Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
Introduction
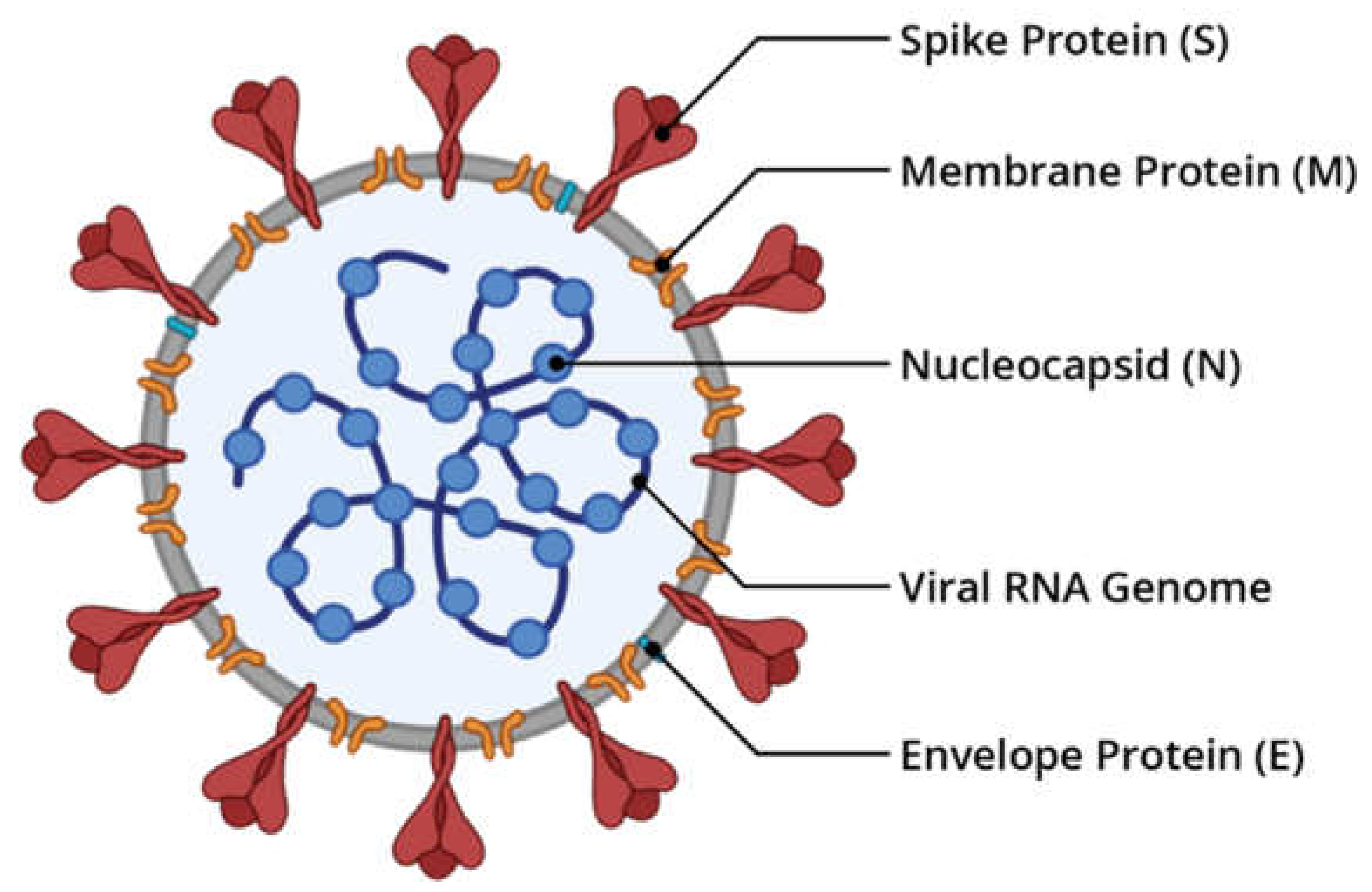
Materials and Methods
Clinical Sample and Processing
Genomic Sequencing
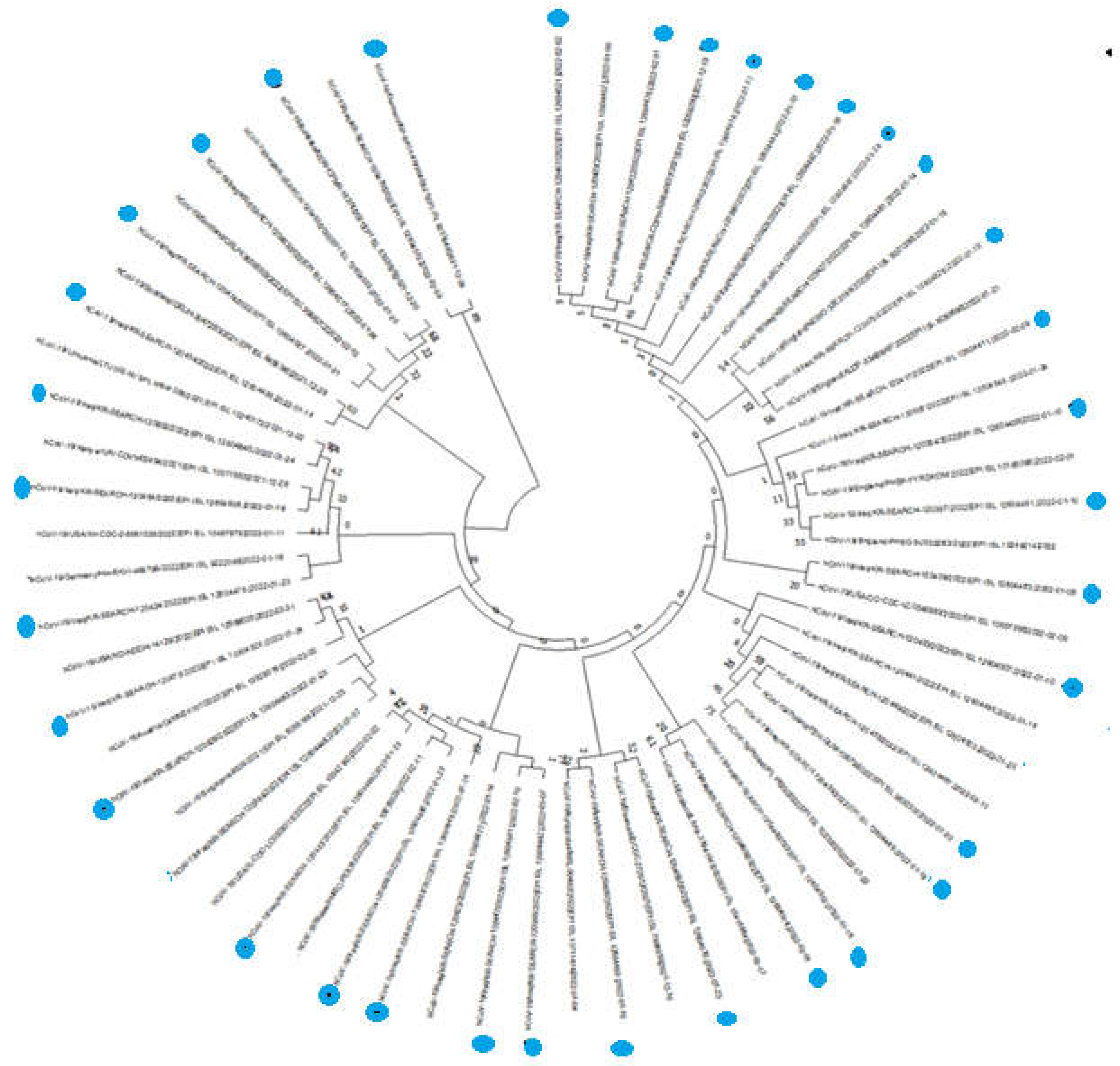
Genome Alignment, and Phylogenetic Analysis
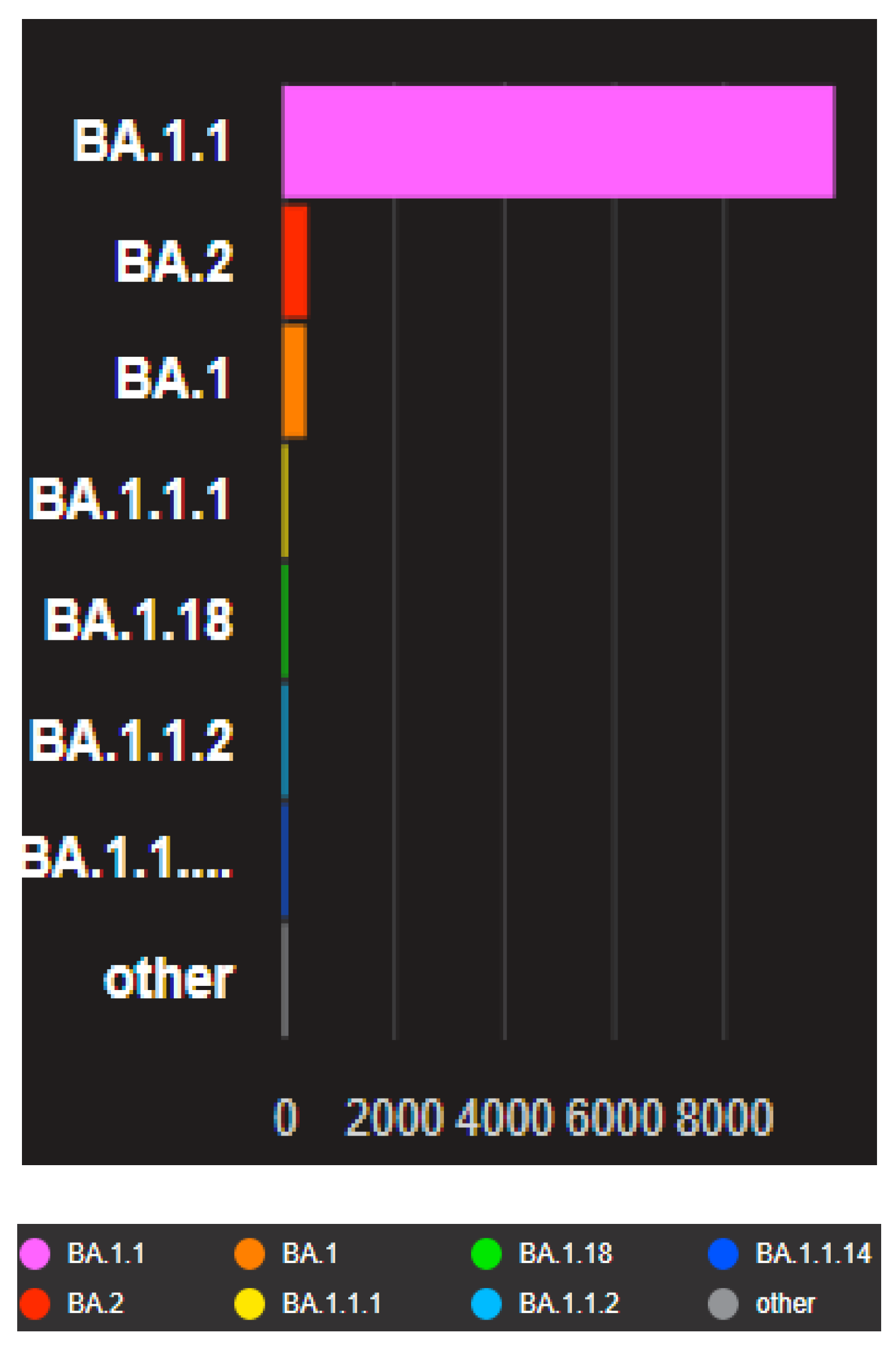
Results and Discussions
| Mutation | Position | Nucleotide change | Code | Amino acid Change | Type of Mutation | ||
|---|---|---|---|---|---|---|---|
| ORF1a (266...13468) | |||||||
| 444 | GTT > GCT | V 60 A | Valin>Alanine | Non-synonymous SNV | |||
| 593 | CAT > TAT | H 110 Y | Histidine>Tyrosine | Non-synonymous SNV | |||
| 670 | AGT > AGG | S 135 R | Serine>Arginine | Non-synonymous SNV | |||
| 1415 | CTT > TTT | L 384 F | Leucine>Phenylalanine | Non-synonymous SNV | |||
| 2790 | ACT > ATT | T 842 I | Threonine>Isoleucine | Non-synonymous SNV | |||
| 2832 | AAG > AGG | K 856 R | Lysine>Arginine | Non-synonymous SNV | |||
| 2883 | TGT > TAT | C 873 Y | Cisteine>Tyrosine | Non-synonymous SNV | |||
| 3896 | GTT > TTT | V 1211 F | Valine>Phenylalanine | Non-synonymous SNV | |||
| 4184 | GGT > AGT | G 1307 S | Glycine>Serine | Non-synonymous SNV | |||
| 4893 | ACA > ATA | T 1543 I | Threonin>Isoleucine | Non-synonymous SNV | |||
| 5007 | ACG > ATG | T 1581 M | Threonin>Methionine | Non-synonymous SNV | |||
| 510 - 518 | ATG > -TG | del82/84 | del82/84 | Non-frame shift deletion | |||
| 519 | ATG > -TG | M 85 V | Methionine>Valine | Non-synonymous SNV | |||
| 6176 | GAT > AAT | D 1971 N | Aspartic acid>Asparagine | Non-synonymous SNV | |||
| 6513 - 6515 | del2083/2083 | del2083/2083 | Non-synonymous deletion | ||||
| 6516 | TTA > -TA | L 2084 I | Leucine>Isoleucine | Non-synonymous SNV | |||
| 7036 | TTA > TTT | L 2257 F | Leucine>Phenylalanine | Non-synonymous SNV | |||
| 7488 | ACT > ATT | T 2408 I | Threonine>Isoleucine | Non-synonymous SNV | |||
| 8393 | GCT > ACT | A 2710 T | Alanine>Threonin | Non-synonymous SNV | |||
| 9344 | CTT > TTT | L 3027 F | Leucine>Phenylalanine | Non-synonymous SNV | |||
| 9474 | GCT > GTT | A 3070 V | Alanine>Valine | Non-synonymous SNV | |||
| 9534 | ACT > ATT | T 3090 I | Threonine>Isoleucine | Non-synonymous SNV | |||
| 9866 | CTT > TTT | L 32201 I | Leucine>Isoleucine | Non-synonymous SNV | |||
| 10029 | ACC > ATC | T 3255 I | Threonin>Isoleucine | Non-synonymous SNV | |||
| 10323 | AAG > AGG | K 3353 R | Lysine>Arginine | Non-synonymous SNV | |||
| 10449 | CCC > CAC | P 3395 H | Proline>Histidine | Non-synonymous SNV | |||
| 11405 | GTC > TTC | V 3714 F | Valine>Phenylalanine | Non-synonymous SNV | |||
| 11285-11293 | del3674/3676 | del3674/3676 | Non-frame shift deletion | ||||
| 11537 | ATT > GTT | I 3758 V | Isoleucine>Valine | Non-synonymous SNV | |||
| 12534 | ACT > ATT | T 409 I | Threonine>Isoleucine | Non-synonymous SNV | |||
| ORF1b (13468...21555) | |||||||
| 13756 | ATA > GTA | I 97 V | Isoleucine>Valine | Non-synonymous SNV | |||
| 14408 | CCT > CTT | P 314 L | Proline>Leucine | Non-synonymous SNV | |||
| 14821 | CCA > TCA | P 452 S | Proline>Serine | Non-synonymous SNV | |||
| 15641 | AAT > AGT | N 725 S | Asparagine>Serine | Non-synonymous SNV | |||
| 15982 | GTA > ATA | V 839 I | Valine>Isoleucine | Non-synonymous SNV | |||
| 16744 | GGT > AGT | G 1093 S | Glycine>Serine | Non-synonymous SNV | |||
| 17410 | GGT > TGT | R 1315 C | Arginine>Cisteine | Non-synonymous SNV | |||
| 18163 | ATA > GTA | I 1566 V | Isoleucine>Valine | Non-synonymous SNV | |||
| 18433 | GAT > CAT | D 165 H | Aspartic acid>Histidine | Non-synonymous SNV | |||
| 19999 | GTT > TTT | V 2178 F | Valine>Phenylalanine | Non-synonymous SNV | |||
| 20003 | GAT > GGT | P 2179 G | Proline>Glycine | Non-synonymous SNV | |||
| S (21563...25384) | |||||||
| 21765 - 21770 | TACATG > - - - | del69/70 | del69/70 | Non-synonymous deletion | |||
| 21789 | ACT > ATT | T 76 I | Threonine>Isoleucine | Non-synonymous SNV | |||
| 21846 | ACT > ATT | T95I | Threonine>Isoleucine | Non-frame shift deletion | |||
| 21987 | GGT > GAT | G142D | Glycine>Aspartic acid | Non-synonymous SNV | |||
| 21987 - 21995 | del142/144 | del142/144 | Non-frame shift deletion | ||||
| 21996 | TAC > -AC | Y 145 D | Tyrosine>Aspartic acid | Non-synonymous SNV | |||
| 22194 - 22196 | AAT > A-- | del211/211 | del211/211 | Non-synonymous deletion | |||
| 22197 | TTA > -TA | L 212 I | Leucine>Isoleucine | Non-synonymous SNV | |||
| 222000 | GTG > GGG | V 213 G | Valine>Glycine | Non-synonymous SNV | |||
| 22578 | GCT > GAT | G339D | Glycine>Aspartic acid | Non-synonymous SNV | |||
| 22599 | AGA > AAA | R346K | Arginine>Lysine | Non-synonymous SNV | |||
| 22673 | T > C | S371L | Serine>Leucine | Non-synonymous SNV | |||
| 22674 | C > T | S 373 P | Serine>Proline | Non-synonymous SNV | |||
| 22686 | TCC > TTC | S 375 F | Serine>Phenylalanine | Non-synonymous SNV | |||
| 22688 | ACT > GCT | T 376 A | Threonine>Isoleucine | Non-synonymous SNV | |||
| 22786 | AGA > AGC | R408S | Arginine>Serine | Non-synonymous SNV | |||
| 22813 | AAG > AAT | K 417 N | Lysine>Asparagine | Non-synonymous SNV | |||
| 22882 | AAT > AAG | N440K | Asparagine>Lysine | Non-synonymous SNV | |||
| 22898 | GGT > AGT | G446S | Glycine>Serine | Non-synonymous SNV | |||
| 23013 | GAA > GCA | E 484 A | Glutamic acid > isoleucine | Non-synonymous SNV | |||
| 22992 | AGC > AAC | S477N | Serine>Asparagine | Non-synonymous SNV | |||
| 22995 | ACA > AAA | T478K | Threonine>Lysine | Non-synonymous SNV | |||
| 23040 | CAA > CGA | Q493R | Glutamine>Arginine | Non-synonymous SNV | |||
| 23048 | G > A | G496S | Glycine>Serine | Non-synonymous SNV | |||
| 23055 | A > G | Q498R | Glutamine>Arginine | Non-synonymous SNV | |||
| 23063 | AAT > TAT | N501Y | Asparagine>Tyrosine | Non-synonymous SNV | |||
| 23075 | TAC > CAC | Y505H | Tyrosine>Histidine | Non-synonymous SNV | |||
| 23202 | ACA > AAA | T547K | Threonine>Lysine | Non-synonymous SNV | |||
| 23403 | GAT > GGT | D614G | Aspartic acid>Glycine | Non-synonymous SNV | |||
| 23525 | CAT > TAT | H655Y | Histidine>Tyrosine | Non-synonymous SNV | |||
| 23599 | T > G | N679K | Asparagine>Lysine | Non-synonymous SNV | |||
| 23604 | CCT > CAT | P681H | Proline>Histidine | Non-synonymous SNV | |||
| 23854 | AAC > AAA | N764K | Asparagine>Lysine | Non-synonymous SNV | |||
| 23948 | GAT > TAT | D796Y | Aspartic acid>Tyrosine | Non-synonymous SNV | |||
| 24130 | ACC > AAA | N856K | Asparagine>Lysine | Non-synonymous SNV | |||
| 24424 | CAA > CAT | Q954H | Glutamine>Histidine | Non-synonymous SNV | |||
| 24469 | AAT > AAA | N969K | Asparagine>Lysine | Non-synonymous SNV | |||
| 24503 | CCT > TTT | L981F | Leucine>Phenylalanine | Non-synonymous SNV | |||
| ORF3a (25393…26220) | |||||||
| 25471 | GAT > TAT | D 27 Y | Aspartic acid>Tyrosine | Non-synonymous SNV | |||
| 26060 | ACT > ATT | T 223 I | Threonine>Isoleucine | Non-synonymous SNV | |||
| M (26523... 27191) | 26530 | GAT > GGT | D 3 G | Aspartic acid>Glycine | Non-synonymous SNV | ||
| 26577 | CAA > GAA | Q 19 E | Glutamine>Glutamic acid | Non-synonymous SNV | |||
| 26709 | GCT > ACT | A 63 T | Alanine>Threonin | Non-synonymous SNV | |||
| ORF6 (27202…27387) | 27269 | AAA > -AA | K 23 * | K23* | Non-synonymous SNV | ||
| 27266 - 27268 | TTA > - - - | del22/23 | del22/23 | Non-frame shift deletion | |||
| ORF9b (28284…28577) | 28311 | CCC > TCC | P 10 S | Proline>Serine | Non-synonymous SNV | ||
| N (28274…29533) | 28881 | AGG > AAA | R 203 K | Arginine>Lysine | Non-synonymous SNV | ||
| 28882 | AGG > AAA | R203 K | Arginine>Lysine | Non-synonymous SNV | |||
| 28883 | GGA > ACG | G 204 R | Glycine>Arginine | Non-synonymous SNV | |||
| 28311 | CCC > CTC | P 13 L | Proline>Leucine | Non-synonymous SNV | |||
| 28725 | CCT > CTT | P 151 L | Proline>Leucine | Non-synonymous SNV | |||
| 29000 | GGC > AGC | G 243 S | Glycine>Serine | Non-synonymous SNV | |||
| 29005 | CAA > CAC | Q 244 H | Glutamine>Histidine | Non-synonymous SNV | |||
| 29510 | AGT > CGT | S 413 R | Serine > Arginine | Non-synonymous SNV | |||
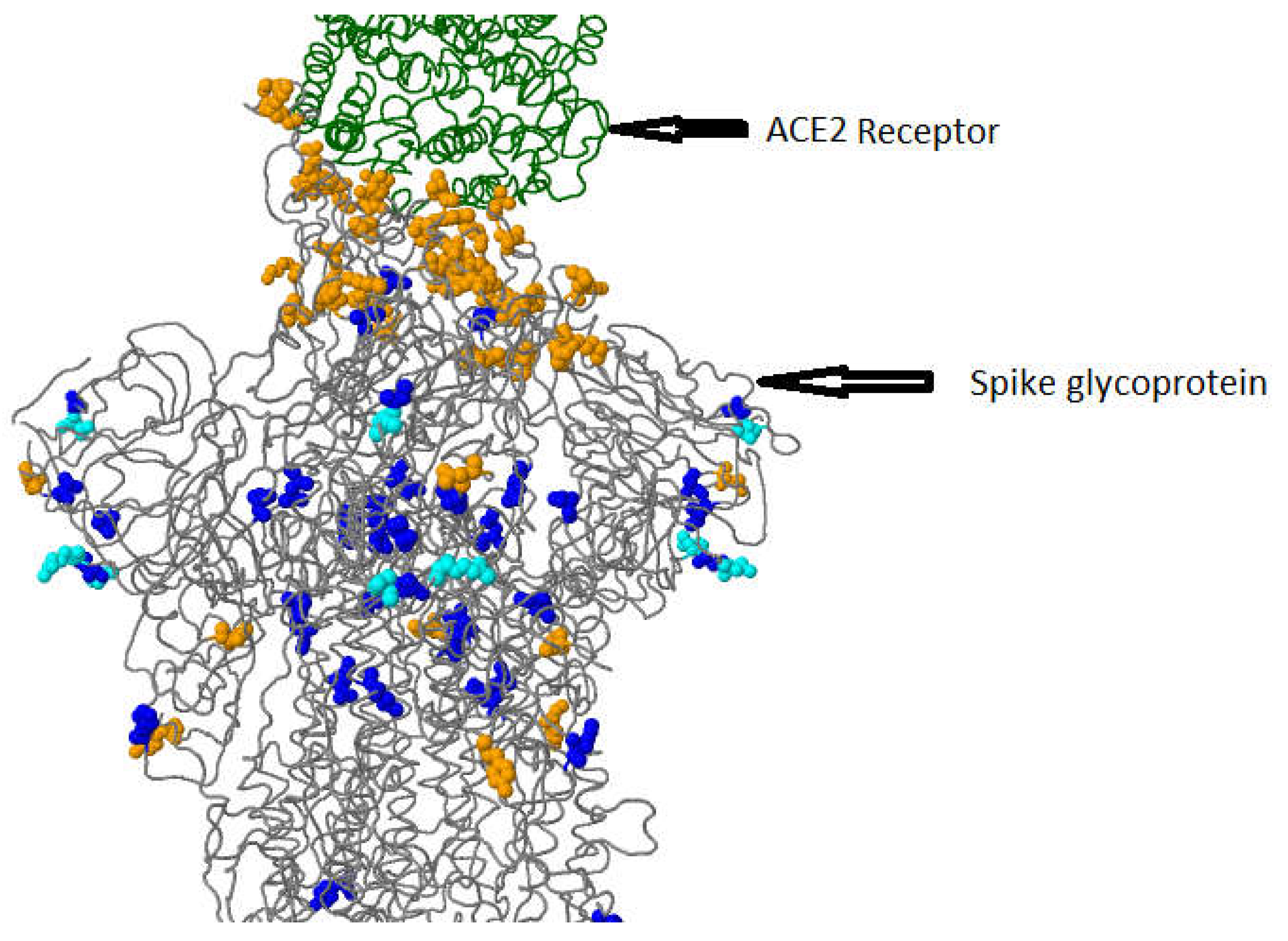
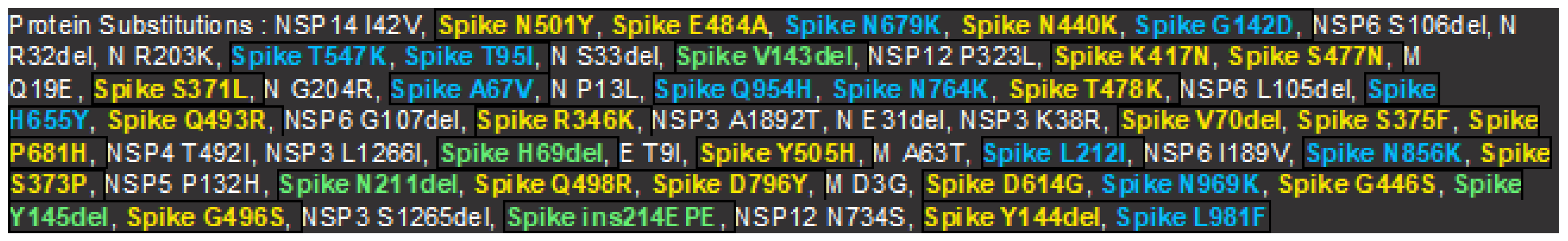
Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Kim D, Lee J-Y, Yang J-S, Kim JW, Kim VN, Chang H, et al. The architecture of SARS-CoV-2 transcriptome. Cell. 2020, 181, 914–921. [Google Scholar] [CrossRef]
- Sidiqi KR, Sabir DK, Ali SM, Kodzius R, et al. Does early childhood vaccination protect against COVID-19? Frontiers in molecular biosciences 2020, 7, 120. [Google Scholar]
- Huremović, D. Psychiatry of pandemics: a mental health response to infection outbreak: Springer; 2019.
- Shaibu JO, Onwuamah CK, James AB, Okwuraiwe AP, Amoo OS, Salu OB, et al. Full length genomic sanger sequencing and phylogenetic analysis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Nigeria. PloS one. 2021, 16, e0243271. [Google Scholar]
- Aldiabat K, Kwekha Rashid A, Talafha H, Karajeh A,et al. The extent of smartphones users to adopt the use of cloud storage. J Comput Sci. 2018, 14, 1588–1598. [Google Scholar] [CrossRef]
- Alhayani B, Abbas ST, Mohammed HJ, Mahajan HB,et al. Intelligent secured two-way image transmission using corvus corone module over WSN. Wireless Personal Communications. 2021, 120, 665–700. [Google Scholar] [CrossRef]
- Hasan HS, Abdallah AA, Khan I, Alosman HS, Kolemen A, Alhayani B,et al. Novel unilateral dental expander appliance (udex): a compound innovative materials. Computers, Materials and Continua. 2021, 3499–3511.
- Mostafaei S, Sayad B, Azar MEF, Doroudian M, Hadifar S, Behrouzi A, et al. The role of viral and bacterial infections in the pathogenesis of IPF: a systematic review and meta-analysis. Respiratory research. 2021, 22, 1–14. [Google Scholar]
- Hui EK-W. Reasons for the increase in emerging and re-emerging viral infectious diseases. Microbes and infection. 2006, 8, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E. Viruses at the edge of adaptation. Virology. 2000, 270, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Taheri M, Rad LM, Hussen BM, Nicknafs F, Sayad A, Ghafouri-Fard S,et al. Evaluation of expression of VDR-associated lncRNAs in COVID-19 patients. BMC Infectious Diseases. 2021, 21, 1–10. [Google Scholar]
- Ibrahim FM, Alkaim A, Kadhom M, Sabir DK, Salih N, Yousif E,et al. Chemistry of selected drugs for SARS-CoV-2 inhibition: tested in vitro and approved by the FDA. Chem Int. 2021, 7, 212–216. [Google Scholar]
- Khan S, Hussain A, Vahdani Y, Kooshki H, Hussen BM, Haghighat S, et al. Exploring the interaction of quercetin-3-O-sophoroside with SARS-CoV-2 main proteins by theoretical studies: A probable prelude to control some variants of coronavirus including Delta. Arabian Journal of Chemistry. 2021, 14, 103353. [Google Scholar] [CrossRef] [PubMed]
- Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Global challenges. 2017, 1, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. others.(2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine.
- Liu J, Dai S, Wang M, Hu Z, Wang H, Deng F,et al. Virus like particle-based vaccines against emerging infectious disease viruses. Virologica Sinica. 2016, 31, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Woo PC, Lau SK, Lam CS, Lai KK, Huang Y, Lee P, et al. Comparative analysis of complete genome sequences of three avian coronaviruses reveals a novel group 3c coronavirus. Journal of virology. 2009, 83, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. Domains and functions of spike protein in Sars-Cov-2 in the context of vaccine design. Viruses. 2021, 13, 109. [Google Scholar] [CrossRef]
- Gupta D, Sharma P, Singh M, Kumar M, Ethayathulla A, Kaur P,et al. Structural and functional insights into the spike protein mutations of emerging SARS-CoV-2 variants. Cellular and Molecular Life Sciences. 2021, 78, 7967–7989. [Google Scholar] [CrossRef]
- Mahajan S, Kode V, Bhojak K, Karunakaran C, Lee K, Manoharan M, et al. Immunodominant T-cell epitopes from the SARS-CoV-2 spike antigen reveal robust pre-existing T-cell immunity in unexposed individuals. Sci Rep. 2021, 11, 13164. [Google Scholar] [CrossRef]
- Wang C, Liu Z, Chen Z, Huang X, Xu M, He T, et al. The establishment of reference sequence for SARS-CoV-2 and variation analysis. Journal of medical virology. 2020, 92, 667–674. [Google Scholar] [CrossRef]
- Zhang L, Jackson CB, Mou H, Ojha A, Peng H, Quinlan BD, et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nature communications. 2020, 11, 19. [Google Scholar]
- Daniloski Z, Jordan TX, Ilmain JK, Guo X, Bhabha G, TenOever BR, et al. The Spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types. Elife. 2021, 10, e65365. [Google Scholar] [CrossRef]
- Zhang J, Cai Y, Xiao T, Lu J, Peng H, Sterling SM, et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science. 2021, 372, 525–530. [Google Scholar] [CrossRef]
- Ahmad, L. Implication of SARS-CoV-2 immune escape spike variants on secondary and vaccine breakthrough infections. Frontiers in immunology. 2021, 2021, 4563. [Google Scholar] [CrossRef] [PubMed]
- https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.
- Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020, 182, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Shah M, Woo HG. Omicron: a heavily mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and potently escapes approved COVID-19 therapeutic antibodies. Frontiers in immunology. 2022, 12, 6031. [Google Scholar]
- Barton MI, MacGowan SA, Kutuzov MA, Dushek O, Barton GJ, Van Der Merwe PA,et al. Effects of common mutations in the SARS-CoV-2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. Elife. 2021, 10, e70658. [Google Scholar] [CrossRef] [PubMed]
- (https://covariants.org/variants/S.E484).
- Khateeb J, Li Y, Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Critical Care. 2021, 25, 1–8. [Google Scholar]
- Barrett CT, Neal HE, Edmonds K, Moncman CL, Thompson R, Branttie JM, et al. Effect of mutations in the SARS-CoV-2 spike protein on protein stability, cleavage, and cell-cell fusion function. bioRxiv. 2021.
- (https://covariants.org/variants/21K.Omicron).
- Mishra SK, Tripathi T. One year update on the COVID-19 pandemic: Where are we now? Acta tropica. 2021, 214, 105778.
- Guruprasad, K. Geographical distribution of amino acid mutations in human SARS-CoV-2 orf1ab poly-proteins compared to the equivalent reference proteins from China. ChemRxiv. 2021. [Google Scholar] [CrossRef]
- Graham RL, Sparks JS, Eckerle LD, Sims AC, Denison MR,et al. SARS coronavirus replicase proteins in pathogenesis. Virus research. 2008, 133, 88–100. [Google Scholar] [CrossRef]
- Thomas, S. Mapping the nonstructural transmembrane proteins of severe acute respiratory syndrome coronavirus 2. Journal of Computational Biology. 2021, 28, 909–921. [Google Scholar] [CrossRef]
- Wolff G, Melia CE, Snijder EJ, Bárcena M,et al. Double-membrane vesicles as platforms for viral replication. Trends in microbiology. 2020, 28, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Diao B, Wen K, Zhang J, Chen J, Han C, Chen Y, et al. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clinical Microbiology and Infection. 2021, 27, 289–e1. [Google Scholar]
- Gao T, Gao Y, Liu X, Nie Z, Sun H, Lin K, et al. Identification and functional analysis of the SARS-COV-2 nucleocapsid protein. BMC microbiology. 2021, 21, 1–10. [Google Scholar]
- Ramesh S, Govindarajulu M, Parise RS, Neel L, Shankar T, Patel S, et al. Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines. 2021, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
- Washington NL, Gangavarapu K, Zeller M, Bolze A, Cirulli ET, Barrett KMS, et al. Emergence and rapid transmission of SARS-CoV-2 B. 1.1. 7 in the United States. Cell. 2021, 184, 2587–2594. [Google Scholar] [CrossRef]
- Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS,et al. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. Journal of virology. 2007, 81, 9812–9824. [Google Scholar] [CrossRef]
- Kannan SR, Spratt AN, Cohen AR, Naqvi SH, Chand HS, Quinn TP, et al. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. Journal of autoimmunity. 2021, 124, 102715. [Google Scholar] [CrossRef]
- Kannan, S.R., Spratt A.N., Quinn T.P., Heng X., Lorson C.L., Sonnerborg A., et al. Infectivity of SARS-CoV2: there is something more than D614G? J. Neuroimmune Pharmacol. 2020, 15, 574–577. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





