Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. General Information on Charcot-Marie-Tooth Disease and Associated Mutations
3. CMT Disease Upon Mutations of the Vitamin-Dependent Enzymes
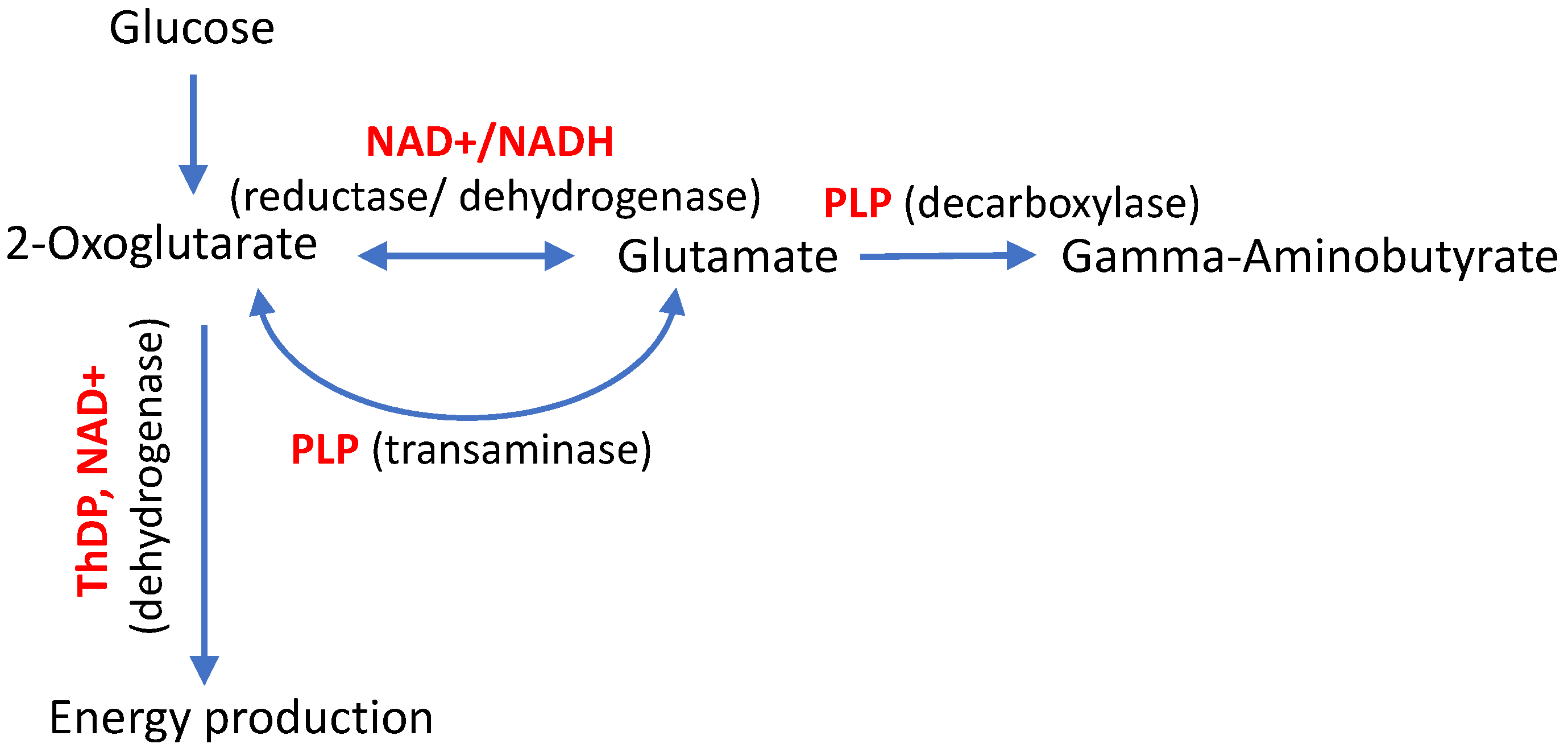
3.1. Pyridoxal Kinase
3.2. Vitamin B1-Dependent Enzymes
3.2.1. Isoenzyme 3 of Kinase of the ThDP-Dependent Pyruvate Dehydrogenase
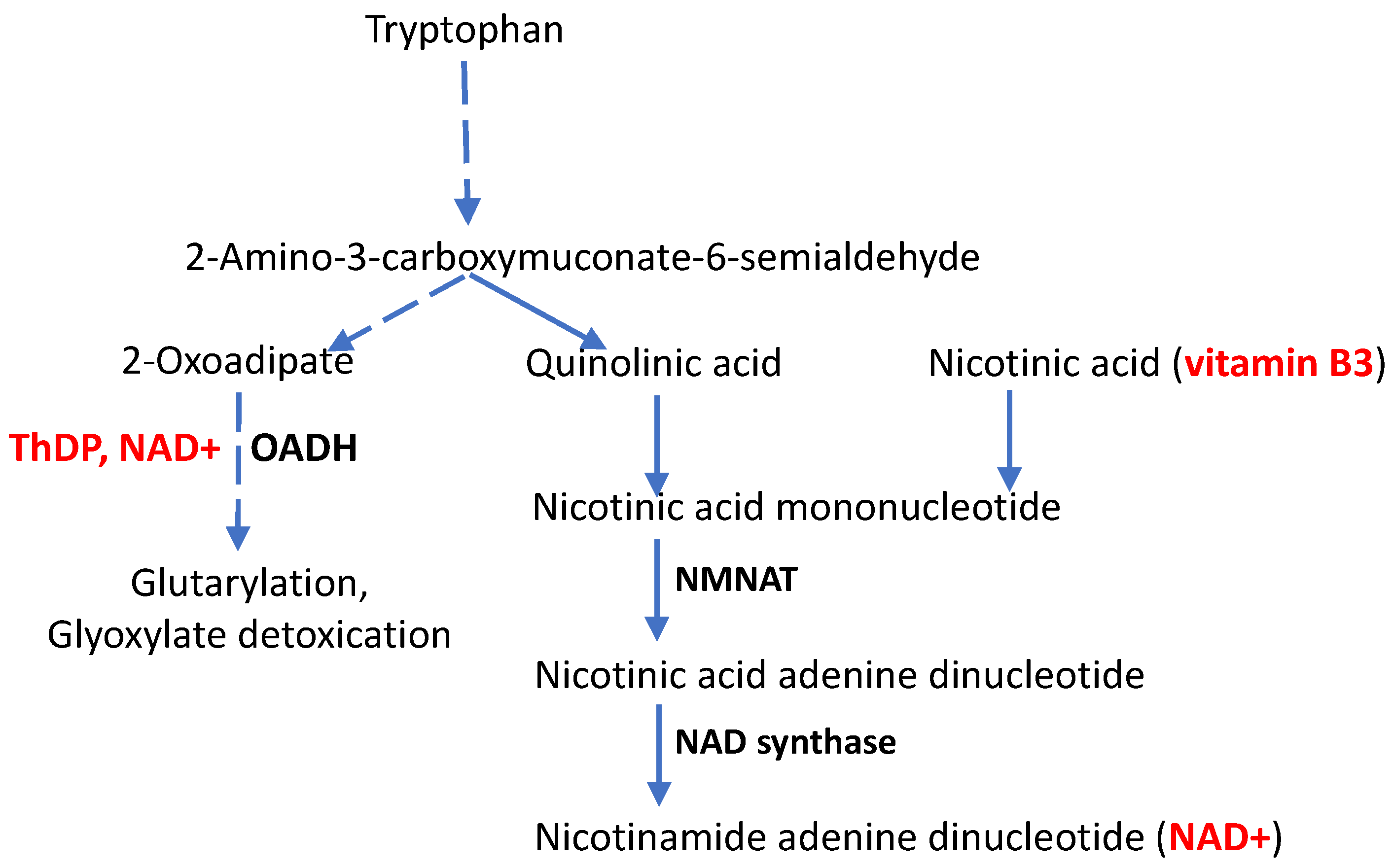
3.2.2. Molecular Mechanisms of CMT Disease Caused by Mutations in the DHTKD1-Encoded ThDP-Dependent 2-Oxoadipate Dehydrogenase
4. CMT Disease, Aging and the Vitamin B3 Derivative NAD+
4. Spatial Specificity of the Impact of Gene Mutations Perturbing the Vitamin-Dependent Processes
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibson, G.E.; Feldman, H.H.; Zhang, S.; Flowers, S.A.; Luchsinger, J.A. Pharmacological thiamine levels as a therapeutic approach in alzheimer's disease. Front Med (Lausanne) 2022, 9, 1033272. [Google Scholar] [CrossRef]
- Fukui, K.O.; Kubota, M.; Terashima, H.; Ishiguro, A.; Kashii, H. Early administration of vitamins b1 and b6 and l-carnitine prevents a second attack of acute encephalopathy with biphasic seizures and late reduced diffusion: A case control study. Brain Dev 2019, 41, 618–624. [Google Scholar] [CrossRef]
- Chelban, V.; Wilson, M.P.; Warman Chardon, J.; Vandrovcova, J.; Zanetti, M.N.; Zamba-Papanicolaou, E.; Efthymiou, S.; Pope, S.; Conte, M.R.; Abis, G.; et al. Pdxk mutations cause polyneuropathy responsive to pyridoxal 5'-phosphate supplementation. Ann Neurol 2019, 86, 225–240. [Google Scholar] [CrossRef]
- Rusch, C.T.; Wortmann, S.B.; Kovacs-Nagy, R.; Grehten, P.; Haberle, J.; Latal, B.; Stettner, G.M. Thiamine pyrophosphokinase deficiency due to mutations in the tpk1 gene: A rare, treatable neurodegenerative disorder. Neuropediatrics 2021, 52, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Qin, J.; Liu, D.; Wang, Y.; Shen, X.; Yang, N.; Zhou, H.; Cai, X.T.; Wang, Z.L.; Yu, D.; et al. Reduced thiamine binding is a novel mechanism for tpk deficiency disorder. Mol Genet Genomics 2019, 294, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V.; Aleshin, V.; Nogues, I.; Kahne, T.; Parroni, A.; Contestabile, R.; Salvo, M.L.; Graf, A.; Tramonti, A. Thiamine-dependent regulation of mammalian brain pyridoxal kinase in vitro and in vivo. J Neurochem 2022, 161, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Lu, M.; Lee, B.Y.; Ugurbil, K.; Chen, W. In vivo nad assay reveals the intracellular nad contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Liu, L.; Moon, P.K.; Joshi, A.U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.A.; Coronado, M.; et al. Macrophage de novo nad(+) synthesis specifies immune function in aging and inflammation. Nat Immunol 2019, 20, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Fei, G.; Lu, J.; Jin, L.; Pan, S.; Chen, Z.; Wang, C.; Sang, S.; Liu, H.; Hu, W.; et al. Measurement of blood thiamine metabolites for alzheimer's disease diagnosis. EBioMedicine 2016, 3, 155–162. [Google Scholar] [CrossRef]
- Pan, X.; Sang, S.; Fei, G.; Jin, L.; Liu, H.; Wang, Z.; Wang, H.; Zhong, C. Enhanced activities of blood thiamine diphosphatase and monophosphatase in alzheimer's disease. PLoS One 2017, 12, e0167273. [Google Scholar] [CrossRef]
- Fattal-Valevski, A.; Kesler, A.; Sela, B.A.; Nitzan-Kaluski, D.; Rotstein, M.; Mesterman, R.; Toledano-Alhadef, H.; Stolovitch, C.; Hoffmann, C.; Globus, O.; et al. Outbreak of life-threatening thiamine deficiency in infants in israel caused by a defective soy-based formula. Pediatrics 2005, 115, e233–e238. [Google Scholar] [CrossRef]
- Luan, C.J.; Guo, W.; Chen, L.; Wei, X.W.; He, Y.; Chen, Y.; Dang, S.Y.; Prior, R.; Li, X.; Kuang, Y.; et al. Cmt2q-causing mutation in the dhtkd1 gene lead to sensory defects, mitochondrial accumulation and altered metabolism in a knock-in mouse model. Acta Neuropathol Commun 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.K.; Brewer, M.H.; Perez-Siles, G.; Ellis, M.; Ly, C.; Burgess, A.; Neumann, B.; Nicholson, G.A.; Vucic, S.; Kennerson, M.L. Charcot-marie-tooth disease causing mutation (p.R158h) in pyruvate dehydrogenase kinase 3 (pdk3) affects synaptic transmission, atp production and causes neurodegeneration in a cmtx6 c. Elegans model. Hum Mol Genet 2021, 31, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Dohrn, M.F.; Glockle, N.; Mulahasanovic, L.; Heller, C.; Mohr, J.; Bauer, C.; Riesch, E.; Becker, A.; Battke, F.; Hortnagel, K.; et al. Frequent genes in rare diseases: Panel-based next generation sequencing to disclose causal mutations in hereditary neuropathies. J Neurochem 2017, 143, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Vaur, P.; Brugg, B.; Mericskay, M.; Li, Z.; Schmidt, M.S.; Vivien, D.; Orset, C.; Jacotot, E.; Brenner, C.; Duplus, E. Nicotinamide riboside, a form of vitamin b(3), protects against excitotoxicity-induced axonal degeneration. FASEB J 2017, 31, 5440–5452. [Google Scholar] [CrossRef]
- McGuinness, H.Y.; Gu, W.; Shi, Y.; Kobe, B.; Ve, T. Sarm1-dependent axon degeneration: Nucleotide signaling, neurodegenerative disorders, toxicity, and therapeutic opportunities. Neuroscientist 2023, 10738584231162508. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.I.; Karlina, I.S.; Zavileyskiy, L.G.; Aleshin, V.A.; Artiukhov, A.V.; Kaehne, T.; Ksenofontov, A.L.; Ryabov, S.I.; Graf, A.V.; Tramonti, A.; et al. Delayed impact of 2-oxoadipate dehydrogenase inhibition on the rat brain metabolism is linked to protein glutarylation. Front Med (Lausanne) 2022, 9, 896263. [Google Scholar] [CrossRef]
- Keller, N.; Mendoza-Ferreira, N.; Maroofian, R.; Chelban, V.; Khalil, Y.; Mills, P.B.; Boostani, R.; Torbati, P.N.; Karimiani, E.G.; Thiele, H.; et al. Hereditary polyneuropathy with optic atrophy due to pdxk variant leading to impaired vitamin b6 metabolism. Neuromuscul Disord 2020, 30, 583–589. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Bunik, V.I. Mechanisms of non-coenzyme action of thiamine: Protein targets and medical significance. Biochemistry (Mosc) 2019, 84, 829–850. [Google Scholar] [CrossRef]
- Boyko, A.; Tsepkova, P.; Aleshin, V.; Artiukhov, A.; Mkrtchyan, G.; Ksenofontov, A.; Baratova, L.; Ryabov, S.; Graf, A.; Bunik, V. Severe spinal cord injury in rats induces chronic changes in the spinal cord and cerebral cortex metabolism, adjusted by thiamine that improves locomotor performance. Front Mol Neurosci 2021, 14, 620593. [Google Scholar] [CrossRef]
- Mkrtchyan, G.V.; Ucal, M.; Mullebner, A.; Dumitrescu, S.; Kames, M.; Moldzio, R.; Molcanyi, M.; Schaefer, S.; Weidinger, A.; Schaefer, U.; et al. Thiamine preserves mitochondrial function in a rat model of traumatic brain injury, preventing inactivation of the 2-oxoglutarate dehydrogenase complex. Biochim Biophys Acta Bioenerg 2018, 1859, 925–931. [Google Scholar] [CrossRef]
- Weidinger, A.; Milivojev, N.; Hosmann, A.; Duvigneau, J.C.; Szabo, C.; Toro, G.; Rauter, L.; Vaglio-Garro, A.; Mkrtchyan, G.V.; Trofimova, L.; et al. Oxoglutarate dehydrogenase complex controls glutamate-mediated neuronal death. Redox Biol 2023, 62, 102669. [Google Scholar] [CrossRef]
- Tiamkao, S.; Boonsong, A.; Saepeung, K.; Kasemsap, N.; Apiwattanakul, M.; Suanprasert, N.; Hemachudha, T.; Pithak, P.; Juntee, K.; Waisaen, C.; et al. An outbreak of peripheral neuropathy in a prison. Case Rep Neurol 2019, 11, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bunik, V. Vitamin-dependent complexes of 2-oxo acid dehydrogenases: Structure, function, regulation and medical implications. Nova Science Publishers: New York, 2017. [Google Scholar]
- Hanberry, B.S.; Berger, R.; Zastre, J.A. High-dose vitamin b1 reduces proliferation in cancer cell lines analogous to dichloroacetate. Cancer Chemother Pharmacol 2014, 73, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Jonus, H.C.; Byrnes, C.C.; Kim, J.; Valle, M.L.; Bartlett, M.G.; Said, H.M.; Zastre, J.A. Thiamine mimetics sulbutiamine and benfotiamine as a nutraceutical approach to anticancer therapy. Biomed Pharmacother 2020, 121, 109648. [Google Scholar] [CrossRef] [PubMed]
- Klyuyeva, A.; Tuganova, A.; Kedishvili, N.; Popov, K.M. Tissue-specific kinase expression and activity regulate flux through the pyruvate dehydrogenase complex. J Biol Chem 2019, 294, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiao, Y.; Meng, F.; Li, Y.; Shi, Z.; Qian, K. Functions and mechanisms of lysine glutarylation in eukaryotes. Front Cell Dev Biol 2021, 9, 667684. [Google Scholar] [CrossRef] [PubMed]
- Kennerson, M.L.; Yiu, E.M.; Chuang, D.T.; Kidambi, A.; Tso, S.C.; Ly, C.; Chaudhry, R.; Drew, A.P.; Rance, G.; Delatycki, M.B.; et al. A new locus for x-linked dominant charcot-marie-tooth disease (cmtx6) is caused by mutations in the pyruvate dehydrogenase kinase isoenzyme 3 (pdk3) gene. Hum Mol Genet 2013, 22, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Perez-Siles, G.; Cutrupi, A.; Ellis, M.; Screnci, R.; Mao, D.; Uesugi, M.; Yiu, E.M.; Ryan, M.M.; Choi, B.O.; Nicholson, G.; et al. Energy metabolism and mitochondrial defects in x-linked charcot-marie-tooth (cmtx6) ipsc-derived motor neurons with the p.R158h pdk3 mutation. Sci Rep 2020, 10, 9262. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Y.; Gu, M.M.; Sun, L.H.; Guo, W.T.; Zhu, H.B.; Ma, J.F.; Yuan, W.T.; Kuang, Y.; Ji, B.J.; Wu, X.L.; et al. A nonsense mutation in dhtkd1 causes charcot-marie-tooth disease type 2 in a large chinese pedigree. Am J Hum Genet 2012, 91, 1088–1094. [Google Scholar] [CrossRef]
- Xu, W.Y.; Zhu, H.; Shen, Y.; Wan, Y.H.; Tu, X.D.; Wu, W.T.; Tang, L.; Zhang, H.X.; Lu, S.Y.; Jin, X.L.; et al. Dhtkd1 deficiency causes charcot-marie-tooth disease in mice. Mol Cell Biol 2018, 38. [Google Scholar] [CrossRef]
- Danhauser, K.; Sauer, S.W.; Haack, T.B.; Wieland, T.; Staufner, C.; Graf, E.; Zschocke, J.; Strom, T.M.; Traub, T.; Okun, J.G.; et al. Dhtkd1 mutations cause 2-aminoadipic and 2-oxoadipic aciduria. Am J Hum Genet 2012, 91, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.; te Brinke, H.; Wanders, R.J.; Knegt, A.C.; Oussoren, E.; Hoogeboom, A.J.; Ruijter, G.J.; Becker, D.; Schwab, K.O.; Franke, I.; et al. Genetic basis of alpha-aminoadipic and alpha-ketoadipic aciduria. J Inherit Metab Dis 2015, 38, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Stiles, A.R.; Venturoni, L.; Mucci, G.; Elbalalesy, N.; Woontner, M.; Goodman, S.; Abdenur, J.E. New cases of dhtkd1 mutations in patients with 2-ketoadipic aciduria. JIMD Rep 2016, 25, 15–19. [Google Scholar] [PubMed]
- Sherrill, J.D.; Kc, K.; Wang, X.; Wen, T.; Chamberlin, A.; Stucke, E.M.; Collins, M.H.; Abonia, J.P.; Peng, Y.; Wu, Q.; et al. Whole-exome sequencing uncovers oxidoreductases dhtkd1 and ogdhl as linkers between mitochondrial dysfunction and eosinophilic esophagitis. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Wang, Y.Z. A chinese pedigree with a novel mutation in gjb1 gene and a rare variation in dhtkd1 gene for diverse charcot-marie-tooth diseases. Mol Med Rep 2019, 19, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, G.M.; Hoyer, H.; Taioli, F.; Cavallaro, T.; Hilmarsen, H.T.; Squintani, G.M.; Zanette, G.; Braathen, G.J. Inherited motor-sensory neuropathy with upper limb predominance associated with the tropomyosin-receptor kinase fused gene. Neuromuscul Disord 2020, 30, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Leandro, J.; Khamrui, S.; Wang, H.; Suebsuwong, C.; Nemeria, N.S.; Huynh, K.; Moustakim, M.; Secor, C.; Wang, M.; Dodatko, T.; et al. Inhibition and crystal structure of the human dhtkd1-thiamin diphosphate complex. ACS Chem Biol 2020, 15, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, G.A.; Foster, W.R.; Bailey, H.J.; Hicks, K.G.; Sauer, S.W.; Dimitrov, B.; McCorvie, T.J.; Okun, J.G.; Rutter, J.; Kolker, S.; et al. Crystal structure and interaction studies of human dhtkd1 provide insight into a mitochondrial megacomplex in lysine catabolism. IUCrJ 2020, 7, 693–706. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Kazantsev, A.V.; Lukashev, N.V.; Bellinzoni, M.; Bunik, V.I. Selective inhibition of 2-oxoglutarate and 2-oxoadipate dehydrogenases by the phosphonate analogs of their 2-oxo acid substrates. Front Chem 2020, 8, 596187. [Google Scholar] [CrossRef]
- Nemeria, N.S.; Nagy, B.; Sanchez, R.; Zhang, X.; Leandro, J.; Ambrus, A.; Houten, S.M.; Jordan, F. Functional versatility of the human 2-oxoadipate dehydrogenase in the l-lysine degradation pathway toward its non-cognate substrate 2-oxopimelic acid. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Bunik, V.I.; Degtyarev, D. Structure-function relationships in the 2-oxo acid dehydrogenase family: Substrate-specific signatures and functional predictions for the 2-oxoglutarate dehydrogenase-like proteins. Proteins 2008, 71, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Ozohanics, O.; Zhang, X.; Nemeria, N.S.; Ambrus, A.; Jordan, F. Probing the e1o-e2o and e1a-e2o interactions in binary subcomplexes of the human 2-oxoglutarate dehydrogenase and 2-oxoadipate dehydrogenase complexes by chemical cross-linking mass spectrometry and molecular dynamics simulation. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Nemeria, N.S.; Zhang, X.; Leandro, J.; Zhou, J.; Yang, L.; Houten, S.M.; Jordan, F. Toward an understanding of the structural and mechanistic aspects of protein-protein interactions in 2-oxoacid dehydrogenase complexes. Life (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Nemeria, N.S.; Gerfen, G.; Nareddy, P.R.; Yang, L.; Zhang, X.; Szostak, M.; Jordan, F. The mitochondrial 2-oxoadipate and 2-oxoglutarate dehydrogenase complexes share their e2 and e3 components for their function and both generate reactive oxygen species. Free Radic Biol Med 2018, 115, 136–145. [Google Scholar] [CrossRef]
- Boyko, A.I.; Artiukhov, A.V.; Kaehne, T.; di Salvo, M.L.; Bonaccorsi di Patti, M.C.; Contestabile, R.; Tramonti, A.; Bunik, V.I. Isoforms of the dhtkd1-encoded 2-oxoadipate dehydrogenase, identified in animal tissues, are not observed upon the human dhtkd1 expression in bacterial or yeast systems. Biochemistry (Mosc) 2020, 85, 920–929. [Google Scholar] [CrossRef]
- Biagosch, C.; Ediga, R.D.; Hensler, S.V.; Faerberboeck, M.; Kuehn, R.; Wurst, W.; Meitinger, T.; Kolker, S.; Sauer, S.; Prokisch, H. Elevated glutaric acid levels in dhtkd1-/gcdh- double knockout mice challenge our current understanding of lysine metabolism. Biochim Biophys Acta Mol Basis Dis 2017, 1863, 2220–2228. [Google Scholar] [CrossRef]
- Leandro, J.; Dodatko, T.; Aten, J.; Nemeria, N.S.; Zhang, X.; Jordan, F.; Hendrickson, R.C.; Sanchez, R.; Yu, C.; DeVita, R.J.; et al. Dhtkd1 and ogdh display substrate overlap in cultured cells and form a hybrid 2-oxo acid dehydrogenase complex in vivo. Hum Mol Genet 2020, 29, 1168–1179. [Google Scholar] [CrossRef]
- Wu, Y.; Williams, E.G.; Dubuis, S.; Mottis, A.; Jovaisaite, V.; Houten, S.M.; Argmann, C.A.; Faridi, P.; Wolski, W.; Kutalik, Z.; et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 2014, 158, 1415–1430. [Google Scholar] [CrossRef]
- Wang, T.J.; Ngo, D.; Psychogios, N.; Dejam, A.; Larson, M.G.; Vasan, R.S.; Ghorbani, A.; O'Sullivan, J.; Cheng, S.; Rhee, E.P.; et al. 2-aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 2013, 123, 4309–4317. [Google Scholar] [CrossRef]
- Plubell, D.L.; Fenton, A.M.; Wilmarth, P.A.; Bergstrom, P.; Zhao, Y.; Minnier, J.; Heinecke, J.W.; Yang, X.; Pamir, N. Gm-csf driven myeloid cells in adipose tissue link weight gain and insulin resistance via formation of 2-aminoadipate. Sci Rep 2018, 8, 11485. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Liu, Z.; Apontes, P.; Feng, D.; Pessin, J.E.; Sauve, A.A.; Angeletti, R.H.; Chi, Y. Dual mode action of mangiferin in mouse liver under high fat diet. PLoS One 2014, 9, e90137. [Google Scholar] [CrossRef] [PubMed]
- Tsepkova, P.M.; Artiukhov, A.V.; Boyko, A.I.; Aleshin, V.A.; Mkrtchyan, G.V.; Zvyagintseva, M.A.; Ryabov, S.I.; Ksenofontov, A.L.; Baratova, L.A.; Graf, A.V.; et al. Thiamine induces long-term changes in amino acid profiles and activities of 2-oxoglutarate and 2-oxoadipate dehydrogenases in rat brain. Biochemistry (Mosc) 2017, 82, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Atherton, P.J.; Larsson, O.; Sood, S.; Blokhin, I.O.; Brogan, R.J.; Volmar, C.H.; Josse, A.R.; Slentz, C.; Wahlestedt, C.; et al. A coding and non-coding transcriptomic perspective on the genomics of human metabolic disease. Nucleic Acids Res 2018, 46, 7772–7792. [Google Scholar] [CrossRef] [PubMed]
- Artiukhov, A.V.; Grabarska, A.; Gumbarewicz, E.; Aleshin, V.A.; Kahne, T.; Obata, T.; Kazantsev, A.V.; Lukashev, N.V.; Stepulak, A.; Fernie, A.R.; et al. Synthetic analogues of 2-oxo acids discriminate metabolic contribution of the 2-oxoglutarate and 2-oxoadipate dehydrogenases in mammalian cells and tissues. Sci Rep 2020, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, H.; Gu, M.; Luo, Q.; Ding, J.; Yao, Y.; Chen, F.; Wang, Z. Dhtkd1 is essential for mitochondrial biogenesis and function maintenance. FEBS Lett 2013, 587, 3587–3592. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Panahi, H.K.S.; Kavyani, B.; Heng, B.; Tan, V.; Braidy, N.; Guillemin, G.J. The role of kynurenine pathway and nad(+) metabolism in myalgic encephalomyelitis/chronic fatigue syndrome. Aging Dis 2022, 13, 698–711. [Google Scholar] [CrossRef]
- Birkisdottir, M.B.; van Galen, I.; Brandt, R.M.C.; Barnhoorn, S.; van Vliet, N.; van Dijk, C.; Nagarajah, B.; Imholz, S.; van Oostrom, C.T.; Reiling, E.; et al. The use of progeroid DNA repair-deficient mice for assessing anti-aging compounds, illustrating the benefits of nicotinamide riboside. Front Aging 2022, 3, 1005322. [Google Scholar] [CrossRef]
- Vreones, M.; Mustapic, M.; Moaddel, R.; Pucha, K.A.; Lovett, J.; Seals, D.R.; Kapogiannis, D.; Martens, C.R. Oral nicotinamide riboside raises nad+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell 2023, 22, e13754. [Google Scholar] [CrossRef]
- Kazamel, M.; Boes, C.J. Charcot marie tooth disease (cmt): Historical perspectives and evolution. J Neurol 2015, 262, 801–805. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. Nad+ and sirtuins in aging and disease. Trends Cell Biol 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Imai, S.I.; Guarente, L. It takes two to tango: Nad(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis 2016, 2, 16017. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Imai, S.I. Nad (+) biosynthesis, aging, and disease. F1000Res 2018, 7, 132. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. Nad(+) in aging: Molecular mechanisms and translational implications. Trends Mol Med 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.I. Nad(+) intermediates: The biology and therapeutic potential of nmn and nr. Cell Metab 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, V.A.; Gromyko, D.V.; Nikiforov, A.A. The regulatory role of nad in human and animal cells. Biochemistry (Mosc) 2018, 83, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Ying Cao, Y.W. , Jing Yang. Nad+-dependent mechanism of pathological axon degeneration. Cell Insight 2022, 1. [Google Scholar]
- Araki, T.; Sasaki, Y.; Milbrandt, J. Increased nuclear nad biosynthesis and sirt1 activation prevent axonal degeneration. Science 2004, 305, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.R.; Hoke, A. Targeting the programmed axon degeneration pathway as a potential therapeutic for charcot-marie-tooth disease. Brain Res 2020, 1727, 146539. [Google Scholar] [CrossRef]
- Barile, A.; Nogues, I.; di Salvo, M.L.; Bunik, V.; Contestabile, R.; Tramonti, A. Molecular characterization of pyridoxine 5'-phosphate oxidase and its pathogenic forms associated with neonatal epileptic encephalopathy. Sci Rep 2020, 10, 13621. [Google Scholar] [CrossRef]
- Gachon, F.; Fonjallaz, P.; Damiola, F.; Gos, P.; Kodama, T.; Zakany, J.; Duboule, D.; Petit, B.; Tafti, M.; Schibler, U. The loss of circadian par bzip transcription factors results in epilepsy. Genes Dev 2004, 18, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Mandal, A.; Roy, D.; Chatterjee, S.; Ghosh, M.K.; Dubey, S.; Lahiri, D.; Finsterer, J.; Ray, B.K. Seizure as a presenting manifestation of wernicke's encephalopathy induced by hyperemesis gravidarum. J Family Med Prim Care 2021, 10, 567–571. [Google Scholar] [PubMed]
- Mengi, T.; Beckmann, Y. Wernicke encephalopathy with epileptic seizures during pregnancy. Neurocase 2022, 28, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Mimouni-Bloch, A.; Goldberg-Stern, H.; Strausberg, R.; Brezner, A.; Heyman, E.; Inbar, D.; Kivity, S.; Zvulunov, A.; Sztarkier, I.; Fogelman, R.; et al. Thiamine deficiency in infancy: Long-term follow-up. Pediatr Neurol 2014, 51, 311–316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




