Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
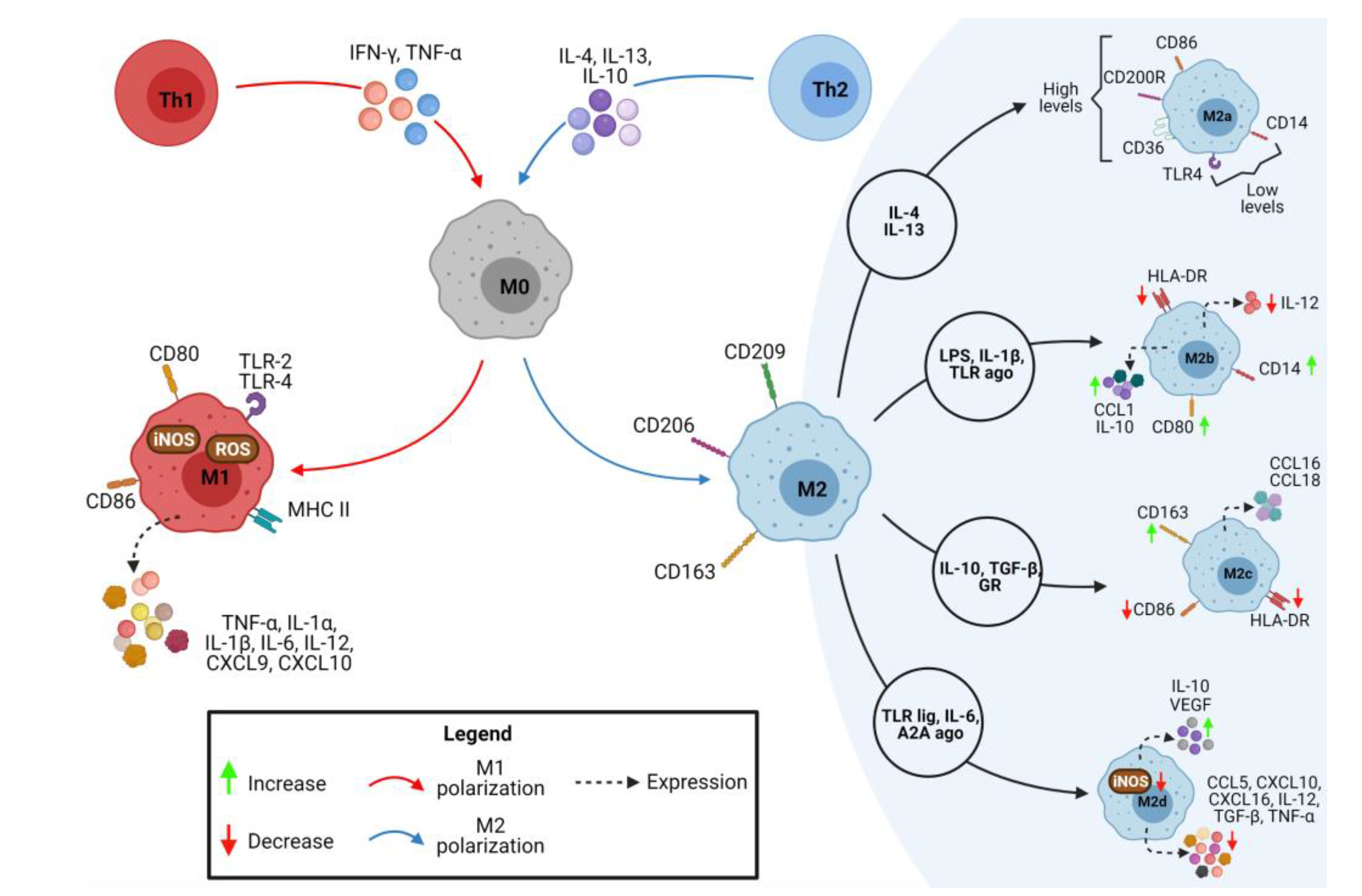
2. Macrophages M1/M2 Polarization
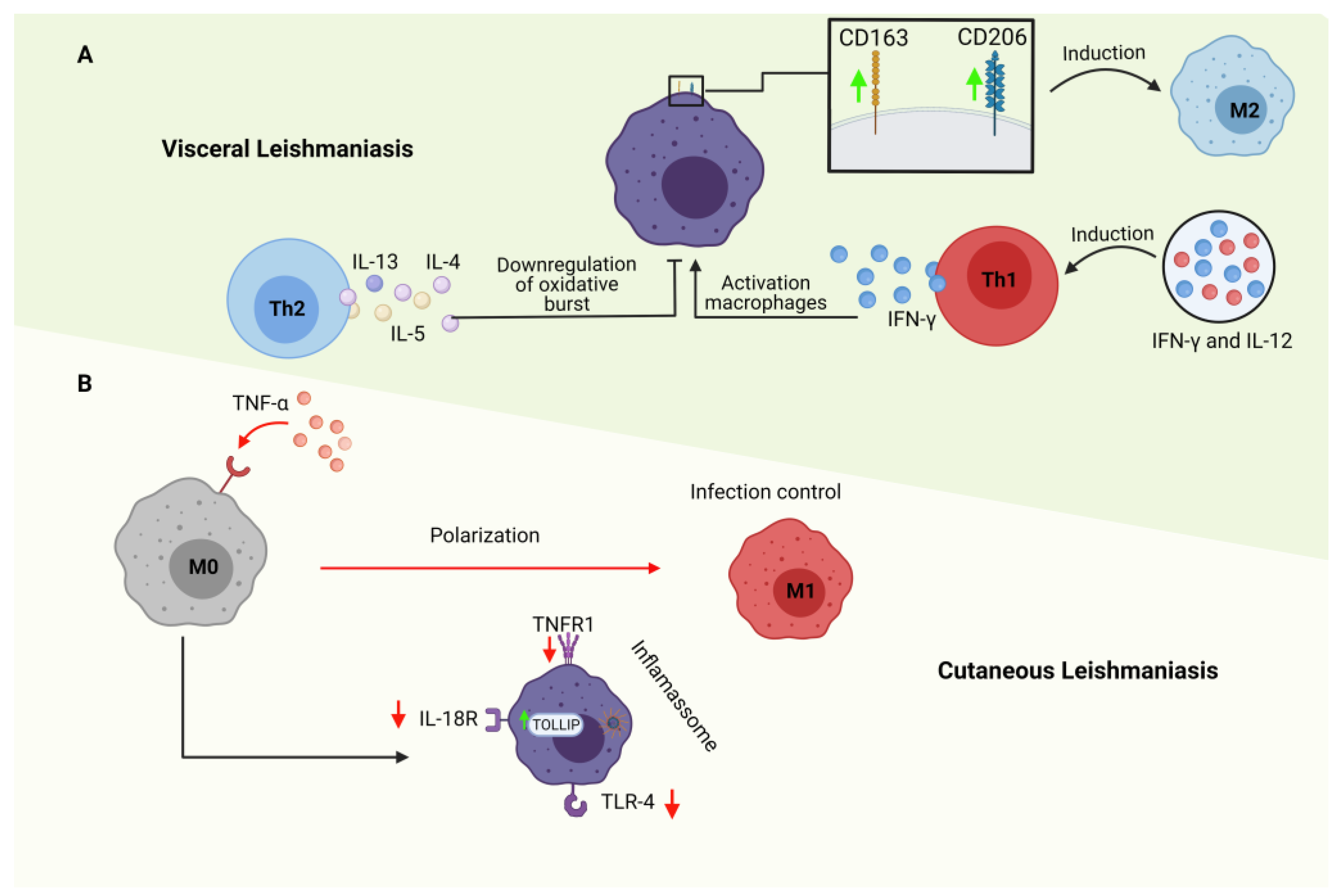
3. M1 and M2 Macrophages in Cutaneous Leishmaniasis
4. M1 and M2 Macrophages in Visceral Leishmaniasis
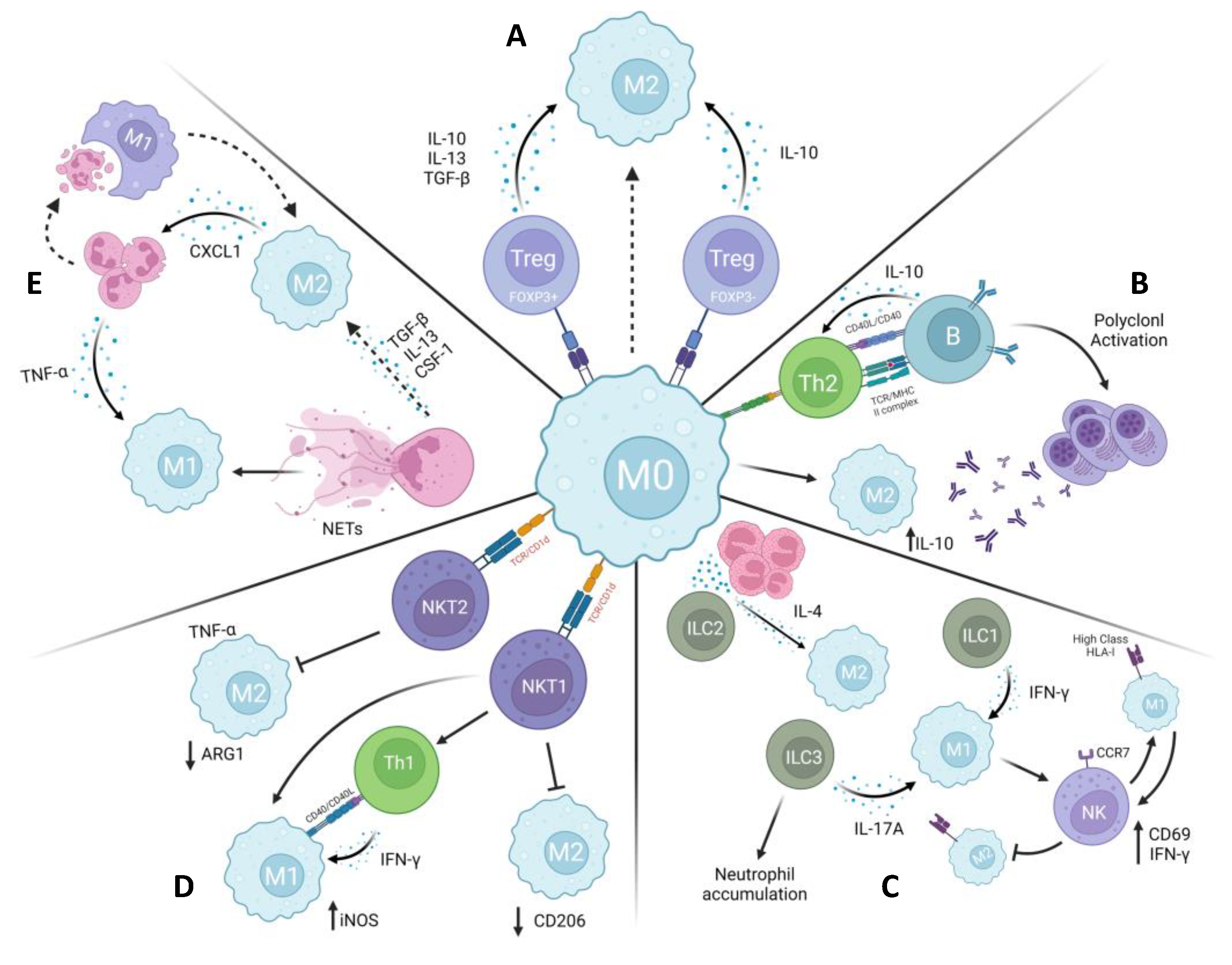
6. Leishmaniasis: Immune Cells Crosstalk in Macrophage Polarization
6.1. ILCs
6.2. NKT
6.3. Neutrophils
6.4. T regulatory cells
6.5. B Cells
7. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rycker, M.; Baragaña, B.; Duce, S.L.; Gilbert, I.H. Challenges and recent progress in drug discovery for tropical diseases. Nature 2018, 559, 498–506. [Google Scholar] [CrossRef]
- Holzmuller, P.; Geiger, A.; Nzoumbou-Boko, R.; Pissarra, J.; Hamrouni, S.; Rodrigues, V.; Dauchy, F.A.; Lemesre, J.L.; Vincendeau, P.; Bras-Gonçalves, R. Trypanosomatid Infections: How Do Parasites and Their Excreted-Secreted Factors Modulate the Inducible Metabolism of l-Arginine in Macrophages? Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The history of leishmaniasis. Parasit. Vectors 2017, 10, 1–10. [Google Scholar] [CrossRef]
- Arenas, R.; Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J. Leishmaniasis: A review. F1000Research 2017, 6. [Google Scholar] [CrossRef]
- Organization, P.A.H. Leishmaniases. Epidemiological Report of the Americas, December 2020. Leishmaniases Report;9, 20 December.
- Herrera, G.; Barragán, N.; Luna, N.; Martínez, D.; De Martino, F.; Medina, J.; Niño, S.; Páez, L.; Ramírez, A.; Vega, L.; et al. An interactive database of Leishmania species distribution in the Americas. Sci. data 2020, 7. [Google Scholar] [CrossRef]
- Kobets, T.; Čepičková, M.; Volkova, V.; Sohrabi, Y.; Havelková, H.; Svobodová, M.; Demant, P.; Lipoldová, M. Novel Loci Controlling Parasite Load in Organs of Mice Infected With Leishmania major, Their Interactions and Sex Influence. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Das, S.; Kamran, M.; Ejazi, S.A.; Ali, N. The pathogenicity and virulence of Leishmania - interplay of virulence factors with host defenses. Virulence 2022, 13, 903. [Google Scholar] [CrossRef]
- WHO Leishmaniasis. World Heal. Organ. 2023.
- Lipoldová, M.; Demant, P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat. Rev. Genet. 2006, 7, 294–305. [Google Scholar] [CrossRef]
- Serafim, T.D.; Coutinho-Abreu, I. V.; Oliveira, F.; Meneses, C.; Kamhawi, S.; Valenzuela, J.G. Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nat. Microbiol. 2018, 3, 548–555. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, S. da C.; Pessoa-e-Silva, R.; Trajano-Silva, L.A.M.; de Goes, T.C.; de Morais, R.C.S.; Oliveira, C.N. d. C.; de Lorena, V.M.B.; de Paiva-Cavalcanti, M. The Equivocal Role of Th17 Cells and Neutrophils on Immunopathogenesis of Leishmaniasis. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- De Menezes, J.P.; Saraiva, E.M.; Da Rocha-Azevedo, B. The site of the bite: Leishmania interaction with macrophages, neutrophils and the extracellular matrix in the dermis. Parasit. Vectors 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.B.; Vaz, L.G.; Afonso, L.C.C.; Horta, M.F.; Vieira, L.Q. Regulation of macrophage subsets and cytokine production in leishmaniasis. Cytokine 2021, 147. [Google Scholar] [CrossRef] [PubMed]
- Tomiotto-Pellissier, F.; Bortoleti, B.T. da S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Malta-Santos, H.; França-Costa, J.; Macedo, A.; Queiroz, A.T.L.; Fukutani, K.F.; Muxel, S.M.; Khouri, R.; Van Weyenbergh, J.; Boaventura, V.; Barral, A.; et al. Differential expression of polyamine biosynthetic pathways in skin lesions and in plasma reveals distinct profiles in diffuse cutaneous leishmaniasis. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Porta, C.; Riboldi, E.; Ippolito, A.; Sica, A. Molecular and epigenetic basis of macrophage polarized activation. Semin. Immunol. 2015, 27, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 7820–7825. [Google Scholar] [CrossRef]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Artyomov, M.N.; Sergushichev, A.; Schilling, J.D. Integrating immunometabolism and macrophage diversity. Semin. Immunol. 2016, 28, 417–424. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.B.; Lopes, M.E.; Hohman, L.S.; Romano, A.; David, B.A.; Kratofil, R.; Kubes, P.; Workentine, M.L.; Campos, A.C.; Vieira, L.Q.; et al. Th1-Th2 Cross-Regulation Controls Early Leishmania Infection in the Skin by Modulating the Size of the Permissive Monocytic Host Cell Reservoir. Cell Host Microbe 2020, 27, 752–768. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [CrossRef]
- van Teijlingen Bakker, N.; Pearce, E.J. Cell-intrinsic metabolic regulation of mononuclear phagocyte activation: Findings from the tip of the iceberg. Immunol. Rev. 2020, 295, 54–67. [Google Scholar] [CrossRef]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.M.; Castoldi, A.; Sanin, D.E.; Flachsmann, L.J.; Field, C.S.; Puleston, D.J.; Kyle, R.L.; Patterson, A.E.; Hässler, F.; Buescher, J.M.; et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 2019, 20, 420–432. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Breda, C.N. de S.; Davanzo, G.G.; Basso, P.J.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26. [Google Scholar] [CrossRef]
- Ryan, D.G.; O’Neill, L.A.J. Krebs cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett. 2017, 591, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Ligthart-Melis, G.C.; Van De Poll, M.C.G.; Boelens, P.G.; Dejong, C.H.C.; Deutz, N.E.P.; Van Leeuwen, P.A.M. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am. J. Clin. Nutr. 2008, 87, 1282–1289. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.S.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Ty, M.C.; Loke, P.; Alberola, J.; Rodriguez-Cortes, A.; Rodriguez-Cortes, A. Immuno-metabolic profile of human macrophages after Leishmania and Trypanosoma cruzi infection. PLoS One 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pandey, R.K.; Siqueira-Neto, J.L.; Kwon, Y.J.; Freitas-Junior, L.H.; Shaha, C.; Madhubala, R. Proteomic-based approach to gain insight into reprogramming of THP-1 cells exposed to Leishmania donovani over an early temporal window. Infect. Immun. 2015, 83, 1853–1868. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Hassani, K.; da Silva Lira Filho, A.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef]
- Soulat, D.; Bogdan, C. Function of Macrophage and Parasite Phosphatases in Leishmaniasis. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Bogdan, C. Macrophages as host, effector and immunoregulatory cells in leishmaniasis: Impact of tissue micro-environment and metabolism. Cytokine X 2020, 2. [Google Scholar] [CrossRef]
- Schleicher, U.; Paduch, K.; Debus, A.; Obermeyer, S.; König, T.; Kling, J.C.; Ribechini, E.; Dudziak, D.; Mougiakakos, D.; Murray, P.J.; et al. TNF-Mediated Restriction of Arginase 1 Expression in Myeloid Cells Triggers Type 2 NO Synthase Activity at the Site of Infection. Cell Rep. 2016, 15, 1062–1075. [Google Scholar] [CrossRef]
- Hu, S.; Marshall, C.; Darby, J.; Wei, W.; Lyons, A.B.; Körner, H. Absence of Tumor Necrosis Factor Supports Alternative Activation of Macrophages in the Liver after Infection with Leishmania major. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Silva, L.L. de L.; Gomes, R.S.; Silva, M.V.T.; Joosten, L.A.B.; Ribeiro-Dias, F. IL-15 enhances the capacity of primary human macrophages to control Leishmania braziliensis infection by IL-32/vitamin D dependent and independent pathways. Parasitol. Int. 2020, 76. [Google Scholar] [CrossRef]
- Lecoeur, H.; Prina, E.; Rosazza, T.; Kokou, K.; N’Diaye, P.; Aulner, N.; Varet, H.; Bussotti, G.; Xing, Y.; Milon, G.; et al. Targeting Macrophage Histone H3 Modification as a Leishmania Strategy to Dampen the NF-κB/NLRP3-Mediated Inflammatory Response. Cell Rep. 2020, 30, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Maran, N.; Gomes, P.S.; Freire-De-Lima, L.; Freitas, E.O.; Freire-De-Lima, C.G.; Morrot, A. Host resistance to visceral leishmaniasis: prevalence and prevention. Expert Rev. Anti. Infect. Ther. 2016, 14, 435–442. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Diro, E. Visceral leishmaniasis. Infect. Dis. Clin. North Am. 2012, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.L.; de Oliveira, F.A.; Santos, M.L.B.; Cunha, L.C.S.; Lino, M.T.B.; de Oliveira, M.F.S.; Bomfim, M.O.M.; Silva, A.M.; de Moura, T.R.; de Jesus, A.R.; et al. The Severity of Visceral Leishmaniasis Correlates with Elevated Levels of Serum IL-6, IL-27 and sCD14. PLoS Negl. Trop. Dis. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Milde, R.; Varin, A.; Melgert, B.N.; Draijer, C.; Thomas, B.; Fabbri, M.; Crawshaw, A.; Ho, L.P.; et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013, 121. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef]
- Maroof, A.; Kaye, P.M. Temporal regulation of interleukin-12p70 (IL-12p70) and IL-12-related cytokines in splenic dendritic cell subsets during Leishmania donovani infection. Infect. Immun. 2008, 76, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Lopez Kostka, S.; Siebenhaar, F.; Moelle, K.; Metz, M.; Knop, J.; Stebut, E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006, 20, 2460–2467. [Google Scholar] [CrossRef]
- Khadem, F.; Uzonna, J.E. Immunity to visceral leishmaniasis: implications for immunotherapy. Future Microbiol. 2014, 9, 901–915. [Google Scholar] [CrossRef]
- Moreira, P.R.R.; Fernando, F.S.; Montassier, H.J.; André, M.R.; de Oliveira Vasconcelos, R. Polarized M2 macrophages in dogs with visceral leishmaniasis. Vet. Parasitol. 2016, 226, 69–73. [Google Scholar] [CrossRef]
- Kong, F.; Saldarriaga, O.A.; Spratt, H.; Osorio, E.Y.; Travi, B.L.; Luxon, B.A.; Melby, P.C. Transcriptional Profiling in Experimental Visceral Leishmaniasis Reveals a Broad Splenic Inflammatory Environment that Conditions Macrophages toward a Disease-Promoting Phenotype. PLoS Pathog. 2017, 13. [Google Scholar] [CrossRef]
- Vivier, E. The discovery of innate lymphoid cells. Nat. Rev. Immunol. 2021 2110 2021, 21, 616–616. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, O.L.; Lugo, D.A.; Cabrera, M.; Sánchez, M.A.; Zerpa, O.; Tapia, F.J. Innate lymphoid cells in peripheral blood of patients with American Cutaneous Leishmaniasis. Exp. Dermatol. 2021, 30, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Arancibia-Cárcamo, C. V.; Fleming, M.P.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.L.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Van Der Gracht, E.; Zahner, S.; Kronenberg, M. When Insult Is Added to Injury: Cross Talk between ILCs and Intestinal Epithelium in IBD. Mediators Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Sasse, C.; Barinberg, D.; Obermeyer, S.; Debus, A.; Schleicher, U.; Bogdan, C. Eosinophils, but Not Type 2 Innate Lymphoid Cells, Are the Predominant Source of Interleukin 4 during the Innate Phase of Leishmania major Infection. Pathog. (Basel, Switzerland) 2022, 11. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Lopes, M.E.; dos Santos, L.M.; Sacks, D.; Vieira, L.Q.; Carneiro, M.B. Resistance Against Leishmania major Infection Depends on Microbiota-Guided Macrophage Activation. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Gimblet, C.; Meisel, J.S.; Loesche, M.A.; Cole, S.D.; Horwinski, J.; Novais, F.O.; Misic, A.M.; Bradley, C.W.; Beiting, D.P.; Rankin, S.C.; et al. Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation. Cell Host Microbe 2017, 22, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Carvalho, A.M.; Sacramento, L.A.; Grice, E.A.; Scott, P. Microbiota instruct IL-17A-producing innate lymphoid cells to promote skin inflammation in cutaneous leishmaniasis. PLoS Pathog. 2021, 17. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Barin, J.G.; Baldeviano, G.C.; Talor, M. V.; Wu, L.; Ong, S.; Quader, F.; Chen, P.; Zheng, D.; Caturegli, P.; Rose, N.R.; et al. Macrophages participate in IL-17-mediated inflammation. Eur. J. Immunol. 2012, 42, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Jin, S.P.; Jang, S.; Choi, J.Y.; Chung, D.H.; Lee, D.H.; Kim, K.H.; Kim, H.Y. IL-17A-Producing Innate Lymphoid Cells Promote Skin Inflammation by Inducing IL-33-Driven Type 2 Immune Responses. J. Invest. Dermatol. 2020, 140, 827–837. [Google Scholar] [CrossRef]
- Alshaweesh, J.; Nakamura, R.; Tanaka, Y.; Hayashishita, M.; Musa, A.; Kikuchi, M.; Inaoka, D.K.; Hamano, S. Leishmania major Strain-Dependent Macrophage Activation Contributes to Pathogenicity in the Absence of Lymphocytes. Microbiol. Spectr. 2022, 10. [Google Scholar] [CrossRef]
- Covre, L.P.; Devine, O.P.; Garcia de Moura, R.; Vukmanovic-Stejic, M.; Dietze, R.; Ribeiro-Rodrigues, R.; Guedes, H.L. de M.; Lubiana Zanotti, R.; Falqueto, A.; Akbar, A.N.; et al. Compartmentalized cytotoxic immune response leads to distinct pathogenic roles of natural killer and senescent CD8+ T cells in human cutaneous leishmaniasis. Immunology 2020, 159, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Bhattacharya, P.; Oljuskin, T.; Dey, R.; Gannavaram, S.; Satoskar, A.R.; Nakhasi, H.L. Determinants of Innate Immunity in Visceral Leishmaniasis and Their Implication in Vaccine Development. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Bellora, F.; Castriconi, R.; Dondero, A.; Reggiardo, G.; Moretta, L.; Mantovani, A.; Moretta, A.; Bottino, C. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 21659–21664. [Google Scholar] [CrossRef]
- Ferraz, R.; Cunha, C.F.; Pimentel, M.I.F.; Lyra, M.R.; Pereira-Da-Silva, T.; Schubach, A.O.; Da-Cruz, A.M.; Bertho, A.L. CD3+CD4negCD8neg (double negative) T lymphocytes and NKT cells as the main cytotoxic-related-CD107a+ cells in lesions of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. Parasit. Vectors 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.F.; Ferraz-Nogueira, R.; Costa, V.F.A.; Pimentel, M.I.F.; Chometon, T.Q.; Lyra, M.R.; Schubach, A.O.; Da-Cruz, A.M.; Bertho, A.L. Contribution of Leishmania braziliensis antigen-specific CD4+ T, CD8+ T, NK and CD3+CD56+NKT cells in the immunopathogenesis of cutaneous leishmaniasis patients: Cytotoxic, activation and exhaustion profiles. PLoS One 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Hentges, F.; Zimmer, J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chhatar, S.; Mishra, A.; Lal, G. Natural killer T cell activation increases iNOS+CD206- M1 macrophage and controls the growth of solid tumor. J. Immunother. cancer 2019, 7. [Google Scholar] [CrossRef]
- Zamora-Chimal, J.; Hernández-Ruiz, J.; Becker, I. NKT cells in leishmaniasis. Immunobiology 2017, 222, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Chimal, J.; Fernández-Figueroa, E.A.; Ruiz-Remigio, A.; Wilkins-Rodríguez, A.A.; Delgado-Domínguez, J.; Salaiza-Suazo, N.; Gutiérrez-Kobeh, L.; Becker, I. NKT cell activation by Leishmania mexicana LPG: Description of a novel pathway. Immunobiology 2017, 222, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.P.; Cruz, M.S.; Cardoso, A.P.; Oliveira, M.J.; Macedo, M.F. Human iNKT Cells Modulate Macrophage Survival and Phenotype. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Shivam, P.; Hansa, J.; Jamal, F.; Singh, M.K.; Bimal, S.; Narayan, S.; Pandey, K.; Das, V.N.R.; Das, P.; et al. CD8dim but not CD8bright cells positive to CD56 dominantly express KIR and are cytotoxic during visceral leishmaniasis. Hum. Immunol. 2018, 79, 616–620. [Google Scholar] [CrossRef]
- Kumari, S.; Shivam, P.; Kumar, S.; Jamal, F.; Singh, M.K.; Bimal, S.; Narayan, S.; Pandey, K.; Das, V.N.R.; Das, P.; et al. Leishmania donovani mediated higher expression of CCL4 induces differential accumulation of CD4+CD56+NKT and CD8+CD56+NKT cells at infection site. Cytokine 2018, 110, 306–315. [Google Scholar] [CrossRef]
- Gois, B.M.; Peixoto, R.F.; Maciel, B.L.L.; Gomes, J.A.S.; de Azevedo, F.L.A.A.; Veras, R.C.; de Medeiros, I.A.; de Lima Grisi, T.C.S.; de Araújo, D.A.M.; do Amaral, I.P.G.; et al. Dual immune effect of iNKT cells considering human cutaneous and visceral leishmaniasis: An example of cell plasticity according to different disease scenarios. Scand. J. Immunol. 2018, 87. [Google Scholar] [CrossRef]
- Cruz, M.S.; Loureiro, J.P.; Oliveira, M.J.; Macedo, M.F. The iNKT Cell-Macrophage Axis in Homeostasis and Disease. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Beattie, L.; Svensson, M.; Bune, A.; Brown, N.; Maroof, A.; Zubairi, S.; Smith, K.R.; Kaye, P.M. Leishmania donovani-induced expression of signal regulatory protein alpha on Kupffer cells enhances hepatic invariant NKT-cell activation. Eur. J. Immunol. 2010, 40, 117–123. [Google Scholar] [CrossRef]
- Grabarz, F.; Aguiar, C.F.; Correa-Costa, M.; Braga, T.T.; Hyane, M.I.; Andrade-Oliveira, V.; Landgraf, M.A.; Câmara, N.O.S. Protective role of NKT cells and macrophage M2-driven phenotype in bleomycin-induced pulmonary fibrosis. Inflammopharmacology 2018, 26, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.B.; Deniset, J.F.; Kubes, P. Neutrophils in homeostasis and tissue repair. Int. Immunol. 2022, 34, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010, 87, 93–106. [Google Scholar] [CrossRef]
- Bouchery, T.; Harris, N. Neutrophil–macrophage cooperation and its impact on tissue repair. Immunol. Cell Biol. 2019, 97, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, W.; Millman, A.; Craft, J.F.; Chen, E.; Patel, N.; Boucher, J.L.; Urban, J.F.; Kim, C.C.; Gause, W.C. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 2014, 15, 938–946. [Google Scholar] [CrossRef]
- Braza, M.S.; Conde, P.; Garcia, M.; Cortegano, I.; Brahmachary, M.; Pothula, V.; Fay, F.; Boros, P.; Werner, S.A.; Ginhoux, F.; et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am. J. Transplant 2018, 18, 1247–1255. [Google Scholar] [CrossRef]
- Marwick, J.A.; Mills, R.; Kay, O.; Michail, K.; Stephen, J.; Rossi, A.G.; Dransfield, I.; Hirani, N. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-κB activation. Cell Death Dis. 2018 96 2018, 9, 1–13. [Google Scholar] [CrossRef]
- D’alessandro, S.; Parapini, S.; Corbett, Y.; Frigerio, R.; Delbue, S.; Modenese, A.; Gramiccia, M.; Ferrante, P.; Taramelli, D.; Basilico, N. Leishmania Promastigotes Enhance Neutrophil Recruitment through the Production of CXCL8 by Endothelial Cells. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Passelli, K.; Billion, O.; Tacchini-Cottier, F. The Impact of Neutrophil Recruitment to the Skin on the Pathology Induced by Leishmania Infection. Front. Immunol. 2021, 12, 446. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Regli, I.B.; Tacchini-Cottier, F. Different Leishmania Species Drive Distinct Neutrophil Functions. Trends Parasitol. 2016, 32, 392–401. [Google Scholar] [CrossRef]
- Conceição, J.; Davis, R.; Carneiro, P.P.; Giudice, A.; Muniz, A.C.; Wilson, M.E.; Carvalho, E.M.; Bacellar, O. Characterization of Neutrophil Function in Human Cutaneous Leishmaniasis Caused by Leishmania braziliensis. PLoS Negl. Trop. Dis. 2016, 10, e0004715. [Google Scholar] [CrossRef] [PubMed]
- Rochael, N.C.; Guimarães-Costa, A.B.; Nascimento, M.T.C.; Desouza-Vieira, T.S.; Oliveira, M.P.; Garciae Souza, L.F.; Oliveira, M.F.; Saraiva, E.M. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Gomes, F.L.; Moniz-de-Souza, M.C.A.; Alexandre-Moreira, M.S.; Dias, W.B.; Lopes, M.F.; Nunes, M.P.; Lungarella, G.; DosReis, G.A. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J. Immunol. 2007, 179, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Y.; Zheng, J. The State of Art of Extracellular Traps in Protozoan Infections (Review). Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Novais, F.O.; Santiago, R.C.; Báfica, A.; Khouri, R.; Afonso, L.; Borges, V.M.; Brodskyn, C.; Barral-Netto, M.; Barral, A.; de Oliveira, C.I. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J. Immunol. 2009, 183, 8088–8098. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, F.L.; Sacks, D. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2012, 2, 59. [Google Scholar] [CrossRef]
- Ricci-Azevedo, R.; Oliveira, A.F.; Conrado, M.C.A.V.; Carvalho, F.C.; Roque-Barreira, M.C. Neutrophils Contribute to the Protection Conferred by ArtinM against Intracellular Pathogens: A Study on Leishmania major. PLoS Negl. Trop. Dis. 2016, 10, e0004609. [Google Scholar] [CrossRef]
- Gagliani, N.; Amezcua Vesely, M.C.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; De Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Newson, J.; Stables, M.; Karra, E.; Arce-Vargas, F.; Quezada, S.; Motwani, M.; Mack, M.; Yona, S.; Audzevich, T.; Gilroy, D.W. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood 2014, 124, 1748–1764. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M.; DiPaolo, R.A.; Andersson, J.; Zhao, D.M.; Stephens, G.L.; Thornton, A.M. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 2006, 212, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Fiorentino, M.; Giunchi, F.; Eriksson, M.; Erlandsson, A.; Sundqvist, P.; Carlsson, J. Infiltration of M2 Macrophages and Regulatory T Cells Plays a Role in Recurrence of Renal Cell Carcinoma. Eur. Urol. open Sci. 2020, 20, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakamura, K.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J. Leukoc. Biol. 2012, 92, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Divenuto, F.; Pavia, G.; Marascio, N.; Barreca, G.S.; Quirino, A.; Matera, G. Role of Treg, Breg and other cytokine sets in host protection and immunopathology during human leishmaniasis: Are they potential valuable markers in clinical settings and vaccine evaluation? Acta Trop. 2023, 240. [Google Scholar] [CrossRef]
- Rostami, M.N.; Khamesipour, A. Potential biomarkers of immune protection in human leishmaniasis. Med. Microbiol. Immunol. 2021, 210, 81–100. [Google Scholar] [CrossRef]
- Jin, Q.; Gui, L.; Niu, F.; Yu, B.; Lauda, N.; Liu, J.; Mao, X.; Chen, Y. Macrophages in keloid are potent at promoting the differentiation and function of regulatory T cells. Exp. Cell Res. 2018, 362, 472–476. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, K.; Rajalingam, R.; Martin, S.; Pal, C. Cytokines in the generation and function of regulatory T cell subsets in leishmaniasis. Cytokine 2021, 147. [Google Scholar] [CrossRef]
- Bunn, P.T.; Montes de Oca, M.; de Labastida Rivera, F.; Kumar, R.; Ng, S.S.; Edwards, C.L.; Faleiro, R.J.; Sheel, M.; Amante, F.H.; Frame, T.C.M.; et al. Distinct Roles for CD4+ Foxp3+ Regulatory T Cells and IL-10-Mediated Immunoregulatory Mechanisms during Experimental Visceral Leishmaniasis Caused by Leishmania donovani. J. Immunol. 2018, 201, 3362–3372. [Google Scholar] [CrossRef] [PubMed]
- Medina-Colorado, A.A.; Osorio, E.Y.; Saldarriaga, O.A.; Travi, B.L.; Kong, F.; Spratt, H.; Soong, L.; Melby, P.C. Splenic CD4+ T Cells in Progressive Visceral Leishmaniasis Show a Mixed Effector-Regulatory Phenotype and Impair Macrophage Effector Function through Inhibitory Receptor Expression. PLoS One 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Roy, S.; Ukil, A. Cytokines and Signaling Networks Regulating Disease Outcomes in Leishmaniasis. Infect. Immun. 2022, 90. [Google Scholar] [CrossRef] [PubMed]
- Proto, W.R.; Coombs, G.H.; Mottram, J.C. Cell death in parasitic protozoa: regulated or incidental? Nat. Rev. Microbiol. 2013, 11, 58–66. [Google Scholar] [CrossRef] [PubMed]
- A, A.; N, M.; F, G. Wound healing in cutaneous leishmaniasis: A double edged sword of IL-10 and TGF-β. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51. [Google Scholar] [CrossRef]
- Matera, G.; Torti, C.; Mazzitelli, M.; Greco, G.; Rania, A.; Peronace, C.; Settembre, P.; Galati, L.; Giancotti, A.; Lamberti, A.G.; et al. Depression of lymphocyte activity during cutaneous leishmaniasis: a case report. Diagn. Microbiol. Infect. Dis. 2018, 92, 230–234. [Google Scholar] [CrossRef]
- Costa, D.L.; Guimarães, L.H.; Cardoso, T.M.; Queiroz, A.; Lago, E.; Roselino, A.M.; Bacellar, O.; Carvalho, E.M.; Silva, J.S. Characterization of regulatory T cell (Treg) function in patients infected with Leishmania braziliensis. Hum. Immunol. 2013, 74, 1491–1500. [Google Scholar] [CrossRef]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef]
- Biomed, D.A.; Sci, J.; Res, T. Trend of Regulatory T-Cells in the Pathogenesisof Leishmania Infection. Biomed. J. Sci. Tech. Res. 2018, 10, 001–003. [Google Scholar] [CrossRef]
- Croft, M.; Joseph, S.B.; Miner, K.T. Partial activation of naive CD4 T cells and tolerance induction in response to peptide presented by resting B cells. J. Immunol. 1997, 159, 3257–3265. [Google Scholar] [CrossRef]
- Cassell, D.J.; Schwartz, R.H. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J. Exp. Med. 1994, 180, 1829–1840. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Mohler, K.M.; Shanebeck, K.D.; Baum, P.R.; Picha, K.S.; Otten-Evans, C.A.; Janeway, C.A.; Grabstein, K.H. Induction of B cell costimulatory function by recombinant murine CD40 ligand. Eur. J. Immunol. 1994, 24, 116–123. [Google Scholar] [CrossRef]
- Hoehlig, K.; Lampropoulou, V.; Roch, T.; Neves, P.; Calderon-Gomez, E.; Anderton, S.M.; Steinhoff, U.; Fillatreau, S. Immune regulation by B cells and antibodies a view towards the clinic. Adv. Immunol. 2008, 98, 1–38. [Google Scholar] [CrossRef]
- Malynn, B.; Romeo, D.; Wortis, H. Antigen-specific B cells efficiently present low doses of antigen for induction of T cell proliferation - PubMed. J Immunol. 1985, 135, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Silva-Barrios, S.; Charpentier, T.; Stäger, S. The Deadly Dance of B Cells with Trypanosomatids. Trends Parasitol. 2018, 34, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Piccirillo, C.A.; Mendez, S.; Shevach, E.M.; Sacks, D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002, 420, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Oukka, M.; Kuchroo, V.J.; Sacks, D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007, 204, 285–297. [Google Scholar] [CrossRef]
- MINOPRIO, P.; BURLEN, O.; PEREIRA, P.; GUILBERT, B.; ANDRADE, L.; HONTEBEYRIE-JOSKOWICZ, M.; COUTINHO, A. Most B cells in acute Trypanosoma cruzi infection lack parasite specificity. Scand. J. Immunol. 1988, 28, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.; Umekita, L.F. The effect of C3 depletion on the clearance of Trypanosoma cruzi induced by IgG antibodies. Immunol. Lett. 1989, 21, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, V.; Posey, C.; Horauf, A.M.; Solbach, W.; Piessens, W.F.; Harn, D.A. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp. Parasitol. 1996, 84, 168–177. [Google Scholar] [CrossRef]
- Sacks, D.; Scott, P.; Asofsky, R.; Sher, F. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway - PubMed. J Immunol. 1984, 132, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Bollig, N.; Brus<monospace>̈</monospace>tle, A.; Kellner, K.; Ackermann, W.; Abass, E.; Raifer, H.; Camara, B.; Brendel, C.; Giel, G.; Bothur, E.; et al. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 8664–8669. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Good-Jacobson, K.L.; Curtis, J.; Light, A.; Tellier, J.; Shi, W.; Smyth, G.K.; Tarlinton, D.M.; Belz, G.T.; Corcoran, L.M.; et al. Transcription factor IRF4 regulates germinal center cell formation through a B cell-intrinsic mechanism. J. Immunol. 2014, 192, 3200–3206. [Google Scholar] [CrossRef] [PubMed]
- Schaut, R.G.; Lamb, I.M.; Toepp, A.J.; Scott, B.; Mendes-Aguiar, C.O.; Coutinho, J.F. V.; Jeronimo, S.M.B.; Wilson, M.E.; Harty, J.T.; Waldschmidt, T.J.; et al. Regulatory IgDhi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis. J. Immunol. 2016, 196, 4100–4109. [Google Scholar] [CrossRef] [PubMed]
- Menezes Cabral, S.; Leal Silvestre, R.; Moreira Santarém, N.; Costa Tavares, J.; Franco Silva, A.; Cordeiro-da-Silva, A. A Leishmania infantum cytosolic tryparedoxin activates B cells to secrete interleukin-10 and specific immunoglobulin. Immunology 2008, 123, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Ronet, C.; Torre, Y.H.-L.; Revaz-Breton, M.; Mastelic, B.; Tacchini-Cottier, F.; Louis, J.; Launois, P. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J. Immunol. 2010, 184, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Smelt, S.C.; Cotterell, S.E.J.; Engwerda, C.R.; Kaye, P.M. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 2000, 164, 3681–3688. [Google Scholar] [CrossRef]
- Bankoti, R.; Gupta, K.; Levchenko, A.; Stäger, S. Marginal zone B cells regulate antigen-specific T cell responses during infection. J. Immunol. 2012, 188, 3961–3971. [Google Scholar] [CrossRef]
- Deak, E.; Jayakumar, A.; Cho, K.W.; Goldsmith-Pestana, K.; Dondji, B.; Lambris, J.D.; McMahon-Pratt, D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur. J. Immunol. 2010, 40, 1355–1368. [Google Scholar] [CrossRef]
- Silva-Barrios, S.; Smans, M.; Duerr, C.U.; Qureshi, S.T.; Fritz, J.H.; Descoteaux, A.; Stäger, S. Innate Immune B Cell Activation by Leishmania donovani Exacerbates Disease and Mediates Hypergammaglobulinemia. Cell Rep. 2016, 15, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Ouellet, M.; Menasria, R.; Gomez, A.M.; Barat, C.; Tremblay, M.J. Leishmania infantum amastigotes trigger a subpopulation of human B cells with an immunoregulatory phenotype. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.A.; Conrad, S.M.; Alves, R.G.; Jeronimo, S.M.B.; Mosser, D.M. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005, 201, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Louzir, H.; Belal-Kacemi, L.; Sassi, A.; Laouini, D.; Ismail, R.B.; Dellagi, K. Natural autoantibodies, IgG antibodies to tetanus toxoid and CD5+ B cells in patients with Mediterranean visceral leishmaniasis. The Leishmania Study Group. Clin. Exp. Immunol. 1994, 95, 479–484. [Google Scholar] [CrossRef]
- Rodrigues, V.; Laforge, M.; Campillo-Gimenez, L.; Soundaramourty, C.; Correia-de-Oliveira, A.; Dinis-Oliveira, R.J.; Ouaissi, A.; Cordeiro-da-Silva, A.; Silvestre, R.; Estaquier, J. Abortive T follicular helper development is associated with a defective humoral response in Leishmania infantum-infected macaques. PLoS Pathog. 2014, 10. [Google Scholar] [CrossRef]
- Bénard, A.; Sakwa, I.; Schierloh, P.; Colom, A.; Mercier, I.; Tailleux, L.; Jouneau, L.; Boudinot, P.; Al-Saati, T.; Lang, R.; et al. B Cells Producing Type I IFN Modulate Macrophage Polarization in Tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 197, 801–813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




