Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction

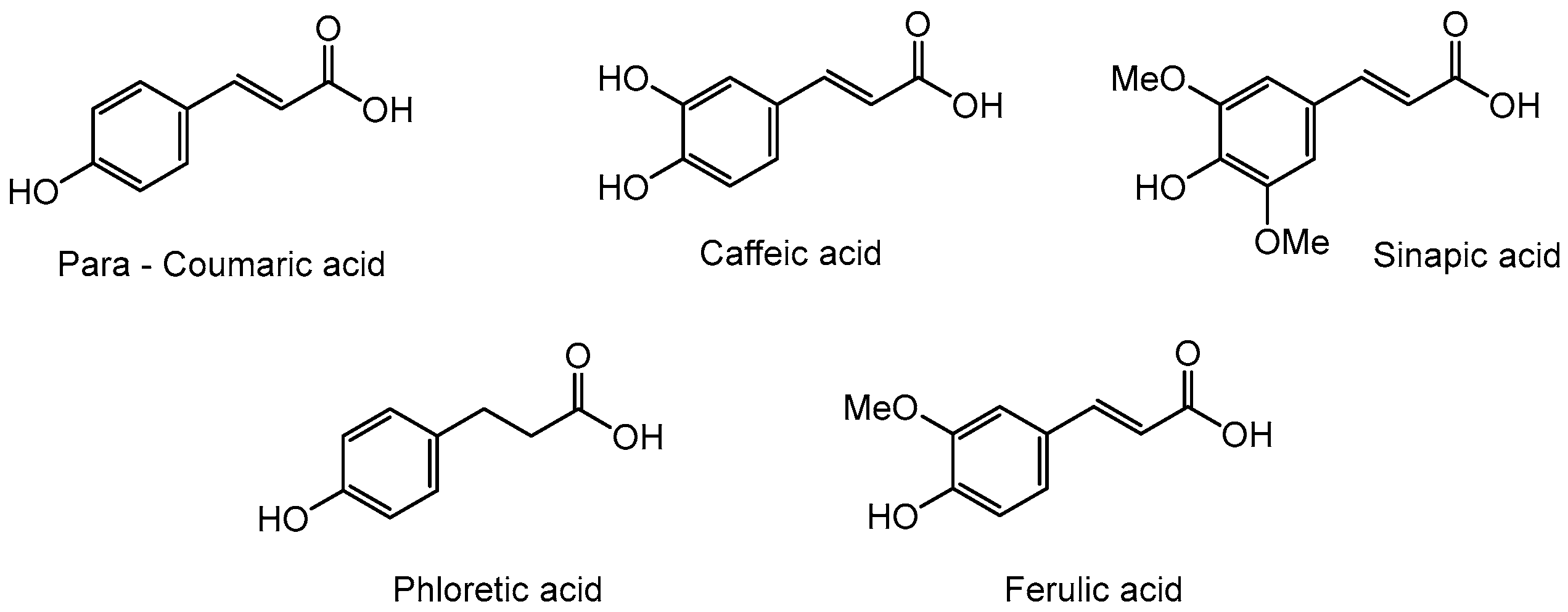
2. Synthesis
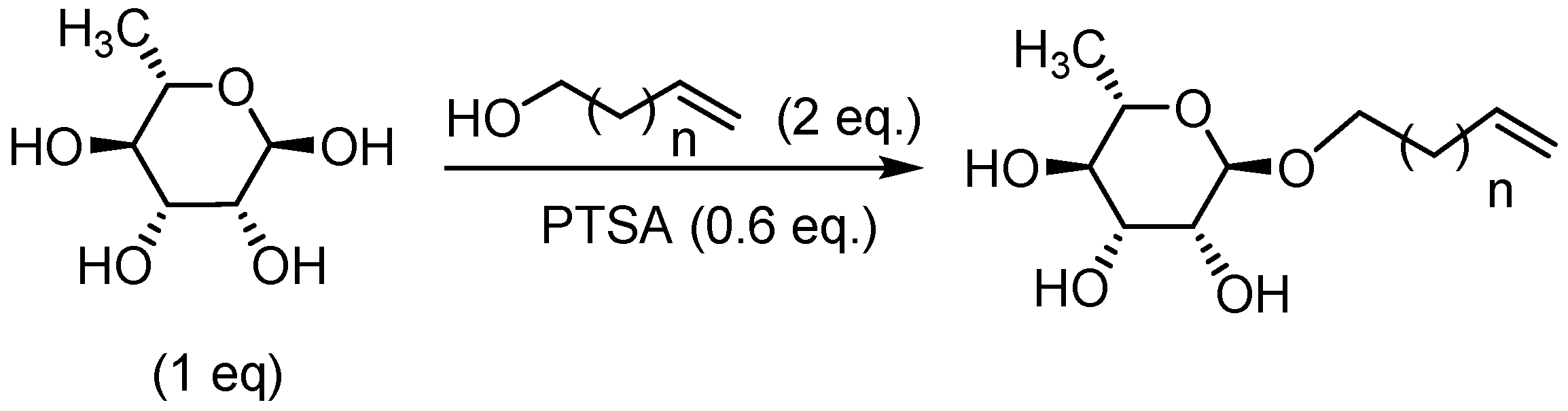
| Entry | Alcohol | Conditions | Isolated yields in corresponding rhamnoside (%) after flash chromatography (CH2Cl2/MeOH : 9/1 ) |
|---|---|---|---|
| 1 | Hex-5-enol | THF, 80°C, 48h | 19 |
| 2 | Dec-9-enol | THF, 80°C, 48h | 10 |
| 3 | Hex-5-enol | THF, 60°C, 48h |
5 |
| 4 | Dec-9-enol | THF, 60°C, 48h |
5 |
| 5 | Hex-5-enol | THF, 60°C, 48h Addition of the catalyst in 3 times |
7 |
| 6 | Dec-9-enol | THF, 60°C, 48h Addition of the catalyst in 3 times |
7 |
| 7 | Hex-5-enol | Neat, 60°C, 5h |
38 |
| 8 | Dec-9-enol | Neat, 60°C, 5h | 40 |
| Entry | Ratio L-rhamnose / Hex-5-enol / PTSA | Conditions | Isolated yields in corresponding rhamnoside (%) (combiflash) |
|---|---|---|---|
| 1 | 1/2/0.6 | 35°C, 5 min, 80 W | - |
| 2 | 1/2/0.6 | 35°C, 10 min, 80 W | 17 |
| 3 | 1/2/0.6 | 80°C, 35 min, 60 W | 40 |
| 4 | 1/2/0.6 | 60°C, 60 min, 60 W | 48 |
| 5 | 1/2/0.6 | 80°C, 60 min, 60 W | 50 |
| 6 | 1/4/0.6 | 60°C, 60 min, 60 W | 58 |
| 7 | 1/4/0.6 | 80°C, 60 min, 60 W | 55 |
| 8 | 1/4/0.6 | 60°C, 120 min, 60 W | 64 |
| 9 | 1/6/0.6 | 60°C, 120 min, 60 W | 62 |
| 10 | 1/6/0.6 | 80°C, 60 min, 60 W | 60 |
| Entry | Alcohol | Solvent (5 mL) | Rhamnoside | Isolated yields (%) (combiflash) |
|---|---|---|---|---|
| 1 | Hex-5-enol | THF | 1a | 64 |
| 2 | Hex-5-enol | γ-valerolactone | 1a | 66 |
| 3 | Hex-5-enol | 2-methyltetrahydrofuran | 1a | 63 |
| 4 | Hept-6-enol | THF | 2a | 60 |
| 5 | Oct-7-enol | THF | 3a | 50 |
| 6 | Non-8-enol | THF | 4a | 53 |
| 7 | Dec-9-enol | THF | 5a | 48 |
| 8 | Dec-9-enol | γ-valerolactone | 5a | 73 |
| 9 | Dec-9-enol | 2-methyltetrahydrofuran | 5a | 55 |
| 10 | Undec-10-enol | THF | 6a | 47 |
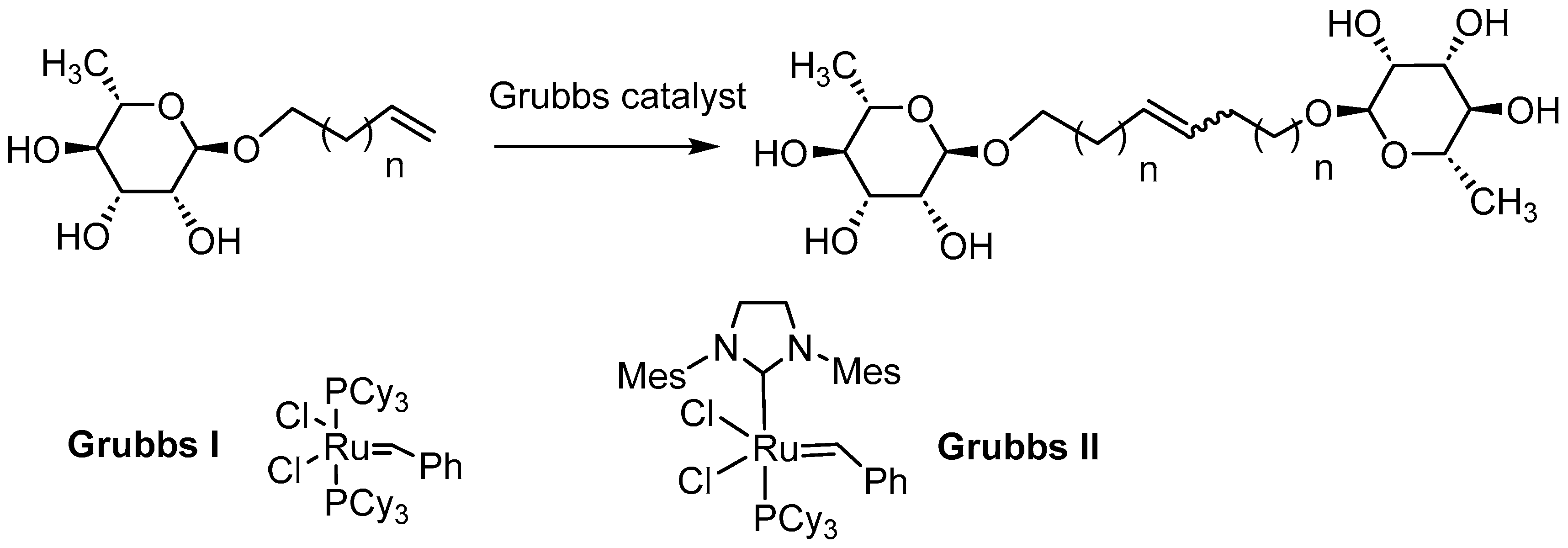
| Entry | Monocatenar Rhamnoside | Conditions | Grubbs Catalyst (0.1 eq.) | Purification | Bolaform rhamonisde Isolated yields (%) |
|---|---|---|---|---|---|
| 1 | 1a | CH2Cl2 | Grubbs I | Silica Chromatography |
7a 19 |
| 2 | 2a | CH2Cl2 | Grubbs I | Silica Chromatography |
8a 13 |
| 3 | 5a | CH2Cl2 | Grubbs I | Silica Chromatography | 9a 10 |
| 4 | 1a | CH2Cl2/MeOH (9/1) | Grubbs I | Combi Flash |
7a 52 |
| 5 | 2a | CH2Cl2/MeOH (9/1) | Grubbs I | Combi Flash |
8a 37 |
| 6 | 5a | CH2Cl2/MeOH (9/1) | Grubbs I | Combi Flash |
9a 31 |
| 7 | 1a | CH2Cl2/MeOH (9/1) | Grubbs II | Combi Flash |
7a 77 |
| 8 | 2a | CH2Cl2/MeOH (9/1) | Grubbs II | Combi Flash |
8a 60 |
| 9 | 5a | CH2Cl2/MeOH (9/1) | Grubbs II | Combi Flash |
9a 56 |
| 10 | 6a | CH2Cl2/MeOH (9/1) | Grubbs II | Combi Flash |
10a 32 |

| Compounds | Conversion of acid (% GC) | Isolated yields (Combiflash) % |
Isolated yields (Flash chromatography) % |
|---|---|---|---|
| 1b | 97 | 95 | 12 |
| 2b | 90 | 88 | 28 |
| 3b | 97 | 96 | 46 |
| 4b | 85 | 79 | 31 |
| Compounds | solvent | Isolated yields (%) |
|---|---|---|
| 5b | 2M2B (40 mL) | 70 |
| 6b | 2M2B (40 mL) | 74 |
| 7b | 2M2B/THF (1/2) (30 mL) | 52 |
| 8b | 2M2B/THF (1/2) (30 mL) | 45 |
| 10b | 2M2B (60 mL) | 52 |
| 11b | 2M2B (60 mL) | 52 |
| 9b | Acetone (30 mL) | 40 |

| Compound | Isolated Yields | |
|---|---|---|
| Grubbs I catalyst | Grubbs II catalyst | |
| 12b | 20 | 56 |
| 13b | 16 | 48 |
| 14b | 18 | 60 |
| 15b | 11 | 42 |
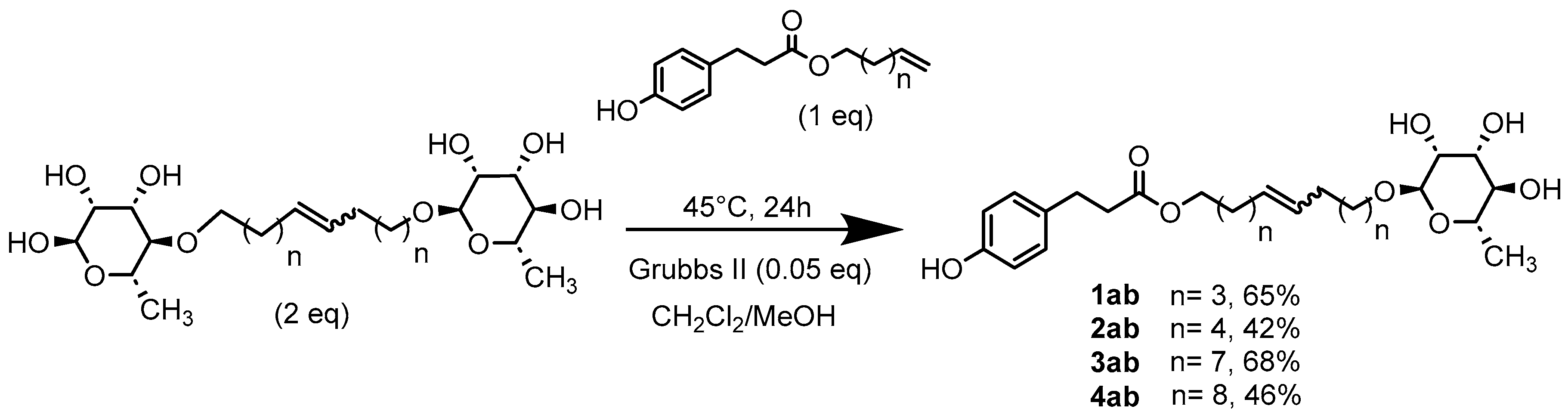
Anti-Oxidant Properties
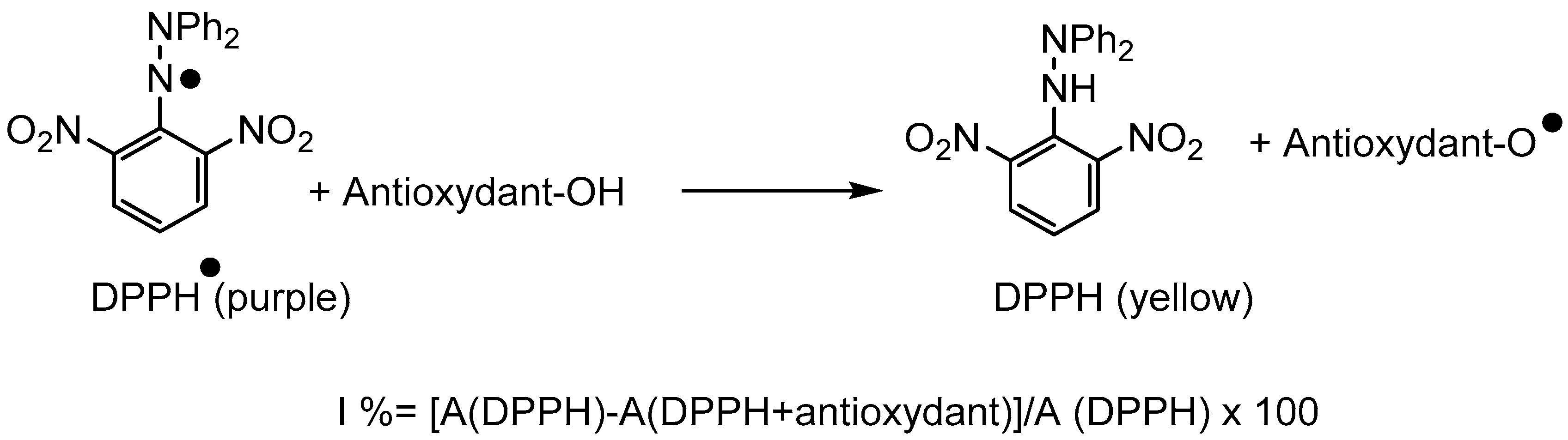
| Entry | Compounds | IC50 (μmol/L) |
|---|---|---|
| 1 | Vitamin C | 34 |
| 2 |  |
45 |
| 3 |  |
29 |
| 4 |  |
31 |
| 5 |  |
47 |
| 6 |  |
32 |
| 7 |  |
32 |
| 8 |  |
36 |
| 9 |  |
35 |
| 10 |  |
27 |
Eliciting Properties
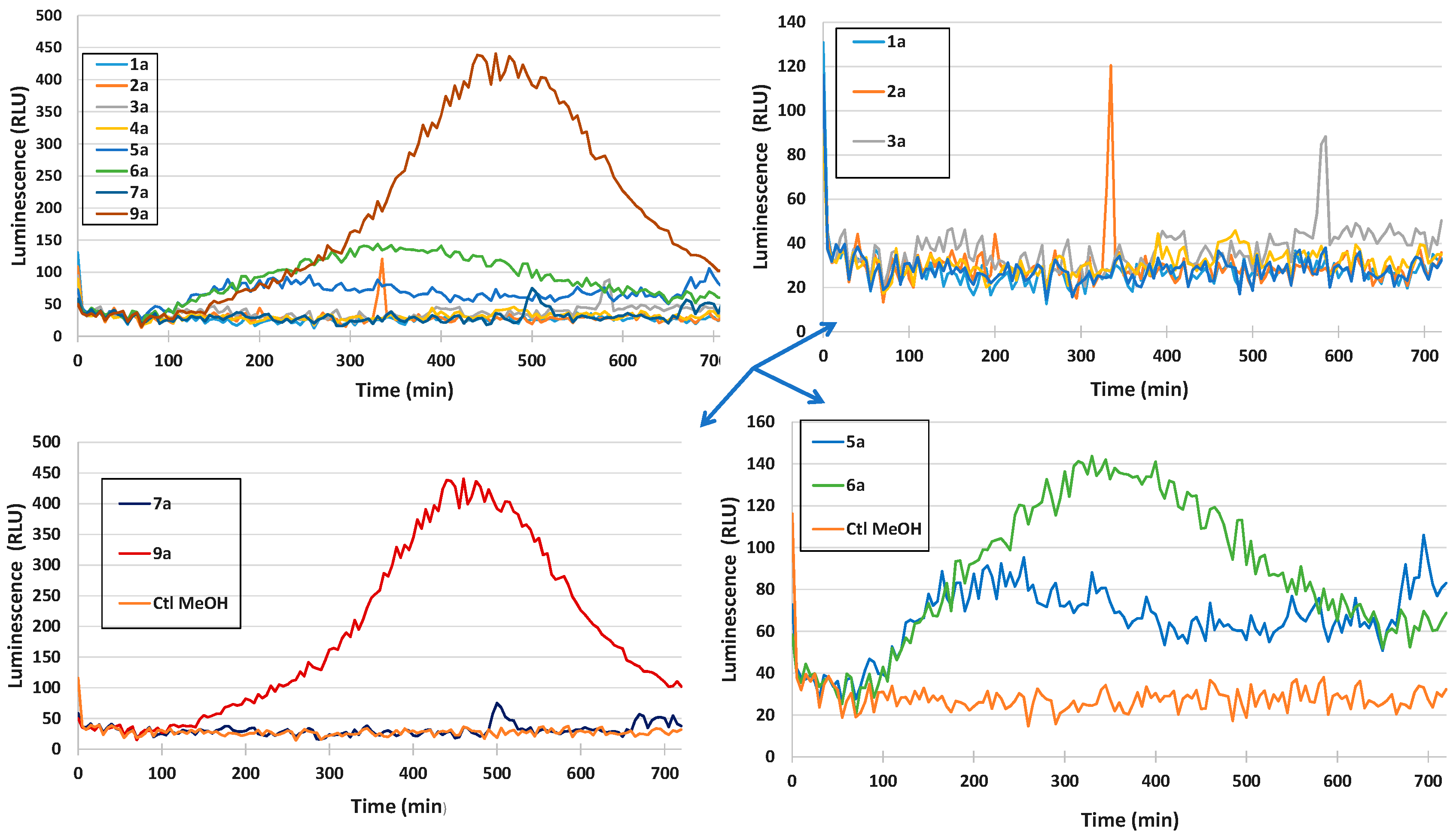
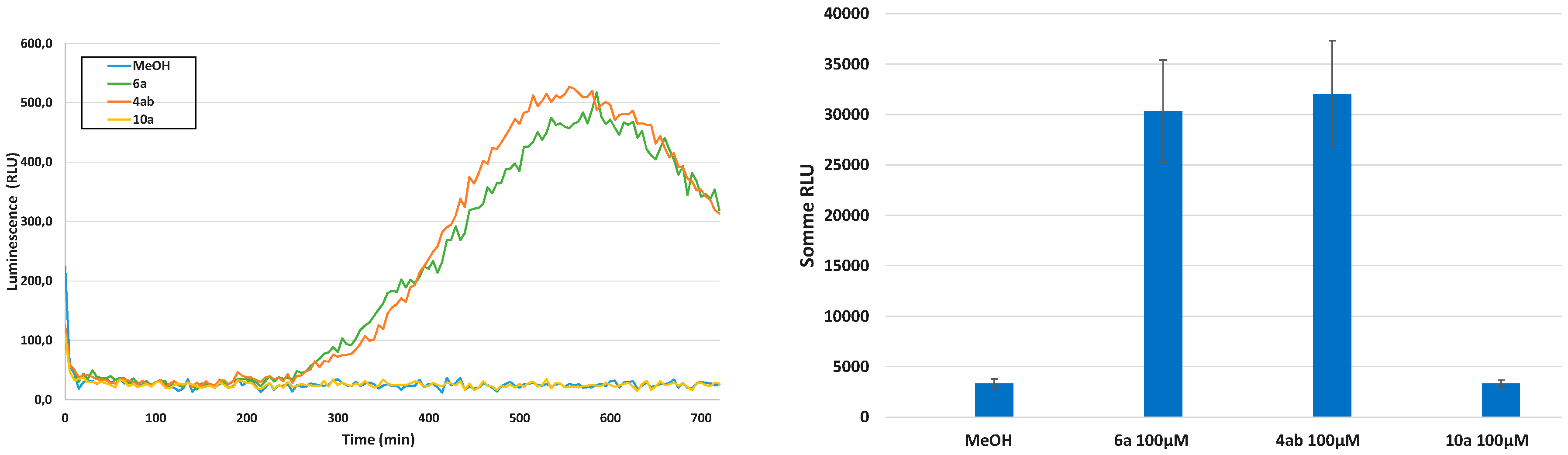

Cytotoxicity Evaluation
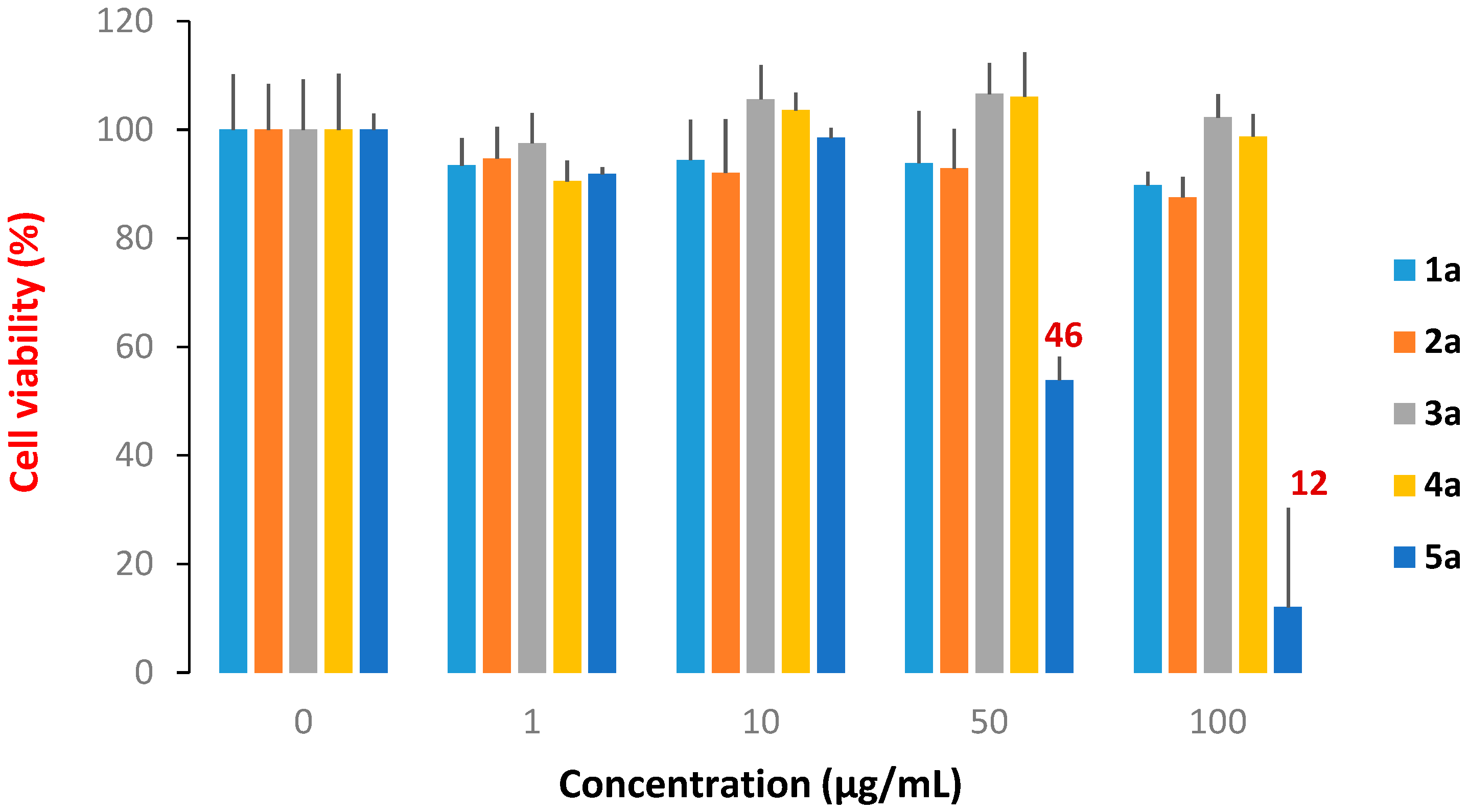
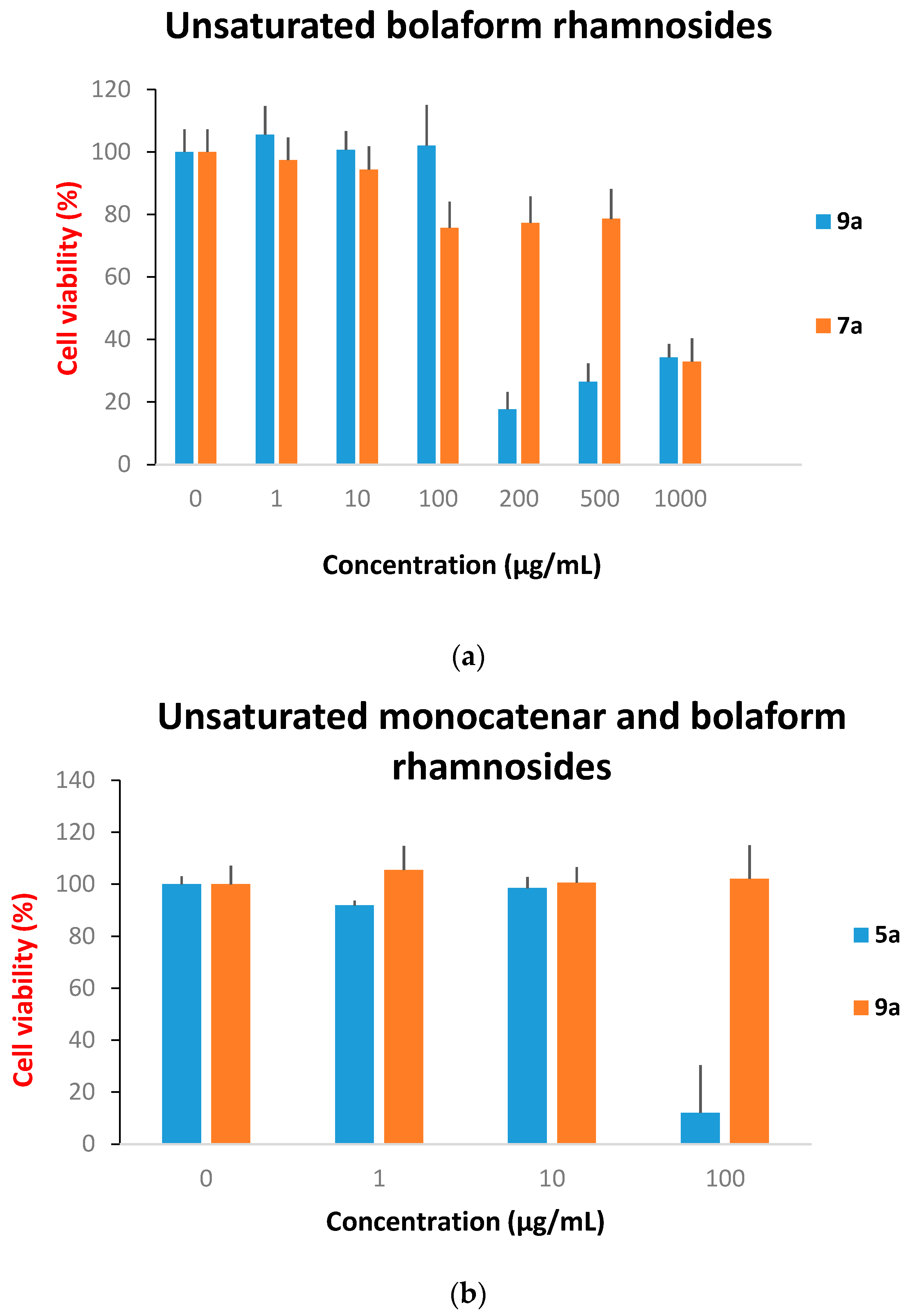

3. Experimental
Cytotoxicity Assays
General Procedure for the Preparation of Rhamnosides under Microwave Activation with Tetrahydrofuran (THF) or 2-methyltetrahydrofuran (2-MeTHF) as Solvent
|
Hex-5’-enyl-α-L-rhamnopyranoside (1a) General Procedure for the preparation of the rhamnosides under microwave activation with L-rhamnose (2 g; 10.35 mmol; 1 eq), 5-Hexen-1-ol (5.27 ml; 41.4 mmol; 4 eq) and PTSA (1.25 g; 6.21 mmol; 0.6 eq). Compound 1a is obtained as a thick yellow liquid with a yield of 64 % (with THF) and 63 % (with 2-MeTHF). |
 |
| νmax (ATR) cm-1 : 3363 (OH), 2926-2856 (C-H), 1641 (C=C), 1384 (CH3), 1265-1231 (C-OHTert.). [α]20D (589 nm, MeOH) = -52.501 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.25 (3H, d, J 7.5 Hz, H6’), 1.50 (2H, m, H3), 1.63 (2H, m, H2), 2.13 (2H, m, H4), 3.17 - 3.58 (5H, m, H1, H2’, H3’, H4’, H5’), 4.52-4.72 (3H, s, 3 OH), 4.98 (2H, d, J 7.2 Hz, H6), 5.80 (1H, m, H5). 13C NMR (125 MHz; DMSO-d6, ppm) δ 18.4 (C6’), 25.4 (C3), 29.1, 33.4 (C2, C4), 66.6 (C1), 68.9-73.9 (C2’, C3’, C4’, C5’), 100.6 (C1’), 115.3 (C6), 139.1 (C5). Analysis (%): calculated for: C12H22O5: C 58.52, H 9.02. Found: C 58.97, H 8.88. | |
|
Hept-6’-enyl-α-L-rhamnopyranoside (2a) General Procedure for the preparation of the rhamnosides under microwave activation with L-rhamnose (0.76 g; 3.93 mmol; 1 eq), 6-hepten-1-ol (3 ml; 15.72 mmol; 4 eq) and PTSA (1.25 g; 6.21 mmol; 0.6 eq). Compound 2a is obtained as a thick yellow liquid with a yield of 60 %. |
 |
| νmax (ATR) cm-1 : 3368 (OH), 2926-2856 (C-H), 1641 (C=C), 1382 (CH3),1227 (C-OHTert.). [α]20D (589 nm, MeOH) = -49.101 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.13 (3H, d, J 7.5 Hz, H6’), 1.36 (4H, m, H3, H4), 1.50 (2H, m, H2), 2.02 (2H, m, H5), 3.17–3.56 (5H, m, H1, H2’, H3’, H4’, H5’), 4.52-4.73 (3H, s, 3 OH), 4.98 (2H, d, J 7.2 Hz, H7), 5.80 (1H, m, H6). 13C NMR (125 MHz; DMSO-d6, ppm) δ 18.38 (C6’), 25.7 (C4), 28.5, 29.3, 33.6 (C3, C2, C5), 66.8 (C1), 68.9-72.5 (C2’, C3’, C4’, C5’), 100.4 (C1’), 115.2 (C7), 139.2 (C6). Analysis (%): calculated for: C13H24O5: C 59.98, H 9.29. Found: C 59.71, H 9.14. | |
|
Oct-7’-enyl-α-L-rhamnopyranoside (3a) General Procedure for the preparation of the rhamnosides under microwave activation with L-rhamnose (0.6 g; 3.11 mmol; 1 eq), 7-octen-1-ol (2.52 ml; 12.4 mmol; 4 eq) and PTSA (1.25 g; 6.21 mmol; 0.6 eq). Compound 3a is obtained as a thick yellow liquid with a yield of 50 %. |
 |
| νmax (ATR) cm-1 : 3371 (OH), 2926-2856 (C-H), 1641 (C=C), 1381 (CH3),1231 (C-OHTert.). [α]20D (589 nm, MeOH) = -46.501 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.13 (3H, d, J 7.5 Hz, H6’), 1.30 (6H, m, (H4, H3, H5), 2.02 (2H, m, H2), 3.18 (2H, m, H6), 3.36 - 3.58 (5H, m, H1, H2’, H3’, H4’, H5’), 4.53 - 5.02 (3H, s, 3 OH), 5.18 (2H, d, J 7.2 Hz, H8), 5.80 (1H, m, H7). 13C NMR (125 MHz; DMSO-d6) 18.4 (C6’), 26.0 (C4), 28.7, 28.8 29.5, 33.6 (C3, C5, C2, C6), 66.78 (C1), 68.9-72.5 (C2’, C3’, C4’, C5’), 100.4 (C1), 115.2 (C8), 139.3 (C7). Analysis (%): calculated for: C14H26O5: C 61.29, H 9.55. Found: C 60.83, H 9.41. | |
|
Non-8’-enyl-α-L-rhamnopyranoside (4a) General Procedure for the preparation of the rhamnosides under microwave activation with L-rhamnose (0.57 g; 2.95 mmol; 1 eq) and 8-nonen-1-ol (2.5 ml; 11.80 mmol; 4 eq), and PTSA (1.25 g; 6.21 mmol; 0.6 eq). Compound 4a is obtained as a thick yellow liquid with a yield of 53 %. |
 |
| νmax (ATR) cm-1 : 3367 (OH), 2926-2856 (C-H), 1641 (C=C), 1382 (CH3),1230 (C-OHTert.). [α]20D (589 nm, MeOH) = -45.001 1H NMR (500,1MHz; CD3OD) δ 1.13 (3H, d, J 7.5 Hz, H6’), 1.32 (8H, m, H5, H6, H4, H3), 1.50 (2H, m, H2), 2.02 (2H, m, H7), 3.15-3.54 (5H, m, H1, H2’, H3’, H4’, H5’), 4.51 - 5.06 (3H, s, 3 OH), 5.27 (2H, d, J 7.2 Hz, H9), 5.80 (1H, m, H8). 13C NMR (125 MHz; DMSO-d6) δ 18.4 (C6’), 26.0 (C5), 26.1, 28.8, 29.1, 29.6, 33.6 (C6, C4, C3, C2, C7), 66.8 (C1), 68.8-73.2 (C2’, C3’, C4’, C5’), 100.5 (C1), 115.1 (C9), 139.3 (C8). Analysis (%): calculated for: C15H28O5: C 62.47, H 9.79. Found: C 62.35, H 9.65. | |
|
Dec-9’-enyl-α-L-rhamno-pyranoside (5a) General Procedure for the preparation of the rhamnosides under microwave activation with L-rhamnose (0.53 g; 2.74 mmol; 1 eq), 9-decen-1-ol (2.5 ml; 10.96 mmol; 4 eq) and PTSA (1.25 g; 6.21 mmol; 0.6 eq). Compound 5a is obtained as a thick yellow liquid with a yield of 48 % (with THF) and 55 % (with 2-MeTHF). |
 |
| νmax (ATR) cm-1 : 3374 (OH), 2926-2856 (C-H), 1641 (C=C), 1382 (CH3),1229 (C-OHTert.). [α]20D (589 nm, MeOH) = -43.701 1H NMR (500,1MHz; CD3OD) δ 1.13 (3H, d, J 7.5 Hz, H6’), 1.32 (10H, m, H5, H6, H4, H7, H3), 1.50 (2H, m, H2), 2.02 (2H, m, H8), 3.15-3.54 (5H, m, H1, H2’, H3’, H4’, H5’), 4.15 - 4.54 (3H, s, 3 OH), 4.97 (2H, d, J 7.2 Hz, H10), 5.80 (1H, m, H9). 13C NMR (125 MHz; DMSO-d6) δ 18.4 (C6’), 26.1 (C5), 28.7, 28.9, 29.2, 29.3, 29.5, 33.6 (C6, C4, C7, C3, C2, C8), 66.8 (C1), 68.9-73.5 (C2’, C3’, C4’, C5’), 100.4 (C1’), 115.1 (C10), 139.3 (C9). Analysis (%): calculated for: C16H30O5: C 63.55, H 10.03. Found: C 63.16, H 9.82. | |
|
Undec-10’-enyl-α-L-rhamnopyranoside (6a) General Procedure for the preparation of the rhamnosides under microwave activation with L-rhamnose (0.5 g; 2.59 mmol; 1 eq), 10-undecen-1-ol (2.5 ml; 10.36 mmol; 4 eq) and PTSA (1.25 g; 6.21 mmol; 0.6 eq). Compound 6a is obtained as a thick yellow liquid with a yield of 47 %. |
 |
| νmax (ATR) cm-1 : 3366 (OH), 2926-2856 (C-H), 1641 (C=C), 1382 (CH3),1229 (C-OHTert.). [α]20D (589 nm, MeOH) = -37.001 1H NMR (500,1MHz; CD3OD) δ 1.13 (3H, d, J 7.5 Hz, H6’), 1.31 (12H, H6, H7, H5, H4, H8, H3), 1.50 (2H, m, H2), 2.01 (2H, m,H9), 3.08 - 3.54 (5H, m, H1, H2’, H3’, H4’, H5’), 4.52 - 4.72 (3H, s, 3 OH), 4.97 (2H, d, J 7.2 Hz, H11), 5.80 (1H, m, H10). 13C NMR (125 MHz; DMSO-d6) δ 18.4 (C6’), 26.1 (C6), 28.7, 28.9, 29.2, 29.3, 29.4, 29.5, 33.6 (C5, C7, C4, C8, C3, C2, C9), 66.80 (C1), 68.9-72.5 (C2’, C3’, C4’, C5’),100.6 (C1’),115.1 (C11), 139.3 (C10). Analysis (%): calculated for C17H32O5: C 64.53, H 10.19. Found: C 63.91, H 10.14. | |
General Procedure for the Preparation of Rhamnosides under Microwave Activation with Gamma-Valerolactone as Solvent
General Procedure for the Preparation of Rhamnoside-Based Boloamphiphiles under Microwave Activation
|
1’,10’-bis-dec-5’-eny-L-rhamnopyranoside (7a) General Procedure for the preparation of the rhamnoside-based bolaamphiphiles with compound 1a (1.5 g, 6.09 mmol, 1eq.) under microwave activation with Grubbs II catalyst (0.5 g, 0.6 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2/MeOH. Compound 7a is obtained as a brown paste with a yield of 77 %. |
 |
| νmax (ATR) cm-1 : 3347 (OH), 2971-2901 (C-H), 1634 (C=C), 1384 (CH3),1128-1048 (C-O-C). [α]20D (589 nm, MeOH) = -34.801 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.24 (6H, d, J 7.5 Hz, H6’), 1.48 (4H, m, H3), 1.61 (4H, m, H2), 2.17(4H, m, H4), 3.56-3.12 (10H, m, H1, H2’, H3’, H4’, H5’), 4.70-4.48 (6H, s, 6 OH), 5.78 (2H, d, J 7.2 Hz, H5). 13C NMR (125 MHz; DMSO-d6, ppm) δ 18.3 (2 C6’), 23.6 (2C3), 30.1, 33.2 (2 C2, 2 C4), 64.3 (2 C1), 67.6-71.9 (2 C2’, 2 C3’, 2 C4’, 2 C5’), 102.1 (2 C1’), 130.1 (CH2CH(Z)=CH(Z)CH2), 130.6 (CH2CH(E)=CH(E)CH2). Analysis (%): calculated for C22H40O10: C 56.88, H 8.68. Found: C 56.47, H 8.96. | |
|
1’,12’-bis-dodec-6’-enyl-L-rhamnopyranoside (8a) General Procedure for the preparation of the rhamnoside-based bolaamphiphiles with compound 2a (0.7 g, 2.69 mmol, 1eq.) under microwave activation with Grubbs II catalyst (0.2 g, 0.27 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2/MeOH. Compound 8a is obtained as a brown paste with a yield of 60 %. |
 |
| νmax (ATR) cm-1 : 3342 (OH), 2970-2913 (C-H), 1632 (C=C), 1386 (CH3), 1124-1051 (C-O-C). [α]20D (589 nm, MeOH) = -41.301 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.14 (6H, d, J 7.5 Hz, H6’), 1.38 (8H, m, H3, H4), 1.51 (4H, m, H2), 2.07(4H, m, H5), 3.36-3.18(10H, m, H1, H2’, H3’, H4’, H5’), 4.71-4.58 (6H, s, 6 OH), 5.78 (2H, d, J 7.2 Hz, H5). 13C NMR (125 MHz; DMSO-d6, ppm) δ 17.3 (2 C6’), 25.6 (2 C3), 29.0, 29.4, 33.6 (2 C3, 2 C2, 2 C5), 65.1 (2 C1), 68.6-72.6 (2 C2’, 2 C3’, 2 C4’, 2 C5’), 100.2 (2 C1’), 138.0 (CH2CH(Z)=CH(Z)CH2), 138.1 (CH2CH(E)=CH(E)CH2). Analysis (%): calculated for: C24H44O10: C 58.52, H 9.00. Found: C 58.87, H 9.19. | |
|
1’, 18’-bis-octadec-9’-enyl -L-rhamnopyranoside (9a) General Procedure for the preparation of the rhamnoside-based bolaamphiphiles with compound 5a (1.45 g, 4.80 mmol, 1eq.) under microwave activation with Grubbs II catalyst (0.4 g, 0.48 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2/MeOH. Compound 9a is obtained as a brown paste with a yield of 56 %. |
 |
| νmax (ATR) cm-1 : 3345 (OH), 2971-2922 (C-H), 1634 (C=C), 1382 (CH3), 1127-1051 (C-O-C). [α]20D (589 nm, MeOH) = -32.601 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.13 (6H, d, J 7.5 Hz, H6’), 1.34 (20H, m, H3, H4, H5, H6, H7), 1.51 (4H, m, H2), 2.09 (4H, m, H8), 3.16-3.58 (10H, m, H1, H2’, H3’, H4’, H5’), 4.31-4.58 (6H, s, 6 OH), 5.70 (2H, d, J 7.2 Hz, H9). 13C NMR (125 MHz; DMSO-d6, ppm) δ 18.3 (2 C6’), 26.2 (2 C5), 28.6, 28.9, 29.4, 29.7, 33.8 (2 C3, 2 C2, 2 C4, 2 C5, 2 C6, 2 C7, 2 C8), 66.8 (2 C1), 68.7 - 72.9 (2 C2’, 2 C3’, 2 C4’, 2 C5’), 100.4 (2 C1’), 130.1 (CH2CH(Z)=CH(Z)CH2), 130.5 (CH2CH(E)=CH(E)CH2). Analysis (%): calculated for C30H56O10: C 62.47, H 9.79. Found: C 62.11, H 9.29. | |
|
1’, 20’-bis-eicosa-10’-enyl- L-rhamnopyranoside (10a) General Procedure for the preparation of the rhamnoside-based bolaamphiphiles with compound 6a (1.09 g, 3.60 mmol, 1eq.) under microwave activation with Grubbs II catalyst (0.3 g, 0.36 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2/MeOH. Compound 10a is obtained as a brown paste with a yield of 32 %. |
 |
| νmax (ATR) cm-1 : 3338 (OH), 2968-2919 (C-H), 1632 (C=C), 1381 (CH3), 1124-1053 (C-O-C). [α]20D (589 nm, MeOH) = -35.001 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.18 (6H, d, J 7.5 Hz, H6’), 1.39 (24H, m, H3, H4, H5, H6, H7, H8), 1.57 (4H, m, H2), 2.15 (4H, m, H9), 3.19-3.61 (10H, m, H1, H2’, H3’, H4’, H5’), 4.28-4.47 (6H, s, 6 OH), 5.74 (2H, d, J 7.2 Hz, H10). 13C NMR (125 MHz; DMSO-d6, ppm) δ 17.5 (2 C6’), 24.9 (2 C5), 27.4, 28.8, 29.1, 29.8, 32.5, 35.0 (2 C3, 2 C2, 2 C4, 2 C5, 2 C6, 2 C7, 2 C8, 2 C9), 66.5 (2 C1), 70.1 - 73.0 (2 C2’, 2 C3’, 2 C4’, 2 C5’), 107.3 (2 C1’), 131.3 (CH2CH(Z)=CH(Z)CH2), 133.1 (CH2CH(E)=CH(E)CH2). Analysis (%): calculated for C32H60O10: C 63.55, H 10.00. Found: C 63.41, H 9.89. | |
General Procedure for the Hydrogenation of Monocatenar Unsaturated Rhamnosides
|
Decyl-α-L-rhamno-pyranoside (5a’) General Procedure for hydrogenation of monocatenar unsaturated rhamnosides with unsaturated L-rhamnoside 5a (0.4 g; 2.04 mmol; 1 eq), palladium on activated charcoal (4.6 mg ; 0.02 eq) and 4 ml ethanol. Compound 5a’ is obtained as a thick yellow liquid with a quantitative yield. |
 |
| νmax (ATR) cm-1 : 3373 (OH), 2928-2854 (C-H), 1381 (CH3), 1227 (C-OHTert.). [α]20D (589 nm, MeOH) = -43.501 1H NMR (500,1MHz; CD3OD) δ 0.86 (3H, t, J = 7.5 Hz, H10);1.14 (3H, d, J 7.5 Hz, H6’), 1.32 (10H, m, H3, H4, H5, H6, H7, H8, H9), 1.51 (2H, m, H2), 3.14-3.56 (5H, m, H1, H2’, H3’, H4’, H5’), 4.12 - 4.57 (3H, s, 3 OH). 13C NMR (125 MHz; DMSO-d6) δ 14.1 (C10), 18.4 (C6’), 28.7, 28.9, 29.2, 29.3, 29.5, 33.6 (C9, C8, C7, C6, C5, C4, C3), 46.2 (C2), 66.8 (C1), 68.9-73.5 (C2’, C3’, C4’, C5’), 100.4 (C1’). Analysis (%): calculated for: C16H32O5: C 63.13, H 10.60. Found: C 63.32, H 10.72. | |
|
Undecyl-α-L-rhamnopyranoside (6a’) General Procedure for the hydrogenation of monocatenar unsaturated rhamnosides with unsaturated L-rhamnoside 6a (4 g; 2.34 mmol; 1eq), palladium on activated charcoal (4.4 mg; Pd/C 10 % w/w; 0.02 eq) Compound 6a’ was obtained as a thick yellow liquid with a quantitative yield. |
 |
| νmax (ATR) cm-1: 3375 (OH), 2927-2854 (C-H), 1383 (CH3),1228 (C-OHTert.). [α]20D (589 nm, MeOH) = -37.03 1H NMR (500,1MHz; CD3OD) δ 0.85 (3H, t, J = 7.5 Hz, H11); 1.17 (3H, d, J 7.5 Hz, H6’), 1.34 (10H, m, H3, H4, H5, H6, H7, H8, H9, H10), 1.54 (2H, m, H2), 3.16-3.67 (5H, m, H1, H2’, H3’, H4’, H5’), 4.14 - 4.55 (3H, s, 3 OH). 13C NMR (125 MHz; DMSO-d6) δ 13.6 (C11), 18.3 (C6’), 27.5, 28.6, 28.9, 29.7, 30.5, 33.9 (C10, C9, C8, C7, C6, C5, C4, C3), 48.1 (C2), 67.1 (C1), 69.8-75.5 (C2’, C3’, C4’, C5’), 103.4 (C1’). Analysis (%): calculated for: C17H34O5: C 64.12, H 10.76. Found: C 64.25, H 10.64. | |
General Procedure for the Preparation of Monocatenar Esters Derived from Phenolic Acids
|
Hex-5’-enyl-3-(4-hydroxyphenyl)propionic (1b) General Procedure for the preparation of the monocatenar ester with 5-hexen-1-ol (0.7 ml; 6.53 mmol; 1 eq) and 3-(4-hydroxyphenyl)propionic acid (6.50 g; 39.18 mmol; 6 eq) in 60 ml of 2-methyl-2-butanol. Compound 1b was obtained as a clear oil with a yield of 95%. |
 |
| νmax (ATR) cm-1 : 3397 (OH), 2932 (C-H), 1732 (C=O), 1614 (C=C) 1H NMR (500 MHz; CD3OD, ppm) δ 1.36 (2H, broad, H3), 1.55 (2H, broad, H4), 2.03 (2H, broad, H2), 2.54 (2H, t, J 7.2 Hz, H2’), 2.77 (2H, t, J 7.2 Hz, H1’), 4.01 (2H, t, J 7.2 Hz, H1), 4.98 (2H, broad, H6), 5.78 (1H, broad, H5), 6.73 (2H, d, J 10 Hz, H4’, H8’), 7.00 (2H, d, J 10 Hz, H5’, H7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.0 (C3), 28.1 (C4), 30.0 (C2), 33.2 (C2’), 36.0 (C1’), 64.0 (C1),115.4 (C4’, C8’), 115.5 (C5’, C7’), 129.5 (C6), 131.0 (C5), 138.9 (C3’), 156.1 (C6’), 172.8 (C=O). Analysis (%): calculated for C15H20O3: C 72.55, H 8.12. Found: C 72.93, H 8.46. | |
|
Hept-6’-enyl-3-(4-hydroxyphenyl)propionic (2b) General Procedure for the preparation of the monocatenar ester with 5-hepten-1-ol (0.47 ml; 3.01 mmol; 1 eq) and 3-(4-hydroxyphenyl)propionic acid (3 g; 18.05 mmol; 6 eq) in 60 ml of 2-methyl-2-butanol. Compound 2b was obtained as a clear oil with a yield of 88%. |
 |
| νmax (ATR) cm-1 : 3392 (OH), 2930 (C-H), 1732 (C=O), 1614 (C=C) 1H NMR (500 MHz; CD3OD, ppm) δ 1.29 (2H, m, H4), 1.38 (2H, m, H3), 1.56 (2H, m, H5), 2.03 (2H, m, H2), 2.55 (2H, t, J 7.2 Hz, H2’), 2.74 (2H, t, J 7.2 Hz, H1’), 3.98 (2H, t, J 7.2 Hz, H1), 5.03 (2H, m, H7), 5.83 (1H, m, H6), 6.66 (2H, d, J 10 Hz, H5’, H7’), 7.02 (2H, d, J 10 Hz, H4’, H8’) 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.3 (C4), 28.3 (C3), 28.4 (C5), 30.0 (C2), 33.5 (C2’), 36.0 (C1’), 64.1 (C1),115.2 (C7), 115.5 (C4’, C8’), 129.5 (C5’, C7’), 131.0 (C3’), 139.1 (C6), 156.1 (C6’), 172.8 (C=O). Analysis (%): calculated for C16 H22O3: C 73.25, H 8.45. Found: C 72.93, H 8.24. | |
|
Dec-9’-enyl-3-(4-hydroxyphenyl)propionic (3b) General Procedure for the preparation of the monocatenar ester with 9-decen-1-ol (1.2 ml; 3.01 mmol; 1 eq) and 3-(4-hydroxyphenyl)propionic acid (6,51 g; 18.06 mmol; 6 eq) in 60 ml of 2-methyl-2-butanol. Compound 3b was obtained as a clear oil with a yield of 96%. |
 |
| νmax (ATR) cm-1 : 3397 (OH), 2925 (C-H), 1733 (C=O), 1614 (C=C) 1H NMR (500 MHz; CD3OD, ppm) δ 1.26 (8H, m, H4, H5, H6, H7), 1.36 (2H, m, H3), 1.55 (2H, m, H8), 2.04 (2H, m, H2), 2.54 (2H, t, J 7.2 Hz, H2’), 2.77 (2H, t, J 7.2 Hz, H1’), 4.00 (2H, t,J 7.2 Hz, H1), 5.00 (2H, m, H10), 5.83 (1H, m, H9), 6.71 (2H, d, J 10 Hz, H5’, H7’), 6.99 (2H, d, J 10 Hz, H4’, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.8 (C5), 28.6 (C6), 28.8 (C4), 28.9 (C7), 29.1 (C3), 29.2 (C8), 30.1 (C2), 33.7 (C2’), 36.1 (C1’), 64.2 (C1), 115.0 (C10), 115.5 (C4’, C8’), 129.5 (C5’, C7’), 130.9 (C3’), 139.2 (C9), 156.1 (C6’), 172.7 (C=O). Analysis (%): calculated for C19 H28O3: C 74.96, H 9.27. Found: C 75.13, H 9.03. | |
|
Undec-10′-enyl-3-(4-hydroxyphenyl)propionic (4b) General procedure for the preparation of the fatty esters with 36.83 mmol of 3-(4-hydroxyphenyl)propionic acid (6.12 g; 6 eq.) and 6.14 mmol of 10-undecen-1-ol (1.045 g; 1 eq.). Compound 4b was obtained as a bright oil with a yield of 79%. |
 |
| νmax (ATR) cm-1 : 3387 (OH), 2931 (C-H), 1732 (C=O), 1613 (C=C) 1H NMR (500 MHz; CD3OD, ppm) δ 1.24 (8H, m, H4, H5, H6, H7, H8), 1.34 (2H, m, H3), 1.58 (2H, m, H9), 2.07 (2H, m, H2), 2.51 (2H, t, J 7.2 Hz, H2’), 2.74 (2H, t, J 7.2 Hz, C1’), 3.97 (2H, t,J 7.2 Hz, H1), 5.21 (2H, m, C11), 5.81 (1H, m, H10), 6.65 (2H, d, J 10 Hz, H5’, H7’), 7.09 (2H, d, J 10 Hz, H4’, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 24.8 (C5), 28.1 (C6), 28.5 (C4), 28.8 (C7), 29.1 (C3), 29.2 (C8), 30.0 (C9), 30.1 (C2), 33.5 (C2’), 35.3 (C1’), 65.1 (C1), 114.0 (C11), 115.6 (C4’, C8’), 130.0 (C5’, C7’), 130.7 (C3’), 140.0 (C10), 154.6 (C6’), 172.1 (C=O). Analysis (%): calculated for C20 H30O3: C 75.43, H 9.50. Found: C 75.37, H 9.49. | |
|
Hex-5’-enyl-3-(4-hydroxyphenyl)prop-2-enoic (5b) General Procedure for the preparation of the monocatenar ester with 5-hexen-1-ol (0.36 ml; 3.04 mmol; 1 eq) and p-coumaric acid (3.28 g; 18.21 mmol; 6 eq) in 40 ml of 2-methyl-2-butanol. Compound 5b was obtained as a pale yellow oil with a yield of 70 %. |
 |
| νmax (ATR) cm-1 : 3397 (OH), 2932 (C-H), 1732 (C=O), 1614 (-CH=CH2-), 1668 (Phenyl-CH=CH-COO) 1H NMR (500 MHz; CD3OD, ppm) δ 1.51 (2H, m, H3), 1.75 (2H, m, H4), 2.15 (2H, m, H2), 4.25 (2H, t, J 7.2 Hz, H1), 5.01 (2H, m, H6), 5.80 (1H, m, H5), 6.35 (1H, d, J 7.2 Hz, H1’), 6.80 (2H, d, J 7.2 Hz, H7’, H5’), 7.40 (1H, d, J 7.2 Hz, H1’), 7.65 (2H, d, J 7.2 Hz, H4’, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 23.2 (C3), 25.2 (C4), 34.2 (C2), 68.3 (C1), 114.2 (C6), 115.0 (C1’), 127.3 (C5), 130.1 (C7’, C5’), 140.1 (C8’, C4’), 145.0 (C3’), 158.0 (C6’), 168.4 (C=O). Analysis (%): calculated for C15H18O3: C 73.15, H 7.37. Found: C 72.77, H 7.66. | |
|
Dec-9’-enyl-3-(4-hydroxyphenyl)prop-2-enoïc (6b) General Procedure for the preparation of the monocatenar ester with 9-decen-1-ol (0.57 ml; 3.2 mmol; 1 eq) and para-coumaric acid (3.15 g; 19.2 mmol; 6 eq) in 40 ml of 2-methyl-2-butanol. Compound 6b was obtained a yellow oil with a yield of 74 %. |
 |
| νmax (ATR) cm-1 : 3397 (OH), 2932 (C-H), 1732 (C=O), 1612 (-CH=CH2-), 1665 (Phenyl-CH=CH-COO) 1H NMR (500 MHz; CD3OD, ppm) δ 11.22 (8H, m, H4, H5, H6, H7), 1.70 (2H, m, H3), 2.10 (2H, m, H8), 3.75 (2H, m, H2), 4.20 (2H, t, J 7.2 Hz, H1), 5.01 (2H, d, J 7.2 Hz, H10), 5.81 (1H, m, H9), 6.32 (1H, d, J 7.2 Hz, H1’), 6.80 (2H, d, J 10 Hz, H7’, H5’), 7.43 (1H, d, J 7.2 Hz, H2’), 7.65 (2H, d, J 10 Hz, H4’, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.1 (C5), 27.5 (C6), 28.4 (C4), 28.9 (C7), 29.1 (C3), 29.5 (C8), 34.0 (C2), 77.2 (C1), 114.1 (C10), 115.2 (C1’), 116.0 (C9), 127.1 (C5’, C7’), 130.3 (C4’, C8’), 140.2 (C9), 145.1 (C2’), 158.7 (C6’), 168.9 (C=O). Analysis (%): calculated for C19 H26O3: C 75.46, H 8.67. Found: C 75.67, H 9.21. | |
|
Hex-5’-enyl-3-(3,5-dihydroxyphenyl)prop-2-enoic (7b) General Procedure for the preparation of the monocatenar ester with 5-hexen-1-ol (0.36 ml; 3.04 mmol; 1 eq) and para-coumaric acid (3.28 g; 18.24 mmol; 6 eq) in 30 ml of 2-methyl-2-butanol/THF (10/20). Compound 7b was obtained a pale yellow oil with a yield of 52 %. |
 |
| νmax (ATR) cm-1 : 3390 (OH), 2930 (C-H), 1734 (C=O), 1615 (-CH=CH2-), 1664 (Phenyl-CH=CH-COO)1H NMR (500 MHz; CD3OD, ppm) δ 1.59 (2H, m, H3), 1.61 (2H, m, H2), 2.21 (2H, m, H4), 4.22 (2H, t, J 7.2 Hz, H1), 5.05 (2H, d, J 7.2 Hz, H6), 5.12 (1H, d, J 7.2 Hz, H1’), 5.85 (1H, m, H5), 6.36 (2H, d, J 7.2 Hz, H2’), 6.79 (1H, d, J 7.2 Hz, H8’), 6.94 (1H, d, J 7.2 Hz, H7’), 7.18 (1H, s, C4’), 7.50 (1H, d, J 7.2 Hz, C2’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 24.9 (C3), 25.4 (C4), 33.9 (C2), 65.1 (C1), 115.2 (C6), 116.4 (C1’), 117.1 (C7’), 122.2 (C8’), 128.1 (C3’), 139.0 (C5), 144.2 (C4’), 147.2 (C5’, C6’), 167.4 (C=O). Analysis (%): calculated for C15H18O4: C 68.69, H 6.92. Found: C 68.24, H 7.98. | |
|
Dec-9’-enyl-3-(4-hydroxyphenyl)prop-2-enoic (8b) General Procedure for the preparation of the monocatenar ester with 9-decen-1-ol (0.74 ml; 3.7 mmol; 1 eq) and para-coumaric acid (4 g; 19.20 mmol; 6 eq) in 30 ml of 2-methyl-2-butanol/THF (10/20). Compound 8b was obtained a pale yellow oil with a yield of 45 %. |
 |
| νmax (ATR) cm-1 : 3375(OH), 2926 (C-H), 1731 (C=O), 1614 (-CH=CH2-), 1661 (Phenyl-CH=CH-COO) 1H NMR (500 MHz; CD3OD, ppm) δ 1.28 (8H, m, H4, H5, H6, H7), 1.44 (2H, m, H3), 1.62 (2H, m, H2), 2.18 (2H, m, H8), 3.99 (2H, t, J 7.2 Hz, H1), 5.02 (2H, d, J 7.2 Hz, H10), 5.17 (1H, d, J 7.2 Hz, H1’), 5.83 (1H, m, H9), 6.30 (1H, d, J 10 Hz, H8’), 6.79 (1H, d, J 7.2 Hz, H7’), 6.95 (1H, d, J 7.2 Hz, H4’), 7.49 (1H, d, J 7.2 Hz, H2’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.52 (C5), 29.32, 29.57, 31.16, 32.48 (C6, C4, C7, C3), 32.97 (C2), 33.93 (C8), 65.31 (C1), 115.25 (C10), 116.48 (C1’), 117.16 (C3’), 122.12 (C8’), 128.17 (C7’), 129.18 (C4’), 139.13 (C9), 144.95 (C1’), 147.23 (C5’, C6’), 167.13 (C=O). Analysis (%): calculated for C19H26O4: C 71.67, H 8.23. Found: C 71.91, H 8.66. | |
|
Hex-5’-enyl-3-(3,5-dimethoxy-4-hydroxyphenyl)prop-2-enoic (9b) General Procedure for the preparation of the monocatenar ester with 5-hexen-1-ol (1 ml; 0.74 mmol; 1 eq) and sinapic acid (1 g; 4.44 mmol; 6 eq) in 30 ml of acetone. Compound 9b was obtained an orange-yellow oil with a yield of 40 %. |
 |
| νmax (ATR) cm-1 : 3392 (OH), 2934 (C-H), 1732 (C=O), 1612 (-CH=CH2-), 1665 (Phenyl-CH=CH-COO). 1H NMR (500 MHz; CD3OD, ppm) δ .58 (2H, m, H3), 1.62 (2H, m, H2), 2.19 (2H, m, H4), 3.83 (6H, t, J 7.2 Hz, (CH3-O)2-Phenyl), 3.99 (2H, d, J 7.2 Hz, H1), 5.12 (1 H, d, J 7.2 Hz, H6), 5.82 (1H, m, H1’), 6.32 (1H, d, J 7.2 Hz, H5), 6.72 (2H, s, H4’, H8’), 7.48 (1H, d, J 7.2 Hz, H2’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.2 (C3), 25.6 (C2), 33.7 (C4), 56.3 ((CH3-O)2-Phenyl), 65.1 (C1), 108.2 (C6), 115.2 (C1’), 116.1 (C3’), 126.1 (C4’, C8’), 136.0 (C6’),139.2 (C5), 146.3 (C2’), 148.1 (C5’, C7’), 167.9 (C=O). Analysis (%): calculated for C17H22O5: C 66.65, H 7.24. Found: C 66.39, H 7.62. | |
|
Hex-5’-enyl-3-(4-hydroxy-3-méthoxyphényl)prop-2-enoïc (10b) General Procedure for the preparation of the monocatenar ester with 5-hexen-1-ol (0.7 ml; 6.53 mmol; 1 eq) and ferulic acid or 3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic (7.61 mg; 39.2 mmol; 6 eq) in 60 ml of 2-methyl-2-butanol. Compound 10b was obtained as a clear oil with a yield of 52%. |
 |
| νmax (ATR) cm-1 : 3392(OH), 2927 (C-H), 1713 (C=O), 1659-1640 (CH=CH2), 1688-1664 (Phenyl-CH=CH-COO) 1H NMR (500 MHz; CD3OD, ppm) δ 1.48 (2H, m, H3), 1.67 (2H, m, H4), 2.09 (2H, m, H2), 3.82 (3H, s, CH3-O-Phenyl), 4.14 (2H, t, J 7.2 Hz, H1), 5.05 (2H, m, H6), 5.86 (1H, m, H5), 6.49 (1H, d, J 7.2 Hz, H7’), 6.80 (1H, d, J 7.2 Hz, H1’), 7.13 (1H, d, J 7.2 Hz, H2’), 7.33 (1H, s, H4’), 7.56 (1H, d, J 7.2, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.2 (C3), 28.3 (C4), 33.2 (C2), 56.2 (CH3-O-Phenyl), 64.0 (C1), 111.6 (C7’), 114.9 (C1’), 115.5 (C6), 115.9 (C4’), 123.6 (C2’), 126.0 (C3’), 138.9 (C5), 145.4 (C8’), 149.8 (C6’), 148.4 (C5’), 167.2 (C=O). Analysis (%): calculated for C16H20O4: C 69.55, H 7.30. Found: C 69.73, H 7.52. | |
|
Dec-9’-enyl-3-enyl-3-(4-hydroxy-3-méthoxyphényl)prop-2-enoïc (11b) General Procedure for the preparation of the monocatenar ester with 9-decen-1-ol (1.2 ml; 3.01 mmol; 1 eq) and 3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid (3,51 mg; 18.06 mmol; 6 eq) in 60 ml of 2-methyl-2-butanol. Compound 11b was obtained as a clear oil with a yield of 52%. |
 |
| νmax (ATR) cm-1 : 3394 (OH), 2926 (C-H), 1724 (C=O), 1660-1640 (CH=CH2), 1690-1664 (Phenyl-CH=CH-COO). 1H NMR (500 MHz; CD3OD, ppm) δ 1.33 (8H, m, H4, H5, H6, H7), 1.55 (2H, m, H3), 1.63 (2H, m, H8), 2.02 (2H, m, H2), 3.82 (3H, s, CH3-O-Phenyl), 4.12 (2H, t, J 7.2 Hz, H1), 4.99 (2H, m, H10), 5.82 (1H, m, H9), 6.47 (1H, d, J 7.2 Hz, H7’), 6.80 (1H, d, J 7.2 Hz, H1’), 7.11 (1H, d, J 7.2 Hz, H2’), 7.31 (1H, s, H4’), 7.56 (1H, d, J 7.2, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 28.9 (C5), 28.9 (C6), 29.0 (C4), 29.1 (C7), 29.2 (C3), 29.3 (C8), 33.6 (C2), 56.1 (CH3-O-Phenyl), 64.2 (C1), 111.5 (C7’), 114.9 (C1’), 115.0 (C6), 115.9 (C4’), 123.5 (C2’), 126.0 (C3’), 139.2 (C5), 145.4 (C8’), 148.4 (C6’), 149.8 (C5’), 167.1 (C=O). Analysis (%): calculated for C20H28O4: C 72.26, H 8.49. Found: C 71.92, H 8.64. | |
General Procedure for the Preparation of the Fatty Ester-Based Bolaamphiphiles under Microwave Activation
|
1’, 10’-bis-dec-5’-enyl-3-(4-hydroxyphenyl)propionic ester (12b) General Procedure for the preparation of the fatty ester-based bolaamphiphiles with compound 1b (1 g, 4.03 mmol, 1 eq.) under microwave activation with Grubbs II catalyst (0.34 g, 0.403 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2. Compound 12b is obtained as a brown paste with a yield of 56 %. |
 |
| νmax (ATR) cm-1 : 3385 (OH), 2927 - 2855 (C-H), 1730 (C=O), 1614 (C=C) 1H NMR (500 MHz; CD3OD, ppm) δ 1.27-1.36 (12H, m, 2C3, 2C4, 2C2), 2.51 (4H, t, J 7.2 Hz, 2C2’), 2.74 (4H, t, J 7.2 Hz, 2C1’), 3.98 (4H, t, J 7.2 Hz, 2C1), 5.74 (2H, d, J 7.2 Hz, 2C5), 6.71 (4H, d, J 10 Hz, 2C4’, 2C8’), 6.96 (4H, d, J 10 Hz, 2C5’, 2C7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.9 (2 C3), 28.2 (2 C4), 30.1 (2 C2), 33.4 (2 C2’), 36.4 (2 C1’), 64.2 (2 C1),116.2 (2 C4’, 2 C8’), 129.5 (2 C5’, 2 C7’), 130.1 (2 C3’), 130.5 (CH2CH(Z)=CH(Z)CH2), 130.9 (CH2CH(E)=CH(E)CH2), 156.1 (2 C6’), 172.8 (C=O). Analysis (%): calculated for: C28H36O6: C 71.77, H 7.74. Found: C 72.04, H 7.44 %. | |
|
1’, 12’-bis-dodec-6’-enyl-3-(4-hydroxyphenyl)propionic ester (13b) General Procedure for the preparation of the fatty ester-based bolaamphiphiles with compound 2b (0.45 g, 1.72 mmol, 1 eq.) under microwave activation with Grubbs II catalyst (0.15 g, 0.172 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2. Compound 13b is obtained as a brown paste with a yield of 48 %. |
 |
| νmax (ATR) cm-1 : 3374 (OH), 2923 - 2835 (C-H), 1732 (C=O), 1621 (C=C). 1H NMR (500 MHz; CD3OD, ppm) δ 1.28-1.32 (12H, m, H3, H4, H5, H2), 2.53 (4H, t, J 7.2 Hz, H2’), 2.72 (4H, t, J 7.2 Hz, H1’), 4.08 (4H, t, J 7.2 Hz, H1), 5.84 (2H, d, J 7.2 Hz, H6), 6.73 (4H, d, J 10 Hz, H4’, H8’), 6.92 (4H, d, J 10 Hz, H5’, H7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 25.9 (2C3), 28.3 (2 C4), 28.6 (2 C5), 32.2 (2 C2), 33.58 (2 C2’), 36.04 (2 C1’), 64.25 (2 C1),115.18 (2 C4’, 2 C8’), 129.4 (2 C5’, 2 C7’), 130.2 (2C3’), 130.5 (CH2CH(Z)=CH(Z)CH2), 130.9 (CH2CH(E)=CH(E)CH2), 156.2 (2 C6’), 173.0 (C=O). Analysis (%): calculated for C30H40O6: C 72.55, H 8.12. Found: C 72.06, H 7.93. | |
|
1’, 18’-bis-octadec-9’-enyl-3-(4-hydroxyphenyl)propionic ester (14b) General Procedure for the preparation of the fatty ester-based bolaamphiphiles with compound 3b (1 g, 3.29 mmol, 1 eq.) under microwave activation with Grubbs II catalyst (0.28 g, 0.329 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2. Compound 14b is obtained as a brown paste with a yield of 60 %. |
 |
| νmax (ATR) cm-1 : 3383 (OH), 2927 - 2854 (C-H), 1731 (C=O), 1614 (C=C). 1H NMR (500 MHz; CD3OD, ppm) δ 1.29 (20H, m, H3, H4, H5, H6, H7), 1.51 (4H, m, J 7.2 Hz, H2), 1.94 (4H, t, J 7.2 Hz, H8), 2.53 (4H, t, J 7.2 Hz, H1’), 2.72 (4H, t, J 7.2 Hz, H2’), 3.97 (4H, t, J 10 Hz, H1), 5.36 (2H, t, J 7.2 Hz, H9), 6.65 (4H, d, J 10 Hz, H5’, H7’), 6.90 (4H, d, J 10 Hz, H4’, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 28.9 (2 C5), 29.1 (2 C6), 29.2 (2 C4), 29.4 (2 C7), 29.4 (2 C3), 30.0 (2 C8), 32.4 (2 C2), 32.4 (2 C2’), 36.0 (2 C1’), 64.2 (2 C1), 115.5 (2 C4’, 2 C8’), 129.5 (2 C5’, 2 C7’), 130.1 (2 C3’), 130.5 (CH2CH(Z)=CH(Z)CH2), 130.9 (CH2CH(E)=CH(E)CH2), 156.1 (C6’), 172.8 (C=O). Analysis (%): calculated for C36H52O6: C 74.45, H 9.02. Found: C 74.07, H 8.78. | |
|
1’, 10’-bis-dec-5’-enyl-3-(4-hydroxyphenyl)prop-2-enoic (15b) General Procedure for the preparation of the fatty ester-based bolaamphiphiles with compound 4b (0.1 g, 4.06 mmol, 1eq.) under microwave activation with Grubbs II catalyst (0.35 g, 0.406 mmol; 0.1 eq.) dissolved in 10 ml CH2Cl2. Compound 14b is obtained as a brown paste with a yield of 42 %. |
 |
| νmax (ATR) cm-1 : 3395 (OH), 2937 (C-H), 1731 (C=O), 1614 (C=C), 1668 (Phenyl-CH=CH-COO). 1H NMR (500 MHz; CD3OD, ppm) δ 1.25-1.51 (12H, m, H3, H4, H2), 4.25 (4H, t, J 7.2 Hz, H1), 5.48 (2H, t, J 7.2 Hz, H5), 6.30 (2H, d, J 7.2 Hz, H2’), 6.81 (4H, d, J 10 Hz, H5’, H7’), 7.30 (2H, d, J 7.2 Hz, H1’), 7.74 (4, d, 10 Hz, H4’, H8’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 24.1 (2 C3), 25.1 (2 C4), 31.3 (2 C2), 32.2 (2 C2’), 65.3 (2 C1), 114.1 (2 C2’), 1115.0 (2 C5’, 2 C7’), 127.2 (2 C3’), 129.6 (2 C4’, 2 C8’), 130.1 (CH2CH(Z)=CH(Z)CH2), 130.3 (CH2CH(E)=CH(E)CH2), 145.4 (2 C6’), 158.2 (2 C1’), 170.5 (C=O). Analysis (%): calculated for C28H32O6: C 72.39, H 6.94. Found: C 72.82, H 7.21. | |
General Procedure for the Preparation of the Unsymmetrical Fatty Ester- and Rhamnoside-Based Bolaamphiphiles by Classic Heating
|
1’,10’-bis-dec-5’-enyl-3-(4-hydroxyphenyl)propionicacid–α–L-rhamnopyranoside (1ab) General Procedure for the preparation of the unsymmetrical fatty ester- and rhamnoside-based bolaamphiphiles with compound 6a (1 g, 2.15 mmol, 2 eq.) and compound 1b (0.267 g, 1.080 mmol) under argon with Grubbs II catalyst (0.09 g, 0.108 mmol; 0.05 eq.) dissolved in 40 ml CH2Cl2/MeOH (35/5). Compound 1ab is obtained as a brown paste with a yield of 65 %. |
 |
| νmax (ATR) cm-1 : 3353 (OH), 2928(C-H), 1732 (C=O), 1615 (C=C). [α]20D (589 nm, MeOH) = -26.601. 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.27 (3H, d, J 7.5 Hz, H6’-ose), 1.41 (4H, m, H3), 1.83 (4H, m, H2), 2.15 (4H, m, H4), 2.53 (2H, t, J 7.2 Hz, H2’-acid), 2.72 (2H, t, J 7.2 Hz, H1’-acid), 3.12 – 3.54 (5H, m, H1’-ose, C2’-ose, C3’-ose, C4’-ose, C5’-ose), 4.01 (2H, t, J 7.2 Hz, H1-acid), 4.52-4.72 (3H, s, 3 OH-ose), 5.76 (2H, t, J 7.2 Hz, 2 H5-C=C-), 6.71 (2H, d, J 10 Hz, H4’, H8’), 6.98 (2H, d, J 10 Hz, H5’, H7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 17.7 (C6’-ose), 25.8 (2 C3), 28.6, 31.7 (2 C2, 2 C4), 32.9 (C2’-acid), 36.1 (C1’-acid), 63.9 (C1-acid), 66.3 (C1-ose), 68.7-74.1 (C2’-ose, C3’-ose, C4’-ose, C5’-ose), 100.4 (C1’-ose), 115.4 (C4’-acid, C8’-acid), 115.5 (C5’-acid, C7’-acid), 131.0 (CH2CH(Z)=CH(Z)CH2), 131.5 (CH2CH(E)=CH(E)CH2), 136.3 (C3’), 156.1 (C6’), 172.8 (C=O). Analysis (%): calculated for C25H38O8: C 64.36, H 8.21. Found: C 64.09, H 8.57. | |
|
1’,12’-bis-dodec-6’-enyl-3-(4-hydroxyphenyl)propionicacid–α–L-rhamnopyranoside (2ab) General Procedure for the preparation of the unsymmetrical fatty ester- and rhamnoside-based bolaamphiphiles with compound 7a (0.20 g, 0.4 mmol, 2 eq.) and compound 2b (0.053 g, 0.2 mmol) under argon with Grubbs II catalyst (0.017 g, 0.02 mmol; 0.05 eq.) dissolved in 40 ml CH2Cl2/MeOH (35/5). Compound 2ab is obtained as a brown paste with a yield of 42 %. |
 |
| νmax (ATR) cm-1 : 3351 (OH), 2923(C-H), 1731 (C=O), 1614 (C=C). [α]20D (589 nm, MeOH) = -16.900. 1H NMR (500 MHz; DMSO-d6, ppm) δ 11.16 (3H, d, J 7.5 Hz, H6’-ose), 1.28 (4H, m, H4) 1.43 (4H, m, H3), 1.80 (4H, m, H2), 2.12 (4H, m, H4), 2.56 (2H, t, J 7.2 Hz, H2’-acid), 2.70 (2H, t, J 7.2 Hz, H1’-acid), 3.14 - 3.52 (5H, m, H1-ose, H2’-ose, H3’-ose, H4’-ose, H5’-ose), 4.13 (2H, t, J 7.2 Hz, H1-acid), 4.42-4.82 (3H, s, 3 OH-ose), 5.74 (2H, t, J 7.2 Hz, H5-C=C-), 6.67 (2H, d, J 10 Hz, H4’, H8’), 7.08 (2H, d, J 10 Hz, H5’, H7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 17.3 (C6’-ose), 25.4 (2 C4), 25.7 (2 C3), 28.6, 31.7 (2 C2, 2 C4), 32.8 (C2’-acid), 36.2 (C1’-acid), 63.9 (C1-acid), 66.1 (C1-ose), 68.5-74.1 (C2’-ose, C3’-ose, C4’-ose, C5’-ose), 100.6 (C1’-ose), 115.1 (C4’-acid, C8’-acid), 114.3 (C5’-acid, C7’-acid), 130.9 (CH2CH(Z)=CH(Z)CH2), 131.6 (CH2CH(E)=CH(E)CH2), 136.3 (C3’), 156.4 (C6’), 172.8 (C=O). Analysis (%): calculated for C27H42O8: C 65.56, H 8.56. Found: C 65.80, H 8.74. | |
|
1’,18’-bis-dodec-9’-enyl-3-(4-hydroxyphenyl)propionicacid–α–L-rhamnopyranoside (3ab) General Procedure for the preparation of the unsymmetrical fatty ester- and rhamnoside-based bolaamphiphiles with compound 8a (1 g, 1.73 mmol, 2 eq.) and compound 3b (0.264 g, 0.867 mmol) under argon with Grubbs II catalyst (0.074 g, 0.086 mmol; 0.05 eq.) dissolved in 40 ml CH2Cl2/MeOH (35/5). Compound 3ab is obtained as a brown paste with a yield of 68 %. |
 |
| νmax (ATR) cm-1 : 3353 (OH), 2925(C-H), 1732 (C=O), 1614 (C=C). [α]20D (589 nm, MeOH) = -32.601. 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.12 (3H, d, J 7.5 Hz, H6’-ose), 1.34 (20H, m, H3, H4, H5, H6, H7), 1.52 (4H, m, H2), 2.03 (4H, m, H8), 2.51 (2H, t, J 7.2 Hz, H2’-acid), 2.74 (2H, t, J 7.2 Hz, H1’-acid), 3.18-3.47 (5H, m, H1-ose, H2’-ose, H3’-ose, H4’-ose, H5’-ose), 4.06 (2H, t, J 7.2 Hz, H1-acide), 4.13 - 4.51 (3H, s, 3 OH-ose), 5.81 (2H, t, J 7.2 Hz, H9-C=C-), 6.72 (2H, d, J 10 Hz, H4’, H8’), 7.02 (2H, d, J 10 Hz, H5’, H7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 18.3 (C6’-ose), 26.1, 28.7, 28.9, 29.2, 29.4 (2 C3, 2 C4, 2 C5, 2 C6, 2 C7), 29.4 (2 C2), 31.8 (2 C8), 33.4 (C2’-acid), 36.2 (C1’-acid), 64.2 (C1-acid), 66.8 (C1-ose), 68.8-73.5 (C2’-ose, C3’-ose, C4’-ose, C5’-ose), 100.7 (C1’-ose), 115.5 (C4’-acid, C8’-acid), 128.3 (C5’-acid, C7’-acid), 130.7 (CH2CH(Z)=CH(Z)CH2), 131.1 (CH2CH(E)=CH(E)CH2), 138.5 (C3’-acid),156.2 (C6’), 171.8 (C=O). Analysis (%): calculated for: C33 H54O8: C 68.48, H 9.40. Found: C 67.98, H 9.78. | |
|
1’,20’-bis-eicosa-10’-enyl-3-(4-hydroxyphenyl)propionicacid–α–L-rhamnopyranoside (4ab) General Procedure for the preparation of the unsymmetrical fatty ester- and rhamnoside-based bolaamphiphiles with compound 10a (0.5 g, 0.872 mmol, 2 eq.) and compound 4b (0.132 g, 0.437 mmol, 1 eq.) under argon with Grubbs II catalyst (0.017 g, 0.019 mmol; 0.05 eq.) dissolved in 40 ml CH2Cl2/MeOH (35/5). Compound 4ab is obtained as a brown paste with a yield of 46%. |
 |
| νmax (ATR) cm-1 : 3350 (OH), 2921(C-H), 1729 (C=O), 1612 (C=C). [α]20D (589 nm, MeOH) = - 20,300 1H NMR (500 MHz; DMSO-d6, ppm) δ 1.15 (3H, d, J 7.5 Hz, H6’-ose), 1.32 (24H, m, H3, H4, H5, H6, H7, H8), 1.50 (4H, m, H2), 2.31 (4H, m, H9), 2.58 (2H, t, J 7.2 Hz, H2’-acid), 2.73 (2H, t, J 7.2 Hz, H1’-acid), 3.14-3.52 (5H, m, H1-ose, H2’-ose, H3’-ose, H4’-ose, H5’-ose), 4.09 (2H, t, J 7.2 Hz, H1-acide), 4.15 - 4.73 (3H, s, 3 OH-ose), 5.95 (2H, t, J 7.2 Hz, H10-C=C-), 6.74 (2H, d, J 10 Hz, H4’, H8’), 7.08 (2H, d, J 10 Hz, H5’, H7’). 13C NMR (125 MHz; DMSO-d6, ppm) δ 17.5 (C6’-ose), 24.8, 27.6, 28.1, 28.9, 29.2, 29.3 (2 C3, 2 C4, 2 C5, 2 C6, 2 C7, 2 C8), 29.7 (2 C2), 32.1 (2 C9), 34.8 (C2’-acid), 36.1 (C1’-acid), 64.5 (C1-acid), 67.0 (C1-ose), 68.1-74.0 (C2’-ose, C3’-ose, C4’-ose, C5’-ose), 105.7 (C1’-ose), 115.0 (C4’-acid, C8’-acid), 129.2 (C5’-acid, C7’-acid), 130.6 (CH2CH(Z)=CH(Z)CH2), 131.1 (CH2CH(E)=CH(E)CH2), 137.7 (C3’-acid),154.5 (C6’), 171.8 (C=O). Analysis (%): calculated for: C35 H58O8: C 69.27, H 9.63. Found: C 69.38, H 9.71. | |
4. Conclusions
References
- Ash, I.; Ash, M. in Encyclopedia of surfactants, Chemical Pub. Co. 1983.
- Damez, C.; Bouquillon, S.; Harakat, D.; Hénin, F.; Muzart, J.; Pezron, I.; Komunjer. L. Alkenyl and alkenoyl amphiphilic derivatives of D-xylose and their surfactant properties. Carbohydr. Res. 2007, 342, 154–163. [CrossRef]
- a) Montesinos, E.; Badosa, E.; Pla, M.; Montesinos, L.; Bonaterra, A. Biocontrol of Plant Disease: Recent Advances and Prospects in Plant Protection 2022, 121-147. b) Kumar, A.; Kumar Singh, S.; Kant, C.; Verma, H.; Kumar, D.; Pratap Singh, P.; Modi, A.; Droby, S.; Singh Kesawat, M.; Alavilli, H.; Kant Bhatia, S.; Dattatraya Saratale, G.; Ganesh Saratale, R.; Chung, S.M.; Kumar, M. Antioxidants 2021, 10, 1472–1498.
- a) Andree, H.; Hessel, J.F.; Meine, G.; Middelhauve, B.; Schmid, K. Alkyl Polyglycosides, technology, properties and applications, (Eds. K. Hill, W. Von Rybinski, G. Stoll,), VCH Publishers Inc., New York, 1996, 99-130. b) Naveen; K.; Rashmi. T.; "Characteristic and Application of Anionic Dimeric Surfactants: A Review" Tenside Surfactants Detergents 2019, 56, 172–179. c) Abe, M.; Schechter, D.; Schechter, R.S.; Wade, H.; Weerasooriya, U.; Yiv, S. Microemulsion formation with branched tail polyoxyethylene sulfonate surfactants J. Colloid Interface Sci.1986, 114, 342-356. d) Sunwoo, C.K.; Wade, W.H. Optimal Surfactant Structures for Cosurfactant-Free Microemulston Systems I. C16 and C14 Guerbet Alcohol Hydrophobes. J. Dispersion Sci. Technol.1992, 13, 491-514. e) Sanchez, J.; del Valle, M. Determination of Anionic Surfactants Employing Potentiometric Sensors-A Review. Crit. Rev. Anal.Chem. 2005, 35, 15–29.
- a) Gonçalves, R. A..; Holmberg, K.; Lindman, B. Cationic surfactants: A review. J. Mol. Liq. 2023, 375, 121335–121364. b) Kaler, E.W.; Herrington, K.L.; Murthy, A.K.; Zasadzinski, J.A. Phase Behavior and Structures of Mixtures of Anionic and Cationic Surfactants. J. Phys. Chem. 1992, 96, 6698–6707. c) Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581–102609. d) Jesus, C.F.; Alves, A.A.S.; Fiuza, S.M.; Murtinho, D.; Antunes, F.E. Mini-review: Synthetic methods for the production of cationic sugar-based surfactants, J. Mol. Liq. 2021, 342, 117389–117398.
- Holmberg, K. in Handbook of applied surface and colloid chemistry, 2002.
- Benvegnu, T.; Sassi, J. F. Oligomannuronates from Seaweeds as Renewable Sources for the Development of Green Surfactants. Top. Curr. Chem. 2010, 294, 143–164.
- Farn R.J. in Chemistry and technology of surfactants, Wiley - blackwell, 2008.
- Gatard, S.; Nasir, M.N.; Deleu, M.; Klai, N.; Legrand, V.; Bouquillon, S. Bolaamphiphiles Derived from Alkenyl L-Rhamnosides and Alkenyl D-Xylosides: Importance of the Hydrophilic Head. Molecules, 2013, 18, 6101–6112. [CrossRef]
- Nasir, M.N.; Lins, L.; Crowet, J.M.; Ongena, M.; Dorey, S.; Dhondt-Cordelier, S.; Clément, C.; Bouquillon, S.; Haudrechy, A.; Sarazin, C.; Fauconnier, M.L.; Nott, K.; Deleu, M. Interactions of sugar-based bolaamphiphiles with biomimetic systems of plasma membranes Biochimie 2016, 130, 23–32.
- Mulligan, C.N. Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 372–378. [CrossRef]
- Deleu, M.; Paquot, M. From renewable vegetables resources to microorganisms: New trends in surfactants. C. R. Chim. 2004, 7, 641– 646. [CrossRef]
- Lin, S.C. Biosurfactants: Recent advances. J. Chem. Technol. Biotechnol. 1996, 66, 109–120. [CrossRef]
- Lang, S. Biological amphiphiles (microbial biosurfactants). Curr. Opin. Colloid Interface Sci. 2002, 7, 12–20. [CrossRef]
- Corma, A.; Hamid, S.B.A.; Iborra, S.; Velty, A. Surfactants from Biomass: A Two-Step Cascade Reaction for the Synthesis of Sorbitol Fatty Acid Esters Using Solid Acid Catalysts. ChemSusChem 2008, 1, 85–90. [CrossRef]
- Angyal, S. J. Complexes of Metal Cations with Carbohydrates in Solution Adv. Carbohydr. Chem. Biochem. 1989, 47, 1–43.
- Garelli-Calvet, R.; Brisset, F.; Godefroy, L.; Lattes, A. Alkyldiamides à double tête de sucre ou dérivé de sucre comme composés amphiphiles. Brevet Européen N° 0 541 467 A2, 1993, FR 9113851.
- Satgé, C.; Granet, R.; Verneuil, B.; Champavier, Y.; Krausz, P. Synthesis and properties of new bolaform and macrocyclic galactose-based surfactants obtained by olefin metathesis. Carbohydr. Res. 2004, 339, 1243–1254. [CrossRef]
- Dzulkefly, K.; Khoh, H.F.; Ahmad, F.B.H.; Adlie Ahmad, S.; Lim, W.H. Solvent-free esterification process for the synthesis of glucose bolaform surfactants. Orient. J. Chem. 2010, 26, 747–752.
- Obounou Akong, F.; Bouquillon, S. Efficient syntheses of bolaform surfactants from l-rhamnose and/or 3-(4-hydroxyphenyl)propionic acid. Green Chem. 2015, 17, 3290–3300.
- Stuerga, D. Microwave-material interaction and dielectric properties, key ingredients for mastery of chemical microwave process in Microwave in organic synthesis, second edition, Ed. A. Loupy, 2006, 1-61.
- Luzuriaga-Loaiza, W.P.; Schellenberger, R.; De Gaetano, Y.; Obounou Akong, F.; Villaume, S.; Crouzet, J.; Haudrechy, A.; Baillieul, F.; Clément, C.; Lins, L.; Allais, F.; Ongena, M.; Bouquillon, S.; Deleu, M.; Dorey, S. Synthetic Rhamnolipid Bolaforms trigger an innate immune response in Arabidopsis thaliana. Sci. Rep. 2018, 8, 8534–8547. [CrossRef]
- Perreux, L.; Loupy, A. A tentative rationalisation of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron, 2001, 57, 9199–9223.
- Bornaghi, F.L.; Poulsen, S.A. Microwave-accelerated Fischer glycosylation. Tetrahedron Lett. 2005, 46, 3485–3488.
- Kerkel, F.; Markiewicz, M.; Stolte, S.; Müller, C.; Kunz, W. The green platform molecule gamma-valerolactone - ecotoxicity, biodegradability, solvent properties, and potential applications. Green Chem. 2021, 23, 2962–2976.
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Hau Ng, Y.; Sik Ok, Y.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts. Chem. Eng. J. 2019, 372, 992–1006. [CrossRef]
- a) Snoussi, M.; Trabelsi, N.; Dehmeni, A.; Benzekri, R.; Bouslama, L.; Hajlaoui, B.; Al-sieni, A.; Papetti, A. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crops Prod. 2016, 89, 533–542. b) Selka, A.; Moutombi, F.J.; N.; Jean-François, J.; Touaibia, M. Hydroxycinnamic Acids and Their Related Synthetic Analogs: An Update of Pharmacological Activities. Mini Rev. Med. Chem. 2022, 22, 1516–1544. c) Abazari, M.F.; Nasiri, N.; Karizi, S.Z.; Nejati, F.; Haghiaminjan, H.; Norouzi, S.; Piri, P.; Estakhr, L.; Faradonbeh, D.R.; Kohandani, M.; Daliri, K.; Sanadgol, N. An updated review of various medicinal applications of p-coumaric acid: From antioxidative and anti-inflammatory properties to effects on cell cycle and proliferation. Mini Rev. Med. Chem. 2021, 21, 2187–2201. d) Kaur, J.; Kaur, R. p-Coumaric Acid: A Naturally Occurring Chemical with Potential Therapeutic Applications. Curr. Org. Chem. 2022, 26, 1333–1349. e) Antonopoulou I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid from Plant Biomass: A Phytochemical With Promising Antiviral Properties. Front. Nutr. 2022, 8, Article 777576.
- a) Suárez-Escobedo, L.; Gotor-Fernández, V. Solvent role in the lipase-catalysed esterification of cinnamic acid and derivatives. Optimisation of the biotransformation conditions. Tetrahedron 2021, 8112, 131873–131879. b) Chen, H.C.; Twu, Y.K.; Chang, C.M.J.; Liu, Y.C.; Shieh, C.J. Optimized synthesis of lipase-catalyzed octyl caffeate by Novozym® 435. Industrial Crops and Products 2010, 32, 522–526.
- Kotha, S.; Dipak, M.K. Strategies and tactics in olefin metathesis Tetrahedron 2012, 68, 397–421.
- Connon S.J.; Blechert, S. Angew. Chem. Int. Ed. 2003, 42, 1900–1923.
- a) López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. b) Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410.
- Ilyasov I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131-1158. [CrossRef]
- Moussaoui, A.L.; Jawhari, F.Z.; Almehdi, A.M.; Elmsellem, H.; Benbrahim, K.F.; Bousta, D.; Bari, A. Antibacterial, antifungal and antioxidant activity of total polyphenols of Withania frutescens.L. Bioorg. Chem. 2019, 93, 103337–103346. [CrossRef]
- Sakurai, S.; Kawakami, Y.; Kuroki, M.; Gotoh, H. Structure/antioxidant activity (oxygen radical absorbance capacity) relationships of phenolic compounds. Struct. Chem. 2022, 33, 1055–1062. [CrossRef]
- Zheng, Y.; Karimi-Maleh, H.; Fu, L. Evaluation of Antioxidants Using Electrochemical Sensors: A Bibliometric Analysis. Sensors 2022, 22, 3238–3263. [CrossRef]
- Chaillou, L.L.; Nazareno, M.A. New Method to Determine Antioxidant Activity of Polyphenols. J. Agric. Food Chem. 2006, 54, 8397–8402. [CrossRef]
- a) Aziz, A.; Kapoor, D. Salicylic Acid: It’s Physiological Role and Interactions. Research J. Pharm. and Tech. 2018, 11, 3171-3177. b) Ullah, C.; Chen, Y.H.; Ortega, M.A.; Tsai, C.J. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Curr. Opin. Plant Biol. 2023, 72, 102349.
- Blaser, H.U.; Malan, C.; Pugin, B.; Spindler, F.; Steiner, H.; Studer, M. Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments. Adv. Synth. Catal. 2003, 345, 103–151. [CrossRef]
- Ishiyama, I.; Shiga, M.; Sasamoto, K. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem. Pharm. Bull. 1993, 41, 1118-1122. [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005, 73, 1907-1916. [CrossRef]
- Paolinia,A.; Porredon Guarch, C.; Ramos-López, D.; de Lapuente, J.; Lascialfari, A.; Guari, Y.; Larionova, J.; Long, J.; Nano, J. Rhamnose-coated superparamagnetic iron-oxide nanoparticles: An evaluation of their in vitro cytotoxicity, genotoxicity and carcinogenicity. J. Appl. Toxicol. 2016, 36, 510–520.
- Chen, Y.; Wang, G.; Wang, H.; Cheng, C.; Zang, G.; Guo, X.; Liu, R.H. Phytochemical Profiles and Antioxidant Activities in Six Species of Ramie Leaves. PLoS ONE 2014, 9, e108140. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





