Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
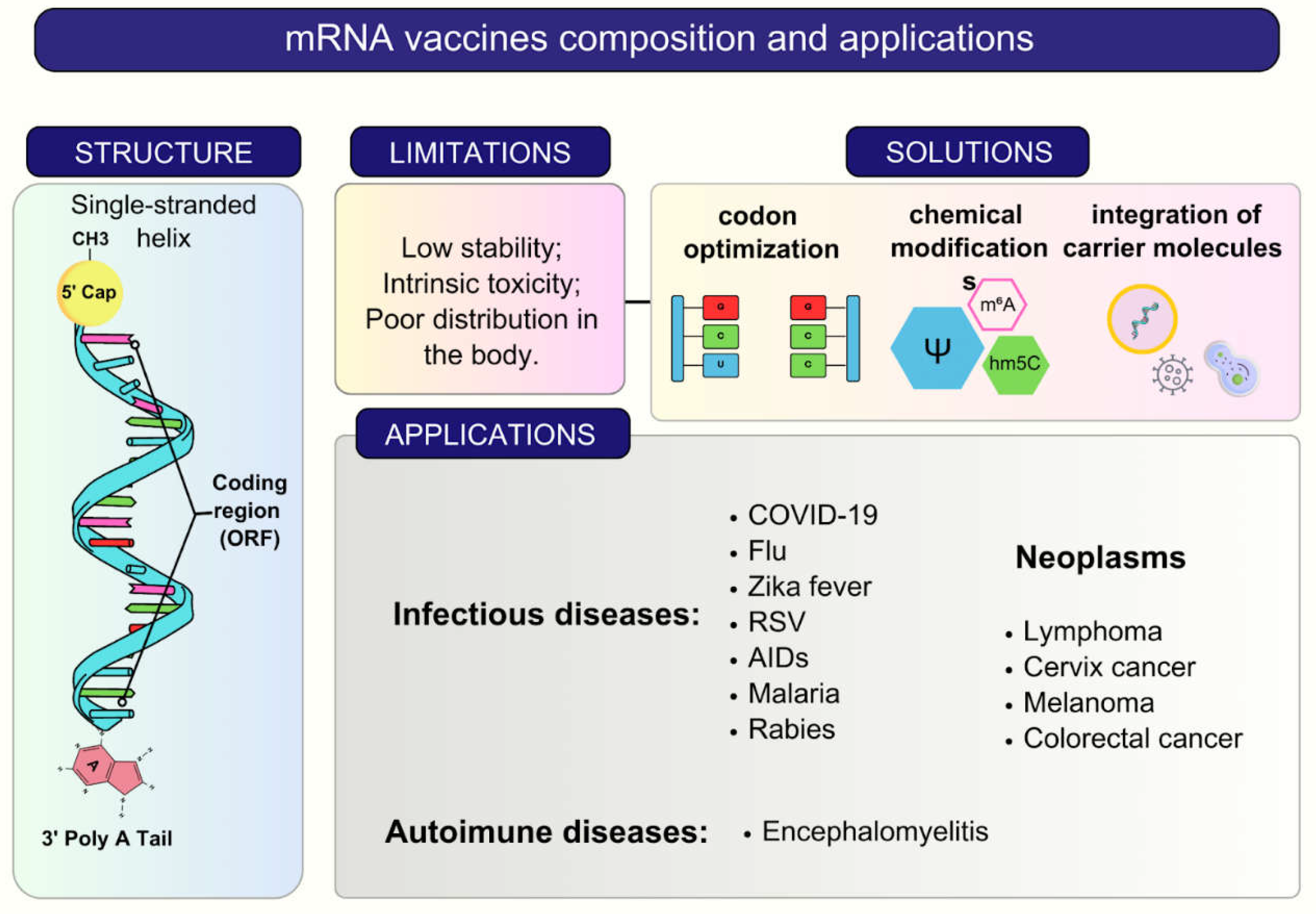
2. RNA Vaccines and Delivery Systems
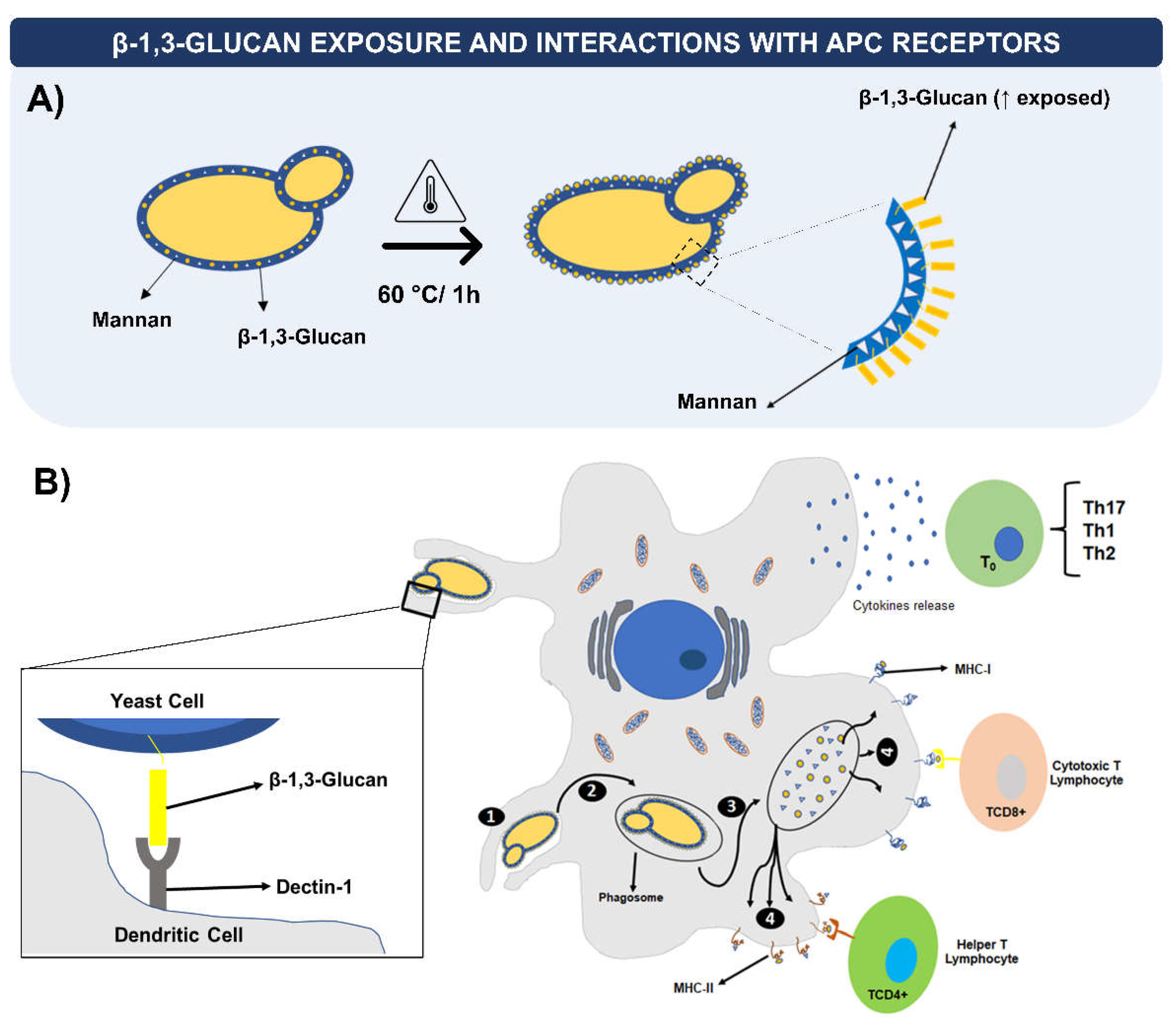
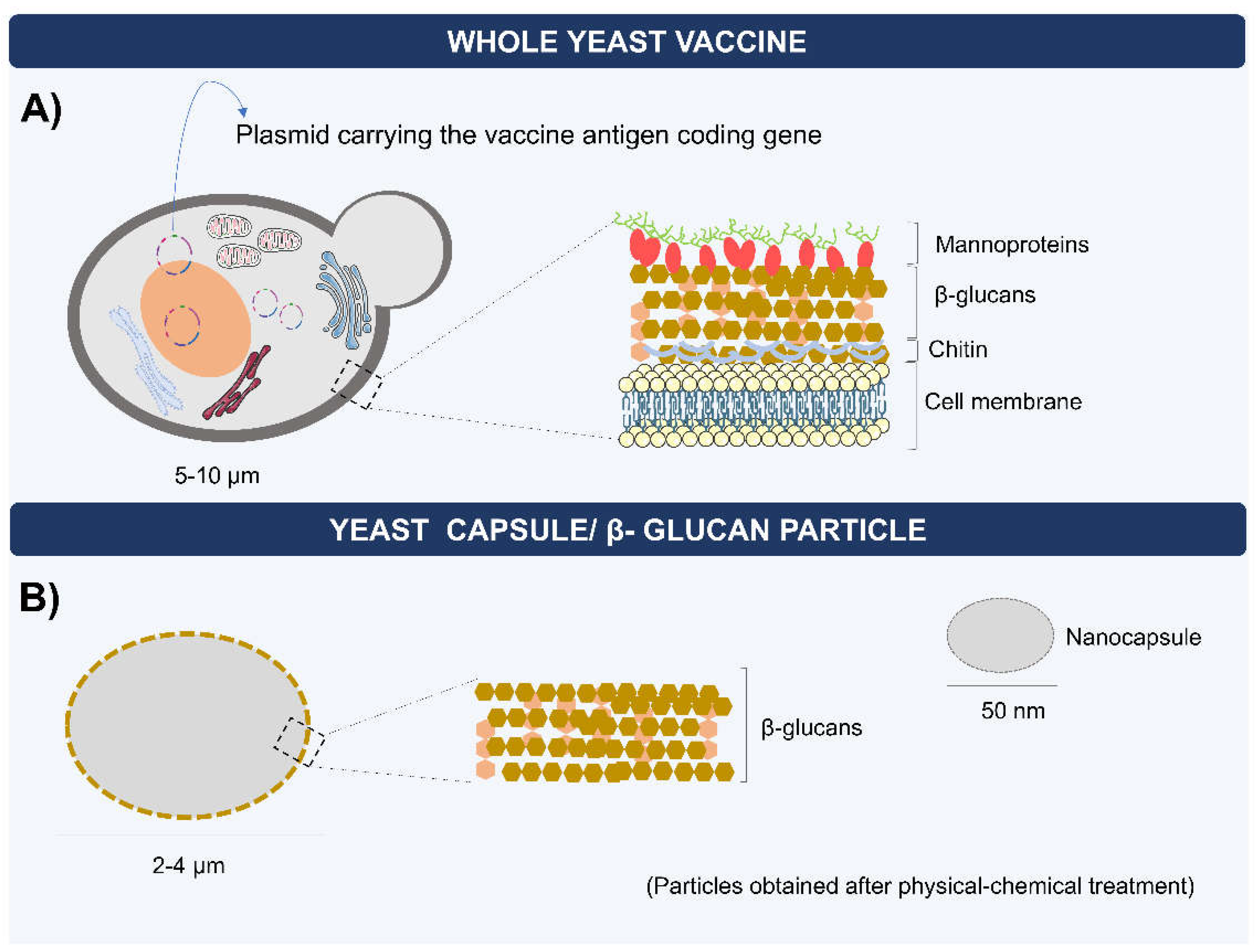
3. Yeasts as Vaccine Carriers: Characteristics and Immunological Aspects
4. Biodelivery of mRNA Vaccines by Yeasts
5. Capsules, Microparticles, and Nanoparticles of Yeast β-Glucans for RNA Delivery
6. Oral Yeast-Based Immunization for RNA Vaccines
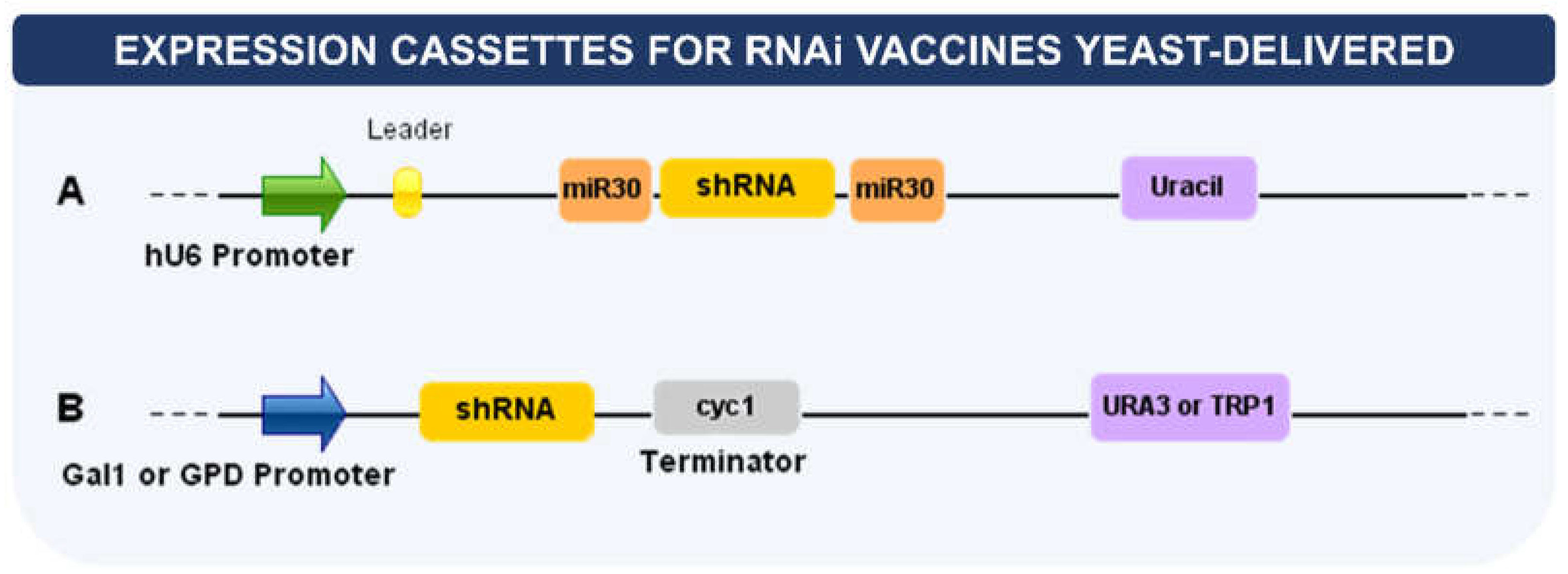
7. Delivery of RNA Interference (RNAi)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current Challenges in Vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.-H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Bashatwah, R.M.; Obeid, M.A.; Mishra, V.; Mishra, Y.; Serrano-Aroca, Á.; Lundstrom, K.; Tambuwala, M.M. Current state of, prospects for, and obstacles to mRNA vaccine development. Drug Discovery Today. 2023, 28, 103458. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-Assembled MRNA Vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for Non-Viral Intracellular Delivery of Messenger RNA Therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef]
- de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; Invenção M da, C.V.; de Sousa, M.M.G.; de Freitas, A.C. Enhancing the Effect of Nucleic Acid Vaccines in the Treatment of HPV-Related Cancers: An Overview of Delivery Systems. Pathogens 2022, 11, 1444. [Google Scholar] [CrossRef]
- Silva, A.J.D.; de Macêdo, L.S.; Leal, L.R.S.; de Jesus, A.L.S.; Freitas, A.C. Yeasts as a promising delivery platform for DNA and RNA vaccines. FEMS Yeast Res. 2021. 21, foab018. [CrossRef]
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; Diken, M.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. MRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. MRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef] [PubMed]
- Freund, I.; Eigenbrod, T.; Helm, M.; Dalpke, A. RNA Modifications Modulate Activation of Innate Toll-Like Receptors. Genes 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Azizian, K.T.; Haque, A.K.M.A.; Henderson, J.M.; Hendel, A.; Shore, S.; Antony, J.S.; Hogrefe, R.I.; Kormann, M.S.D.; Porteus, M.H.; et al. Uridine Depletion and Chemical Modification Increase Cas9 mRNA Activity and Reduce Immunogenicity without HPLC Purification. Mol. Ther. Nucleic Acids 2018. 12, 530–542. [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Matsumura, T.; Takano, T.; Takahashi, Y. Immune responses related to the immunogenicity and reactogenicity of COVID-19 mRNA vaccines. Int. Immunol. 2022, dxac064. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Shende, P.; Basarkar, V. Recent trends and advances in microbe-based drug delivery systems. DARU J. Pharm. Sci. 2019, 27, 799–809. [Google Scholar] [CrossRef]
- Silva, A.J.D.; Rocha CK da, S.; de Freitas, A.C. Standardization and Key Aspects of the Development of Whole Yeast Cell Vaccines. Pharmaceutics 2022, 14, 2792. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Heterologous Protein Expression in Pichia pastoris : Latest Research Progress and Applications. ChemBioChem 2018, 19, 7–21. [Google Scholar] [CrossRef]
- Bal, J.; Luong, N.N.; Park, J.; Song, K.D.; Jang, Y.S.; Kim, D.H.l. Comparative immunogenicity of preparations of yeast-derived dengue oral vaccine candidate. Microb. Cell Fact. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018, 10, 373–397. [Google Scholar] [CrossRef]
- Brown, G.D. Innate antifungal immunity: The key role of phagocytes. Annu. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Bazan, S.B. , Breinig, T., Schmitt, M.J., Breinig, F. Heat treatment improves antigen-specific T cell activation after protein delivery by several but not all yeast genera. Vaccine 2014, 32, 2591–2598. [Google Scholar] [CrossRef]
- Bazan, S.B.; Geginat, G.; Breinig, T.; Schmitt, M.J.; Breinig, F. Uptake of various yeast genera by antigen-presenting cells and influence of subcellular antigen localization on the activation of ovalbumin-specific CD8 T lymphocytes. Vaccine 2011, 29, 8165–8173. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef]
- Bazan, S.B.; Walch-Rückheim, B.; Schmitt, M.J.; Breinig, F. Maturation and cytokine pattern of human dendritic cells in response to different yeasts. Med. Microbiol. Immunol. 2018, 207, 75–81. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Arlen, P.M.; Rauckhorst, M.; Apelian, D.; Tsang, K.Y.; Tucker, J.A.; Jochems, C.; Schlom, J.; Gulley, J.L.; et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol. Immunother. 2014, 63, 225–234. [Google Scholar] [CrossRef]
- Seif, M.; Hoppstädter, J.; Breinig, F.; Kiemer, A.K. Yeast-mediated mRNA delivery polarizes immuno-suppressive macrophages towards an immuno-stimulatory phenotype. Eur. J. Pharm. Biopharm. 2017, 117, 1–13. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, R.; Zhang, H.; Sun, R.; Li, S.; Xia, C.; Li, Z.; Zhang, L.; Guo, Y.; Huang, J. Recombinant hemagglutinin protein and DNA-RNA-combined nucleic acid vaccines harbored by yeast elicit protective immunity against H9N2 avian influenza infection. Poultry Sci. 2023, 102, 102662. [Google Scholar] [CrossRef]
- Breinig, F.; Breinig, T.; Schmitt, M.J. mRNA Delivery to Human Dendritic Cells by Recombinant Yeast and Activation of Antigen-Specific Memory T Cells, in: Rabinovich, P.M. (Ed.), Synthetic Messenger RNA and Cell Metabolism Modulation, Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 163–184. [Google Scholar] [CrossRef]
- Evstafieva, A.G.; Beletsky, A.V.; Borovjagin, A.V.; Bogdanov, A.A. Internal Ribosome Entry Site Ofencephalomyocarditis Virus RNA Is Unable to Direct Translation in Saccharomyces Cerevisiae. FEBS Lett. 1993, 335, 273–276. [Google Scholar] [CrossRef]
- Walch-Rückheim, B.; Kiefer, R.; Geginat, G.; Schmitt, M.J.; Breinig, F. Coexpression of Human Perforin Improves Yeast-Mediated Delivery of DNA and MRNA to Mammalian Antigen-Presenting Cells. Gene Ther. 2016, 23, 103–107. [Google Scholar] [CrossRef]
- He, L.; Bai, Y.; Xia, L.; Pan, J.; Sun, X.; Zhu, Z.; Ding, J.; Qi, C.; Tang, C. Oral Administration of a Whole Glucan Particle (WGP)-Based Therapeutic Cancer Vaccine Targeting Macrophages Inhibits Tumor Growth. Cancer Immunol. Immunother. 2022, 71, 2007–2028. [Google Scholar] [CrossRef]
- Xu, T.; Yu, S.; Zhang, J.; Wu, S. Dysregulated Tumor-Associated Macrophages in Carcinogenesis, Progression and Targeted Therapy of Gynecological and Breast Cancers. J. Hematol. Oncol. 2021, 14, 181. [Google Scholar] [CrossRef]
- Berner, V.K.; Sura, M.E.; Hunter, K.W. Conjugation of Protein Antigen to Microparticulate β-Glucan from Saccharomyces Cerevisiae: A New Adjuvant for Intradermal and Oral Immunizations. Appl. Microbiol. Biotechnol. 2008, 80, 1053–1061. [Google Scholar] [CrossRef]
- De Smet, R.; Allais, L.; Cuvelier, C.A. Recent Advances in Oral Vaccine Development: Yeast-Derived β-Glucan Particles. Human. Vaccines Immunother. 2014, 10, 1309–1318. [Google Scholar] [CrossRef]
- Tipper, D.J.; Szomolanyi-Tsuda, E. Scaffolded Antigens in Yeast Cell Particle Vaccines Provide Protection against Systemic Polyoma Virus Infection. J. Immunol. Res. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Dinarvand, R.; Cesar De Morais, P.; D’Emanuele, A. Nanoparticles for Targeted Delivery of Active Agents against Tumor Cells. J. Drug Deliv. 2012, 2012, 1–2. [Google Scholar] [CrossRef]
- Zhou, X.; Ling, K.; Liu, M.; Zhang, X.; Ding, J.; Dong, Y.; Liang, Z.; Li, J.; Zhang, J. Targeted Delivery of Cisplatin-Derived Nanoprecursors via a Biomimetic Yeast Microcapsule for Tumor Therapy by the Oral Route. Theranostics 2019, 9, 6568–6586. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Feng, M.; Zhang, W.; Li, Y. Yeast Microcapsule-Mediated Oral Delivery of IL-1β ShRNA for Post-Traumatic Osteoarthritis Therapy. Mol. Ther. - Nucleic Acids 2021, 23, 336–346. [Google Scholar] [CrossRef]
- Liu, H.; Meng, Z.; Wang, H.; Zhang, S.; Huang, Z.; Geng, X.; Guo, R.; Wu, Z.; Hong, Z. Robust Immune Responses Elicited by a Hybrid Adjuvant Based on β-Glucan Particles from Yeast for the Hepatitis B Vaccine. ACS Appl. Bio Mater. 2021, 4, 3614–3622. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, M.; Huang, J.; Fan, Y.; Long, H.; Chen, Q.; Ren, Z.; Wu, C.; Wang, Y. Single-Helical Formyl β-Glucan Effectively Deliver CpG DNA with Poly(DA) to Macrophages for Enhanced Vaccine Effects. Int. J. Biol. Macromol. 2022, 223, 67–76. [Google Scholar] [CrossRef]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Muta, T. Molecular Basis for Invertebrate Innate Immune Recognition of (1→3)-β- D-Glucan as A Pathogen-Associated Molecular Pattern. CPD 2006, 12, 4155–4161. [Google Scholar] [CrossRef]
- Soto, E.R.; Ostroff, G.R. Characterization of Multilayered Nanoparticles Encapsulated in Yeast Cell Wall Particles for DNA Delivery. Bioconjugate Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, S.; Zhang, L.; Ji, M.; Liu, S.; Wang, S.; Liu, R. An Indoleamine 2, 3-Dioxygenase SiRNA Nanoparticle-Coated and Trp2-Displayed Recombinant Yeast Vaccine Inhibits Melanoma Tumor Growth in Mice. J. Control. Release 2018, 273, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust Stimulation of Humoral and Cellular Immune Responses Following Vaccination with Antigen-Loaded β-Glucan Particles. mBio 2010, 1, e00164–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, Q.; Zhang, Y.; Fei, Z.; Sun, Y.; Fan, Q.; Liu, B.; Bai, J.; Yu, Y.; Chu, J.; et al. Yeast-Derived Nanoparticles Remodel the Immunosuppressive Microenvironment in Tumor and Tumor-Draining Lymph Nodes to Suppress Tumor Growth. Nat. Commun. 2022, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Min, D.; Choi, Y.; Yoon, S.; Jang, J.; Hwang, J.; Jeon, H.; Cho, Y.W.; Choi, J. Self-Assembling β-Glucan Nanomedicine for the Delivery of SiRNA. Biomedicines 2020, 8, 497. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, K.; Gilad, Assaf. A.; Choi, J. Synthesis of Beta-Glucan Nanoparticles for the Delivery of Single Strand DNA. Biotechnol. Bioproc E 2018, 23, 144–149. [Google Scholar] [CrossRef]
- Zinkhan, S.; Ogrina, A.; Balke, I.; Reseviča, G.; Zeltins, A.; De Brot, S.; Lipp, C.; Chang, X.; Zha, L.; Vogel, M.; et al. The Impact of Size on Particle Drainage Dynamics and Antibody Response. J. Control. Release 2021, 331, 296–308. [Google Scholar] [CrossRef]
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, Adjuvants and Autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Zhang, P.; Andorko, J.I.; Jewell, C.M. Impact of Dose, Route, and Composition on the Immunogenicity of Immune Polyelectrolyte Multilayers Delivered on Gold Templates. Biotechnol. Bioeng. 2017, 114, 423–431. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 Vaccines: From Bench to Bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Alexander, E. Yeasts in Nanotechnology-Enabled Oral Vaccine and Gene Delivery. Bioengineered 2021, 12, 8325–8335. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.R.; Farazuddin, M.; Wong, P.T.; O’Konek, J.J. The Unfulfilled Potential of Mucosal Immunization. J. Allergy Clin. Immunol. 2022, 150, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kour, P.; Rath, G.; Sharma, G.; Goyal, A.K. Recent Advancement in Nanocarriers for Oral Vaccination. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally Delivered SiRNA Targeting Macrophage Map4k4 Suppresses Systemic Inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Wang, L.; Shao, S.; Chen, Z.; Zhang, Z. In Vivo Targeted Delivery of CD40 ShRNA to Mouse Intestinal Dendritic Cells by Oral Administration of Recombinant Sacchromyces Cerevisiae. Gene Ther. 2014, 21, 709–714. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Zhang, W.; Li, Y.; Liu, L.; Leng, T. Yeast Cell Wall Particle Mediated Nanotube-RNA Delivery System Loaded with MiR365 Antagomir for Post-Traumatic Osteoarthritis Therapy via Oral Route. Theranostics 2020, 10, 8479–8493. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W.Y. RNAi Therapeutics: A Potential New Class of Pharmaceutical Drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic SiRNA: State of the Art. Sig Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Karagiannis, T.C.; El-Osta, A. RNA Interference and Potential Therapeutic Applications of Short Interfering RNAs. Cancer Gene Ther. 2005, 12, 787–795. [Google Scholar] [CrossRef]
- Mahmoodi Chalbatani, G.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rezvani Rad, M.; Marmari, V. Small Interfering RNAs (SiRNAs) in Cancer Therapy: A Nano-Based Approach. IJN 2019, 14, 3111–3128. [Google Scholar] [CrossRef]
- Huang, D.T.-N.; Lu, C.-Y.; Shao, P.-L.; Chang, L.-Y.; Wang, J.-Y.; Chang, Y.-H.; Lai, M.-J.; Chi, Y.-H.; Huang, L.-M. In Vivo Inhibition of Influenza A Virus Replication by RNA Interference Targeting the PB2 Subunit via Intratracheal Delivery. PLoS ONE 2017, 12, e0174523. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, F.; Limani, S.W.; Mnyandu, N.; Maepa, M.B.; Ely, A.; Arbuthnot, P. Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses 2020, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Min, D.; Choi, Y.; Yoon, S.; Jang, J.; Hwang, J.; Jeon, H.; Cho, Y.W.; Choi, J. Self-Assembling β-Glucan Nanomedicine for the Delivery of SiRNA. Biomedicines 2020, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Khan, D.N.; Singh, T.; Shukla, S.; Prakash, S.; Amita, J. Effect of SiRNA Targeting Dengue Virus Genes on Replication of Dengue Virus: An in Vitro Experimental Study. VirusDis. 2021, 32, 518–525. [Google Scholar] [CrossRef]
- Fujita, Y.; Takeshita, F.; Kuwano, K.; Ochiya, T. RNAi Therapeutic Platforms for Lung Diseases. Pharmaceuticals 2013, 6, 223–250. [Google Scholar] [CrossRef]
- Wu, S.; Liu, C.; Bai, S.; Lu, Z.; Liu, G. Broadening the Horizons of RNA Delivery Strategies in Cancer Therapy. Bioengineering 2022, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Setten, R.L.; Rossi, J.J.; Han, S. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Xiang, S.; Fruehauf, J.; Li, C.J. Short Hairpin RNA–Expressing Bacteria Elicit RNA Interference in Mammals. Nat. Biotechnol. 2006, 24, 697–702. [Google Scholar] [CrossRef]
- Bochicchio, S.; Dalmoro, A.; Barba, A.; Grassi, G.; Lamberti, G. Liposomes as SiRNA Delivery Vectors. CDM 2015, 15, 882–892. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors Applied for RNAi-Based Antiviral Therapy. Viruses 2020, 12, 924. [Google Scholar] [CrossRef]
- Duman-Scheel, M. Saccharomyces Cerevisiae (Baker’s Yeast) as an Interfering RNA Expression and Delivery System. CDT 2019, 20, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Barreby, E.; Sulen, A.; Aouadi, M. Glucan-Encapsulated SiRNA Particles (GeRPs) for Specific Gene Silencing in Adipose Tissue Macrophages. In Lipid-Activated Nuclear Receptors; Gage, M.C., Pineda-Torra, I., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, 2019; ISBN 978-1-4939-9129-7. [Google Scholar]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-Kill Yeast Interfering RNA Larvicides Targeting Neural Genes in the Human Disease Vector Mosquito Aedes Aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Harper, E.I.; Chen, Y.; Eggleson, K.K.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Wei, N.; et al. Yeast Interfering RNA Larvicides Targeting Neural Genes Induce High Rates of Anopheles Larval Mortality. Malar. J. 2017, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-Infrared Fluorescence: Application to in Vivo Molecular Imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Tesz, G.J.; Aouadi, M.; Prot, M.; Nicoloro, S.M.; Boutet, E.; Amano, S.U.; Goller, A.; Wang, M.; Guo, C.-A.; Salomon, W.E.; et al. Glucan Particles for Selective Delivery of SiRNA to Phagocytic Cells in Mice. Biochem. J. 2011, 436, 351–362. [Google Scholar] [CrossRef]
- Zakria, H.M.; Han, B.; Yue, F.; Mu, L.; Fang, Y.; Li, X.; Xu, K.; Zhang, Z. Significant Body Mass Increase by Oral Administration of a Cascade of ShIL21-MSTN Yeast-Based DNA Vaccine in Mice. Biomed. Pharmacother. 2019, 118, 109147. [Google Scholar] [CrossRef]

| Type of RNA | Antigen | Vehicle type | Main findings | Ref. |
|---|---|---|---|---|
| siRNA | Tnf-α and Map4k4 | Yeast capsule | - Macrophages in the GALT internalize orally absorbed GeRPs, undergo siRNA-mediated gene silencing, and migrate into tissues throughout the body.- Oral gavage with Map4k4-siRNA-containing GeRPs significantly protects mice from LPS/d-GalN-induced lethality through inhibition of TNF-α and Il-1β production in macrophages. | [65] |
| mRNA | human pp65 | Yeast-derived microparticles | - Using CMV pp65 as a model antigen, showed that recombinant yeast cells can deliver functional mRNA very efficiently to murine macrophages and, importantly, to human DCs. | [39] |
| shRNA | CD40 | Whole yeast | - A shRNA driven by the U6 promoter along with a U6 snRNA leader sequence was delivered into intestinal DCs via orally administered yeast and effectively repressed the target gene (CD40) in vivo.- Compared with the control group, all shRNAs had a significant effect on IL-6, IL-10, IL-12, and TNF-α (P<0.01). And, the inhibition of the CD40 gene by CD40 shRNA1656-induced INF-γ increased expression (P<0.05). Thus, the inhibition of CD40 via orally administered recombinant yeast may be used in immunotherapy approaches. | [66] |
| shRNA | IL-1β | Yeast microcapsule | - The expression of IL-1β in small intestinal macrophage was downregulated with the treatment of recombinant IL-1β shRNA yeast;- The results showed that yeast microcapsule-mediated targeted delivery of IL-1β shRNA successfully, downregulating the intestinal inflammatory response in PTOA mice; | [47] |
| miRNA | miR365 antagomir | Yeast cell wall particle (YCWP) | - The results showed that most of YCWP can effectively resist the corrosion of SGF;- Evidence suggests that yeast was engulfed by gut-associated lymphatic tissue macrophages. These macrophages may traffic away from the gut and infiltrate other reticuloendothelial and mononuclear phagocyte tissues; | [67] |
| mRNA | rH9-DNA-RNA | Whole yeast | - Yeast vaccines elicited humoral and cellular immune responses;- The expression of the eGFP and mCherry in phagocytes of the duodenum was detected after the oral administration. The results suggested that both DNA and RNA cassettes were successfully delivered. The vaccine antigen, HA protein, was also expressed. | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





