in the sample. In case of a negative sample, only the control line (C) shows a red line.
2.3. Data Acquisition Using a Smartphone Camera and Image Analysis
We used a smartphone to capture images of the LFA strips 10 minutes after sample application. Images were captured using the rear-facing camera of a Samsung Galaxy S21 smartphone (Samsung Electronics, Seoul, South Korea) running on Android 12.0.0, with autofocus and flash turned off. The images were then uploaded to a server for analysis by an artificial intelligence algorithm. The algorithm quickly analyzed the transferred image and generated a user report with the results and statistical information.
Figure 2 shows the workflow of SCAISY.
2.4. Feature Extraction
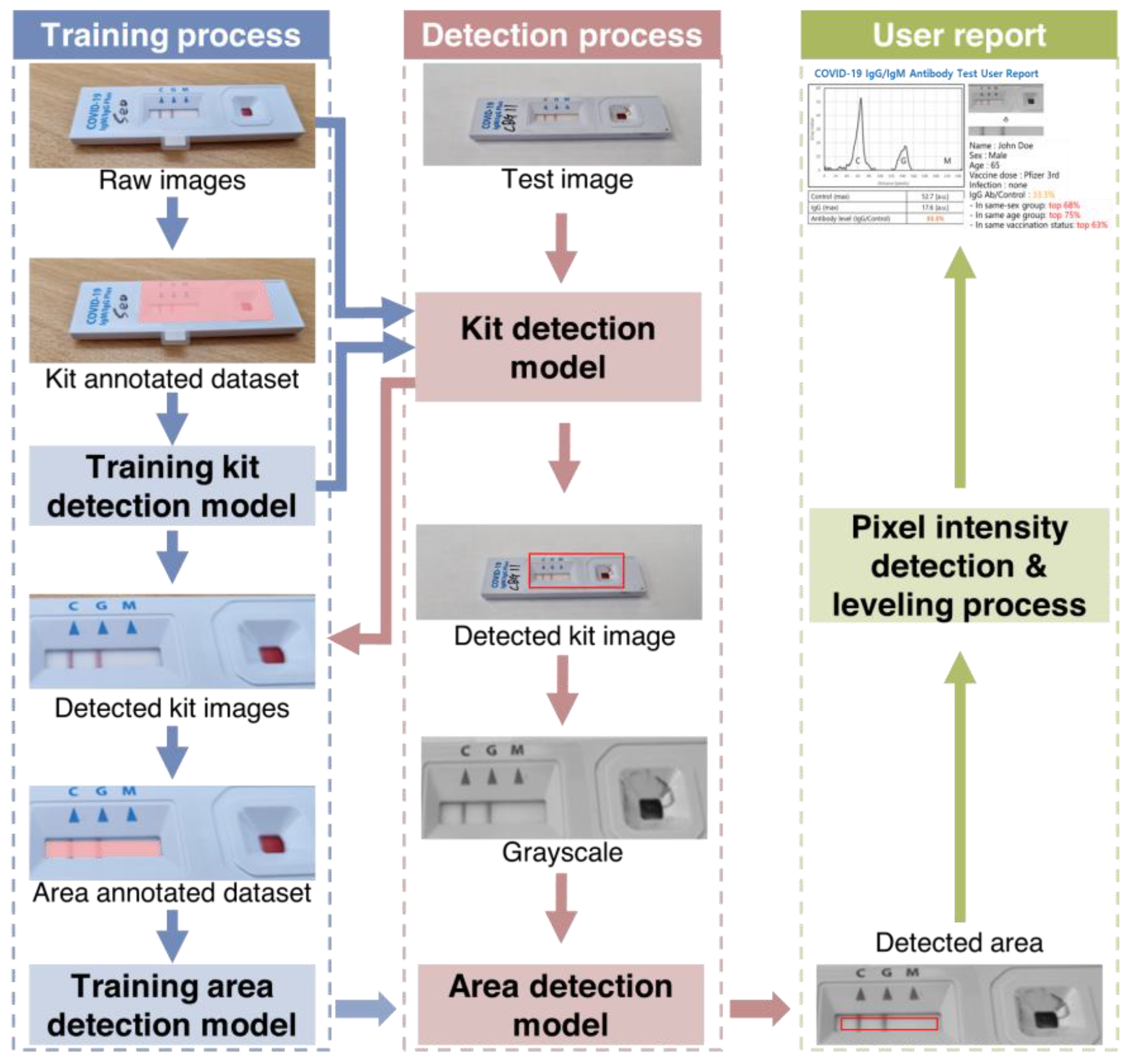
The specialized server for LFA kit evaluation consists of two main components: a web server that receives the kit image and user data after the LFA test, and an analysis server responsible for kit evaluation (
Figure S1). During the training process, a range detection model was used to determine the range of the analysis result window. Then, a kit detection model was used to determine whether the image was from a particular LFA kit based on images from different kits. A region of interest containing both test and control lines was automatically extracted from the image. Black and white images were created from the color images and an area detection model was used to extract pixel values from the resulting window image. The user was then presented with a graphical representation of the pixel intensities.
2.5. Test to Control Line Signal Intensity (T/C) Quantification using AI
Figure 2 shows a schematic representation of image processing of rapid immunodiagnostic kits for quantitative evaluation using SCAISY. Two object recognition models based on the Mask Region-Based Convolutional Neural Network (Mask-R-CNN) algorithm were used to quantitatively analyze images of kits taken with a camera. The first model recognized the area of the kit in the photo and was trained using images in which the kit area was masked as a polygon. Approximately 700 photos were taken under different environmental conditions to account for factors such as angle and background.
The second model recognized the area used for quantitative analysis within the detected kit area. To improve accuracy, a reference angle was defined for the training dataset and the accuracy of the recognition model was checked when the image was rotated. The area with the highest accuracy among the rotated areas was selected as the final result and converted to a numerical value using a quantification function. A leveling algorithm was used to calibrate this value and correct deviations caused by shadows on the photo.
3. Results
3.1. Capabilities of SCAISY
The primary objective of this study was to perform quantitative measurements of IgG and IgM antibodies, with a focus on developing a rapid, efficient, and cost-effective system for semi-quantitative detection of IgG antibodies. After training SCAISY, we used it to determine the antibody status of more than 240 individuals.
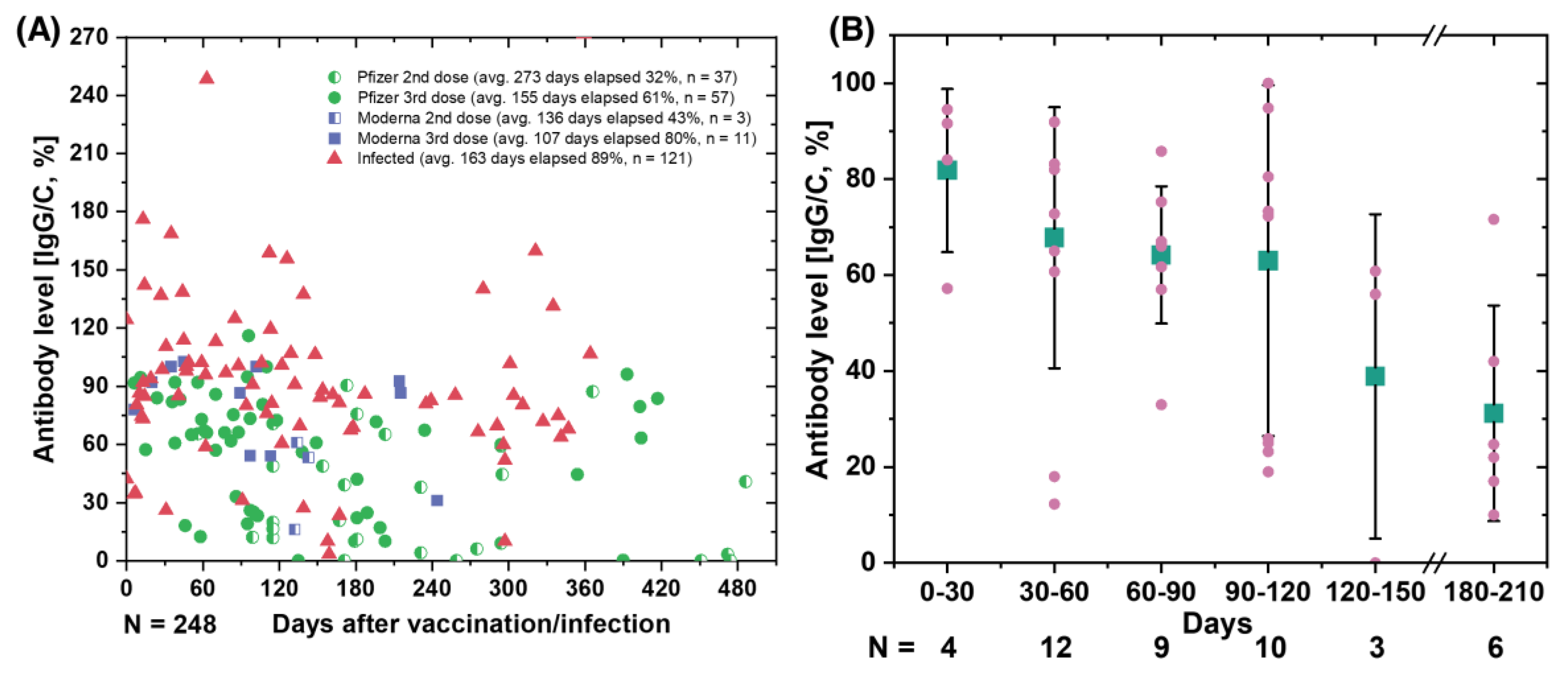
Figure 3A shows the SARS-CoV-2 antibody retention rate (IgG/C) over time for various users based on vaccine type and sequence. Our data show that the specific antibody retention rate (IgG/C) for the third dose of Pfizer was 61%, which was 19% lower than that for the third dose of Moderna (80%). In addition, individuals who received a second dose of Pfizer or Moderna (average 273 and 155 elapsed days, respectively) had lower antibody retention rates than individuals who received a third vaccination (average 100–107 elapsed days).
For individuals who received a third dose of Pfizer vaccine, we grouped the data by 30-day intervals, starting at 0 to 210 days.
Figure 3B shows a comparison of antibody levels in different groups of individuals after receiving the third dose of Pfizer, grouped by 30-day intervals. Our data show that relative protection against infection decreased from 81.8% one month after vaccination to 63% three months after the third dose of Pfizer BNT162b2 mRNA COVID -19 vaccine [
35]. We observed a gradual decline in antibody levels nearly six months post- vaccination, suggesting that vaccine efficacy declines over time and shows similar trends to previous studies [
35].
We want to clarify that our goal is not to draw conclusions about the efficacy of a particular vaccine, but to demonstrate that our method can be used to quantify antibody levels and track them over time. Controlled, randomized trials are needed to reach definitive conclusions about vaccine efficacy and immunity status. By using our method to track an individual’s antibody levels, particularly in the post- COVID-19 era, individuals with low antibody levels may be prioritized for vaccination. This could reduce pressure on the supply chain and ensure an even distribution of vaccinations. In addition, this technology could enable a wider range of individuals to participate in vaccination trials and studies by allowing them to submit their data from the comfort of their own home.
3.2. Monitoring the antibody against SARS-CoV-2
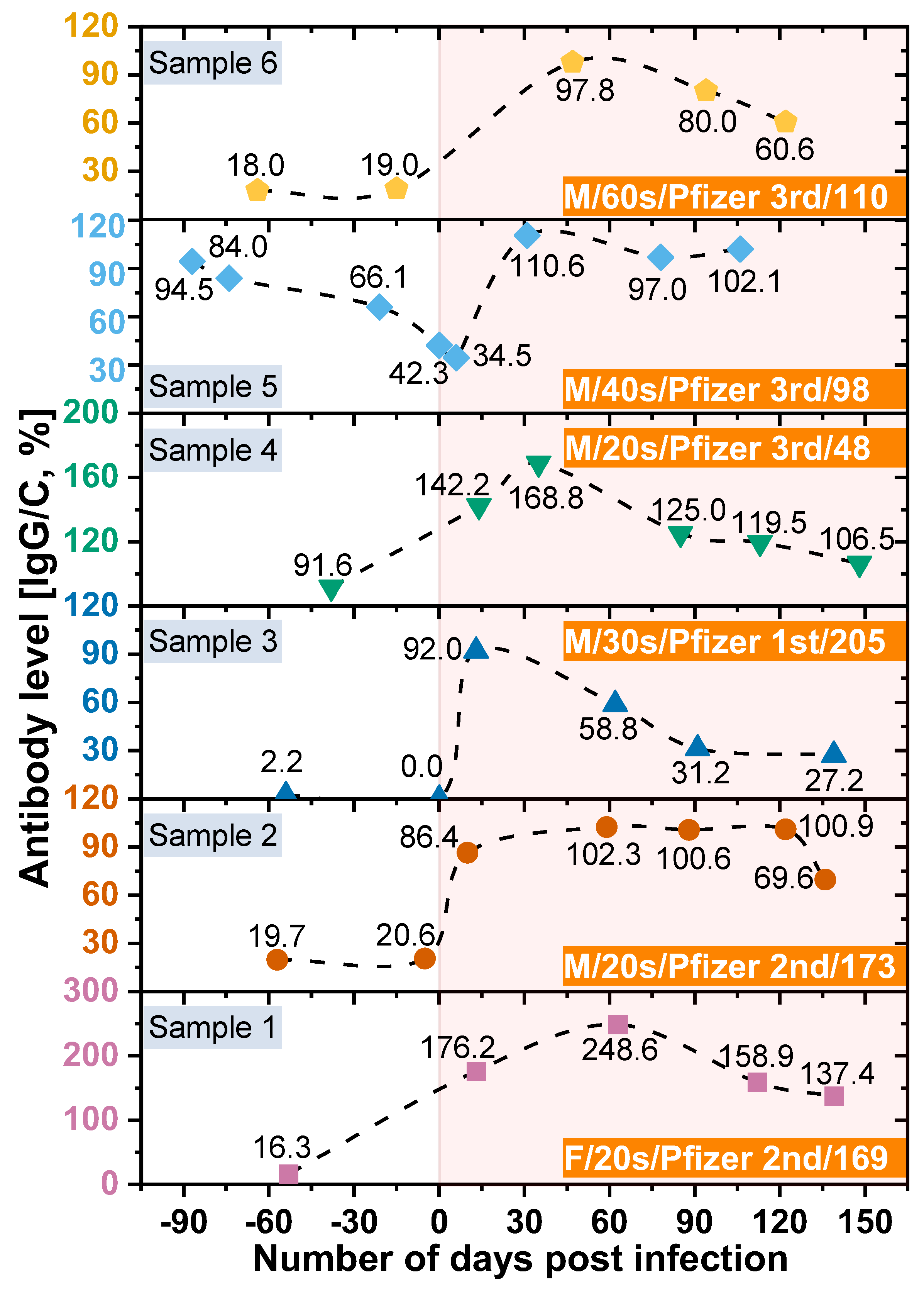
In this study, we also analyzed the changes in antibody retention rate of confirmed COVID -19 cases using the SCAISY. As shown in
Figure 4, the IgG/C antibody retention rate increased significantly after the COVID-19 confirmation date (day zero) and decreased gradually over time. These results suggest that the immune response to COVID-19 may vary depending on the duration of infection. Interestingly, we observed that antibody levels decreased more slowly in individuals who received the second or third dose of the vaccine, suggesting that vaccination may contribute to the maintenance of long-term immunity against COVID-19. Our results are consistent with previous studies that have found a decline in antibody response to SARS-CoV-2 over time after infection [
36]. However, the potential impact of vaccination on the duration and strength of the immune response to COVID-19 is the subject of ongoing investigation [
37,
38]. Our study provides further evidence that vaccination may contribute to the maintenance of long-term immunity against COVID-19.
In addition, individuals who received only the first dose of the vaccine had lower peak antibody levels after infection than those who received the second or third dose. The highest antibody level was observed within 15–60 days after infection and gradually decreased after 60 days [
37]. These results are consistent with previous studies, including Swartz et al. who found that the expected antibody response after COVID-19 infection persisted for more than 500 days after natural infection [
38,
39]. COVID-19 deaths have been strongly correlated with age. People aged ≥ 80 years have a 20-fold higher risk of death than people aged < 50 years [
40]. Furthermore, we found no significant differences in antibody levels between age groups, suggesting that antibody production does not contribute to age-related mortality. However, we acknowledge that underlying health conditions, genetics, and lifestyle factors may also be important for antibody production and function and should be considered when interpreting study results.
In addition, our study used LFA kits to measure antibody levels, which cannot distinguish between the effects of immunization and the effects of COVID-19 infection. Nonetheless, our focus was on measuring IgG and IgM levels in COVID-19 patients rather than distinguishing between these two effects. We are aware of this limitation and suggest that future studies address this issue. Interestingly, our results are consistent with those of [
41], who used an expensive instrument to measure antibody levels in a bead-based multiplex assay (MILLIPLEX® SARS-CoV-2 Antigen Panel 1 IgG; Merck KGaA, Darmstadt, Germany). However, it is important to note that our study was based on a small sample size and further investigation is required to confirm or refute our results.
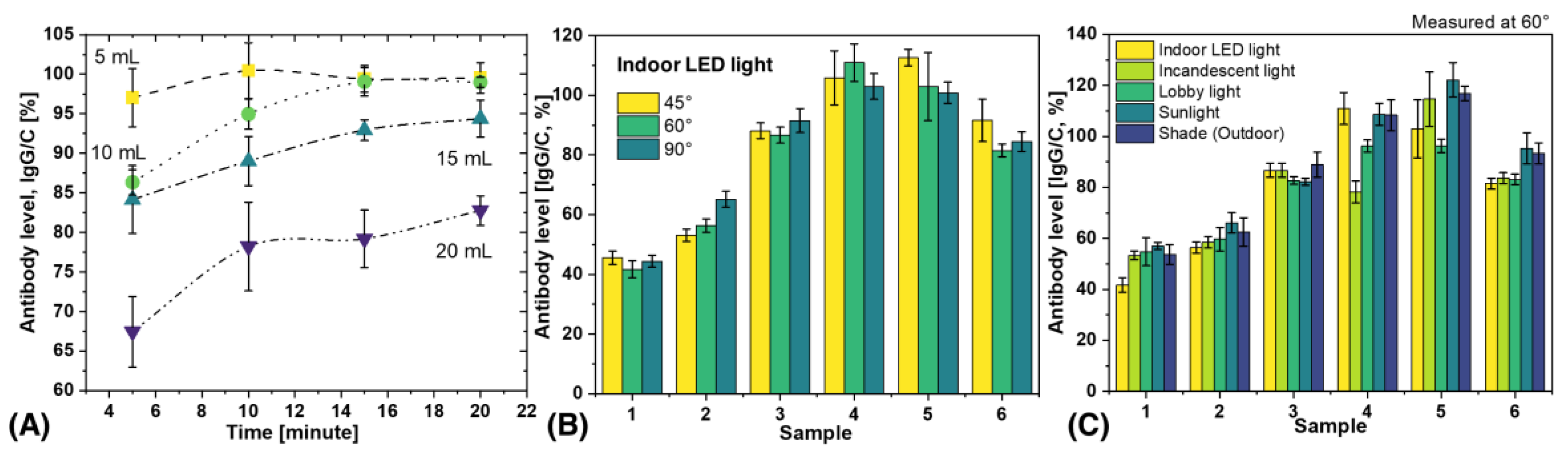
3.3. Effect of Blood Volume and Measurement Time on Antibody Level
Determining the effects of blood volume and measurement time on antibody results is critical for practical applications and also important for scientific replication. In this study, blood volumes of 5 to 20 µL were applied to the inlet of the test strip, and measurements were performed at intervals of 5 to 20 min, as shown in
Figure 5A. The results showed that antibody levels increased with time and reached a plateau after 10 min. Notably, the results of 5 µL and 10 µL blood were almost identical after 15 min, while the antibody levels decreased when the blood volume was increased to 20 µL. This decrease in antibody levels at a blood volume of 20 µL could be due to the fact that the chemical components of the LFA kit flow through the porous materials and do not reach a steady state. The formation of immune complexes in the test and control lines depends on the finite time for the components to be sufficiently close to each other to combine. This time is determined by the capillary flow rate. Xia et al. have previously reported that sample volume can affect flow rate and that there is an inverse relationship between the effective concentration of analyte in the sample and the square of the change in flow rate [
42,
43]. Consequently, a larger volume may result in a lower effective antibody concentration as the flow rate of the analyte increases [
43]. Therefore, 5–10 µL is the recommended volume and 15 min is the optimal time to obtain reproducible results.
3.4. Analysis of Variability Caused by Different Lighting Conditions and Shooting Angles
POCT devices are highly portable and automated diagnostic instruments that can be used in various settings such as hospitals, private homes, outpatient clinics, and remote locations [
44,
45,
46]. These devices can be used in real-world scenarios such as outdoor or indoor camps and by individuals or groups in different environments. For practical applications, it is important to understand the variations that can occur due to different external lighting conditions and image acquisition angles. To evaluate the performance of SCAISY in real-world situations, six different LFA samples were used and data were collected under five different lighting conditions (indoor LED light, incandescent light, lobby light, sunlight, and shade) and three different camera positions (45°, 60°, and 90°). The influence of the acquisition angle on the results under indoor LED light is shown in
Figure 5B, while the influence of the acquisition angle on the results under different lighting conditions is shown in
Figure S2. The results for all three angles were almost identical, with a standard deviation (SD) of less than 10%. Thus, SCAISY is a robust system that provides accurate results with a low SD for images captured between 45° and 90°, making it possible to capture images in real-world scenarios without worrying about the camera's field of view or capture angle.
The effect of different illumination conditions on the performance of SCAISY was evaluated at two different angles.
Figure 5C shows the results obtained at 60° illumination, while
Figure S3 shows a comparison of the results under different illumination conditions at 90°. The data show that all illumination conditions gave similar results within the SD. However, in some cases, antibody levels were slightly lower under incandescent light. However, this deviation is considered negligible since incandescent bulbs are no longer widely used and can be replaced by LED light on a smartphone to avoid this effect. In addition, the reproducibility of the results was evaluated for seven different samples, as shown in
Figure S4. For each sample, N images were acquired to test the reproducibility, and the obtained results were transmitted to SCAISY for analysis. The SD was less than 10% under the same illumination conditions, indicating high reproducibility. Furthermore, the repeatability of the results was investigated for four different samples, as shown in
Figure S5. The results obtained were consistent, demonstrating the robustness of SCAISY. This feature is particularly useful for medical professionals as it allows them to store digital photos in databases and analyze the results later if needed.
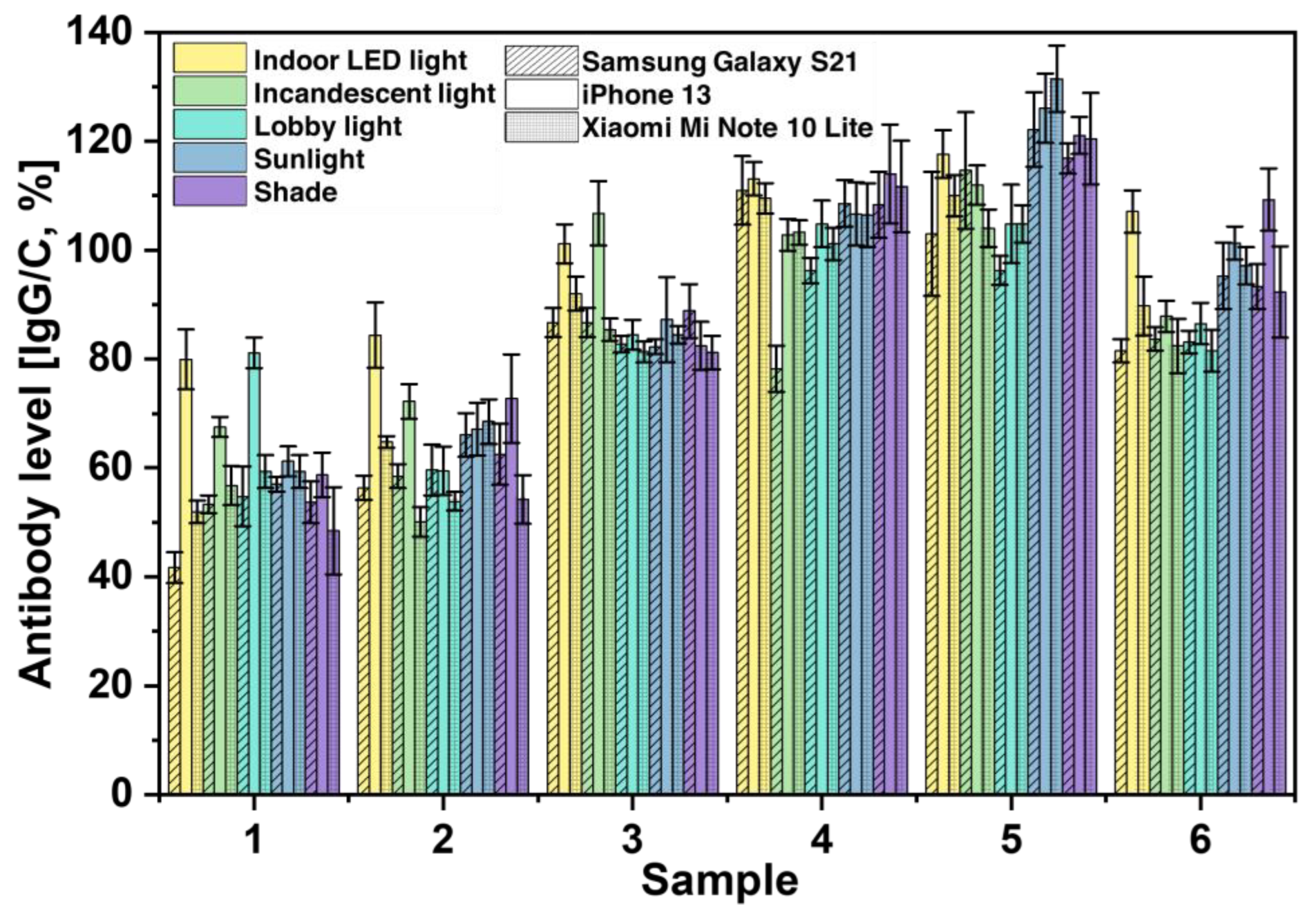
3.5. Analysis of Variability Caused by Smartphone Cameras
Finally, we investigated the effects of different cameras on the performance of SCAISY by taking images of six different samples under different lighting conditions (see
Figure 6). For the study, we used the rear cameras of two popular Android smartphones, the Samsung Galaxy S21 (Samsung Electronics, Seoul, South Korea) and the Mi Note 10 Lite (Xiaomi Inc., Beijing, China), and an iPhone 13 (Apple Inc., California, USA). All phones were equipped with autofocus and the flash was turned off during image capture. Our results showed that Samsung and Xiaomi achieved almost identical results in all lighting scenarios, as shown by the stripes and dotted grid, respectively. In contrast, the iPhone predicted slightly higher antibody levels, especially in indoor and incandescent lighting. These performance differences could be due to the unique image processing and sharpening implemented in the iPhone OS. However, this potential source of error can be mitigated by incorporating this information into the AI system and using machine learning to train the AI to detect and account for these factors.
It is worth noting that SCAISY has the potential to be a versatile tool for quantifying different types of LFA kits beyond the scope of this study. By generating a calibration curve via regression analysis using the T/C ratio, SCAISY can estimate the analyte concentration in a sample by analyzing an LFA kit image. Further studies are needed to explore the effectiveness of this approach with different LFA kit types and analytes. Nevertheless, our results are promising evidence for the usefulness of SCAISY as a general approach for quantification of LFA kits and open new possibilities for the application of this technology in clinical and research settings.
4. Conclusions
In summary, we have developed SCAISY, a novel and easy-to-use method for quantification of SARS-CoV-2 IgG antibodies from LFA using a smartphone camera, which can aid in rapid COVID -19 serodiagnosis. Our study provides a basis for quantifying and tracking antibody levels over time, comparing levels between individuals, and monitoring immune responses to SARS-CoV-2 exposure or vaccination. We found that images acquired between 45° and 90° provided accurate results with a small standard deviation and that all illumination conditions provided essentially identical results within the standard deviation. In addition, the iPhone camera results should be interpreted with caution, as they yielded higher antibody levels compared to Android phones. Therefore, we recommend that only Android phones be used to estimate antibody levels at this time. Although this is a proof-of-concept study, we emphasize that randomized controlled clinical trials are needed to ensure accurate quantification of antibody levels for calibration before routine use. Larger sample sizes and more comprehensive assessments of diagnostic accuracy are needed to further validate and optimize the performance of SCAISY. Once the system is calibrated with absolute counts of antibodies, dedicated antibody counting equipment will no longer be needed. SCAISY offers several advantages over traditional methods for detecting antibodies, such as ELISA. Firstly, the system is simple and easy to use, requiring only a smartphone and an LFA kit. Secondly, the SCAISY system provides quantitative data on antibody production rates, allowing accurate tracking of changes in immune responses over time. Finally, the SCAISY system can be deployed in resource-limited environments, making it a valuable tool for public health and real-time health monitoring. Therefore, SCAISY has tremendous potential for use in clinical and community health settings in developing countries, as well as for direct-to-consumer market. In conclusion, SCAISY is a promising tool for rapid and accurate serodiagnosis of COVID -19 and has the potential to facilitate early detection of infection, guide vaccination interventions, and monitor the effectiveness of interventions.
Supplementary Materials
Supplementary data to this article can be found online.
Author Contributions
Samir Kumar: Investigation, data curation, formal analysis, visualization, writing–original draft preparation, reviewing, and editing. Taewoo Ko: Investigation, data curation, formal analysis, visualization, reviewing, and editing. Yeonghun Chae: Methodology, software, writing–review, and editing. Ahyeon Lee: Data curation and formal analysis. Sanghoon Shin: Data curation and formal analysis. Myung-Hyun Nam: Methodology, validation, IRB approval, reviewing, and editing. Byung Soo Kim: Methodology, validation, IRB approval, reviewing, and editing. Hyun Sik Jun: Conceptualization, formal analysis, manuscript writing, writing–final draft, reviewing, and editing. Sungkyu Seo: conceptualization, formal analysis, visualization, supervision, writing–final draft, reviewing and editing, funding acquisition, and project administration.
Acknowledgments
Authors acknowledge the supports of the Basic Science Research Program (Grant#: 2020R1A2C1012109) funded by the National Research Foundation (NRF) of Korea, the Korea Medical Device Development Program funded by the Korean Government (the Ministry of Science and ICT, the Ministry of Trade, Industry, and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Grant#: RS-2020-KD000142), and the Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries of Korea (Grant#: 20210660).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 April 2023).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yau, H.S.; Yu, M.Y.; Tsang, H.F.; Chan, L.W.C.; Cho, W.C.S.; Shing Yu, A.C.; Yuen Yim, A.K.; Li, M.J.W.; Wong, Y.K.E.; et al. The diagnostic methods in the COVID-19 pandemic, today and in the future. Expert Rev. Mol. Diagn. 2020, 20, 985–993. [Google Scholar] [CrossRef]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Cassaniti, I.; Novazzi, F.; Giardina, F.; Salinaro, F.; Sachs, M.; Perlini, S.; Bruno, R.; Mojoli, F.; Baldanti, F. ; Members of the San Matteo Pavia COVID-19 Task Force Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020, 92, 1724–1727. [Google Scholar] [CrossRef]
- Kierkegaard, P.; McLister, A.; Buckle, P. Rapid point-of-care testing for COVID-19: quality of supportive information for lateral flow serology assays. BMJ Open 2021, 11, e047163. [Google Scholar] [CrossRef]
- Rasmi, Y.; Li, X.; Khan, J.; Ozer, T.; Choi, J.R. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: current progress, challenges, and future prospects. Anal. Bioanal. Chem. 2021, 413, 4137–4159. [Google Scholar] [CrossRef]
- Uwamino, Y.; Wakui, M.; Aoki, W.; Kurafuji, T.; Yanagita, E.; Morita, M.; Nagata, M.; Inose, R.; Noguchi, M.; Yokota, H.; et al. Evaluation of the usability of various rapid antibody tests in the diagnostic application for COVID-19. Ann. Clin. Biochem. 2021, 58, 174–180. [Google Scholar] [CrossRef]
- Xu, J.; Kerr, L.; Jiang, Y.; Suo, W.; Zhang, L.; Lao, T.; Chen, Y.; Zhang, Y. Rapid antigen diagnostics as frontline testing in the COVID-19 pandemic. Small Sci 2022, 2200009. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajslova, J.; Elliott, C.T. Smartphone-Based Optical Assays in the Food Safety Field. Trends Analyt. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef]
- Tong, H.; Cao, C.; You, M.; Han, S.; Liu, Z.; Xiao, Y.; He, W.; Liu, C.; Peng, P.; Xue, Z.; et al. Artificial Intelligence-Assisted Colorimetric Lateral Flow Immunoassay for Sensitive and Quantitative Detection of COVID-19 Neutralizing Antibody. Biosens. Bioelectron. 2022, 213, 114449. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat Hum Behav 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Vaccine Equity Available online:. Available online: https://www.who.int/campaigns/vaccine-equity (accessed on 12 April 2023).
- COVID Vaccines: Widening Inequality and Millions Vulnerable. Available online: https://news.un.org/en/story/2021/09/1100192 (accessed on 12 April 2023).
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 - 22 December 2021. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---22-december-2021 (accessed on 12 April 2023).
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- Paradiso, A.V.; De Summa, S.; Loconsole, D.; Procacci, V.; Sallustio, A.; Centrone, F.; Silvestris, N.; Cafagna, V.; De Palma, G.; Tufaro, A.; et al. Rapid Serological Assays and SARS-CoV-2 Real-Time Polymerase Chain Reaction Assays for the Detection of SARS-CoV-2: Comparative Study. J. Med. Internet Res. 2020, 22, e19152. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. Journal of Saudi Chemical Society 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Ding, L.; Huang, X.; Xiong, Y. Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Analyt. Chem. 2021, 145, 116452. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Labmedica Expo. Available online: https://mobile.labmedica.com/expo/product/5344/fluorescent-reader-model-hrdr-300 (accessed on 12 August 2022).
- Microplate Readers: Multi-Mode and Absorbance Readers. Available online: https://www.biotek.com/products/detection/ (accessed on 4 August 2022).
- Ag, T.T. Microplate Readers. Available online: https://lifesciences.tecan.com/microplate-readers (accessed on 12 April 2023).
- Varioskan LUX Multimode Microplate Reader - KR. Available online: https://www.thermofisher.com/kr/ko/home/life-science/lab-equipment/microplate-instruments/plate-readers/models/varioskan.html (accessed on 4 August 2022).
- xMAP® Technology. Available online: https://www.luminexcorp.com/xmap-technology/ (accessed on 22 August 2022).
- Cooper, D.; Cooper, D.; Callahan, B.; Callahan, P.; Burnett, L. Mobile image ratiometry: A new method for instantaneous analysis of rapid test strips. Derm. Helv. 2012. [Google Scholar] [CrossRef]
- Eltzov, E.; Guttel, S.; Low Yuen Kei, A.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays - from paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Foysal, K.H.; Seo, S.E.; Kim, M.J.; Kwon, O.S.; Chong, J.W. Analyte Quantity Detection from Lateral Flow Assay Using a Smartphone. Sensors 2019, 19. [Google Scholar] [CrossRef]
- Lee, S.; O’Dell, D.; Hohenstein, J.; Colt, S.; Mehta, S.; Erickson, D. NutriPhone: a mobile platform for low-cost point-of-care quantification of vitamin B12 concentrations. Sci. Rep. 2016, 6, 28237. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of Cobalt Ferrite Nanoparticles in Biomedical Nanotechnology. Nanomedicine (Lond.) 2018, 13, 1221–1238. [Google Scholar] [CrossRef]
- Lee, S.; Oncescu, V.; Mancuso, M.; Mehta, S.; Erickson, D. A smartphone platform for the quantification of vitamin D levels. Lab Chip 2014, 14, 1437–1442. [Google Scholar] [CrossRef]
- Srinivasan, B.; O’Dell, D.; Finkelstein, J.L.; Lee, S.; Erickson, D.; Mehta, S. ironPhone: Mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens. Bioelectron. 2018, 99, 115–121. [Google Scholar] [CrossRef]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Xiao, M.; Chen, L.; Zhao, Y.; Zhang, H.; Long, P.; Zhou, Y.; Xu, X.; Lei, Y.; et al. Dynamics of the SARS-CoV-2 Antibody Response up to 10 Months after Infection. Cell. Mol. Immunol. 2021, 18, 1832–1834. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef]
- Swartz, M.D.; DeSantis, S.M.; Yaseen, A.; Brito, F.A.; Valerio-Shewmaker, M.A.; Messiah, S.E.; Leon-Novelo, L.G.; Kohl, H.W.; Pinzon-Gomez, C.L.; Hao, T.; et al. Antibody duration after infection from SARS-CoV-2 in the Texas Coronavirus Antibody Response Survey. J. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Antibody Persistence through 6 Months after the Second Dose of MRNA-1273 Vaccine for Covid-19. N. Engl. J. Med. 2022, 386, 500. [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Young, M.K.; Kornmeier, C.; Carpenter, R.M.; Natale, N.R.; Sasson, J.M.; Solga, M.D.; Mathers, A.J.; Poulter, M.D.; Qiang, X.; Petri, W.A. IgG antibodies against SARS-CoV-2 correlate with days from symptom onset, viral load and IL-10. medRxiv 2020. [Google Scholar] [CrossRef]
- Xia, G.; Wang, J.; Liu, Z.; Bai, L.; Ma, L. Effect of sample volume on the sensitivity of lateral flow assays through computational modeling. Anal. Biochem. 2021, 619, 114130. [Google Scholar] [CrossRef]
- Nuntawong, P.; Putalun, W.; Tanaka, H.; Morimoto, S.; Sakamoto, S. Lateral flow immunoassay for small-molecules detection in phytoproducts: a review. J. Nat. Med. 2022, 76, 521–545. [Google Scholar] [CrossRef]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Analyt. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Seo, D.; Han, E.; Kumar, S.; Jeon, E.; Nam, M.-H.; Jun, H.S.; Seo, S. Field-Portable Leukocyte Classification Device Based on Lens-Free Shadow Imaging Technique. Biosensors 2022, 12. [Google Scholar] [CrossRef]
- Shin, S.; Oh, S.; Seo, D.; Kumar, S.; Lee, A.; Lee, S.; Kim, Y.-R.; Lee, M.; Seo, S. Field-Portable Seawater Toxicity Monitoring Platform Using Lens-Free Shadow Imaging Technology. Water Res. 2023, 230, 119585. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).