Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
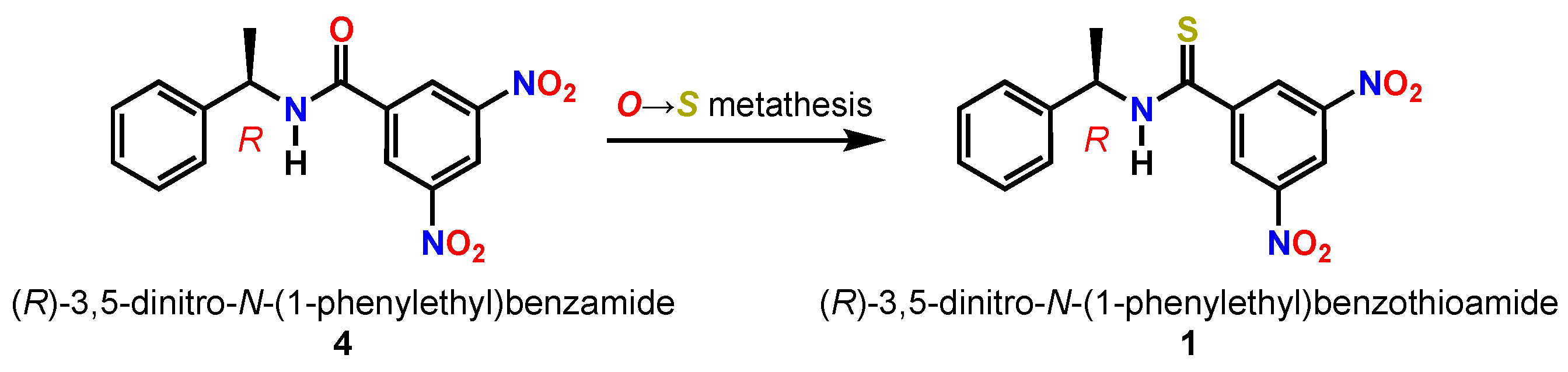
2. Results and Discussion
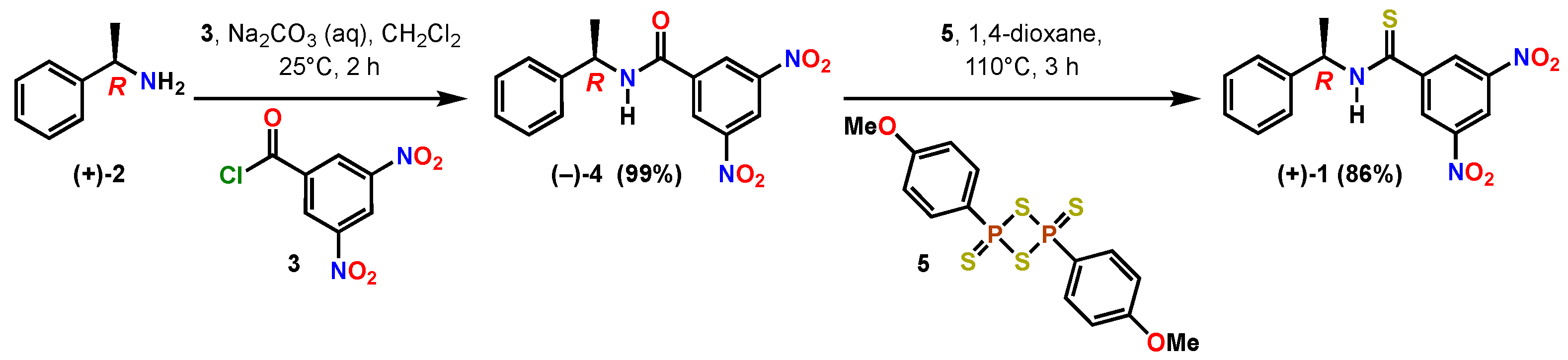
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Instrumentation and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, M.S. Recent Advances in Multinuclear NMR Spectroscopy for Chiral Recognition of Organic Compounds. Molecules 2017, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Wilcox, J.D. Chiral Reagents for the Determination of Enantiomeric Excess and Absolute Configuration Using NMR Spectroscopy. Chirality 2003, 15, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Gal, J. Molecular Chirality: Language, History and Significance. In Differentiation of Enantiomers I, Springer: New York, USA, 2013; pp. 1-21. [CrossRef]
- Parker, D. NMR Determination of Enantiomeric Purity. Chem. Rev. 1991, 91, 1441–1457. [Google Scholar] [CrossRef]
- Deshmukh, M.; Duñach, E.; Juge, S.; Kagan, H.B. A Convenient Family of Chiral Shift Reagents for Measurement of Enantiomeric Excesses of Sulfoxides. Tetrahedron Lett. 1984, 25, 3467–3470. [Google Scholar] [CrossRef]
- Wolf, C.; Cook, A.M.; Dannatt, J.E. Enantiodifferentiation of Multifunctional Tertiary Alcohols by NMR Spectroscopy with a Whelk-O Type Chiral Solvating Agent. Tetrahedron: Asymmetry 2014, 25, 163–169. [Google Scholar] [CrossRef]
- Iwaniuk, D.P.; Wolf, C. A Versatile and Practical Solvating Agent for Enantioselective Recognition and NMR Analysis of Protected Amines. J. Org. Chem. 2010, 75, 6724–6727. [Google Scholar] [CrossRef] [PubMed]
- Duñach, E.; Kagan, H.B. A Simple Chiral Shift Reagent for Measurement of Enantiomeric Excesses of Phosphine Oxides. Tetrahedron Lett. 1985, 26, 2649–2652. [Google Scholar] [CrossRef]
- Pakulski, X.; Demchuk, O. M.; Kwiatosz, R.; Osinski, P.W.; Swierczynska, W.; Pietrusiewicz, K.M. The Classical Kagan’s Amides are Still Practical NMR Chiral Shift Reagents: Determination of Enantiomeric Purity of P-Chiroenic Phospholene Oxides. Tetrahedron: Asymmetry 2003, 14, 1459–1462. [Google Scholar] [CrossRef]
- Abboud, J.L.M.; Mo, O.; de Paz, J.L.G.; Yanez, M.; Esseffar, M.; Bouab, W.; El-Mouhtadi, M.; Mokhlisse, R.; Ballesteros, E.; Herreros, M.; Homan, H.; Lopez-Mardomingo, C.; Notario, R. Thiocarbonyl versus Carbonyl Compounds: A Comparision of Intrinsic Reactivities. J. Am. Chem. Soc. 1993, 115, 12468–12476. [Google Scholar] [CrossRef]
- Madarász, A.; Dósa, Z.; Varga, S.; Soós, T.; Csámpai, A.; Pápai, I. Thiourea Derivatives as Bronsted Acid Organocatalysts. ACS Catal. 2016, 6, 4379–4387. [Google Scholar] [CrossRef]
- Rombola, M.; Sumaria, C.S.; Montgomery, T.D.; Rawal, V.H. Development of Chiral, Bifunctional Thiosquaramides: Enantioselective Michael Additions of Barbituric Acids to Nitroalkenes. J. Am. Chem. Soc. 2017, 139, 5297–5300. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Seguin, T.J.; Guan, Y.; Doney, A.C. Noncovalent Interactions in Organocatalysis and the Prospect of Computational Catalyst Desgin. Acc. Chem. Res. 2016, 49, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.M.F.; Prechtl, M.H.G.; Pombeiro, A.J.L. Non-Covalent Interactions in Enantioselective Organocatalysis: Theoretical and Mechanistic Studies of Reactions Mediated by Dual H-Bond Donors, Bifunctional Squaramides, Thioureas and Related Catalysts. Catalysts 2021, 11, 569. [Google Scholar] [CrossRef]
- Truter, M.R. An Accurate Determination of the Crystal Structure of Thioacetamide. J. Chem. Soc. 1960, 0, 997–1007. [Google Scholar] [CrossRef]
- Zieliński, T.; Jurczak, J. Thioamides versus Amides in Anion Binding. Tetrahedron 2005, 61, 4081–4089. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium Acidities in Dimethyl Sulfoxide Solution. Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, Y.S.; Lee, K.B.; Park, J.; Yoon, C.J. Hydrogen Bonding Abilities of Thioamide. J. Phys. Chem. A 2002, 106, 7010–7017. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoá, E.; Riguera, R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–118. [Google Scholar] [CrossRef]
- Yde, B.; Yousif, N.M.; Pedersen, U.; Thomsen, I.; Lawesson, S.O. Studies on Organophosphorus Compounds XLVII: Preparation of Thiated Synthons of Amides, Lactams and Imides by Use of Some New P,S-Containing Reagents. Tetrahedron 1984, 40, 2047–2052. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s Reagent in Organic Synthesis. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef]
- Spinner, E. Detection of Thiocarbonyl Groups by Infrared Spectroscopy. J. Org. Chem. 1958, 23, 2037–2038. [Google Scholar] [CrossRef]
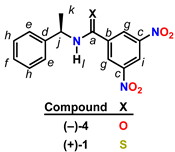 | ||||
| 13C NMR Data 1 | 1H NMR Data 2 | |||
| Position 3 | 4𝛅C | 1𝛅C | 4𝛅H | 1𝛅H |
| a | 161.8 (s) | 191.7 (s) | — | — |
| b | 148.6 (s) | 144.5 (s) | — | — |
| c | 141.8 (s) | 148.1 (s) | — | — |
| d | 137.9 (s) | 140.4 (s) | — | — |
| e | 129.0 (d) | 129.0 (d) | 7.41-7.35 (m, 2H) | 7.43-7.29 (m, 2H) |
| f | 128.0 (d) | 128.3 (d) | 7.33-7.28 (m, 1H) | 7.43-7.29 (m, 1H) |
| g | 127.1 (d) | 126.7 (d) | 8.93 (d, J=2.04 Hz, 2H) | 8.81 (d, J=2.04 Hz, 2H) |
| h | 126.3 (d) | 126.8 (d) | 7.41-7.35 (m, 2H) | 7.43-7.29 (m, 2H) |
| i | 121.1 (d) | 119.9 (d) | 9.14 (t, J=2.08 Hz, 1H) | 8.99 (t, J=2.04 Hz, 1H) |
| j | 50.3 (d) | 56.2 (d) | 5.34 (dq, J=7.16, 7.12 Hz, 1H) | 5.83 (dq, J=7.20, 7.00 Hz, 1H) |
| k | 21.4 (q) | 19.9 (q) | 1.67 (d, J=6.92 Hz, 3H) | 1.76 (d, J=6.92 Hz, 3H) |
| l | — | — | 6.64 (s, 1H) | 8.12 (s, 1H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





