Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Setting
2.3. Interventions
2.4. Participants
2.5. Variables
2.6. Data collection/measurement
2.7. Bias
2.8. Study size
2.9. Statistical methods
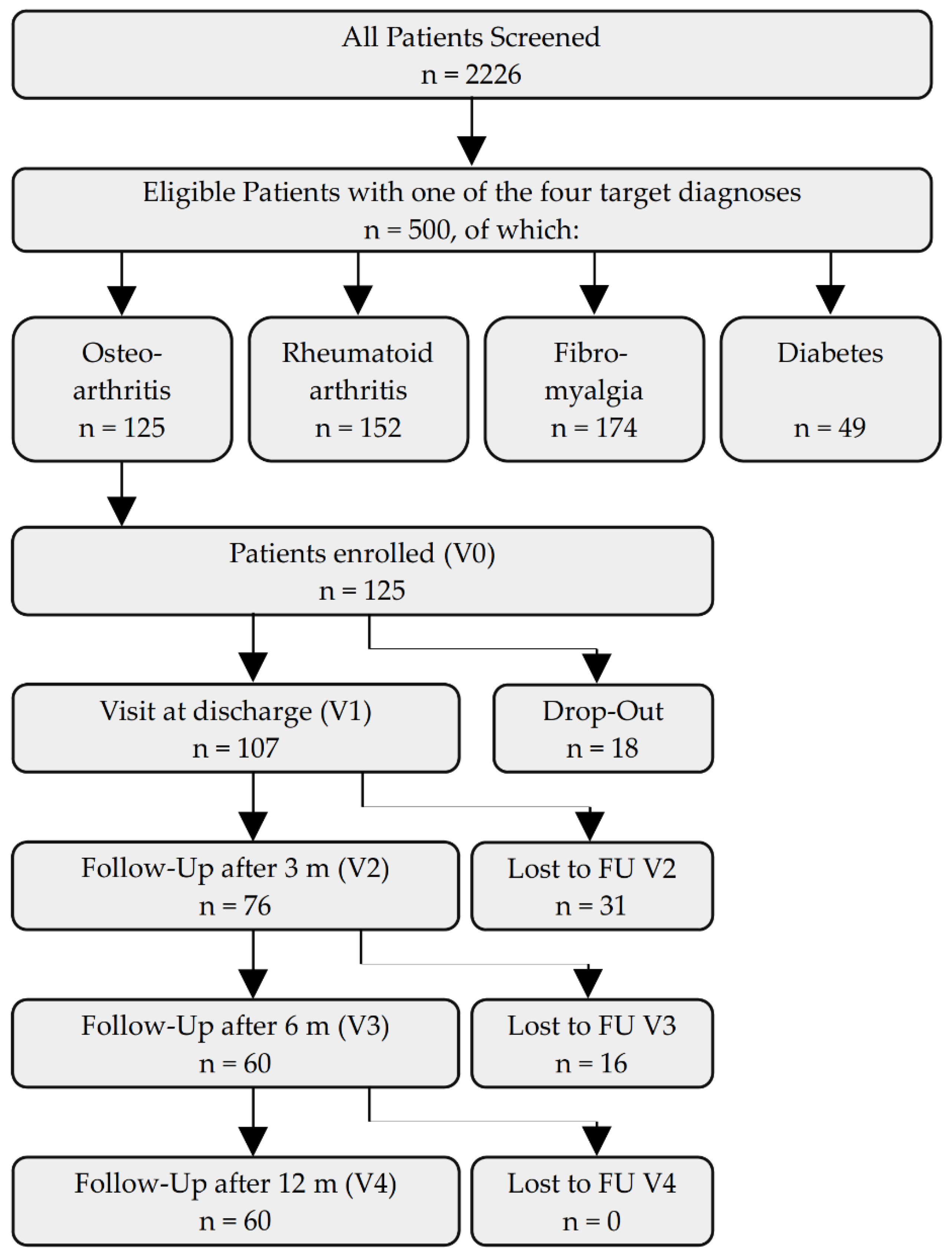
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- RKI. 12-Monats-Prävalenz von Arthrose in Deutschland: RKI; 2017 [Available from: https://www.rki.de/DE/Content/Gesundheitsmonitoring/Gesundheitsberichterstattung/GBEDownloadsJ/FactSheets/JoHM_03_2017_Praevalenz_Arthrose.pdf.
- Knapik JJ, Pope R, Orr R, Schram B. Osteoarthritis: Pathophysiology, Prevalence, Risk Factors, and Exercise for Reducing Pain and Disability. J Spec Oper Med. 2018;18(3):94-102. [CrossRef]
- Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160-7. [CrossRef]
- Wei N, Dai Z. The Role of Nutrition in Osteoarthritis: A Literature Review. Clin Geriatr Med. 2022;38(2):303-22. [CrossRef]
- Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770-5. [CrossRef]
- Michalsen, A. Prolonged fasting as a method of mood enhancement in chronic pain syndromes: a review of clinical evidence and mechanisms. Curr Pain Headache Rep. 2010;14(2):80-7. [CrossRef]
- Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS One. 2019;14(1):e0209353. [CrossRef]
- Drinda S, Grundler F, Neumann T, Lehmann T, Steckhan N, Michalsen A, et al. Effects of Periodic Fasting on Fatty Liver Index-A Prospective Observational Study. Nutrients. 2019;11(11). [CrossRef]
- Hasanpour-Dehkordi A, Kabiri F, Dris F. Comparing the Effects of Massage Therapy and Aromatherapy on Knee Pain, Morning Stiffness, Daily Life Function, and Quality of Life in Patients with Knee Osteoarthritis. Complement Med Res. 2021;28(4):292-9. [CrossRef]
- Ring RM, Eisenmann C, Kandil FI, Steckhan N, Demmrich S, Klatte C, et al. Mental and Behavioural Responses to Baha'i Fasting: Looking behind the Scenes of a Religiously Motivated Intermittent Fast Using a Mixed Methods Approach. Nutrients. 2022;14(5). [CrossRef]
- Schmidt S, Stange R, Lischka E, Kiehntopf M, Deufel T, Loth D, et al. [Uncontrolled clinical study of the efficacy of ambulant fasting in patients with osteoarthritis]. Forsch Komplementmed. 2010;17(2):87-94. [CrossRef]
- Kjeldsen-Kragh J, Sumar N, Bodman-Smith K, Brostoff J. Changes in glycosylation of IgG during fasting in patients with rheumatoid arthritis. Br J Rheumatol. 1996;35(2):117-9. [CrossRef]
- Kjeldsen-Kragh J, Mellbye OJ, Haugen M, Mollnes TE, Hammer HB, Sioud M, et al. Changes in laboratory variables in rheumatoid arthritis patients during a trial of fasting and one-year vegetarian diet. Scand J Rheumatol. 1995;24(2):85-93. [CrossRef]
- Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. 1991;338(8772):899-902. [CrossRef]
- Muller H, de Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol. 2001;30(1):1-10. [CrossRef]
- Wilhelmi de Toledo F, Buchinger A, Burggrabe H, Holz G, Kuhn C, Lischka E, et al. Fasting therapy - an expert panel update of the 2002 consensus guidelines. Forsch Komplementmed. 2013;20(6):434-43.
- Fischer JM, Kandil FI, Kessler CS, Nayeri L, Zager LS, Rocabado Hennhofer T, et al. Stress Reduction by Yoga versus Mindfulness Training in Adults Suffering from Distress: A Three-Armed Randomized Controlled Trial including Qualitative Interviews (RELAX Study). J Clin Med. 2022;11(19). [CrossRef]
- Kessler CS, Jeitler M, Dhiman KS, Kumar A, Ostermann T, Gupta S, et al. Ayurveda in Knee Osteoarthritis-Secondary Analyses of a Randomized Controlled Trial. J Clin Med. 2022;11(11). [CrossRef]
- Bringmann HC, Michalsen A, Jeitler M, Kessler CS, Brinkhaus B, Brunnhuber S, et al. Meditation-based lifestyle modification in mild to moderate depression-A randomized controlled trial. Depress Anxiety. 2022;39(5):363-75. [CrossRef]
- Jeitler M, Michalsen A, Schwiertz A, Kessler CS, Koppold-Liebscher D, Grasme J, et al. Effects of a Supplement Containing a Cranberry Extract on Recurrent Urinary Tract Infections and Intestinal Microbiota: A Prospective, Uncontrolled Exploratory Study. J Integr Complement Med. 2022;28(5):399-406. [CrossRef]
- Jeitler M, Wottke T, Schumann D, Puerto Valencia LM, Michalsen A, Steckhan N, et al. Ayurvedic vs. Conventional Nutritional Therapy Including Low-FODMAP Diet for Patients With Irritable Bowel Syndrome-A Randomized Controlled Trial. Front Med (Lausanne). 2021;8:622029. [CrossRef]
- Jeitler M, Michalsen A, Frings D, Hubner M, Fischer M, Koppold-Liebscher DA, et al. Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review. Front Pharmacol. 2020;11:580656. [CrossRef]
- Jeitler M, Roth S, Steckhan N, Meier L, Koppold-Liebscher DA, Kandil FI, et al. Therapeutic Phlebotomy in Patients with Grade 1 Hypertension: A Randomized-Controlled Trial. J Integr Complement Med. 2022;28(6):530-9. [CrossRef]
- Hohmann CD, Stange R, Steckhan N, Robens S, Ostermann T, Paetow A, et al. The Effectiveness of Leech Therapy in Chronic Low Back Pain. Dtsch Arztebl Int. 2018;115(47):785-92. [CrossRef]
- Michalsen A, Ludtke R, Cesur O, Afra D, Musial F, Baecker M, et al. Effectiveness of leech therapy in women with symptomatic arthrosis of the first carpometacarpal joint: a randomized controlled trial. Pain. 2008;137(2):452-9. [CrossRef]
- Michalsen A, Klotz S, Ludtke R, Moebus S, Spahn G, Dobos GJ. Effectiveness of leech therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2003;139(9):724-30. [CrossRef]
- Michalsen A, Moebus S, Spahn G, Esch T, Langhorst J, Dobos GJ. Leech therapy for symptomatic treatment of knee osteoarthritis: results and implications of a pilot study. Altern Ther Health Med. 2002;8(5):84-8.
- Michalsen A, Ludtke R, Buhring M, Spahn G, Langhorst J, Dobos GJ. Thermal hydrotherapy improves quality of life and hemodynamic function in patients with chronic heart failure. Am Heart J. 2003;146(4):728-33. [CrossRef]
- Jeitler M, Jaspers J, von Scheidt C, Koch B, Michalsen A, Steckhan N, et al. Mind-body medicine and lifestyle modification in supportive cancer care: A cohort study on a day care clinic program for cancer patients. Psychooncology. 2017;26(12):2127-34. [CrossRef]
- Hartmann AM DOM, Spoo M, Fischer JM, Steckhan N, Jeitler M, Häupl T, Kandil FI, Michalsen A, Koppold-Liebscher DA and Kessler C. To eat or not to eat—an exploratory randomized controlled.
- trial on fasting and plant-based diet in rheumatoid arthritis (NutriFast-study).. Front Nutr 2022;9:1030380.
- Jeitler M, Lauche R, Hohmann C, Choi KA, Schneider N, Steckhan N, et al. A Randomized Controlled Trial of Fasting and Lifestyle Modification in Patients with Metabolic Syndrome: Effects on Patient-Reported Outcomes. Nutrients. 2022;14(17). [CrossRef]
- Cramer H, Hohmann C, Lauche R, Choi KA, Schneider N, Steckhan N, et al. Effects of Fasting and Lifestyle Modification in Patients with Metabolic Syndrome: A Randomized Controlled Trial. J Clin Med. 2022;11(16). [CrossRef]
- Koppold-Liebscher DA, Klatte C, Demmrich S, Schwarz J, Kandil FI, Steckhan N, et al. Effects of Daytime Dry Fasting on Hydration, Glucose Metabolism and Circadian Phase: A Prospective Exploratory Cohort Study in Baha'i Volunteers. Front Nutr. 2021;8:662310.
- Hartmann AM, Dell'Oro M, Kessler CS, Schumann D, Steckhan N, Jeitler M, et al. Efficacy of therapeutic fasting and plant-based diet in patients with rheumatoid arthritis (NutriFast): study protocol for a randomised controlled clinical trial. BMJ open. 2021;11(8):e047758. [CrossRef]
- Maifeld A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Markó L, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021;12(1):1970. [CrossRef]
- Koppold-Liebscher D, Kessler CS, Steckhan N, Bahr V, Kempter C, Wischnewsky M, et al. Short-term fasting accompanying chemotherapy as a supportive therapy in gynecological cancer: protocol for a multicenter randomized controlled clinical trial. Trials. 2020;21(1):854. [CrossRef]
- Bahr LS, Bock M, Liebscher D, Bellmann-Strobl J, Franz L, Pruss A, et al. Ketogenic diet and fasting diet as Nutritional Approaches in Multiple Sclerosis (NAMS): protocol of a randomized controlled study. Trials. 2020;21(1):3. [CrossRef]
- Li C, Sadraie B, Steckhan N, Kessler C, Stange R, Jeitler M, et al. Effects of A One-week Fasting Therapy in Patients with Type-2 Diabetes Mellitus and Metabolic Syndrome - A Randomized Controlled Explorative Study. Exp Clin Endocrinol Diabetes. 2017;125(9):618-24. [CrossRef]
- Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016;15(10):2136-46. [CrossRef]
- Michalsen A, Li C. Fasting therapy for treating and preventing disease - current state of evidence. Forsch Komplementmed. 2013;20(6):444-53. [CrossRef]
- Stange R, Pflugbeil C, Michalsen A, Uehleke B. Therapeutic fasting in patients with metabolic syndrome and impaired insulin resistance. Forsch Komplementmed. 2013;20(6):421-6. [CrossRef]
- Li C, Ostermann T, Hardt M, Ludtke R, Broecker-Preuss M, Dobos G, et al. Metabolic and psychological response to 7-day fasting in obese patients with and without metabolic syndrome. Forsch Komplementmed. 2013;20(6):413-20. [CrossRef]
- Michalsen A, Li C, Kaiser K, Ludtke R, Meier L, Stange R, et al. In-Patient Treatment of Fibromyalgia: A Controlled Nonrandomized Comparison of Conventional Medicine versus Integrative Medicine including Fasting Therapy. Evid Based Complement Alternat Med. 2013;2013:908610. [CrossRef]
- Michalsen A, Li C, Kaiser K, Lüdtke R, Meier L, Stange R, et al. In-Patient Treatment of Fibromyalgia: A Controlled Nonrandomized Comparison of Conventional Medicine versus Integrative Medicine including Fasting Therapy. Evid Based Complement Alternat Med. 2013;2013:908610. [CrossRef]
- Abendroth A, Michalsen A, Ludtke R, Ruffer A, Musial F, Dobos GJ, et al. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch Komplementmed. 2010;17(6):307-13. [CrossRef]
- Michalsen A, Frey UH, Merse S, Siffert W, Dobos GJ. Hunger and mood during extended fasting are dependent on the GNB3 C825T polymorphism. Ann Nutr Metab. 2009;54(3):184-8. [CrossRef]
- Michalsen A, Kuhlmann MK, Ludtke R, Backer M, Langhorst J, Dobos GJ. Prolonged fasting in patients with chronic pain syndromes leads to late mood-enhancement not related to weight loss and fasting-induced leptin depletion. Nutr Neurosci. 2006;9(5-6):195-200. [CrossRef]
- Michalsen A, Hoffmann B, Moebus S, Backer M, Langhorst J, Dobos GJ. Incorporation of fasting therapy in an integrative medicine ward: evaluation of outcome, safety, and effects on lifestyle adherence in a large prospective cohort study. J Altern Complement Med. 2005;11(4):601-7. [CrossRef]
- Michalsen A, Riegert M, Lüdtke R, Bäcker M, Langhorst J, Schwickert M, et al. Mediterranean diet or extended fasting's influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: an observational study. BMC Complement Altern Med. 2005;5:22. [CrossRef]
- Michalsen A, Schlegel F, Rodenbeck A, Ludtke R, Huether G, Teschler H, et al. Effects of short-term modified fasting on sleep patterns and daytime vigilance in non-obese subjects: results of a pilot study. Ann Nutr Metab. 2003;47(5):194-200. [CrossRef]
- Michalsen A, Schneider S, Rodenbeck A, Ludtke R, Huether G, Dobos GJ. The short-term effects of fasting on the neuroendocrine system in patients with chronic pain syndromes. Nutr Neurosci. 2003;6(1):11-8. [CrossRef]
- Michalsen A, Weidenhammer W, Melchart D, Langhorst J, Saha J, Dobos G. [Short-term therapeutic fasting in the treatment of chronic pain and fatigue syndromes--well-being and side effects with and without mineral supplements]. Forsch Komplementarmed Klass Naturheilkd. 2002;9(4):221-7.
- Clement ND, Bardgett M, Weir D, Holland J, Gerrand C, Deehan DJ. What is the Minimum Clinically Important Difference for the WOMAC Index After TKA? Clin Orthop Relat Res. 2018;476(10):2005-14.
- Ariani A, Bazzichi L, Sarzi-Puttini P, Salaffi F, Manara M, Prevete I, et al. The Italian Society for Rheumatology clinical practice guidelines for the diagnosis and management of fibromyalgia Best practices based on current scientific evidence. Reumatismo. 2021;73(2):89-105. [CrossRef]
- Oudmaijer CAJ, Minnee RC, Pol RA, van den Boogaard WMC, Komninos DSJ, van de Wetering J, et al. Fasting before living-kidney donation: effect on donor well-being and postoperative recovery: study protocol of a multicenter randomized controlled trial. Trials. 2022;23(1):18. [CrossRef]
- Hofer SJ, Carmona-Gutierrez D, Mueller MI, Madeo F. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol Med. 2022;14(1):e14418. [CrossRef]
- Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford). 2015;54(4):588-600.
- Morales-Ivorra I, Romera-Baures M, Roman-Vinas B, Serra-Majem L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients. 2018;10(8). [CrossRef]
- Tu C, He J, Wu B, Wang W, Li Z. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine. 2019;113:1-12. [CrossRef]
- Gabriel S, Ncube M, Zeiler E, Thompson N, Karlsen MC, Goldman DM, et al. A Six-Week Follow-Up Study on the Sustained Effects of Prolonged Water-Only Fasting and Refeeding on Markers of Cardiometabolic Risk. Nutrients. 2022;14(20). [CrossRef]
- Andersson M, Haglund E, Aili K, Bremander A, Bergman S. Associations between metabolic factors and radiographic knee osteoarthritis in early disease - a cross-sectional study of individuals with knee pain. BMC Musculoskelet Disord. 2022;23(1):938. [CrossRef]
- Papathanasiou I, Anastasopoulou L, Tsezou A. Cholesterol metabolism related genes in osteoarthritis. Bone. 2021;152:116076. [CrossRef]
- Song Y, Liu J, Zhao K, Gao L, Zhao J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33(10):1911-25. [CrossRef]
- Ertürk C, Altay MA, Bilge A, Çelik H. Is there a relationship between serum ox-LDL, oxidative stress, and PON1 in knee osteoarthritis? Clin Rheumatol. 2017;36(12):2775-80.
- Fond G, Macgregor A, Leboyer M, Michalsen A. Fasting in mood disorders: neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013;209(3):253-8. [CrossRef]
- Watkins E, Serpell L. The Psychological Effects of Short-Term Fasting in Healthy Women. Front Nutr. 2016;3:27. [CrossRef]
| Parameter | Value | All Patients | Knee OA | Hip OA |
|---|---|---|---|---|
| Total | 125 (100.0%) | 97 (100.0%) | 28 (100.0%) | |
| Sex | Female | 107 (85.6%) | 81 (83.5%) | 26 (92.9%) |
| Male | 18 (14.4%) | 16 (16.5%) | 2 (7.1%) | |
| Age Group (years) | 18-35 | 2 (1.6%) | 2 (2.1%) | 0 (0.0%) |
| 36-50 | 8 (6.4%) | 5 (5.2%) | 3 (10.7%) | |
| 51-65 | 77 (61.6%) | 64 (66.0%) | 13 (46.4%) | |
| 66-80 | 38 (30.4%) | 26 (26.8%) | 12 (42.9%) | |
| Marital Status | single | 16 (12.8%) | 14 (14.4%) | 2 (7.1%) |
| married | 69 (55.2%) | 51 (52.6%) | 18 (64.3%) | |
| separated or divorced | 29 (23.2%) | 23 (23.7%) | 6 (21.5%) | |
| widowed | 9 (7.2%) | 7 (7.2%) | 2 (7.1%) | |
| other | 2 (1.6%) | 2 (2.1%) | 0 (0.0%) | |
| Household | single | 43 (34.4%) | 36 (37.1%) | 7 (25.0%) |
| with partner | 61 (48.8%) | 43 (44.3%) | 18 (64.3%) | |
| single with children | 4 (3.2%) | 4 (4.1%) | 0 (0.0%) | |
| with partner and children | 15 (12.0%) | 12 (12.4%) | 3 (10.7%) | |
| other | 2 (1.6%) | 2 (1.2%) | 0 (0.0%) | |
| Highest Educational Level | primary schooling | 8 (6.4%) | 6 (6.2%) | 2 (7.1%) |
| secondary schooling | 34 (27.2%) | 26 (26.8%) | 8 (28.6%) | |
| high school | 21 (16.8%) | 17 (17.5%) | 4 (14.3%) | |
| university degree | 56 (44.8%) | 43 (44.3%) | 13 (46.4%) | |
| other | 6 (4.8%) | 5 (5.2%) | 1 (3.6%) | |
| Occupation | self-employed | 12 (9.6%) | 10 (10.3%) | 2 (7.1%) |
| civil servant | 5 (4.0%) | 4 (4.1%) | 1 (3.6%) | |
| employed | 39 (31.2%) | 31 (32.0%) | 8 (28.6%) | |
| worker | 2 (1.6%) | 1 (1.0%) | 1 (3.6%) | |
| homemaker | 3 (2.4%) | 3 (3.1%) | 0 (0.0%) | |
| unemployed | 5 (4.0%) | 5 (5.2%) | 0 (0.0%) | |
| retired | 43 (34.4%) | 30 (30.9%) | 13 (46.4%) | |
| permanently disabled | 12 (9.6%) | 10 (10.3%) | 2 (7.1%) | |
| other | 4 (3.2%) | 3 (3.1%) | 1 (3.6%) | |
| Annual Gross Salary |
< 20.000 Euros | 49 (39.2%) | 40 (41.2%) | 9 (32.1%) |
| 20-40.000 Euro | 42 (33.6%) | 33 (34.0%) | 9 (32.1%) | |
| 40-60.000 Euro | 21 (16.8%) | 14 (14.4%) | 7 (25.0%) | |
| 60-80.000 Euro | 11 (8.8%) | 8 (8.2%) | 3 (10.7%) | |
| > 80.000 Euro | 2 (1.6%) | 2 (2.1%) | 0 (0.0%) | |
| Subjective Physical Health Status | not impaired | 1 (0.8%) | 1 (1.0%) | 0 (0.0%) |
| mildly impaired | 16 (12.8%) | 11 (11.3%) | 5 (17.9%) | |
| impaired | 76 (60.8%) | 59 (60.8%) | 17 (60.7%) | |
| strongly impaired | 32 (25.6%) | 26 (26.8%) | 6 (21.4%) | |
| Subjective Psychological Health Status | not impaired | 21 (16.8%) | 15 (15.5%) | 6 (21.4%) |
| mildly impaired | 49 (39.2%) | 36 (37.1%) | 13 (46.4%) | |
| impaired | 38 (30.4%) | 32 (33.0%) | 6 (21.4%) | |
| strongly impaired | 17 (13.6%) | 14 (14.4%) | 3 (10.7%) | |
| Psychotherapy | none so far | 49 (39.2%) | 37 (38.1%) | 12 (42.9%) |
| ealier | 57 (45.6%) | 45 (46.4%) | 12 (42.9%) | |
| currently | 19 (15.2%) | 15 (15.5%) | 4 (14.3%) | |
| Integrative Medicine | familiar with concept | 86 (68.8%) | 65 (67.0%) | 21 (75.0%) |
| Stay at this clinic | first | 83 (66.4%) | 67 (69.1%) | 16 (57.1%) |
| second | 23 (18.4%) | 16 (16.5%) | 7 (25.0%) | |
| third | 12 (9.6%) | 8 (8.2%) | 4 (14.3%) | |
| fourth | 7 (5.6%) | 6 (6.2%) | 1 (3.6%) | |
| Fasting Experience | never | 56 (44.8%) | 44 (45.4%) | 12 (42.9%) |
| once | 17 (13.6%) | 12 (12.4%) | 5 (17.9%) | |
| twice | 18 (14.4%) | 14 (14.4%) | 4 (14.3%) | |
| 3 times | 9 (7.2%) | 7 (7.2%) | 2 (7.1%) | |
| 4 times | 5 (4.0%) | 5 (5.2%) | 0 (0.0%) | |
| 5 times and more | 20 (16.0%) | 15 (15.5%) | 5 (17.9%) | |
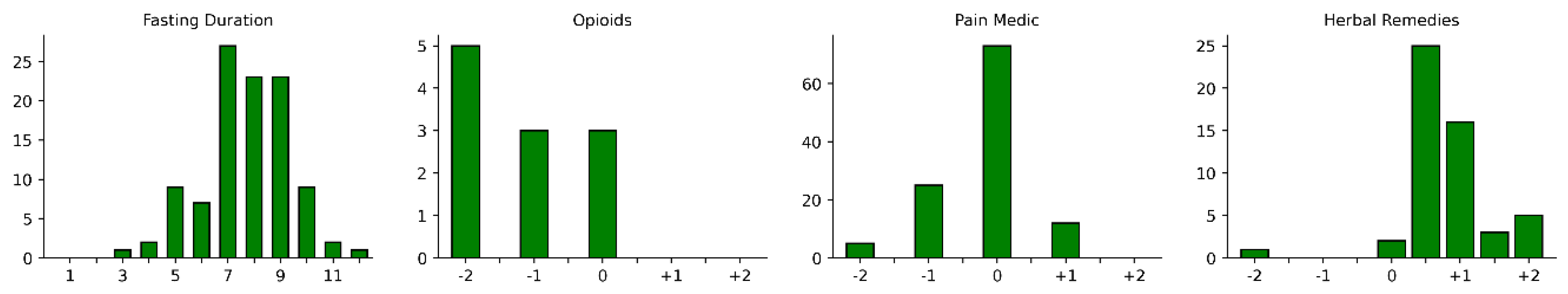
| Medication atadmission | Opioids | 11 (8.8%) | 8 (8.2%) | 3 (10.7%) |
| Pain Medication | 115 (92.0%) | 88 (90.7%) | 27 (96.4%) | |
| Herbal Remedies | 52 (41.6%) | 40 (41.2%) | 12 (42.9%) | |
| Subjective Impairment by OA | NRS [0-10]: M±SD | 6.1 (± 1.6) | 6.2 (± 1.6) | 5.9 (± 1.5) |
| Anticipation of Efficacy | NRS [0-10]: M±SD | 6.4 (± 2.0) | 6.4 (± 1.9) | 6.4 (± 2.5) |
| Difference between V1 and V0 * | |||||||||
| Parameter | Visit | M | SD | n | M | SD | T | p | d |
| Cholesterol [mg/dL] | V0 | 239.2 | 44.86 | 84 | |||||
| V1 | 201.1 | 48.85 | 70 | -38.4 | 31.20 | 10.23 | <0.001 | 0.80 | |
| LDL [mg/dL] |
V0 | 155.9 | 38.27 | 81 | |||||
| V1 | 129.8 | 46.91 | 63 | -24.5 | 30.72 | 6.29 | <0.001 | 0.56 | |
| HDL [mg/dL] |
V0 | 58.2 | 13.80 | 81 | |||||
| V1 | 49.2 | 12.61 | 62 | -8.0 | 7.74 | 8.12 | <0.001 | 0.59 | |
| Triglycerides [mg/dL] |
V0 | 133.7 | 66.42 | 84 | |||||
| V1 | 113.1 | 47.25 | 66 | -24.5 | 67.43 | 2.92 | 0.005 | 0.42 | |
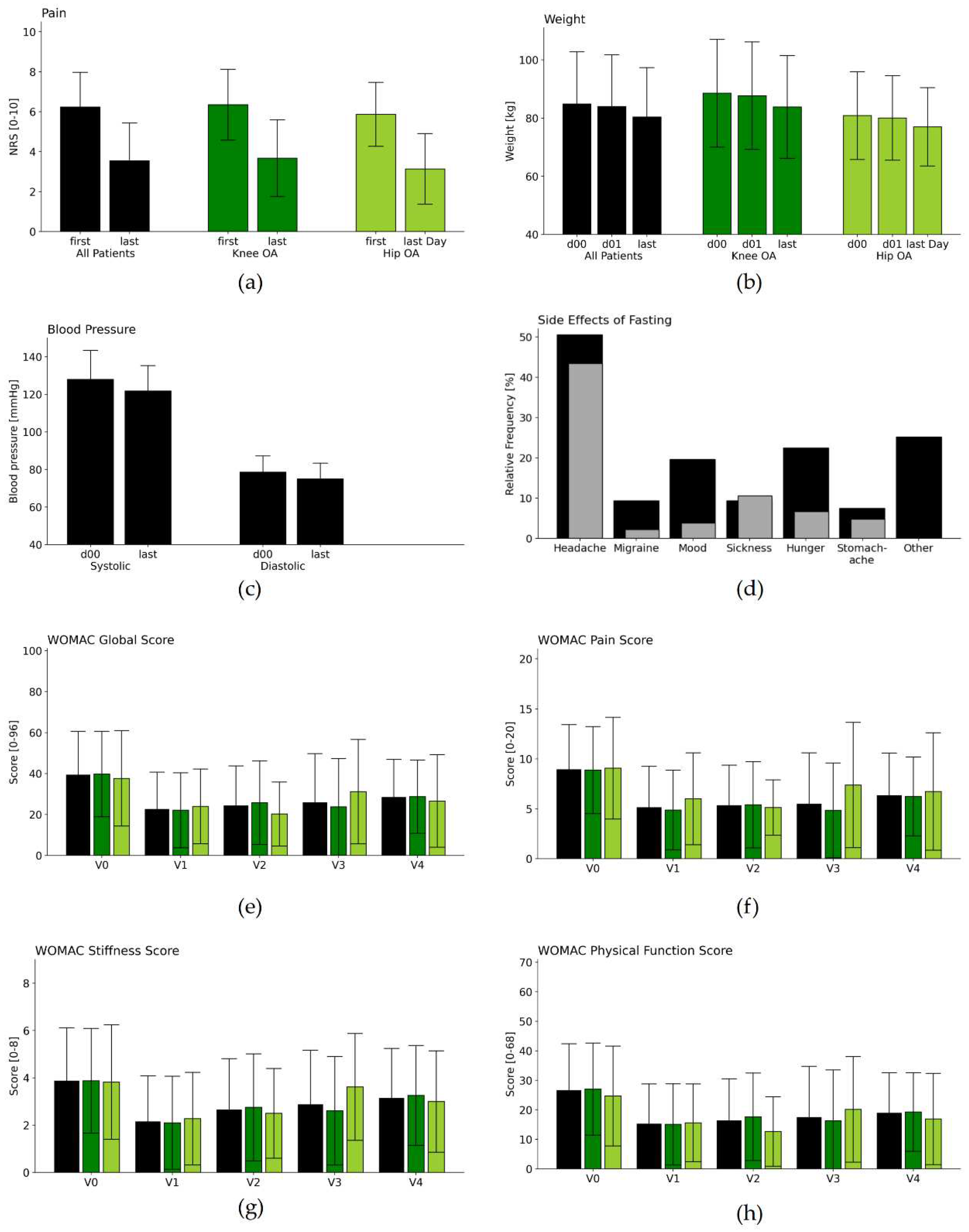
| NRSPain [scale 0-10] | V0 | 6.2 | 1.72 | 90 | |||||
| V1 | 3.5 | 1.87 | 64 | -2.7 | 1.98 | 10.8 | <0.001 | 1.48 | |
| Weight [kg] | V0 | 84.8 | 17.9 | 115 | |||||
| D 01 | 83.9 | 17.74 | 115 | ||||||
| V1 | 80.3 | 16.88 | 115 | -3.6 | 1.65 | 23.29 | <0.001 | 0.21 | |
| Systolic BP [mmHg] | V0 | 128.0 | 15.34 | 115 | |||||
| V1 | 121.8 | 13.42 | 115 | -6.2 | 15.93 | 4.15 | <0.001 | 0.43 | |
| Diastolic BP [mmHg] | V0 | 78.6 | 8.61 | 115 | |||||
| V1 | 74.9 | 8.4 | 115 | -3.7 | 10.55 | 3.70 | <0.001 | 0.43 | |
| Difference to V0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Visit | M | SD | n | M | SD | T | p | d |
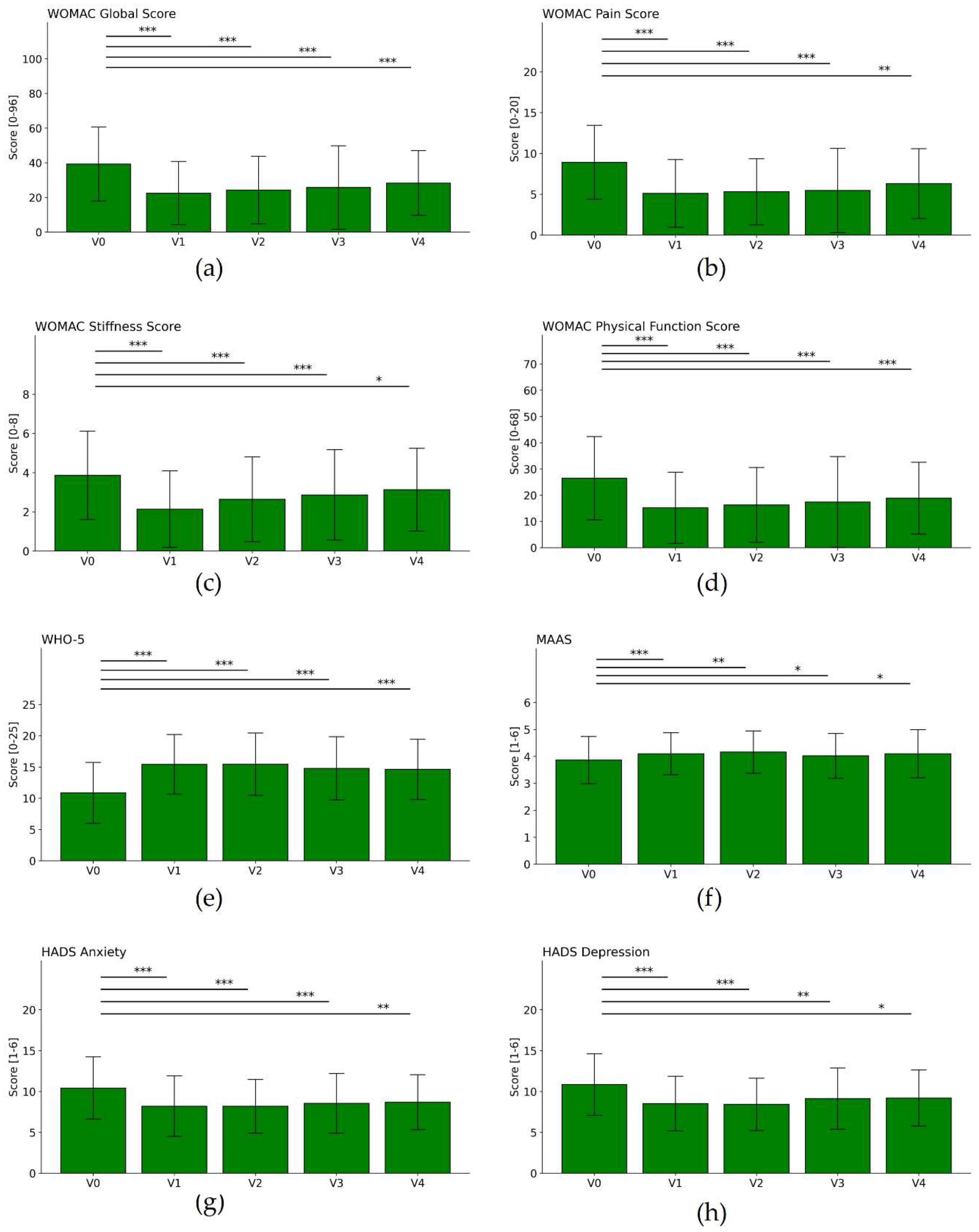
| WHO 5 | V0 | 11 | 4.74 | 107 | |||||
| V1 | 15.4 | 4.75 | 107 | 4.5 | 4.94 | 9.29 | <0.001 | 0.94 | |
| V2 | 15.3 | 4.96 | 68 | 3.6 | 4.27 | 7 | <0.001 | 0.74 | |
| V3 | 14.9 | 5.04 | 56 | 3.4 | 4.54 | 5.57 | <0.001 | 0.67 | |
| V4 | 14.3 | 4.61 | 53 | 2.7 | 5.05 | 3.8 | <0.001 | 0.56 | |
| MAAS | V0 | 3.9 | 0.87 | 107 | |||||
| V1 | 4.1 | 0.77 | 107 | 0.2 | 0.61 | 4.07 | 0.001 | 0.29 | |
| V2 | 4.2 | 0.79 | 68 | 0.2 | 0.6 | 3.28 | 0.002 | 0.28 | |
| V3 | 4 | 0.81 | 56 | 0.1 | 0.57 | 1.87 | 0.067 | 0.18 | |
| V4 | 4 | 0.91 | 53 | 0.2 | 0.71 | 1.98 | 0.053 | 0.21 | |
| HADS Depression | V0 | 10.9 | 3.74 | 107 | |||||
| V1 | 8.5 | 3.32 | 107 | -2.3 | 3.01 | 7.94 | <0.001 | 0.65 | |
| V2 | 8.5 | 3.17 | 68 | -2 | 3.13 | 5.35 | <0.001 | 0.59 | |
| V3 | 9.1 | 3.64 | 56 | -1.5 | 3.3 | 3.29 | 0.002 | 0.37 | |
| V4 | 9.5 | 3.37 | 53 | -0.9 | 2.76 | 2.32 | 0.024 | 0.24 | |
| HADS Anxiety | V0 | 10.3 | 3.73 | 107 | |||||
| V1 | 8.2 | 3.69 | 107 | -2.1 | 2.91 | 7.28 | <0.001 | 0.55 | |
| V2 | 8.3 | 3.18 | 68 | -1.7 | 2.4 | 5.91 | <0.001 | 0.52 | |
| V3 | 8.6 | 3.6 | 56 | -1.7 | 2.84 | 4.38 | <0.001 | 0.45 | |
| V4 | 9 | 3.36 | 53 | -1.1 | 2.96 | 2.76 | 0.008 | 0.32 | |
| WOMAC Global Score [0-96] |
V0 | 37.7 | 19.33 | 107 | |||||
| V1 | 22.5 | 18.16 | 101 | -14.9 | 13.37 | 11.18 | <0.001 | 0.79 | |
| V2 | 23.3 | 17.25 | 68 | -12.9 | 13.84 | 7.65 | <0.001 | 0.71 | |
| V3 | 24.6 | 22.98 | 56 | -11.4 | 20.17 | 4.19 | <0.001 | 0.53 | |
| V4 | 28 | 17.98 | 53 | -10 | 18.5 | 3.89 | <0.001 | 0.55 | |
| WOMAC Pain Score [0-20] |
V0 | 8.5 | 3.98 | 107 | |||||
| V1 | 5.1 | 4.12 | 101 | -3.4 | 3.61 | 9.29 | <0.001 | 0.82 | |
| V2 | 5.1 | 3.64 | 68 | -3.1 | 3.67 | 6.98 | <0.001 | 0.81 | |
| V3 | 5.2 | 5.02 | 56 | -2.7 | 4.42 | 4.58 | <0.001 | 0.59 | |
| V4 | 6.3 | 4.24 | 53 | -2 | 4.57 | 3.1 | 0.003 | 0.47 | |
| WOMAC Subscale Stiffness Score [0-8] |
V0 | 3.8 | 2.18 | 107 | |||||
| V1 | 2.1 | 1.94 | 101 | -1.6 | 1.81 | 8.79 | <0.001 | 0.76 | |
| V2 | 2.6 | 2.03 | 68 | -1.2 | 1.83 | 5.26 | <0.001 | 0.56 | |
| V3 | 2.7 | 2.14 | 56 | -1 | 1.92 | 3.73 | <0.001 | 0.46 | |
| V4 | 3.2 | 2.11 | 53 | -0.6 | 2.42 | 1.91 | 0.062 | 0.31 | |
| WOMAC Physical Function Score [0-68] |
V0 | 25.4 | 14.5 | 107 | |||||
| V1 | 15.2 | 13.5 | 101 | -10 | 9.8 | 10.22 | <0.001 | 0.71 | |
| V2 | 15.6 | 12.73 | 68 | -8.6 | 9.93 | 7.09 | <0.001 | 0.64 | |
| V3 | 16.6 | 16.54 | 56 | -7.7 | 14.96 | 3.81 | <0.001 | 0.49 | |
| V4 | 53 | -7.3 | 13.08 | 4.05 | <0.002 | 0.55 | |||
| Change | -2 | -1 | -0.5 | 0 | +0.5 | +1 | +1.5 | +2 |
|---|---|---|---|---|---|---|---|---|
| Opioids | 5 | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| Pain Medication | 5 | 25 | 0 | 73 | 0 | 12 | 0 | 0 |
| Herbal Remedies | 1 | 0 | 0 | 2 | 25 | 16 | 3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





