1. Introduction
Hybridization can be defined as reproduction between members of genetically distinct populations [
1,
2]. Hybridization may be the result of interactions involving a wide range of types and levels of genetic divergence between the parental forms [
2]. Hybridization has been considered as one of the mechanisms that influences the process of speciation [
2,
3]. The hybrid can reproduce either with its parental lineages (backcrossing or introgression) or only with similar hybrids [
3]. In both cases, hybridization can lead to the emergence of novel features as well as new species altogether. Reticulate evolution caused by hybridization has played an important role in diversification of the several anthozoan genera [
4,
5,
6,
7]. Much less is known about the importance of hybridization in Medusozoa taxa including Hydrozoa [
8,
9,
10,
11,
12,
13] and Scyphozoa [
14].
The complex life cycle of Hydrozoa includes pelagic medusa and sessile polyp stages [
15,
16,
17,
18]. Free-swimming medusae detach from benthic polyps or colonies, grow and spawn gametes shortly after maturation. Ciliated larva, planula, develops from the fertilized egg, settles and undergoes metamorphosis into the new polyp. However, reduction of the medusa stage is a widespread evolutionary trend among Hydroidolina and occurred independently in many phylogenetic lineages [
19,
20,
21]. Reduced gonophores lose many features of the medusa and usually form gametes staying attached to the mother colony.
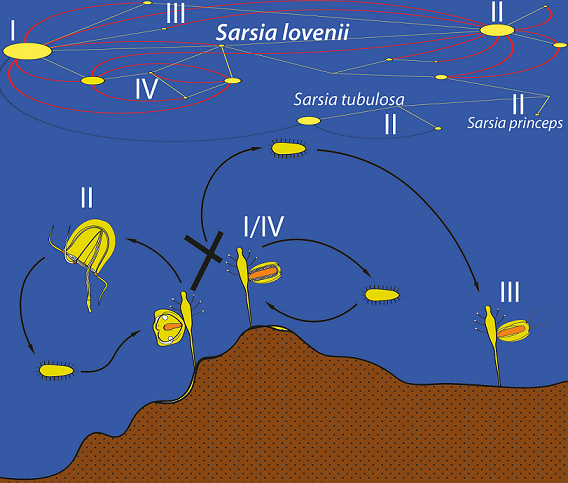
Recently, an unusual morpho-genetic polymorphism was found in the hydroid
S. lovenii (Corynidae) [
13]. According to traditional views, colonies of
S. lovenii produce reduced medusae named medusoids [
22]. Medusoids form gonads without breaking away from the mother colony and lack ocelli and tentacles. Recently it was demonstrated, that
S. lovenii has two morphotypes of gonophores: some colonies produce free-swimming medusae, while others produce medusoids [
13]. The studied morphotypes belong to different genetic haplogroups, but the genetic distances between these haplogroups are minimal and correspond to the level of intraspecific variability. The possibility of hybridization between these haplogroups has also been experimentally proven. The results obtained were interpreted as a case of incipient speciation [
13]. However, little attention was paid to hybridization of different lineages
S. lovenii in the sea.
The aim of our work is a detailed analysis of the morphogenetic diversity of hydrozoans Sarsia in the White Sea, including the search for natural hybridization of lineages of S. lovenii using region of internal transcribed spacers of the ribosomal operon (ITS1-5.8S-ITS2).
2. Materials and Methods
Sampling and experimental cultures
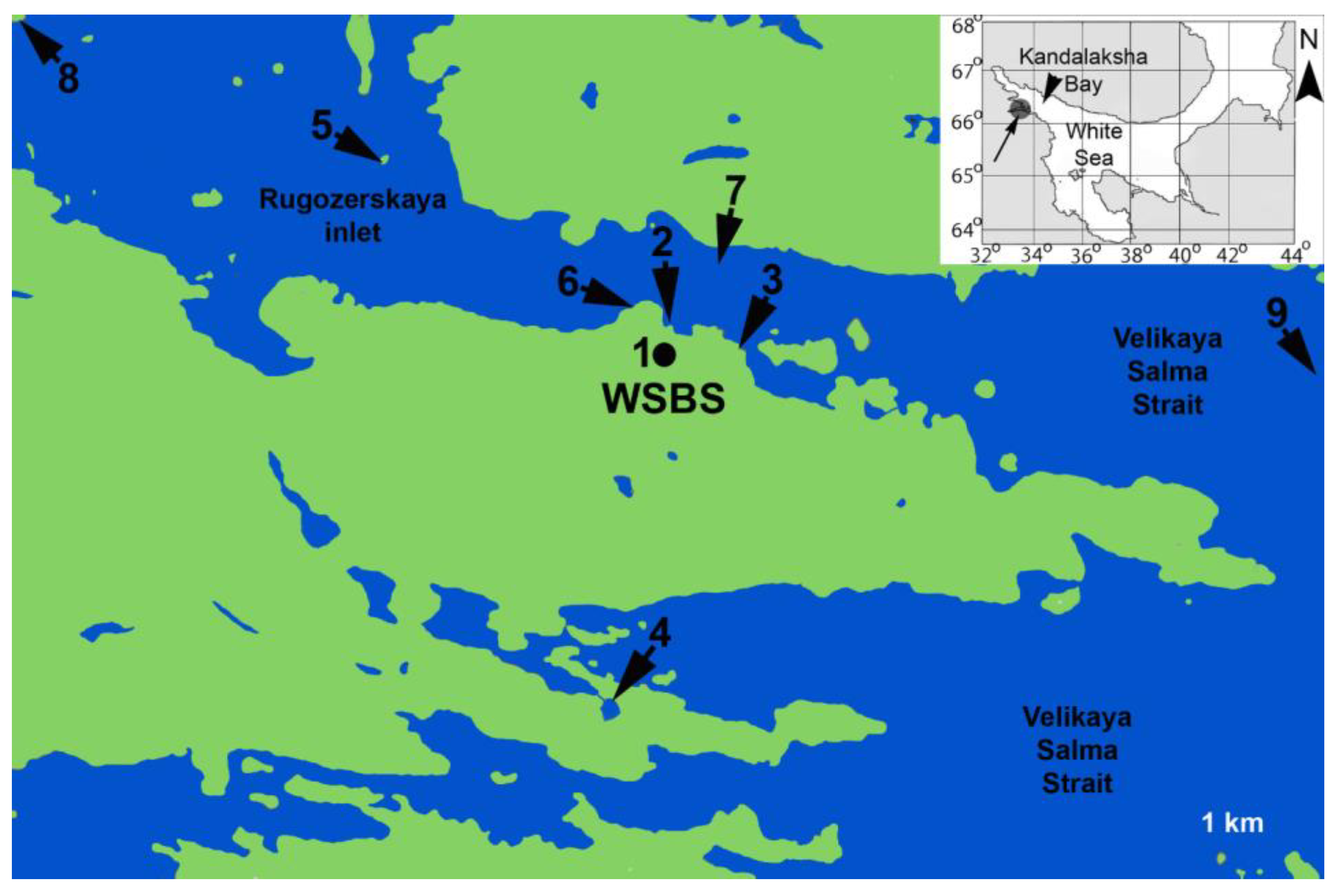
The material was collected near the Pertsov White Sea Biological Station (Lomonosov Moscow State University) (66°34′N, 33°08′E) in 2015-2021 (
Figure 1,
Table S1).
Colonies were collected on different substrates near the shore. Medusae were collected manually from the surface of the water. Two hydroids were collected outside the White Sea: colony of
Sarsia lovenii with medusoids was collected in the Barents Sea (Dalnezelenetskaya inlet) and medusa of
Sarsia sp. was collected in the Bering Sea (Senyavin Strait). Medusae and colonies were photographed alive and fixed in ethanol (96%) for molecular phylogenetic analysis. Additionally, the collected specimens were used for experimental cultures or for hybridization experiments. For experimental crossing, ready-to-spawn medusae and medusoids of
Sarsia spp. were collected and maintained in small containers (200 ml) with filtered (0.2 microns) seawater at a temperature of 10-12° C. Females of
S. lovenii with "medusoid" morphotype were placed together with male medusae of
S. lovenii, and female medusae of
S. lovenii were placed with males medusoids of
S. lovenii (
Table 1). In addition,
S. tubulosa were crossed with
S. lovenii medusoids.
New COI and ITS sequences for 140 specimens were obtained (
Table S1). Previously obtained sequences were also used for analysis (the list of specimens see in [
13]). In total, 183 specimens were used for analysis (
Table 2): 143 specimens collected in the sea (including 42 medusa-specimens and 101 polyp-specimens), 40 specimens maintained in the laboratory (including 18 specimens obtained by experimental hybridization). For 99 specimens, we observed the mature gonophores (including medusa specimens) or experimentally induced their formation.
Morphological Analysis
To distinguish different morphotypes of gonophores in collected specimens and experimental colonies we analyzed: if gonophore detaches from the mother polyp as a free-swimming medusa or not; if edges of the bell with tentacles are bent inward or not, if bell unfolds before detachment or not; the presence of tentacles and ocelli on the tentacle bulbs; the presence of an incomplete nematocyst ring in epidermal part of bulbs; the shape of the bell and the size of the manubrium, the presence or absence of gonads at the gonophore before the detachment.
Molecular Analysis
DNA was isolated with a Diatom kit (Diatom DNA Prep 100 kit, Isogen Laboratory, Moscow, Russia) according to the manufacturer's protocol. The cytochrome c oxidase (COI) subunit fragment I and internal transcribed spacers of the ribosomal operon 18S-ITS1-5.8S-ITS2-28S rRNA (ITS) were amplified from isolated DNA with the following primers pairs: SR6R (AAGWAAAAGTCGTAACAAGG) and LR1 (GGTTGGTTTCTTTTCCT) for 18S-ITS1-5.8S-ITS2-28S rRNA [23, 24] with program 95 °C for 5 min; followed by 34 cycles of 15 s at 94 °C, 30 s at 52 °C and 60 s at 72°C and then a final extension of 5 min at 72 °C; and SAR-F (TTTGGGGCTTTCGCCGGTAT) and SAR-R (CAGGATCACCTCCTCCTGC) for COI (
Sarsia-specific primers, current study) with program 95 °C for 5 min; followed by 34 cycles of 15 s at 95 °C, 30 s at 50 °C and 60 s at 72°C and then a final extension of 5 min at 72 °C. The polymerase chain reaction was carried out in a reaction volume of 20 µl, which included 4 µl of 5x Screen Mix solution (Eurogen, Moscow, Russia), 0.5 µl of each primer, 1 µl of DNA and 14 µl of sterile water. Amplification was also used in a volume of 25 ml, which included 5 ml of 5x Taq Red Buffer (Evrogen Lab), 0.5 ml of polymerase (HS-Taq Polymerase by Eurogen Lab), 0.5 ml of dNTP (50 µM stock), 0.3 ml of each primer (10 µM stock), 1 ml of DNA and 17.7 ml of sterile water (MilliQ). Sequencing was carried out at Evrogen (Russia) in an ABI Prism 3500 Genetic Analyzer (Applied Biosystems). Accession numbers of the sequences generated in the present study are listed in
Table S1: accession numbers COI (OQ859724 – OQ859863),
accession numbers ITS will be provided during review.
DNA Cloning
Specimen H122 is an experimental hybrid of female medusa S. lovenii and male medusa Sarsia sp. Specimen H144 is an experimental hybrid of female medusa S. lovenii and male medusoid S. lovenii. ITS of H122 and H144 specimens were isolated from the genome DNA samples with gene-specific SR6R and LR1 primers pair. Amplified fragments were cloned into the pAL-TA vector (Evrogen, Russia). Three clones have been sequenced from each plate.
Phylogenetic Analysis
Sequences were assembled and checked for improper base calling with CodonCode Aligner software (
www.codoncode.com/aligner). Sequences were aligned using the MUSCLE [
25] algorithm in MEGA 6 software [
26]. The final alignments resulted in dataset comprising of 624 bp for the COI. JModelTest 2 [
27] was used to estimate the best substitution model for each partition based on the Bayesian information criterion (BIC). The GTR+G model was found to be optimal for the COI dataset. Bayesian phylogenetic trees were built in PhyloBayes 3.3 [
28]. The analysis was performed with random starting trees and 10 million generations. Two MCMC chains were run in parallel, and the analyses were stopped when the maximum discrepancy of bipartitions between chains was below 0.01. Final phylogenetic tree images were rendered in FigTree 1.4.0. Maximum Likelihood Phylogenetic analysis was performed in IQTree v.2.0-rc2 software [
29] with standard algorithm. The best model of nucleotide substitution (GTR+F+G4) was chosen using ModelFinder [
30] according to Bayesian information criterion (BIC). One thousand bootstrap replicates were generated for the analysis.
A haplotype network for COI dataset was constructed using the TCS network inference method [
31] within PopART software (
http://popart.otago.ac.nz/index.shtml). According to a constraint of the method, we used a reduced COI dataset without undefined states of nucleotides. Haplogroups of
S. lovenii were identified in accordance to [
13] or according to the morphology of the specimens.
For the analysis of ITS-sequences with heterozygotes we used Champuru v. 1.1 [
32] (
https://eeg-ebe.github.io/Champuru/input.html) to determine the haplotypes of heterozygous individuals without cloning, simply by analyzing the patterns of double peaks in the forward and reverse chromatograms (marked in results as phase-1 and phase-2). This method is well suited for separating haplotypes that differ only in one deletion locus, which is typical for different interlineage hybrids of
S. lovenii. Sequences with one heterozygote we manually divided into two alleles (marked in results as allel-1 and allel-2). We trimmed the ends with unknown bases to align the length of all sequences. In addition, we excluded from analysis several short sequences less than 500 bp (H150, H157, H161, H170) and sequences with two single heterozygotes (H97, H118, H153, H184). ITS fragment of several
S. tubulosa specimens, containing many single heterozygotes, also cannot be divided to haplotypes. The final alignments resulted in dataset comprising of 509 bp for the 214 sequences/ 152 specimens. We export dataset in RDF-format (Roehl Data File) using DnaSP 5.10 software with option «considered sites with gaps/missing» [
33]. A haplotype network was constructed using the median joining algorithm [
34] within NETWORK 10.2.0.0 software (Fluxus Technology Ltd,
www.fluxus-engineering.com).
3. Results
3.1. Morphotypes of Gonophores in Specimens of Sarsia spp
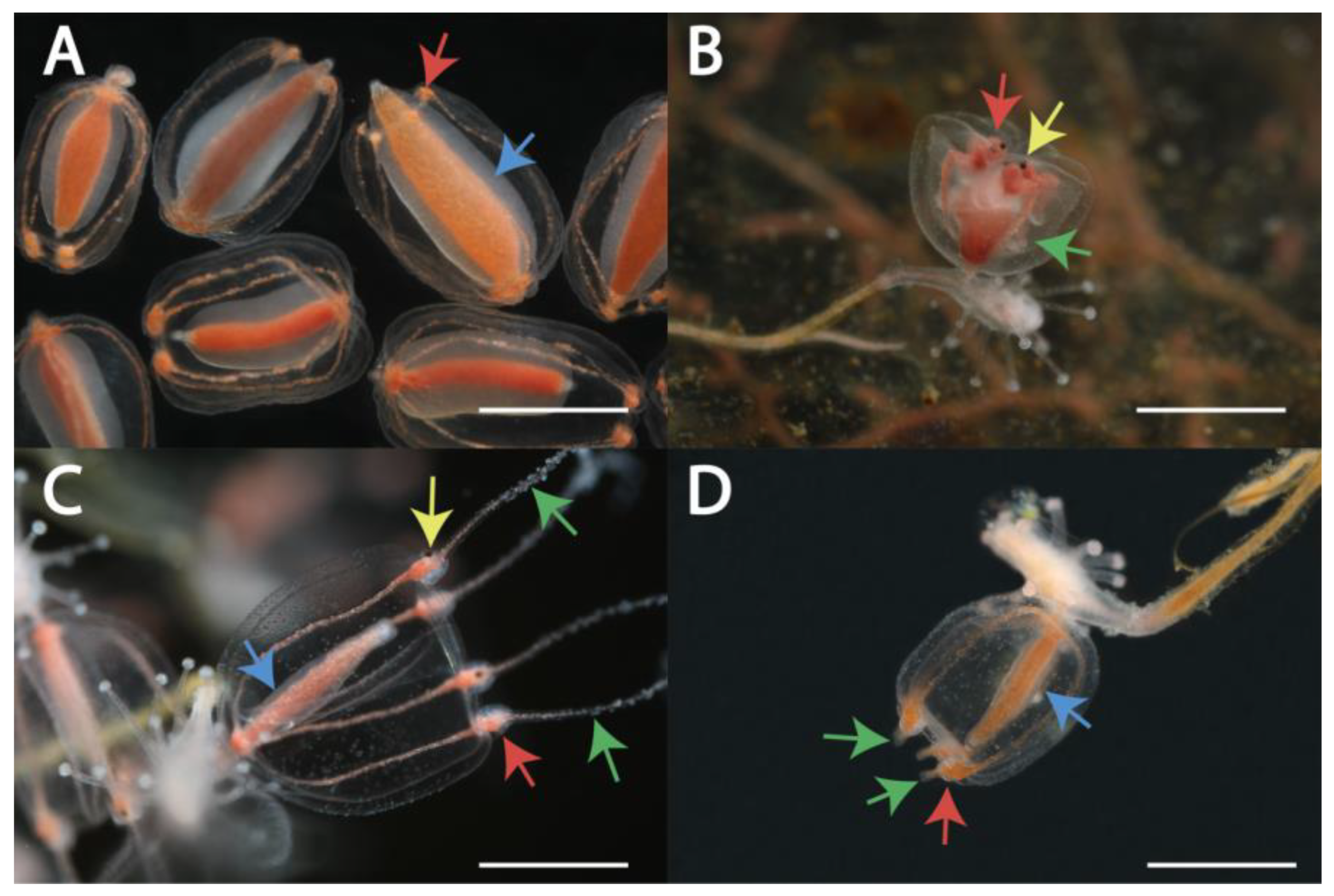
We identified four morphotypes of gonophores: free-swimming medusa (53 specimens), medusoid (17 specimens), attached medusa (17 specimens) and "abnormal medusoid" (12 specimens) (
Figure 1,
Figure S1,
Table S1, [
13]).
Morphotype I "medusoid" is characterized by the absence of tentacles and eyes on tentacle bulbs (
Figure 2A). Tentacular bulbs are present, but they are significantly reduced compared to other morphotypes, they lack a C-shaped nematocyst zone. The bell is oval, noticeably narrowed in the distal part. The manubrium is encircled by gonads. Manubrium with gonads occupies a large part of the subumbrellar cavity. Gonads on the manubrium appear early in the development, sometimes they cover even the most distal part of the manubrium. There is no functional mouth, the medusoid does not feed. When medusoid is ripe, it is possible to observe a series of bell contractions, due to which gametes are expelled from the subumbrellar cavity. Colonies of
S. lovenii with ripe medusoids were collected in the sea from June till July, and also medusoids were produced by some experimental colonies (
Figure S1,
Table S1).
Morphotype II, medusa, detaches from the mother colony when it is completely developed. Further growth and maturation of gonads occurs in the feeding free-swimming medusa. Medusae of
Sarsia spp. can be identified by morphological characters such as size of bell, morphology of tentacle bulbs and apical knob, position of gonads over manubrium [
13,
22]. We observed the development of medusa buds in
S. lovenii. Late medusa buds are characterized by the presence of tentacles and eyes on tentacular bulbs, the edges of the bell are bent inward, and tentacles are inside the bell (
Figure 2B). The medusa turns the tentacles out shortly before the detachment from the mother polyp, when the bell begins to contract. Medusae or medusa buds of
Sarsia spp. were collected at the sea from March till July, and also obtained on some experimental colonies (
Figure S1,
Table S1).
Morphotype III, "attached medusa", is similar to a new-born free-swimming medusa by shape of the bell, however, gonophores usually do not detach from the mother colony (
Figure 2C). Such attached medusae have relatively short tentacles and tentacle bulbs with ocellus. Late gonophores have a fully expanded bell, outward located tentacles, and are capable of periodic contraction. Mouth opening locates at the end of the manubrium. Gonads cover manubrium as a tube and can be observed in proximal and middle parts of manubrium. If mother polyp is resorbed, the gonophore may broke away from the mother colony and swim near the bottom and even be able to feed. Morphotype “attached medusa” were formed on colonies that were obtained as a result of experimental crossing between the morphotype "medusa" and the morphotype "medusoid", both in the case of crossing individuals of
S. lovenii, and in the case of interspecific crossing between the medusoid
S. lovenii and the medusa
S. tubulosa. Moreover, gonophores with such a morphotype were found in the sea on 12 May 2021 (
Figure S1,
Table S1).
Morphotype IV, "abnormal medusoid", differs from the typical medusoid by well-developed tentacular bulbs often elongated into short rod-shaped tentacles (
Figure 2D). C-shaped zone of nematocysts is also visible at bulbs. The shape of the bell is the most similar to the morphotype "attached medusa". However, such gonophores lack ocelli on tentacular bulbs. Abnormal medusoids could break away from the mother colony because of a mechanical impact and move near the bottom of the experimental bowl. Abnormal medusoids were found on colonies of
Sarsia sp. in the sea on 12 May 2021 (
Figure S1,
Table S1).
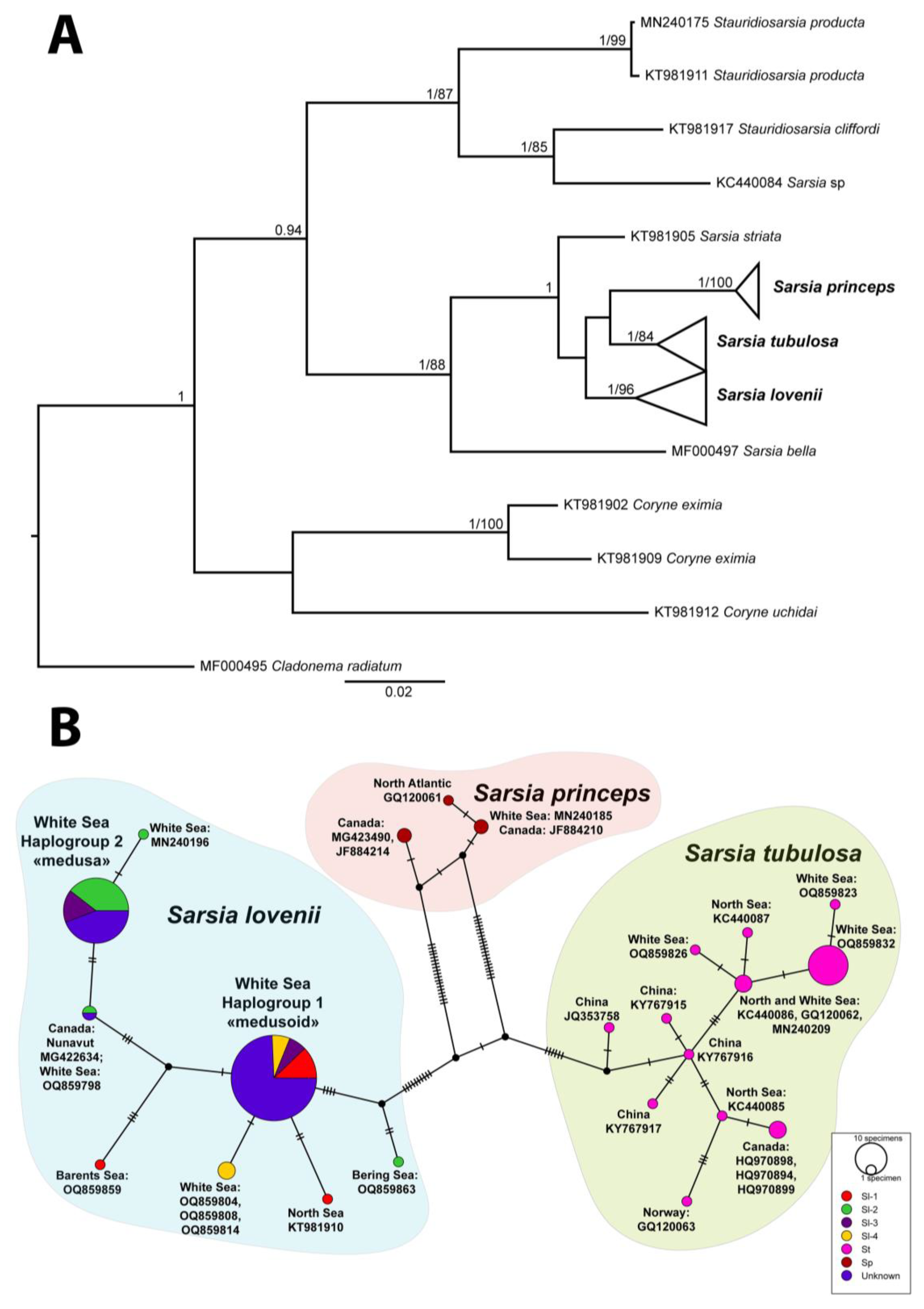
Analysis of COI
The analysis of the molecular phylogenetic tree and the haplotype net of the mitochondrial marker COI allowed us to divide the collected specimens into three species:
S. tubulosa,
S. lovenii,
S. princeps (
Figure 3,
Table S2). Moreover, the specimens of
S. lovenii form two haplogroups: haplogroup COI-1 and haplogroup COI-2 (
Figure 3B; see also [
13]). Haplogroup COI-1 includes specimens of morphotype I, III and IV (medusoid, attached medusa and abnormal medusoid). Haplogroup COI-2 includes specimens with morphotypes II and III (medusa, attached medusa).
Most specimens from the White Sea within each haplogroups have identical COI haplotypes. Some haplotypes of
S. lovenii that have unique substitutions are mainly from other locations, such as the Barents Sea (OQ859859), North Sea (KT981910), Canada: Nunavut (MG422634). Specimen H248 (OQ859798) found in the deep–water part of the White Sea has also haplotype dissimilar from littoral specimens but identical with medusa-specimen from Canada. We assign it to haplogroup COI-2 because the specimen from Canada is a medusa. There are also some unique haplotypes of
S. lovenii collected in the shallow part of the White Sea, which are adjacent to one or another haplogroup. Medusa-specimen from the Bering Sea (H97) has unique haplotype (OQ859863) dissimilar with other medusa-haplotypes
S. lovenii. Being closer to haplogroup COI-1, it nevertheless has the morphotype II. Specimens of
S. tubulosa from the White Sea group with some haplotypes from the North Sea (
Figure 3B). Specimen of
S. princeps from the White Sea groups with some haplotypes from North Atlantic, including Canada’s water and Iceland Sea (
Figure 3B).
Analysis of ITS
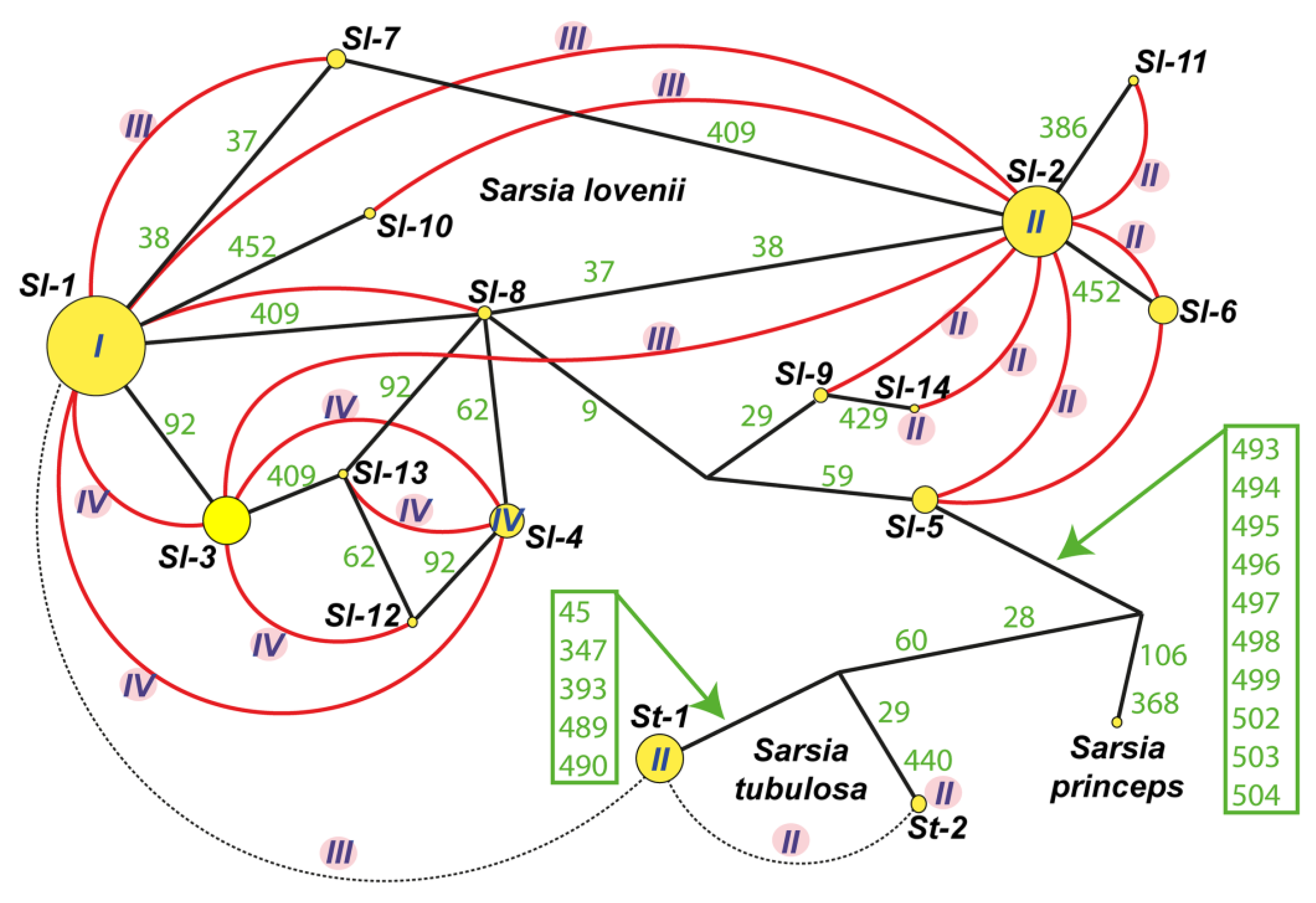
For the analysis, a dataset with a length of 509 nucleotides was built. Within dataset, 31 positions were variable (
Figure 4,
Table S3). We distinguish groups of specimens belonging to certain haplotypes, as well as specimens with heterozygous states of certain loci, which may indicate the processes of hybridization between individuals with different haplotypes in populations.
Heterozygous specimens of
Sarsia tubulosa have ITS-sequences with several single heterozygous loci. Such sequences could be the result of a combination of potential alleles, and the number of combinations increases rapidly with the increase in the number of heterozygotes. Therefore, we use only specimens of two main haplotypes without heterozygous loci (st-1 and st-2) in the network of haplotypes (
Figure 4,
Table S4). The differences between them were in seven loci. Heterozygotes from these loci found in 10 specimens probably indicate hybridization between lineages st-1 and st-2 (
Figure 4,
Table S4). Two haplotypes of
Sarsia tubulosa and heterozygous specimens have gonophores of morphotype II (free-swimming medusa). We did not find any significant differences in the morphology of medusae of different haplotypes.
A large number of heterozygous loci were obtained for an experimental interspecies hybrid between
S. tubulosa and
S. lovenii (
Figure 4: hybrid Sl-1/St-1). Due to the presence of several deletion zones, we could not determine the state of some loci when separating alleles by Champuru v. 1.1 [
32]. Such hybrid colonies produced attached medusa (morphotype III) (DNA isolate H163:
Figure S1,
Table S1).
Several heterozygous specimens of
Sarsia lovenii have ITS-sequences with group of double peaks in chromatograms. We managed to separate alleles of specimens with heterozygotes using Champuru v. 1.1 [
32], as well as by cloning of two DNA isolates. As a result, we identified 14 haplotypes for
Sarsia lovenii (
Figure 4). Only three haplotypes (Sl-1, Sl-2 and Sl-4) include specimens having ITS-sequences without heterozygotes. The remaining specimens have ITS-sequences with heterozygotes and became part of different haplotypes, being divided into alleles. Hydroids with the Sl-1 haplotype produced normal medusoids (morphotype I). The Sl-2 haplotype was found in free-swimming medusae or in hydroids that produce free-swimming medusae. In addition, free-swimming medusae were registered in specimens with a mix of the allele Sl-2 and the alleles Sl-6, Sl-11, Sl-5, Sl-9, Sl-14.
A hybrid specimen was obtained in experimental crossing between the medusa with haplotype Sl-2 and the medusa with haplotype Sl-14 (DNA isolate H122). The resulted hybrid colony produced free-swimming medusae (morphotype II). Experimental hybridization of specimens with haplotypes Sl-1 and Sl-2 resulted in hybrid colonies that produced attached medusae (morphotype III) (
Table 1). Colonies of hydroids with such a morphotype of gonophores were also found in the sea in May (DNA isolates H335, H337, H338). In addition, attached medusae were registered in specimens with a mix of the allele Sl-2 and allele Sl-10 or in mix of allele Sl-1 and allele Sl-7.
Colonies of hydroids with the haplotype Sl-4 produced medusoids of abnormal structure (morphotype IV). Several more heterozygous specimens for ITS had the same morphotype. Being separated into alleles, these sequences became part of the haplotypes: Sl-1, Sl-3, Sl-4, Sl-12, Sl-13 (
Figure 4).
4. Discussion
4.1. Morphotypes of Gonophores in Sarsia spp. in the White Sea
In this work, we found four morphotypes of gonophores in
Sarsia spp. (medusoid, free-swimming medusa, attached medusa and abnormal medusoid) compared to the three that were described earlier [
13]. The structure of medusoids is quite variable. They differ in size, color, shape of the bell, the size of the manubrium and gonads. Morphotype IV, which we called "abnormal medusoids", differs from typical medusoids in the presence of short tentacles and a nematocyst zone at the tentacle bulbs (
Figure 2D). On the other hand, abnormal medusoids do not have ocelli at tentacle bulbs and thus differ from attached medusae. This morphotype has not been previously described for
Sarsia hydroids and, thus, it is not yet known whether it occurs outside the White Sea.
4.1. Species and COI-Haplogroups of Sarsia spp. in the White Sea
According to COI analysis, collected specimens can be attributed to three species:
S. lovenii,
S. tubulosa,
S. princeps (
Figure 3A). This result completely coincides with the previous analysis [
13]. Analysis of haplotypes also recovered three groups that corresponds to three species.
Sarsia lovenii specimens can be attributed to two main haplogroups. Previously, we divided these groups according to the morphotype of gonophores. Specimens with medusoid were assigned to haplogroup COI-1 and specimens with free-swimming medusa were assigned to haplogroup COI-2 [
13]. However, here we demonstrated that each haplogroup includes specimens with several types of gonophores (
Figure 3B). In each haplogroup there are specimens with an intermediate type of gonophore (morphotype III), which corresponds to hybrid forms. Given that mitochondrial genes are inherited on the maternal side, we believe that hybridization between haplogroups goes in both directions. In addition, haplogroup COI-1 also includes specimens with the morphotype of gonophore IV. A special position is occupied by a specimen from the Bering Sea, which has a medusa as a gonophore, but is more closely related to haplogroup COI-1. Perhaps the medusa in the evolution of the species
S. lovenii could be reduced to a medusoid and then recover again. However, to understand the phylogeography of the species
S. lovenii, more specimens from different locations is required.
4.3. Hybridization or Intragenomic Polymorphism?
Nuclear ribosomal DNA (nrDNA) is the genomic region, in which the RNA components of ribosomes are encoded [
35,
36,
37,
38,
39]. Eukaryotic nrDNA comprises a multigene family including transcribable rRNA genes (18S rRNA, 28S rRNA and 5.8S rRNA) separated by internal transcribed spacers (ITS1 and ITS2) and an intergenic spacer (IGS) that are located downstream of the 18S rRNA gene and upstream of 28S rRNA gene. These genes cluster in large tandems located on certain chromosomes to form nucleolus organising regions. Ribosomal genes and associated spacers are arranged in one or more large arrays consisting of hundreds or thousands of tandemly repeated copies. During evolution, the coding regions (18S and 28S rRNA) have remained more conserved than the non-coding regions (ITS and IGS). There is a considerable precedent for the use of ITS sequence divergence to infer relationships at or below the species level in a wide variety of taxonomic groups, most notably plants and fungi [
40,
41,
42,
43]. Sometimes ITS sequence were used in recovering phylogeny of cnidarian taxa such as corals [
44] and hydrozoans [45–50). In addition, ITS region is used to study intraspecific genetic heterogeneity [
51]. In our study, we present a result of detailed analysis of the ITS marker in 183 specimens of
Sarsia from the White Sea. We found three pure haplotypes of
S. lovenii, two haplotypes of
S. tubulosa and 1 haplotype of
S. princeps. All these haplotypes did not contain heterozygotes. We also found intra-individual polymorphism in the structure of ITS for
S. lovenii and
S. tubulosa.
Two widely acknowledged problems with the usage of the ribosomal ITS region as a phylogenetic marker are intragenomic variation and alignment ambiguities [
44]. ITS region can be hypervariable and prone to insertions and deletions, which can result in alignment ambiguities [
44,
51]. We have previously shown that ITS marker is suitable for the separation of haplotypes in
S. lovenii [
13]. However, when analyzing chromatograms, we encountered ambiguities of peaks in some specimens. While some of the substitutions, present in single specimens, may be PCR artifacts, the frequent occurrence of common patterns between specimens indicates that most of the sequence variations reflect real ITS heterogeneity. Since two main haplotypes of
S. lovenii (Sl-1 and Sl-2) differ by deletion of two nucleotides (positions in the dataset 37-38), hybridization of these lineages results in hybrids with wide areas of double peaks in ITS chromatograms. The presence of parental ITS alleles in experimental hybrids was proved by cloning (sample H144) and by separation of alleles using Champuru software.
Tandemly arranged gene families tend to exhibit concerted evolution, a term used to describe the phenomenon when multiple copies of a gene family tend to be homogeneous, leading to greater sequence similarities among the paralogues within a genome than among orthologues among species [
52,
53]. Recombinant processes such as gene conversion and unequal crossover etc. are thought to be the homogenizing mechanisms [
53,
54,
55,
56]. Despite concerted evolution, intragenomic ITS variation has been found in many different types of invertebrates [
57,
58,
59,
60,
61,
62], indicating consideration has to be given for intra-individual rDNA variation. The simplest reason for the appearance of intra-individual rDNA variation is hybridization between different species or haplotypes of the same species [
43]. Significant variation between copies within a species has been also attributed to introgression from hybridization, pseudogenes, separately evolving chromosomal lineages, slowed rates of lineage sorting of ancestral alleles [
51,
58,
63,
64,
65]. Interspecific hybridization and intragenomic rDNA polymorphism are often difficult to distinguish [
66].
We suppose that ITS polymorphism in S. lovenii and S. tubulosa is primarily associated with hybridization. Sequence data from ITS indicate that rDNA arrays are homogeneous in specimens related to haplotypes Sl-1, Sl-2, Sl-4 and St-1, St-2. Though we did not perform mass cloning of our DNA-samples, we assume that intragenomic polymorphism is absent or insignificant for these specimens. In addition, the polymorphism of many specimens might be explained by the presence of hybrid forms between known haplotypes. Here, we experimentally proved that polymorphism is a result of crossing. Thus, questions remain for those specimens with ITS polymorphism for which we have not found potential parental haplotypes. Vegetative reproduction is likely to be a reason for maintenance of parental ITS sequences in the hybrids. It seems unlikely to us that intragenomic polymorphism occurs in some lineages of S. lovenii, but is absent in other lineages. However, the presence of a network of interconnected haplotypes in S. lovenii suggests the presence of genetic connectivity between them and the transfer of genetic material through recombinant processes. Thus, potential introgression due to hybridization, as a necessary component of reticulate evolution, may be an interesting direction for further research.
4.4. Hybridization Experiments
Here we experimentally confirmed the possibility of hybridization between different haplotypes of
S. lovenii, as well as the possibility of interspecific hybridization between
S. lovenii and
S. tubulosa (
Table 1,
Figure S1). The possibility of hybridization between medusa and medusoid of
S. lovenii has already been proven [
13], but here we confirmed the result in several repetitions. We also performed a backcrossing between the first generation hybrid (F1) and the medusa
S. lovenii. The crossing was successful. However, the poor survival of the resulting hybrids-F2 did not allow quantitative analysis of different alleles in the descendants. Nevertheless, our results confirm the possibility of such hybridization in the sea and support the possibility of introgression due to hybridization.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Photographs of specimens used for phylogenetic analyses. Full description of specimens presents in Table S1. Scale Bars 1 mm; Table S1: List of
Sarsia specimens from the White Sea used for phylogenetic analyses: collection data and GenBank accession numbers. Abbreviations: Exp – Experiment. Species: Sl –
Sarsia lovenii, St –
Sarsia tubulosa. Sex: Fem – female, male. Gonophore type: 1 – medusoid, 2 – medusa, 3 – attached medusa, 4 – “abnormal medusoid”. Stage: p – polyp, m –medusa, pm – medusoid. Locality (see
Figure 1): W1 – Aquarium at WSBS; W2 – Pier of WSBS; W3 – Eremeevskie rapids; W4 – saline lake at the Green Cape; W5 – location “Luda”; W6 – location “Krest”; W7 – Rugozerskaya inlet, depth 5-15 m; W8 – Polovye islands; W9 – Velikaya Salma Strait, depth 40-60 m. Table S2: Specimens, haplotypes and haplogroups COI of
S. lovenii,
S. tubulosa and
S. princeps visualized at haplotypes net (
Figure 3B). Sequences excluded from analysis of COI-haplotypes denoted in table as “exc”. Morphotypes for
S. lovenii: medusoid, medusa, attached medusa, abnormal medusoid, unknown morphotype. Table S3: Alleles of significant loci of ITS dataset associated with interspecies and haplotypes differences (see
Figure 4). Table S4: Specimens and ITS-haplotypes of
Sarsia lovenii (Sl-1 – Sl-14),
Sarsia tubulosa (St-1, St-2) and
Sarsia princeps visualized at haplotype network (
Figure 4). Abbreviations: allel _1 and allel_2 – haplotypes separated manually from sequences with one heterozygote. Phase1 and phase2 - haplotypes separated from sequences by means of Champuru v. 1.1 (Flot, 2007). St-1-add - specimens
S. tubulosa of haplotype 1 with additive heterozygotes in some loci. St-1/2 – specimens with many single heterozygotes. Specimens of
S. tubulosa with heterozygotes (St-1-add and St-1/2) were not included in haplotype analyses.
Author Contributions
Conceptualization, A.P.; methodology, A.P.; software, A.P.; validation, A.P.; formal analysis, A.P.; investigation, A.P., A.V. and S.K.; resources, A.P. and S.K.; data curation, A.P.; writing—original draft preparation, A.P.; writing—review and editing, A.P. and A.V.; visualization, A.P.; supervision, A.P.; project administration, A.P.; funding acquisition, A.P. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Russian Science Foundation, grant number 21-74-00129 (Stanislav Kremnyov) and the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University, grants number 121032300118–0 (Andrey Prudkovsky). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and
Supplementary Materials) or are available from the corresponding authors upon reasonable request.
Acknowledgments
We thank to Ivan Fedutin and Olga Filatova for collecting specimen in Bering Sea and we thank to Nikolai Neretin, Glafira Kolbasova, Anna Mihlina and Boris Osadchenko for collecting specimen in Barents Sea. We like to acknowledge the staff of N.F. Pertzov White Sea Biological Station of Lomonosov Moscow State University, Russia, for providing opportunity for the research and equipment usage of the Center of microscopy WSBS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barton, N.H.; Hewitt, G.M. Analysis of hybrid zones. Annu. Rev. Ecol. Evol. Syst., 1985, 16, 113–148. https://www.jstor.org/stable/2097045.
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; Butlin R. K.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26(2), 229–246. [CrossRef]
- Gontier, N. Reticulate evolution; Springer: Cham Heidelberg New York Dordrecht London. 2015; pp. 1–40. ISBN 978-3-319-16344-4.
- Richards, Z.T.; van Oppen, M.J.; Wallace, C.C.; Willis, B.L.; Miller, D.J. Some rare Indo-Pacific coral species are probable hybrids. PloS one 2008, 3(9), e3240. [CrossRef]
- Fogarty, N.D.; Vollmer, S.V.; Levitan, D.R. Weak prezygotic isolating mechanisms in threatened Caribbean Acropora corals. PLoS One 2012, 7(2), e30486. [CrossRef]
- Richards, Z.T.; Hobbs, J.P.A. Hybridisation on coral reefs and the conservation of evolutionary novelty. Curr. Zool. 2015, 61(1), 132–145. [CrossRef]
- Hobbs, J.P.A.; Richards, Z.T.; Popovic, I.; Lei, C.; Staeudle, T.M.; Montanari, S. R.; DiBattista, J.D. Hybridisation and the evolution of coral reef biodiversity. Coral Reefs 2022, 41(3), 535–549. [CrossRef]
- Miller, R.L. Sperm chemotaxis in the hydromedusae. I. Species-specificity and sperm behavior. Mar. Biol. 1979, 53, 99–113. [CrossRef]
- Miller, R.L. Identification of sibling species within the “Sarsia tubulosa complex” at Friday Harbor, Washington (Hydrozoa: Anthomedusae). J. Exp. Mar. Biol. Ecol. 1982, 62(2), 153–172. [CrossRef]
- Kubota, S. Crossing-experiments between Japanese populations of three hydrozoans symbiotic with bivalves. Hydrobiologia 1991, 216, 429–436. [CrossRef]
- Soong, K.; Ch,o, L.C. Synchronized release of medusae from three species of hydrozoan fire corals. Coral Reefs 1998, 17, 145–154. [CrossRef]
- Brinckmann-Voss, A. Reproductive barriers and early development from hybridization experiments in two sympatric species of the genus Sarsia (Cnidaria, Hydrozoa, Anthoathecatae, Corynidae). Vie et Milieu/Life & Environment, 2002, 121–130. https://hal.sorbonne-universite.fr/hal-03198882.
- Prudkovsky, A.A.; Ekimova, I.A.; Neretina, T.V. A case of nascent speciation: unique polymorphism of gonophores within hydrozoan Sarsia lovenii. Sci. Rep. 2019, 9(1), 15567. [CrossRef]
- Schroth, W.; Jarms, G.; Streit, B.; Schierwater, B. Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evol. Biol. 2002, 2(1), 1–10. [CrossRef]
- Daly, M.; Brugler, M.R.; Cartwright, P.; Collins, A.G.; Dawson, M.N.; Fautin, D.G.; France, S.C.; McFadden, C.S.; Opresko, D.M.; Rodriguez, E.; Romano, S.L.; Stake, J.L. The phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. In Linnaeus Tercentenary: Progress in Invertebrate Taxonomy, Z.-Q. Zhang and W. A. Shear, editors. Zootaxa 2007, 1668, 127–182. http://hdl.handle.net/1808/13641.
- Kayal, E.; Bentlage, B.; Sabrina Pankey, M.; Ohdera, A.H.; Medina, M.; Plachetzki, D. C.; Collins A.G.; Ryan, J.F. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 2018, 18, 1–18. [CrossRef]
- Mills, C.E.; Marques, A.C.; Migotto, A.E.; Calder, D.R.; Hand, C. Hydrozoa: polyps, hydromedusae, and siphonophora. In The Light and Smith manual: intertidal invertebrates from central California to Oregon. Carlton, J.T., Ed.; University of California Press: Berkeley, 2007; ISBN 9780520239395.
- Bouillon, J.; Gravili, C.; Gili, J.M.; Boero, F. An introduction to Hydrozoa. Publications Scientifiques du Muséum, Paris, 2006.
- Cornelius, P.F. Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated life-cycles among their remote-island faunae: An interim review. Sci. Mar. 1992, 56, 245–261.
- Leclère, L.; Schuchert, P.; Cruaud, C.; Couloux, A.; Manuel, M. Molecular phylogenetics of Thecata (Hydrozoa, Cnidaria) reveals long-term maintenance of life history traits despite high frequency of recent character changes. Syst. Biol. 2009, 58, 509–526, . [CrossRef]
- Miglietta, M.P.; Cunningham, C.W. Evolution of life cycle, colony morphology, and host specificity in the family Hydractiniidae (Hydrozoa, Cnidaria). Evol. Int. J. Org. Evol. 2012, 66, 3876–3901. [CrossRef]
- Schuchert, P. Survey of the family Corynidae (Cnidaria, Hydrozoa). Rev. suisse Zool. 2001, 108, 739–878. [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. Mycol. 1990, 172, 4238–4246. [CrossRef]
- Vilgalys R. Conserved primer sequences for PCR amplification of fungal rDNA. 2018 unpubl.: Available online: https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/ (accessed on 2019).
- Edgar, R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729, . [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [CrossRef]
- Lartillot, N.; Lepage, T.; Blanquart, S. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinform.2009, 25, 2286–2288, . [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37(5), 1530–1534. [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. methods 2017, 14(6), 587–589. [CrossRef]
- Clement, M.; Snell, Q.; Walke, P.; Posada, D.; Crandall, K. TCS: estimating gene genealogies. Proc. 16th Int. Parallel Distributed Process. Symp. 2002, 3, 0184. [CrossRef]
- Flot, J.-F. Champuru 1.0: a computer software for unraveling mixtures of two DNA sequences of unequal lengths. Mol. Ecol. Notes 2007, 7(6), 974–977. [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinform. 2009, 25(11), 1451–1452. [CrossRef]
- Bandelt, H-J; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol.1999, 16, 37–48. [CrossRef]
- Dyomin, A.G.; Koshel, E.I.; Kiselev, A.M.; Saifitdinova, A.F.; Galkina, S.A.; Fukagawa, T.; Kostareva, A.A.; Gaginskaya, E.R. Chicken rRNA gene cluster structure. PloS one 2016, 11(6), e0157464. [CrossRef]
- Schlötterer, C. Ribosomal DNA probes and primers. In Molecular Tools for Screening Biodiversity; Karp, A., Isaac, P.G., Ingram, D.S., Eds.; Chapman & Hall: London, 1998; pp. 267–276. ISBN-13: 978-94-010-6496-5.
- Ki, J.S.; Kim, I.C.; Lee, J.S. Comparative analysis of nuclear ribosomal DNA from the moon jelly Aurelia sp. 1 (Cnidaria: Scyphozoa) with characterizations of the 18S, 28S genes, and the intergenic spacer (IGS). In Proc. Second Int. Jellyfish Blooms Symp., held at the Gold Coast, Queensland, Australia, 24–27 June, 2007. Springer: Netherlands. 2009; pp. 229-239.
- Li, X.; Xu, J.; He, Y.; Shen, S.; Zhu, J.; Shen, Z. The complete nuclear ribosomal DNA (nrDNA) cistron sequence of Pyropia yezoensis (Bangiales, Rhodophyta). J. Appl. Phycol. 2016, 28, 663–669. [CrossRef]
- Guo, Z.; Han, L.; Ding, Y.; Hou, X.; Liang, Z. Molecular characterisation of the complete nuclear ribosomal DNA sequence of the blacklip abalone Haliotis rubra. N. Z. J. Mar. Freshw. Res. 2018, 52(3), 430–443. [CrossRef]
- Baldwin, B.G.; Sanderson, M.J.; Porter, J.M.; Wojciechowski, M.F.; Campbell, C.S.; Donoghue, M.J. The ITS region of nuclear ribosomal DNA: a valuable source of evidence on angiosperm phylogeny. Ann. Mo. Bot. Gard. 1995, 82(2), 247–277. [CrossRef]
- Hilário, S.; Santos, L.; Phillips, A. J.; Alves, A. Caveats of the internal transcribed spacer region as a barcode to resolve species boundaries in Diaporthe. Fungal biol. 2022, 126(1), 54-74. [CrossRef]
- Kuninaga, S.; Natsuaki, T.; Takeuchi, T.; Yokosawa, R. Sequence variation of the rDNA ITS regions within and between anastomosis groups in Rhizoctonia solani. Curr. Genet. 1997, 32, 237–243. [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Documentation of reticulate evolution in peonies (Paeonia) using internal transcribed spacer sequences of nuclear ribosomal DNA: implications for biogeography and concerted evolution. PNAS, 1995, 92(15), 6813–6817. [CrossRef]
- Forsman, Z.H.; Hunter, C.L.; Fox, G.E.; Wellington, G.M. Is the ITS region the solution to the “species problem” in corals? Intragenomic variation and alignment permutation in Porites, Siderastrea and outgroup taxa. Proc. 10th Int. Coral Reef Symp. 2006, 1, 14–23.
- Campbell, R.D.; Iniguez, A.R.; Iniguez, A.J.; Martínez, D. E. Hydra of Hawaii: phylogenetic relationships with continental species. Hydrobiologia, 2013, 713, 199–205. [CrossRef]
- Cunha, A.F.; Genzano, G.N.; Marques, A.C. Reassessment of morphological diagnostic characters and species boundaries requires taxonomical changes for the genus Orthopyxis L. Agassiz, 1862 (Campanulariidae, Hydrozoa) and some related campanulariids. PLoS One, 2015, 10(2), e0117553.
- Postaire, B.; Magalon, H.; Bourmaud, C.A.F.; Gravier-Bonnet, N.; Bruggemann, J.H. Phylogenetic relationships within Aglaopheniidae (Cnidaria, Hydrozoa) reveal unexpected generic diversity. Zoologica Scripta 2016, 45(1), 103-114. [CrossRef]
- Schuchert, P. High genetic diversity in the hydroid Plumularia setacea: a multitude of cryptic species or extensive population subdivision? Mol. Phylogenetics Evol. 2014, 76, 1–9. [CrossRef]
- Schuchert, P. DNA barcoding of some Pandeidae species (Cnidaria, Hydrozoa, Anthoathecata). Rev. suisse Zool. 2018, 125(1), 101–127. [CrossRef]
- Prudkovsky, A.A.; Neretina, T.V. The life cycle of Catablema vesicarium (A. Agassiz, 1862) (Hydrozoa, Pandeidae). Polar Biol. 2016, 39, 533–542. [CrossRef]
- Reimer, J.D.; Takishita, K.; Ono, S.; Tsukahara, J.; Maruyama, T. Molecular evidence suggesting interspecific hybridization in Zoanthus spp.(Anthozoa: Hexacorallia). Zool. Sci. 2007, 24(4), 346–359. [CrossRef]
- Liao, D.; Pavelitz, T.; Kidd, J.R.; Kidd, K.K.; Weiner, A.M. Concerted evolution of the tandemly repeated genes encoding human U2 snRNA (the RNU2 locus) involves rapid intrachromosomal homogenization and rare interchromosomal gene conversion. EMBO J. 1997, 16(3), 588–598. [CrossRef]
- Elder Jr, J.F.; Turner, B.J. Concerted evolution of repetitive DNA sequences in eukaryotes. Q. Rev. Biol. 1995, 70(3), 297–320.
- Hillis, D.M.; Dixon, M.T. Ribosomal DNA: molecular evolution and phylogenetic inference. Q. Rev. Biol. 1991, 66(4), 411–453. [CrossRef]
- Wang, S.; Zhang, L.; Zhan, A.; Wang, X.; Liu, Z.; Hu, J.; Bao, Z. (2007). Patterns of concerted evolution of the rDNA family in a natural population of zhikong scallop, Chlamys farreri. J. Mol. Evol. 2007, 65, 660–667. [CrossRef]
- Naidoo, K.; Steenkamp, E.T.; Coetzee, M.P.; Wingfield, M.J.; Wingfield, B.D. Concerted evolution in the ribosomal RNA cistron. PLoS one 2013, 8(3), e59355. [CrossRef]
- Harris, D.J.; Crandall, K.A. Intragenomic variation within ITS1 and ITS2 of freshwater crayfishes (Decapoda: Cambaridae): implications for phylogenetic and microsatellite studies. Mol. Biol. Evol. 2000, 17(2), 284–291. [CrossRef]
- Vollmer, S.V.; Palumbi, S.R. Testing the utility of internally transcribed spacer sequences in coral phylogenetics. Mol. Ecol., 2004, 13(9), 2763-2772. [CrossRef]
- Wei, N.W.V.; Wallace, C.C.; Dai, C.F.; Pillay, K.R.M.; Chen, C.A. Analyses of the Ribosomal Internal Transcribed Spacers (ITS) and the 5.8 S Gene Indicate that Extremely High rDNA Heterogeneity is a Unique Feature in the Scleractinian Coral Genus Acropora (Scleractinia; Acroporidae). Zool. Stud. 2006, 45(3), 404–418.
- Sánchez, J.A.; Dorado, D. Intragenomic ITS2 variation in Caribbean seafans. In Proc. 11th Int. Coral Reef Symp. Ft. Lauderdale, Florida, 7-11 July 2008; pp. 1383-1387.
- Gong, L.; Shi, W.; Yang, M.; Si, L.; Kong, X. Non-concerted evolution in ribosomal ITS2 sequence in Cynoglossus zanzibarensis (Pleuronectiformes: Cynoglossidae). Biochem. Syst. Ecol. 2016, 66, 181–187. [CrossRef]
- Xu, B.; Zeng, X.M.; Gao, X.F.; Jin, D.P.; Zhang, L.B. ITS non-concerted evolution and rampant hybridization in the legume genus Lespedeza (Fabaceae). Sci. Rep. 2017, 7(1), 40057. [CrossRef]
- O'Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the Fungus fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7(1), 103–116. [CrossRef]
- Muir, G.; Fleming, C.C.; Schlötterer, C. (2001). Three divergent rDNA clusters predate the species divergence in Quercus petraea (Matt.) Liebl. and Quercus robur L. Mol. Biol. Evol. 2001, 18(2), 112-119. [CrossRef]
- Van Oppen, M.J.H.; Wörheide, G.; Takabayashi, M. Nuclear markers in evolutionary and population genetic studies of scleractinian corals and sponges. In Proc. 9th Int. Coral Reef Symp. Bali, Indonesia 23-27 October 2000; 2002; Volume 1, pp. 131–138.
- Aguilar, C.; & Reimer, J.D. Molecular phylogenetic hypotheses of Zoanthus species (Anthozoa: Hexacorallia) using RNA secondary structure of the internal transcribed spacer 2 (ITS2). Mar. Biodivers. 2010, 40(3), 195–204. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).