Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
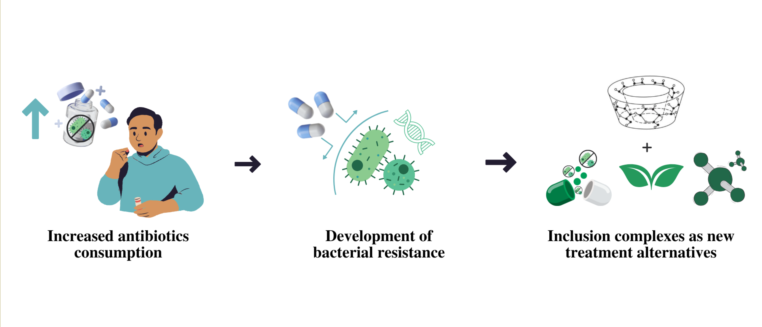
1. Introduction
2. Materials and Methods
Search Strategy
Study Selection
Data Extraction
3. Results and Discussion
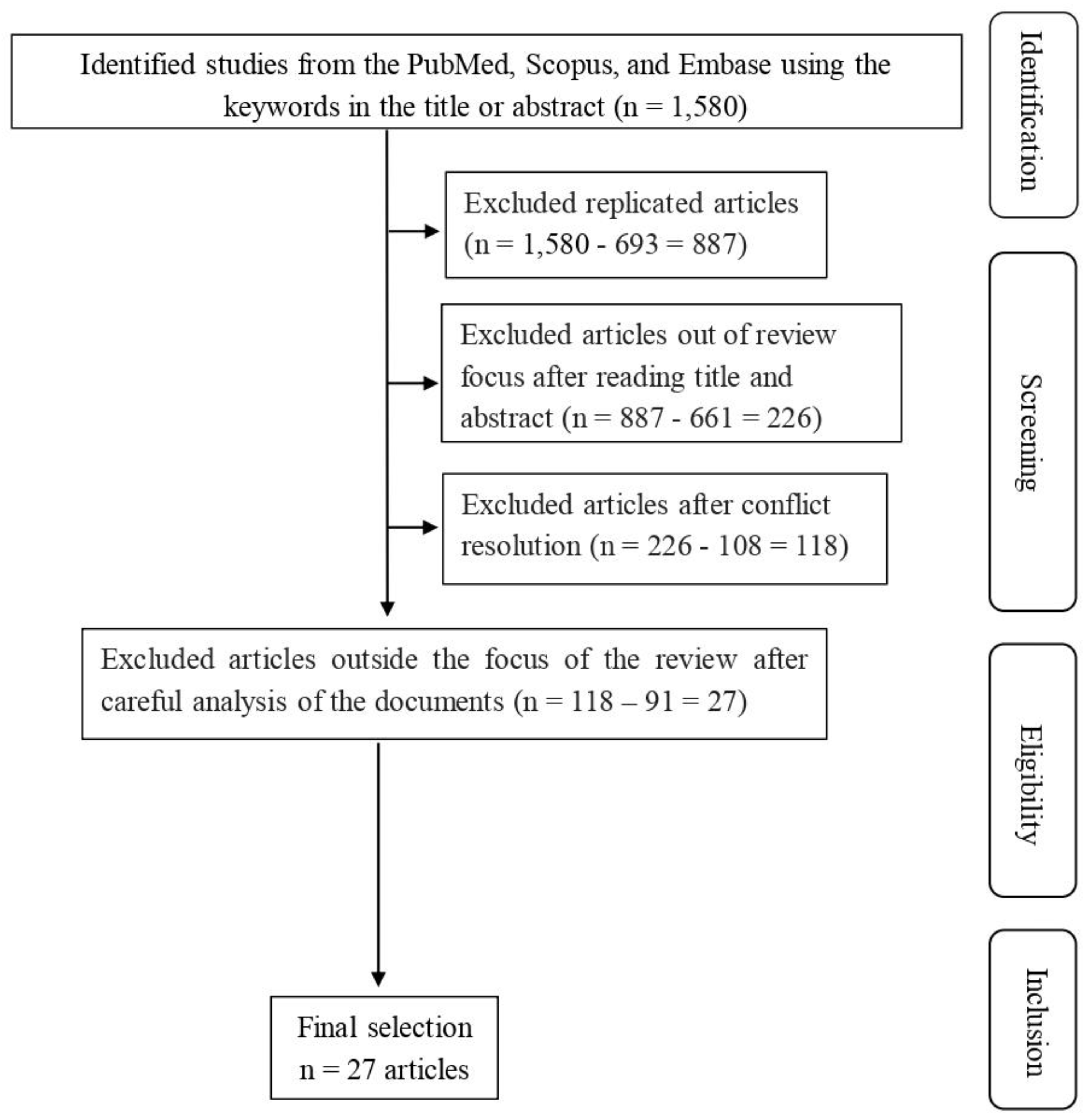
3.1. Search Results
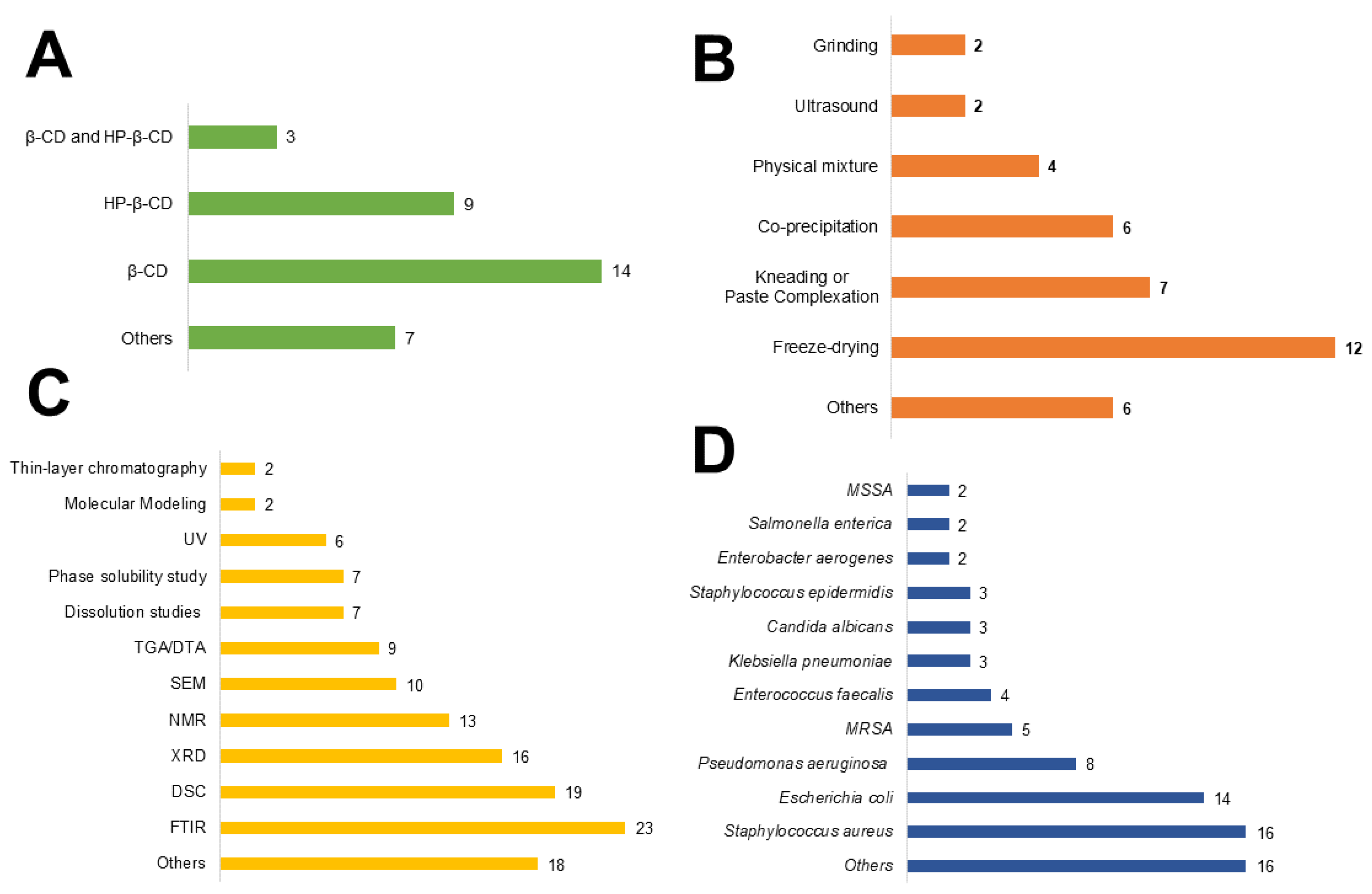
3.2. Study description
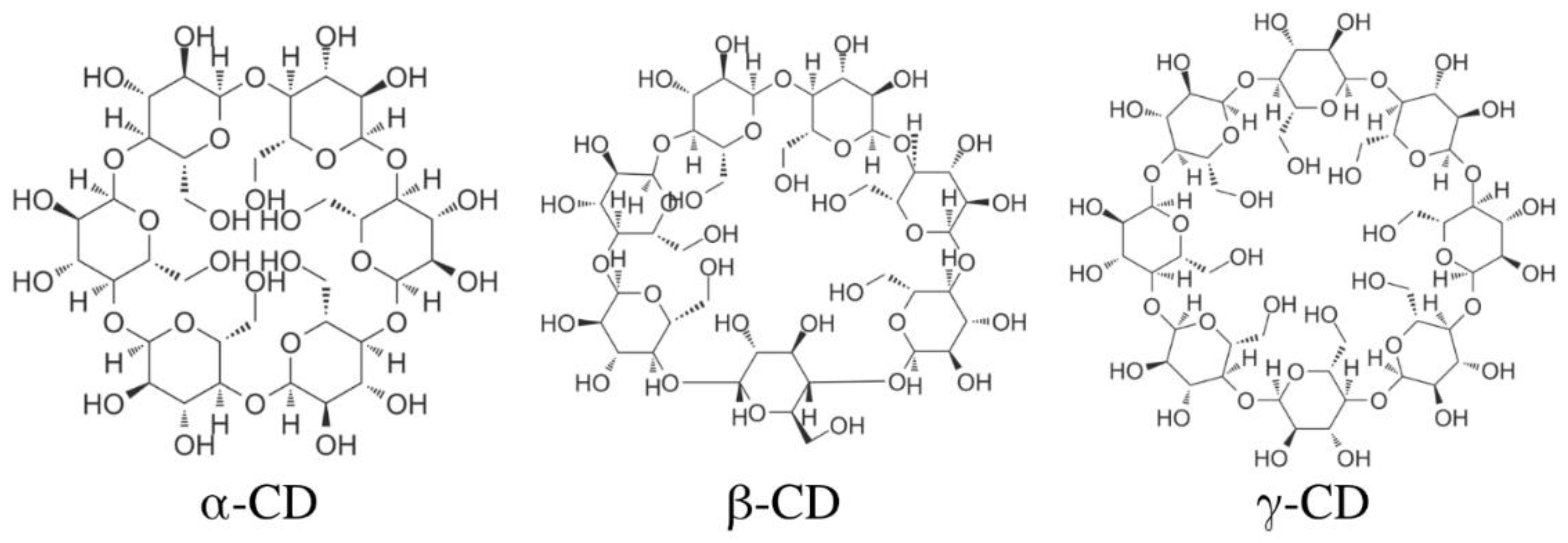
3.3. Cyclodextrins
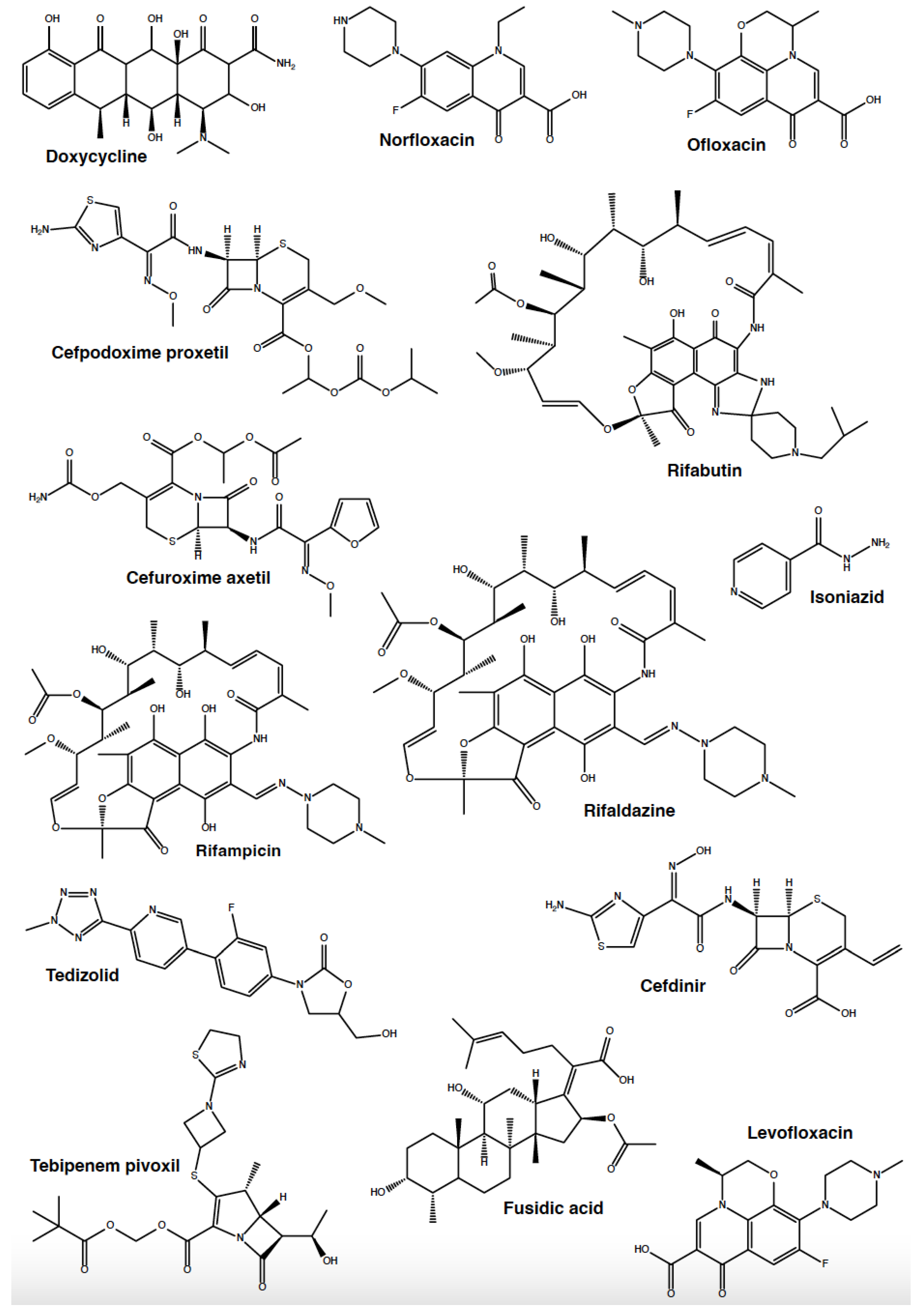
3.4. Cyclodextrins Enhance the Antibacterial Activity of Antibiotics
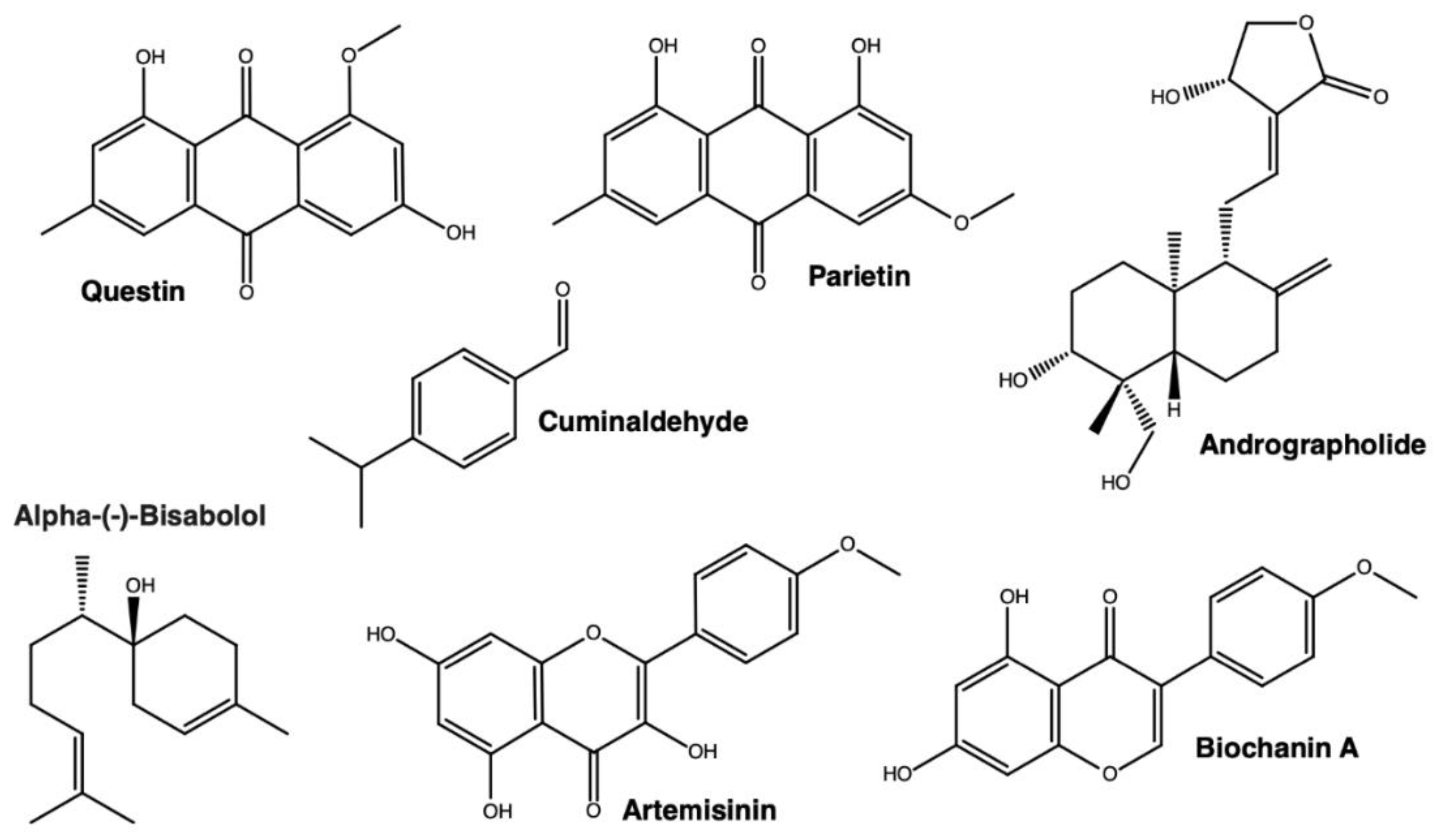
3.4. Cyclodextrins Enhance the Antibacterial Activity of Natural Products
3.5. Cyclodextrins Enhance the Antibacterial Activity of Synthetic Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osman M, al Mir H, Rafei R, Dabboussi F, Madec JY, Haenni M, et al. Epidemiology of antimicrobial resistance in Lebanese extra-hospital settings: An overview. J Glob Antimicrob Resist 2019;17:123–9. [CrossRef]
- Prescott, JF. The resistance tsunami, antimicrobial stewardship, and the golden age of microbiology. Vet Microbiol 2014;171:273–8. [CrossRef]
- Vikesland P, Garner E, Gupta S, Kang S, Maile-Moskowitz A, Zhu N. Differential Drivers of Antimicrobial Resistance across the World. Acc Chem Res 2019;52:916–24. [CrossRef]
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 2022;399:629–55. [CrossRef]
- Song Y, Han Z, Song K, Zhen T. Antibiotic Consumption Trends in China: Evidence From Six-Year Surveillance Sales Records in Shandong Province. Front Pharmacol 2020;11:1. [CrossRef]
- Qiao M, Ying GG, Singer AC, Zhu YG. Review of antibiotic resistance in China and its environment. Environ Int 2018;110:160–72. [CrossRef]
- 2019 Antibiotic Resistance Threats Report | CDC n.d. https://www.cdc.gov/drugresistance/biggest-threats.html (accessed November 22, 2022).
- Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev 2013;65:1803–15. [CrossRef]
- Zarandona I, Barba C, Guerrero P, de la Caba K, Maté J. Development of chitosan films containing β-cyclodextrin inclusion complex for controlled release of bioactives. Food Hydrocoll 2020;104:105720. [CrossRef]
- Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018;23. [CrossRef]
- Zhang D, Lv P, Zhou C, Zhao Y, Liao X, Yang B. Cyclodextrin-based delivery systems for cancer treatment. Materials Science and Engineering C 2019;96:872–86. [CrossRef]
- Mahjoubin-Tehran M, Kovanen PT, Xu S, Jamialahmadi T, Sahebkar A. Cyclodextrins: Potential therapeutics against atherosclerosis. Pharmacol Ther 2020;214. [CrossRef]
- Chen G, Zhou Y, Zhang W, Qin Y, Wei B, Sun Y, et al. Methyl-β-cyclodextrin suppresses the monocyte-endothelial adhesion triggered by lipopolysaccharide (LPS) or oxidized low-density lipoprotein (oxLDL). Pharm Biol 2021;59:1034. [CrossRef]
- Boczar D, Michalska K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022;14. [CrossRef]
- Andrade TA, Freitas TS, Araújo FO, Menezes PP, Dória GAA, Rabelo AS, et al. Physico-chemical characterization and antibacterial activity of inclusion complexes of Hyptis martiusii Benth essential oil in β-cyclodextrin. Biomedicine and Pharmacotherapy 2017;89:201–7. [CrossRef]
- Menezes P dos P, Andrade T de A, Frank LA, de Souza EPBSS, Trindade G das GG, Trindade IAS, et al. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int J Pharm 2019;559:312–28. [CrossRef]
- Gandhi SR, Quintans JDSS, Gandhi GR, Araújo AADS, Quintans Júnior LJ. The use of cyclodextrin inclusion complexes to improve anticancer drug profiles: a systematic review. Https://DoiOrg/101080/1742524720201776261 2020;17:1069–80. [CrossRef]
- de Oliveira MG, Guimarães AG, Araújo AA, Quintans JS, Santos MR, Quintans-Júnior LJ. Cyclodextrins: Improving the therapeutic response of analgesic drugs: A patent review. Expert Opin Ther Pat 2015;25:897–907. [CrossRef]
- Lima B dos S, Shanmugam S, Quintans J de SS, Quintans-Júnior LJ, Araújo AA de S. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: a systematic review. Phytochemistry Reviews 2019;18:1337–59. [CrossRef]
- del Valle EMM. Cyclodextrins and their uses: a review. Process Biochemistry 2004;39:1033–46. [CrossRef]
- Otero-Espinar FJ, Luzardo-Alvarez A, Blanco-Mendez J. Cyclodextrins: More than Pharmaceutical Excipients. Mini-Reviews in Medicinal Chemistry 2010;10:715–25. [CrossRef]
- Jansook P, Ogawa N, Loftsson T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int J Pharm 2018;535:272–84. [CrossRef]
- Tian B, Hua S, Liu J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr Polym 2020;232. [CrossRef]
- Santos AC, Costa D, Ferreira L, Guerra C, Pereira-Silva M, Pereira I, et al. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv Transl Res 2021;11:49–71. [CrossRef]
- Carneiro SB, Duarte FÍC, Heimfarth L, Quintans JDSS, Quintans-Júnior LJ, Júnior VFDV, et al. Cyclodextrin−Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int J Mol Sci 2019;20. [CrossRef]
- Di L, Kerns EH. Formulation. Drug-Like Properties, Elsevier; 2016, p. 497–510. [CrossRef]
- Cid-Samamed A, Rakmai J, Mejuto JC, Simal-Gandara J, Astray G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem 2022;384. [CrossRef]
- Alopaeus JF, Göbel A, Breitkreutz J, Sande SA, Tho I. Investigation of hydroxypropyl-β-cyclodextrin inclusion complexation of two poorly soluble model drugs and their taste-sensation - Effect of electrolytes, freeze-drying and incorporation into oral film formulations. J Drug Deliv Sci Technol 2021;61. [CrossRef]
- da Silva Júnior WF, de Oliveira Pinheiro JG, Moreira CDLFA, de Souza FJJ, de Lima ÁAN. Alternative Technologies to Improve Solubility and Stability of Poorly Water-Soluble Drugs. Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics 2017:281–305. [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J Pharm Biomed Anal 2015;113:226–38. [CrossRef]
- Szente L, Szemán J, Sohajda T. Analytical characterization of cyclodextrins: History, official methods and recommended new techniques. J Pharm Biomed Anal 2016;130:347–65. [CrossRef]
- Tiwari G, Tiwari R, Rai AK. Cyclodextrins in delivery systems: Applications. J Pharm Bioallied Sci 2010;2:72. [CrossRef]
- Goluszko P, Nowicki B. Membrane Cholesterol: a Crucial Molecule Affecting Interactions of Microbial Pathogens with Mammalian Cells. Infect Immun 2005;73:7791. [CrossRef]
- Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021;12:547–69. [CrossRef]
- Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 2019;17:203–18. [CrossRef]
- Asokan G, v. , Ramadhan T, Ahmed E, Sanad H. WHO global priority pathogens list: A bibliometric analysis of medline-pubmed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med J 2019;34:184–93. [CrossRef]
- Karginov, VA. Cyclodextrin derivatives as anti-infectives 2013. [CrossRef]
- Fedoce Lopes J, Boczar D, Michalska K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems-A Pharmaceutical Perspective 2022. [CrossRef]
- Santos AM, Santos MM, Nascimento Júnior JAC, Brito JRLR, de Araújo Andrade T, Frank LA, et al. Mapping of new pharmacological alternatives in the face of the emergence of antibiotic resistance in COVID-19 patents treated for opportunistic respiratory bacterial pathogens. Recent Advances in Anti-Infective Drug Discovery 2022;17. [CrossRef]
- Soleymani F, Taheri F, Haerizadeh M, Haerizadeh M, Karimi A. Evaluation of medicine prescription pattern using World Health Organization prescribing indicators in Iran: A cross-sectional study. J Res Pharm Pract 2014;3:39. [CrossRef]
- Imperiale JC, Sosnik AD. Cyclodextrin complexes for treatment improvement in infectious diseases. Nanomedicine 2015;10:1621–41. [CrossRef]
- Popielec A, Loftsson T. Effects of cyclodextrins on the chemical stability of drugs. Int J Pharm 2017;531:532–42. [CrossRef]
- Onufrak NJ, Forrest A, Gonzalez D, Author CT. Pharmacokinetic and Pharmacodynamic Principles of Anti-Infective Dosing HHS Public Access Author manuscript. Clin Ther 2016;38:1930–47. [CrossRef]
- Zhu C, Jiang L, Chen TM, Hwang KK. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur J Med Chem 2002;37:399–407. [CrossRef]
- Breda SA, Jimenez-Kairuz AF, Manzo RH, Olivera ME. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int J Pharm 2009;371:106–13. [CrossRef]
- Yu X, Zipp GL, Davidson GWR. The effect of temperature and pH on the solubility of quinolone compounds: estimation of heat of fusion. Pharm Res 1994;11:522–7. [CrossRef]
- Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 2004;3:1023–35. [CrossRef]
- Wong CE, Dolzhenko A v., Lee SM, Young DJ. Cyclodextrins: A Weapon in the Fight Against Antimicrobial Resistance. J Mol Eng Mater 2017;05:1740006. [CrossRef]
- Zhang G, Yuan C, Sun Y. Effect of Selective Encapsulation of Hydroxypropyl-β-cyclodextrin on Components and Antibacterial Properties of Star Anise Essential Oil. Molecules 2018;23. [CrossRef]
- Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev 2007;59:645–66. [CrossRef]
- Agrawal G, Bhargava S. Preparation & Characterization of Solid Inclusion Complex of Cefpodoxime Proxetil with β- Cyclodextrin. Curr Drug Deliv 2008;5:1–6. [CrossRef]
- Lin X, Kück · Ulrich. Cephalosporins as key lead generation beta-lactam antibiotics. Appl Microbiol Biotechnol 2022;106:8007–20. [CrossRef]
- Aleem O, Kuchekar B, Pore Y, Late S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J Pharm Biomed Anal 2008;47:535–40. [CrossRef]
- Mizera M, Szymanowska D, Stasiłowicz A, Si D, Lewandowska K, Miklaszewski A, et al. Computer-Aided Design of Cefuroxime Axetil/Cyclodextrin System with Enhanced Solubility and Antimicrobial Activity 2019. [CrossRef]
- Paczkowska-walendowska M, Rosiak N, Tykarska E, Michalska K, Płazińska A, Płaziński W, et al. Tedizolid-Cyclodextrin System as Delayed-Release Drug Delivery with Antibacterial Activity. Int J Mol Sci 2020;22:1–15. [CrossRef]
- Quilaqueo M, Millao S, Luzardo-Ocampo I, Campos-Vega R, Acevedo F, Shene C, et al. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. Food Hydrocoll 2019;91:143–52. [CrossRef]
- Suárez DF, Consuegra J, Trajano VC, Gontijo SML, Guimarães PPG, Cortés ME, et al. Structural and thermodynamic characterization of doxycycline/β-cyclodextrin supramolecular complex and its bacterial membrane interactions. Colloids Surf B Biointerfaces 2014;118:194–201. [CrossRef]
- Erwin ER, Addison AP, John SF, Olaleye OA, Rosell RC. Pharmacokinetics of isoniazid: The good, the bad, and the alternatives. Tuberculosis (Edinb) 2019;116S:S66–70. [CrossRef]
- Slayden RA, Lee RE, Barry CE. Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol Microbiol 2000;38:514–25. [CrossRef]
- Teixeira MG, de Assis J v., Soares CGP, Venâncio MF, Lopes JF, Nascimento CS, et al. Theoretical and Experimental Study of Inclusion Complexes Formed by Isoniazid and Modified β-Cyclodextrins: 1 H NMR Structural Determination and Antibacterial Activity Evaluation. J Phys Chem B 2014;118:81–93. [CrossRef]
- Lee H, Ahn S, Hwang NY, Jeon K, Kwon J, Huh HJ, et al. Treatment outcomes of rifabutin-containing regimens for rifabutin-sensitive multidrug-resistant pulmonary tuberculosis 2017. [CrossRef]
- Shanmuga Priya A, Sivakamavalli J, Vaseeharan B, Stalin T. Improvement on dissolution rate of inclusion complex of Rifabutin drug with β-cyclodextrin. Int J Biol Macromol 2013;62:472–80. [CrossRef]
- Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, et al. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin Pharmacol Ther 2012;91:881–8. [CrossRef]
- Tan Q, He D, Wu M, Yang L, Ren Y, Liu J, et al. Characterization, activity, and computer modeling of a molecular inclusion complex containing rifaldazine. Int J Nanomedicine 2013;8:477–84. [CrossRef]
- Abulfathi AA, Decloedt EH, Svensson EM, Diacon AH, Donald P, Reuter H. Clinical Pharmacokinetics and Pharmacodynamics of Rifampicin in Human Tuberculosis. Clin Pharmacokinet 2019;58:1103–29. [CrossRef]
- Dan Córdoba A v, Aiassa V, Longhi MR, Quevedo MA, Zoppi A. Improved Activity of Rifampicin Against Biofilms of Staphylococcus aureus by Multicomponent Complexation. AAPS PharmSciTech 2020;21:163. [CrossRef]
- Schlievert PM, Strandberg KL, Lin Y-C, Peterson ML, Leung DYM. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. Journal of Allergy and Clinical Immunology 2010;125:39–49. [CrossRef]
- Nikolic P, Mudgil P, Harman DG, Whitehall J. Untargeted lipidomic differences between clinical strains of methicillin-sensitive and methicillin-resistant Staphylococcus aureus. Infect Dis (Lond) 2022;54:497–507. [CrossRef]
- Loftus RW, Dexter F, Robinson ADM. Methicillin-resistant Staphylococcus aureus has greater risk of transmission in the operating room than methicillin-sensitive S aureus. Am J Infect Control 2018;46:520–5. [CrossRef]
- Verbist, L. The antimicrobial activity of fusidic acid. J Antimicrob Chemother 1990;25 Suppl B:1–5. [CrossRef]
- Whitby, M. Fusidic acid in the treatment of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 1999;12 Suppl 2. [CrossRef]
- Marian E, Tita B, Duteanu N, Vicas L, Ciocan S, Jurca T, et al. Antimicrobial activity of fusidic acid inclusion complexes. International Journal of Infectious Diseases 2020;101:65–73. [CrossRef]
- Paczkowska M, Szymanowska-Powałowska D, Mizera M, Siąkowska D, Błaszczak W, Piotrowska-Kempisty H, et al. Cyclodextrins as multifunctional excipients: Influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil 2019. [CrossRef]
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413–20. [CrossRef]
- Mendes C, Wiemes BP, Buttchevitz A, Christ AP, Ribas KG, Adams AIH, et al. Investigation of β-cyclodextrin-norfloxacin inclusion complexes. Part 1. Preparation, physicochemical and microbiological characterization. Expert Rev Anti Infect Ther 2015;13:119–29. [CrossRef]
- Fan YL, Wu JB, Cheng XW, Zhang FZ, Feng LS. Fluoroquinolone derivatives and their anti-tubercular activities. Eur J Med Chem 2018;146:554–63. [CrossRef]
- Amaro BR, Alves CCS, Ferreira GF, Carvalho PE, da Silva JG, Souza CA, et al. Multifunctionality of βcD/ofloxacin and HPβCD/ofloxacin complexes: Improvement of the antimicrobial activity and apoptosis induction on lung adenocarcinoma A549 cells. J Braz Chem Soc 2020;31:2628–37. [CrossRef]
- Bientinesi R, Murri R, Sacco E. Efficacy and safety of levofloxacin as a treatment for complicated urinary tract infections and pyelonephritis. Expert Opin Pharmacother 2020;21:637–44. [CrossRef]
- Li Y, Zhou J, Gu J, Shao Q, Chen Y. Enhanced antibacterial activity of levofloxacin/ hydroxypropyl-β-cyclodextrin inclusion complex: In vitro and in vivo evaluation 2022. [CrossRef]
- Zhang G, Yuan C, Sun Y. Effect of Selective Encapsulation of Hydroxypropyl-β-cyclodextrin on Components and Antibacterial Properties of Star Anise Essential Oil. Molecules 2018;23:1126. [CrossRef]
- Guo L, Cao X, Yang S, Wang X, Wen Y, Zhang F, et al. Characterization, solubility and antibacterial activity of inclusion complex of questin with hydroxypropyl-β-cyclodextrin. 3 Biotech 2019;9:123. [CrossRef]
- Ayoub AM, Gutberlet B, Preis E, Abdelsalam AM, Abu Dayyih A, Abdelkader A, et al. Parietin Cyclodextrin-Inclusion Complex as an Effective Formulation for Bacterial Photoinactivation. Pharmaceutics 2022;14:357. [CrossRef]
- Cui H, Siva S, Lin L. Ultrasound processed cuminaldehyde/2-hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization and antibacterial activity. Ultrason Sonochem 2019;56:84–93. [CrossRef]
- Nikolic IL, Savic IM, Popsavin MM, Rakic SJ, Mihajilov-Krstev TM, Ristic IS, et al. Preparation, characterization and antimicrobial activity of inclusion complex of biochanin A with (2-hydroxypropyl)-β-cyclodextrin. Journal of Pharmacy and Pharmacology 2018;70:1485–93. [CrossRef]
- Oliveira F de S, Freitas TS de, Cruz RP da, Costa M do S, Pereira RLS, Quintans-Júnior LJ, et al. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomedicine & Pharmacotherapy 2017;92:1111–8. [CrossRef]
- Lin L, Mao X, Sun Y, Cui H. Antibacterial mechanism of artemisinin / beta-cyclodextrins against methicillin-resistant Staphylococcus aureus ( MRSA ). Microb Pathog 2018;118:66–73. [CrossRef]
- Zhang T, Zhu L, Li M, Hu Y, Zhang E, Jiang Q, et al. Inhalable Andrographolide-β-cyclodextrin Inclusion Complexes for Treatment of Staphylococcus aureus Pneumonia by Regulating Immune Responses. Mol Pharm 2017;14:1718–25. [CrossRef]
- Li M, Zhu L, Zhang T, Liu B, Du L, Jin Y. Pulmonary delivery of tea tree oil-β-cyclodextrin inclusion complexes for the treatment of fungal and bacterial pneumonia. Journal of Pharmacy and Pharmacology 2017;69:1458–67. [CrossRef]
- Siva S, Li C, Cui H, Meenatchi V, Lin L. Encapsulation of essential oil components with methyl-β-cyclodextrin using ultrasonication: Solubility, characterization, DPPH and antibacterial assay. Ultrason Sonochem 2020;64:104997. [CrossRef]
- de Almeida Magalhães TSS, de Oliveira Macedo PC, Kawashima Pacheco SY, Silva SS da, Barbosa EG, Pereira RR, et al. Development and Evaluation of Antimicrobial and Modulatory Activity of Inclusion Complex of Euterpe oleracea Mart Oil and β-Cyclodextrin or HP-β-Cyclodextrin. Int J Mol Sci 2020;21:942. [CrossRef]
- Zhang D, Lv P, Zhou C, Zhao Y, Liao X, Yang B. Cyclodextrin-based delivery systems for cancer treatment. Mater Sci Eng C Mater Biol Appl 2019;96:872–86. [CrossRef]
- Fair RJ, Tor Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect Medicin Chem 2014;6:25. [CrossRef]
- Briñez-Ortega E, de Almeida VL, Lopes JCD, Burgos AE. Partial inclusion of bis(1,10-phenanthroline) silver(I) salicylate in β-cyclodextrin: Spectroscopic characterization, in vitro and in silico antimicrobial evaluation. An Acad Bras Cienc 2020;92:20181323. [CrossRef]
- Brancaccio D, Pizzo E, Cafaro V, Notomista E, de Lise F, Bosso A, et al. Antimicrobial peptide Temporin-L complexed with anionic cyclodextrins results in a potent and safe agent against sessile bacteria. Int J Pharm 2020;584. [CrossRef]
- Vandera KKA, Picconi P, Valero M, González-Gaitano G, Woods A, Zain NMM, et al. Antibiotic-in-Cyclodextrin-in-Liposomes: Formulation Development and Interactions with Model Bacterial Membranes. Mol Pharm 2020;17:2354–69. [CrossRef]
- Santos AM, Júnior CCS, Júnior JACN, Andrade T de A, Shanmugam S, Thangaraj P, et al. Antibacterial drugs and cyclodextrin inclusion complexes: a patent review. Https://DoiOrg/101080/1742524720232175815 2023;20:349–66. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




