Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
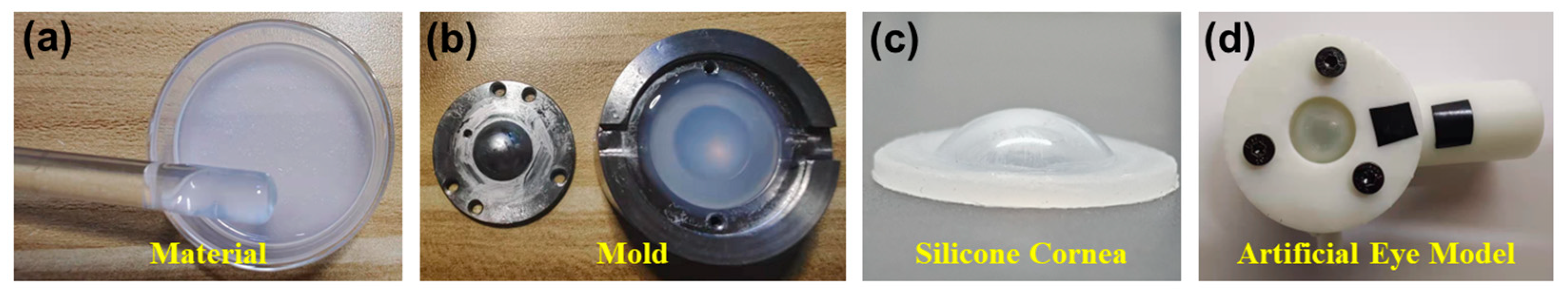
2.1. Artificial Eye Model
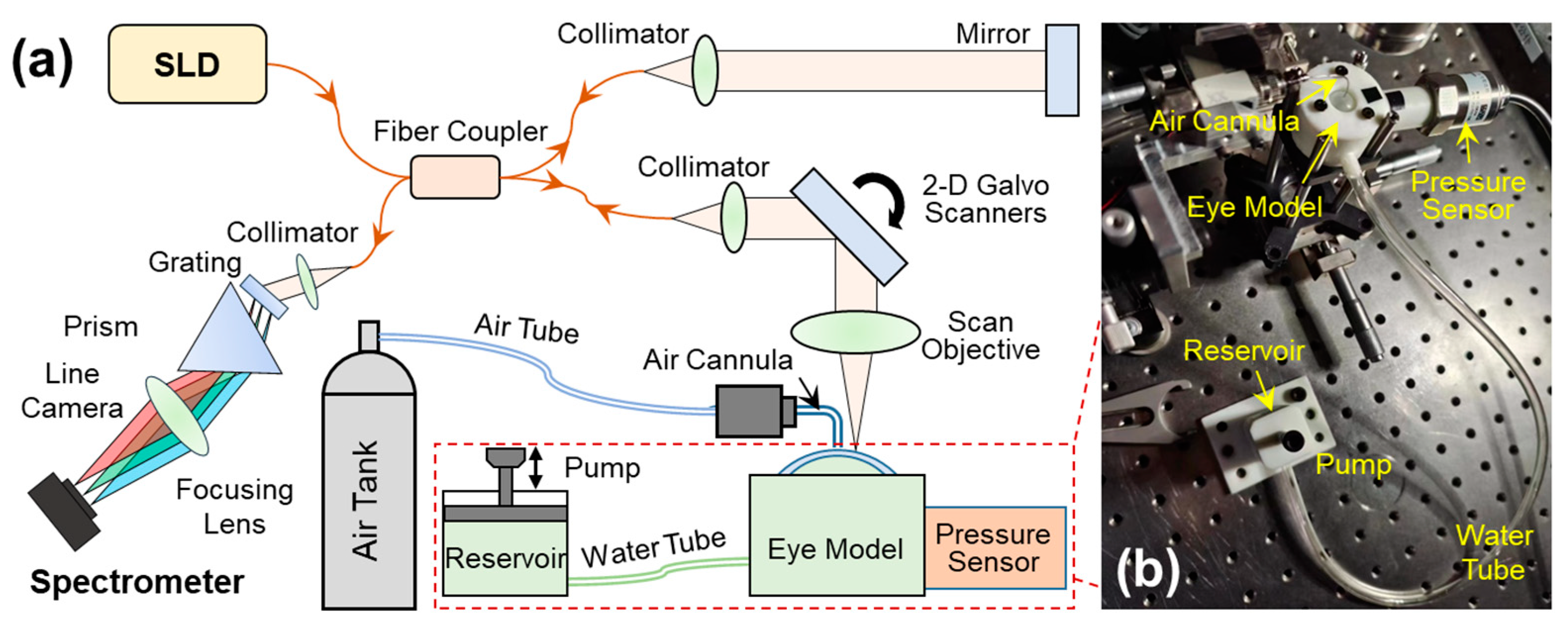
2.2. OCE System Set-Up
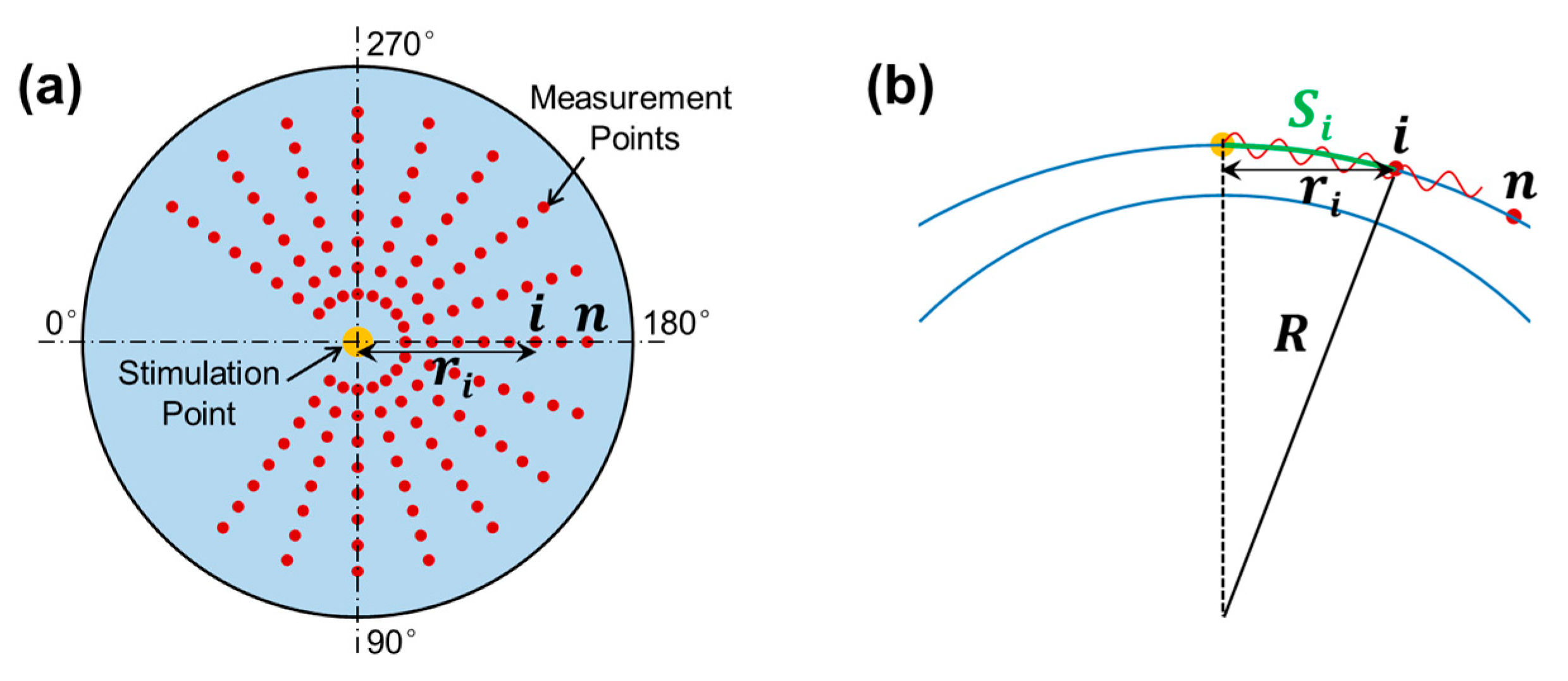
2.3. M-B Mode Radial Scan Patten
3. Results
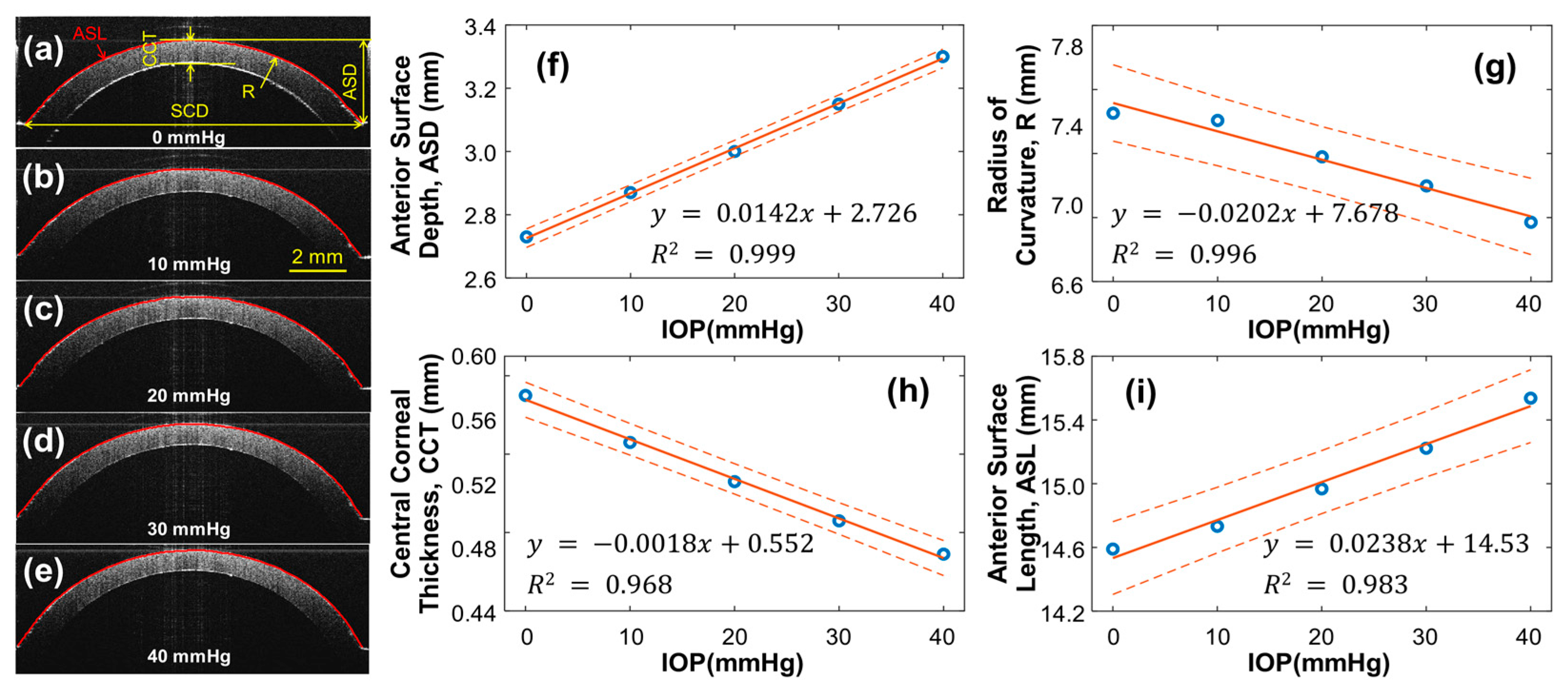
3.1. Geometry of the Silicone Cornea in Association with IOP Values
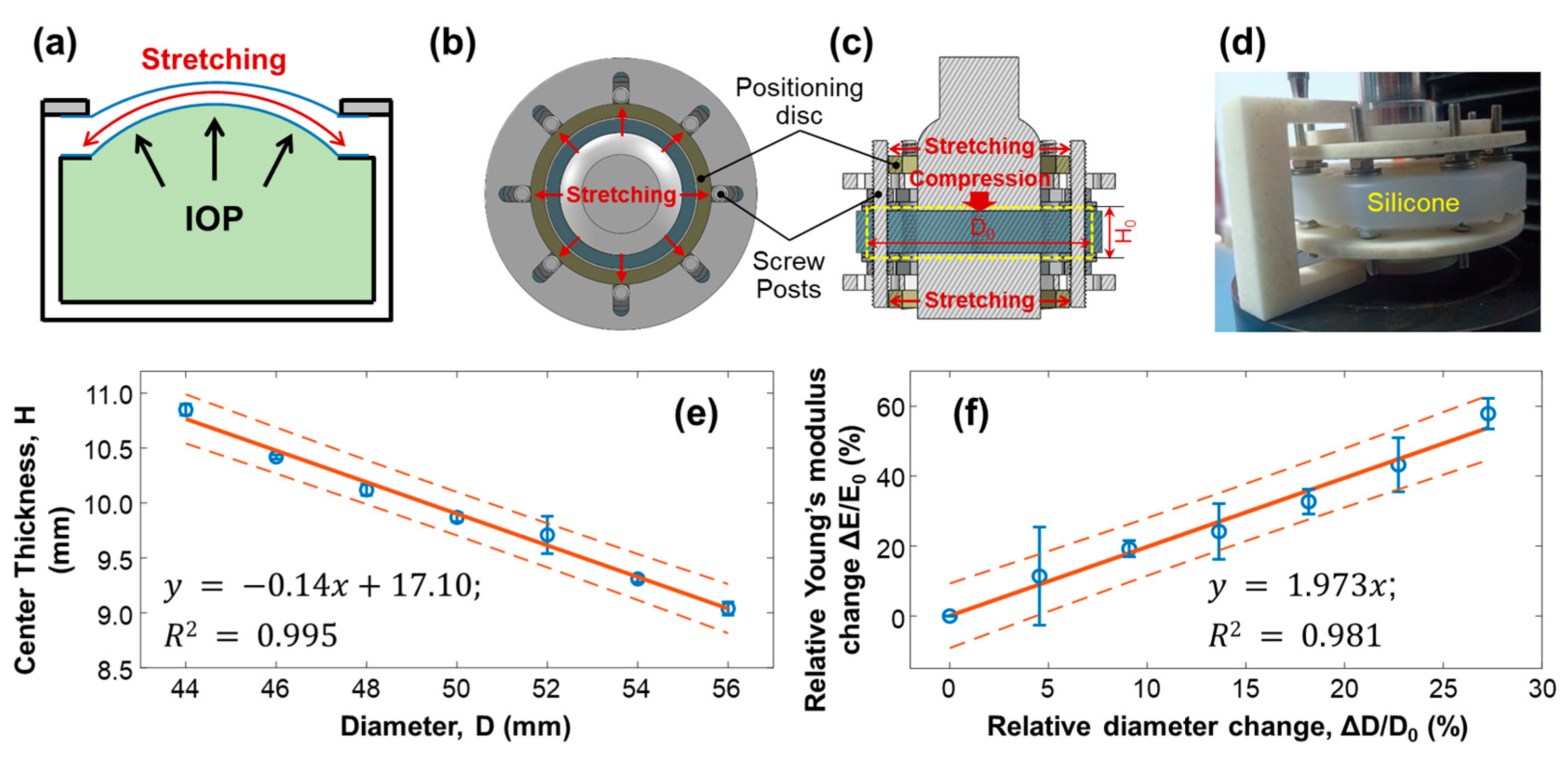
3.2. Effect of Stretching on Young’s Modulus of Silicone Material
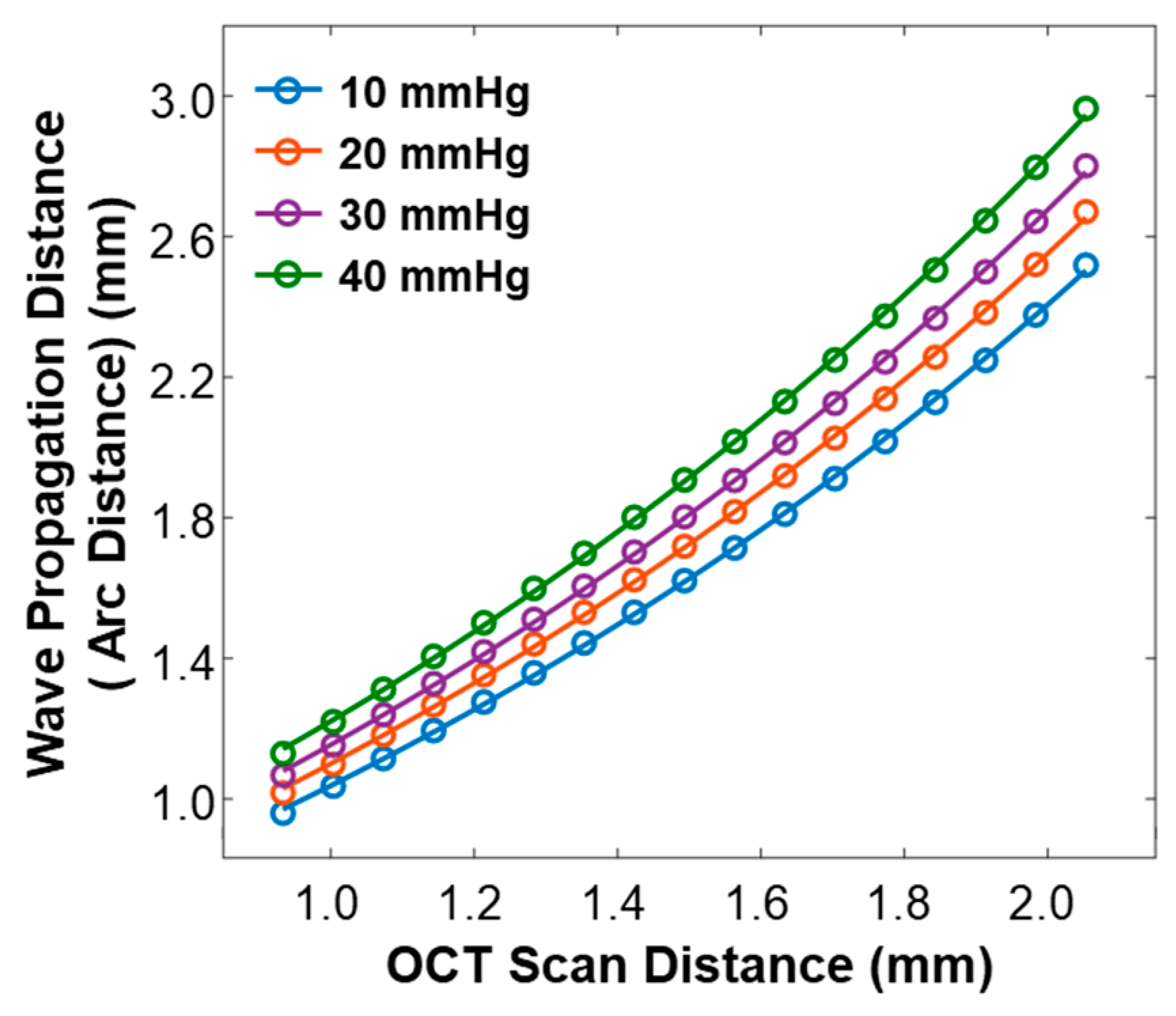
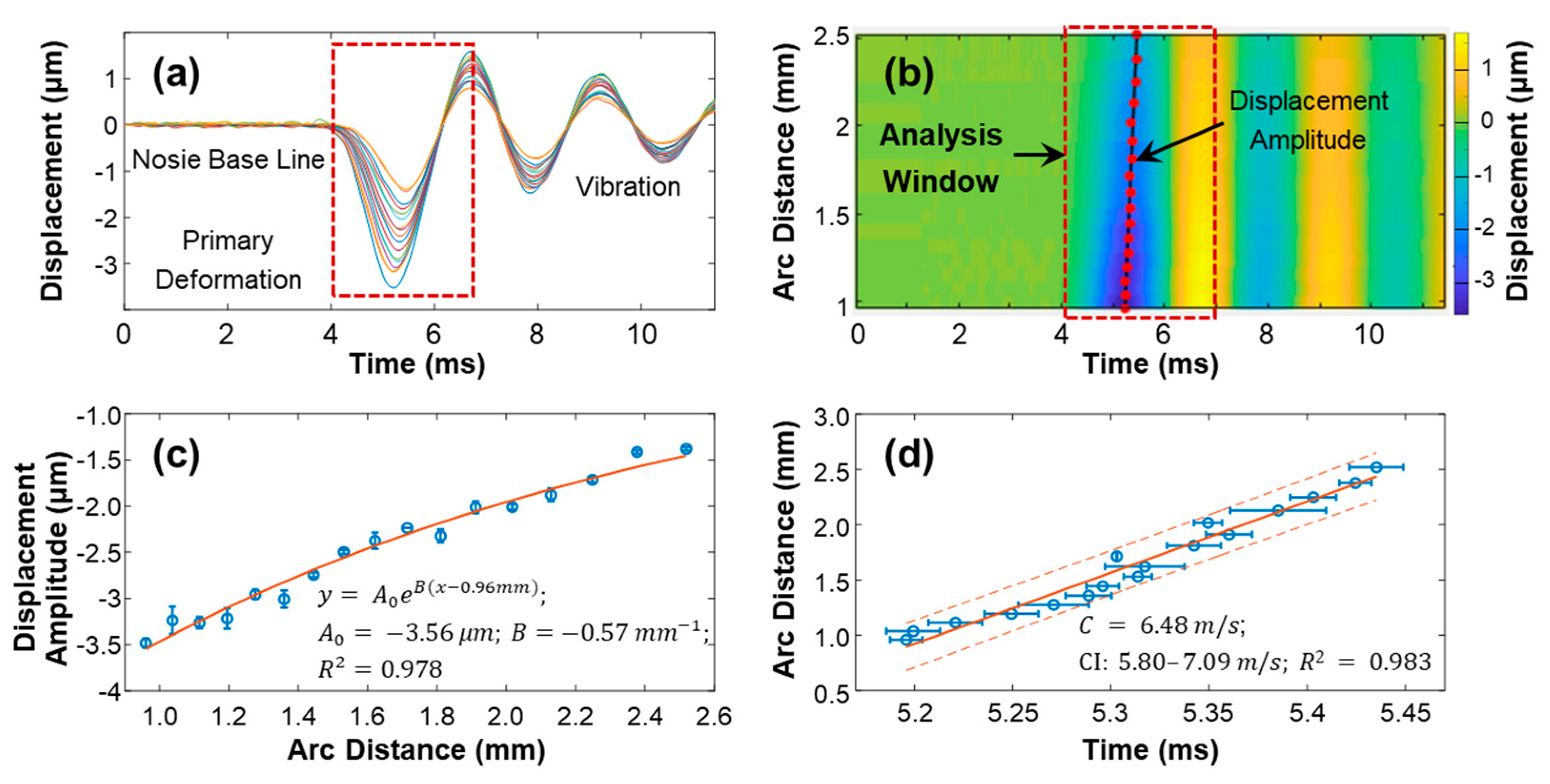
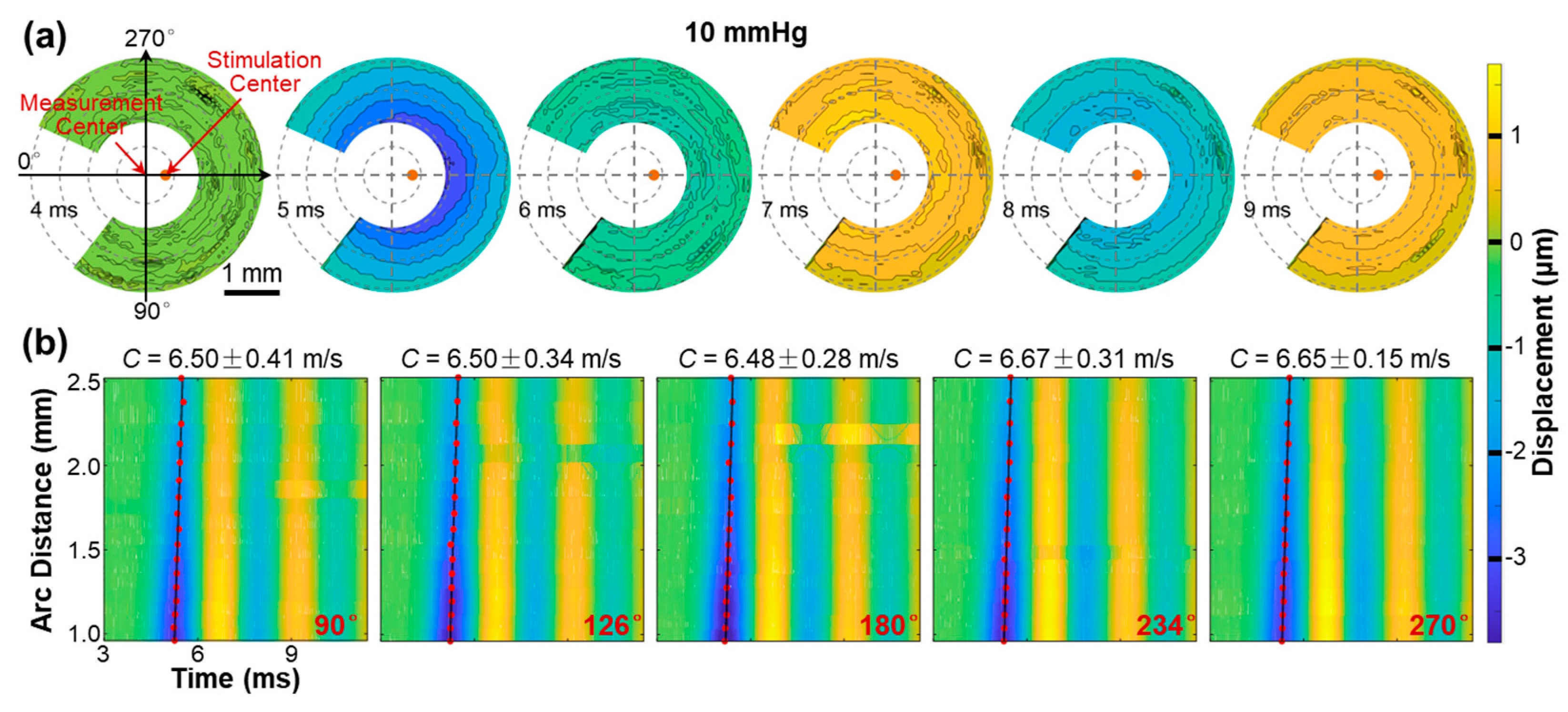
3.3. Surface Wave Characterization in the Silicone Cornea
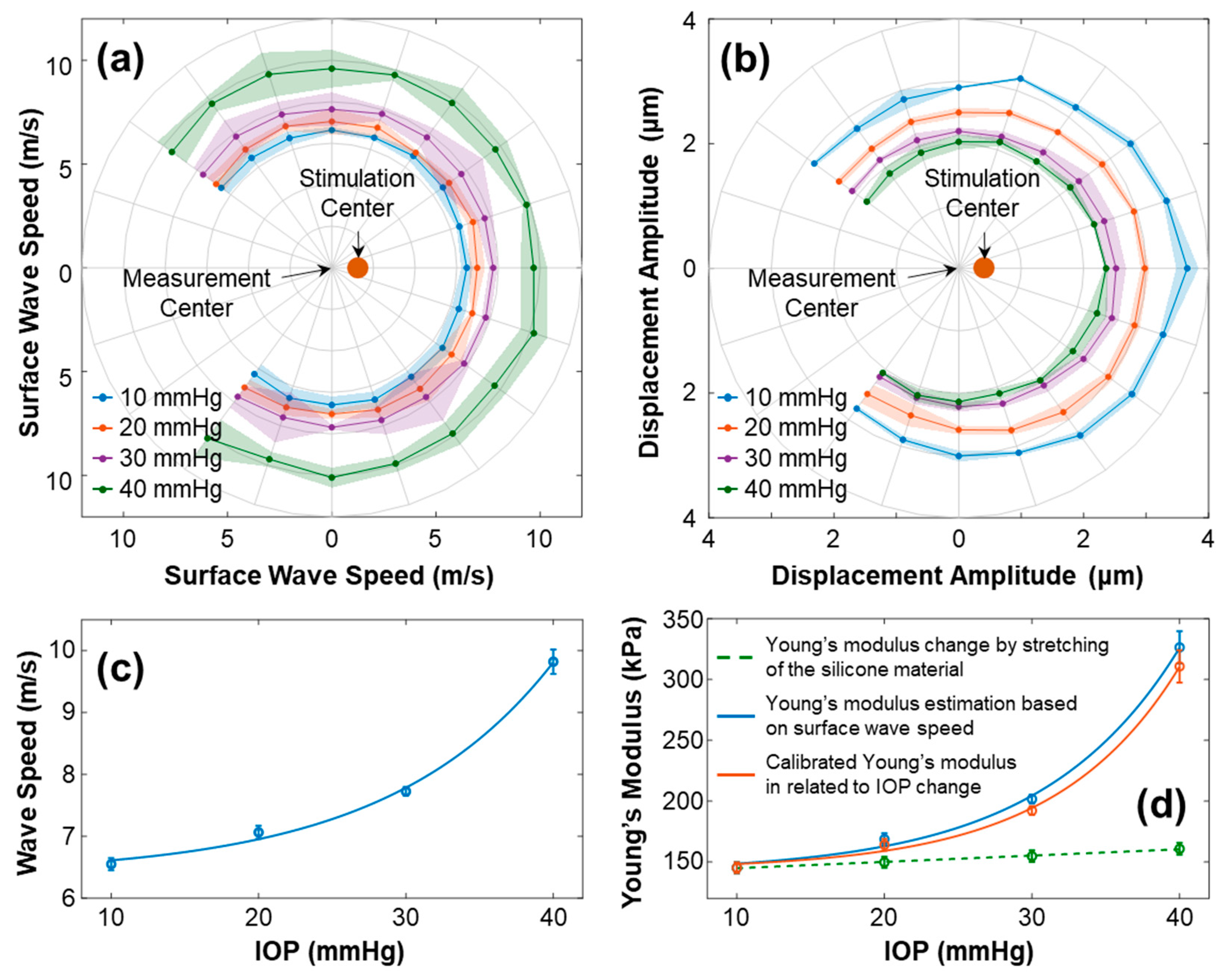
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, G.; Twa, M.D.; Song, C.; Feng, J.; Huang, Y.; Xu, J.; Qin, J.; An, L.; Wei, X. In Vivo Corneal Elastography: A Topical Review of Challenges and Opportunities. Comput. Struct. Biotechnol. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Safeen, S.; Shah, S.; Laiquzzaman, M. Changes of corneal biomechanics with keratoconus. Cornea 2012, 31, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Congdon, N.G. Corneal structure and biomechanics: impact on the diagnosis and management of glaucoma. Curr. Opin. Ophthalmol. 2006, 17, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Pascolini, D.; Mariotti, S.P.; Pokharel, G.P. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull. World Health Organ. 2008, 86, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Fan, F.; Xue, A.; Wang, J.; Zhou, X.; Lu, F. Biomechanical properties of the cornea in high myopia. Vision Res. 2008, 48, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Chansangpetch, S.; Panpruk, R.; Manassakorn, A.; Tantisevi, V.; Rojanapongpun, P.; Hurst, C.P.; Lin, S.C. Impact of myopia on corneal biomechanics in glaucoma and nonglaucoma patients. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4990–4996. [Google Scholar] [CrossRef]
- Kim, T.I.; Alió Del Barrio, J.L.; Wilkins, M.; Cochener, B.; Ang, M. Refractive surgery. Lancet 2019, 393, 2085–2098. [Google Scholar] [CrossRef]
- Guo, H.; Hosseini-Moghaddam, S.M.; Hodge, W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: a systematic review and meta-analysis. BMC ophthalmol. 2019, 19, 1–20. [Google Scholar] [CrossRef]
- Wang, D.; Liu, M.; Chen, Y.; Zhang, X.; Xu, Y.; Wang, J.; To, C.-h.; Liu, Q. Differences in the corneal biomechanical changes after SMILE and LASIK. J. Refract. Surg. 2014, 30, 702–707. [Google Scholar] [CrossRef]
- Shetty, R.; Francis, M.; Shroff, R.; Pahuja, N.; Khamar, P.; Girrish, M.; Nuijts, R.M.; Roy, A.S. Corneal biomechanical changes and tissue remodeling after SMILE and LASIK. Invest. Ophthalmol. Vis. Sci. 2017, 58, 5703–5712. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Richoz, O.; Mosquera, S.A.; Tabibian, D.; Hoogewoud, F.; Hafezi, F. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Invest. Ophthalmol. Vis. Sci. 2014, 55, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Besner, S.; Pineda, R.; Yun, S.H.; science, v. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest. Ophthalmol. Vis. Sci. 2014, 55, 4490–4495. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Laiquzzaman, M.; Bhojwani, R.; Mantry, S.; Cunliffe, I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest. Ophthalmol. Vis. Sci. 2007, 48, 3026–3031. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J. Post-LASIK ectasia. Ophthalmology 2007, 114, 1230–1230. [Google Scholar] [CrossRef]
- Binder, P.S.; Lindstrom, R.L.; Stulting, R.D.; Donnenfeld, E.; Wu, H.; McDonnell, P.; Rabinowitz, Y. Keratoconus and corneal ectasia after LASIK. J. Refract. Surg. 2005, 21, 749–752. [Google Scholar] [CrossRef]
- Seven, I.; Vahdati, A.; De Stefano, V.S.; Krueger, R.R.; Dupps, W.J., Jr. Comparison of Patient-Specific Computational Modeling Predictions and Clinical Outcomes of LASIK for Myopia. Invest. Ophthalmol. Vis. Sci. 2016, 57, 6287–6297. [Google Scholar] [CrossRef]
- Schmitt, J. OCT elastography: imaging microscopic deformation and strain of tissue. Opt Express 1998, 3, 199–211. [Google Scholar] [CrossRef]
- Zvietcovich, F.; Larin, K.V. Wave-based optical coherence elastography: the 10-year perspective. Prog Biomed Eng 2021, 4. [Google Scholar] [CrossRef]
- Ramier, A.; Eltony, A.M.; Chen, Y.; Clouser, F.; Birkenfeld, J.S.; Watts, A.; Yun, S.-H. In vivo measurement of shear modulus of the human cornea using optical coherence elastography. Scientific Reports 2020, 10, 17366. [Google Scholar] [CrossRef]
- Hepburn, M.S.; Wijesinghe, P.; Chin, L.; Kennedy, B.F. Analysis of spatial resolution in phase-sensitive compression optical coherence elastography. Biomed. Opt. Express 2019, 10, 1496–1513. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Nair, A.; Aglyamov, S.R.; Larin, K.V. Compressional optical coherence elastography of the cornea. Photonics 2021, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Akca, B.I.; Chang, E.W.; Kling, S.; Ramier, A.; Scarcelli, G.; Marcos, S.; Yun, S.H. Observation of sound-induced corneal vibrational modes by optical coherence tomography. Biomed Opt Express 2015, 6, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, G.; Huang, Z.; Johnstone, M.; Wang, R.K. Noncontact all-optical measurement of corneal elasticity. Opt Lett 2012, 37, 1625–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Larin, K.V.; Li, J.; Vantipalli, S.; Manapuram, R.K.; Aglyamov, S.; Emelianov, S.; Twa, M.D. A focused air-pulse system for optical-coherence-tomography-based measurements of tissue elasticity. Laser Phys Lett 2013, 10, 075605. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, C.; Pascual, D.; Perez-Merino, P.; Kling, S.; Marcos, S. Dynamic OCT measurement of corneal deformation by an air puff in normal and cross-linked corneas. Biomed Opt Express 2012, 3, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Singh, M.; Aglyamov, S.R.; Larin, K.V. Heartbeat OCE: corneal biomechanical response to simulated heartbeat pulsation measured by optical coherence elastography. J Biomed Opt 2020, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Singh, M.; Larin, K.V.; Twa, M.D. Common-path phase-sensitive optical coherence tomography provides enhanced phase stability and detection sensitivity for dynamic elastography. Biomed. Opt. Express 2017, 8, 5253–5266. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, S.; Dai, Y.; Bao, C.; Ye, S.; Zhou, Y.; Wang, Y.; Huang, S.; Wang, Y.; Shen, M.; et al. In vivo noninvasive measurement of spatially resolved corneal elasticity in human eyes using Lamb wave optical coherence elastography. J. Biophotonics 2020, 13, e202000104. [Google Scholar] [CrossRef]
- Lan, G.; Aglyamov, S.R.; Larin, K.V.; Twa, M.D.J.O.; Science, V. In Vivo Human Corneal Shear-wave Optical Coherence Elastography. Optom. Vis. Sci. 2021, 98, 58–63. [Google Scholar] [CrossRef]
- Song, S.; Huang, Z.; Nguyen, T.M.; Wong, E.Y.; Arnal, B.; O'Donnell, M.; Wang, R.K. Shear modulus imaging by direct visualization of propagating shear waves with phase-sensitive optical coherence tomography. J Biomed Opt 2013, 18, 121509. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Larin, K.V. Shear wave imaging optical coherence tomography (SWI-OCT) for ocular tissue biomechanics. Opt Lett 2014, 39, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, J.W.; Sinha Roy, A.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu Rev Biomed Eng 2011, 13, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Gu, B.; Larin, K.V.; Twa, M.D. Clinical corneal optical coherence elastography measurement precision: effect of heartbeat and respiration. Transl Vis Sci Technol 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Li, J.; Han, Z.; Wu, C.; Aglyamov, S.R.; Twa, M.D.; Larin, K.V. Investigating Elastic Anisotropy of the Porcine Cornea as a Function of Intraocular Pressure With Optical Coherence Elastography. Journal of refractive surgery (Thorofare, N.J. : 1995) 2016, 32, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Aglyamov, S.; Larin, K.V.; Twa, M.D. In vivo human corneal natural frequency quantification using dynamic optical coherence elastography: repeatability and reproducibility. J. Biomech. 2021, 121, 110427. [Google Scholar] [CrossRef] [PubMed]
- Maczynska, E.; Rzeszewska-Zamiara, J.; Jimenez Villar, A.; Wojtkowski, M.; Kaluzny, B.J.; I., G. Air-Puff-Induced Dynamics of Ocular Components Measured with Optical Biometry. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1979–1986. [Google Scholar] [CrossRef]
- Li, W.; Feng, J.; Wang, Y.; Shi, Q.; Ma, G.; Aglyamov, S.; Larin, K.V.; Lan, G.; Twa, M. Micron-scale hysteresis measurement using dynamic optical coherence elastography. Biomed Opt Express 2022, 13, 3021–3041. [Google Scholar] [CrossRef]
- Lan, G.; Shi, Q.; Wang, Y.; Ma, G.; Cai, J.; Feng, J.; Huang, Y.; Gu, B.; An, L.; Xu, J. Spatial assessment of heterogeneous tissue natural frequency using micro-force optical coherence elastography. Frontiers in Bioengineering and Biotechnology 2022, 382. [Google Scholar] [CrossRef]
- Lan, G.; Xu, J.; Hu, Z.; Huang, Y.; Wei, Y.; Yuan, X.; Liu, H.; Qin, J.; Wang, Y.; Shi, Q.; et al. Design of 1300-nm spectral domain optical coherence tomography angiography system for iris microvascular imaging. J Phys D Appl Phys 2021, 8. [Google Scholar] [CrossRef]
- Hu, Z.; Rollins, A.M. Fourier domain optical coherence tomography with a linear-in-wavenumber spectrometer. Opt Lett 2007, 32, 3525–3527. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Li, G. Design of a k-space spectrometer for ultra-broad waveband spectral domain optical coherence tomography. Sci Rep 2017, 7, 42353. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, Z.; Wang, R.K. Tracking mechanical wave propagation within tissue using phase-sensitive optical coherence tomography: motion artifact and its compensation. J Biomed Opt 2013, 18, 121505–121505. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Aubry, J.F.; Fink, M.; Bercoff, J.; Tanter, M. In vivo evidence of porcine cornea anisotropy using supersonic shear wave imaging. Investigative ophthalmology & visual science 2014, 55, 7545–7552. [Google Scholar] [CrossRef]
- Zvietcovich, F.; Singh, M.; Ambekar, Y.S.; Aglyamov, S.R.; Twa, M.D.; Larin, K.V. Micro Air-Pulse Spatial Deformation Spreading Characterizes Degree of Anisotropy in Tissues. IEEE journal of selected topics in quantum electronics : a publication of the IEEE Lasers and Electro-optics Society 2021, 27, 6800810. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.G.; Son, T.; Crutison, J.; Guaiquil, V.; Lin, S.; Nammari, L.; Klatt, D.; Yao, X.; Rosenblatt, M.I.; Royston, T.J. Optical coherence elastography for assessing the influence of intraocular pressure on elastic wave dispersion in the cornea. Journal of the mechanical behavior of biomedical materials 2022, 128, 105100. [Google Scholar] [CrossRef]
- Wang, S.; Larin, K.V. Optical coherence elastography for tissue characterization: a review. Journal of biophotonics 2015, 8, 279–302. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Singh, M.; Twa, M.D.; Larin, K.V. Differentiating untreated and cross-linked porcine corneas of the same measured stiffness with optical coherence elastography. J. Biomed. Opt. 2014, 19, 110502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



