Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Features and Visceral Involvement
2.3. Serological Parameters
2.4. Procedures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varga J, Trojanowska M, Kuwana M (2017) Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord 2:137–152. [CrossRef]
- Korman B. Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Transl Res. 2019 Jul;209:77-89. Epub 2019 Feb 23. PMID: 30876809; PMCID: PMC6545260. [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699.
- Lee, S.W.; Kim, B.K.; Park, J.Y.; et al. Disease duration and Medsger’s severity score are associated with significant liver fibrosis in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2015, 33, S68–S74.
- Bergmann C, Distler JH. Epigenetic factors as drivers of fibrosis in systemic sclerosis. Epigenomics. 2017 Apr;9(4):463-477. Epub 2017 Mar 27. PMID: 28343418. [CrossRef]
- Efe C, Ozaslan E, Nasiroglu N, Tunca H, Purnak T, Altiparmak E. The development of autoimmune hepatitis and primary biliary cirrhosis overlap syndrome during the course of connective tissue diseases: report of three cases and review of the literature. Dig Dis Sci. 2010;55:2417-21. [CrossRef]
- Marí-Alfonso B, Simeón-Aznar CP, Guillén-Del Castillo A, Rubio-Rivas M, Trapiella-Martínez L, Todolí-Parra JA, Rodríguez Carballeira M, Marín-Ballvé A, Iniesta-Arandia N, Colunga-Argüelles D, Castillo-Palma MJ, Sáez-Comet L, Egurbide-Arberas MV, Ortego-Centeno N, Freire M, Vargas Hitos JA, Chamorro AJ, Madroñero-Vuelta AB, Perales-Fraile I, Pla-Salas X, Fernández-De-La-Puebla RA, Fonollosa-Pla V, Tolosa-Vilella C; RESCLE Investigators; Systemic Autoimmune Diseases Study Group (GEAS). Hepatobiliary involvement in systemic sclerosis and the cutaneous subsets: Characteristics and survival of patients from the Spanish RESCLE Registry. Semin Arthritis Rheum. 2018;47:849-857. [CrossRef]
- Barr R, Ferraioli G, Palmeri M, et al. (2016) Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Ultrasound Q 32(2):94–107. [CrossRef]
- Vetrano E, Rinaldi L, Mormone A, Giorgione C, Galiero R, Caturano A, Nevola R, Marfella R, Sasso FC. Non-alcoholic Fatty Liver Disease (NAFLD), Type 2 Diabetes, and Non-viral Hepatocarcinoma: Pathophysiological Mechanisms and New Therapeutic Strategies. Biomedicines. 2023 Feb 6;11(2):468. PMID: 36831004; PMCID: PMC9953066. [CrossRef]
- van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative..Ann Rheum Dis. 2013 Nov;72(11):1747-55. [CrossRef]
- Cutolo M, Sulli A, Pizzorni C, et al. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol 2000; 27: 155–160.
- Valentini G, Della Rossa A, Bombardieri S, et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann Rheum Dis 2001; 60(6): 592–598.
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015 Jul;63(1):237-64. Epub 2015 Apr 21. PMID: 25911335. [CrossRef]
- Valente G, Rinaldi L, Moggio G, Piai G. Point shear wave elastography and vibration controlled transient elastography for estimating liver fibrosis in a cohort of liver transplant patients. Eur Rev Med Pharmacol Sci. 2020 Jul;24(13):7357-7365. PMID: 32706074. [CrossRef]
- Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011 Apr;54(4):650-9. Epub 2010 Sep 24. PMID: 21146892. [CrossRef]
- Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J, Oberti F, Charbonnier M, Fouchard-Hubert I, Rousselet MC, Calès P, de Lédinghen V. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016 Sep;65(3):570-8. Epub 2016 May 2. PMID: 27151181. [CrossRef]
- Boursier J, Decraecker M, Bourlière M, Bureau C, Ganne-Carrié N, de Lédinghen V. Quality criteria for the measurement of liver stiffness. Clin Res Hepatol Gastroenterol 2022; 46: 101761. [CrossRef]
- Kim SU, Kim JK, Park JY, Ahn SH, Lee JM, Baatarkhuu O, Choi EH, Han KH, Chon CY, Kim DY. Variability in liver stiffness values from different intercostal spaces. Liver Int 2009; 29: 760–766. [CrossRef]
- Lee J, Kang HJ, Yoon JH, Lee JM. Ultrasound-guided transient elastography and two-dimensional shear wave elastography for assessment of liver fibrosis: emphasis on technical success and reliable measurements. Ultrasonography 2021; 40: 217-227. [CrossRef]
- Gatos I, Yarmenitis S, Theotokas I, Koskinas J, Manesis E, Zoumpoulis SP, Zoumpoulis PS. Comparison of Visual Transient Elastography, Vibration Controlled Transient Elastography, Shear Wave Elastography and Sound Touch Elastography in Chronic liver Disease assessment using liver biopsy as 'Gold Standard'. Eur J Radiol 2022; 157: 110557. [CrossRef]
- Mendes LC, Ferreira PA, Miotto N, Zanaga L, Gonçales ESL, Pedro MN, Lazarini MS, Júnior FLG, Stucchi RSB, Vigani AG. Elastogram quality assessment score in vibration-controlled transient elastography: Diagnostic performance compared to digital morphometric analysis of liver biopsy in chronic hepatitis C. J Viral Hepat 2018; 25: 335-343. [CrossRef]
- Harris R, Card TR, Delahooke T, Aithal GP, Guha IN. The XL probe: A luxury or a necessity? Risk stratification in an obese community cohort using transient elastography. United European Gastroenterol J 2018; 6: 1372-1379. [CrossRef]
- Xia B, Wang F, Friedrich-Rust M, Zhou F, Zhu J, Yang H, Ruan W, Zeng Z. Feasibility and Efficacy of Transient Elastography using the XL probe to diagnose liver fibrosis and cirrhosis: A meta-analysis. Medicine (Baltimore) 2018; 97: e11816 [PMID: 30278481. [CrossRef]
- Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017 May;152(6):1544-1577. PMID: 28442120. [CrossRef]
- Tapper EB, Castera L, Afdhal NH. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin Gastroenterol Hepatol. 2015 Jan;13(1):27-36. [CrossRef]
- Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012 May;142(6):1293-1302.e4. PMID: 22537436. [CrossRef]
- Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010 Nov;36(11):1825-35. Epub 2010 Sep 27. PMID: 20870345. [CrossRef]
- Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019 May;156(6):1717-1730. Epub 2019 Jan 25. PMID: 30689971. [CrossRef]
- Kim JK, Lee KS, Choi JR, Chung HJ, Jung DH, Lee KA, Lee JI. Usefulness of the controlled attenuation parameter for detecting liver steatosis in health checkup examinees. Gut Liver. 2015 May 23;9(3):405-10. PMID: 25717046; PMCID: PMC4413975. [CrossRef]
- Caturano A, Galiero R, Loffredo G, Vetrano E, Medicamento G, Acierno C, Rinaldi L, Marrone A, Salvatore T, Monda M, Sardu C, Marfella R, Sasso FC. Effects of a Combination of Empagliflozin Plus Metformin vs. Metformin Monotherapy on NAFLD Progression in Type 2 Diabetes: The IMAGIN Pilot Study. Biomedicines. 2023 Jan 23;11(2):322.
- Huang, Z.; Ng, K.; Chen, H.; et al. Validation of Controlled Attenuation Parameter Measured by FibroScan as a Novel Surrogate Marker for the Evaluation of Metabolic Derangement. Front. Endocrinol. 2022, 12, 739875.
- 32. Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis - a lethal component of systemic sclerosis. Nat Rev Rheumatol. 2014;10:390-402. [CrossRef]
- Brown M, O'Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin Exp Immunol. 2019;195:310-321. [CrossRef]
- McMahan ZH, Hummers LK. Gastrointestinal involvement in systemic sclerosis: diagnosis and management. Curr Opin Rheumatol. 2018;30:533-540. [CrossRef]
- Assassi S, Fritzler MJ, Arnett FC et al.: Primary biliary cirrhosis (PBC), PBC autoantibodies, and hepatic parameter abnormalities in a large population of systemic sclerosis patients. J Rheumatol 2009; 36: 2250-6.
- D’Angelo WA, Fries JF, Masi AT, et al. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty eight autopsy cases and fifty-eight matched controls. Am J Med 1969; 46: 428-40.
- Chiappini F., Coilly A., Kadar H., et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci. Rep. 2017;7:46658. [CrossRef]
- Maffoni, S.; Brazzo, S.; De Giuseppe, R.; et al. Lifestyle changes and body mass index during COVID-19 pandemic lockdown: An Italian online-survey. Nutrients 2021, 13, 1117.
- Koehler EM.; Plompen E.P.C.; Schouten JN.L; et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study Hepatology 63(1):p 138-147, January 2016. |. [CrossRef]
- Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectros-copy and transient elastography. Gut 2012; 61:409–415.
- Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011; 60:977–984).
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [CrossRef]

| Clinical and Epidemiological Data | |
|---|---|
| Age (years), median [IQR] | 51 [20–80] |
| Sex, n (%) M/F | 6 (10.2%)/53 (89.8%) |
| BMI (kg/m2 ), median [IQR] | 24.77 [18.93-36.51] |
| SSc characteristics | |
| Duration of disease (years), median [IQR] | 6 [1-17] |
| Activity index, median [IQR] | 0.5 [0-4] |
| mRSS, mean (SD) | 2.24 (2.95) |
| Cutaneous involvement, subset, n (%) Sine Limited Diffuse |
59 (100%) 16 (27.1%) 35 (59.3%) 8 (13.5%) |
| E/A ratio, n (%) normal/abnormal (54) | 36 (66.6%)/18 (33.3%) |
| PAPs, median [IQR] | 25 [0-65] |
| FVC, median [IQR] | 98.5 [57.4–126] |
| DLCO, median [IQR] | 85.5 [38–138] |
| Videocapillaroscopic pattern, n (%) Normal Early Active Late |
54 (91.5%) 9 (16.7%) 24 (44.4%) 15 (27.8%) 6 (11.1) |
| Gastrointestinal involvement, n (%) | 33 (55.9%) |
| Scleroderma renal crisis, n (%) | - |
| ILD, n (%) (57 pts) | 20 (35%) |
| ANA positive, n (%) | 59 (100%) |
| ENA, n (%) No autoantibodies anti-centromere anti-Scl70 anti-RNA polymerase III |
59 (100%) 10 (16.9%) 20 (33.9%) 24 (40.7%) 5 (8.5%) |
| Ulcers, n (%) | 1 (1.7%) |
| Pitting scars, n (%) | 7 (11.9%) |
| Telangiectasias, n (%) | 28 (48%) |
| Fibroscan results | |
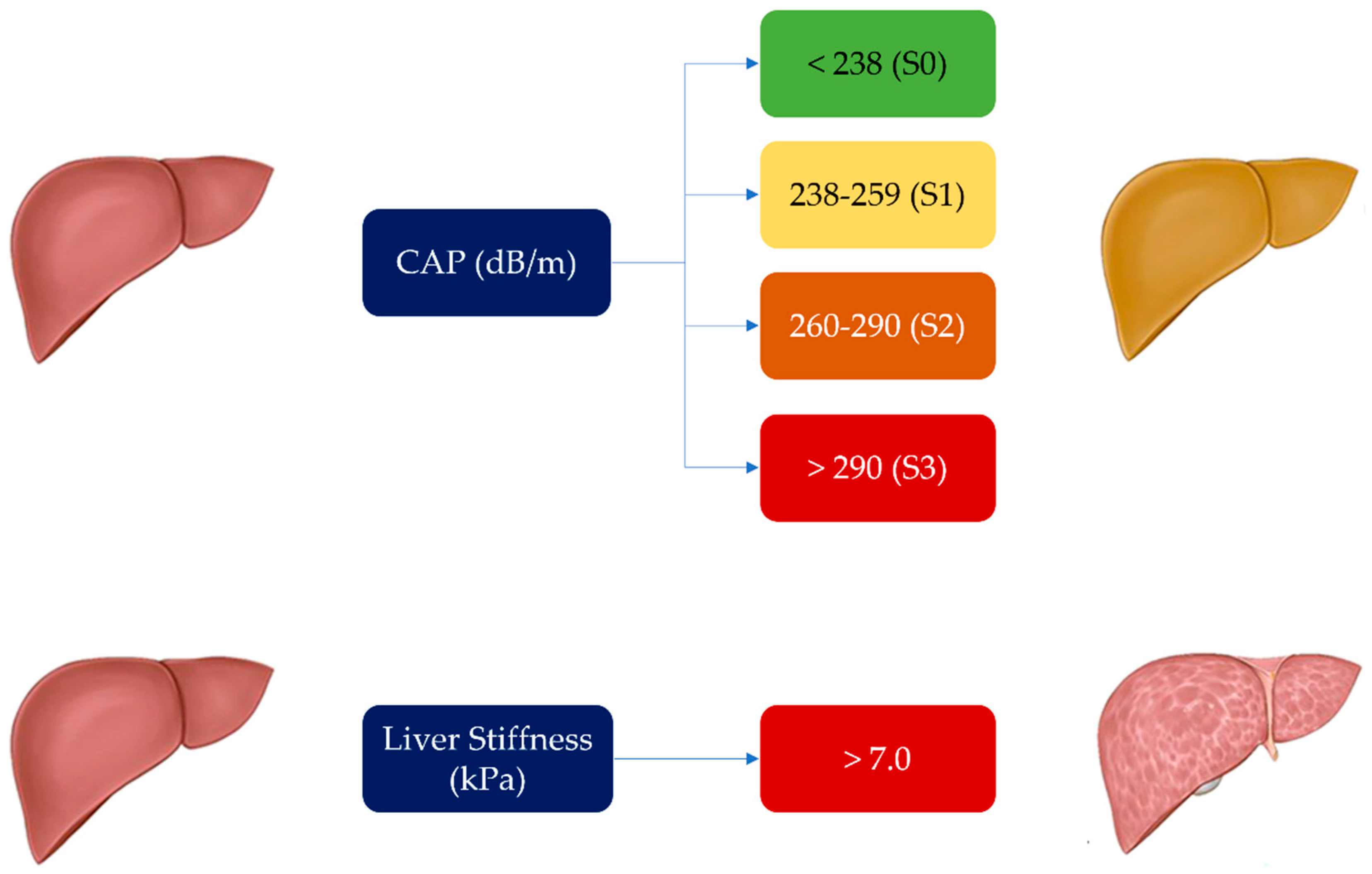
| CAP median [IQR] | 223 [164-343] |
| LS median [IQR] | 4.5 [2.9-8.3] |
| Concomitant therapies | |
| Corticosteroids, n (%) | 38 (64.4%) |
| Hydroxychloroquine, n (%) | 12 (20.3%) |
| Immunosoppressants, n (%) Azatioprine, n (%) Micofenolate, n (%) |
32 (54.2%) 14 (23.7%) 18 (30.5%) |
| Laboratory parameters | |
| Total cholesterol (mg/dl) median [IQR] (52 pts) HDL-cholesterol, (mg/dl) median [IQR] (40 pts) LDL- cholesterol(mg/dl) median [IQR] (36 pts) |
182.5 [101-307] 64.5 [37-130] 102.5 [40-172] |
| Triglycerides (mg/dl) median [IQR] (46 pts) | 87 [33-268] |
| Vitamin D (UI) median [IQR] (47 pts) | 28.5[7.7-53.9] |
| Parameter | ||||||
|---|---|---|---|---|---|---|
| LS | CAP | |||||
| Correlation Coefficient | 95 % CI | P | rho | 95% CI | p | |
|
Subset sine/L/D L/D |
0.24 0.24 |
-0.02 to 0.47 -0.01 to 0.48 |
0.068 0.059 |
0.06 0.02 |
-0.19 to0.32 -0.24 to 0.28 |
0.6 0.9 |
| Gender | 0.32 | 0.06 to 0.54 | 0.013 | 0.17 | -0.10 to 0.41 | 0.2 |
| HDL-cholesterol | -0.38 | -0.63 to -0.07 | 0.014 | 0.11 | -0.21 to 0.42 | 0.4 |
| TG | 0.40 | 0.11 to 0.62 | 0.006 | 0.27 | -0.03 to 0.53 | 0.06 |
| ILD | 0.23 | -0.04 to 0.46 | 0.09 | 0.23 | -0.04 to 0.47 | 0.09 |
| Telangiectasias | 0.26 | -0.001 to 0.49 | 0.045 | -0.09 | -0.35 to 0.18 | 0.5 |
| DLCO | -0.23 | -0.46 to 0.04 | 0.087 | -0.12 | -0.37 to 0.15 | 0.4 |
| Activity index | 0.16 | -0.11 to 0.40 | 0.2 | 0.34 | 0.09 to 0.56 | 0.007 |
| PAPs | -0.08 | -0.35 to 0.20 | 0.5 | 0.31 | 0.04 to 0.54 | 0.023 |
| E/A | 0.14 | -0.14 to 0.40 | 0.3 | 0.41 | 0.15 to 0.62 | 0.002 |
| BMI | 0.09 | -0.18 to 0.34 | 0.5 | 0.50 | 0.27 to 0.67 | <0.0001 |
| Age | -0.17 | -0.42 to 0.09 | 0.2 | 0.52 | 0.29 to .69 | <0.0001 |
| Immunosuppressive treatment MMF AZA |
0.14 0.16 0.05 |
-0.12 to 0.38 -0.10 to 0.41 -0.21 to 0.3 |
0.3 0.22 0.7 |
0.24 0.32 -0.08 |
-0.01 to 0.47 0.07 to 0.53 -0.34 to 0.17 |
0.06 0.013 0.5 |
| Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LS | CAP | ||||||||
| Coefficient | Std. Error | t | p | Coefficient | std. Error | t | p | ||
| sesso | 0.43 | 0.82 | 0.53 | 0.6 | |||||
| HDL -Chol | -0.01 | 0.01 | -1.23 | 0.23 | |||||
| Triglycerides | 0.01 | 0.004 | 2.43 | 0.02 | |||||
| teleangectasie | 0.32 | 0.34 | 0.95 | 0.35 | |||||
| Activity index | 4.61 | 4.12 | 1,12 | 0.26 | |||||
| PAPs | 0.33 | 0.31 | 1,08 | 0.28 | |||||
| E/A | 11.7 | 10.17 | 1,12 | 0.25 | |||||
| BMI | 2.28 | 0.96 | 2.36 | 0.023 | |||||
| Age | 0.77 | 0.33 | 2,37 | 0.022 | |||||
| MMF | 9.72 | 8.35 | 1.16 | 0.25 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





