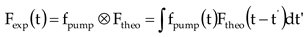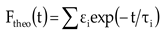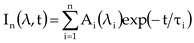1. Introduction
Cyanobacteria, or Cyanophyta, are aquatic gram-negative bacteria [
1]. It is widely accepted that cyanobacteria are the first organisms known to have created oxygen via photosynthesis. In addition, by creating and evolving the molecular oxygen as a byproduct of photosynthesis, the live and multiply of cyanobacteria has changed the early oxygen-poor and allowed for the emergence of diverse life forms on earth [
2]. Unlike purple and other bacteria that perform anoxygenic photosynthesis, cyanobacterial thylakoid membranes are not continuous with the plasma membrane but have separate compartments [
3]. These thylakoid membranes have an important function in photosynthesis, as they host PBS which functions as a primary light-harvesting antenna for the photosystems [
4]. Those PBS are composed of chromophorylated protein stacks, referred to as phycobiliproteins (PBPs) and linker proteins. The PBS assumes a classical structure, wherein the allophycocyanin (APC) performs the core of the model, surrounded by several outwardly oriented rods made up of stacked disks of phycocyanin (PC) and phycoerythrin (PE). In an antenna-like assembly, the geometrical arrangement of the PBPs is exquisite. This intricate arrangement facilitates high efficacy in the transmission of excitation of PBS [
5].
To provide an enhanced understanding of the energy transfer process in PBS, scientists have explored the protein’s energy distribution and energy transfer model through steady-state and transient spectra, concerning the unique spectral property of different PBPs [
6,
7]. PBPs' spectral properties are primarily determined by their prosthetic groups, which are linear tetrapyrroles known as phycobilin, including phycocyanobilin (PCB), phycoerythrobilin (PEB), and phycourobilin (PUB) [
8]. Each PBP has a distinct maximum absorption and fluorescence emission in the visible range of light. As a result of their presence and the specific arrangement within the PBS, light energy can be absorbed and transferred uni-directionally to the chlorophyll of photosystem II. In this way, the PBPs take advantage of available light wavelengths (in the 500-650 nm range) that chlorophyll cannot access and use their energy for photosynthesis [
9,
10]. This is especially beneficial deeper in the water column, where longer wavelength light is less transmitted and thus less available directly to chlorophyll. The light-trapping function of phycobilisome and phycobilin arises from variations in protein arrangement and microenvironment of prosthetic groups. Sole reliance on phycobilin spectral results falls short in explaining the intricate energy transfer process, which must be understood through a synergistic approach combining spectral properties, protein structure, and composition to derive functional insights from structural features. It is much to be regretted that only five detailed architectures of PBS have been described by cryo-electron microscopy (cryo-EM) with great resolution at the near-atom level: two red alga species
Griffithsia pacifica [
11],
Porphyridium purpureum [
12], three cyanobacterial species,
Anabaena 7120,
Synechococcus 7002 [
13] and
Thermosynechococcus vulcanus 2134 [
14]. Therefore, it is challenging to interpret the complex energy transfer process and mechanism in phycobilisomes.
As mentioned above, the energy transfer dynamics of thermophilic cyanobacterium
T.2134 are controversial in the absence of a high-resolution protein structure. In 2003, Adir et al. discovered a minor fraction of PC that absorbed maximally at 612 nm during the isolation of APC from
T. 2134 [
15]. This unique PC
612 trimmer was thought to cover the space between the lower base core cylinders and the higher cylinder in the APC core at the ends of two of the rods. Based on this structural model, they discovered a quick energy transfer pathway that took 888 fs to go from the rod to the core via linker proteins, and then 17 ps to get from the core to the terminal emitters (L
CM) [
16]. Then, using ps fluorescence and fs pump-probe spectroscopies, Kawakami et al. discovered the energy transfer of 7.3 ps between PC trimers in rods, 53 ps from PC rod to APC core, and 180 ps in the APC core complex [
17]. They also confirmed that the APC trimer, not the PC
612 trimer, is located between the lower base core A cylinders and the higher B cylinder [
18]. Until recently, the APC core and PC rod of
T. 2134 were resolved at 3.7 and 4.2 resolutions, respectively, yielding the results that the PBS molecule of
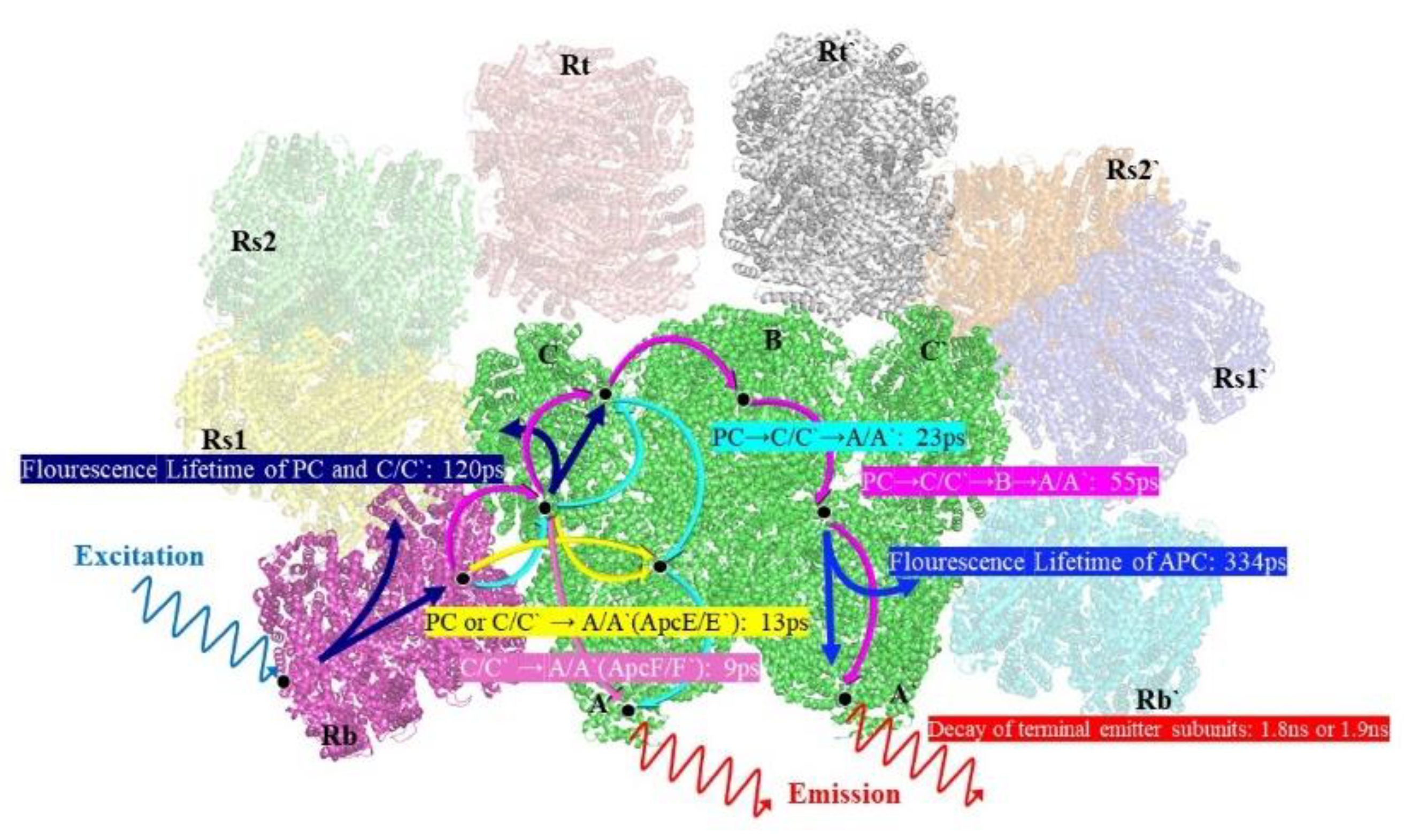
T. 2134 consists of 5 APC core cylinders (A, A’, B, C, C’) surrounded by 8 PC rods (Rb, Rb', Rt, Rt', Rs1, Rs1', Rs2, Rs2'), as shown in
Figure 1, which provide the structural basis for energy transfer research. Hence, with a focus on the PBS rod-core assembly [
19], we aim to re-identified the energy transfer path of the thermophilic cyanobacterium
T. 2134 via fs time-resolved fluorescence spectra and high-resolution structures.
3. Results and Discussion
The steady-state absorption spectra of the isolated PC trimer and APC core from
T.2134, as well as the entire PBS, have been resolved at room temperature [
19]. The absorption band of the intact PBS peaked at 620 nm. The absorption band of the isolated PC rod also peaked at 620nm but exhibited narrower than that of the intact PBS, whereas, the absorption band of the APC core performed as a broadening on the red side of the PC trimer, peaking at 650 nm with a shoulder at 600 nm. The PC rod then exhibited a fluorescence peak at 650 nm, while the peak of the APC fluorescence was around 675 nm. We further measured the spectra property of the C-cylinder of the core, with the absorption band peaked at 612nm and the emission band peaked at 645nm [
26]. In steady state, the emissions from various fluorescence components combine to form the fluorescence spectra, therefore, to investigate the overall energy transfer dynamics in PBS, the time-resolved fluorescence spectra were performed, and the femtosecond laser at the wavelength of 498 nm and 570 nm was selected to excite the PBS sample, respectively. The steady-state absorption spectrum measurements show that only the PC trimer was excited under the wavelength of 500nm as the APC core has no absorption at the wavelength of 498 nm [
19,
27], however, both the PC trimer and APC core can be exited under the wavelength of 570 nm.
The time-resolved fluorescence spectra of
T.2134 PBS at 77 K were thoroughly examined. High signal-to-noise ratios are made possible by the ability to ignore solvent and molecule vibration effects at 77 K, which ensures that the results of time-resolved fluorescence spectroscopy closely match the energy transfer assumptions required in theoretical models [
28].
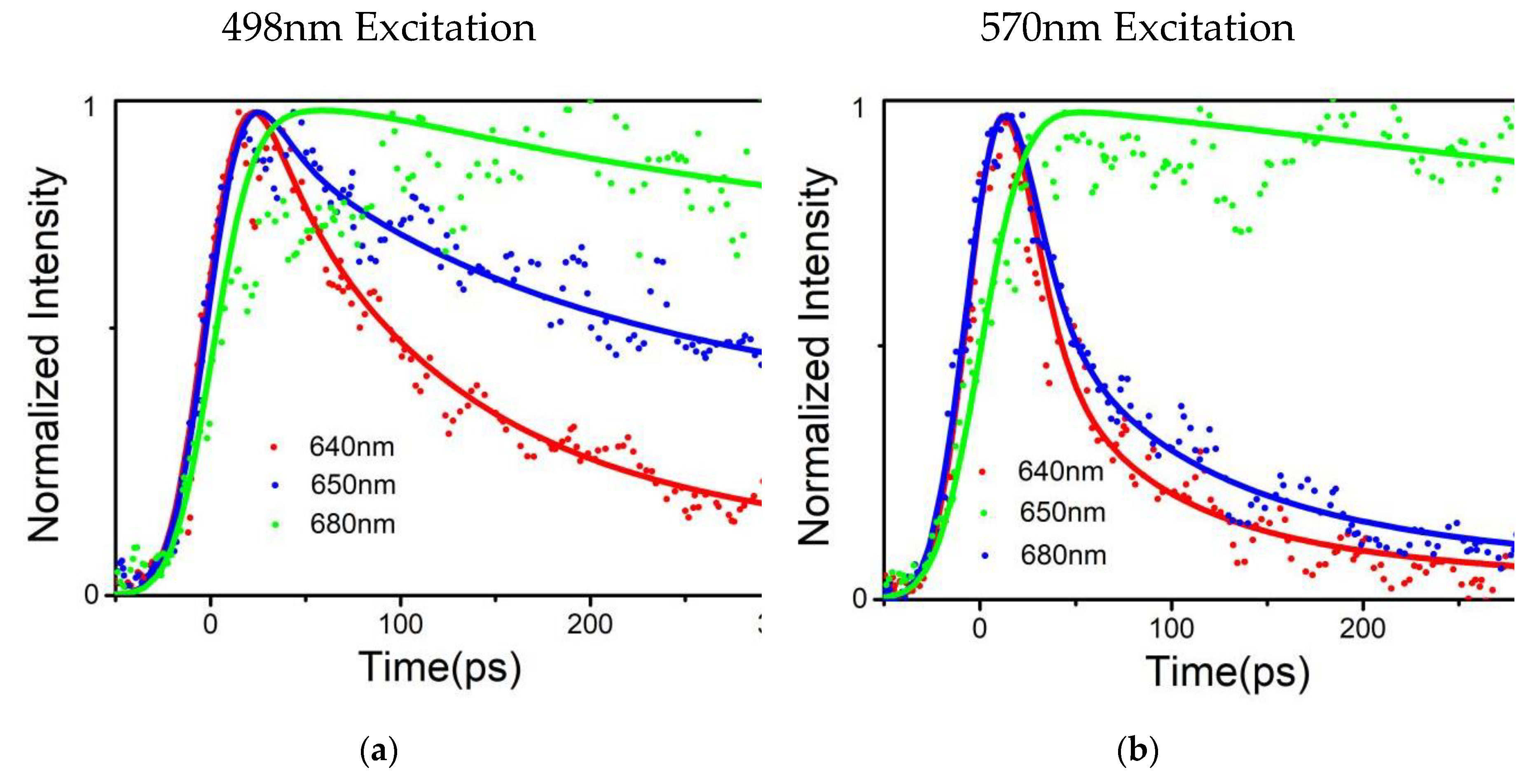
Figure 3a exhibits the reconstructed three-dimensional time-resolved fluorescence spectra of PBS from
T. 2134 excited at 498nm. The expression aids us in conceptualizing the energy flow. Following excitation at 498 nm, which preferentially excited the PCBs of β155 in PC rods [
27], the time-resolved fluorescence spectra of PBS revealed the PC fluorescence band with a central wavelength of around 655 nm, followed immediately by the fluorescence band of the C-cylinder at 645 nm, and the fluorescence band of the APC core at around 675 nm rose concurrently with the decay of C-cylinder. The immediate evidence of the energy transfer from PC→C-cylinder→APC is the changes in the relative intensities of fluorescence components. Different from the results of the 498 nm excitation time-resolved fluorescence spectra, the PC rod and APC core were exited simultaneously under the excitation wavelength of 570 nm. The time-resolved fluorescence spectra result shows that the PC and APC emissions were both discovered at the same time, as shown in
Figure 3b.
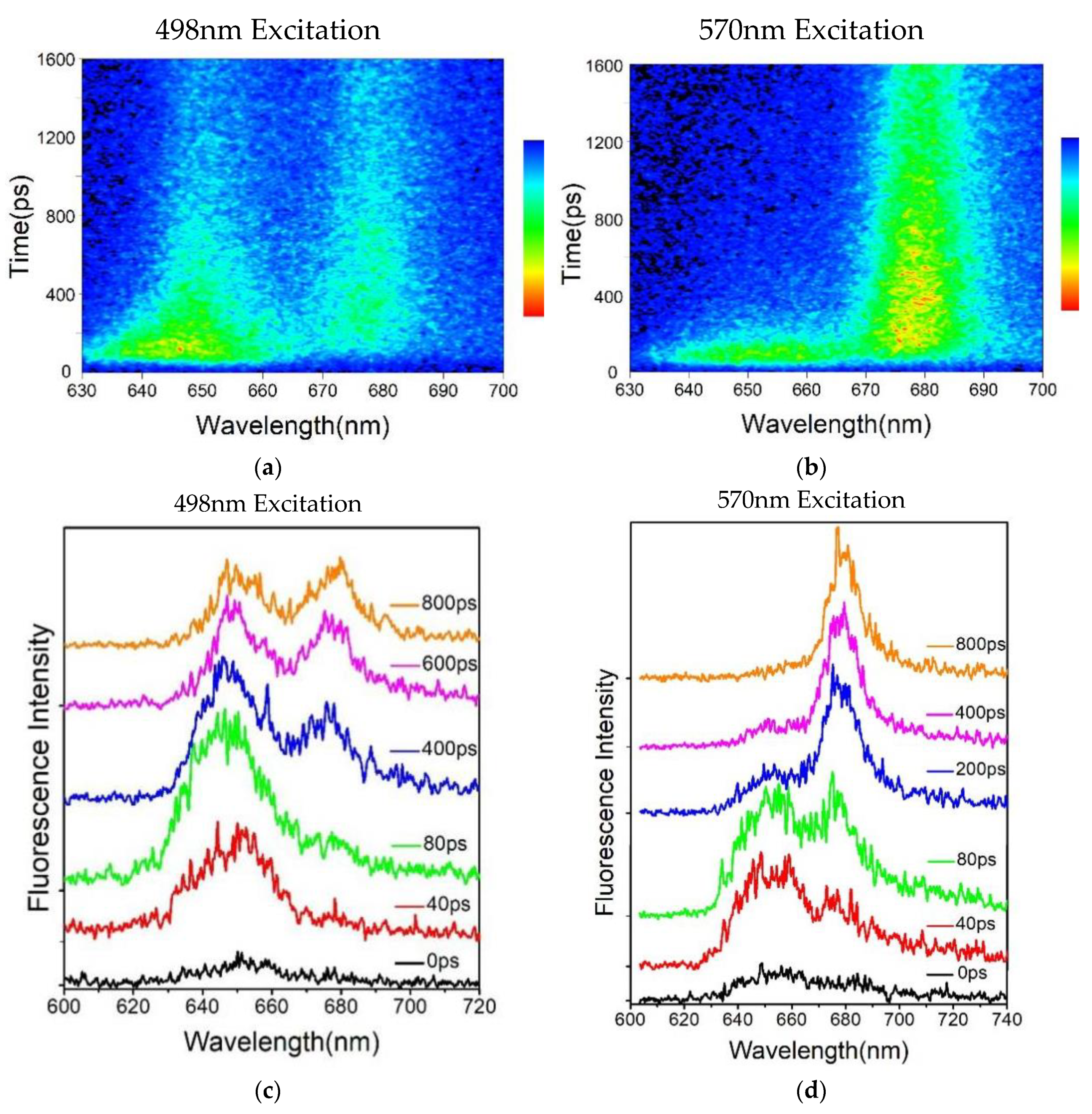
The variations in the fluorescence spectrum were seen to be more obvious in
Figure 3c. Time-resolved fluorescence was discovered using synchronscan technology, and the 650 nm emission was initially clearly identified. And the APC emission appears at 40 ps after excitation, indicating that the terminal emitter in the core accepts the energy transferred from the PC rod within 40 ps. However, the PC emission quickly decayed and nearly disappeared at 400 ps after the excitation (
Figure 3d), indicating that the energy transfer process between PC and APC was completed within 400 ps.
Global optimization deconvolution was used to find out the fluorescence decay constants in the PBS during energy transferring. Multi-exponential deconvolution was used to resolve the fluorescence decay curves at various measured wavelengths, and the Monte-Carlo approach was used to analyze the experimental data. An energy acceptor is represented by the component (A%) with a negative amplitude of the fluorescence's rising phase, while an energy donor is represented by the component (A%) with a positive pre-exponential of the fluorescence's falling phase.
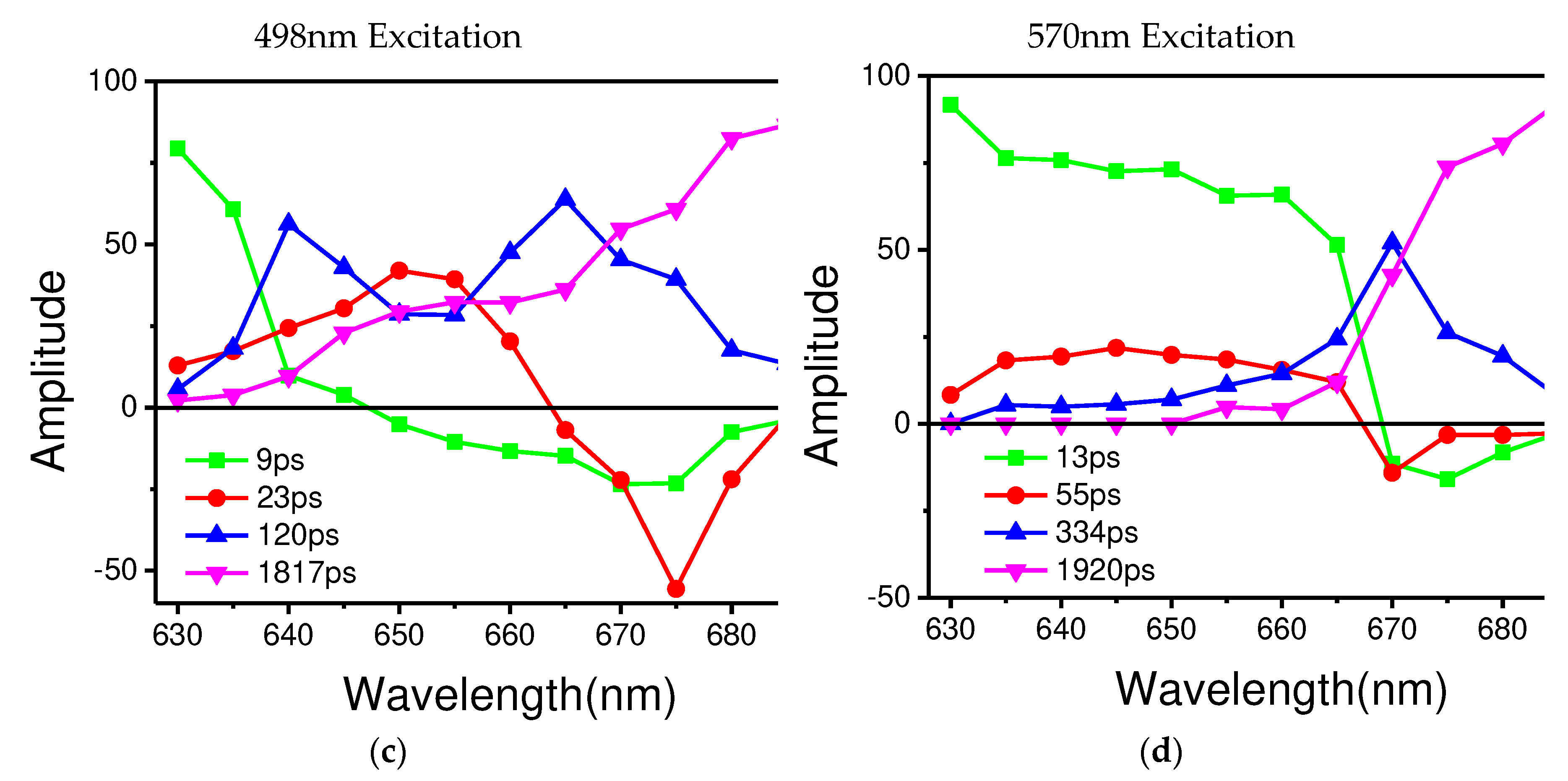
Based on the results of the global multiexponential analysis,
Figure 4c-d show the fluorescence decay-associated spectra (FDAS) obtained by the phycobilisome of
T.2134 under excitation at 498nm and 570nm, respectively. In combination with the high-resolution model, FDAS can further help to reveal the energy transfer in PBS. Typically, to explore the decay kinetics of the same decay constant in different wavelength regions, it is necessary to consider the kinetic increase and reduction of the excited component, that is the change of the amplitude of the kinetic phase. Moreover, the sign of the FDAS factor with the same decay constant is inverted, indicating that there is a coupling occurring between the positive and negative bands, i.e., that there is energy transferred from the complex of the former band that gives energy to the latter band.
From the results, the two excitation wavelengths produce two different sets of decay constants, 10 ps, 20 ps, 120 ps, and 1817 ps for 498 nm excitation, and 13 ps, 55 ps, 344 ps and 1920 ps for 570 nm excitation. In the support material, the fluorescence decay curves and the correspondent fitting results of PBS at different wavelengths are depicted in Figures S1 and S2, and Tables S1 and S2 show the amplitudes of each time component. Among these results, the four rapidly decaying DAS components, 9 ps, 13 ps, 23 ps and 55 ps, exhibit sign change from positive to negative, which are indicative of the decay of one fluorescence band in parallel with the rise of another, showing the characteristics of EET. The other four components had positive peaks without sign changes, indicating that their decay only occurred during the trapping or relaxation. In addition, considering the position of the sign shift and the peak in the horizontal direction, the four decay constants are clearly distinguished. The sign change of 9 ps appeared between 645 nm and 650 nm, while the sign change of 13 ps occurs between 665 nm and 670nm; similarly, the sign change of 23 ps occurs between 660 nm and 665 nm, 55 ps between 665 nm and 670 nm. The positive peaks occur at decay constants of 120 ps and 334 ps, while the decay constants of 1817 ps and 1920 ps gradually increase with increasing wavelength. Therefore, combined with the steady-state spectra and structural information, the FDAS results allow us to assign the following decay constants:
The 9 ps and 13 ps correspond to the fastest decay constants in
Figure 4c-d, respectively. The 9 ps were constant of fluorescence decay in
T. 2134 PBS could be assigned to the time of energy transfer from the C/C’-cylinders direct to the terminal emitter ApcF/F’ in A/A’ cylinders of the APC core, owing to the fact that its amplitude began positively on the blue side before swiftly turning negative and having a minimum at 670 nm (as shown in
Figure 4c). The negative amplitude corresponds to a fluorescence increase and energy transfer from donor to acceptor. Moreover, the latest APC Cryo-EM structure shows that there are strongly coupled pigment pairs between the C/C’ cylinders and the A/A’ cylinders,
Cα
84–
Aα
84. The transferring of energy from the C/C’ cylinders to A/A’ cylinders occurring via
Cα
84–
Aα
84 (35 Å), results in a fast energy transfer time of 9 ps [
14]. Therefore, the quick transfer of energy between C/C'-cylinders and terminal emitter ApcF/F' can be assigned a decay time of 9 ps. The 13 ps constant of the fluorescence decay curve of
T. 2134 PBSs could be attributed to the process of energy transfer from the rod Rb/Rb’ and the C/C’ cylinders to the terminal emitter ApcE/E’ in A/A’ cylinders with the same transfer constant, Shown in
Figure 4d, from 630 to 660 nm, its amplitude was positive. It then started to decline at 665 nm, turned negative at 670 nm, and finally reached a minimum at 675 nm. The following is a description of this amplitude variation: an acceptor with an emission peak of 675 nm receives energy from a donor with an emission peak between 630 and 660 nm. The light absorbed by PCBs in the rod Rb/Rb’ was transferred directly to the ApcE/E’ in A/A’ cylinders via rod-core linker L
RC. At the same time, the light absorbed by PCBs in C/C’ cylinders was transferred to the terminal emitter ApcE/E’ in A/A’ cylinders via core linker L
C. Thus, combining the fluorescence spectra and the cryo-EM structure of the APC core, it is reasonable to assign the decay time of 13 ps to the energy transfer time from the rod or the C/C’ cylinders or B cylinder to the terminal emitter ApcE/E’ in A/A’ cylinders with same transfer constant.
For the decay constants of 23 ps and 55 ps, the experimental results at different excitation wavelengths can clearly see that the terminal receives the energy flow transferred from the PC rod or C/C’ cylinders after 40 ps. Therefore, the 23 ps constant of fluorescence decay in
T. 2134 PBS could be assigned to the energy transfer time from the PC rod Rs/Rs’
→ C/C’ cylinders
→terminal emitter ApcE/E’ in A/A’ cylinders because at the blue side, the amplitude of the FDAS is positive with a maximum wavelength of around 650 nm, while at the red side, the amplitude shifts to the negative with a minimum at about 675 nm (
Figure 4c). Compared with the constants of 9 ps, the higher amplitude of the FDAS curve of the constant of 23 ps shows that the energy transfer pathway with an energy transfer time of 23 ps takes to dominate in the energy transfer from the rods to the core. This agrees with the cryo-EM findings, which show that the C/C' cylinder serves as a conduit for energy transfer from the PC rods to the terminal emitters. Therefore, combining the fluorescence spectra and the cryo-EM structure of the PC rods and APC core, it is appropriate to provide the energy transfer time between Rs/Rs' rods and terminal emitter ApcE/E', a decay time of 23 ps. The 55 ps constant of fluorescence decay curve in
T. 2134 PBS could be attributed to the process of energy transfer time from the PC rod Rs/Rs’
→the C/C’ cylinders
→the B cylinder
→ the terminal emitter ApcD/D’ in A/A’ cylinders, which is a long energy transfer pathway from the rod to the terminal emitter. Because on the blue side, the amplitude of the FDAS is positive, having a maximum of 645 nm, while on the red side, the amplitude shifts to the negative with a minimum of about 670 nm. Moreover, the amplitude of 55 ps is low compared with that of 13 ps, indicating this energy transfer pathway is not the main pathway of the energy transfer from the rod to the core. Therefore, combining the fluorescence spectra and the cryo-EM structure of the PC rods and APC core, it is appropriate to give a decay time of 55 ps to the energy transfer from the PC rod Rs/Rs’
→the C/C’ cylinder
→the B cylinder
→the terminal emitter ApcD/D’ in A/A’ cylinders.
For the four decay constants without EET, the possible energy transfer paths are assigned according to their peak and the trend of the FDAS factor. The fluorescence isotropic decay constants of 1920 ps and 1817 ps reflect the emission of the terminal emitter ApcE (ApcE’). The 120 ps constant of fluorescence decay possibly reflects the emission of the rods and the C/C’ cylinders, because a positive band with two peaks at 640 nm and 665nm was resolved in the FDAS. Due to the fact that only a positive band with a maximum at 670 nm was determined, the fluorescence decay constants of 334 ps may represent the emission of the APC core. The energy transfer dynamics of the intact PBS obtained in this study is summarized in
Figure 5.