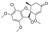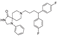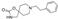In memory of Prof. Mikhail Krasavin
In commemoration of the 300th anniversary of St Petersburg State University’s founding
2. Griseofulvin
Griseofulvin is an antifungal agent used to treat fungal infections of the fingernails and toes. It is one of the earliest spirocyclic drugs which was approved in 1959. The exact mechanism of its action remains unclear with putative targets including tubulin beta chain and keratin, type I cytoskeletal 12 through which griseofulvin interferes with fungal mitosis.
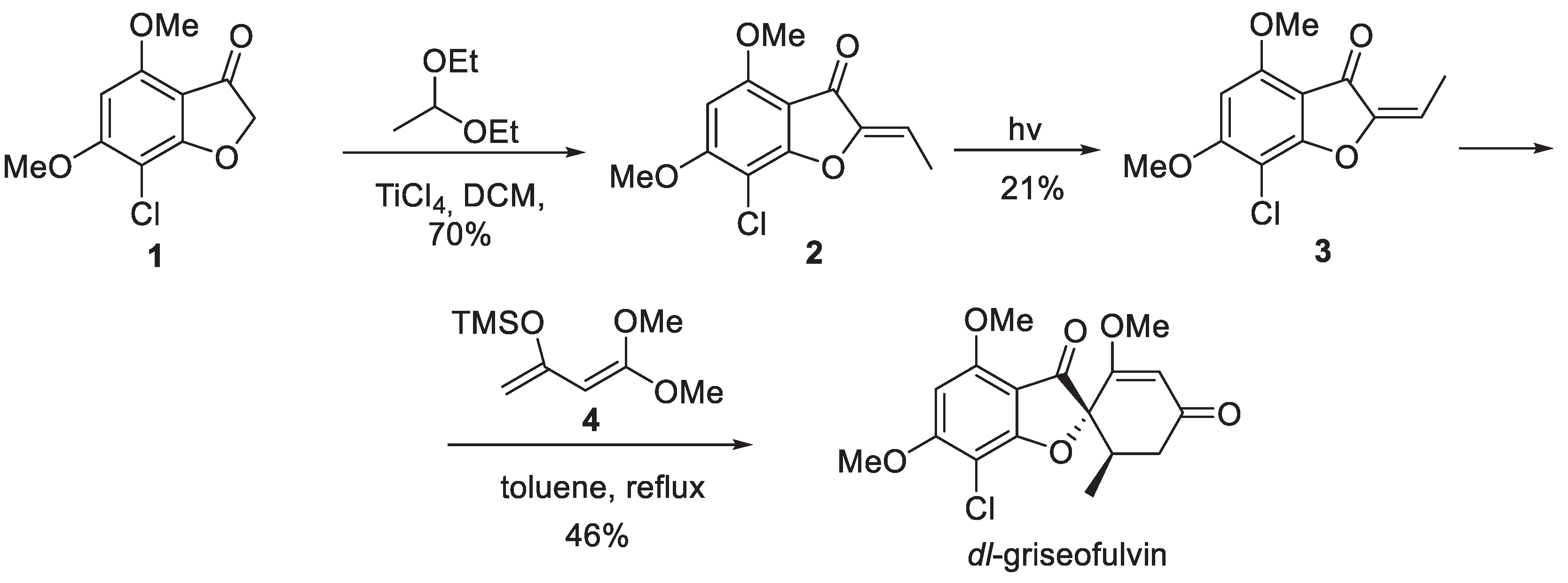
Two total syntheses of griseofulvin were accomplished in the 1990s. Yamoto and co-workers reported the synthesis of racemic griseofulfin in 1990 [
3]. The synthesis commenced with 7-chloro-4,6-dimethoxy-3(2
H)-benzofuranone (
1) which was condensed with acetaldehyde diethyl acetal in the presence of Ti(IV) chloride to give (Z)-configured ethylidene derivative
2. In order to bring the olefin configuration to the desired (
E), compound
2 was subjected to somewhat low-yielding photochemical isomerization. The (
E)-configured olefin
3 was now set for a Diels-Alder reaction with diene
4 (10 equiv.) which was accomplished in refluxing toluene to furnish racemic griseofulvin in 46% yield (
Scheme 1).
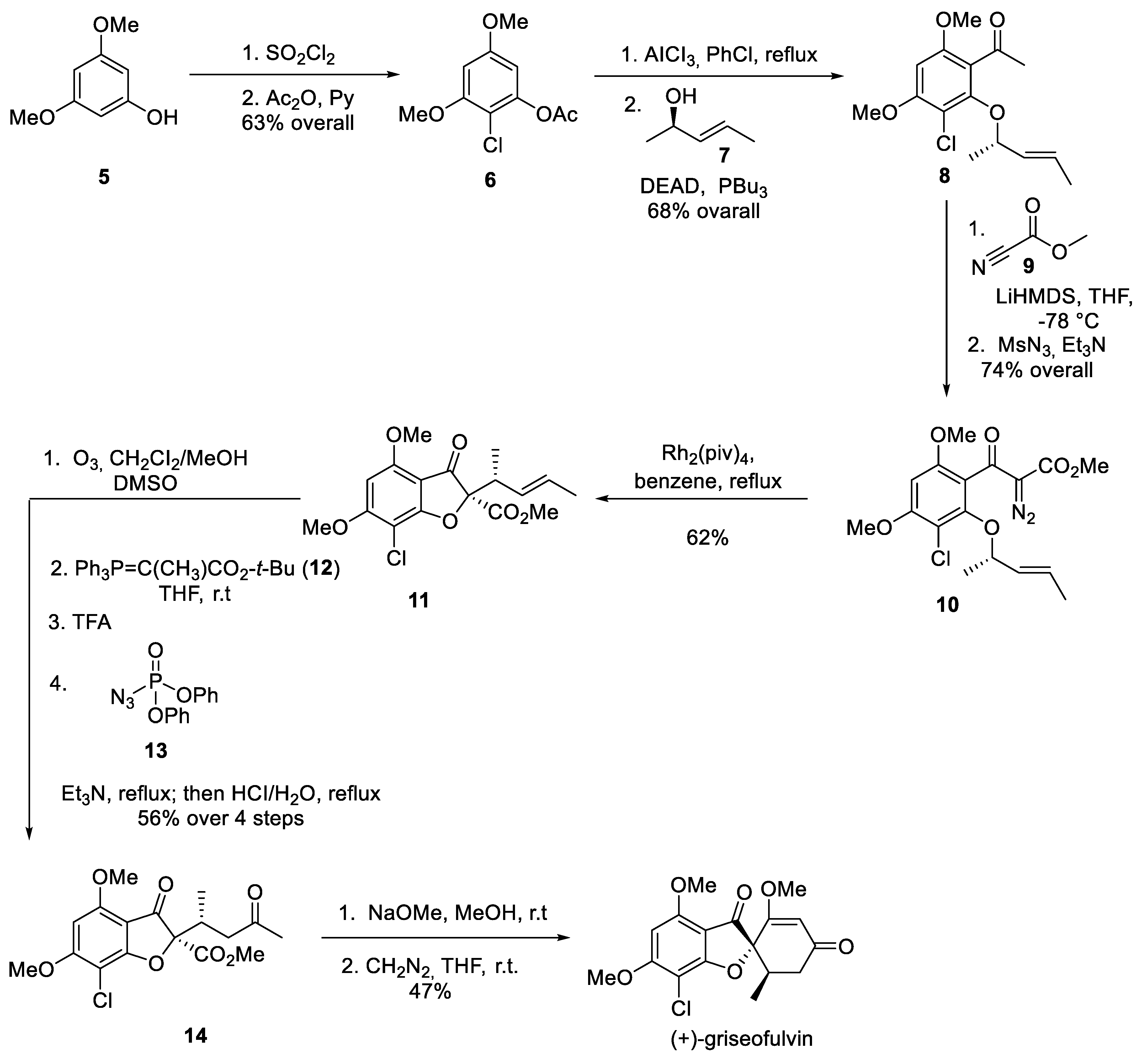
Pirrung and co-workers reported the first asymmetric synthesis of natural enantiomer (+)-griseofulvin in 1991 [
4]. Commercially available phenol
5 was regiospecifically chlorinated to provide intermediate
6. The latter underwent a Fries rearrangement in the presence of aluminum chloride and the liberated phenol was involved in the Mitsunobu coupling with non-racemic allylic alcohol
7 to give rise to pentasubstituted benzene building block
8. The acetyl group in the latter was deprotonated and methoxycarbonylated by treatment with Mander’s reagent (
9) and the resulting -keto ester was subjected to Regitz diazo transfer to provide diazo compound
10. Decomposition of the latter was accomplished with the use of rhodium pivalate catalyst and the resulting rhodium carbene underwent intramolecular trapping with the nearby oxygen atom followed by sigmatropic rearrangement to give benzofuranone
11. Conversion of the latter to non-racemic methyl ketone
14 was accomplished via ozonolysis, Wittig reaction with phosphonium ylide
12,
tert-butyl ester cleavage with TFA and decarboxylation on treatment with diphenylphosphoryl azide (
13) followed by exposure to refluxing aqueous hydrochloric acid. Finally, methyl ketone
14 was subjected to treatment with sodium methoxide on which it underwent the Dieckmann cyclization. The resulting cyclohexane-1,3-dione (griseofulvinic acid) was
O-methylated with diazomethane to furnish (+)-griseofulvin (
Scheme 2).
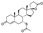
3. Spironolactone
Spironolactone is a relatively old multi-target drug that is primarily used to treat high blood pressure and heart failure. Additional therapeutic applications of this drug include edema, cirrhosis of the liver and primary aldesteronism. The mechanism of action of spironolactone includes binding to intracellular mineralocorticoid receptors (MRs) in kidney epithelial cells which leads to the inhibition of aldosterone binding [
5].
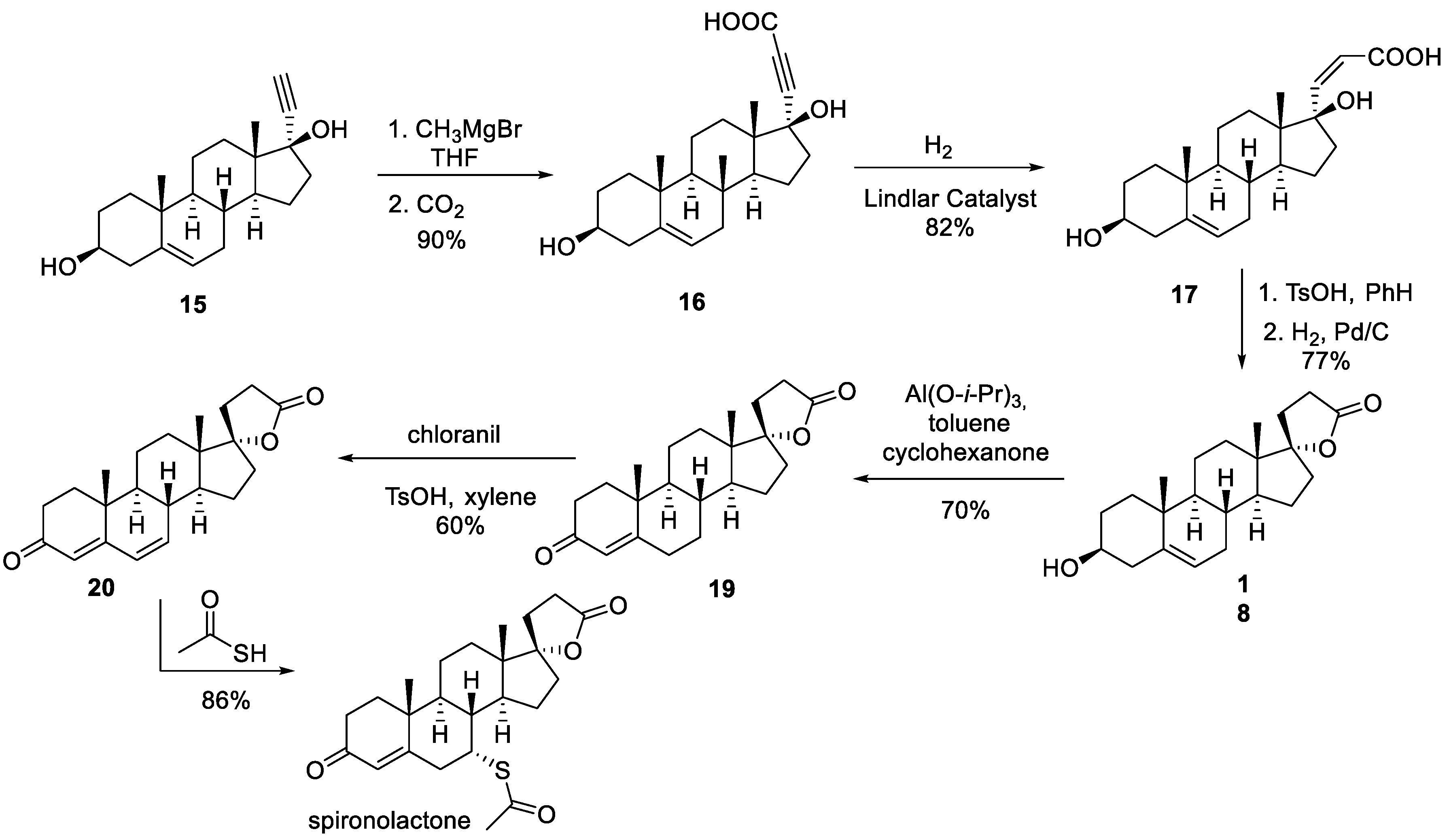
The first industrial synthesis of spironolactone was reported in 1957-1959 by Cella and co-workers of G. D. Searle and Co. [6-8]. The synthesis commences with previously reported ethynyl steroid building block 15. It was carbonylated at the alkyne terminus by treatment with methyl magnesium bromide followed by quenching with carbon dioxide. Hydrogenation of alkyne 16 over Lindlar catalyst elaborated the propionic acid side chain in 17 required for the formation of the spirocyclic lactone at the next step. Cyclization on treatment with p-toluenesulfonic acid yielded unsaturated lactone which was hydrogenated over palladium on carbon to yield spirocyclic lactone 18.
Oppenauer oxidation of the alcohol functionality in
18 was accompanied by the migration of the double bond to give α,
β-unsaturated ketone
19. The conjugated system in
19 was extended by γ,δ -oxidation with chloranil to give compound
20 and set the scene for the conjugate addition of thioacetic acid at the d-position which proceeded with high diastereoselectivity and furnished spironolactone in 86% yield (
Scheme 3).
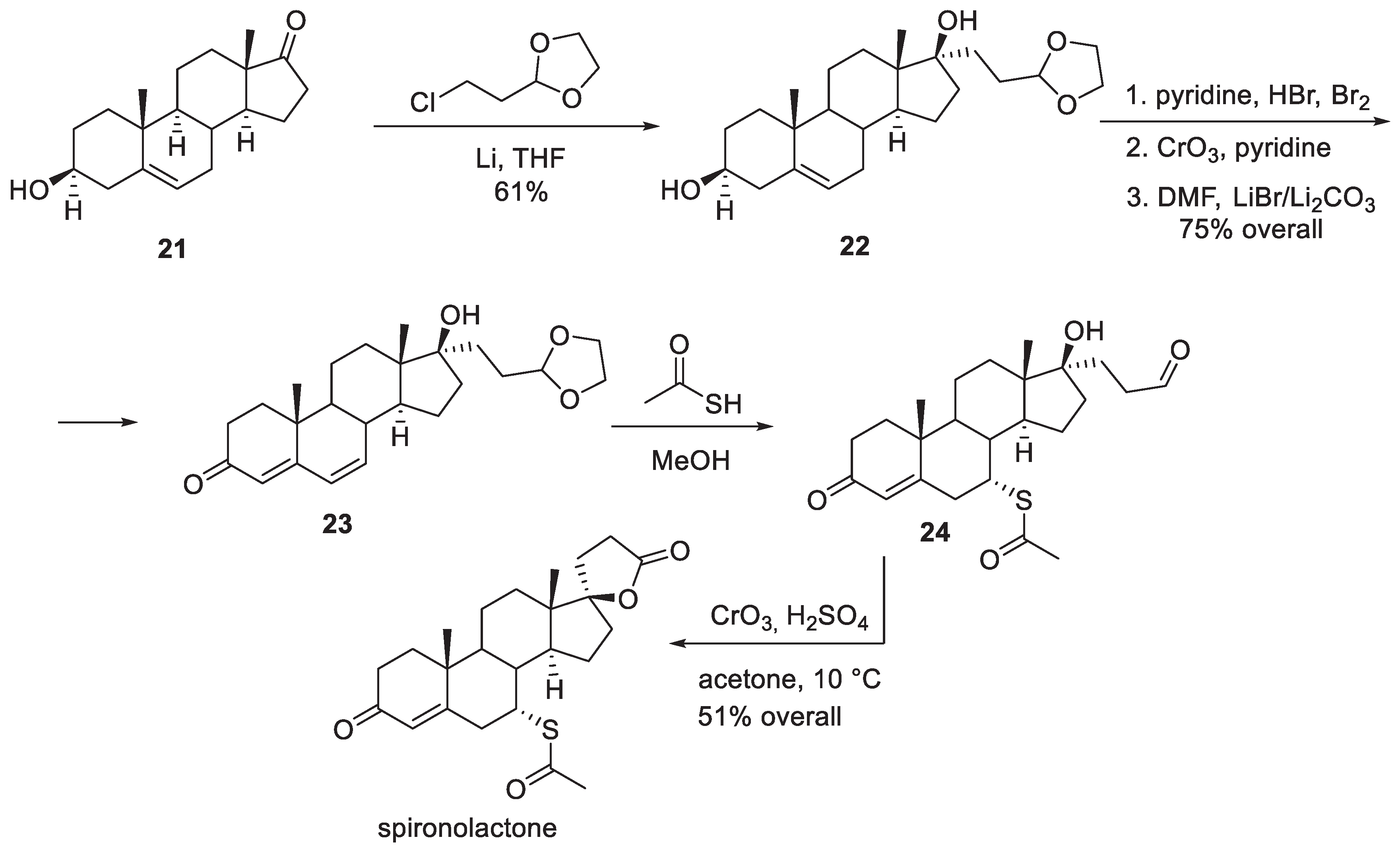
Another industrial synthesis of spironolactone was patented in 1980 by Ciba Geigy scientists [
9]. The synthesis stems from dehydroepiandrosterone (
21) which was treated with 3-chloropropionaldehyde ethylene acetal and lithium to furnish tertiary alcohol
22 in diastereoselective fashion. The following sequence of steps (which proceeded in 75% overall yield) included bromination of the double bond, oxidation of the secondary alcohol to ketone and, finally, base-promoted double dehydrobromination, which led to α,
β,γ,δ-unsaturated ketone (androstadienone)
23. Interestingly, the conjugate addition of thioacetic acid was in this case performed prior to the elaboration of the spirocyclic lactone in the last step. Thioacetic acid also caused the deprotection of the aldehyde to furnish
24. Oxidation of the aldehyde to the carboxylic acid in the presence of sulfuric acid brought about the closure of the lactone ring and led to the formation of spironolactone in 51% yield from
23 (
Scheme 4).
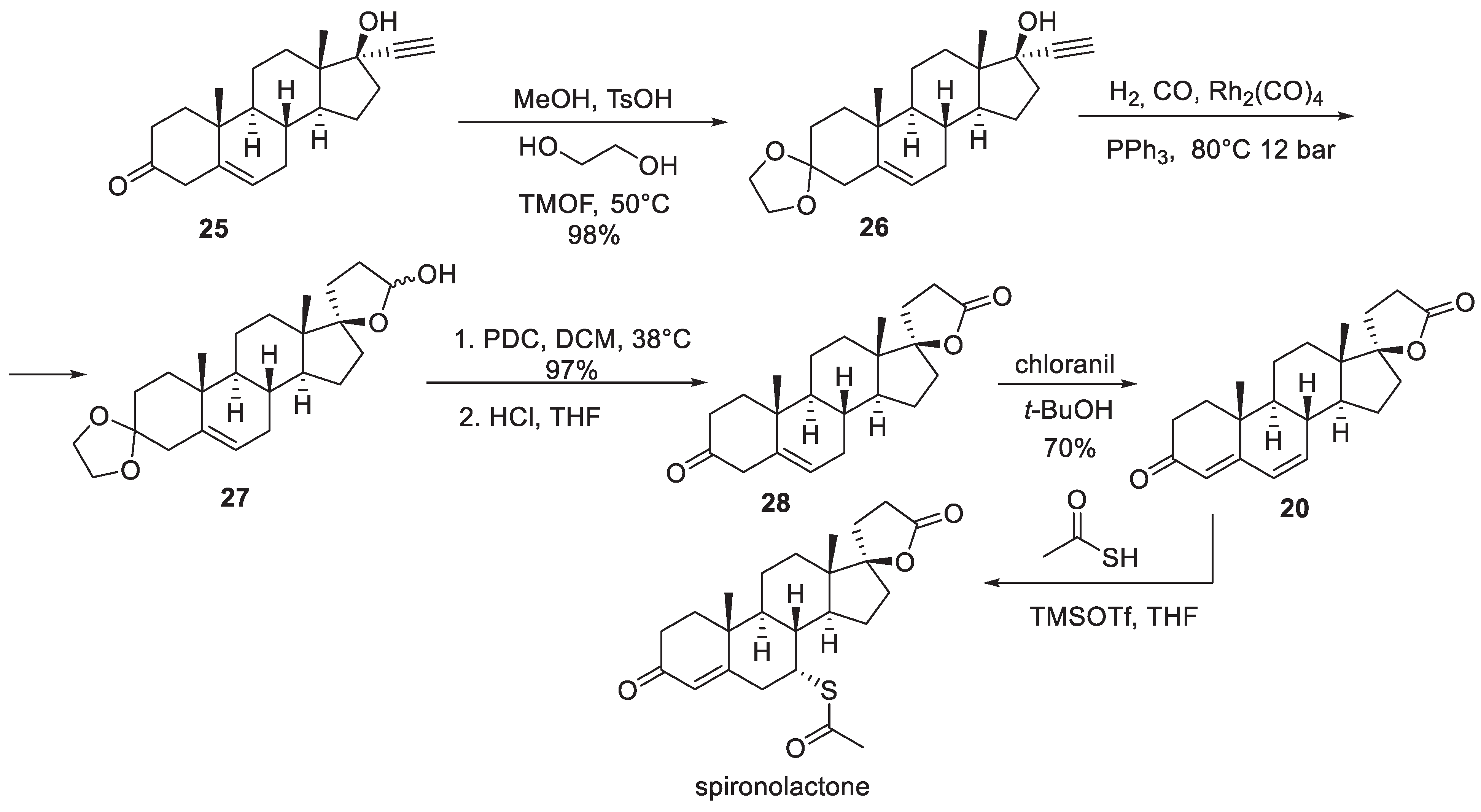
A fundamentally different industrial route to spironolactone was developed by the Chemical Process Research and Development scientists at The Upjohn Co. in the late-1980s [
10]. The synthesis relied on the readily available ethynyl steroid building block
25 the ketone group of which was protected as ethylene glycol ketal in the first step. The key transformation which led to the formation of spirocyclic lactol intermediate
27 was rhodium-catalyzed hydroformylation at elevated temperature and pressure. Oxidation of
27 to lactone was achieved with PDC and the ketal protecting group was removed to afford
28. The formation of α,
β,γ,δ -unsaturated ketone
20 was achieved with chloranil (
vide supra) and conjugate addition of thioacetic acid (catalyzed by TMSOTf) at the -position furnished spironolactone (
Scheme 5).
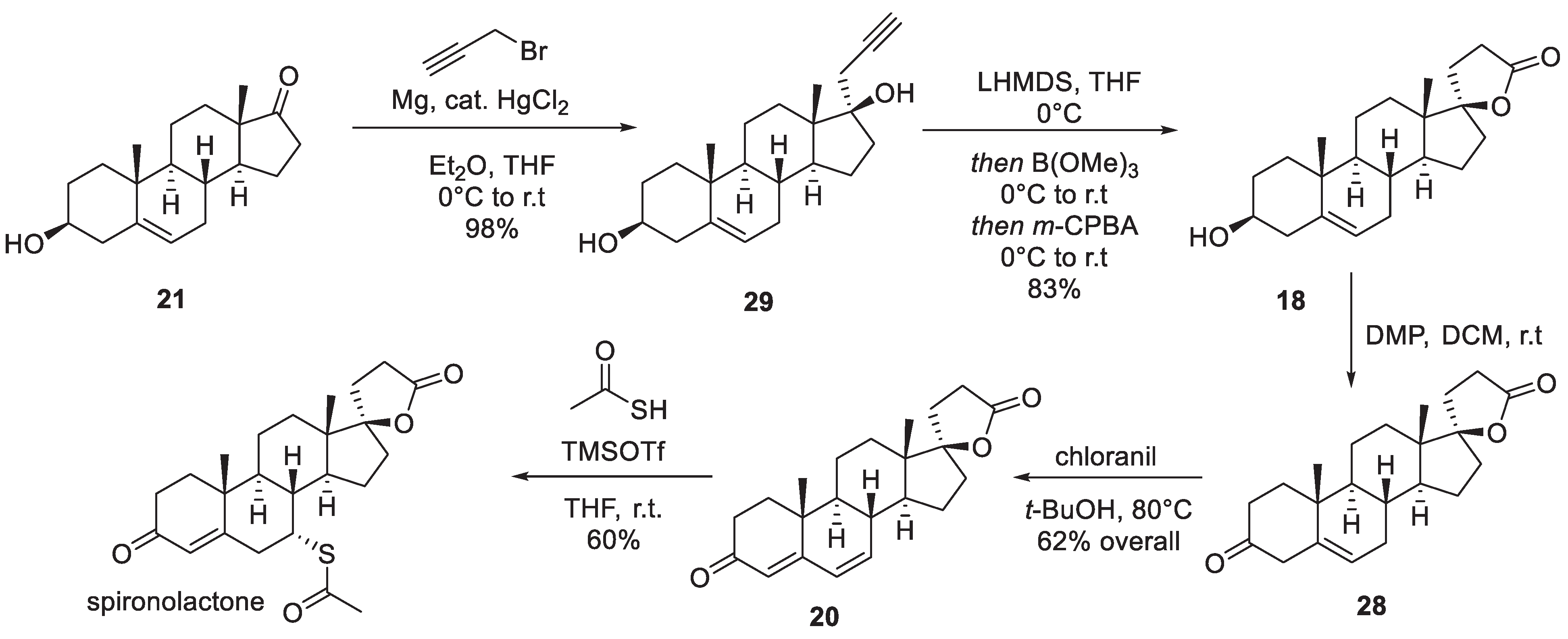
Among many non-industrial syntheses of spironolactone described in the literature [
5] the most recent one reported by Nagamitsu, Ohtawa and co-workers is quite notable [
11]. The synthesis commences with dehydroepiandrosterone (
21) and is reliant on the innovative approach to -lactonization of homopropargyl alcohols which involved terminal borylation of a homopropargyl moiety followed by
m-CPBA oxidation. The latter sequence leads to the transformation of the alkyne moiety into a ketene intermediate which is trapped intramolecularly to give a -lactone. Hence, starting material
21 was treated with propargyl magnesium bromide (under Hg(II) catalysis) to give homopropargyl alcohol
29 in nearly quantitative yield. Application of the -lactonization protocol described above led to the formation of spirocyclic lactone
18. Oxidation of the secondary alcohol moiety in the latter with Dess-Martin periodinane (DMP) gave -unsaturated ketone
28. Transformation of the latter into spironolactone was analogous to the Upjohn Co. synthesis described above. Oxidation with chloranil installed the polyunsaturated ketone system (
20) which is suitable to the conjugate addition of thioacetic acid (also TMSOTf-catalyzed in this case) resulting in the formation of spironolactone (
Scheme 6).
4. Fluspirilene
This spirocyclic antipsychotic drug was discovered at Janssen Pharmaceutica in 1963 and by 1970 it was approved for the treatment of schizophrenia [
12]. The primary molecular targets of fluspirilene in the central nervous system are dopamine D
2 and serotonin 5HT
2A receptors as well as 1 subunit of voltage-dependent calcium channel [
13].
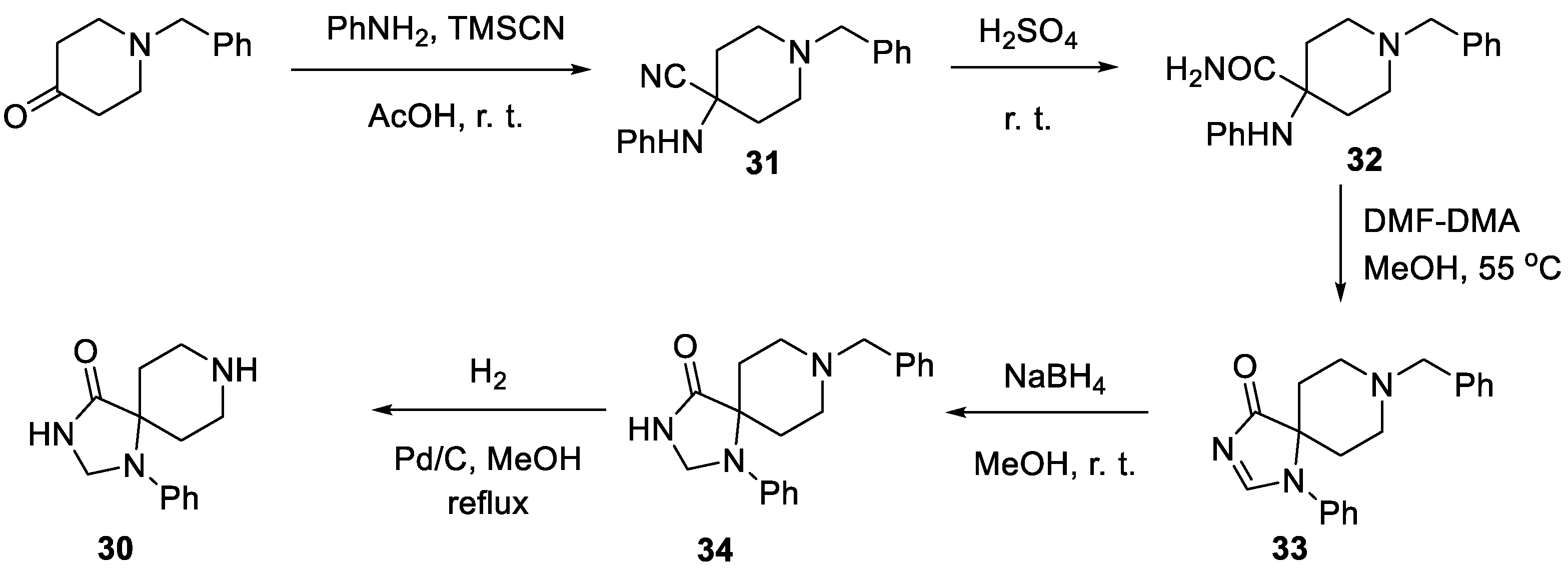
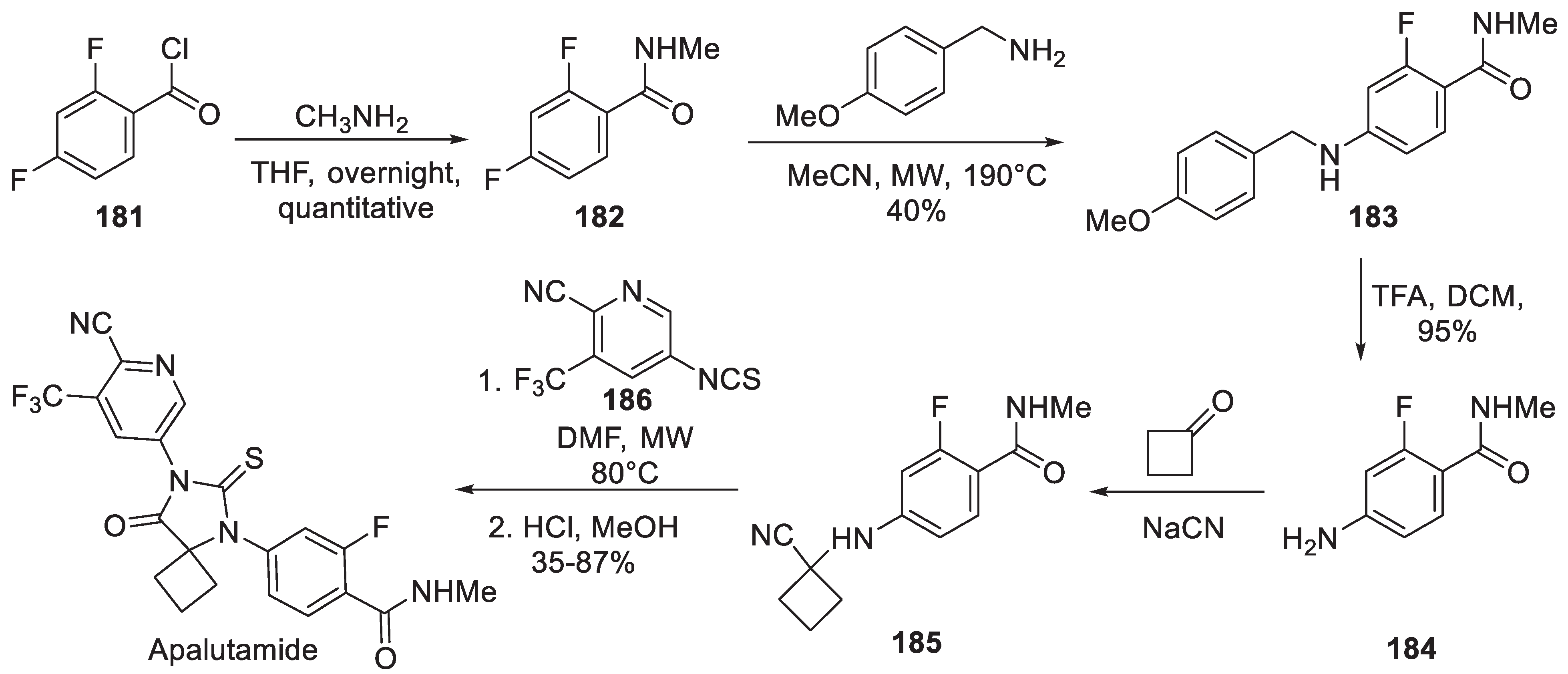
Fluspirilene is an old drug and reviewing the vast patent literature on its synthesis would be tedious. Instead, we will focus on the most notable cases of fluspirilene assemble from relatively recent literature. For instance, an efficient synthesis of the key spirocyclic building block
30 needed for the synthesis of drug was published in 2018 by a team of Italian and Polish scientists [
14]. The synthesis commenced with 1-benzyl piperidin-4-one which was involved in the Strecker reaction with aniline and trimethylsilyl cyanide to give nitrile
31. The latter was hydrated in sulfuric acid and resulting a-anilino carboxamide
32 was condensed with
N,N-dimethylformamide dimethyl acetal to form spirocyclic imidazolinone
33. The C-N double bond in
33 was reduced to give imidazolidinone
34 and the benzyl group was removed by hydrogenation to give the target spirocycle
30 (
Scheme 7).
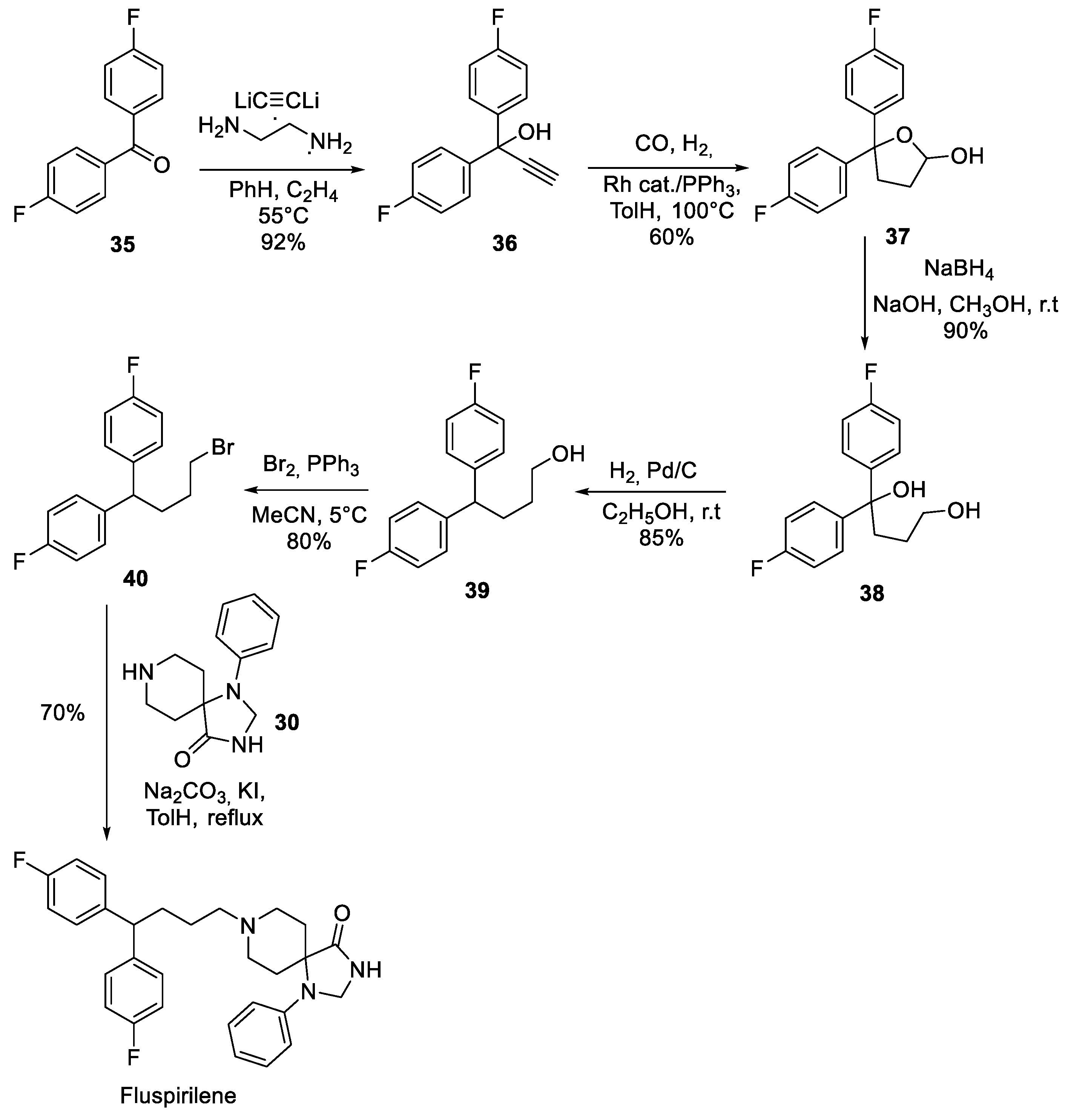
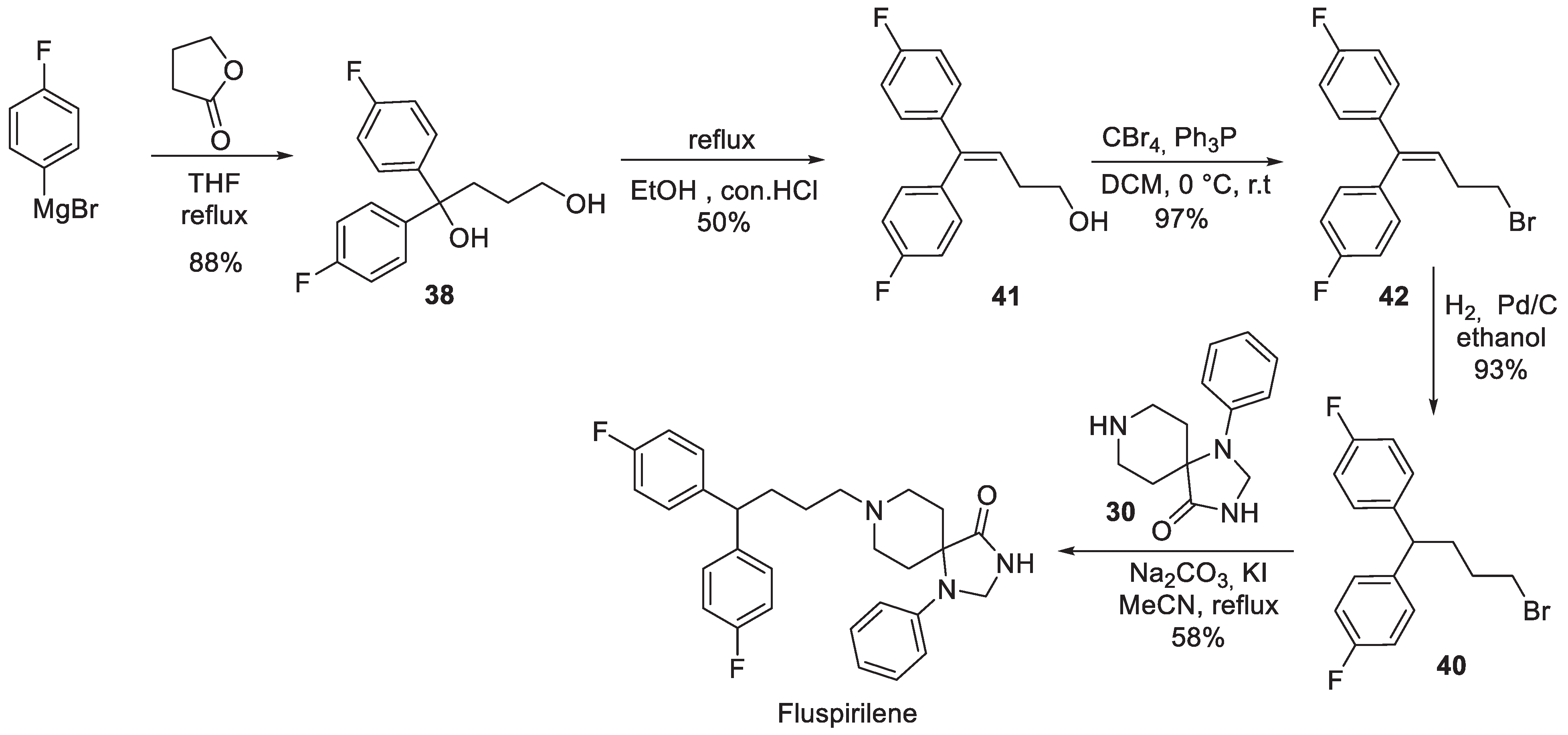
Interestingly, it is the synthetic assembly of the 4,4-bis(4-fluorophenyl)butyl halide building block (for the alkylation of key spirocycle
30) that received much of the synthetic route development. For instance, in 2001, an Italian group reported an interesting synthesis of fluspirilene starting from 4,4’-difluorobenzophenone (
35). Acetylene bis-lithium salt was added to the keto group of
35 in the presence of ethylene diamine to give allylic alcohol
36. The latter was hydroformylated in the presence of a rhodium catalysts cyclic hemiacetal
37. Sodium borohydride reduction of the latter furnished diol
38 which lost its doubly benzylic tertiary alcohol hydroxy group on hydrogenation. Alcohol
39 was brominated to alkyl halide
40 which then was used in the completion of fluspirilene synthesis (
Scheme 8) [
15].
A different approach to bromide
40 was reported in 2011 by a Shanghai Institute of Organic Chemistry group [
16]. Diol
38 was obtained by reacting -butyrolactone with excess 4-fluorophenyl magnesium bromide. Thereupon, it was dehydrated in refluxing aqueous hydrochloric acid to give olefinic alcohol
41, bromination of which gave unsaturated alkyl bromide
42. Hydrogenation of the latter resulted in double bond saturation and went without a loss of the bromide to give alkylating agent
40 which was used in the preparation of fluspirilene (
Scheme 9).
5. Fenspiride
This spirocyclic oxazolidinone, acting as an antagonist of a
1-adrenergic receptors, was first approved in selected European countries for medical use in the 1970s for the treatment of otolaryngological or ENT (ear, nose and throat) organ diseases as well as respiratory tract diseases [
17]. The structure of fenspiride comprises a well-established feature for binding to the hERG channel (aromatic ring at a certain distance from a basic amine center) [
18]. Unfortunately, it does bind to this off-target and causes prolongation of QT interval. For this reason, i. e. the increased risk of torsades de pointes, fenspiride was withdrawn from the pharmaceutical market in most markets in the World [
19].
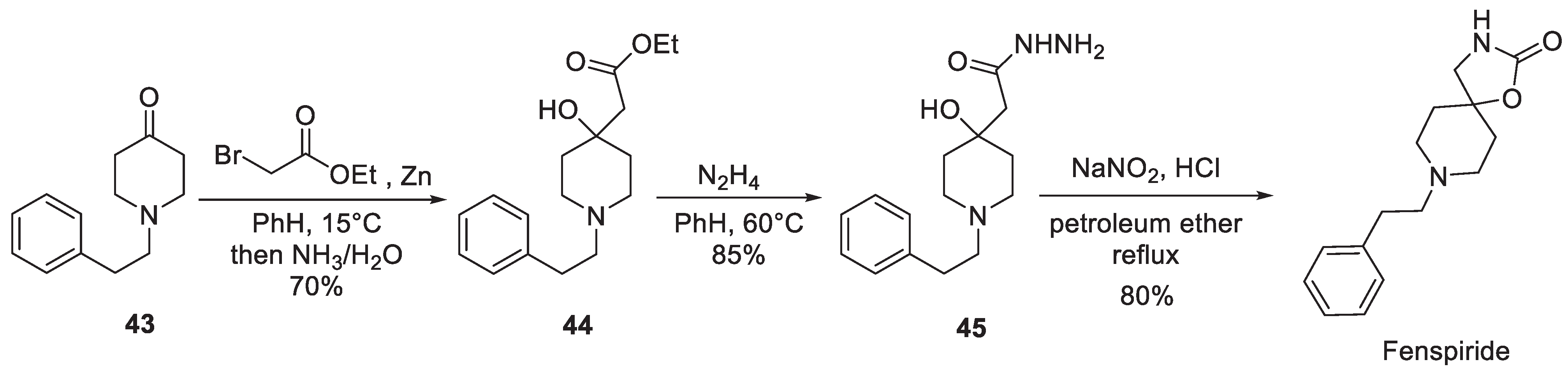
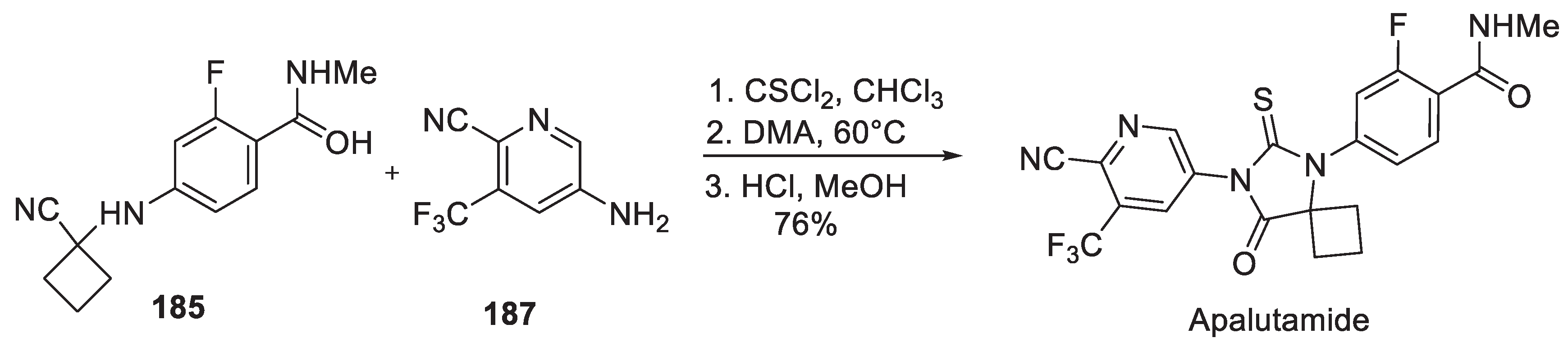
One of the early patents, obtained by Italian Voghera company for the synthesis of fenspiride, describes the assembly of the target molecule starting from 1-phenethyl piperidin-4-one (
43) which was involved in the Reformatsky reaction with ethyl bromoacetate. After the conversion of resulting -hydroxy ester
44 to hydrazide
45, the latter was diazotized which triggered a Curtius rearrangement of the intermediate acyl azide and the isocyanate product of the rearrangement was trapped, intramolecularly, by the nearby hydroxy group to give fenspiride (
Scheme 10) [
20].
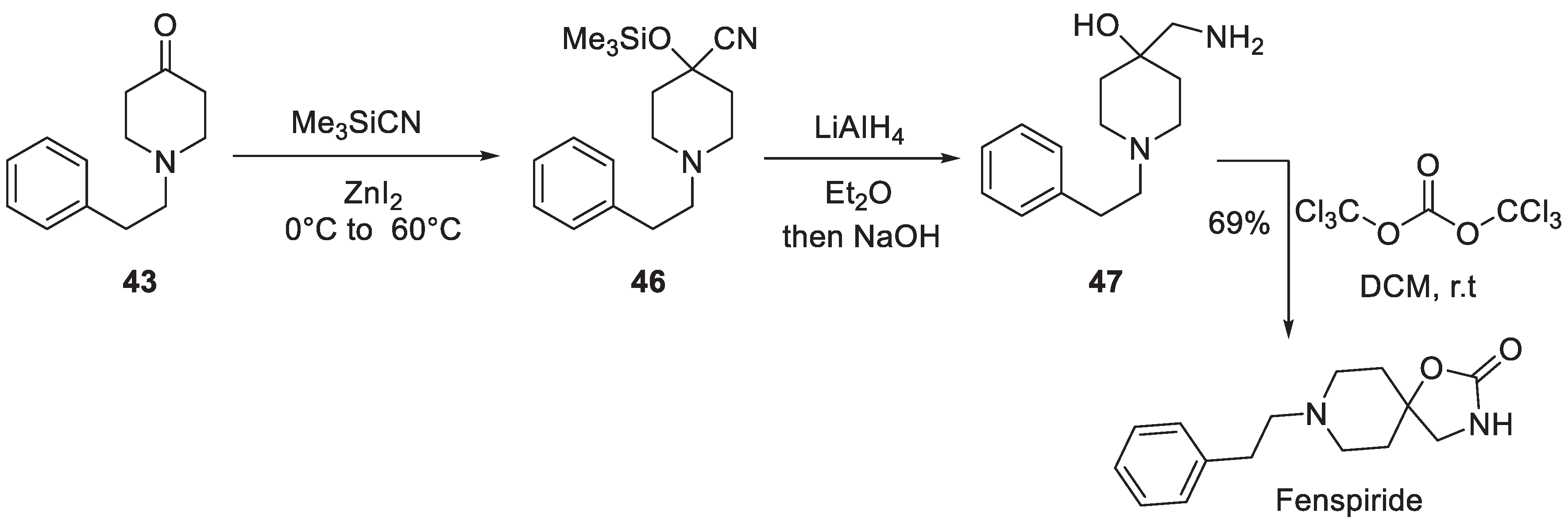
In 1994, a Mexican group disclosed an alternative approach to elaborating the spirocyclic oxazolidinone based on 1-phenethyl piperidin-4-one which includes the Strecker-type reaction of the ketone with trimethylsilyl cyanide. Resulting
O-silylated a-hydroxy nitrile
46 was subjected to reduction with lithium alumohydride to give -amino alcohol
47. The latter was treated with trichloromethyl carbonate which donated the carbonyl group and resulted in the closure of the oxazolidinone ring of fenspiride (
Scheme 11) [
21].
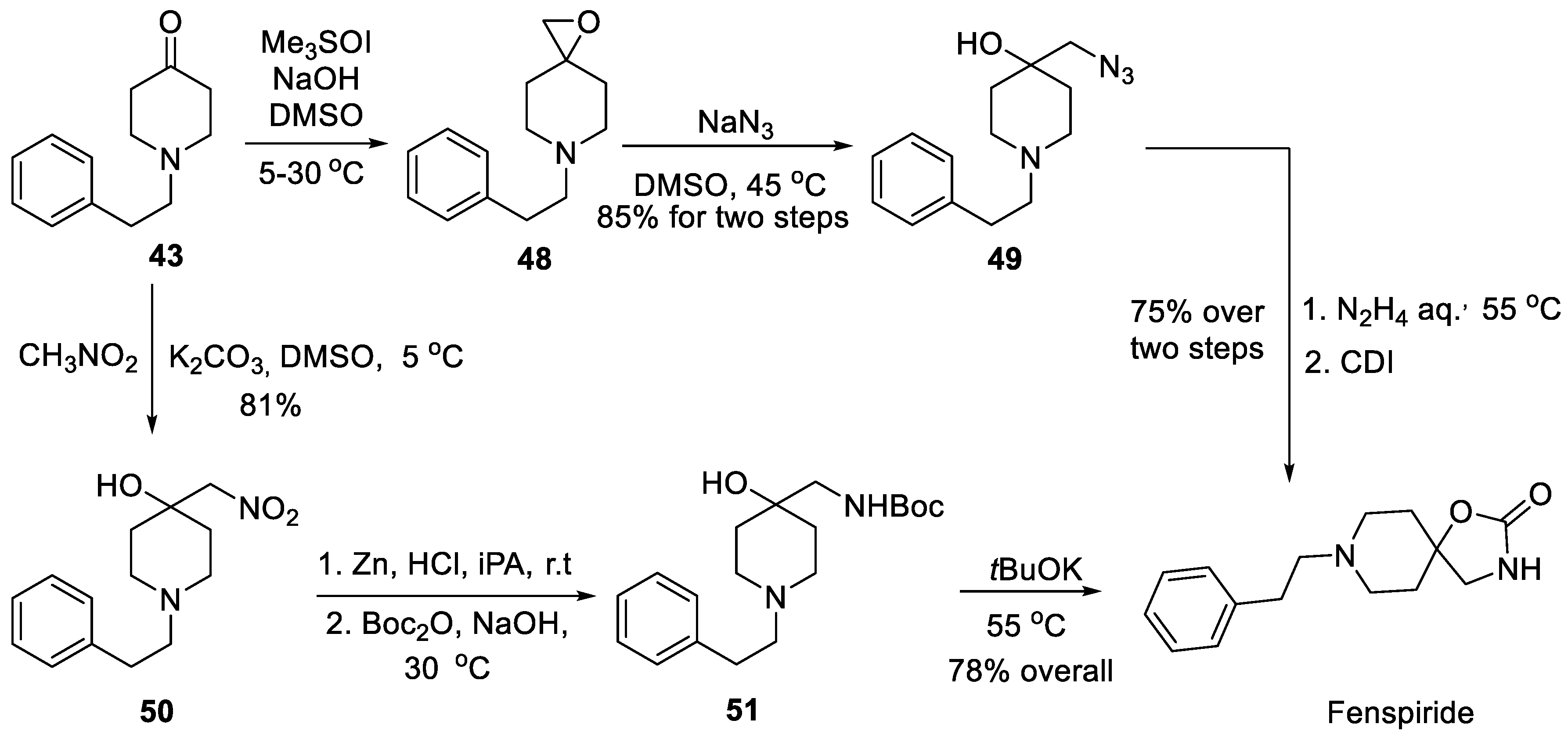
In 2019, Indian company Emcure Pharmaceuticals jointly with a University of Pune group published a completely novel route to fenspiride which is based on nitro aldol reaction and is amenable to industrial scale production of the drug substance [
22]. In fact, the researchers disclosed two possible approaches to fenspiride for comparison and made a conclusion that the nitro aldol route was preferred from the standpoint of pharmaceutical production. In the first approach, Corey-Chaykovsky epoxidation gave epoxide
48 which was opened up by sodium azide to give -azido alcohol
49. Reduction by hydrazine (presumably, to alcohol
47) and treatment with CDI (donor of the carbonyl) afforded fenspiride. In the second, preferred, approach, ketone
43 was condensed with nitromethane anion and resulting nitro compound
50 was reduced to the respective amine and Boc-protected to afford intermediate
51. Deprotonation of the hydroxy group in the latter with potassium
tert-butoxide triggered intramolecular displacement of the
tert-butoxy group and ring closure to fenspiride (
Scheme 12).
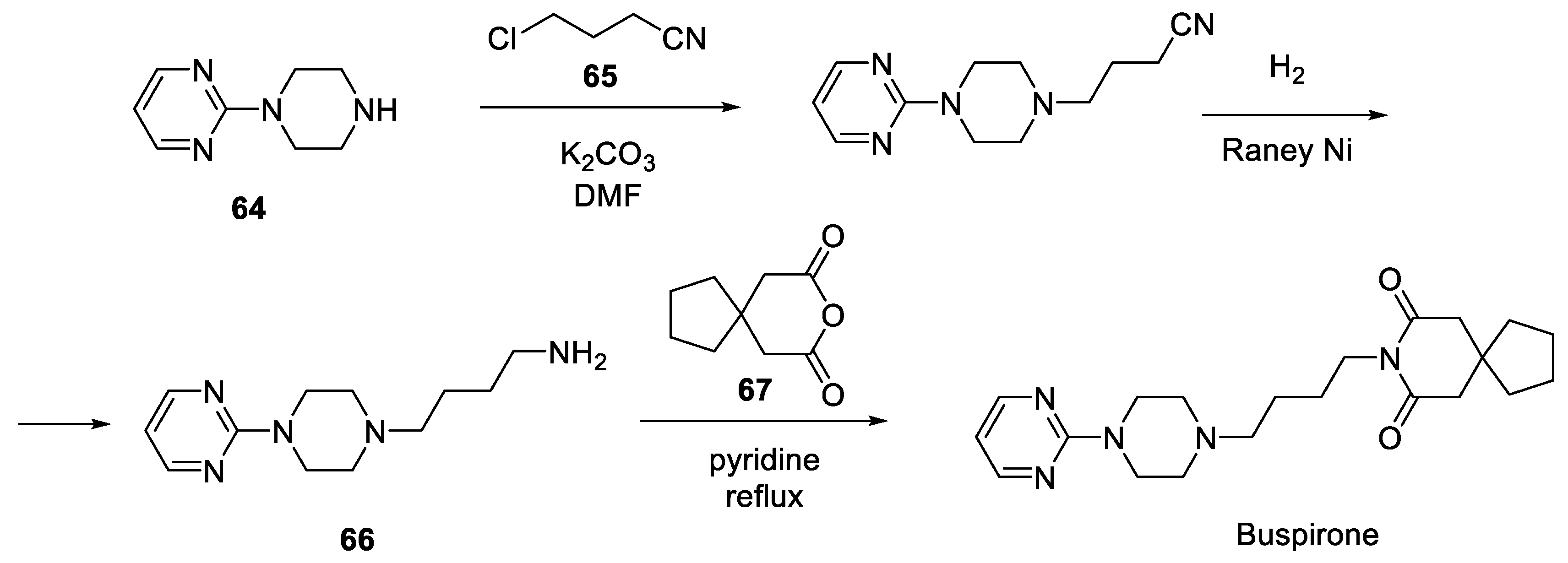
8. Buspirone
First synthesized in 1966 and approved for medical use in 1986, this spirocyclic serotonin 5-HT
1A receptor agonist is primarily used to treat anxiety disorders [
30]. Several patented protocols for the preparation of buspirone [31-33], including industry synthesis, have been reported in the literature.
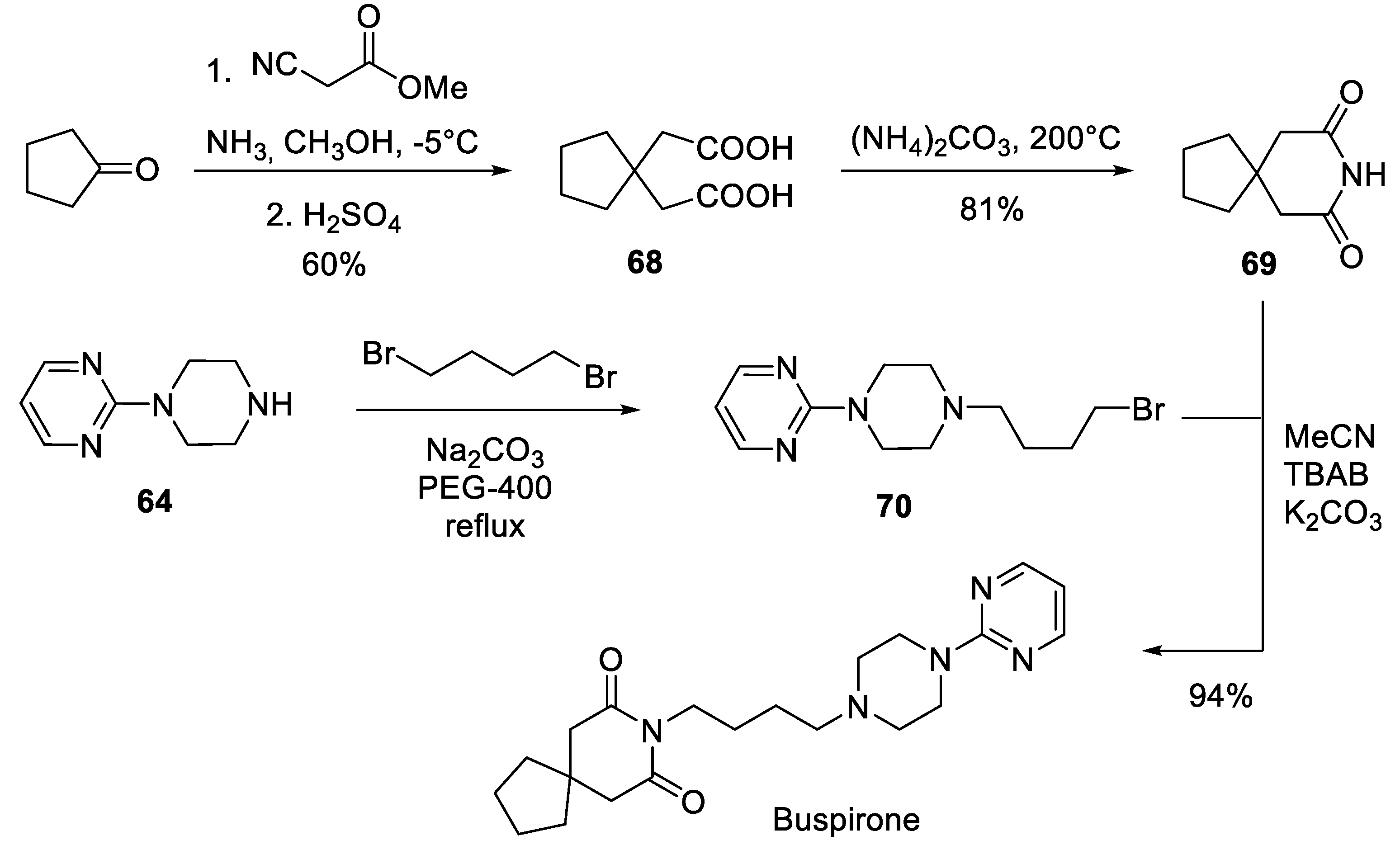
On the plant scale, the following route was initially employed for the production of buspirone. 1-(Pyrimidin-2-yl)piperazine (
64) was alkylated with 4-chlorobutyronitrile (
65) and the nitrile group was hydrogenated over Raney nickel catalyst to give butylamine
66. The latter was condensed with commercially available 3,3-tetramethylene glutaric anhydride (
67) in refluxing pyridine to give, after crystallization, buspirone [
32] (
Scheme 16).
Over more than three decades following buspirone approval for medical use, several newer synthetic routes appeared in the journal literature, some of which will be discussed below.
In 2008, Wei and co-workers from China University of Mining and Technology reported a new process for the production of buspirone. In the first step, condensation of cyclopentanone with methyl isocyanoacetate catalyzed by ammonia followed by acidic ester hydrolysis afforded cyclopentane-1,1-diacetic acid (
68). Heating of the latter with ammonium carbonate at 200 ℃ afforded 3,3-tetramethyleneglutarimide (
69). The latter was alkylated with butyl bromide
70 prepared, in turn, from 1-(pyrimidin-2-yl)piperazine (
64) by alkylation with 1,4-dibromobutane. The last step afforded buspirone in nearly quantitative yield (
Scheme 17) [
34].
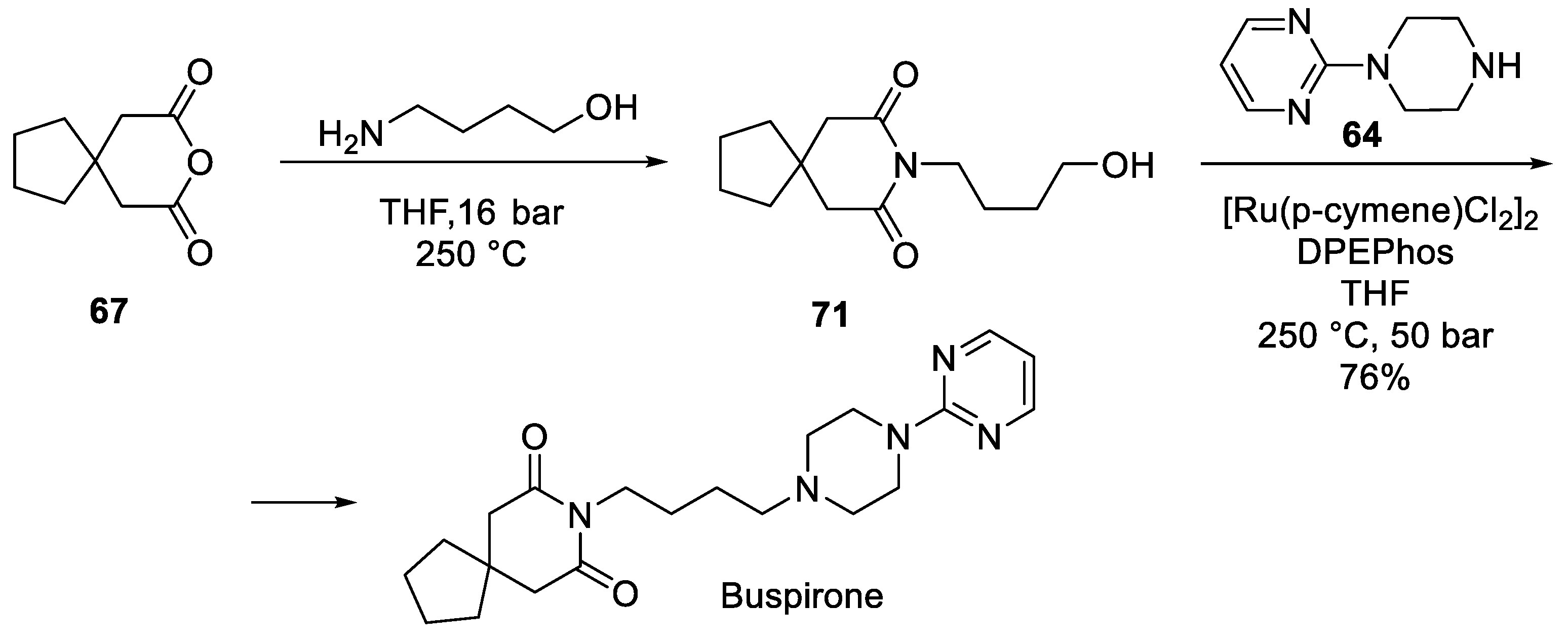
In 2019, Steven Ley and co-workers reported a continuous flow method to prepare buspirone which involves direct alkylation of 1-(pyrimidin-2-yl)piperazine (
64) with alcohol
71 via a Ru(II)-catalyzed hydrogen borrowing approach (
Scheme 18) [
35].
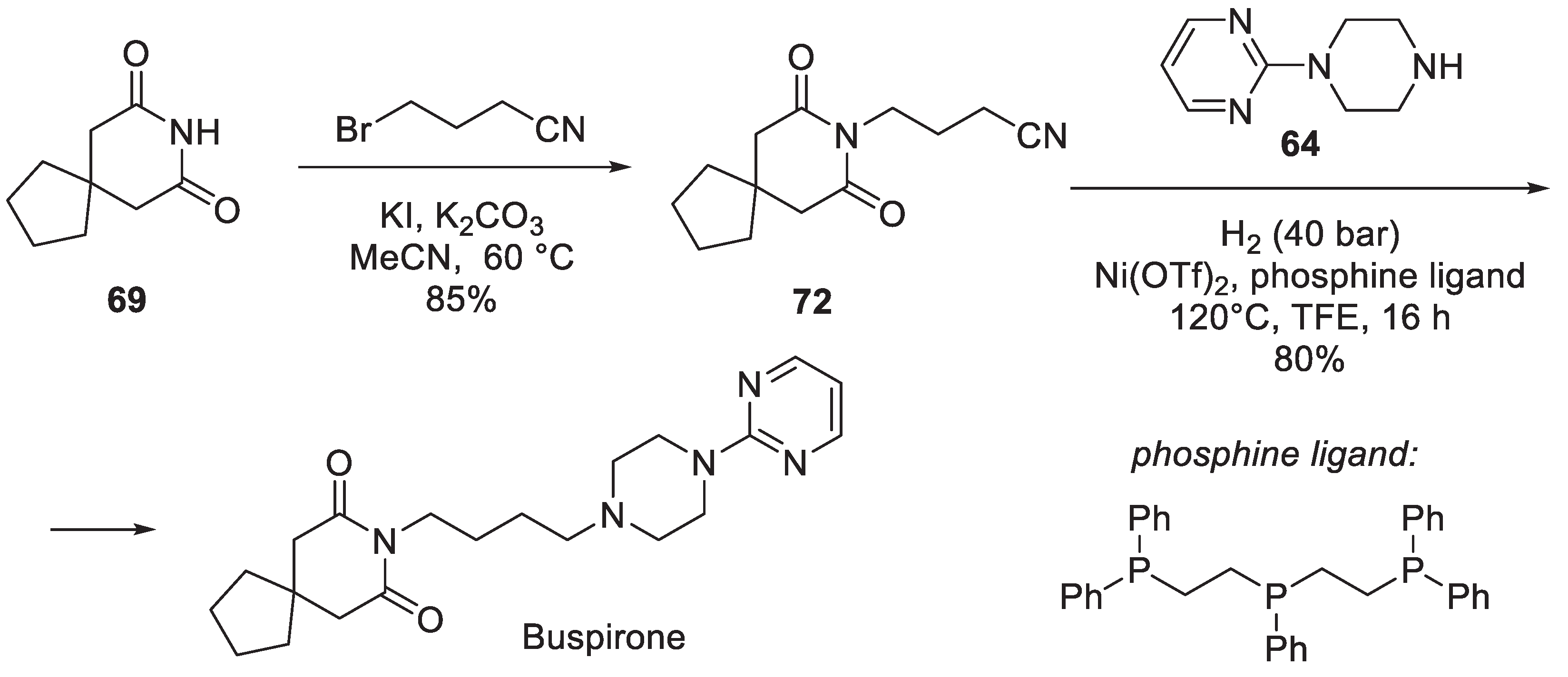
In 2022, Beller and co-workers reported a remarkable, Ni-catalyzed reductive cross-coupling of nitriles with primary and secondary amines. In order to exemplify the scope of their methodology, a novel synthesis of buspirone was presented. Nitrile
72 prepared by alkylation of 3,3-tetramethylene glutarimide (
69) with 4-bromobutyronitrile reacted 1-(pyrimidin-2-yl)piperazine (
64) under 40 bar of hydrogen in the presence of nickel triflate and a special triphosphine ligand to give 80% yield of buspirone (
Scheme 19) [
36].
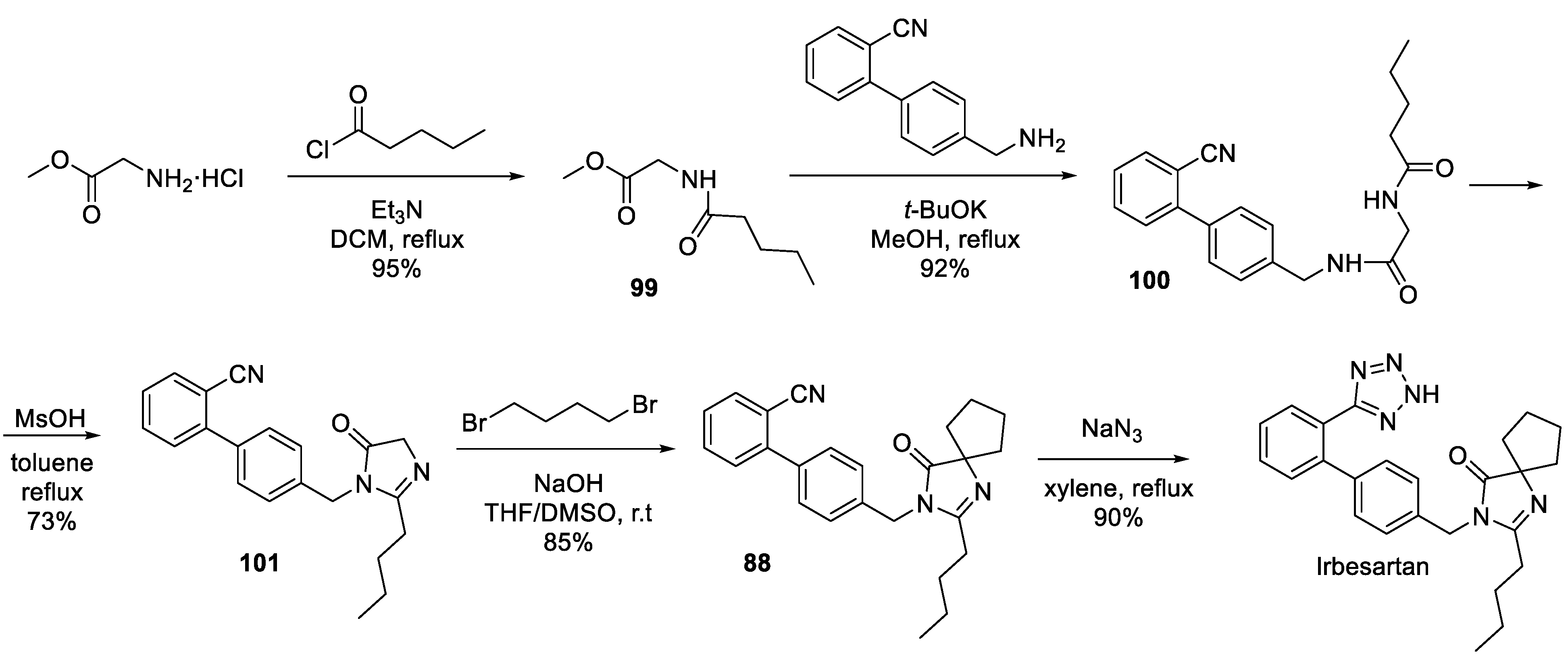
12. Irbesartan
This spirocyclic antihypertensive drug was developed by Sanofi Research (now Sanofi-Aventis) and approved for medical use in 1997. The compound is a typical representative of the sartan family of hypertensives which act by blocking the angiotensin type 1 (AT
1) receptor and preventing its activation and downstream events leading the blood pressure increase [
53]. Irbesartan possesses four key elements of structure which are typical of sartans and could be traced back to losartan, the first-in-class AT
1 receptor blocker: biphenyl substituted with tetrazole in the
ortho position of the distal ring, five-membered nitrogen heterocycle (in this case, imidazoline-5-one) and
n-butyl side chain [
54].
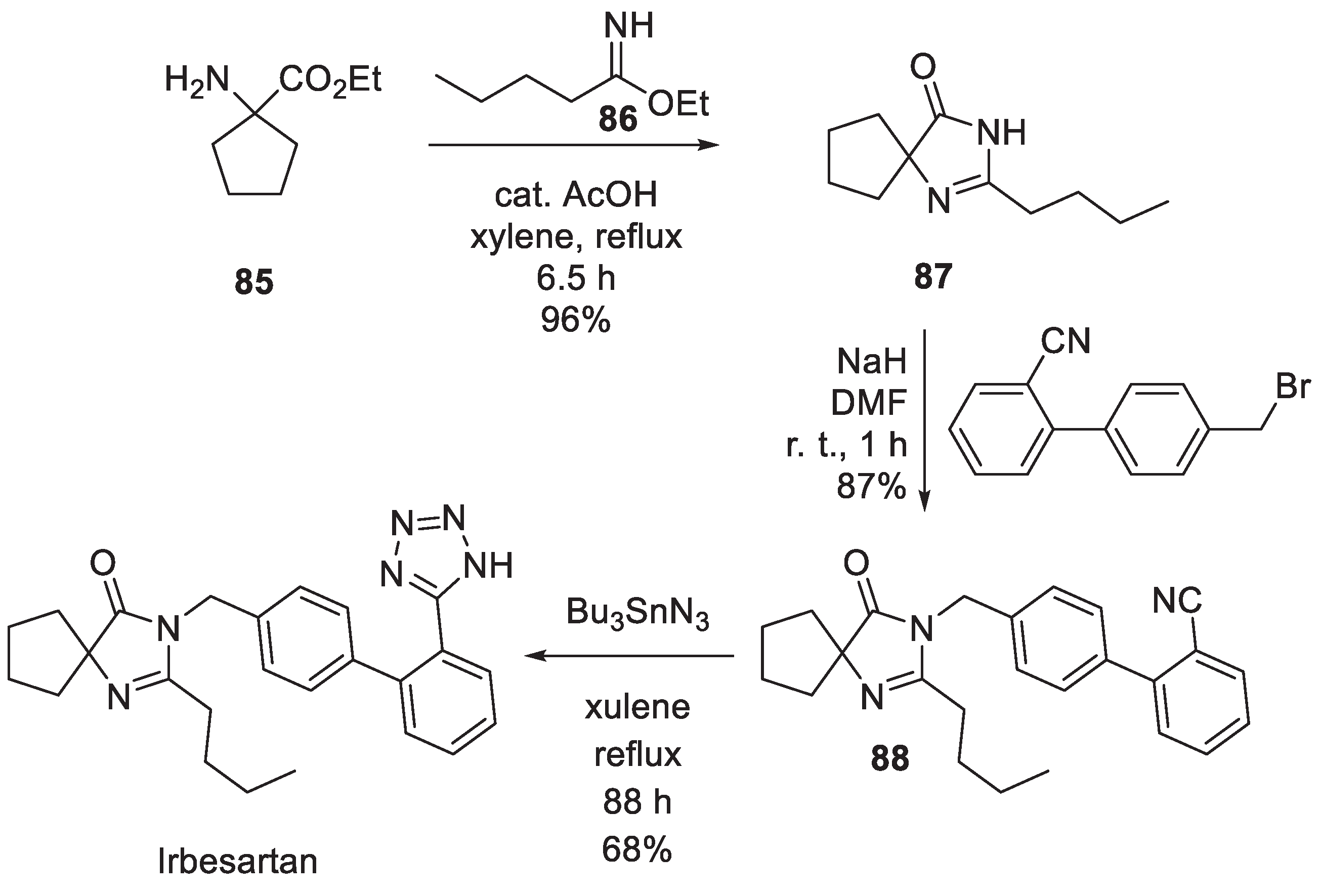
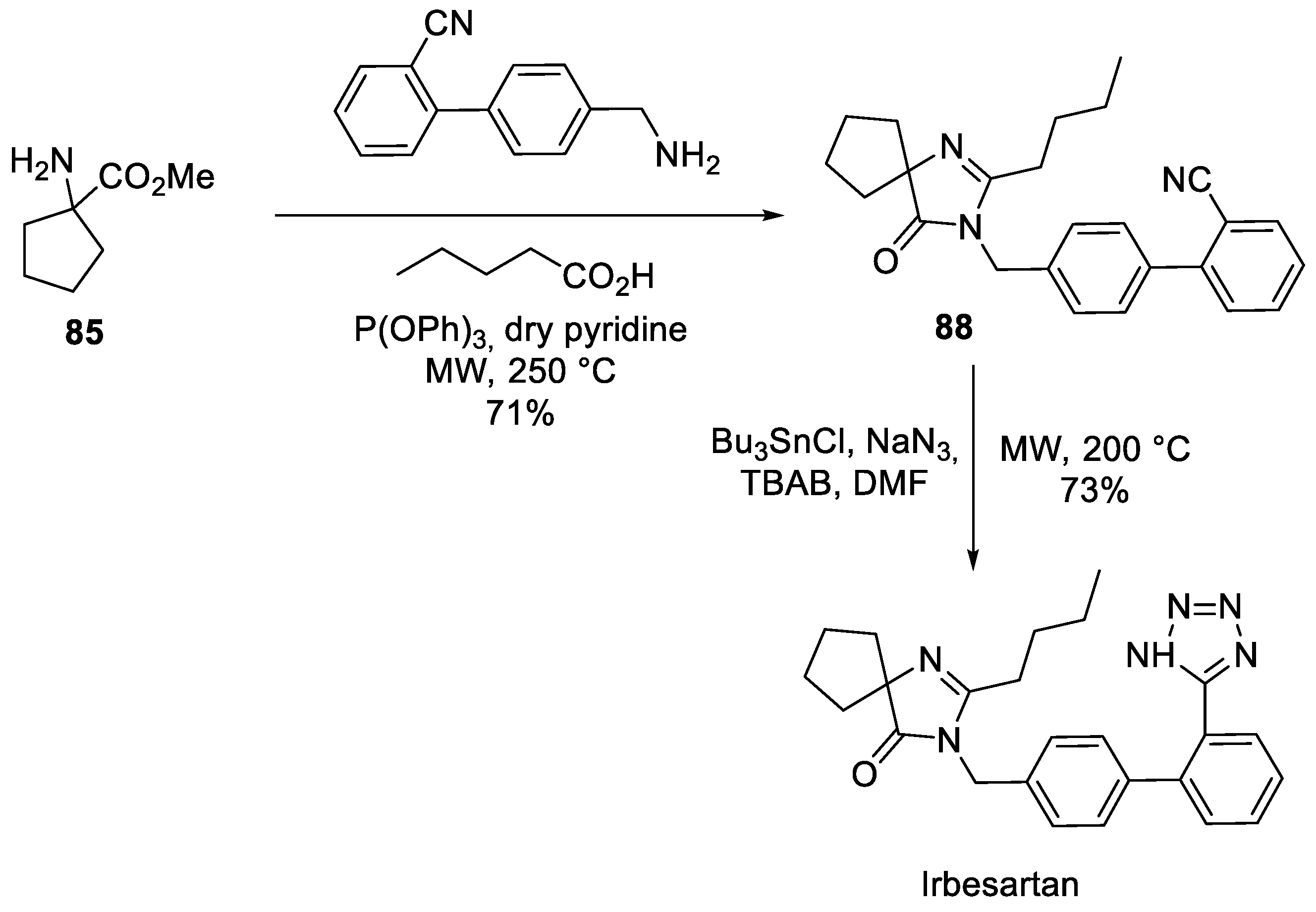
The original synthetic route to irbesartan was described in detail in their 1995 publication [
55]. The synthesis relies on commercially available tetramethylene glycine ethyl ester (
85) as the starting material. Amino ester
85 was condensed with imidate
86 in the presence of a catalytic amount of acetic acid in refluxing xylene to give imidazolin-5-one
87. The latter was alkylated with 4'-(bromomethyl)-[1,1'-biphenyl]-2-carbonitrile and the resulting derivative
88 was treated with tributyl tin azide in refluxing xylene to give irbesartan (
Scheme 24).
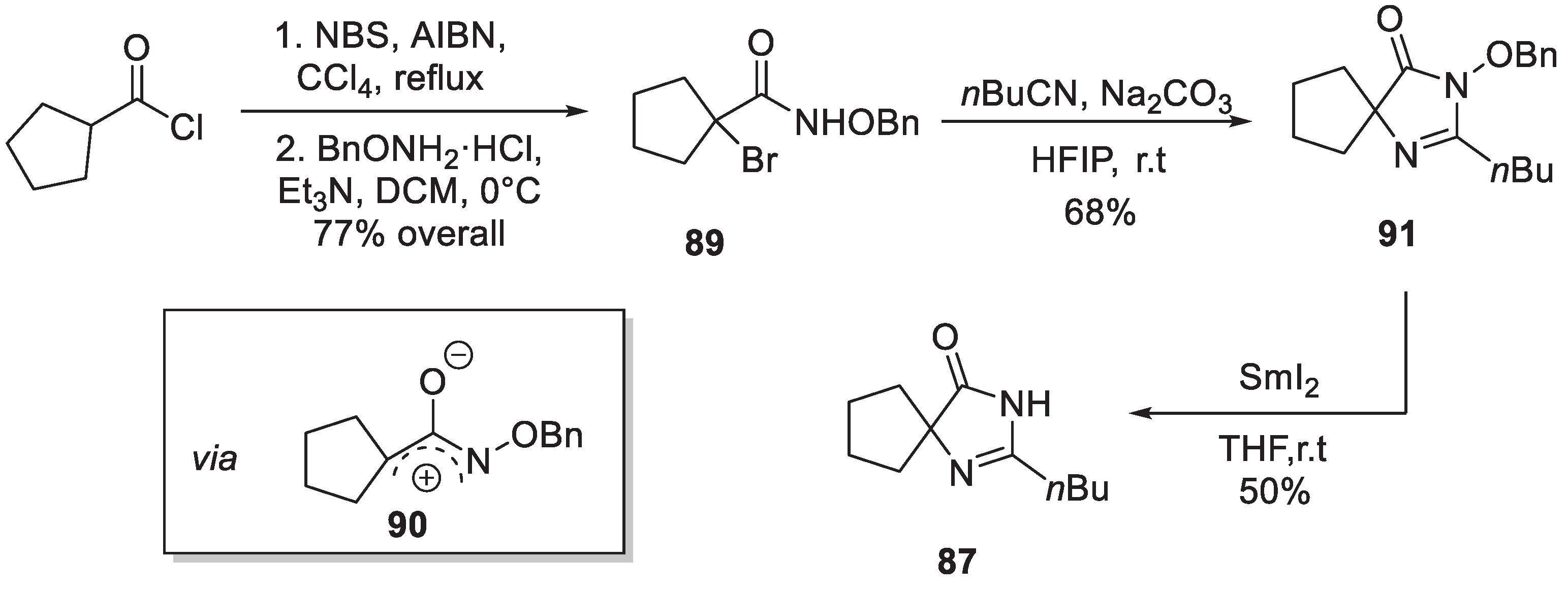
Interestingly, the key spirocyclic imidazolin-5-one building block
87 was recently accessed by Wu and DiPoto
via a novel annulation of azaoxyallyl cations. Cyclopentanoyl chloride was first transformed into
O-benzyl hydroxamic acid
89. The latter was treated with the base to generate azaoxyallyl cation
90 which reacted with pentanenitrile to give benzyloxy-substituted imidazolin-5-one
91.
N-Deoxygenation with samarium iodide gave
87 thereby completing the formal synthesis of irbesartan (
Scheme 25) [
56].
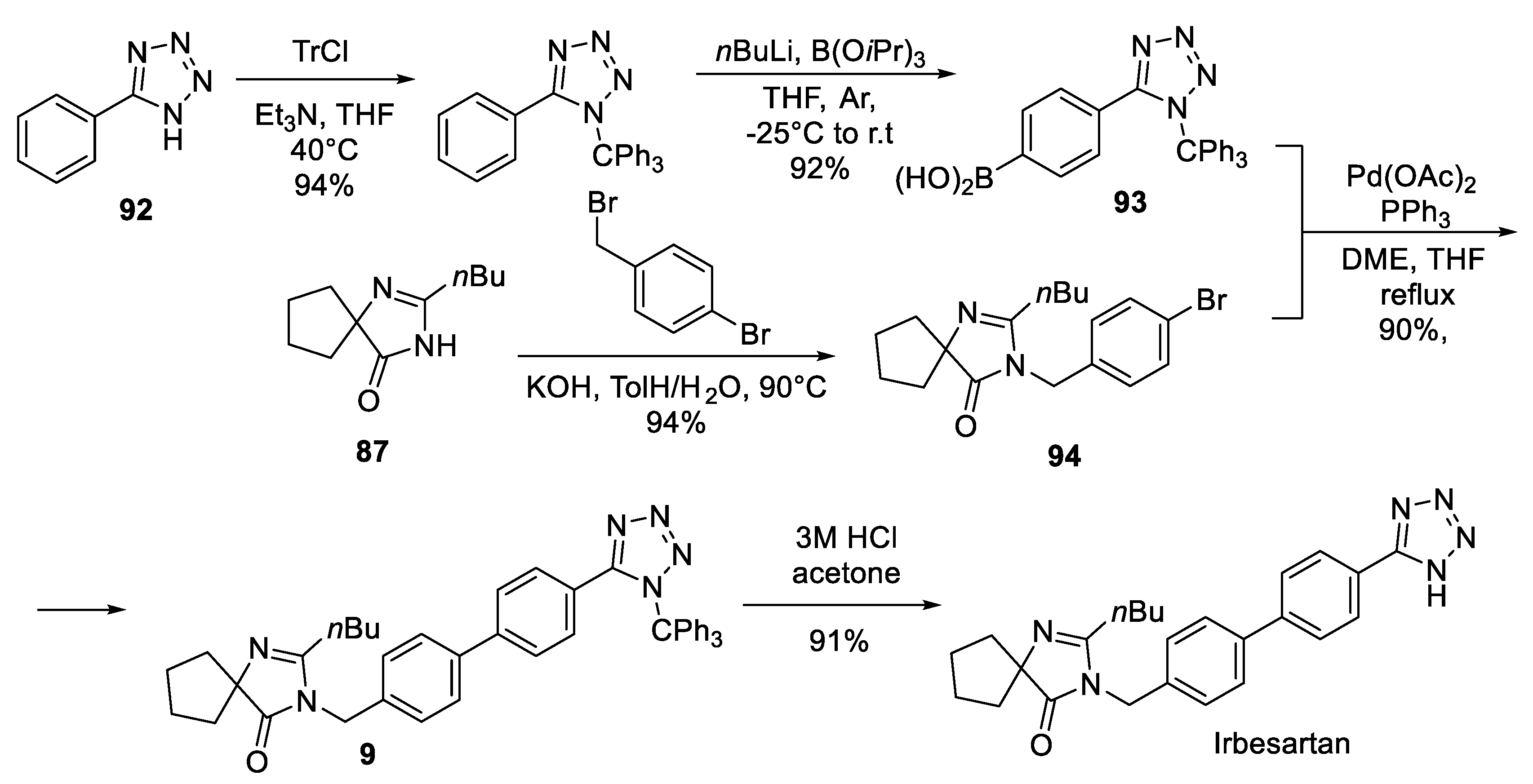
Intermediate
87 was also key in the synthesis of irbesartan patented by a group of Israeli scientists in 2004 [
57]. The convergent route started with
C-phenyl tetrazole (
92) which was protected with a trityl group and borylated in the
para position via lithiation to give boronic acid
93. In parallel, intermediate
87 was
N-alkylated with
p-bromobenzyl bromide and resulting intermediate
94 was involved in Suzuki coupling with boronic acid
93 to give trityl-protected irbesartan
95. Removal of the trityl group furnished the target molecule (
Scheme 26).
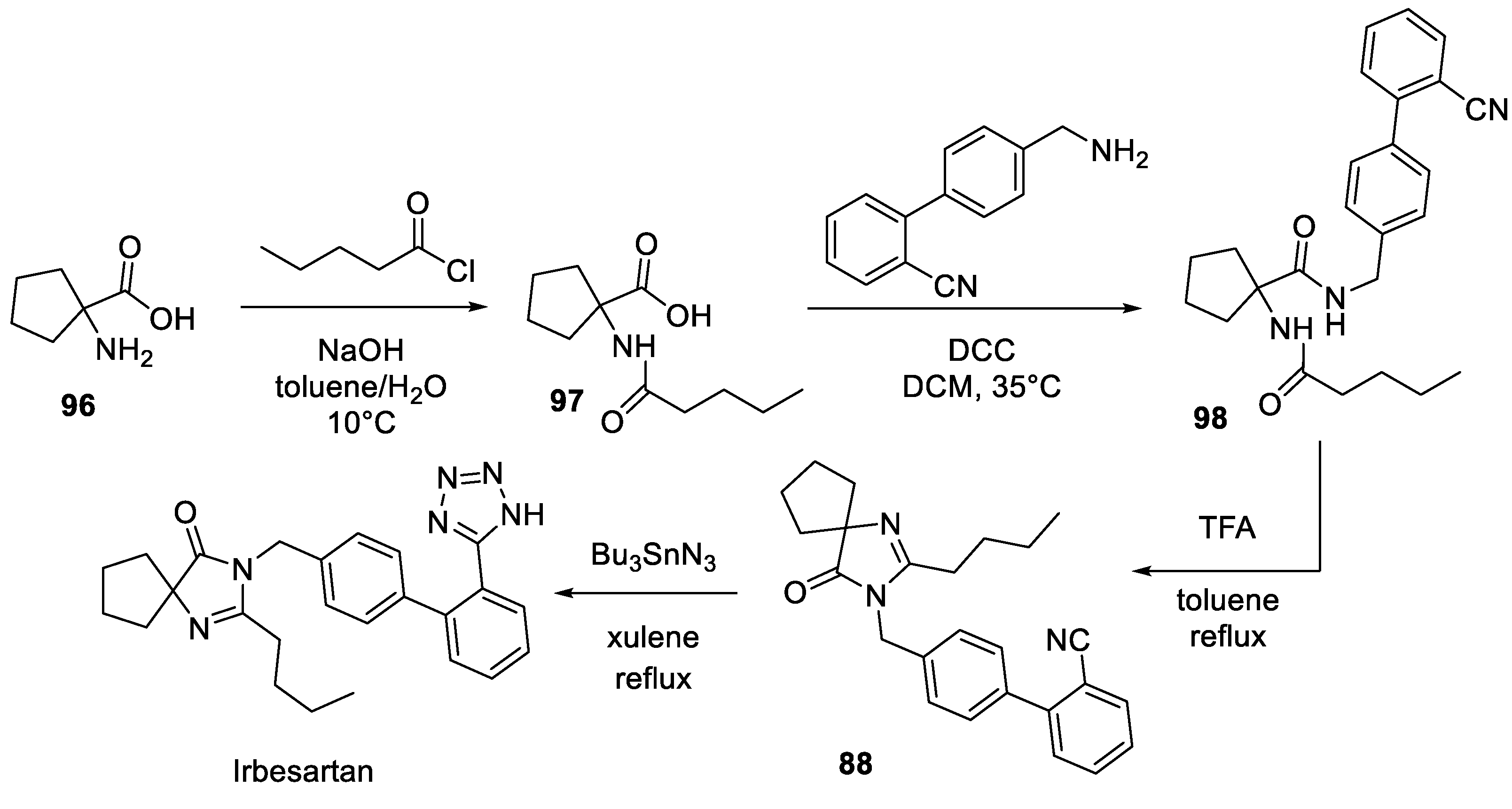
Among the synthetic routes to irbesartan featured in the patent literature, the following synthesis from Dr. Reddy’s Laboratories is notable. Similarly to the Sanofi route (
vide supra), the synthesis starts with tetramethylene glycine (
96) which was acylated with pentanoyl chloride under phase transfer conditions to give carboxylic acid
97 which, in turn, was amidated with 4'-(aminomethyl)-[1,1'-biphenyl]-2-carbonitrile to give ‘fully decorated’ precursor
98. The latter was cyclodehydrated in refluxing toluene in the presence of trifluoroacetic acid to give nitrile
88 and, after [3+2] cycloaddition with tributyltin azide, irbesartan (
Scheme 27) [
58].
An approach rather similar in spirit to the Dr. Reddy’s route was described in 2006 by ArQule scientists [
59]. It is based on three-component, microwave-promoted cyclodehydrative synthesis of irbesartan’s key precursor
88 from ester
85, 4'-(aminomethyl)-[1,1'-biphenyl]-2-carbonitrile and pentanoic acid in the presence of triphenoxyphosphine. The conversion of nitrile
88 to irbesartan was achieved
via in situ generation of tributyltin azide (
Scheme 28).
Finally, a fundamentally different approach to irbesartan was developed in 2022 at Beijing University of Chemical Technology [
60]. In this route, the spirocyclic scaffold of the drug was elaborated at a more advanced stage of the synthesis as shown in
Scheme 29. Glycine methyl ester was acylated by pentanoyl chloride and ester
99 was amidated with 4'-(aminomethyl)-[1,1'-biphenyl]-2-carbonitrile under forcing, base-promoted conditions to give diamide
100. The latter was cyclodehydrated in refluxing toluene in the presence of methanesulfonic acid to give intermediate
101 which was found to be sufficiently C-H acidic to be doubly alkylated with 1,4-dibromobutane to furnish spirocyclic imidazoline-5-one
88. Notably, the nitrile-to-tetrazole conversion was achieved without the use of the toxic tin reagent (used in all previously described syntheses) which makes this 2022 route quite environmentally friendly and amenable to the plant scale production of the drug.
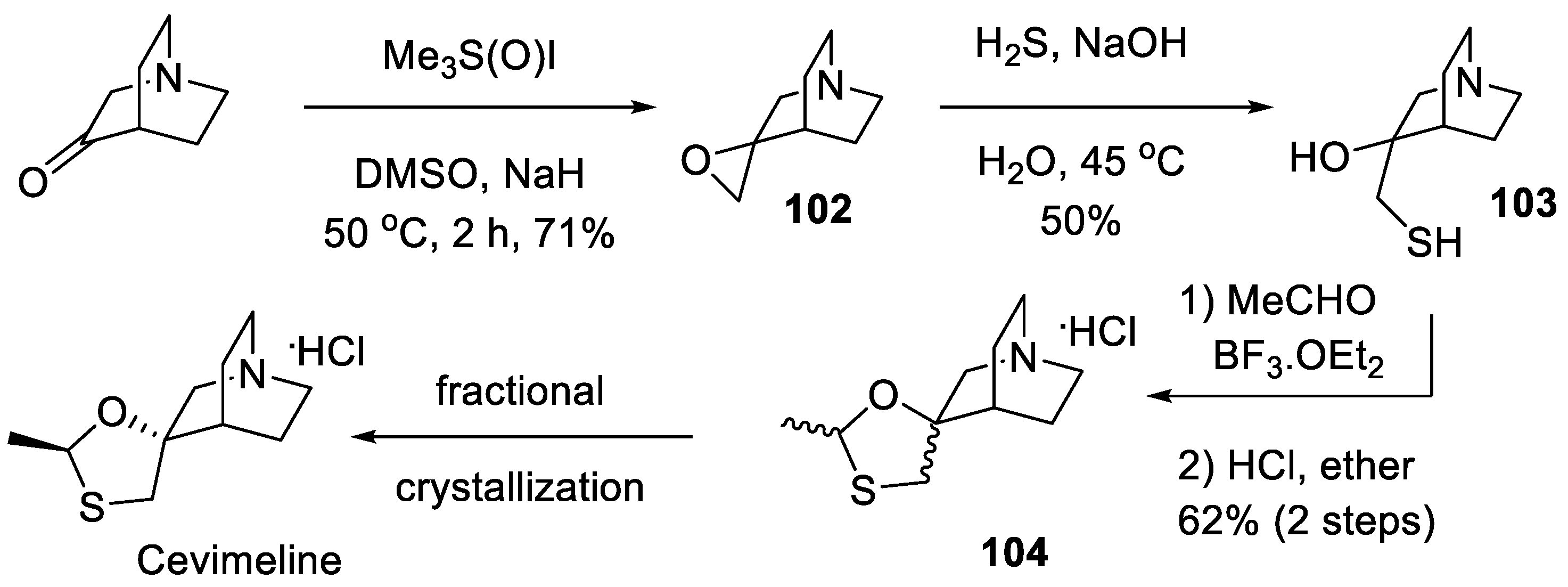
13. Cevimeline
This spirocyclic derivative of quinuclidine acts as an agonist of muscarinic M
1 and M
3 receptors [
61]. Originated from Israel Institute of Biological Research, the drug was developed by Daiichi Sankyo company and gained regulatory approval in 2000 for the treatment of dry mouth and Sjögren's syndrome [
62]. Recently, the drug has been investigated on such therapeutic areas as cognitive impairment and neurodegenerative diseases [
63].
In the original synthesis of cevimeline patented by the Israeli group [
64], commercially available quinuclidin-3-one was converted to epoxide
102 using the Corey-Chaykovsky reaction with trimethyloxosulfonium iodide (Me
3S
+(O)I
-) and sodium hydride. Resulting epoxide
102 was treated with hydrogen sulfide in basic aqueous medium, which led to epoxide opening and the formation of hydroxy thiol
103. The latter was subjected to thio/oxa acetal formation on treatment with acetaldehyde in the presence of boron trifluoride etherate. Cevimeline contained in the diastereomeric mixture of products
104 was separated by fractional crystallization (
Scheme 30). The patent [
64] presented a rather elaborate scheme for the fractional crystallization.
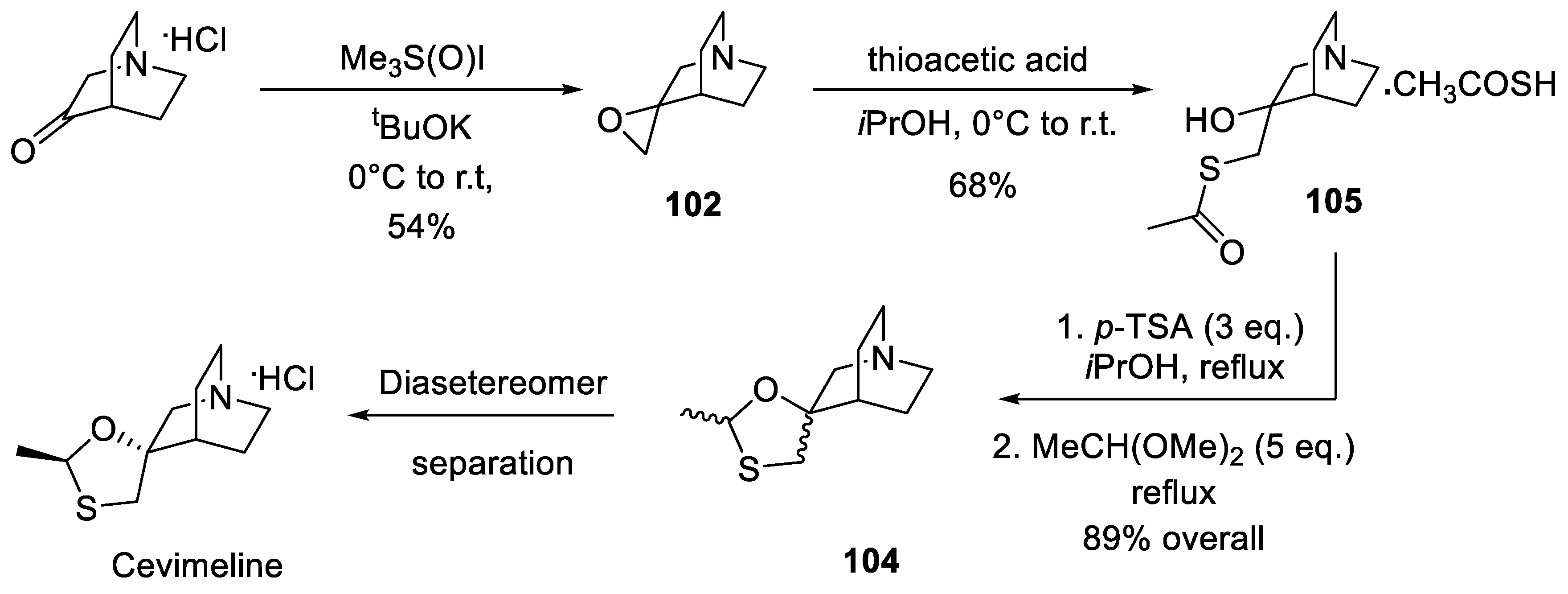
In 2008, Canadian company Apotex Pharmachem, Inc. filed a patent application on an improved synthesis of cevimeline which is similar in spirit to the original Israeli route. The Corey-Chaykovsky epoxidation of quinuclidine-3-one was performed with potassium
tert-butoxide and the opening of epoxide
102 was achieved with thioacetic acid in lieu of hydrogen sulfide gas. Thioacetate
105 was condensed directly with acetaldehyde dimethyl acetal which gave an excellent yield of diastereomeric mixture
104 from which cevimeline was isolated by diastereomer separation (
Scheme 31) [
65].
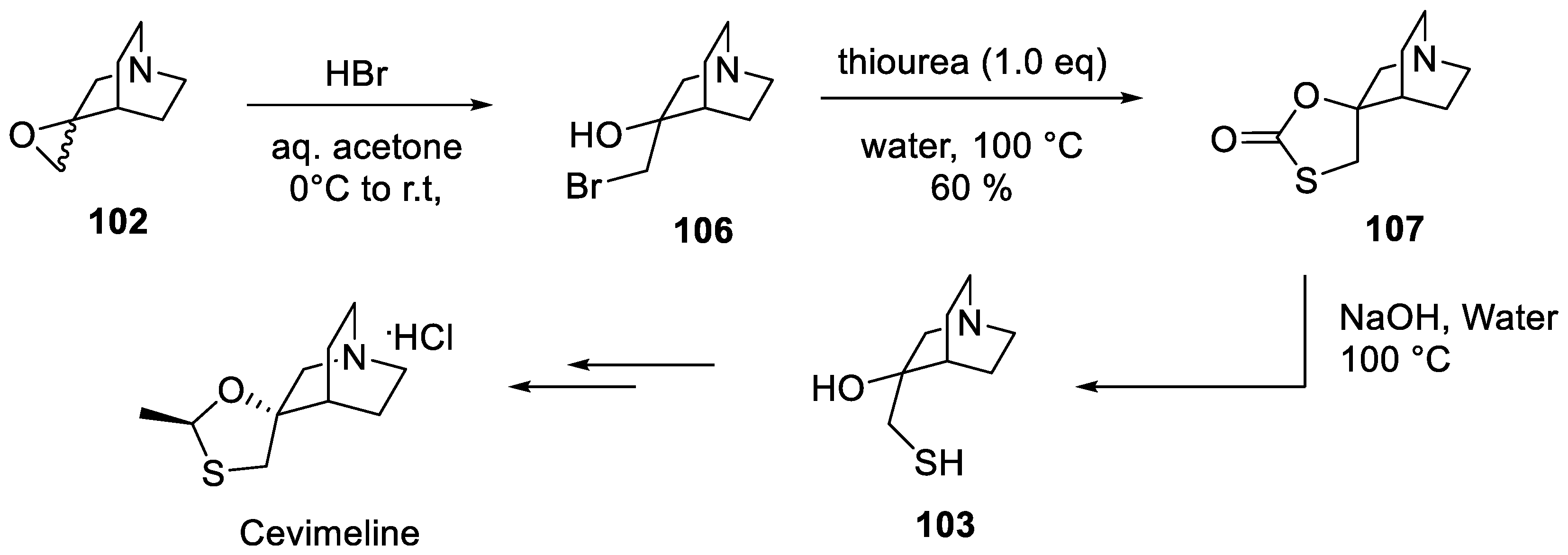
In 2013, the Active Pharmaceutical Ingredient R&D Center of Indian company Emcure Pharmaceuticals, Ltd. published a yet further improved route to cevimeline in which safe and odorless thiourea is utilized as a thiolating reagent. Epoxide
102 was first converted to hydroxy bromide
106 which was then refluxed with thiourea in aqueous medium to furnish thiolactone
107. The latter was hydrolyzed with aqueous sodium hydroxide to give key hydroxy thiol
103 which was converted to cevimeline using the same routine as was developed in the original synthesis (
vide supra) (
Scheme 32) [
66].
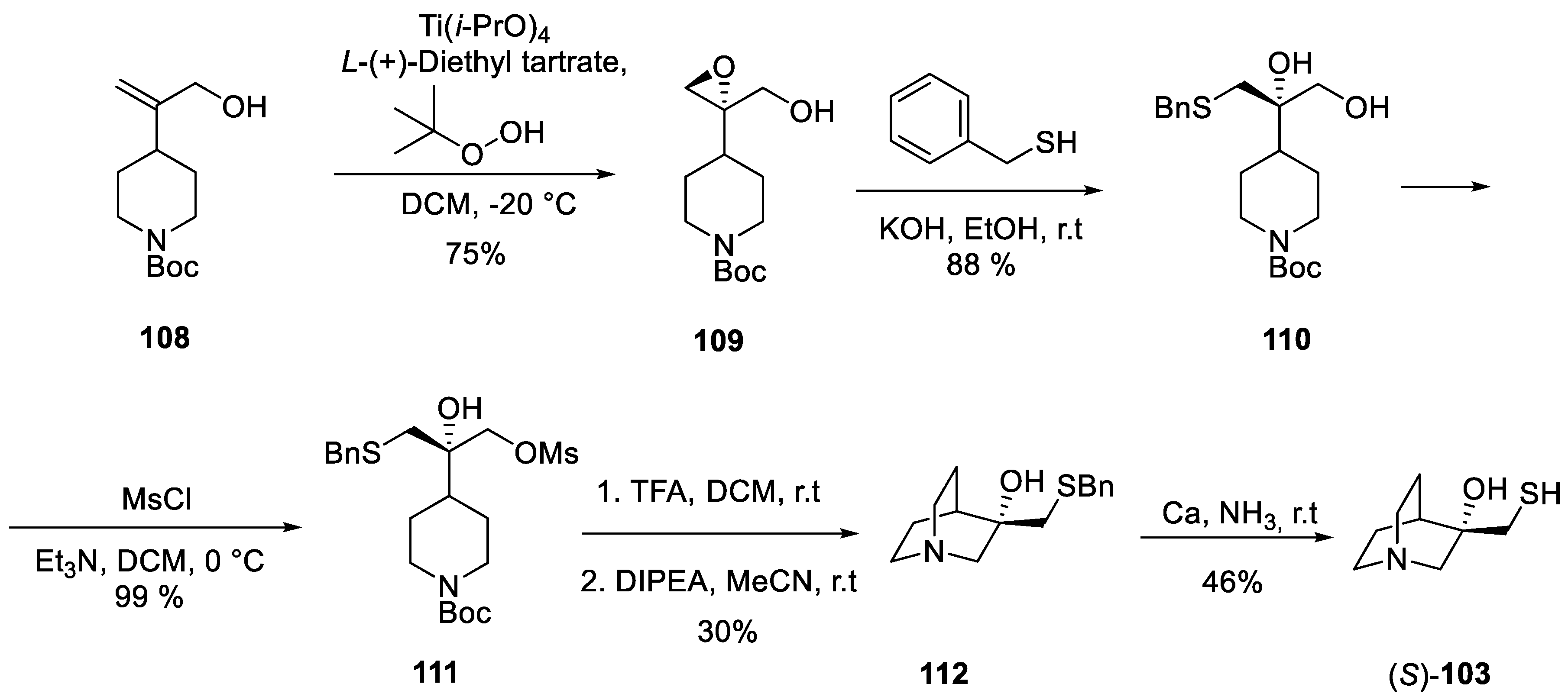
An intriguing, stand-alone approach to enantiomerically pure hydroxy thiol (
S)-
103 was published by F. Hoffmann-La Roche, Ltd. six years prior to cevimeline approval for medical use [
67]. In this synthesis, the quinuclidine nucleus was elaborated quite late into the synthetic route. The synthesis relied on olefin
108 synthesized from pyridine-4-carboxaldehyde in a few trivial steps. Sharpless epoxidation gave enantiomerically pure (
ee 94%) epoxide
109 which was opened with benzyl mercaptan to give diol
110. Mesylation of the primary hydroxy group set the scene for the intramolecular S
N2 reaction to form the quinuclidine core. This was achieved by removal of the Boc group from the piperidine nitrogen atom in
111 and triggering the nucleophilic substitution by DIPEA. The bnzyl group was removed from intermediate
112 by dissolving metal (Ca) reduction in ammonia (
Scheme 33).
14. Eplerenone
This spirocyclic antagonist of mineralocorticoid receptors developed by Ciba-Geigy was approved in 2002 for the treatment of hypertension and hear failure [
68]. It is related to spironolactone but displays better selectivity to its primary target over the affinity to androgen, progestogen, glucocorticoid, or estrogen receptors and thus is devoid of undesired sexual side effects typical for spironolactone which limit its use [
69].
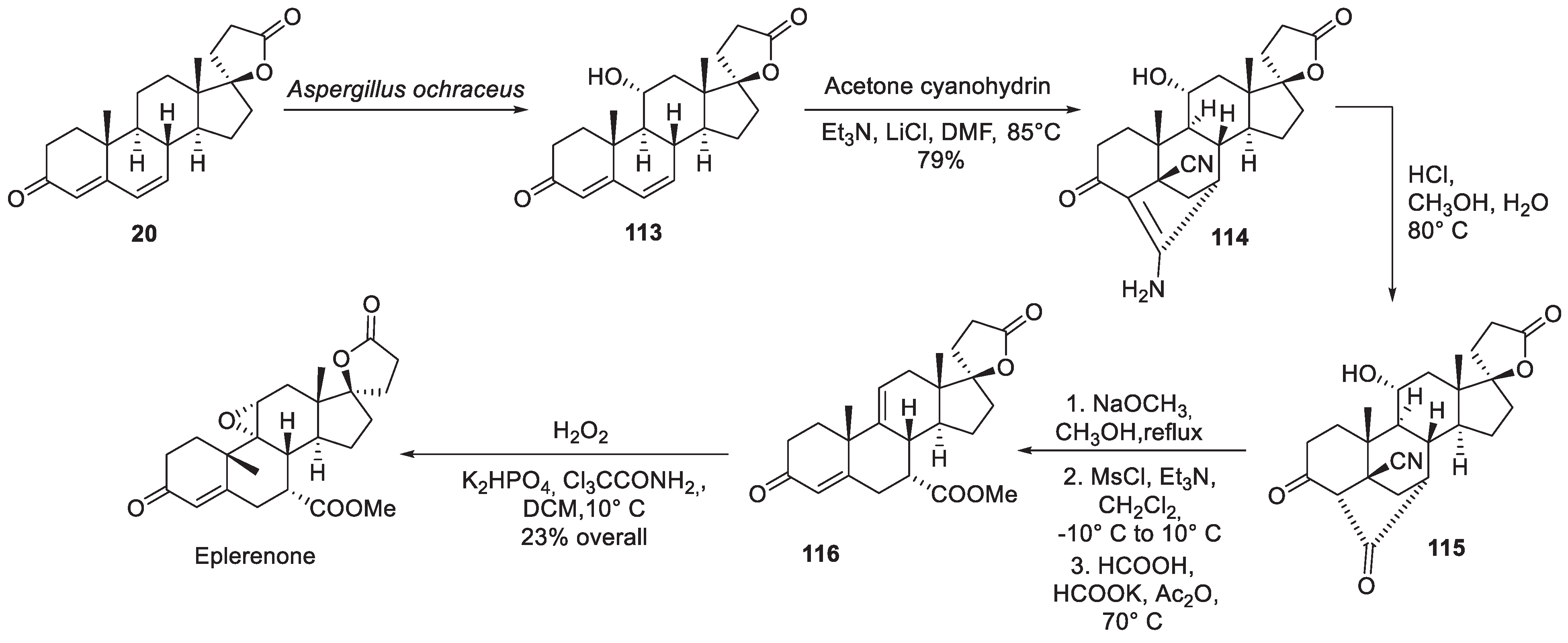
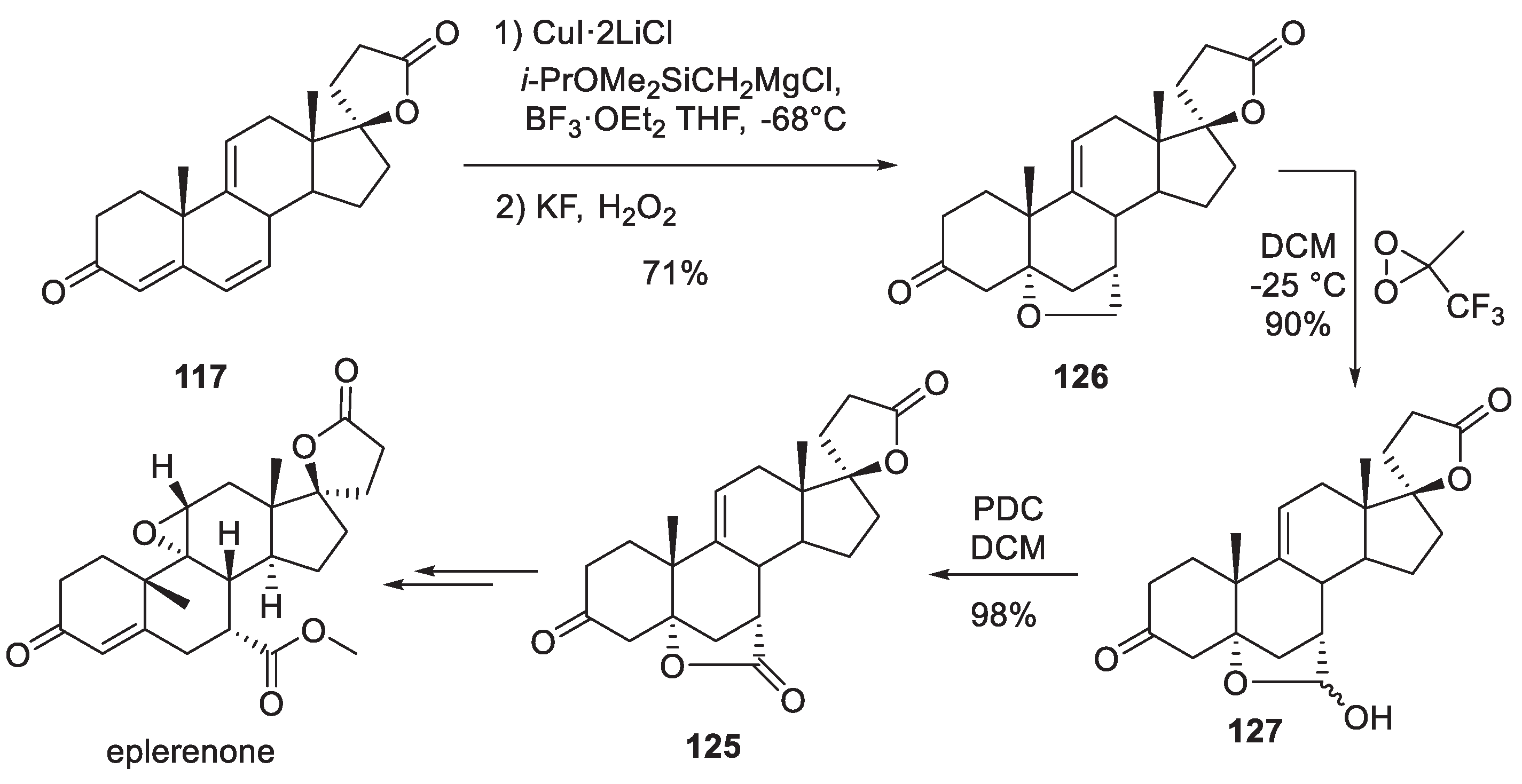
Among industrial syntheses of eplerenone, the route patented in 1998 by Searle & Co. is quite notable [
70]. The synthesis starts with steroid intermediate
20 (also useful in the synthesis of spironolactone,
vide supra) which is subjected to regiospecific and stereoselective hydroxylation by fermentation with
Aspergillus ochraceus mold. The hydroxy group in
113 serves as a kind of masked double bond which is unmasked later into the synthesis. Condensation with two equivalents of cyanide (in the form of acetone cyanohydrin) results in the formation of polycyclic enamine
114. The latter is hydrolyzed to ketone
115 which was then put through a sequence of steps including treatment with sodium methoxide (opening the ketone linkage as the methyl ester with simultaneous elimination of the cyanide), mesylation of the hydroxy group and elimination of methane sulfonic acid to give olefinic intermediate
116. The latter was epoxidized on treatment with hydrogen peroxide in phosphate buffered solution to give eplerenone (
Scheme 34).
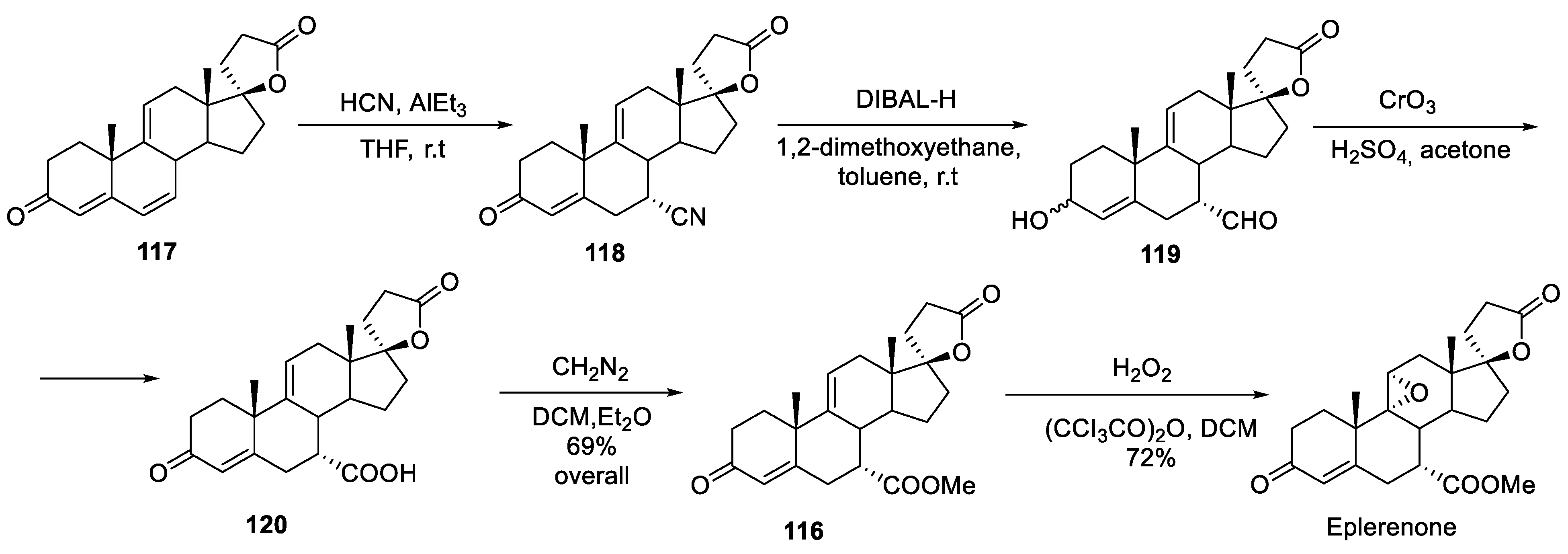
In 1997, Ciba-Geigy chemists published their synthetic route to eplerenone which basically provides another approach to the synthesis of intermediate
116 starting from spirocyclic steroid lactone
117. Conjugate addition of hydrogen cyanide at the -position proceeded stereoselectively and gave intermediate
118. Reduction with DIBAL-H gave aldehyde alcohol
119 which was oxidized into keto carboxylic acid
120. Esterification with diazomethane gave intermediate
116 (at which point the overall yield over four steps was a remarkable 69%). Finally, epoxidation with hydrogen peroxide gave eplerenone (
Scheme 35) [
71].
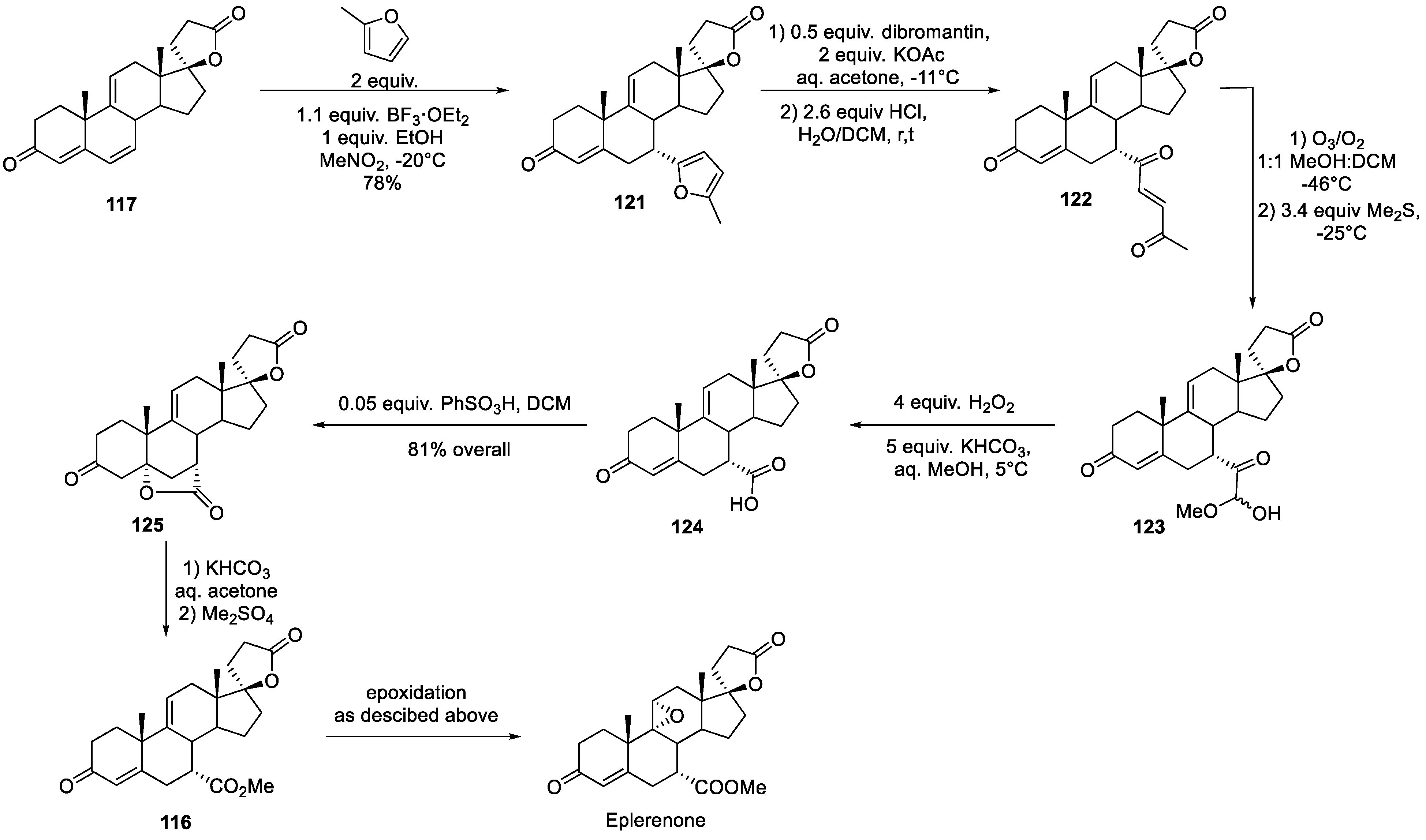
An interesting eplerenone synthesis was published in 2006 by Pfizer’s process chemistry team [
72]. The synthesis commences with the same spirocyclic lactone
117 as was employed by Ciba-Geigy (
vide supra). 2-Methylfuran was employed as a chemical equivalent of the carboxylic group. On Lewis acid catalysis, it underwent arylation of the distal conjugated double bond. The furan moiety in
121 was hydrolyzed to enedione
122 and the latter, without isolation, was ozonolyzed to give hemiacetal
123. The latter was subjected to Beyer-Villiger oxidation to give carboxylic acid
124 which was forced to close to isolable bis-lactone
125. The strained lactone in
125 was opened up
via elimination and intermediate carboxylic acid was esterified with methyl sulfate to give ester
116. The latter is only one epoxidation step away from eplerenone (
Scheme 36).
Intermediate
125 is clearly key to the large-scale synthesis of eplerenone. In 2011, a Chinese Academy of Sciences group published an interesting approach to it from triene
117 which involves conjugate addition of silyl methyl cuprate, Tamao oxidation and another intramolecular oxa-Michael addition to give cyclic ether
126. Curiously, methyltrifluoromethyl dioxirane was found to selectively oxidize ether
126 to lactol
127. PDC oxidation of the latter gave a nearly quantitative yield of lactone
125 which could now be opened and epoxidized to eplerenone as described by Pfizer (
vide supra) (
Scheme 37) [
73].
15. Drospirenone
This is another steroid spirocyclic lactone drug structurally related to spironolactone and eplerenone, antagonists of mineralocorticoid receptors. However, pharmacologically drospirenone is different from the latter two drugs. In addition to high affinity to mineralocorticoid receptors, it binds rather tightly to progesterone receptors and less tightly to androgen receptors [
74], which underlines its use as a birth control medication approved in 2000 [
75].
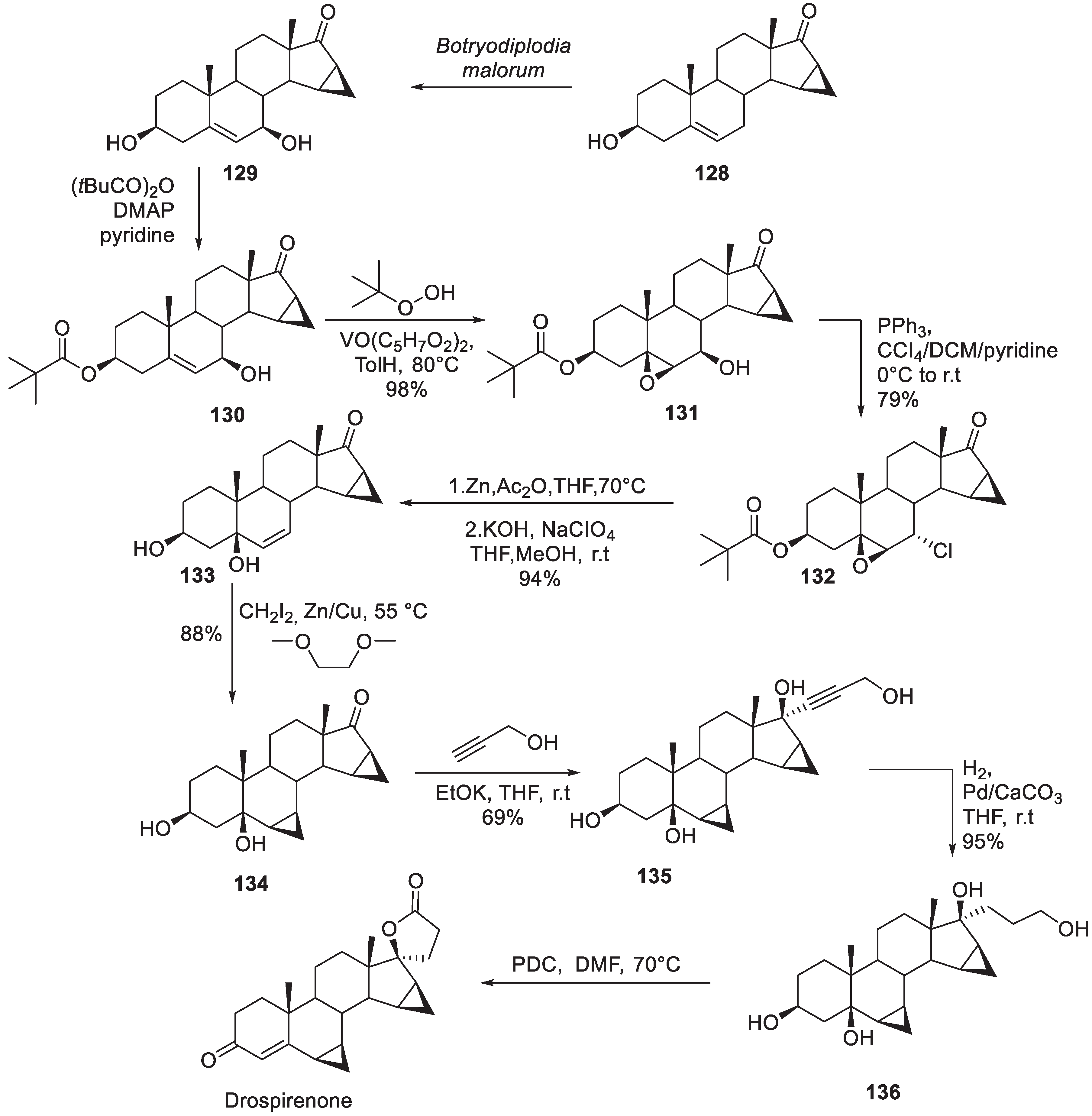
The earliest synthesis of drospirenone was published by Schering AG in 1982 [
76]. The synthesis commenced with compound
128 obtained from 3-hydroxy-5,15-androstadien-17-one by Corey cyclopropanation. Microbial hydroxylation of
128 gave diol
129 which was mono-pivaloylated at the less hindered hydroxy group to give allylic alcohol
130. The olefinic portion of
130 was epoxidized with
tert-butyl hydroperoxide under vanadium(IV) oxide-acetylacetonate catalysis. Resulting epoxide
131 which was converted to chloride
132 by treatment with Ph
3P/CCl
4 in pyridine. Reductive elimination of the chlorine atom on treatment with zinc in acetic acid followed by saponification produced diol
133. The latter was subjected to Simmons-Smith cyclopropanation to establish the basic core of the target compound (
134). Now the scene was set to establish the spirocyclic lactone moiety. Propargylic alcohol was doubly deprotonated and added to the ketone portion of
134 to give propargylic tetraol
135. The triple bond in
135 was hydrogenated over calcium carbonate. Resulting compound
136 was treated with PDC which triggered three events: oxidation of the primary alcohol moiety to carboxylic acid and lactonization, oxidation of the secondary alcohol to ketone and dehydration to form -unsaturated system of drospirenone (
Scheme 38).
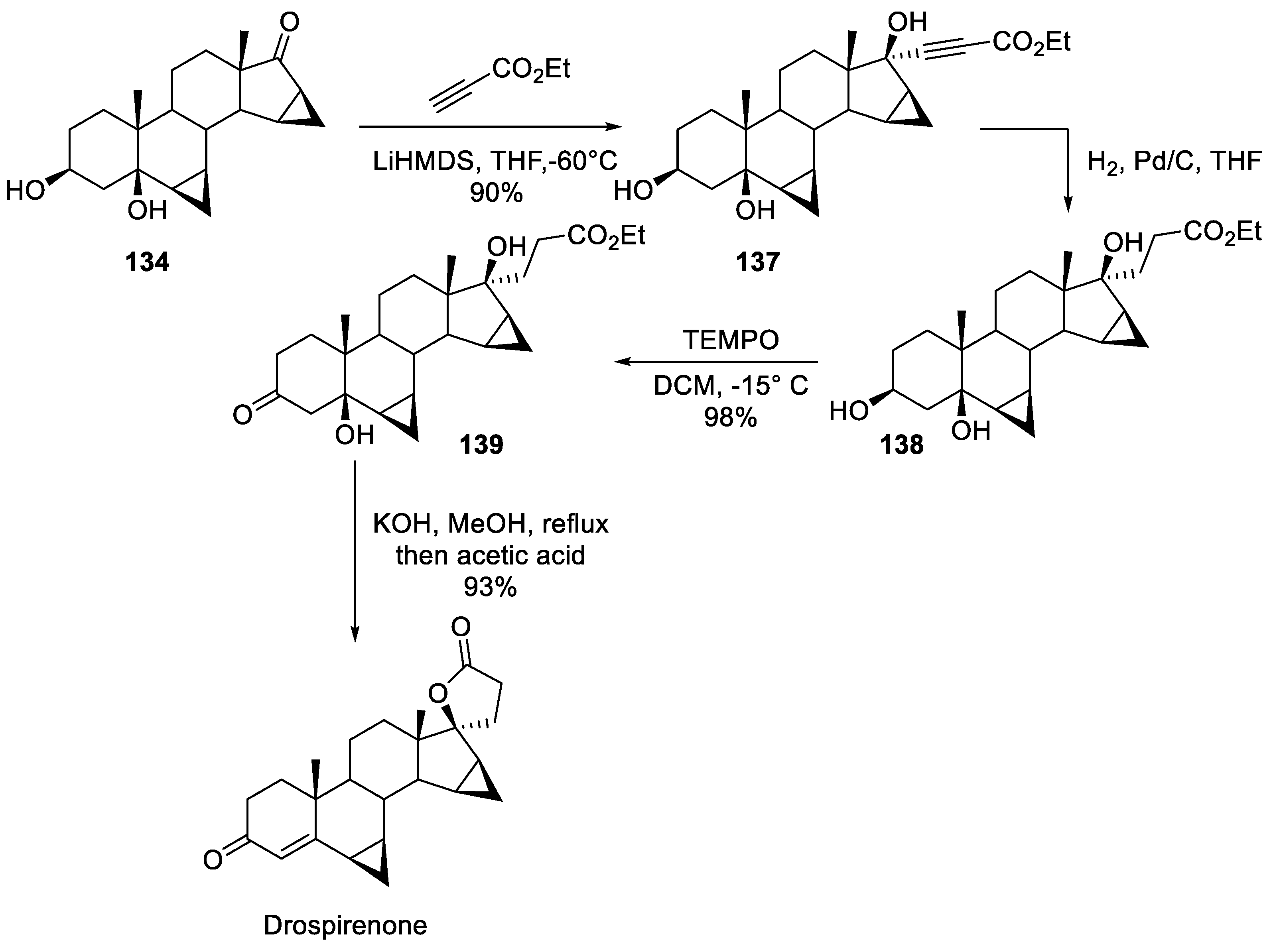
An approach similar in spirit to the synthesis of drospirenone by Schering AG was patented in 2010 by Evestra, Inc. [
77]. The synthesis starts with bis-cyclopropane
134 to the carbonyl group of which
C-anion of ethyl propiolate was added. Hydrogenation of the triple bond in resulting intermediate
137 gave triol
138 in which the secondary alcohol moiety was oxidized by TEMPO. Treatment of ketone
139 with methanolic potassium hydroxide followed by acidification with acetic acid led to the formation of spirocyclic lactone and dehydration of the -hydroxy cyclohexanone moiety which resulted in drospirenone (
Scheme 39).
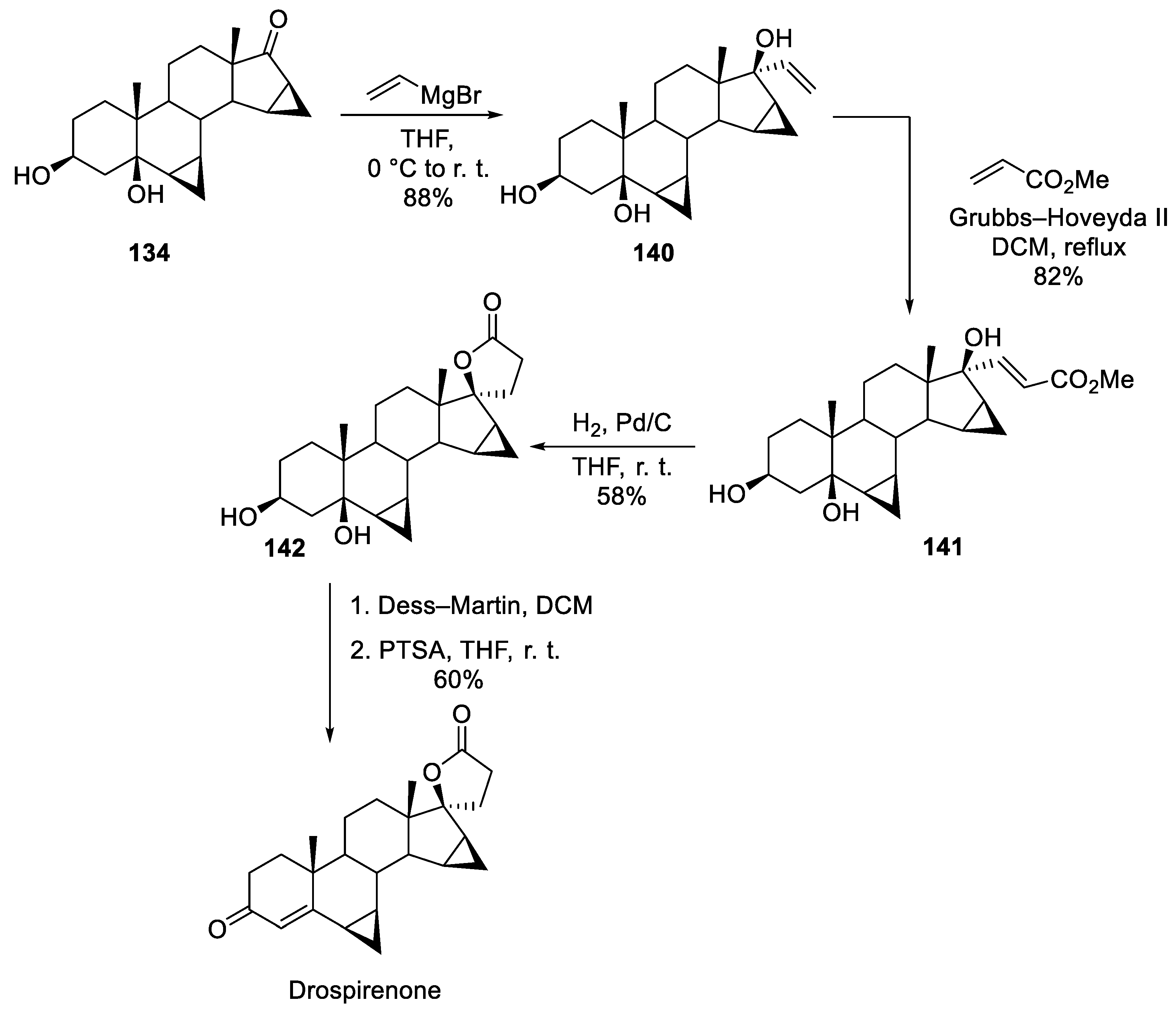
A different approach to the construction of drospirenone’s spirocyclic lactone motif was reported in 2008 by a University of Bologna group [
78]. Ketone
134 was treated with excess vinyl magnesium bromide. Resulting tertiary allylic alcohol
140 was involved in olefin metathesis reaction with methyl acrylate catalyzed by 2
nd generation Grubbs-Hoveyda catalyst. Resulting intermediate
141 having all requisite carbon atoms necessary for the formation of spirocyclic -lactone was hydrogenated over palladium on charcoal which resulted in the reduction of the double bond and the lactone ring closure to give
142. Final steps toward drospirenone were Dess-Martin oxidation of the secondary alcohol moiety and dehydration of the resulting ketone (
Scheme 40).
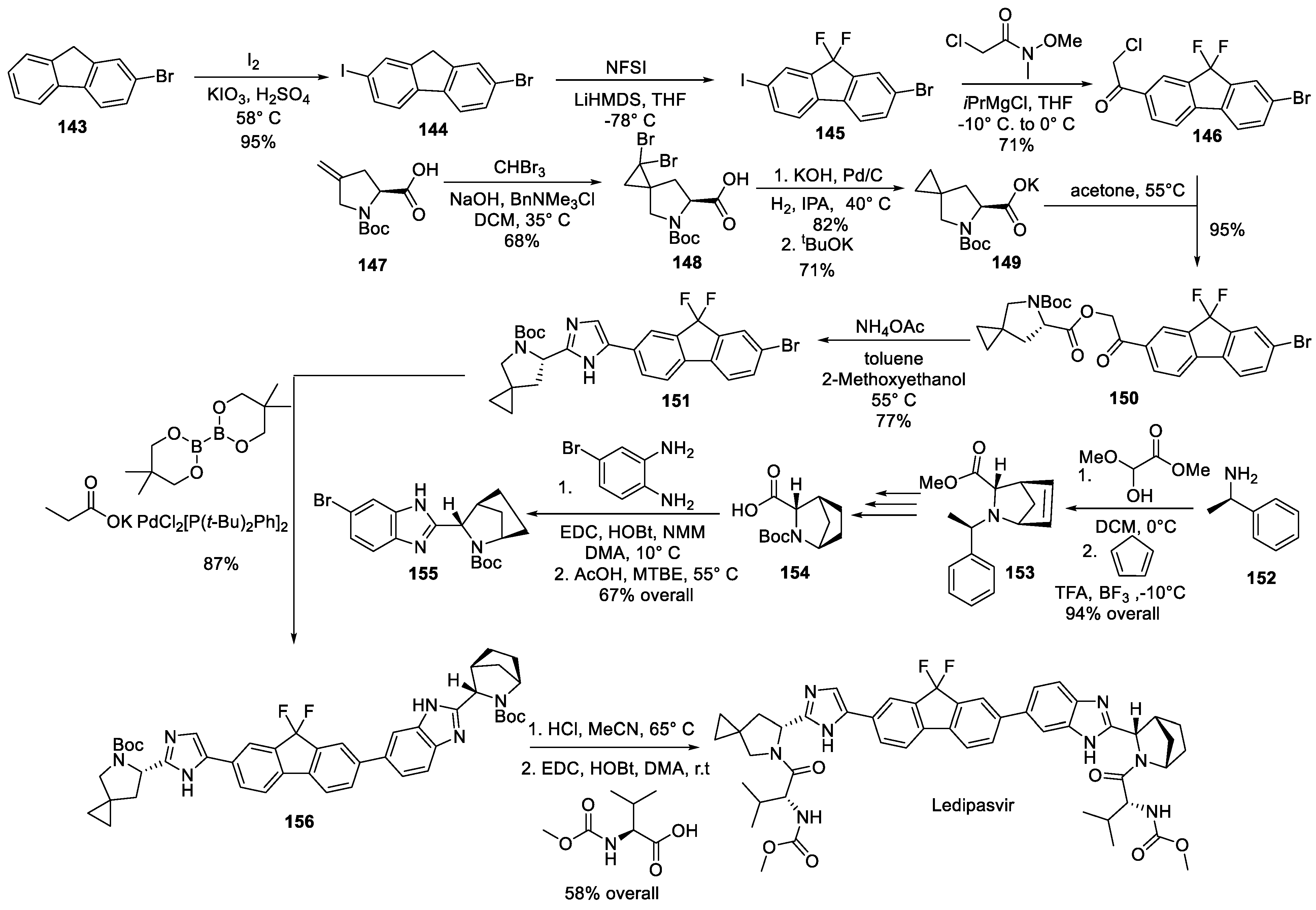
16. Ledipasvir
This inhibitor of hepatitis C virus non-structural protein 5A (HCV NS5A) was developed by Gilead Sciences and was approved in 2014 for the treatment of genotype 1 HCV in combination with sofosbuvir [
79]. The pertinence of this drug to the present review is determined by the spirocyclic pyrrolidine moiety in its structure.
The complexity of ledipasvir’s structure mandates a lengthy synthetic route to assemble the drug molecule. The original synthesis patented by Gilead in 2013 [
80] is presented in
Scheme 41. The synthetic scheme includes the assembly of requisite building blocks from four different directions, thereby being remarkably convergent. From one direction, 2-bromofluorene (
143) was iodinated in position 7 to give
144. The methylene group in the latter was bis-fluorinated with
N-fluorobenzenesulfonimide (NFSI) to give compound
145 which was converted to an aryl magnesium species at the more reactive, iodo side and coupled with chloroacetic acid Weireb amide to give building block
146. From another direction, Boc-protected 4-methylene
L-proline (
147) was converted to spiro dibromo cyclopropane
148 which was dibrominated and converted to potassium carboxylate
149. The latter was involved in a nucleophilic substitution reaction with chloroacetyl compound
146 to give keto ester
150 which was reacted with ammonium acetate to give imidazole
151. Assembly of bicyclic pyrrolidine portion of ledipasvir started with enantiopure -methyl benzylamine (
152) which was used to form a Schiff base with methyl glyoxylate hemiacetal. The Schiff base was involved in a Lewis acid-catalyzed Diels-Alder reaction with cyclopentadiene and the desired bicyclic structure was established (compound
153). Three trivial transformations (debenzylation with double bond reduction, Boc-protection and ester hydrolysis) gave carboxylic acid
154 which was employed in benzimidazole synthesis to give compound
155. Now the two halves of ledipasvir molecule (
151 and
155) were brought together
via a Suzuki coupling involving
in situ formation of aryl boronic acid intermediate. Resulting intermediate
156 was deprotected and acylated at both liberated nitrogen atoms with
N-methoxycarbonyl valine to give lidepasvir.
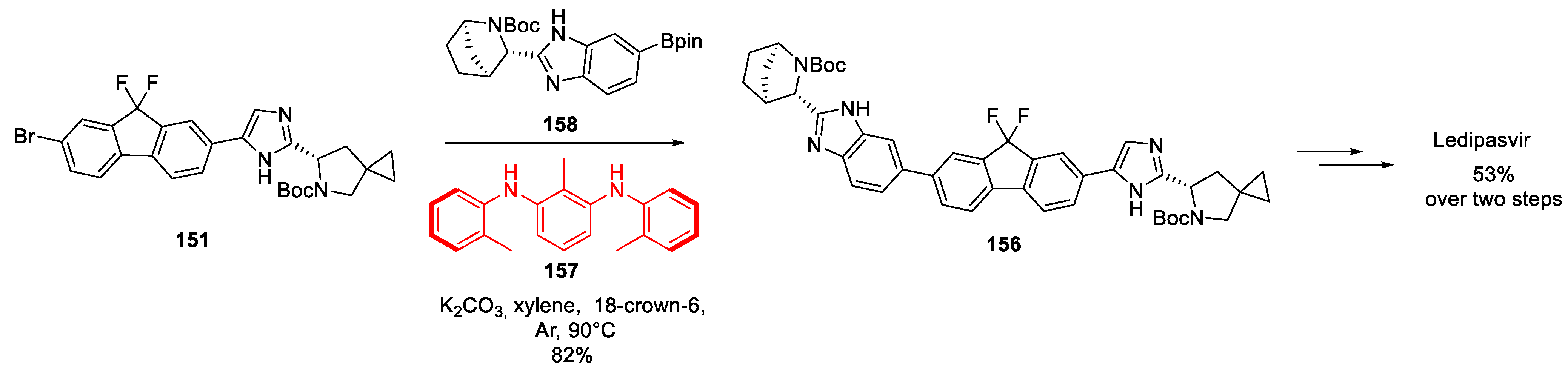
The Gilead Sciences route to ledipasvir may as well be quite optimal, considering that there have not been any attempts to improve it as judged by the literature on the subject. However, in 2021, a Suzuki-Miyaura coupling of the two halves of ledipasvir molecule was selected as a drug synthesis example to illustrate the power of novel aryl halide to aryl boronic acid coupling protocol where no palladium catalyst is employed. The two research groups from China reported the use of organocatalyst
157 to bring about the coupling of aryl bromide
151 with boronic acid pinacol ester
158. Considering the high yield of advanced ledipasvir precursor
156 reported for this coupling, this palladium-free approach appears much more amenable to large-scale pharmaceutical production than the original Gilead Sciences route (
Scheme 42) [
81].
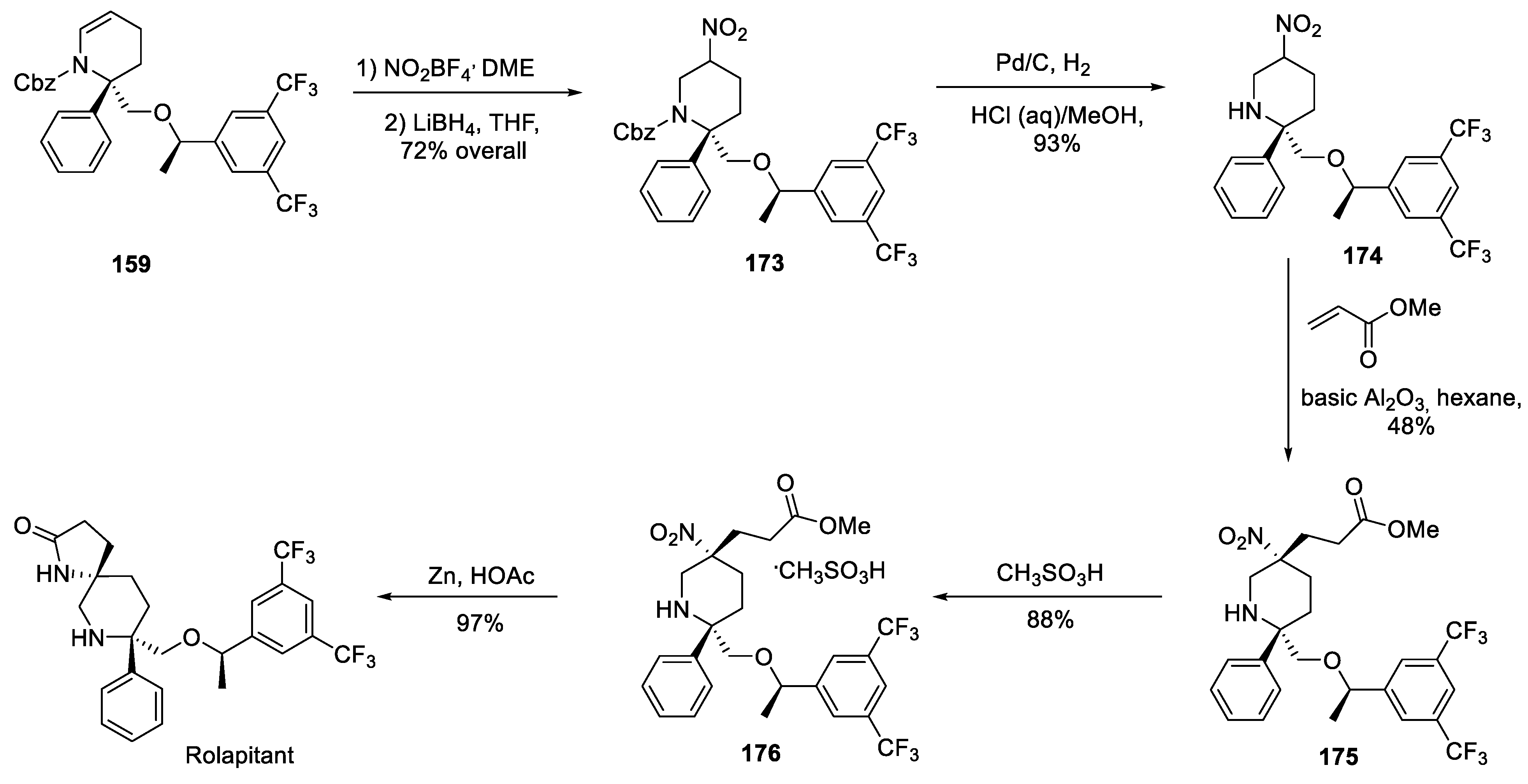
17. Rolapitant
This spirocyclic neurokinin NK1 receptor antagonist comprising three stereogenic centers originated in the laboratories of Schering-Plough and was approved for the treatment of chemotherapy-induced nausea and vomiting in 2015 [
82]. The drug’s syntheses are primarily featured in the patent literature.
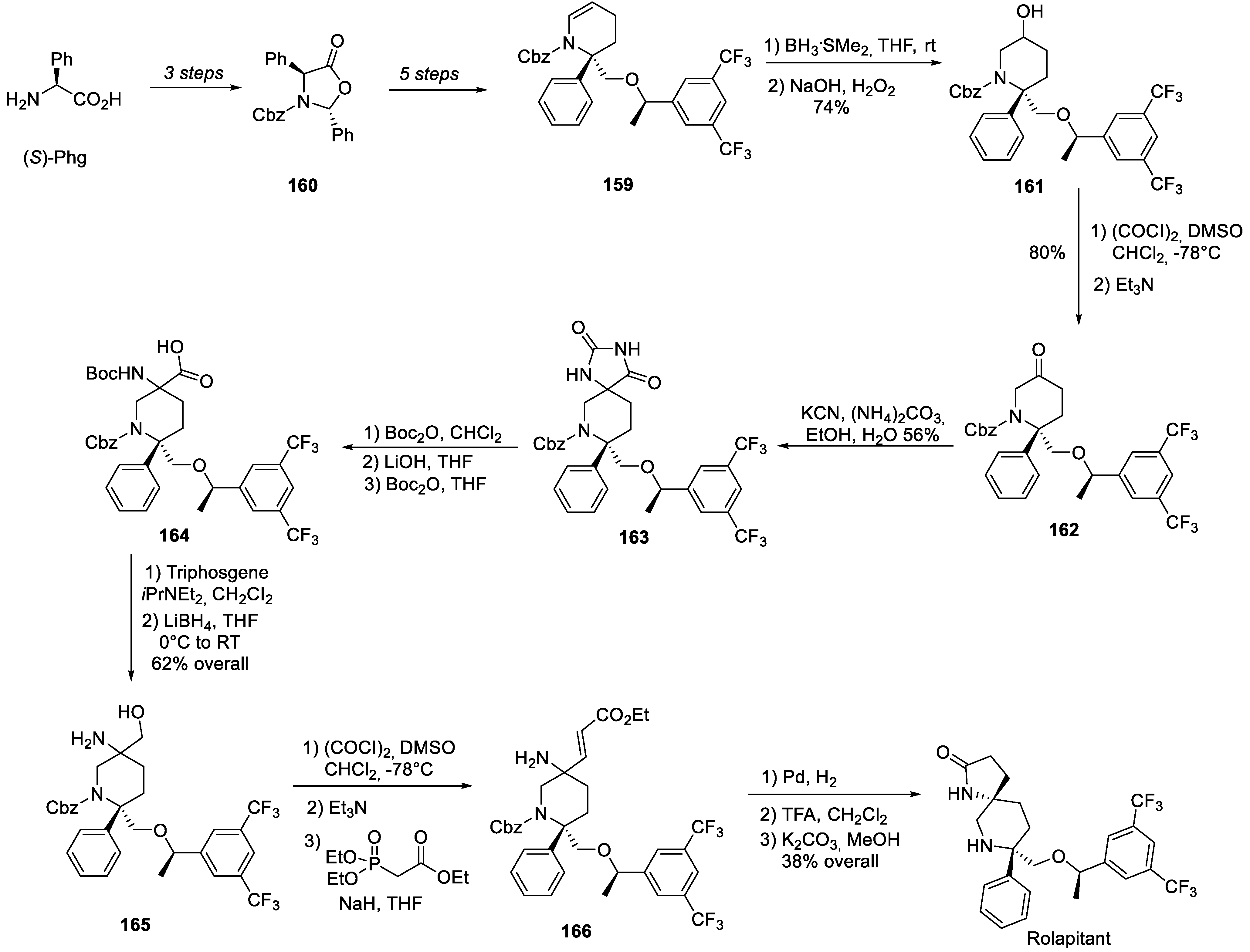
The original synthesis of rolapitant patented by Schering-Plough [
83] relies of the supply of key building block
159. Synthetically, the latter can be traced back to (
S)-phenylglycine over 8 synthetic operations, including the preparation of oxazolidinone
160, as detailed further below. Cyclic enamine
159 was subjected to standard hydroboration-oxidation conditions to afford alcohol
161 which was oxidized to ketone
162 under the Swern conditions. Ketone
162 was involved in a standard hydantoin synthesis with potassium cyanide and ammonium carbonate to afford spirocyclic compound
163. The latter was Boc-protected, hydrolyzed and Boc-protected again to give -amino acid
164. The carboxylic group in
164 was reduced
via mixed anhydride to give amino alcohol
165 which was oxidized (again under the Swern conditions) and involved, without protection of the amino group, in a Horner-Wadsworth-Emmons olefination to give acrylate ester
166. The latter turned out to be a direct precursor of rolapitant and was converted to the drug by hydrogenation (accompanied by lactam formation) and protecting group removal (
Scheme 43).
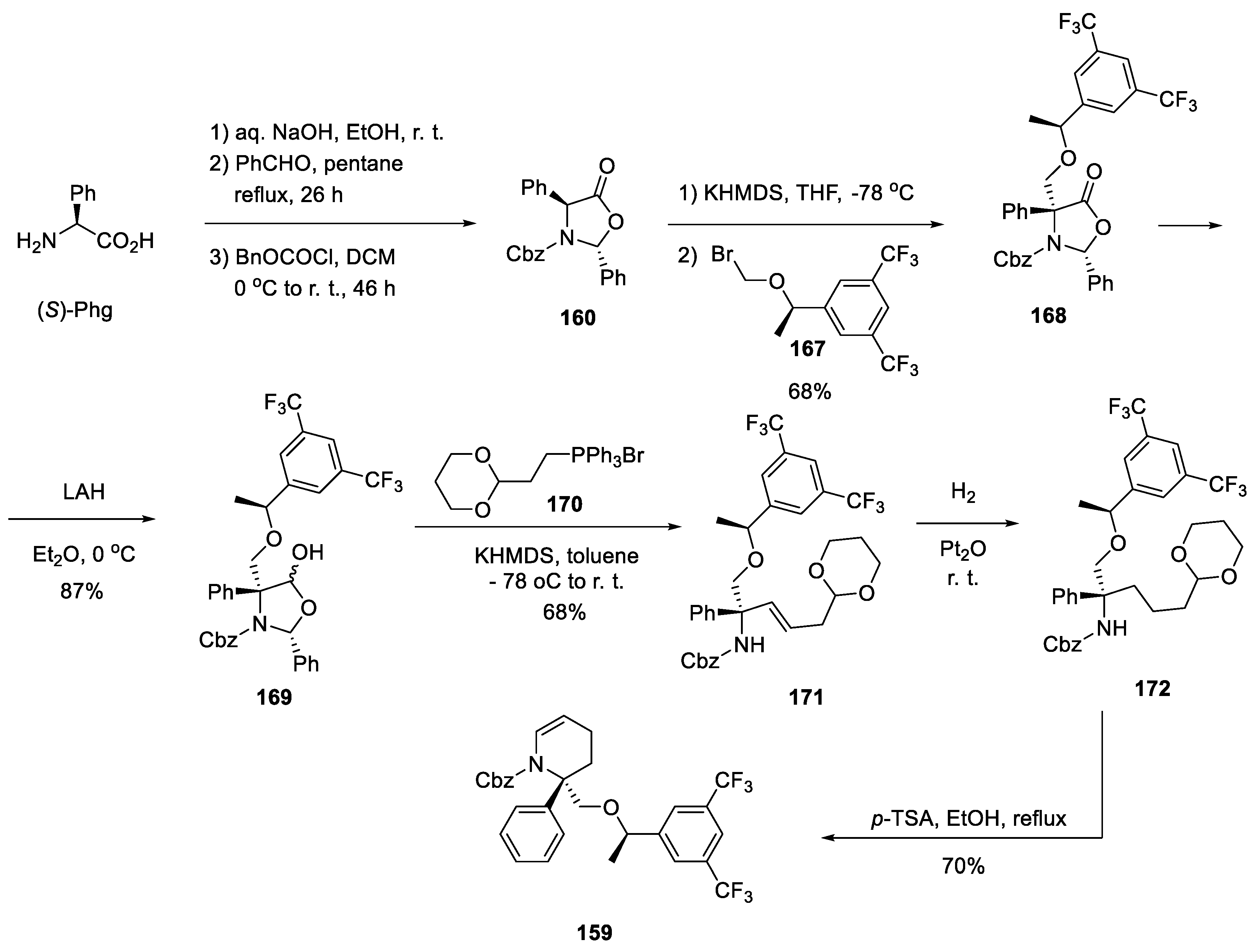
As to the synthesis of key building block
159 from (
S)-phenylglycine ((
S)-Phg) it was accomplished as follows. (
S)-Phg was converted to diastereomerically pure oxazolidinone
160 on condensation with benzaldehyde and Cbz-protection [
84]. Oxazolidinone
160 was
C-alkylated is diastereoselective fashion with chiral bromide
167. The carbonyl group of resulting intermediate
168 was reduced by lithium alumohydride and lactol
169 was obtained. The aldehyde form of the latter reacted with phosphonium ylide generated from alkyl phosphonium bromide
170 and elaborate olefinic building block
171 was obtained. It was hydrogenated over platinum dioxide and resulting acetal
172 was treated with
p-toluene sulfonic acid in refluxing ethanol. This led to the removal of the acetal protecting group and the cyclization of the Cbz-protected amino group onto the liberated aldehyde carbonyl group to give target key intermediate
159 (
Scheme 44) [
83].
In 2013, Opko Health, Inc. patented an alternative route to rolapitant which is also based on key building block
159 [
85]. This intermediate was nitrated and reduced by lithium borohydride to give nitro alkane
173. Removal of the Cbz group (to give
174) and subsequent alkylation of the nitro alkane portion with methyl acrylate over basic alumina gave compound
175 which was converted to methanesulfonic acid salt
176 for easy isolation. Reduction of
176 with zinc metal in acetic acid triggered lactamization and gave rolapitant (
Scheme 45). Thus, the Opko Health, Inc. synthesis appears to be shorter than the original Schering-Plough route and more amenable to large scale API production.
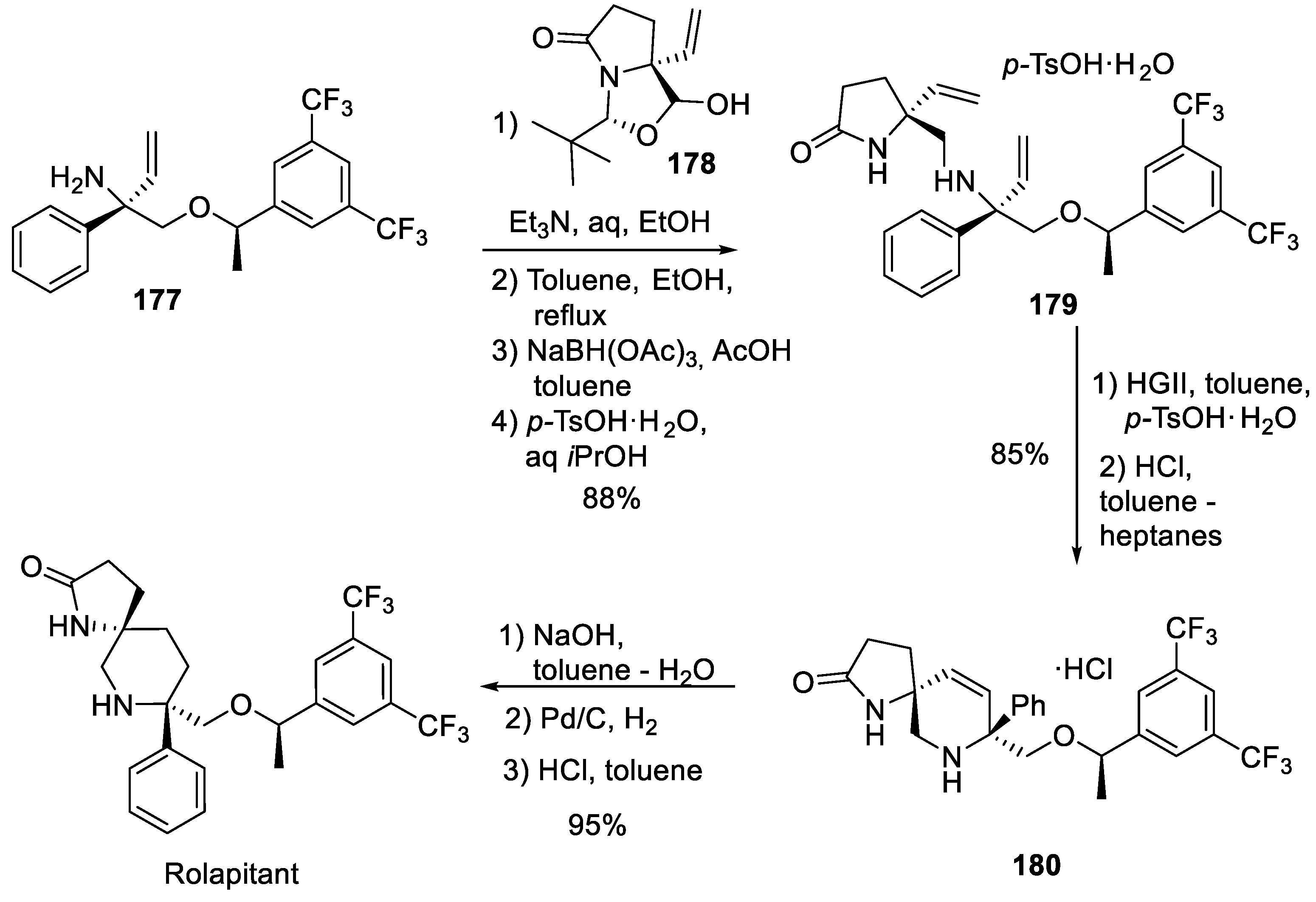
In 2016, Opko Health, Inc. patented a fundamentally different route to rolapitant which relies of chiral building block
177 (for the sake of brevity, we do not discuss its synthesis) [
86]. It was reductively alkylated with masked aldehyde building block
178 to give bis-olefinic compound
179. In the latter, a scene was set for intramolecular olefin metathesis which was successfully brought about using Hoveyda-Grubbs 2
nd generation ruthenium catalysts (HGII) and resulting spirocycle
180 was isolated as hydrochloride salt. The latter advanced intermediate was free-based by aqueous sodium hydroxide and hydrogenated over palladium on charcoal to give rolapitant (
Scheme 46).
20. Ubrogepant
Migraine is a debilitating disease which was traditionally treated only symptomatically by serotonin receptor modulators or non-steroidal anti-inflammatory drugs. However, more recent in-depth investigation of the mechanisms underlying the onset of migraine established calcitonin gene related peptide (CGRP) as a primary player in this pathological process. Produced in peripheral and central neurons, CGRP is a potent vasodilator and mediator of nociception. Thus, antagonizing the binding of CGRP to its receptor was established as a novel approach to the treatment of acute migraine at the fundamental level [
96]. Ubrogepant is the first-in-class drug developed by Merck and Co. and approved in 2020 [
97].
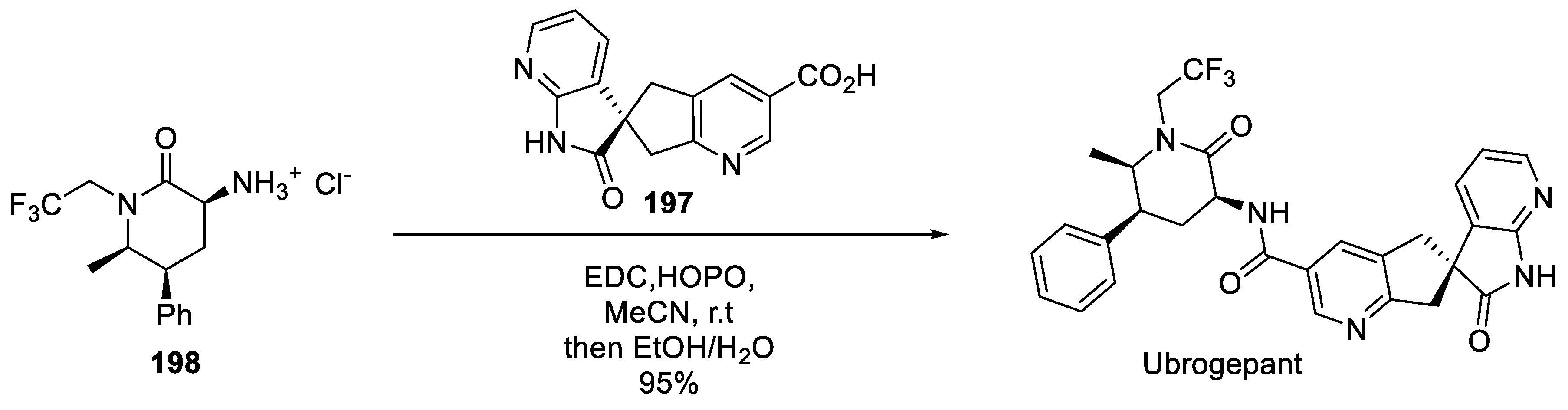
The detailed process chemistry route towards ubrogepant was published by the Merck team in 2017 [
98]. The final step in the assembly of the target molecule was amidation of enantiopure spirocyclic carboxylic acid
197 with enantiopure amine
198 hydrochloride salt.
Scheme 51.
Final amidation in the assembly of ubrogepant.
Scheme 51.
Final amidation in the assembly of ubrogepant.
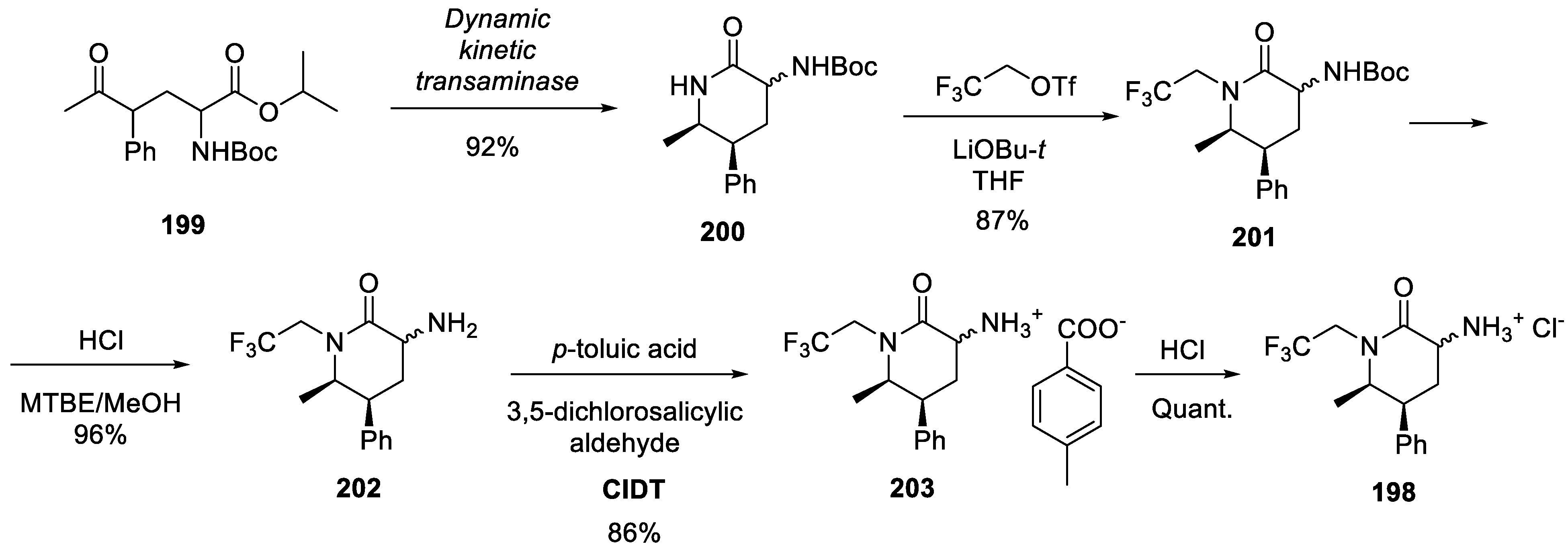
Therefore, it is the assembly of enantiopure building blocks
197 and
198 which required most of the innovation. The synthesis of amine
198 started with a diastereomeric mixture of keto esters
199. The latter was subjected to dynamic kinetic transaminase-mediated cyclization (using an evolved enzyme strain) to give piperidone
200 as a 3:2 mixture of epimers.
N-Alkylation of the latter with 3,3,3-trifluoroethyl triflate afforded predominantly compound
201 (separated in 87% yield from the unreacted starting material and bis-alkylation product). Removal of the Boc group gave epimeric amine
202 which was subjected to crystallization induced diastereoselective transformation (CIDT) with p-toluic acid and 3,5-dichlorosalicylic aldehyde to give building block
203 which was converted to hydrochloride salt
198 (
Scheme 52).
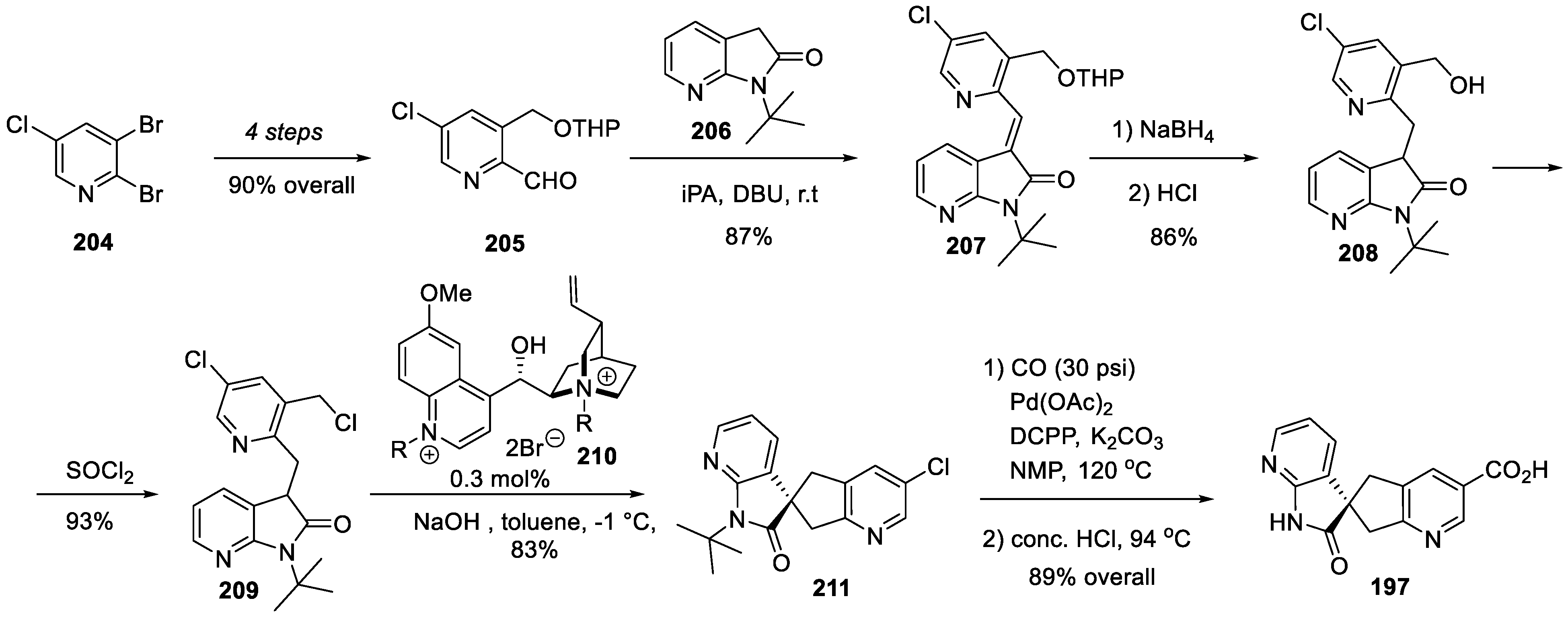
Spirocyclic carboxylic acid
197 was assembled as follows. 2,3-Dibromo-5-chloropyridine (
204) was transformed in four steps (involving two additions of pyridine magnesium species to DMF) to tetrahydropyran-protected aldehyde
205. The latter was condensed with 7-azaindolin-2-one
206 to give olefin
207 which was reduced by sodium borohydride and the THP group was removed to give alcohol
208. Conversion of
208 into alkyl chloride
209 set the scene for spirocyclization. On the action of aqueous sodium hydroxide, in the presence of cinchona alkaloid-derived chiral catalyst
210, chloride
209 underwent an efficient and enantioselective intramolecular
C-alkylation to form spirocycle
211. Palladium-catalyzed carbonylation of the chloropyridine portion in the latter followed by removal of the
tert-butyl group from the lactam nitrogen atom afforded key spirocyclic carboxylic acid
197 (
Scheme 53).
An alternative approach to spirocyclic carboxylic acid
197 was reported in 2022 by Hu and co-workers [
99]. It involves direct asymmetric spiroannulation of Boc-protected 7-azaindolin-2-one (
213) with doubly benzylic bis-bromide
212. Thus, from cheap and readily available starting materials, spirocycle
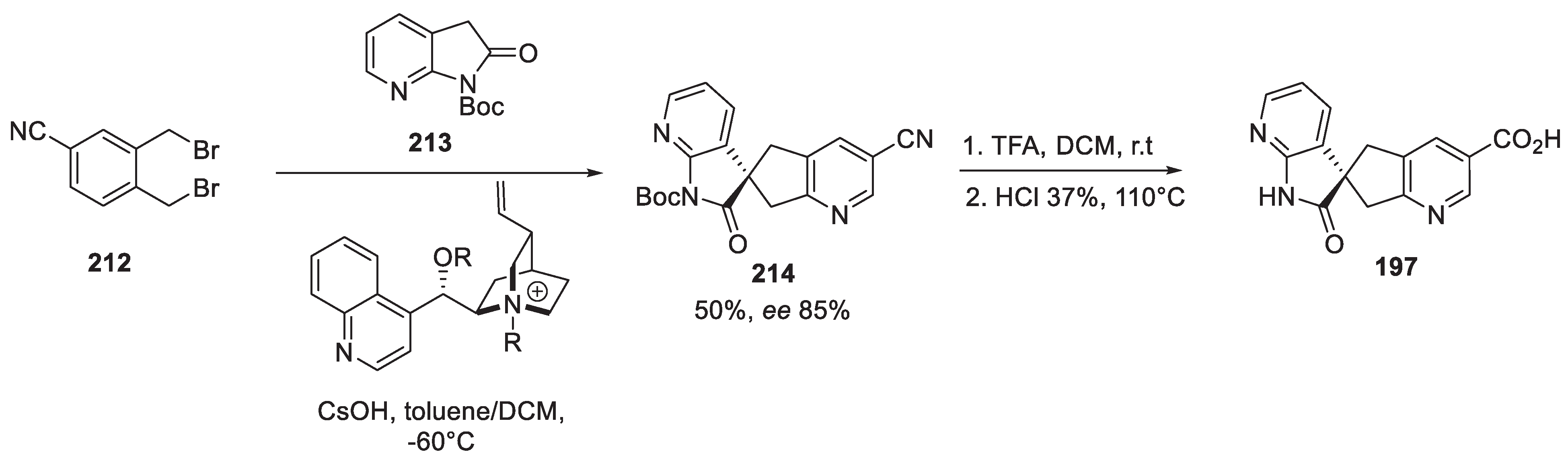
214 was obtained in good yield and enantioselectivity. Removal of the Boc group followed by nitrile hydrolysis gave requisite spiro acid
197 thus completing a formal synthesis of ubrogepant (
Scheme 54).
22. Risdiplam
This spirocyclic drug is the first oral medication to treat spinal muscular atrophy, an orphan disease [
104]. The drug was developed by PTC Therapeutics and Spinal Muscular Atrophy Foundation and received FDA approval in 2020 for the treatment of the disease in patients aged 2 months or older [
105]. The drug acts by modifying mRNA splicing by binding to survival of motor neuron 2 (SMN2) [
106]. It is currently marketed by Genentech, a subsidiary of Roche.
The synthetic routes to risdiplam disclosed in the original process chemistry patents by Roche rely on the commercial supply of the spirocylic piperazine building block and thus do not involve the formation of the spirocyclic moiety in the course of the synthesis.
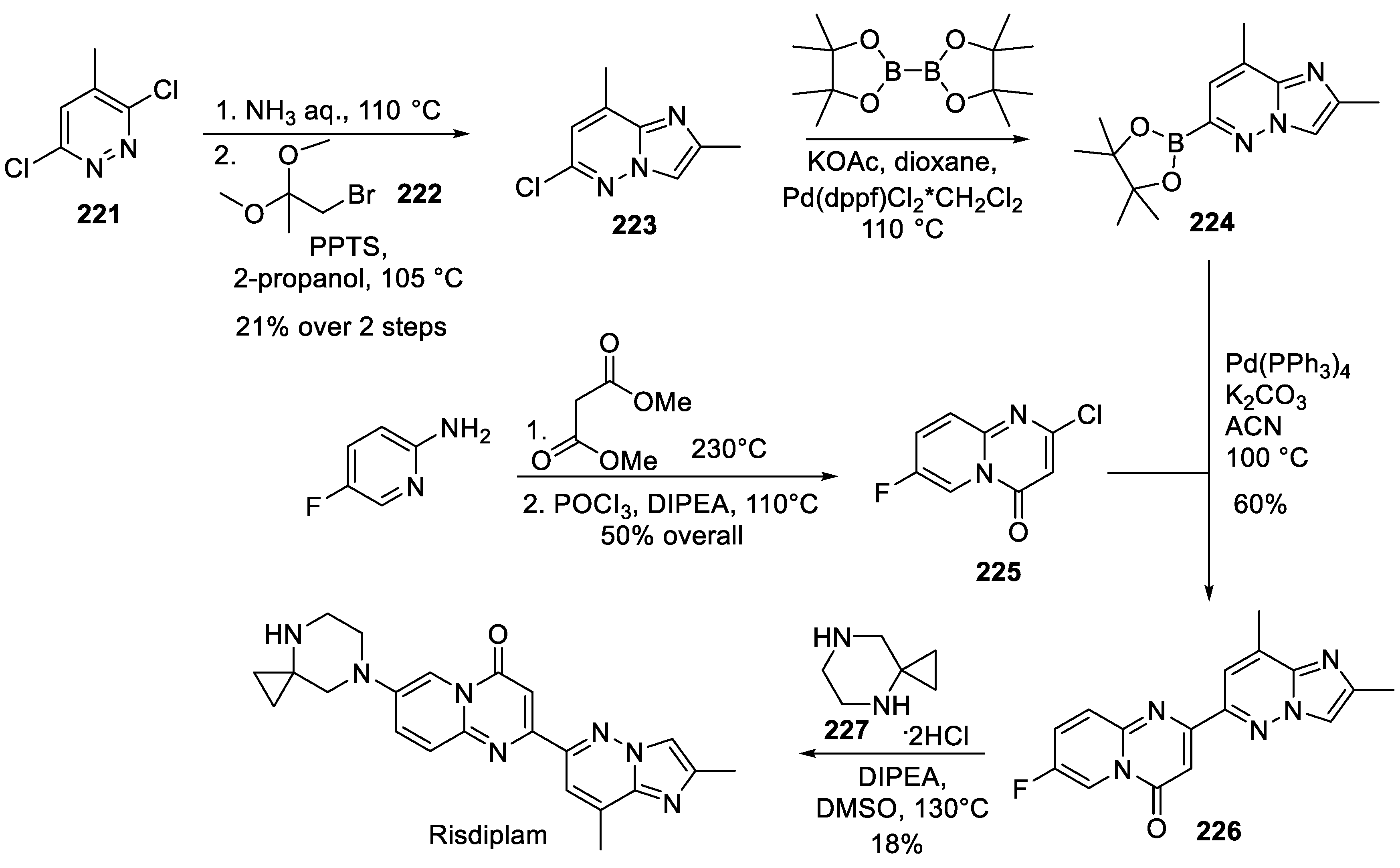
In their 2015 patented route, PTC Therapeutics and Roche scientists started with 3,6-dichloro-5-methylpyridazine (
221) in which the less accessible chlorine atom was substituted with ammonia and the imidazo ring was formed on condensation with bromoacetone dimethyl ketal (
222). The overall yield of resulting imidazo pyridazine
223 was obtained in relatively low yield (unsurprisingly, considering the unfavorable course of the initial nucleophilic aromatic substitution). Compound
223 was coupled, under palladium(II) catalysis, with bis(pinacolato)diboron to give boronic acid
224. The latter was coupled again, in Suzuki fashion, with pyrido pyrimidone building block
225 (obtained, in turn, from 2-amino-5-fluoropyridine on condensation with dimethyl malonate). The Susuki coupling
224 +
225 resulted in core risdiplam scaffold
226 which was decorated with spirocyclic piperazine
227 under thermal conditions to give risdiplam (
Scheme 56) [
107].
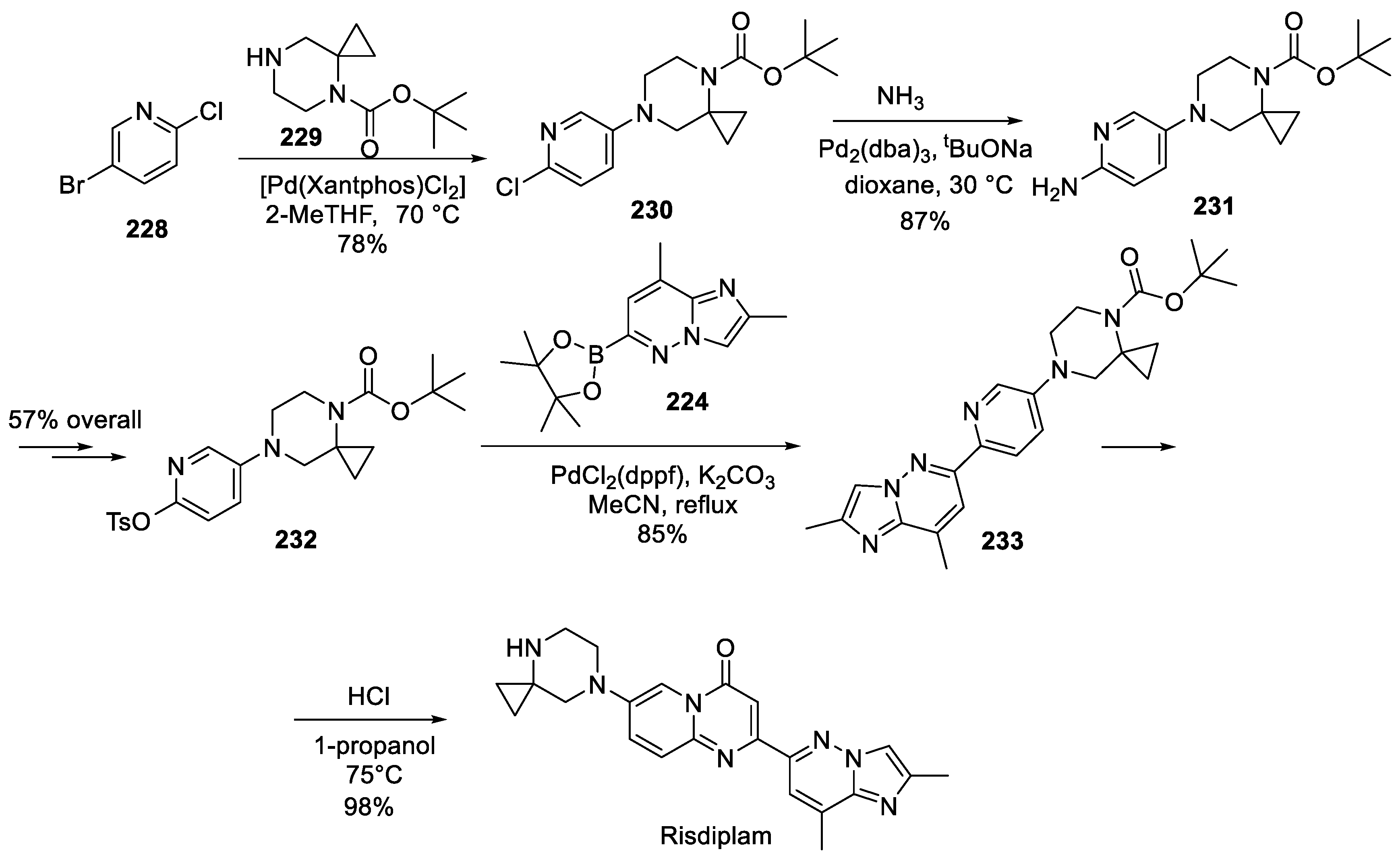
The more recent route from their 2019 patent application, Roche scientists started by involving 5-bromo-2-chloropyridine (
228) in Buchwald-Hartwig amination with Boc-protected spirocyclic piperazine (
229). The unreacted, under Pd(II)-catalyzed conditions, chlorine atom in
230 was displaced by ammonia under Pd(0) catalysis and the amino portion of resulting intermediate 231 was converted in two steps into tosylate
232. The latter was coupled with boronic acid
224 (prepared as described in the previous Roche patent) in Suzuki fashion and the Boc group in resulting coupling products
233 was removed to give risdiplam (
Scheme 57) [
108].
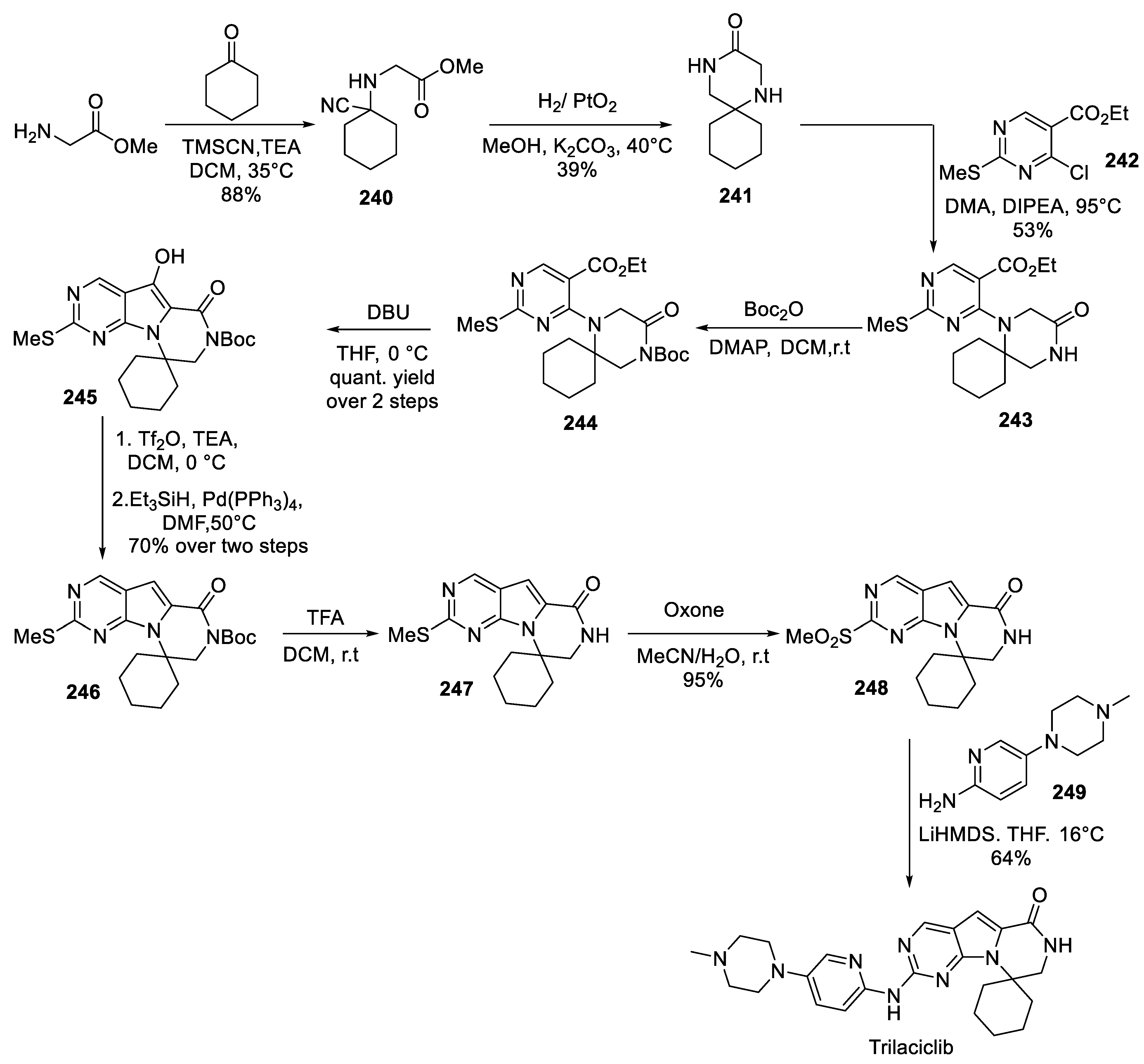
24. Trilaciclib
This spirocyclic inhibitor of cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) was developed by G1 Therapeutics. The drug was indicated for cancer patients receiving certain types of chemotherapy to protect the bone marrow from chemotherapy-induced damage. As trilaciclib was the first-in-class drug developed for this important indication, it received a priority review and a breakthrough therapy designation from the FDA. It was approved for medical use in 2021 [
111].
The drug’s originator patented two synthetic routes to the compound both involving an intramolecular
N-acylation as the key step in the construction of the spirocyclic moiety [112-113]. Of these two approaches, the later one [
113] is described in much greater detail and is most likely the API production route which we chose to discuss herein.
The synthesis commences with an efficient and high-yielding Strecker reaction of cyclohexanone with glycine methyl ester and trimethylsilyl cyanide to afford amino nitrile
240. The latter was hydrogenated over platinum(IV) oxide. This caused reduction of the nitrile to aminomethyl compound which underwent lactamization to afford key spirocyclic building block
241. Without protection, sterically hindered secondary amine
241 was used to displace the labile chlorine atom in pyrimidine template
242. The nucleophilic aromatic substitution reaction proceeded under forcing conditions and afforded spirocycle
243 which was now set for the formation of the pyrrolo portion of the target molecule. As this step would require C-H deprotonation of the piperazinone moiety, the lactam nitrogen of intermediate
243 was Boc-protected to give compound
244. Treatment of the latter with DBU at 0 ℃ in THF triggered the desired deprotonation and intramolecular cyclization and gave 3-hydroxypyrrolo compound
245 in quantitative yield over the last two steps (including the preceding Boc-protection). Clearly, the hydroxy group in
245 had to be removed, which was achieved by
O-triflation of the compound with subsequent palladium(0)-catalyzed reductive coupling with triethylsilane. The two steps proceeded in 70% combined yield and afforded spirocyclic pyrrolo pyrimidine
246 which was deprotected with trifluoroacetic acid to give advanced trilaciclib precursor
247. To complete the synthesis, the methylthio group in
247 was oxidized to the better leaving, methylsulfonyl group with oxone in aqueous acetonitrile to give
248 in excellent yield. The final nucleophilic aromatic substitution step between spirocyclic template
248 and pyridine amine
249 (prepared, in turn via nucleophilic aromatic substitution with 5-halopyridine precursor and
N-methylpiperazine) required the use of a strong base (LiHMDS) and gave trilaciclib (
Scheme 59).
Scheme 1.
Synthesis of dl-griseofulvin by Yamoto and co-workers.
Scheme 1.
Synthesis of dl-griseofulvin by Yamoto and co-workers.
Scheme 2.
Synthesis of (+)-griseofulvin by Pirrung and co-workers.
Scheme 2.
Synthesis of (+)-griseofulvin by Pirrung and co-workers.
Scheme 3.
Spironolactone synthesis by G. D. Searle and Co.
Scheme 3.
Spironolactone synthesis by G. D. Searle and Co.
Scheme 4.
Ciba Geigy industrial synthesis of spironolactone.
Scheme 4.
Ciba Geigy industrial synthesis of spironolactone.
Scheme 5.
The Upjohn Co. synthesis of spironolactone.
Scheme 5.
The Upjohn Co. synthesis of spironolactone.
Scheme 6.
Synthesis of spironolactone via -lactonization of homopropargyl alcohol.
Scheme 6.
Synthesis of spironolactone via -lactonization of homopropargyl alcohol.
Scheme 7.
A 2018 synthesis of the core piperidine spirocycle needed for fluspirilene assembly.
Scheme 7.
A 2018 synthesis of the core piperidine spirocycle needed for fluspirilene assembly.
Scheme 8.
A 2001 synthesis of fluspirilene from an Italian group.
Scheme 8.
A 2001 synthesis of fluspirilene from an Italian group.
Scheme 9.
Fluspirilene synthesis by Shanghai Institute of Organic Chemistry.
Scheme 9.
Fluspirilene synthesis by Shanghai Institute of Organic Chemistry.
Scheme 10.
Synthesis of fenspiride by Voghera company.
Scheme 10.
Synthesis of fenspiride by Voghera company.
Scheme 11.
Synthesis of fenspiride by a Mexican group.
Scheme 11.
Synthesis of fenspiride by a Mexican group.
Scheme 12.
Synthesis of fenspiride by Emcure Pharmaceuticals.
Scheme 12.
Synthesis of fenspiride by Emcure Pharmaceuticals.
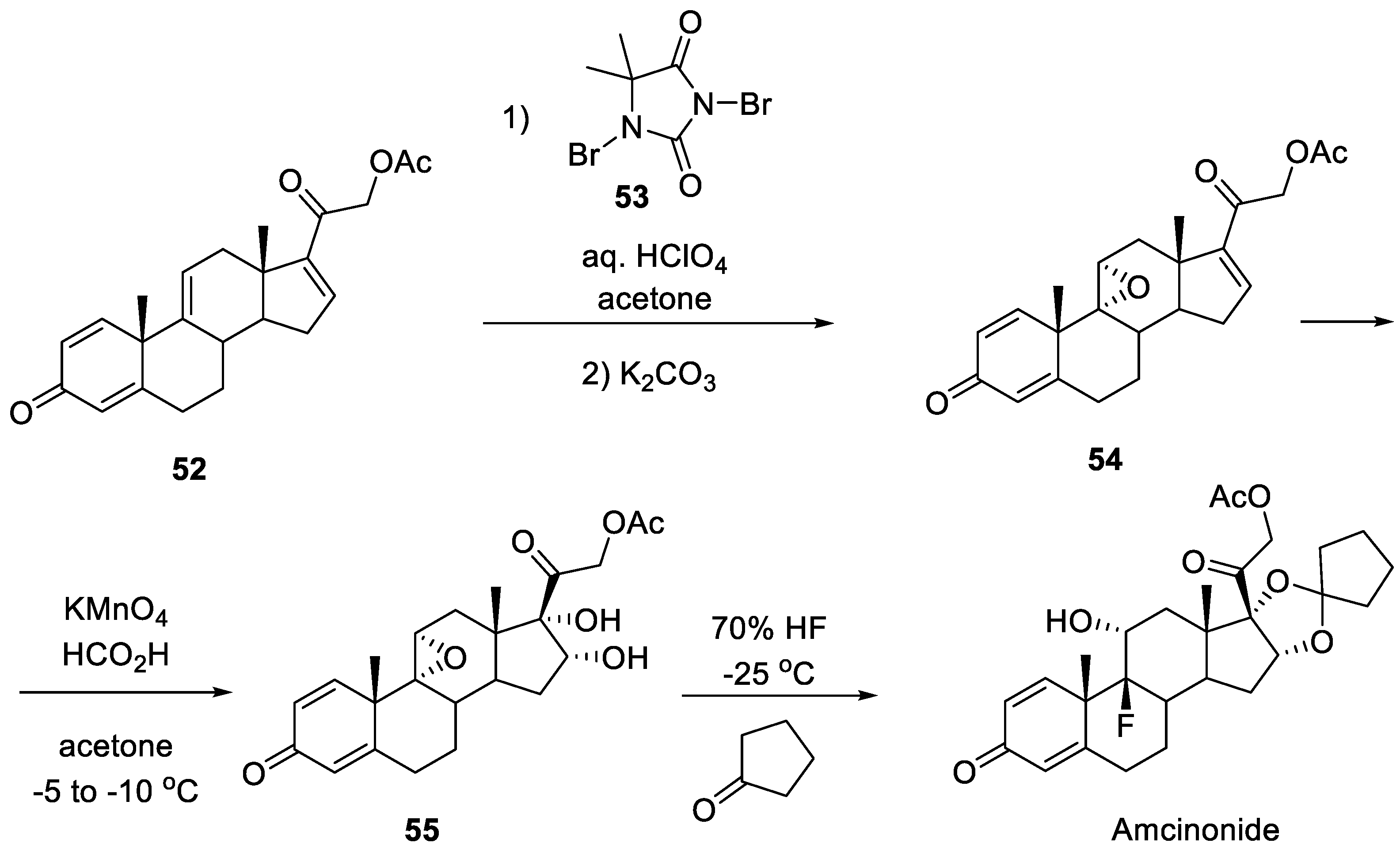
Scheme 13.
Synthesis of amcinonide.
Scheme 13.
Synthesis of amcinonide.
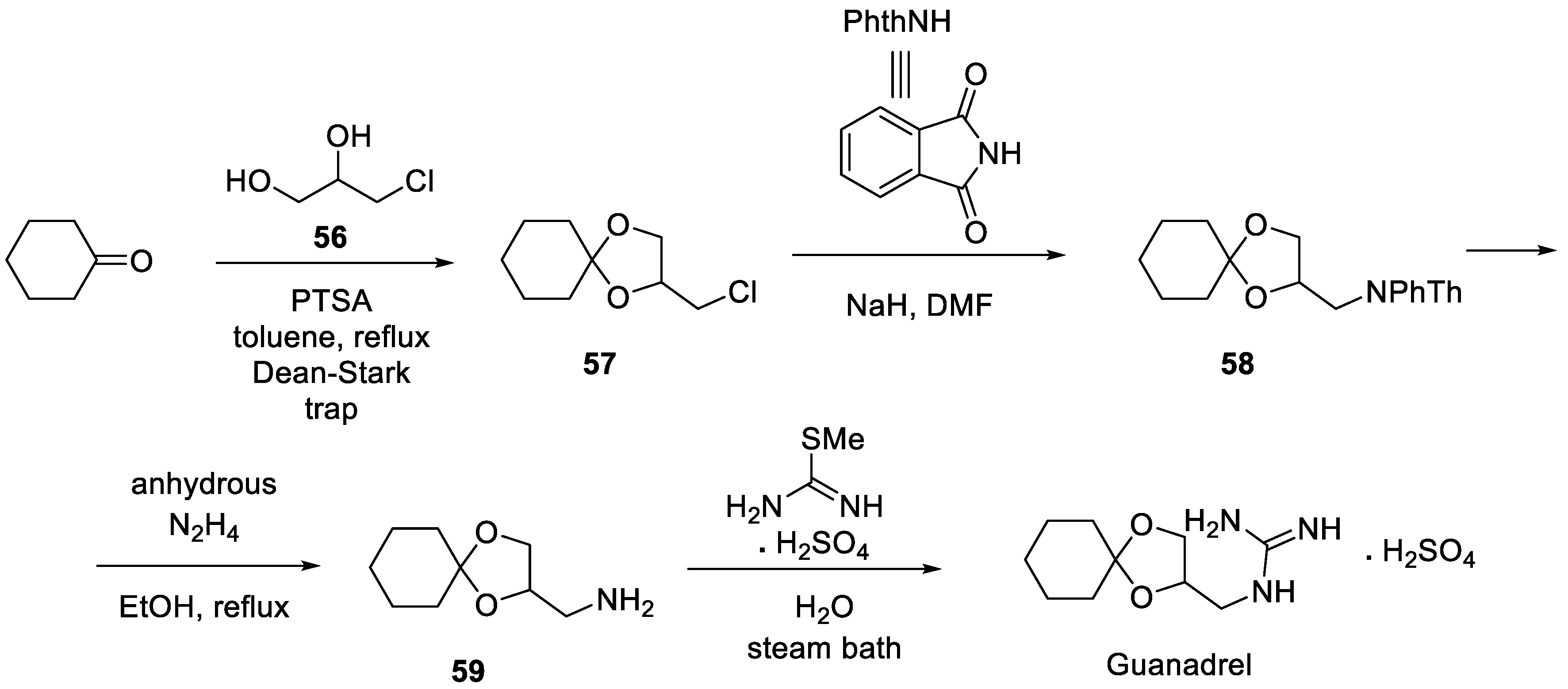
Scheme 14.
Industrial synthesis of guanadrel.
Scheme 14.
Industrial synthesis of guanadrel.
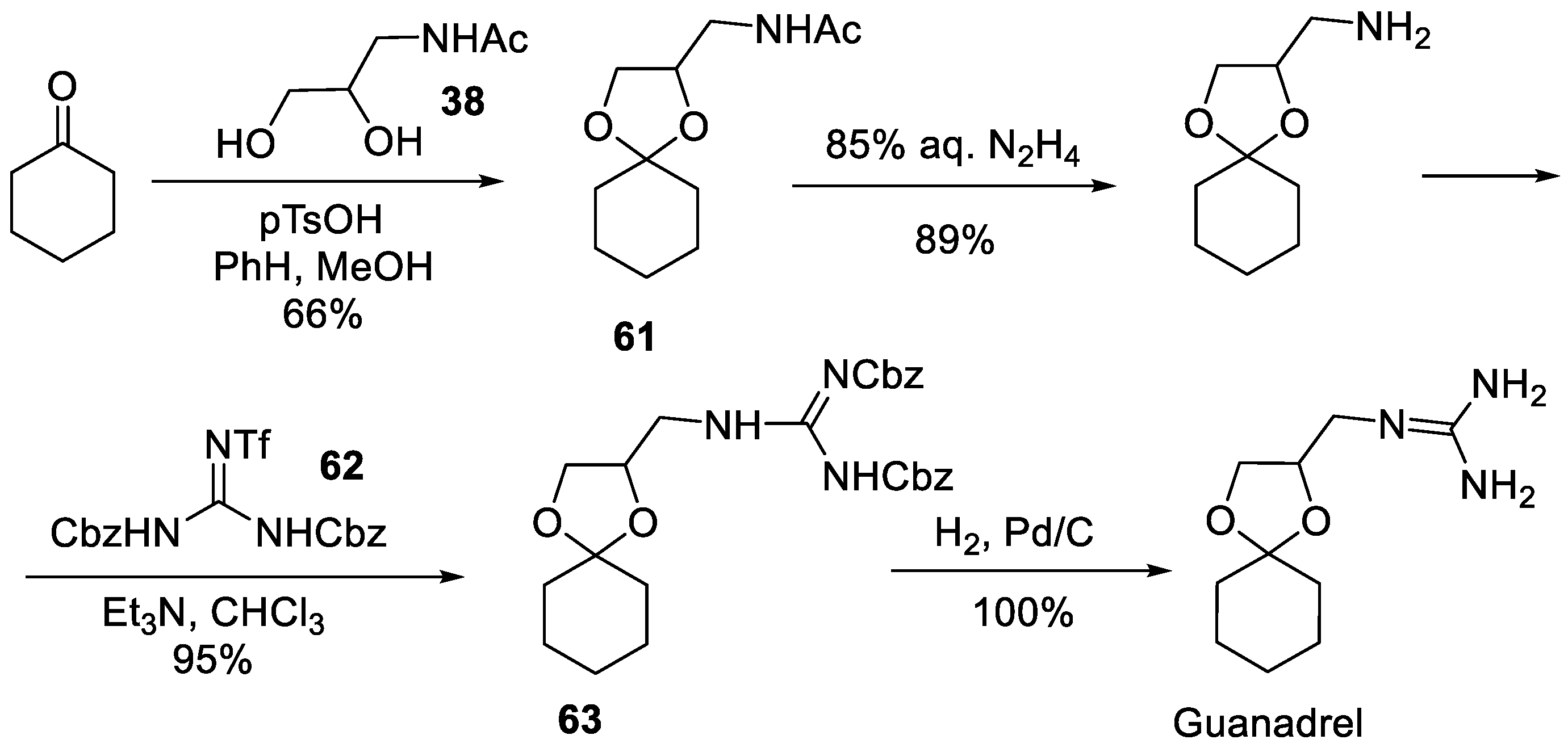
Scheme 15.
Synthesis of guanadrel by Goodman.
Scheme 15.
Synthesis of guanadrel by Goodman.
Scheme 16.
Industry synthesis of buspirone.
Scheme 16.
Industry synthesis of buspirone.
Scheme 17.
Synthesis of buspirone by Wei and co-workers (2008).
Scheme 17.
Synthesis of buspirone by Wei and co-workers (2008).
Scheme 18.
Preparation of buspirone involving piperazine alkylation via hydrogen borrowing.
Scheme 18.
Preparation of buspirone involving piperazine alkylation via hydrogen borrowing.
Scheme 19.
Preparation of buspirone involving reductive cross-coupling of 64 with nitrile 72.
Scheme 19.
Preparation of buspirone involving reductive cross-coupling of 64 with nitrile 72.
Scheme 20.
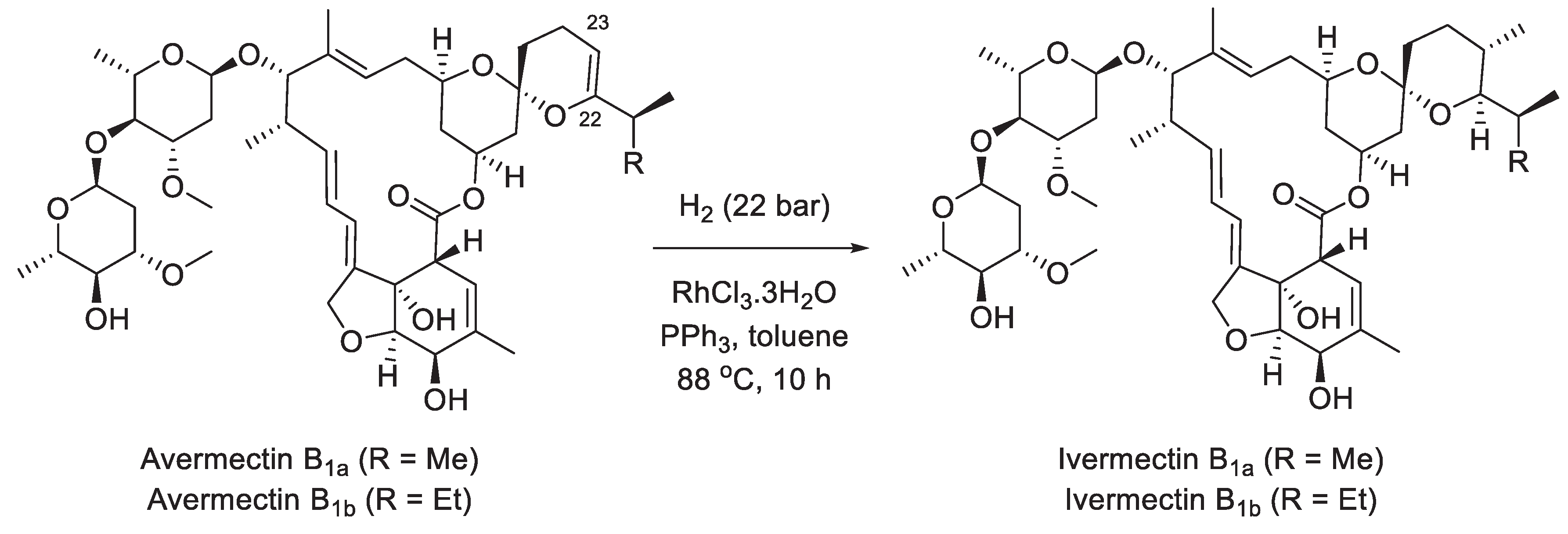
Hydrogenation of avermectins B1a and B1b.
Scheme 20.
Hydrogenation of avermectins B1a and B1b.
Scheme 21.
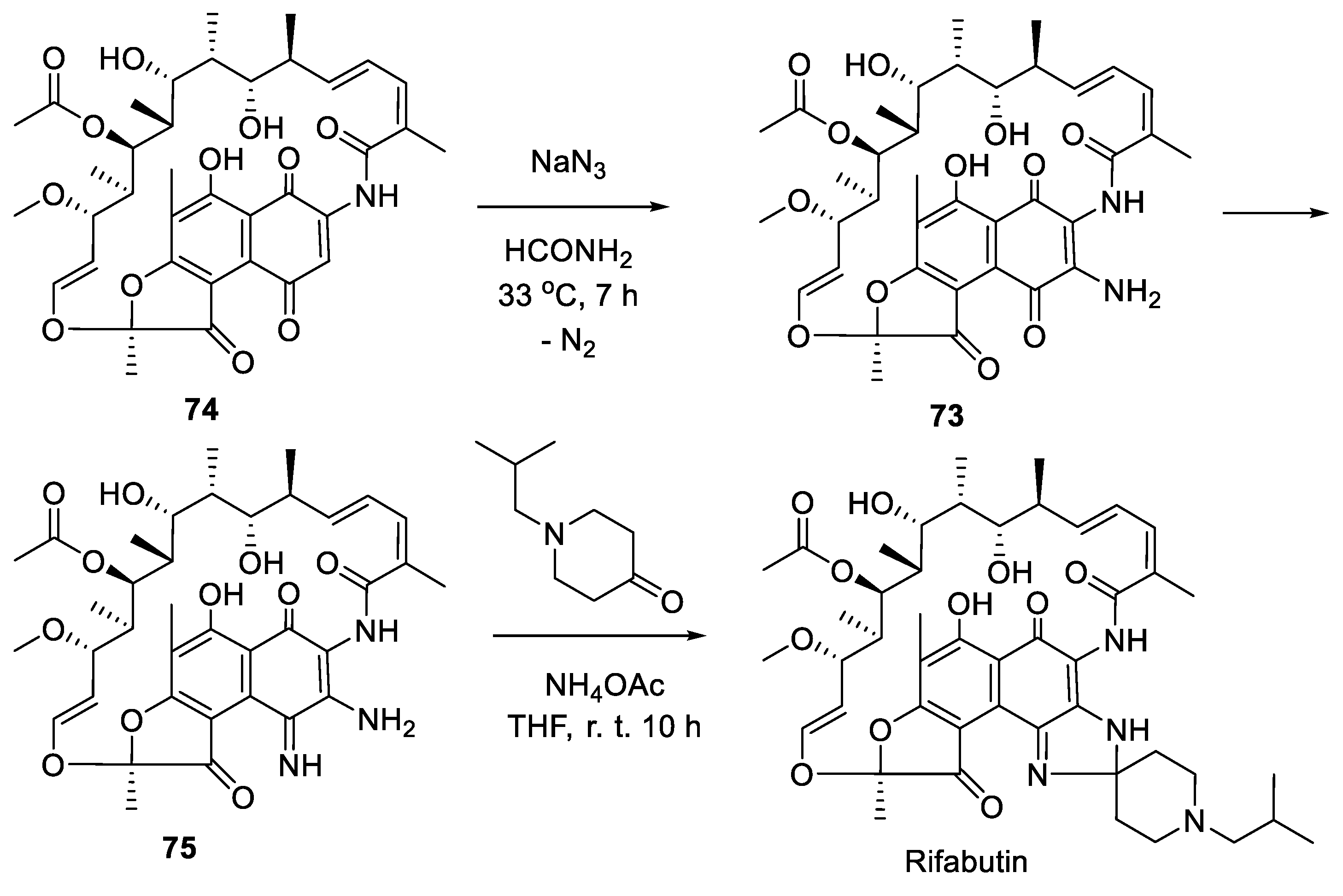
Farmitalia synthesis of rifabutin.
Scheme 21.
Farmitalia synthesis of rifabutin.
Scheme 22.
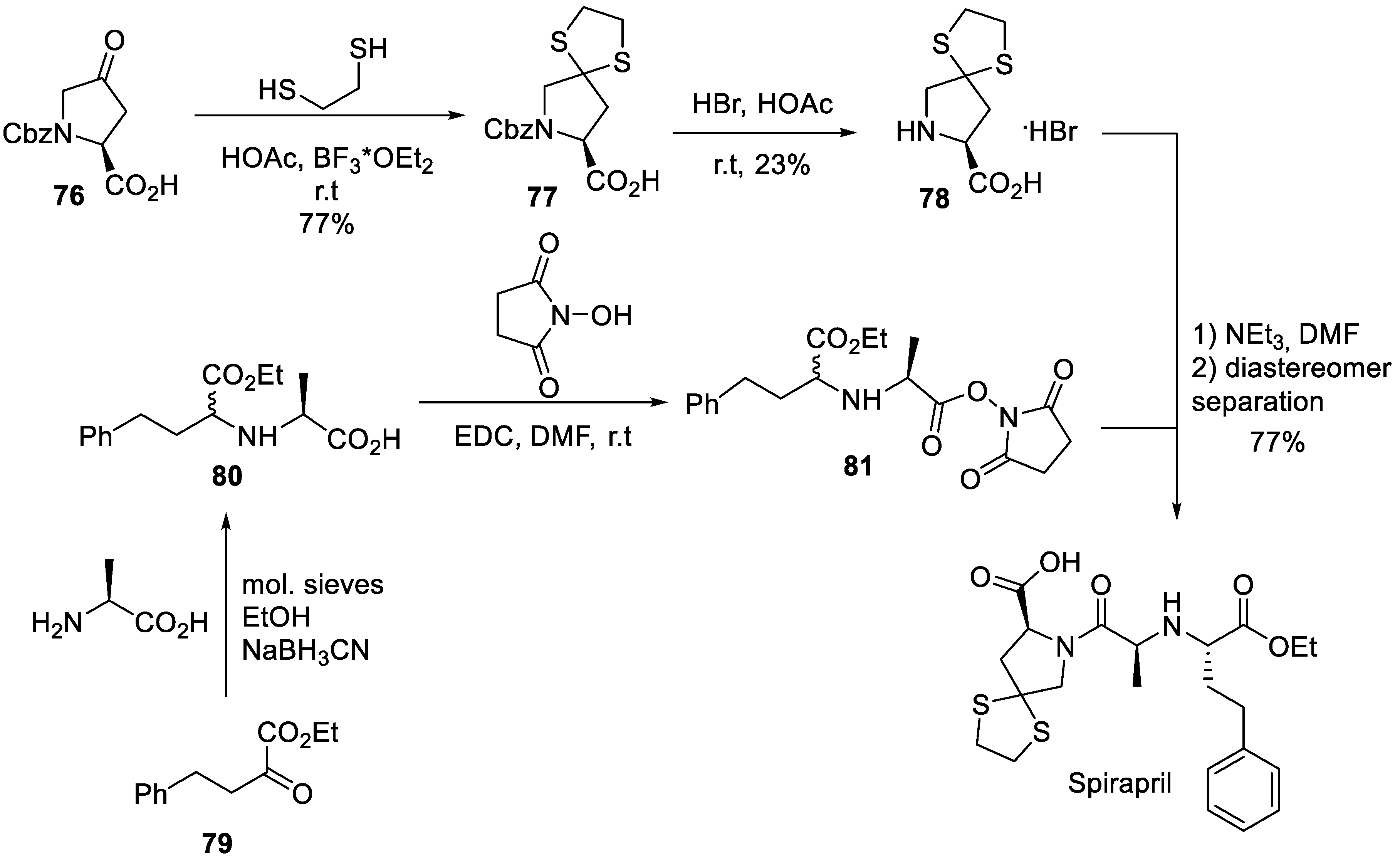
Synthesis of spirapril by Schering-Plough Corporation.
Scheme 22.
Synthesis of spirapril by Schering-Plough Corporation.
Scheme 23.
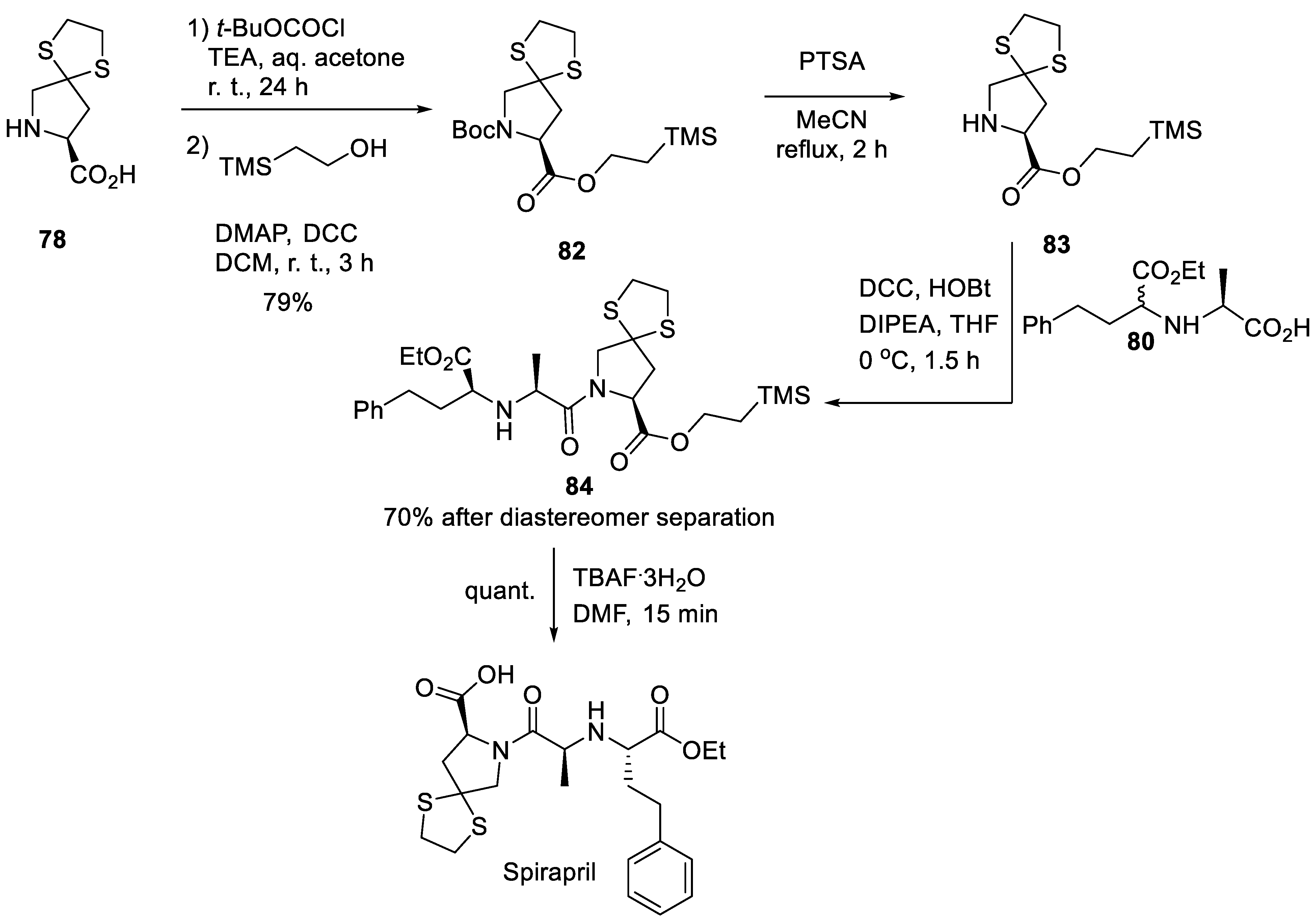
Synthesis of spirapril by Squibb Corporation.
Scheme 23.
Synthesis of spirapril by Squibb Corporation.
Scheme 24.
Original route to irbesartan by Sanofi Research. .
Scheme 24.
Original route to irbesartan by Sanofi Research. .
Scheme 25.
Synthesis of key building block 87.
Scheme 25.
Synthesis of key building block 87.
Scheme 26.
Synthesis of irbesartan by Israeli group.
Scheme 26.
Synthesis of irbesartan by Israeli group.
Scheme 27.
Dr. Reddy’s Laboratories synthesis of irbesartan.
Scheme 27.
Dr. Reddy’s Laboratories synthesis of irbesartan.
Scheme 28.
ArQule synthesis of irbesartan.
Scheme 28.
ArQule synthesis of irbesartan.
Scheme 29.
Beijing University of Chemical Technology route to irbesartan.
Scheme 29.
Beijing University of Chemical Technology route to irbesartan.
Scheme 30.
Original synthesis of cevimeline.
Scheme 30.
Original synthesis of cevimeline.
Scheme 31.
Cevimeline synthesis by Apotex Pharmachem, Inc.
Scheme 31.
Cevimeline synthesis by Apotex Pharmachem, Inc.
Scheme 32.
Cevimeline synthesis by Emcure Pharmaceuticals, Ltd.
Scheme 32.
Cevimeline synthesis by Emcure Pharmaceuticals, Ltd.
Scheme 33.
Synthesis of key intermediate (S)-103 by F. Hoffmann-La Roche, Ltd.
Scheme 33.
Synthesis of key intermediate (S)-103 by F. Hoffmann-La Roche, Ltd.
Scheme 34.
Synthesis of eplerenone by Searle and Co.
Scheme 34.
Synthesis of eplerenone by Searle and Co.
Scheme 35.
Synthesis of eplerenone by Ciba-Geigy.
Scheme 35.
Synthesis of eplerenone by Ciba-Geigy.
Scheme 36.
Synthesis of eplerenone by Pfizer.
Scheme 36.
Synthesis of eplerenone by Pfizer.
Scheme 37.
Synthesis of eplerenone by Chinese Academy of Sciences.
Scheme 37.
Synthesis of eplerenone by Chinese Academy of Sciences.
Scheme 38.
Synthesis of drospirenone by Schering AG.
Scheme 38.
Synthesis of drospirenone by Schering AG.
Scheme 39.
Evestra, Inc. synthesis of drospirenone.
Scheme 39.
Evestra, Inc. synthesis of drospirenone.
Scheme 40.
University of Bologna synthesis of drospirenone.
Scheme 40.
University of Bologna synthesis of drospirenone.
Scheme 41.
Gilead Sciences synthesis of ledipasvir.
Scheme 41.
Gilead Sciences synthesis of ledipasvir.
Scheme 42.
Palladium-free Suzuki-Miyaura-type coupling towards ledipasvir.
Scheme 42.
Palladium-free Suzuki-Miyaura-type coupling towards ledipasvir.
Scheme 43.
Schering-Plough synthesis of rolapitant.
Scheme 43.
Schering-Plough synthesis of rolapitant.
Scheme 44.
Preparation of key building block 159 for the synthesis of rolapitant.
Scheme 44.
Preparation of key building block 159 for the synthesis of rolapitant.
Scheme 45.
A 2013 Opko Health, Inc. synthesis of rolapitant.
Scheme 45.
A 2013 Opko Health, Inc. synthesis of rolapitant.
Scheme 46.
A 2016 Opko Health, Inc. synthesis of rolapitant.
Scheme 46.
A 2016 Opko Health, Inc. synthesis of rolapitant.
Scheme 47.
University of California original synthesis of apalutamide.
Scheme 47.
University of California original synthesis of apalutamide.
Scheme 48.
Apalutamide synthesis with thiophosgene from Sloan-Kettering.
Scheme 48.
Apalutamide synthesis with thiophosgene from Sloan-Kettering.
Scheme 49.
Apalutamide synthesis by Hangzhou Cheminspire Technologies Co., Ltd.
Scheme 49.
Apalutamide synthesis by Hangzhou Cheminspire Technologies Co., Ltd.
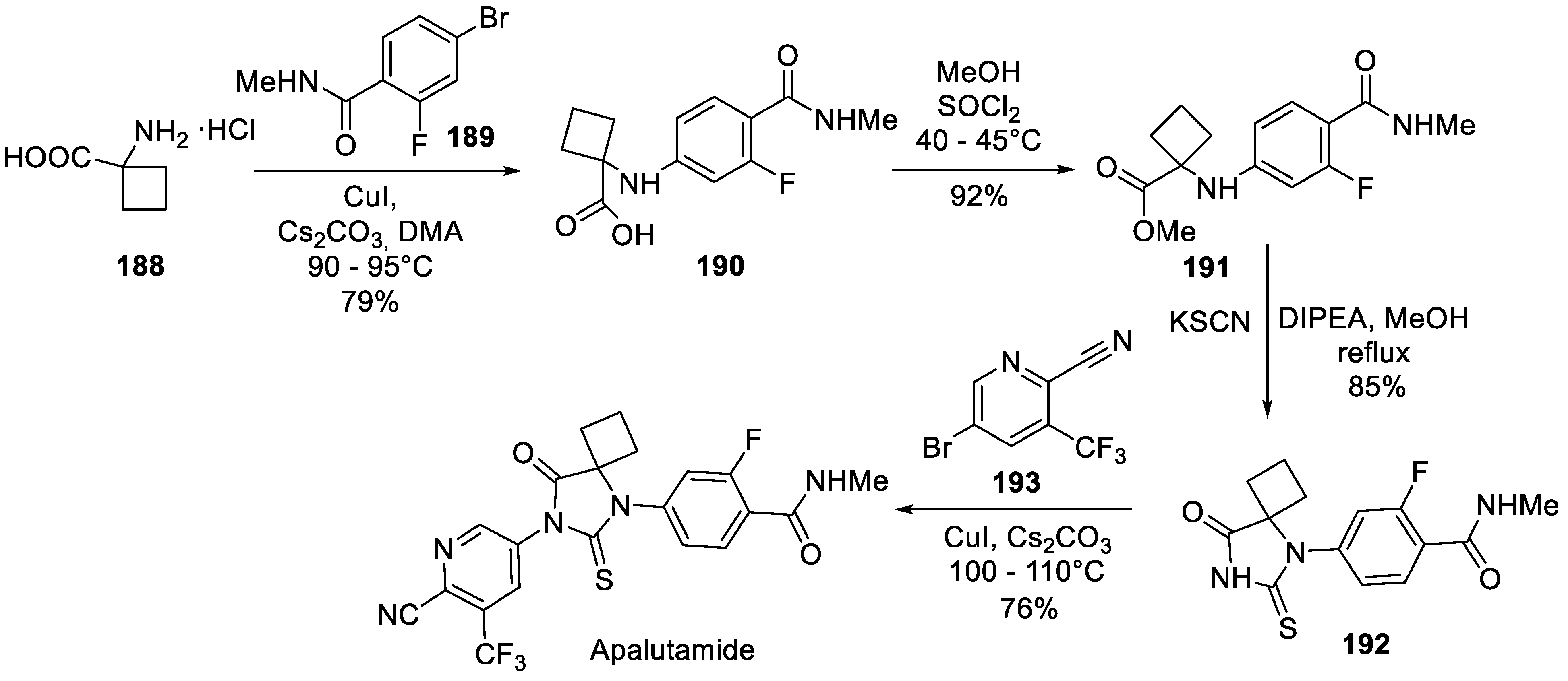
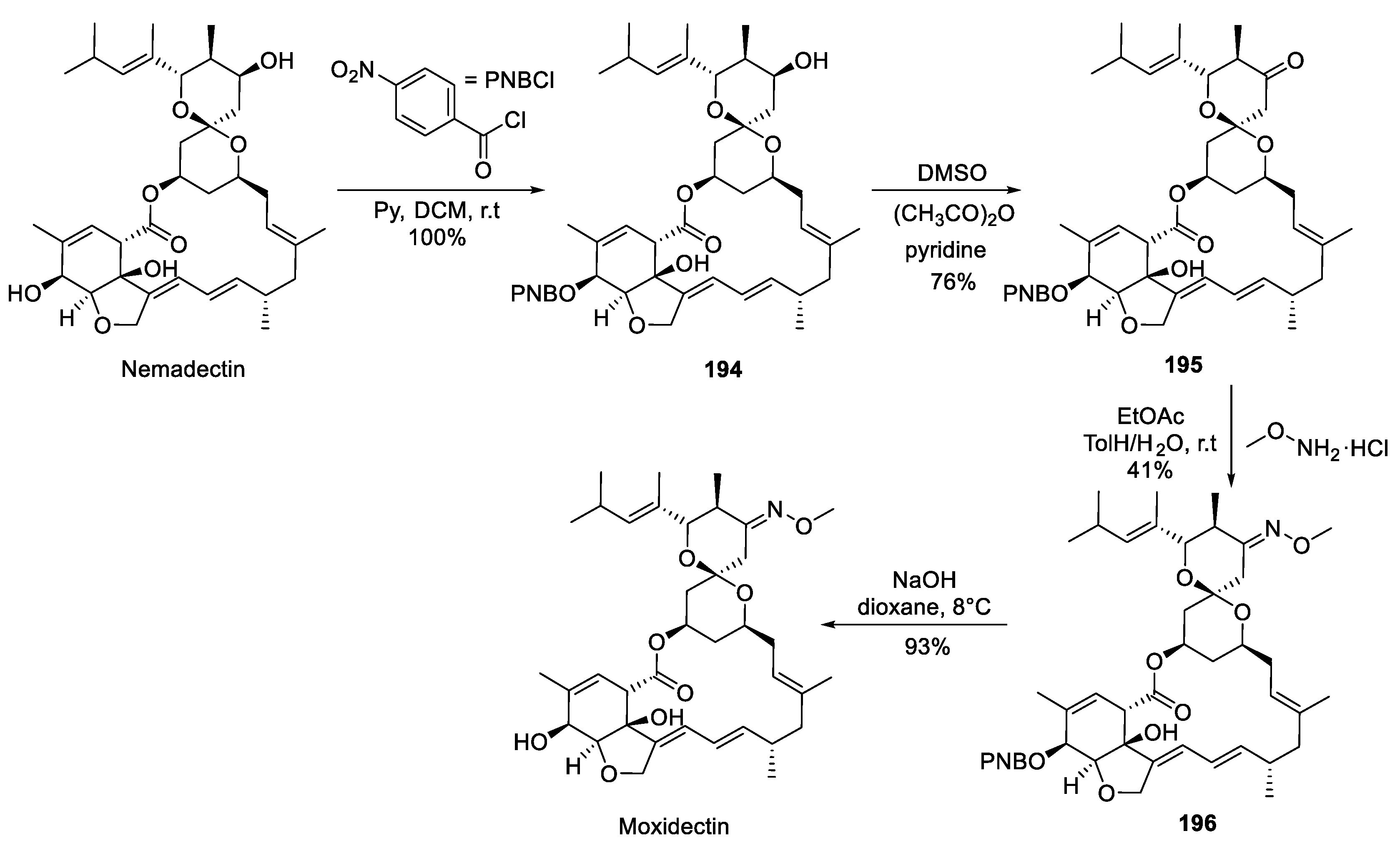
Scheme 50.
Semisynthesis of moxidectin from nemadectin.
Scheme 50.
Semisynthesis of moxidectin from nemadectin.
Scheme 52.
Merck synthesis of enantiopure 3-amino piperidone 198.
Scheme 52.
Merck synthesis of enantiopure 3-amino piperidone 198.
Scheme 53.
Merck synthesis of enantiopure spiro acid 197.
Scheme 53.
Merck synthesis of enantiopure spiro acid 197.
Scheme 54.
Phase-transfer-catalyzed asymmetric synthesis of spiro acid 197.
Scheme 54.
Phase-transfer-catalyzed asymmetric synthesis of spiro acid 197.
Scheme 55.
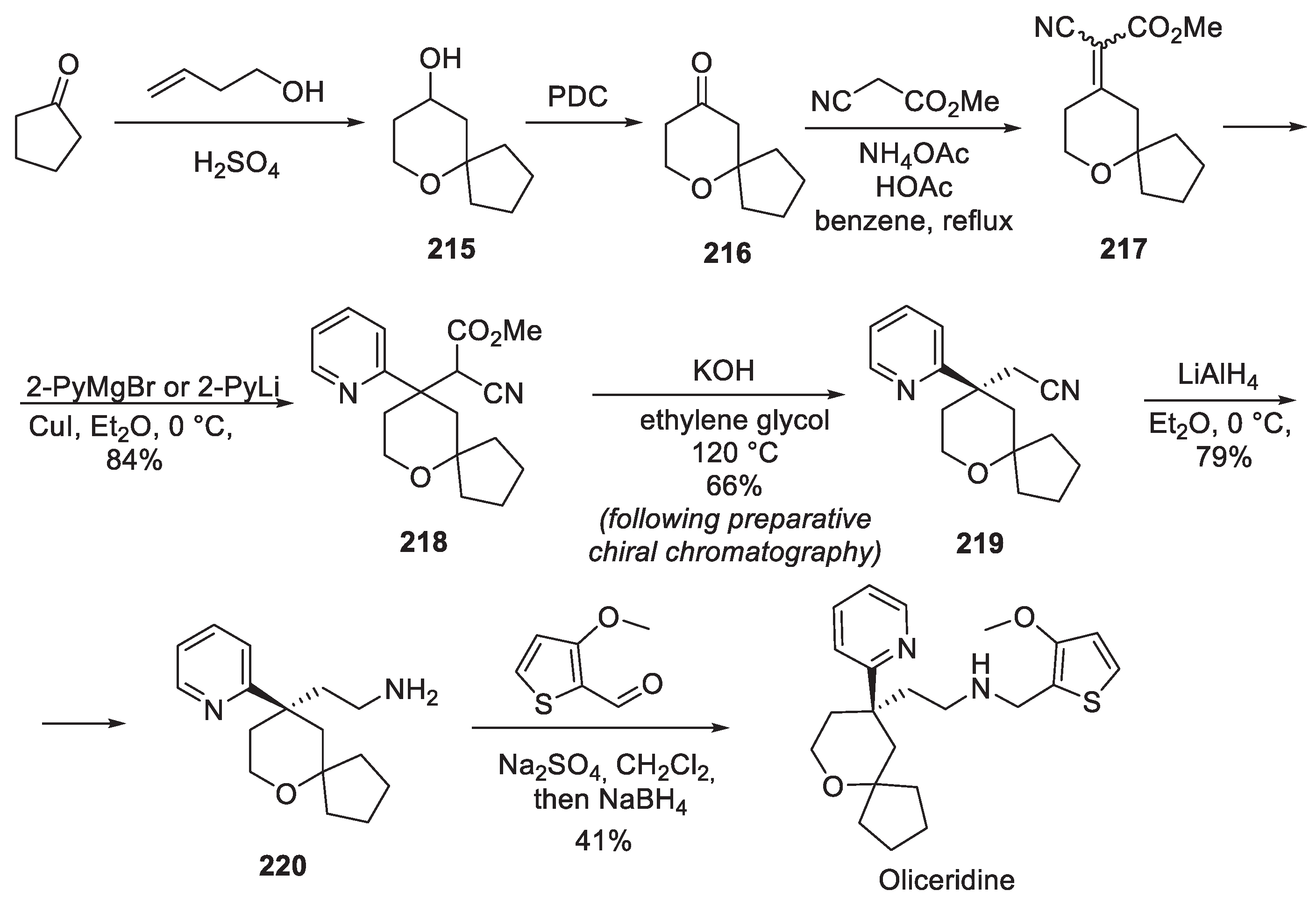
Synthesis of Oliceridine by Trevena, Inc.
Scheme 55.
Synthesis of Oliceridine by Trevena, Inc.
Scheme 56.
A 2015 synthesis of risdiplam by Roche.
Scheme 56.
A 2015 synthesis of risdiplam by Roche.
Scheme 57.
A 2019 synthesis of risdiplam by Roche.
Scheme 57.
A 2019 synthesis of risdiplam by Roche.
Scheme 58.
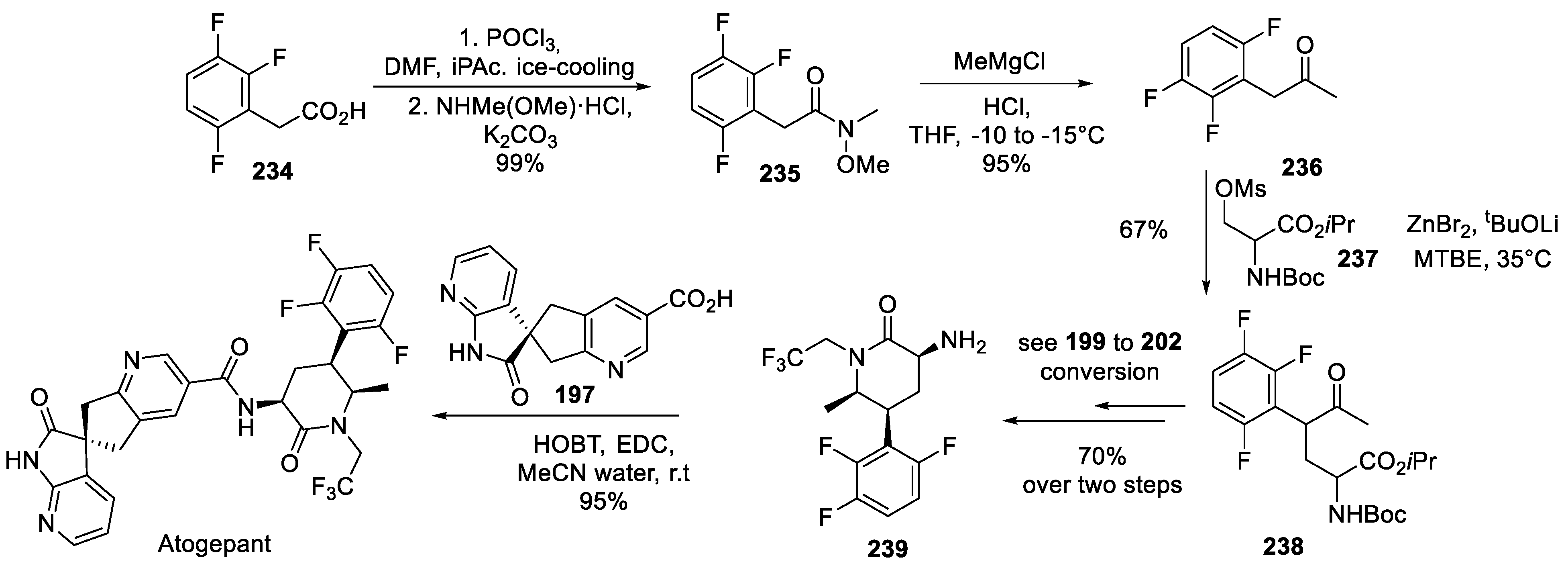
Synthesis of atogepant by Merck.
Scheme 58.
Synthesis of atogepant by Merck.
Scheme 59.
G1 Therapeutics industrial synthesis of trilaciclib.
Scheme 59.
G1 Therapeutics industrial synthesis of trilaciclib.
Table 1.
Summary of approved spirocyclic drugs: year of approval and origin of spirocyclic moiety.
Table 1.
Summary of approved spirocyclic drugs: year of approval and origin of spirocyclic moiety.
| Entry |
Drug name |
Year of approval |
Origin of spirocycle |
| 1 |
Griseofulvin |
1959 |
Cycloaddition, intramolecular Claisen condensation |
| 2 |
Spironolactone |
1960 |
Lactonization |
| 3 |
Fluspirilene |
1970 |
Cyclocondensation |
| 4 |
Fenspiride |
1970 |
Lactamization |
| 5 |
Amcinonide |
1979 |
Ketalization |
| 6 |
Guanadrel |
1979 |
Ketalization |
| 7 |
Buspirone |
1986 |
Commercially available spiro building block |
| 8 |
Ivermectin |
1986 |
Biosynthesis |
| 9 |
Rifabutin |
1992 |
Cyclocondensation |
| 10 |
Spirapril |
1995 |
Thioketalization |
| 11 |
Irbesartan |
1997 |
Cyclocondensation |
| 12 |
Cevimeline |
2000 |
Acetalization |
| 13 |
Eplerenone |
2002 |
Biosynthesis |
| 14 |
Drospirenone |
2000 |
Lactonization |
| 15 |
Ledipasvir |
2014 |
Cyclopropanation |
| 16 |
Rolapitant |
2015 |
Lactamization, ring-closing metathesis |
| 17 |
Apalutamide |
2018 |
Lactonization |
| 18 |
Moxidectin |
2018 |
Biosynthesis |
| 19 |
Ubrogepant |
2020 |
Intramolecular C-alkylation |
| 20 |
Oliceridine |
2020 |
Prins reaction |
| 21 |
Risdiplam |
2020 |
Commercially available spiro building block |
| 22 |
Atogepant |
2021 |
Intramolecular C-alkylation |
| 23 |
Trilaciclib |
2021 |
Intramolecular Claisen condensation |