Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Study population
Sample collection
Histopathology
Microbiological assessment
Statistical analysis
Ethical considerations
3. Results
Appendiceal lumen outcomes
Peritoneal cavity outcomes
Interpretation of the culture outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viniol, A.; Keunecke, C.; Biroga, T.; Stadje, R.; Dornieden, K.; Bosner, S.; Donner-Banzhoff, N.; Haasenritter, J.; Becker, A. Studies of the symptom abdominal pain—A systematic review and metaanalysis. Family Practice 2014, 31, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.C.; Rangel, S.J. Overview, and diagnosis of acute appendicitis in children. Semin. Pediatr. Surg. 2016, 25, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Mc Cabe, K.; Babl, F.E.; Dalton, S. ; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Management of children with possible appendicitis: a survey of emergency physicians in Australia and New Zealand. Emerg. Med. Australas. 2014, 26, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.M.; Bachur, R.; Kharbanda, A.T. Presentation of appendicitis in preadolescent children. Pediatr. Emerg. Care. 2007, 23, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, A.B.; Taylor, G.A.; Fishman, S.J.; Bachur, R.G. A clinical decision rule to identify children at low risk for appendicitis. Pediatrics 2005, 116, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Sakellaris, G.; Sinopidis, X.; Zachos, K.; Spyridakis, I. Acute appendicitis in children: Causes and treatment. In: Appendicitis-Causes and treatments, Intechopen, Eds., London, United Kingdom, 2023; eBook. [CrossRef]
- Lee, S.L.; Stark, R.; Yaghoubian, A.; Shekherdimian, S.; Kaji, A. Does age affect the outcomes and management of pediatric appendicitis? J. Pediatr. Surg. 2011, 46, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Alloo, J.; Gerstle, T.; Shilyansky, J.; Ein, S.H. Appendicitis in children less than 3 years of age: a 28-year review. Pediatr. Surg. Int. 2004, 19, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Panagidis, A.; Sinopidis, X.; Zachos, K.; Alexopoulos, V.; Vareli, A.; Varvarigou, A.; Georgiou, G. Neonatal perforated Amyand’s hernia presenting as an enterocutaneous scrotal fistula. Asian J. Surg. 2015, 38, 177–179. [Google Scholar] [CrossRef]
- Zachos, K.; Fouzas, S.; Kolonitsiou, F.; Skiadopoulos, S.; Gkentzi, D.; Karatza, A.; Marangos, M.; Dimitriou, G.; Georgiou, G.; Sinopidis, X. Prediction of complicated appendicitis risk in children. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7346–7353. [Google Scholar] [CrossRef]
- Jones, B.; Demetriades, D.; Segal, I.; Burkitt, D.P. The prevalence of appendiceal fecaliths in patients with and without appendicitis. A comparative study from Canada and South Africa. Ann. Surgery 1985, 202, 80–82. [Google Scholar] [CrossRef]
- Nitecki, S.; Karmeli, R.; Sarr, M. Appendiceal calculi and fecaliths as indications for appendectomy. Surg. Gynecol. Obstet. 1990, 171, 185–188. [Google Scholar] [PubMed]
- Deng, Y.; Chang, D.C.; Zhang, Y.; Webb, J.; Gabre-Kidan, A.; Abdullah, F. Seasonal and day of the week variations of perforated appendicitis in US children. Pediatr. Surg. Int. 2010, 26, 691–696. [Google Scholar] [CrossRef]
- Andersson, R.; Hugander, A.; Thulin, A.; Nyström, P.O.; Olaison, G. Clusters of acute appendicitis further evidence for an infectious aetiology. Int. J. Epidemiol. 1995, 24, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Kastritsi, O.; Sinopidis, X.; Barbagadakis, S.; Sakellaris, S.; Matzakanis, G.; Kastritsi, E.D.; Sakellaris, G. Non-operative management of acute appendicitis in children: single center, cohort study. Chirurgia 2022, 35, 138–142. [Google Scholar] [CrossRef]
- Di Saverio, S.; Podda, M.; De Simone, B.; Ceresoli, M.; Augustin, G.; Gori, A.; Boermeester, M.; Sartelli, M.; Coccolini, F.; Tarasconi, A.; De' Angelis, N.; Weber, D.G.; Tolonen, M.; Birindelli, A.; Biffl, W.; Moore, E.E.; Kelly, M.; Soreide, K.; Kashuk, J.; Ten Broek, R.; Gomes, C.A.; Sugrue, M.; Davies, R.J. Damaskos, D; Leppäniemi, A.; Kirkpatrick, A.; Peitzman, A.B.; Fraga, G.P.; Maier, R.V.; Coimbra, R.; Chiarugi, M.; Sganga, G.; Pisanu A.; De' Angelis, G.L.; Tan, E.; Van Goor, H.; Pata, F.; Di Carlo, I.; Chiara, O.; Litvin, A.; Campanile, F.C.; Sakakushev, B.; Tomadze, G.; Demetrashvili, Z.; Latifi, R.; Abu-Zidan, F.; Romeo, O.; Segovia-Lohse, H.; Baiocchi, G.; Costa, D.; Rizoli, S.; Balogh, Z.J.; Bendinelli, C.; Scalea, T.; Ivatury, R.; Velmahos, G.; Andersson, R.; Kluger, Y.; Ansaloni, L.; Catena, F. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J. Emerg. Surg. 2020, 15, 27. [Google Scholar] [CrossRef]
- Gomes, C.A.; Nunes, T.A.; Chebli, J.M.F.; Junior, C.S.; Gomes, C.C. Laparoscopic grading system of acute appendicitis: new insight for future trials. Surg. Laparosc. Endosc. Percutan. Tech. 2012, 22, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Kroiča, J.; Reinis, A.; Mohit, K.; Delorme, M.; Broks, R.; Asare, L.; Berezovska, M.; Jansins, V.; Zviedre, A.; Engelis, A.; Saxena, A.; Petersons, A. Culture based evaluation of microbiota in children with acute appendicitis. Proceed. Latv. Acad. Sci. 2020, 74, 100–105. [Google Scholar] [CrossRef]
- Mariage, M.; Sabbagh, C.; Grelpois, G.; Prevot, F.; Darmon, I.; Regimbeau, J.M. Surgeon’s definition of complicated appendicitis: a prospective video survey study, Euroasian J. Hepatogastroenterol. 2019, 9, 1–4. [Google Scholar] [CrossRef]
- Vaos, G.; Dimopoulou, A.; Gkioka, E.; Zavras, N. Immediate surgery or conservative treatment for complicated acute appendicitis in children? A meta-analysis. J. Pediatr. Surg. 2019, 54, 1365–1371. [Google Scholar] [CrossRef]
- Coccolini, F.; Fugazzola, P.; Sartelli, M.; Cicuttin, E.; Sibilla, M.G.; Leandro, G.; De’ Angelis, G.L.; Gaiani, F.; Di Mario, F.; Tomasoni, M.; Catena, F.; Ansaloni, L. Conservative treatment of acute appendicitis. Acta Biomed. 2018, 89, 119–134. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, M.J.; Lee, B.H. Randomized clinical trial of antibiotic therapy for uncomplicated appendicitis. Br. J. Surg. 2017, 104, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L. The vermiform cecal appendix, expendable or essential? A narrative review. Curr. Opin. Gastroenterol. 2022, 38, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.E. The natural history and traditional management of appendicitis revisited: spontaneous resolution and predominance of prehospital perforations imply that a correct diagnosis is more important than an early diagnosis. World J. Surg. 2007, 31, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Chen, J.; Clarke, S. The vermiform appendix: an immunological organ sustaining a microbiome inoculum. Clin. Sci. (Lond.) 2019, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Randal Bollinger, R.; Barbas, A.S.; Bush, E.L.; Lin, S.S.; Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 2007, 21, 249, 826–831. [Google Scholar] [CrossRef]
- Cai, S.; Fan, Y.; Zhang, B.; Lin, J.; Yang, X.; Liu, Y.; Liu, J.; Ren, J.; Xu, H. Appendectomy is associated with alteration of human gut bacterial and fungal communities. Front. Microbiol. 2021, 12, 724980. [Google Scholar] [CrossRef]
- Maita, S.; Andersson, B.; Svensson, J.F.; Wester, T. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr. Surg. Int. 2020, 36, 261–269. [Google Scholar] [CrossRef]
- Fugazzola, P.; Coccolini, F.; Tomasoni, M.; Stella, M.; Ansaloni, L. Early appendectomy vs. conservative management in complicated acute appendicitis in children: A meta-analysis. J. Pediatr. Surg. 2019, 54, 2234–2241. [Google Scholar] [CrossRef]
- Köhler, F.; Müller, S.; Hendricks, A.; Kastner, C.; Reese, L.; Boerner, K.; Flemming, S.; Lock, J.F. ; Germer, G-T.; Wiegering, A. Changes in appendicitis treatment during the COVID-19 pandemic – A systematic review and meta-analysis. Int. J. Surg. 2021, 95, 106148. [Google Scholar] [CrossRef]
- Köhler, F.; Acar, L.; van den Berg, A.; Flemming, S.; Kastner, C.; Müller, S.; Diers, J. ; Germer, C-T.; Lock, J.F.; L'hoest, H.; Marschall, U.; Wiegering, A. Impact of the COVID-19 pandemic on appendicitis treatment in Germany - a population - based analysis. Langenbecks Arch. Surg. 2021, 406, 377–383. [Google Scholar] [CrossRef]
- Willms, A.G.; Oldhafer, K.J.; Conze, S.; Thasler, W.E.; von Schassen, C.; Hauer, T.; Huber, T.; Germer, C.T.; Günster, S.; Bulian, D.R.; Hirche, Z.; Filser, J.; Stavrou, G.A.; Reichert, M.; Malkomes, P.; Seyfried, S.; Ludwig, T.; Hillebrecht, H.C.; Pantelis, D.; Brunner, S.; Rost, W.; Lock, J.F. ; CAMIN Study Group. Langenbecks Arch. Surg. 2021, 406, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Gerardi, C.; Cillara, N.; Fearnhead, N.; Gomes, C.A.; Birindelli, A.; Mulliri, A.; Davies, R.J.; Di Saverio, S. Antibiotic treatment and appendectomy for uncomplicated acute appendicitis in adults and children: a systematic review and meta-analysis. Ann. Surg. 2019, 270, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Guillet-Caruba, C.; Cheikhelard, A.; Guillet, M.; Bille, E.; Descamps, P.; Yin, L.; Khen-Dunlop, N.; Berche, P.; Ferroni, A. Bacteriologic epidemiology and empirical treatment of pediatric complicated appendicitis. Diagn. Microbiol. Infect. Dis. 2011, 69, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Clermidi, P.; Dorsi, M.; Cocquerelle, V.; Gomes, C.F.; Becmeur, F. Bacterial studies of complicated appendicitis over a 20-year period and their impact on empirical antibiotic treatment. J. Pediatr. Surg. 2012, 47, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, M.M. Appendectomy in pediatrics: the value of peritoneal fluid smear and its bacteriological profile. Open J. Med. Microbiol. 2012, 2, 147–152. [Google Scholar] [CrossRef]
- Fallon, S.C.; Hassan, S. F, Larimer, E.L. Modification of an evidence-based protocol for advanced appendicitis in children. J. Surg. Res. 2013, 185, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Obinwa, O.; Casidy, M.; Flynn, J. The microbiology of bacterial peritonitis due to appendicitis in children. Ir. J. Med. Sci. 2014, 183, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Dabaja-Younis, H.; Farah, H.; Miron, R.; Geffen, Y.; Slijper, N.; Steinberg, R.; Kassis, I. The intraperitoneal bacteriology and antimicrobial resistance in acute appendicitis among children: a retrospective cohort study between the years 2007-2017. Eur. J. Pediatr. 2021, 180, 2091–2098. [Google Scholar] [CrossRef]
- Plattner, A.S.; Newland, J.G.; Wallendorf, M.J.; Shakhsheer, B.A. Management and microbiology of perforated appendicitis in pediatric patients: A 5-year retrospective study. Infect. Dis. Ther. 2021, 10, 2247–2257. [Google Scholar] [CrossRef]
- Zhong, D.; Brower-Sinning, R.; Firek, B.; Morowitz, M.J. Acute appendicitis in children is associated with an abundance of bacteria from the phylum Fusobacteria. J. Pediatr. Surg. 2014, 49, 441–446. [Google Scholar] [CrossRef]
- Jackson, H.T.; Mongodin, E.F.; Davenport, K.P.; Fraser, C.M.; Sandler, A.D.; Zeichner, S.L. Culture-independent evaluation of the appendix and rectum microbiomes in children with and without appendicitis. PLoS One 2014, 9, e95414. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Brower-Sinning, R.; Firek, B.; Zhong, D.; Morowitz, M.J. Acute appendicitis in children is associated with a local expansion of fusobacteria. Clin. Infect. Dis. 2016, 63, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Salö, M.; Marungruang, N.; Roth, B.; Sundberg, T.; Stenstrom, P.; Arnbjörnsson, E.; Fak, F.; Ohlsson, B. Evaluation of the microbiome in children's appendicitis. Int. J. Colorectal Dis. 2017, 32, 19–28. [Google Scholar] [CrossRef] [PubMed]
- The, S.M.L.; Bakx, R.; Budding, A.E.; de Meij, T.G.J.; van der Lee, J.H.; Bunders, M.J.; Poort, L.; Heij, H.A.; van Heurn, L.W.E.; Gorter, R.R. Microbiota of children with complex appendicitis: different composition and diversity of the microbiota in children with complex compared with simple appendicitis. Pediatr. Infect. Dis. J. 2019, 38, 1054–1060. [Google Scholar] [CrossRef]
- Schülin, S.; Schlichting, N.; Blod, C.; Opitz, S.; Suttkus, A.; Stingu, C.S.; Barry, K.; Lacher, M.; Buhligen, U.; Mayer, S. The intra- and extraluminal appendiceal microbiome in pediatric patients: A comparative study. Medicine (Baltimore) 2017, 96, e9518. [Google Scholar] [CrossRef]
- Hardwick, R.H; Taylor, A.; Thompson, M.H.; Jones, E.; Roe, A.M. Association between Streptococcus milleri and abscess formation after appendicitis. Ann. R. Coll. Surg. Engl. 2000, 82, 24–26. [Google Scholar]
- Martin, B.; Subramanian, T.; Arul, S.; Patel, M.; Jester, I. Using microbiology culture in pediatric appendicitis to risk stratify patients: a cohort study. Surg. Infect. (Larchmt). 2023, 24, 183–189. [Google Scholar] [CrossRef]
- Theodorou, C.M.; Stokes, S.C.; Hegazi, M.S.; Brown, E.G. , Saadai, P. Is Pseudomonas infection associated with worse outcomes in pediatric perforated appendicitis? J. Pediatr. Surg. 2021, 56, 1826–1830. [Google Scholar] [CrossRef]
- Dreznik, Y.; Feigin, E.; Samuk, I.; Kravarusic, D.; Baazov, A.; Levy, I.; Livni, G.; Freud, E. Dual versus triple antibiotics regimen in children with perforated acute appendicitis. Eur. J. Pediatr. Surg. 2018, 28, 491–494. [Google Scholar] [CrossRef]
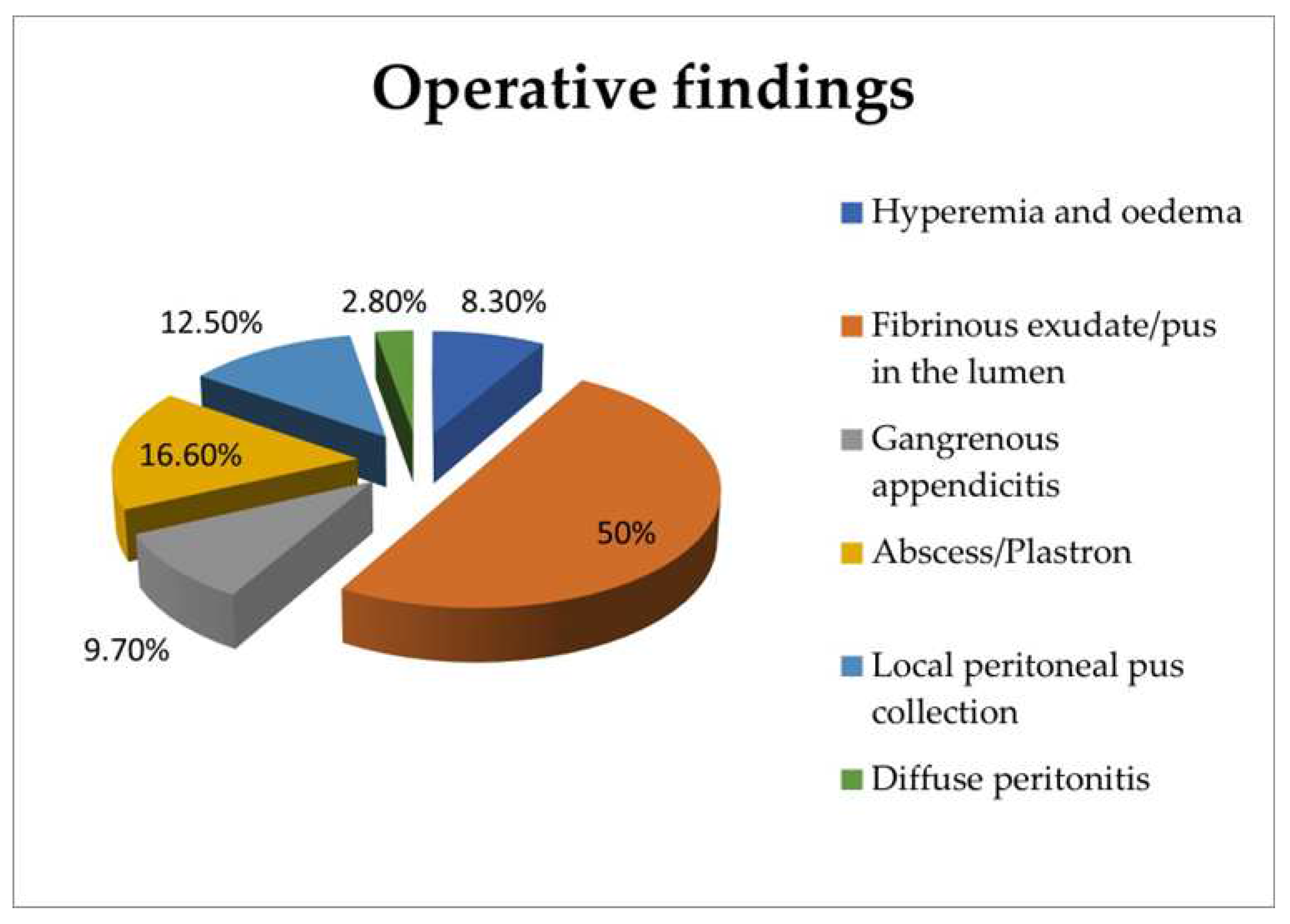
| Variables | Uncomplicated appendicitis | Complicated appendicitis | p-value |
|---|---|---|---|
| n | 42 | 30 | |
| Female | 16 | 13 | 0.444 |
| Male | 26 | 17 | 0.069 |
| Age (years) | 10.8 ± 2.4 | 10.4±3.0 | 0.449 |
| Weight (kg) | 41.5 ± 13.4 | 41.2±15.7 | 0.932 |
| Height (cm) | 151.5 ± 13.7 | 148.7±19 | 0.467 |
| BMI (kg/m2) | 17.6 ± 3.6 | 17.9±3.2 | 0.515 |
| RLQ tenderness | 41 (97.6%) | 30 (100%) | 0.583 |
| Excessive RLQ tenderness | 21 (50%) | 19 (63.3%) | 0.262 |
| Pain migration | 18 (42.9%) | 14 (46.7%) | 0.748 |
| Anorexia | 31 (73.8%) | 25 (83.3%) | 0.338 |
| Nausea/emesis | 28 (66.7%) | 27 (90%) | 0.022 |
| Temperature max (oC) | 37.3±0.8 | 38.0 ± 0.7 | <0.001 |
| WBC (10³/μL) | 14.0±3.5 | 16.0 ± 4.2 | 0.017 |
| Neutrophil count (10³/μL) | 11±3.6 | 13.5 ± 3.9 | 0.007 |
| Neutrophils % | 77.6 ± 8.8 | 83.6 ± 4,6 | <0.001 |
| Hb (g/dL) | 12.96 ± 1.02 | 12.93 ± .18 | 0.685 |
| CRP (mg/dL) | 3.4 ± 4.6 | 6.9 ± 5.9 | 0.015 |
| Bacteria | % |
|---|---|
| Escherichiacoli | 34.7 |
| Escherichia coli + Pseudomonas aeruginosa | 12.5 |
| Escherichia coli + Streptococcus spp | 9.7 |
| Escherichia coli + Enterococcus faecalis | 8.3 |
| Klebsiella pneumoniae | 6.9 |
| Escherichia coli + Bacteroides spp (non-fragilis) | 5.6 |
| Pseudomonas aeruginosa | 2.8 |
| Escherichia coli + Bacteroides fragilis | 2.8 |
| Bacteroides fragilis | 1.4 |
| Escherichia coli + Klebsiella pneumonia | 1.4 |
| Escherichia coli + Enterococcus avium | 1.4 |
| Escherichia coli + Enterococcus gallinarum | 1.4 |
| Escherichia coli + Proteus mirabilis | 1.4 |
| Streptococcus spp + Bacteroides spp | 1.4 |
| Escherichia coli + Propionebacterium spp | 1.4 |
| Escherichia coli + Pseudomonas aeruginosa + Enterococcus faecalis | 1.4 |
| Escherichia coli + Providencia rettgeri + Clostridium spp | 1.4 |
| Pseudomonas aeruginosa + Providencia rettgeri + Bacteroides ovatus | 1.4 |
| Pseudomonas aeruginosa + Klebsiella pneumoniae + Enterococcus faecalis | 1.4 |
| Escherichia coli + Streptococcus spp + Βacteroides spp + Enterobacter aerogenes | 1.4 |
| Bacteria | % |
|---|---|
| Uncomplicated appendicitis | |
| Escherichiacoli | 35.7 |
| Klebsiella pneumoniae | 11.9 |
| Escherichia coli + Pseudomonas aeruginosa | 11.9 |
| Escherichia coli + Enterococcus faecalis | 9.5 |
| Escherichia coli + Bacteroides spp (non-fragilis) | 7.1 |
| Pseudomonas aeruginosa | 4.8 |
| Escherichia coli + Streptococcus spp | 4.8 |
| Bacteroides fragilis | 2.4 |
| Escherichia coli + Enterococcus gallinarum | 2.4 |
| Escherichia coli + Bacteroides fragilis | 2.4 |
| Pseudomonas aeruginosa + Pseudomonas rettgeri + Bacteroides ovatus | 2.4 |
| Pseudomonas aeruginosa + Klebsiella pneumoniae + Enterococcus faecalis | 2.4 |
| Escherichia coli + Streptococcus spp + Bacteroides spp (non-fragilis) + Enterobacter aerogenes | 2.4 |
| Complicated appendicitis | |
| Escherichiacoli | 33.3 |
| Escherichia coli + Streptococcus spp | 16.7 |
| Escherichia coli + Pseudomonas aeruginosa | 13.3 |
| Escherichia coli + Enterococcus faecalis | 6.7 |
| Escherichia coli + Klebsiella pneumoniae | 3.3 |
| Escherichia coli + Bacteroides spp (non-fragilis) | 3.3 |
| Escherichia coli + Enterococcus avium | 3.3 |
| Escherichia coli + Enterococcus gallinarum | 3.3 |
| Escherichia coli + Bacteroides fragilis | 3.3 |
| Streptococcus spp + Bacteroides spp | 3.3 |
| Escherichia coli + Propionebacterium spp | 3.3 |
| Escherichia coli + Pseudomonas aeruginosa + Enterococcus faecalis | 3.3 |
| Escherichia coli + Providencia rettgeri + Clostridium spp | 3.3 |
| Bacteria | % |
|---|---|
| Escherichiacoli | 45.2 |
| Escherichia coli + Streptococcus spp | 12.9 |
| Escherichia coli + Pseudomonas aeruginosa | 9.7 |
| Enterococcus faecalis | 3.2 |
| Bacteroides fragilis | 3.2 |
| Klebsiella pneumoniae | 3.2 |
| Providencia rettgeri | 3.2 |
| Streptococcus spp | 3.2 |
| Escherichia coli + Enterococcus faecalis | 3.2 |
| Escherichia coli + Enterococcus avium | 3.2 |
| Escherichia coli + Enterococcus gallinarum | 3.2 |
| Streptococcus spp + Pseudomonas vesicularis | 3.2 |
| Klebsiella pneumoniae + Enterococcus faecalis | 3.2 |
| Bacteria | % |
|---|---|
| Uncomplicated appendicitis | |
| Escherichiacoli | 45.5 |
| Enterococcus faecalis | 9.1 |
| Bacteroides fragilis | 9.1 |
| Klebsiella pneumoniae | 9.1 |
| Providencia rettgeri | 9.1 |
| Escherichia coli + Pseudomonas aeruginosa | 9.1 |
| Enterococcus faecalis + Klebsiella pneumoniae | 9.1 |
| Complicated appendicitis | |
| Escherichiacoli | 45 |
| Escherichiacoli + Streptococcus spp | 20 |
| Escherichia coli + Pseudomonas aeruginosa | 10 |
| Streptococcus spp | 5 |
| Escherichia coli + Enterococcus faecalis | 5 |
| Escherichia coli + Enterococcus avium | 5 |
| Escherichia coli + Enterococcus gallinarum | 5 |
| Streptococcus spp + Pseudomonas vesicularis | 5 |
| Variable | Model value | Number of patients | |||
| Operative findings | Complicated appendicitis (2) | 30 | |||
| Uncomplicated appendicitis (1) | 42 | ||||
| Total | 72 | ||||
| Prediction coefficient | Coef. | SE Coef. | Z | p-value | Odds Ratio |
| Variable | -1.39208 | 1.57541 | -0.88 | 0.377 | |
| Peritoneal fluid culture (2) | 1.35473 | 0.385695 | 3.51 | 0.001 | 3.88 |
| Appendiceal lumen culture (2) | 0.658258 | 0.544083 | 1.21 | 0.226 | 1.93 |
| 95% CI | |||||
| Prediction coefficient | Lower | Upper | |||
| Variable | |||||
| Peritoneal fluid culture (2) | 1.82 | 8.25 | |||
| Appendiceal lumen culture (2) | 0.66 | 5.61 | |||
| Log-Likelihood = -41.802 | |||||
| Test that all slopes are zero: G = 22.504, DF = 4, p-value = 0.000 | |||||
| Goodness-of-fit tests | |||||
| Method | Chi square | DF | p-value | ||
| Pearson | 64.9860 | 56 | 0.192 | ||
| Deviance | 71.4668 | 56 | 0.080 | ||
| Hosmer-Lemeshow | 6.9920 | 8 | 0.537 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





