1. Introduction
In Colombia, dairy production is one of the main agroeconomic activities carried out, being recognized as the fourth country with the highest milk production in Latin America, with a per capita consumption of 143 liters/inhabitant [
1]. One of the problems that affects milking cows is the manifestation of mastitis that causes high economic losses to the owners, due to the decrease in the quality and quantity of milk, increase in treatment costs, veterinary services, and loss of discarded animals [
2,
3]. This disease is relevant to public health, since contaminated milk can transmit zoonotic diseases such as tuberculosis, brucellosis, and streptococcal pharyngitis [
4,
5].
Subclinical Mastitis (SCM) is an inflammatory process of the mammary gland, in one or more quarters, usually imperceptible in the appearance of the mammary system and in the organoleptic characteristics of the milk [
6]. This pathological condition translates into a reduction in milk production in the affected mammary quarter that will depend on the state of lactation and the dairy production potential of the animal [
6,
7]. At the farm level, the majority of SCM affected animals go undetected in clinical evaluation, because animals with health problems are not recognized [
8]. Cows are milked normally, because this condition does not lead to visible changes in the milk or udder, but does lead to altered milk composition and reduced production [
9,
10]. The presence of SCM is difficult to eradicate since it is caused by multiple factors including: environmental, physical, mechanical, management, production system, production level, as well as nutritional and/or infectious processes, associated with both the cow and its environment [
11,
12]. The high prevalence values of mastitis indicate a risk to human health, mainly due to the consumption of raw milk [
13].
Under field conditions in different countries, a high prevalence of mastitis in cows has been reported since farmers do not perform periodic tests for SCM detection. In Asian countries, values of 35.25% by mammary quarter and 45% for animals are reported [
14]. In the Caribbean islands, a 60% prevalence in the herd was found [
9]. Studies carried out in South American countries indicate a general prevalence of SCM of 39.9% to 42% under hand milking conditions [
15,
16]. Traditionally, the control of SCM is carried out with antibiotics treatment and other antimicrobials that bring with them economic losses due to the less milk, the purchase of medicines, and additionally generate problems of resistance to antibiotics, which constitutes a public health concern [
17]. Poor milking practices are the common cause of spread and prevalence of mastitis on farms [
18].
In Colombia, SCM has been the depressing factor affecting both the quantity and quality of milk; however, most cattle breeders ignore the problem or consider it a transitory effect given the little commitment to carry out sanitary processes for the control and prevention of the disease [
8]. Studies conducted in Colombia report values prevalence of SCM 11.3% to 21.6% for total mammary quarters in dual purpose livestock system [
8,
19]; however, there is a need to investigate dual-purpose livestock systems that are developed with
Bos taurus-Bos indicus crosses in the Colombian Orinoquia.
In Arauca, Colombia, milk production comes mainly from the dual purpose livestock system that is developed under tropical conditions, with low inputs and technological levels. Animal genetic components correspond to multiracial groups as a product of crossbreeding of zebu cattle with European breeds. The udder health status of milking animals is unknown, due to the scarce reports available in the area. The performance of microscopic examinations for the SCM detection is difficult due to problems for access to farms due to the lack of passable access roads, public order problems and distance from urban centers.
At the farm level, the California Mastitis Test (CMT) has been used for decades and is the most used test in field conditions for mastitis diagnosis in cattle [
20,
21,
22,
23,
24]. The CMT is a rapid, practical, and low cost test, with reliable results [
16,
25,
26]. The test does not provide a numerical result, but rather a categorical result, so any result above a vestigial reaction is considered suspicious, presenting validity for the SCM diagnosis [
27].
Another widely used and highly efficient method for SCM diagnosis is the measurement of Electric Conductivity (EC), used since 1942 with different types of conductivity meters [
22,
28,
29,
30]. The EC test has been used in recent decades to monitor and diagnose SCM at the farm level [
20,
25,
27,
31]. The EC test detects milk changes due to the increase in chlorine and sodium ions that occur in inflammatory processes, which increases the electrolytic component of milk [
17,
20,
27].
Since the mentioned tests are used in mastitis diagnosis under field conditions, the aim of the study was to assess the prevalence of SCM in dual purpose livestock systems in Arauca, Colombian Orinoquia, through the analysis of the values found by the CMT and EC tests.
2. Material and methods
2.1. Study site
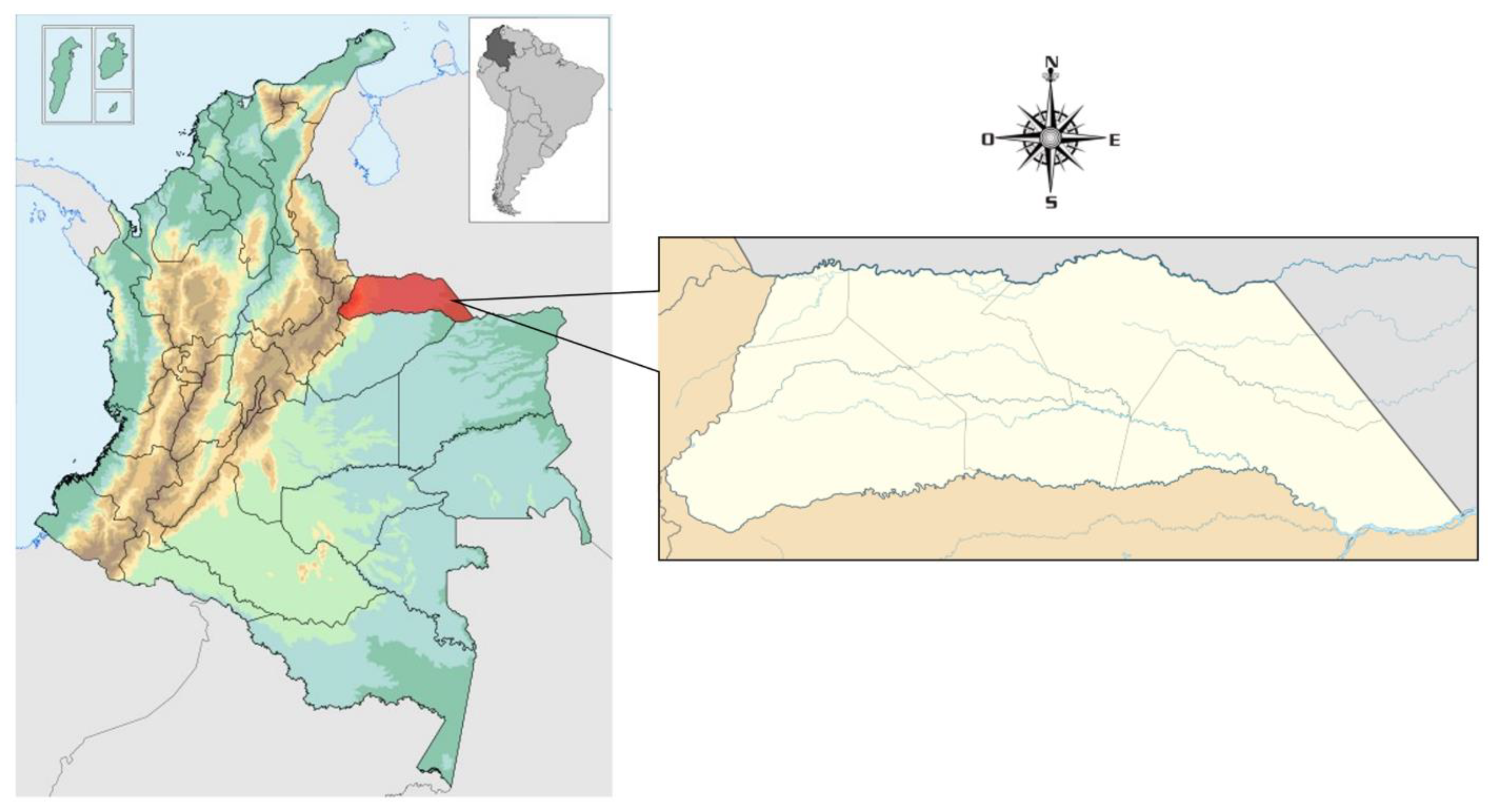
This research was carried out in Arauca, a tropical region of Orinoquia, in eastern Colombia (Latitude: 7º 5’ 5" N; Longitude: 70º 45’ 32.7’’ W) (
Figure 1). The climatic regime corresponds to a period of drought and a period of rains. Annual rainfall is less than 1500 mm, with an ambient temperature of 35º C; a relative humidity of 65% in March, 85% in June and July [
32,
33].
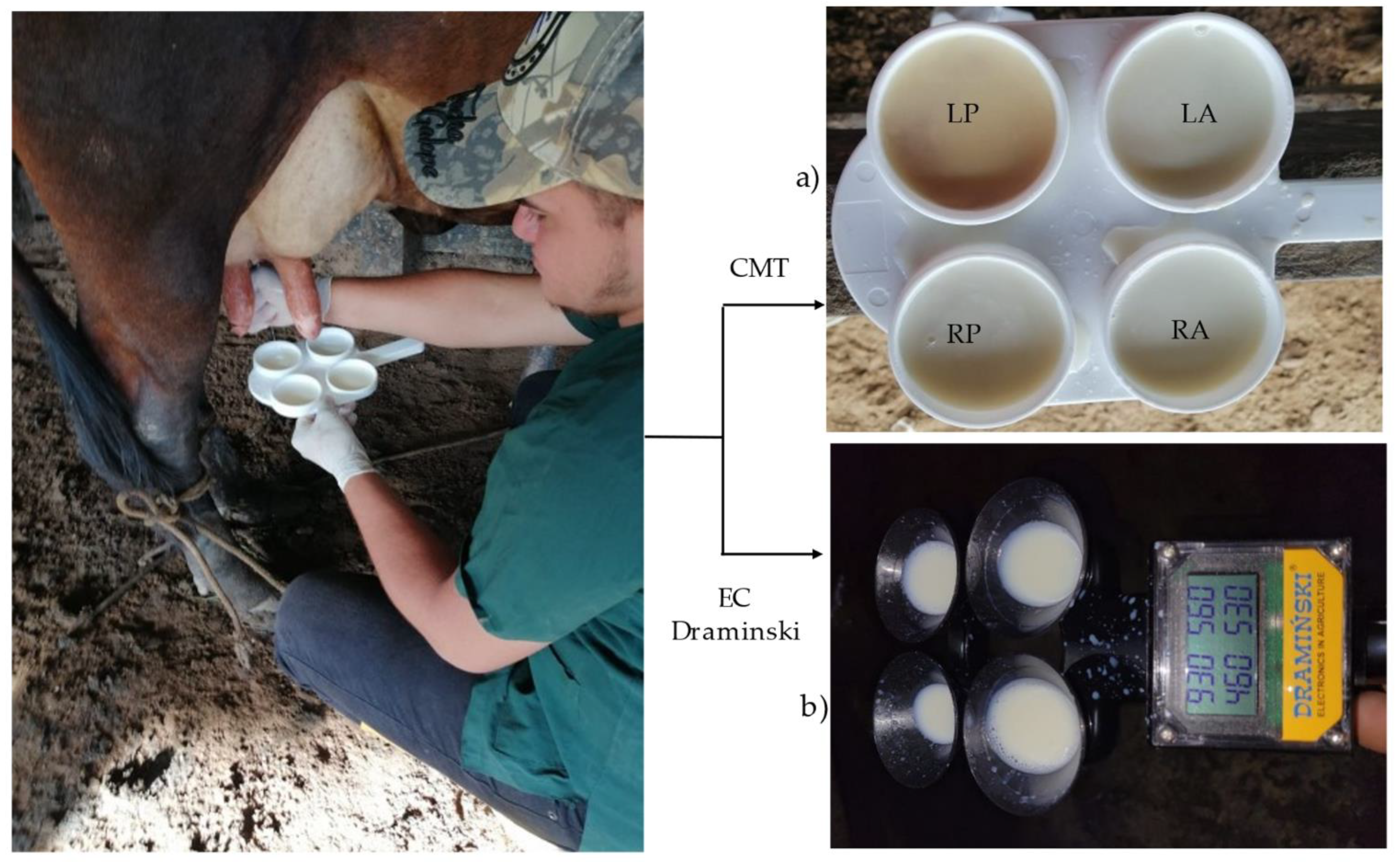
The farms where the animals were sampled belong to small producers with a low technological level. Milking is done by hand, with the presence of the calf (
Figure 2a) and it is done under covered stables or in open environments. Sanitary control is scarce and the destination of most of the milk is for self-consumption, home or artisan processing, reception in collection centers or direct sale to consumers [
34]. On most farms animal feed is based on extensive grazing systems with native grasses and legumes (
Figure 2b). In some systems, Sodium chloride (white salt) is supplied and to a lesser extent mineralized salts are provided.
2.2. Evaluated Animals
Individual milk samples from 1924 mammary quarter belonging to 481 multiracial dairy cows (indefinite
Bos indicus x Bos taurus crosses,
Figure 2b) ranging in age from 3 to 10 years were evaluated. Only milking cows with functional mammary quarters and without antibiotic treatments during the last three months were included in the study.
Animal mammary quarters were sampled during morning milking (4:00 am to 6:00 am) from June 2021 to March 2022, following the available recommendations [
5]. Mammary quarters were identified, and samples were taken in that order (
Figure 3a).
2.3. California Mastitis Test (CMT).
The CMT is a hand test that measures the quantity of somatic cells in milk generated by inflammatory processes [
35,
36]. Somatic cells migrate from blood to milk due to infection response [
37]. The CMT test consists of adding a detergent to the milk (sodium alkylauryl sulfonate) causing leukocytes present in the udder release their DNA content that in combination with milk protein agents forms a gelatinous substance [
20,
38]. The CMT identifies the inflammatory response based on the gel viscosity. The test contains a dye (bromocresol purple) that indicates pH changes that occur in milk, as a result of inflammation [
39].
To sample each mammary quarter, 2 ml of milk were deposited in each receptacle of the plastic test paddle and mixed with 2 ml of CMT reagent (Mastit read) (
Figure 3a), following the standard procedure [
35,
36,
39]. The mixture was homogenized for 10 seconds with circular movements [
35] and with a 45° inclination, the test results were read. Interpretation of results was based on the standard procedure following the CMT grades: 0= Negative; Trace= possible infection; + = positive grade 1(infected); ++ = positives grade 2 (infected); +++ = positives grade 3 (infected) [
35,
36,
37,
39]. In the study, all cases were counted as CMT positive, suggesting a grade of inflammation [
16].
2.4. Electric Conductivity Test (EC)
The EC test for SCM detection is based on the differences in salts concentration (chlorine and sodium ions) between infected and non-infected mammary quarters of the same cow [
29,
30,
40]. The EC test measures the conduction capacity of electrical currents between two electrodes that corresponds to mS/cm (milliSiemens/centimeters) and the result is expressed in units [
29,
41,
42,
43].
For the EC test, the Draminski Mastitis Detector MD4x4Q equipment (Draminski S.A., Owocowa, PL) was used [
43]. The equipment has a platform with four measuring containers (cups) to deposit the "milk squirts" and a measurement and reading block with a special liquid crystal display, where the results of the four mammary quarters are read for three seconds. The equipment reflects the differences between mammary quarters (
Figure 3b). This test was performed before the CMT since according to the equipment user instructions, the first milk squirts from each mammary quarter are required to provide a better result.
The results were interpreted according to the manufacturer’s instructions available in the ISO 9001 equipment user manual [
43]: 1) readings below 250 units indicate “subclinical inflammation of the mammary quarter or a high risk of becoming acute 2) a difference greater than 40-50 units between the highest and lowest quarter scores of the examined cow indicates SCM. In general terms, it can be assumed that a result between 250 and 300 units indicates an intermediate phase between SCM and a healthy mammary quarter.
Example of EC results interpretation among mammary quarters:
| Right Posterior (RP): |
370u |
| Left Posterior (LP): |
310u |
| Right Anterior (RA): |
380u |
| Left Anterior (LA): |
370u |
Interpretation: the mammary quarter affected with SCM is the left posterior (LP) (380u-310u = 70u), the other mammary quarters remain normal.
2.5. Prevalence Determination
Prevalence refers to the number of cases a disease or an infectious event occurs in a given place and time. It is an indicator of existence or "stock", since it considers all present cases, whether new or old [
44]. The prevalence of SCM for cows (with at least one affected mammary quarter), total mammary quarters and individual mammary quarters was processed according to the following mathematical formulas [
16,
45,
46]:
General prevalence = (Number of positive cows / Total number of sampled cows) x 100
Prevalence in the total number of mammary quarters = (Total number of positive mammary quarters / Total number of sampled mammary quarters) x 100
Prevalence in individual mammary quarters = (Total number of positive mammary quarters per position / Total number of mammary quarters per position) x 100
2.6. Statistical Analysis
The tests result at the farm level were compiled and organized in Excel format for their subsequent analysis according to the following phases:
First phase. To compare the prevalence using the two tests, three databases were generated: a) Considering negative SCM values, those negative results obtained with the CMT test (CMT 0); b) Considering negative SCM values, those negative and trace results obtained with CMT test (CMT 0 and T) and c) the database obtained with the EC test.
Frequency tables were estimated for the variables related to the number of positive animals, number of positive quarters and positivity per mammary quarters (RP, LP, RA, LA) obtained in each method of analysis. The relationship between the SCM prevalence values obtained in the evaluated methods (CMT 0 and T; CMT 0, and EC) was studied. To identify the existence of statistical differences in the prevalence by mammary quarters, a one-way ANOVA was performed, where the fixed effects were the analyzed methods (CMT 0 and T; CMT 0, and EC) and the repetitions were the SCM prevalence observed in each mammary quarter (RP, LP, RA, LA). When statistical differences were found, Tukey’s test was used for the means differentiation (p < 0.05).
Second phase. Spearman correlations (rs) were estimated between the aforementioned variables in the first phase to evaluate the relationship between the SCM positivity values found by the tests. The correlations were significant with a p < 0.05.
Third phase. Confusion matrices were established to assess the concordance between the methods and to identify whether the CE test classified the animals as positive or negative in a similar way to the CMT test (0 and T; or 0). In this case, the values obtained with the CMT test were considered as the reference values. With this information, the following parameters were estimated: a) sensitivity (probability that animals classified as positive for SCM with the CMT test, have also been classified positive by the EC test), b) specificity (probability that animals classified as negative for SCM with the CMT test, have also been classified as negative by the EC test), c) false negatives (probability that animals classified as positive for SCM with the CMT test , have been classified as negative by the EC test) and d) false positives (probability that animals classified as negative for SCM with the CMT test, have been classified as positive by the EC test). The objective of the analysis is to achieve values close to 1 in sensitivity and specificity (agreement in the classification between tests) and close to zero in classification errors (false positives and false negatives). The formulas used to estimate the parameters are shown below [
47]:
|
|
|
|
Se= Sensitivity; Sp= Specificity; VP= True Positives; FN= False Negatives; TN= True Negatives; FP= False Positives.
All data was processed in InfoStat software [
48]
.
3. Results
A total of 481 animals corresponding to 1924 mammary quarters were sampled. The distribution of the animals number with SCM-affected mammary quarters, obtained with the CMT and EC methods within the evaluated sample is shown in
Table 1.
Results showed that 94% (n=451), 69% (n=330) and 70% (n=338) of the analyzed cows did not present any mammary quarter affected, with the CMT (0 and T), CMT (0) and EC test respectively. This suggests that 30, 151, and 143 of the animals presented some degree of positivity to SCM according to the methods used. Similar results were found between the CMT (0) and EC test, so the results discussion was based on these techniques.
The average difference in the animals classification with 0, 1, 2, and 3 affected quarters was 11.3%. However, large differences between techniques (86.4%) were observed when classifying animals with 4 mammary quarters affected.
The SCM prevalence per animal and mammary quarters (individual and total) are presented in
Table 2. The prevalence per-animal indicates whether a cow presented the disease or at least one of its mammary quarters was positive to some degree. The general prevalence values were 31.4% (151/481) and 29.7% (143/481) for the CMT (0) and EC tests respectively, without significant differences. The CMT (0) method showed a higher prevalence in the RP (16.0%) and RA (14.8%) mammary quarters, while the EC method showed it in the LA (12.1%) and RP (11.9%) mammary quarters. When evaluating the prevalence in the total mammary quarters, significant differences were found between the analyzed methods (p < 0.05), although the values were close: 14.2% (274/1924) for CMT (0) and 11.3% (218 /1924) for EC.
Spearman’s correlations between CMT (0) and EC tests per mammary quarter, positive animals, and number of positive mammary quarters are presented in
Table 3. The correlations were significant (p < 0.05), with values between 0.20 – 0.25 for the analyzed methods. These results suggest that the tests showed low concordance in detecting the same animals with or without the presence of SCM. The CMT 0 and EC analysis methods showed similar prevalence. However, some animals that were positive for one test were not for the other.
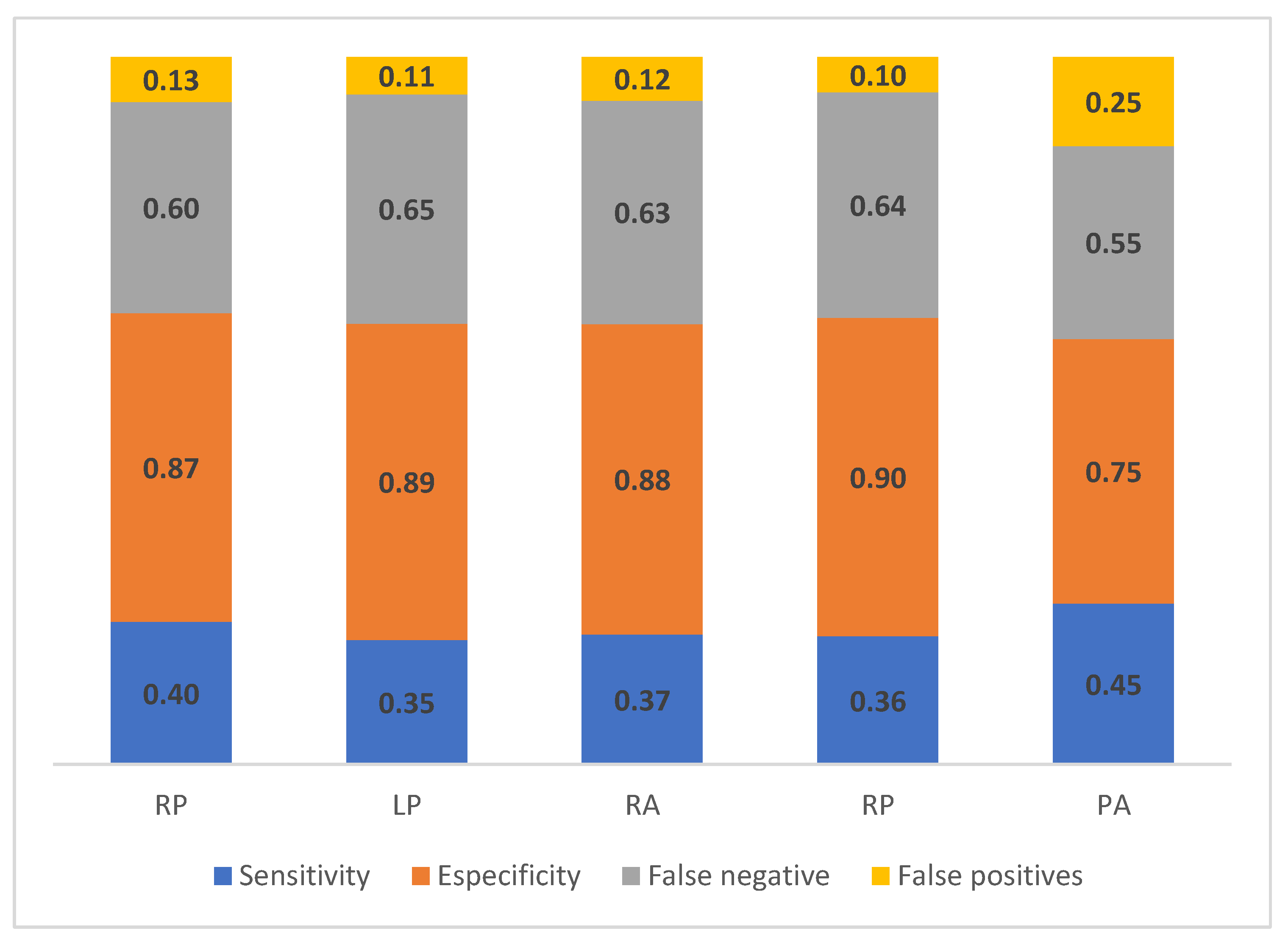
The values of sensitivity, specificity, false negatives, and false positives between the EC and CMT 0 test are shown in
Figure 4.
Values of sensitivity and specificity ranged between 0.35 to 0.45 and 0.75 to 0.90 respectively, between the methods. These results suggest that both methods were similar, classifying animals and their mammary quarters with negative SCM values; however, when classifying animals and their mammary quarters as SCM positive, the concordance between the techniques was low. This was reflected in many classification errors, with false negatives ranging among 0.55 - 0.65, while false positives presented lower values between 0.10 - 0.25.
4. Discussion
4.1. Comparable SCM estimation between CMT and EC
In this study, the result of general prevalence with CMT (31.4%) was higher compared to EC (29.7%) but did not reflect a statistical difference (p > 0.05). The prevalence result per mammary quarter presented a statistical difference (p < 0.05) and was higher for CMT (14.2%) compared to EC (11.3%). A similar and lower prevalence was observed between individual mammary quarters with the EC test compared with CMT. This result is not consistent with those of another study, where the CMT and EC tests were used as farm diagnostic methods, in which the test results were not significant (p > 0.05) [
49]. The values of general prevalence and mammary quarters are lower than those reported in other studies in Colombia in dual purpose livestock systems [
8,
19].
In the CMT test, a higher prevalence in the RP (16.0%) and RA (14.8%) mammary quarter was found, while the EC test findings showed the highest prevalence in the LA (12.1%) and RP (11.9%) mammary quarters. The above may be due to the fact that cows during their rest period take a sternal decubitus position frequently to the right side, allowing the mammary gland to be more vulnerable to environmental microorganisms infection.
Another factor may be related with the form of how manual milking is performed, since the milking usually starts with the two right mammary quarters, or in a crossed way beginning with the RP and the LA mammary quarters. Milking in a crossed way was observed in most of the milkers present in the study. Study reports the findings of SCM prevalence are caused by problems in milking hygiene, lack of education and incentives for milkers, as well as failures in the supply of adequate materials for a good performance of their work [
50], the factors that were observed in this study.
As can be seen, the general value for SCM reported in the present study is lower than the information found in literature, possibly because most of the studies have been carried out in specialized breeds for milk production, while our study was carried out in crossbred breeds (
Bos taurus x Bos indicus). On the other hand, milking with the presence of the calf causes a greater amount of milk to be sucked from the mammary gland, and due to the bactericidal effect of the saliva it can prevent the microorganisms proliferation [
51]. Under farm conditions, it has been pointed out that the prevalence of MSC individually and per mammary quarter is due to the fact that cattle breeders do not practice periodic tests for the detection of the disease [
14]. It is also indicated that the presence of MSC in dual purpose systems (
Bos taurus -Bos indicus) is common in cows with a high degree of European purity [
52].
The CMT test presents limitations such as the variation of interpretation by the operators, factors that influence the reading, such as, the time elapsed after combining the reagent with the milk, the homogenization of the samples, the time elapsed from the taking to its analysis, and the quality of the reagent [
53]. Hence, the CMT is a visual test for subjective evaluation, which despite its simplicity, requires training in order to reduce the variability between operators, which is the most frequent source of variation [
10]. On the other hand, the evaluation of the CMT results (which is the viscosity produced by the mixture of milk and reagent), varies depending on the amount of reagent in the mixture and is affected by the pH of the milk [
10]. The determination of reactive degrees to CMT constitutes a valid criterion to indicate an emergency situation of SCM in a herd due to the presence of high-risk pathogens. This test allows a rapid diagnosis in the farm and without many technical requirements [
53].
In a SCM detection study in different crossbred cows, it was found that 65.2% of the sampled animals were positive using EC, so cattle breeders can easily use the technique to determine the presence of SCM in their dairy cows [
54]. In another study, they conclude that the EC test showed similarity to CMT in the detection of SCM and its reliability would increase even more when used together with the other diagnostic methods [
55]. The results of this study correspond with those found by previous authors.
Past studies indicate that the EC test is efficient in detecting SCM; in comparison, with the CMT test [
56], likewise reliable results are reported in the diagnosis of SCM, when both tests are used [
31]. In general, similar results are reported, when using the two tests SCM detection [
57]. Thus, the main advantage of the EC test is that diagnostic results can be achieved with objective, immediate measurements, and a relatively lower cost, compared to other mastitis detection methods [
29].
Although it is necessary to know the various factors that affect the results of the EC test and the small differences between a healthy gland and an infected halves impose that the conductivity meter must be easy and with an automatic calibration of the conductivity meter [
41]; that is, that the results the EC test may be masked by the equipment calibration [
29]. The EC test is useful for early identification of animals with intramammary infection, it is inexpensive and shows immediate results [
42]
. In several countries, portable equipment has been used for the determination of SCM through EC, evaluating the concordance levels with the CMT test [
30]. Obtaining variable results is one of the questions regarding the determination of EC, despite the fact that it is one of the most widely used methods as a mastitis predictor [
58].
4.2. CMT and EC test Concordance
In this study, the correlation results between CMT and EC test showed low concordance in the detection capacity of the same animals, with or without the SCM infection. That is, despite the fact that the two tests present similar SCM prevalence, some animals that were positive for one test were not for the other. The low correlation between the tests indicates that the methods did not show the same results for the SCM diagnosis; however, they help to identify the health status of the mammary gland. However, a study in Cuba comparatively evaluated the EC test with the CMT test, found similar results obtained with the two tests [
30].
The sensitivity and specificity ranges (0.35 - 0.45 and 0.75 - 0.90) obtained with the EC method respect the CMT test, indicated that both methods presented a good agreement classifying animals and their mammary quarters as SCM negative; however, in the classification of positive cases the concordance was low. These results can be attributed to the fact that the data was not balanced between positive and negative cases (there were more negative than positive cases). In this way, the probability that EC classified the animals and mammary quarters as SCM negative was higher than the classification of positive cases. To corroborate these results, it is necessary to evaluate the concordance between the techniques, considering more balanced information between positive and negative cases. The sensitivity values obtained in this study are different from the values (0.97) reported in another study in livestock systems; while the specificity values were similar (0.93) to those reported in the same study [
25]. Studies carried out report a 50% false positive rate and have found estimated values of sensitivity and specificity of EC not very high [
42], similar information to that found in our study.
5. Conclusions
The EC and CMT diagnostic tests showed a low general prevalence of SCM in the livestock systems evaluated. The EC test for the diagnosis of SCM in small producer farms is a feasible alternative. It is low cost and quick to perform, without the effects of visual subjectivity of CMT that can occur in operators. The two tests were consistent in detecting the same animals with or without the infection presence, although some animals that were positive for one test were not the other. The EC Test classified the animals and their mammary quarters as SCM positive or negative in a manner more similar to that obtained with CMT. The low correlation between the tests indicate that they did not show the same results for the diagnosis of SCM, but they help to determine the health status of the mammary gland at the farm level. The SCM diagnosis with any of the two tests should be complemented with sampling for microbiological analysis to determine the causative agent of the degree of infection in each quarter of the mammary gland.
Author Contributions
Writing, Preparation of the Original Manuscript, Methodology and Formal Analysis, A.S.-C.; M.V.-T., R.T.; Statistic Analysis: M.V.-T.; R.T.; Review, Edition and Conceptualization, R.J.-J., P.M.P-C., J. N. A-L. All Authors have read and accepted the published version of the manuscript.
Funding
This research was funded by the Research Committee—CONADI (ID2958; ID3058)—of the Universidad Cooperativa de Colombia.
Institutional Review Board Statement
Bioethical Concept No. BIO163 issued by the Research Bioethics Subcommittee of the Bucaramanga Sectional Universidad Cooperativa de Colombia (Act No. 4, November 04, 2021).
Informed Consent Statement
The study did not require informed consent from the owners since the study subjects were cows that are milked daily on the farms. No animal was subjected to abuse, complying with animal welfare guidelines, there was no alteration to the animal since the milk samples were taken prior to the start of milking.
Data Availability Statement
Data is available upon reasonable request to the second author.
Acknowledgments
The Authors thank the auxiliary students who helped in the collection of field data. To the farmers for providing the animals for taking milk samples. To the anonymous referees for their valuable comments on the manuscript. All persons included in this section have consented to the acknowledgments.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this research.
References
- PROCOLOMBIA. Aliado estrategico del sector lácteos. Mincomercio Industria y Turismo, 2018. Available online: https://bit.ly/3MpNIji (accessed on 22 February 2023).
- Tomasinsig, L.; De Conti, G.; Skerlavaj, B.; Piccinini, R.; Mazzilli, M.; D’Este, F.; Tossi, A.; Zanetti, M. Broad-spectrum activity against bacterial mastitis pathogens and activation of mammary epithelial cells support a protective role of Neutrophil Cathelicidins in Bovine Mastitis. Infect. Immunity 2010, 78, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Mera Andrade, R.; Muñoz Espinoza, M.; Artieda Rojas, J. R.; Ortíz Tirado, P.; González Salas, R.; Vega Falcón, V. Mastitis bovina y su repercusión en la calidad de la leche. Revi. Elect. Vet. 2017, 8, 1–16. [Google Scholar]
- Aguilar Aldrete, A.; Bañuelos Pineda, J.; Pimienta Barrios, E.; Aguilar Flores, A.; Torres Moran, P. Prevalencia de mastitis subclínica en la Región de Ciénaga del Estado de Jalisco. Abanico Vet. 2014, 4, 24–31. [Google Scholar]
- Wolter, W.; Castañeda, V. H.; Kloppert, B.; Zschöck, M. La Mastitis Bovina. Instituto Estatal de Investigaciones de Hesse; 2004; p 68.
- Florio-Luis de Pineda, J.; Pineda, M.; Polanco, M.; J, M.; Díaz, N.; Florio-Luis, G. Estrategias de prevención y control de mastitis como apoyo para preservar un rebaño bovino en los Llanos Centrales, Venezuela. Actas Iberoam. Conserv. Anim. 2015, 6, 598–616. [Google Scholar]
- Ponce, P.; Ribot, A.; Capdevila, J. Z.; Villoch, A. Manual aprendiendo de la leche PROCAL. San José de las Lajas, Cuba: CENSA, Minagricultura, 2010.
- Mendoza, J. A.; Vera, Y. A.; Peña, L. C. Prevalencia de mastitis subclinica, microorganismos asociados y factores de riesgo identificdos en hatos de la provinvia de Pamplona, Norte de Santander. Rev. Med. Vet. Zoot. 2017, 64, 11–24. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Sánchez-Santana, T.; López-Vigoa, O.; Benítez- Álvarez, M. Á. Prevalencia de mastitis subclínica y microorganismos asociados a esta. Pastos y Forrajes 2018, 41, 35–40. [Google Scholar]
- Ruíz-García, L. F.; Sandoval-Monzón, R. S. Diagnóstico de mastitis subclínica de vacunos lecheros mediante el conteo de células somáticas empleando dos métodos diagnósticos. Rev. Científ. 2018, 28, 129–135. [Google Scholar]
- Andresen, H. Mastitis: prevención y control. Rev. Inv. Vet. Perú 2001, 12, 55–64. [Google Scholar] [CrossRef]
- Ruegg, P. L. A 100-year review: Mastitis detection, management, and prevention. J. Dairy Sc. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Murphy, S. C.; Martin, N. H.; Barbano, D. M.; Wiedmann, M. Influence ofraw milk quality on processed dairyproducts: how do raw milk quality testresults relate to product quality and yield? J. Dairy Sc. 2016, 99, 10128–10149. [Google Scholar] [CrossRef]
- Bachaya, H. A.; Raza, M. A.; Murtaza, S.; Akbar, U. R. Subclinical bovine mastitis in Muzaffar Garh district of Punjab (Pakistan). J. Anim. Plant Sci. 2011, 21, 16–19. [Google Scholar]
- Ruiz, A.; Ponce, P.; Gomes, G.; Mota, R.; Sampaio, E.; Lucena, E.; Benone, S. Prevalencia de mastitis bovina subclínica y microorganismos asociados: comparación entre ordeño manual y mecánico, en Pernambuco, Brasil. Rev. Salud Anim 2011, 33, 57–64. [Google Scholar]
- Gómez-Quispe, O. E.; Santivañez-Ballón, C. S.; Arauco-Villa, F.; Espezua-Flores, O. H.; Manrique-Meza, J. Criterios de Interpretación para California Mastitis Test en el Diagnóstico de Mastitis Subclínica en Bovinos. Rev. Inv. Vet. Perú 2015, 26, 86–95. [Google Scholar] [CrossRef]
- Acosta Moreno, A.; Mira Hernandez, J.; Posada Arias, S. Tópicos en mastitis bovina: desde la etiología hasta algunas terapias alternativas. J. Agricult. Anim. Sci. 2017, 6, 42–58. [Google Scholar] [CrossRef]
- Pinzón, T. A.; Moreno, F. C.; Rodríguez, M. G. Efectos de la mastitis subclínica en algunos hatos de la cuenca lechera del Alto Chicamocha (departamento de Boyacá). Rev. Medic. Vet. 2009, 17, 23–35. [Google Scholar]
- Calderon, R. A.; Rodríguez, R. V.; Arrieta, B. G.; Mattar, V. S. Prevalence of mastitis in dual purpose cattle farms in Monteria (Colombia): etiology and antibacterial susceptibility. Rev. Colomb. Cienc. Pecu. 2011, 24, 19–28. [Google Scholar]
- Medina, C. M.; Montaldo, V. H. El uso de la prueba de conductividad eléctrica y su relación con la prueba de California para mastitis. CNM. V Congreso Nacional de Control de Mastitis, Aguascalientes, México. 29-31 de mayo, 2003.
- AL-Edany, A. A.; Khudor, M. H.; AL-Mousawi, K. S. Comparison of three indirect tests for the diagnosis of bovine subclinical mastitis caused by coagulase negative staphylococci with their susceptibility to seven antibiotics. Bas. J. Vet. Res. 2012, 11, 75–83. [Google Scholar] [CrossRef]
- Maldonado-Arias, D. F.; Santos-Calderón, C. R.; Quilapanta-Guamán, A. E.; Mena-Miño, L. A. Diagnosis of Subclinical Mastitis Using Three Methods for Control and Treatment. Dom. Cien. 2022, 8, 773–790. [Google Scholar]
- Deb, R.; Kumar, A.; Chakraborty, S.; Kumar Verma, A.; Tiwuari, R.; Dhama, K.; Singh, U.; Kumar, S. Trends in Diagnosis and Control of Bovine Mastitis: A review. Pakist. J. Biol. Sci. 2013, 16, 1653–1661. [Google Scholar] [CrossRef]
- Badiuzzaman, M.; Samad, M. A.; Siddiki, S. H. M. F.; Islam, M. T.; Saha, S. Subclinical mastitis in lactating cows: Comparison of four screening tests and effect of animal factors on its occurrence. Bangl. J. Vet. Med. 2015, 13, 41–50. [Google Scholar] [CrossRef]
- Bedoya, C. C.; Castañeda, V. H.; Wolter, W. Métodos de detección de la mastitis bovina. Rev. Electrón. Vet. 2007, 8, 1–17. [Google Scholar]
- Sanford, C. J.; Keefe, G. P.; Sanchez, J.; Dingwell, R. T.; Barkema, H. W.; Leslie, K. E.; Dohoo, I. R. Test characteristics from latent-class models of the California Mastitis Test. Prev. Vet. Med. 2006, 77, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J. M.; Bedoya, J. L. Importancia del conteo de células somáticas en la calidad de la leche. Rev. Elect. Vet. 2008, 9, 1–35. [Google Scholar]
- Musser, J. M.; Anderson, J. L.; Caballero, M.; Amaya, D.; Maroto-Puga, J. Evaluation of a hand-held electrical conductivity meter for detection of subclinical mastitis in cattle. Am. J. Vet. Res. 1998, 59, 1087–1091. [Google Scholar] [PubMed]
- Romero, G.; Díaz, J. R.; Sabater, J. M.; Pérez, C. Evaluation of Commercial Probes for On-Line Electrical Conductivity Measurements during Goat Gland Milking Process. Sensors 2012, 12, 4493–4513. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruano, M.; Tarafa-Zambrana, L. Evaluación del equipo Mas-D-Tec en el diagnóstico de campo de mastitis subclinica en el ganado bovino. Rev. Salud Anim. 2017, 39, 1–7. [Google Scholar]
- Saltijeral, J.; Cordova, A.; Ruiz, G.; Alonso, U. Comparative study of electrical conductivity and CMT of quarter milk samples in dairy farm in Mexico. International Congress of the Society for Animal Hygiène, Saint-Malo, 2004.
- IDEAM. Instituto de Hidrología, Meteorología y Estudios Ambientales. Información Aeronáutica. Climatología. Available online: http://bart.ideam.gov.co/cliciu/arauca/precipitacion.htm (accessed on 23 December 2022).
- Arauca. Clima: Arauca, Aeropuerto Santiago Pérez (En línea). Available online: https://es.allmetsat.com/clima/venezuela.php?code=80099 (accessed on 23 December 2022).
- Salamanca-Carreño, A.; Tamasaukas, R.; Giraldo-Forero, J. C.; Quintero, A. D.; Hernández-Rodríguez, M. E. Interacción entre factores ambientales y raciales sobre la prevalencia de hemotrópicos en hembras bovinas doble propósito en sabanas inundables araucanas, Colombia. Rev. Científ. FCV-LUZ 2018, 28, 52–62. [Google Scholar]
- Mellenberger, R.; Roth, C. J. Hoja de Información de la Prueba de Mastitis California (CMT), 2000. Available online: https://bit.ly/43iegJq (accessed on 23 December 2022).
- NMC. National Mastitis Council, Inc. Laboratory Handbook on Bovine Mastitis., Third printing ed.; Minnesota, 2017; p 151.
- Philpot, W. N.; Nickerson, S. N. Winning the Fight Against Mastitis; Estados Unidos de América, 2001; p 192.
- Bonifaz, N.; Conlago, F. Prevalencia e incidencia de mastitis bovina mediante la prueba de California Mastitis Test con identificación del agente etiológico, en Paquiestancia, Ecuador. La Granja: Rev. Cienc. Vida 2016, 24, 43–52. [Google Scholar] [CrossRef]
- Guízar, J. I.; Bedolla, J. L. C. Determinación de la prevalencia de mastitis bovina en el municipio de Tarímbaro, Michoacán, mediante la prueba de California. Rev. Electrón. Vet. 2008, 9, 1–35. [Google Scholar]
- Zaninelli, M.; Rossi, L.; Tangorra, F. M.; Costa, A.; Agazzi, A.; Savoini, G. On-Line Monitoring of Milk Electrical Conductivity by Fuzzy Logic Technology to Characterise Health Status in Dairy Goats. Ital. J. Anim. Sc. 2014, 13, 340–346. [Google Scholar] [CrossRef]
- Ying, C.; Yang, C.-B.; Hsu, J.-T. Relationship of Somatic Cell Count, Physical, Chemical and Enzymatic Properties to the Bacterial Standard Plate Count in Different Breeds of Dairy Goats. Asian-Aust. J. Anim. Sci. 2004, 17, 554–559. [Google Scholar] [CrossRef]
- Elizalde, E. F.; Signorini, M. L.; Canavesio, V. R.; Cuatrin, A.; Tarabla, H. D.; Calvinho, L. F. Medición de la Conductividad Eléctrica en Leche como Método Diagnóstico de Mastitis Subclínica Bovina. Rev. FAVE - Cienc. Vet. 2019, 8, 15–28. [Google Scholar] [CrossRef]
- Draminski. Mastitis Detector. Electrónica en Agricultura. Manual de uso. ISO 9001 CE. Available online: https://www.draminski.es/agri/detectores-de-mastitis/.
- Manterola, C.; Otzen, T. Valoración Clínica del Riesgo, Interpretación y Utilidad Práctica. Int. J. Morphol. 2015, 33, 842–849. [Google Scholar] [CrossRef]
- Santivañez-Ballón, C. S.; Gómez-Quispe, O. E.; Cárdenas-Villanueva, L. A.; Escobedo-Enríquez, M. H.; Bustinza-Cardenas, R. H.; Peña-Sánchez, J. Prevalencia y factores asociados a la mastitis subclínica bovina en los Andes peruanos. Vet. Zootec. 2013, 7, 92–104. [Google Scholar] [CrossRef]
- Ramírez Sánchez, J. M. Prevalencia y factores predisponentes a mastitis subclínica en establos lecheros de la provincia de Trujillo. Rev. CEDAMAZ 2015, 5, 12–23. [Google Scholar]
- Tarabla, H.; Signorini, M. Epidemiología diagnóstica, 1st ed.; Universidad Nacional del Litoral, UNL: Santa Fe Argentina, 2020. [Google Scholar]
- InfoStat. InfoStat Versión 2020. Manual del Usuario. Universidad Nacional de Córdoba, Argentina., 2020. Available online: https://www.infostat.com.ar/index.php?mod=page&id=46.
- Reyes Sánchez, E. A.; Argüello Sánchez, J. S. Estudio comparativo entre los métodos diagnósticos para mastitis subclínicas, California Test y DRAMINSKI 4Q en vacas Jersey, Diriamba - Carazo, Agosto-Octubre, 2015.; Tesis Médico Veterinario; Universidad Nacional Agraria: Managua, Nicaragua, 2015. [Google Scholar]
- Trujillo, C. M.; Gallego, A. F.; Ramírez, N.; Palacio, L. G. Prevalencia de mastitis en siete hatos lecheros del oriente antioqueño. Rev. Colomb. Cienc. Pecu. 2011, 24, 11–18. [Google Scholar]
- Sánchez-Herencia, D.; Mamani-Mango, G. D. Mastitis subclínica bovina y factores de riesgo ambientales en pequeños productores de ganado lechero criado en alta montaña. Rev. Inv. Vet. Perú 2022, 33, e20466. [Google Scholar] [CrossRef]
- Castillo, M.; Suniaga, J.; Rojas, G.; Hernández, J. A. Prevalencia de mastitis subclinica en la zona alta del estdo Mérida. Estudio preliminar. Agricult. Andina 2007, 13, 65–70. [Google Scholar]
- Cepero, R. O.; Castillo, J. C.; Salado, R. J.; Monteaguado, J. E. Detección de la mastitis subclínica mediante diferentes técnicas diagnósticas en unidades bovinas. Rev. Produc. Anim. 2006, 18, 1–11. [Google Scholar]
- Shahid, M.; Sabir, N.; Ahmed, I.; Khan, R. W.; Irshad, M.; Rizwan, M.; Ahmed, S. Diagnosis of subclinical mastitis in bovine using conventional methods and electronic detector. ARPN J. Agric. Biol. Sci. 2011, 6, 18–22. [Google Scholar]
- Kaşikci, G.; Cetin, O.; Bingol, B. E.; Gunduz, M. C. Relations between electrical conductivity, somatic cell count, California mastitis test and some quality parameters in the diagnosis of subclinical mastitis in dairy cows. Turk. J. Vet. Anim. Sci. 2012, 36, 49–55. [Google Scholar] [CrossRef]
- Mohammadsadegh, M.; Gharagoslu, F.; Bokaii, S. Study the efficacy of an electric conductivity detector (Mas D Tec) in sub clinical mastitis diagnosis. Vet. J. Islam. 2007, 4, 15–22. [Google Scholar]
- Petzer, I. M.; Karzis, J.; Meyer, I. A.; van der Schans, T. J. A cost-benefit model comparing the California Milk Cell Test and Milk Electrical Resistance Test. Onderstepoort J. Vet. Res. 2013, 80, 1–14. [Google Scholar] [CrossRef]
- Khatun, M.; Clark, C. E. F.; Lyons, N. A.; Thomson, P. C.; Kerrisk, K. L.; García, S. C. Early detection of clinical mastitis from electrical conductivity data in an automatic milking system. Anim. Produc. Sc. 2017, 57, 1226–1232. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).