1. Introduction
The limbal ophthalmic scratch assay is an easy, low-cost and well-developed method to measure cell migration
in vitro. The basic steps involve creating a "scratch" in a cell monolayer, capturing the images at the beginning and at regular intervals during cell migration to close the scratch, and comparing the images to quantify the migration rate of the cells. Compared to other methods, the in vitro scratch assay is particularly suitable for studies on the effects of cell-matrix and cell-cell interactions on cell migration, during wound healing in vivo and are compatible with imaging of live cells during migration to monitor intracellular events if desired [
1]. We have used originally the in vitro scratch model: a) to see how antibiotics delay in vitro human limbal stem cell regrowth [
2], b) the regeneration of limbal stem cells in the presence of silver and gold nanoparticles [
3]. In this paper we have extended the in vitro scratch to an in vivo mouse model and investigated how multi-walled carbon nanotubes (MWCNT) interfere with corneal re-epithelization.
The ocular system involves the visual system of the eye: cornea, lens, fuids (
Figure 1). The function of the ocular system is to transduce light into visual signals by means of epithelial cells (primarily limbal cells), keratocytes, fibroblasts, and trabecular meshwork cells. Ultrafine particles also known as nanoparticles between 1 and 100 nm in size disturb the function of the visual system. Exponentially growing research efforts contributed to the potential human health hazard posed by nanoparticles, yet understanding regarding their genotoxicity remained limited. Carbon nanotubes are organized graphene sheets consisting of one or more layers [
4]. The size of nanotubes used in this study was 10-30 nanometer outer diameter and 10-30 µm length corresponding to the size of often occurring man-made pollutants. Such nanoparticles are present in the combustion products of fuels, which makes contact with them unavoidable. The biological impact of nanoparticles is poorly understood, but likely to be unique and characteristic to the type of nanoparticle. The best-studied fullerene carbon nanotubes include single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) [
5]. SWCNTs and MWCNTs are currently under intensive investigation. SWCNTs are promising candidates for cancer chemotherapy delivery, especially when functionalized with anticancer drugs that induce apoptosis (e.g. combretastatin A4, CA4). Experimental data suggest that SWCNT-CA4 has anticancer activity superior to that of free CA4 [
6]. However, functionalization of SWCNTs by binding to proteins may significantly impact the cytotoxicity of nanotubes. The binding of BSA to SWCNTs reduced cytotoxicity and the degraded nanotubes induced less cytotoxicity than non-degraded nanotubes [
7].
Multi-walled carbon nanotubes are known to cause potentially hazardous effects on intracellular and extracellular pathways. Bronchial epithelial cells and mesothelial cells are crucial targets for the safety assessment of inhalation of carbon nanotubes, which resemble asbestos particles in shape [
8]. When the cytotoxicity of two different types of MWCNTs in A549 lung epithelial cells and HepG2 hepatocytes was investigated, heat-treated MWCNTs showed significantly stronger adverse effects compared to the non-treated MWCNTs. Moreover, heat-treated MWCNTs induced a dose-dependent cell cycle arrest in A549 cells and leading to a decrease in surface defects [
9]. The topical application of low doses of MWCNTs in mice induced keratinocyte cytotoxicity and exacerbation of allergic skin conditions in a carboxylation dependent manner. However, neither functionalized (doped) MWCNTs increased the level of in vitro reactive oxygen species in HaCaT keratinocyte cell line [
10]. Formononetin (FMN), and functionalized multi-walled carbon nanotube composites (MWCNT-COOH, MWCNT-FMN) were used to induce apoptosis via reactive oxygen species (ROS) production in murine fibroblast 3T3 and HeLa cells. The in vitro cytotoxicity assay was performed using water-soluble tetrazolium assay and demonstrated that FMN, MWCNT-COOH, and MWCNT-FMN had no significant effects on the proliferation and viability of mouse fibroblast 3T3 cells while the cell growth inhibition of the three samples showed concentration-dependence for HeLa cells [
11].
Pulmonary exposure to MWCNTs gave contradictory results. MWCNTs could generate lung inflammation, fibrosis, and cancer among those exposed to it. However, there are currently no effective biomarkers for detecting lung fibrosis or predicting the risk of lung cancer resulting from MWCNT exposure [
12]. MWCNTs have been shown to induce lung fibrosis in animal models [
13]. To the contrary, gene-specific DNA methylation status was tested upon treatment of human lung cells with SWCNTs, MWCNTs + asbestos and asbestos alone. MWCNT-exposed cells showed significant global DNA hypomethylation of cytosine and global RNA hypomethylation of adenosine. SWCNT, MWCNT, and amosite (asbestos) exposure decreased the DNA methylation pattern [
14].The applications of multi-walled nanocarbon tubes (MWCNTs) in the composite-material industry raised questions about their short and long-term health effects. Although studies of rats upon inhalation of MWCNTs produced no systemic toxicity [
15], conclusive results were not obtained. MWCNTs toxicity tests need comparable and standardized conditions concerning cell line, animal species, exposure conditions [
16]. as well as size, shape, purity, and functionalization of nanotubes [
17]. The diameter was found to be inversely related to the bioactivity of MWCNTs preparations, and carboxyl- or amino-functionalization could be at least in part, attributable to the greater tendency of functionalized MWCNTs to form large agglomerates in protein-rich biological fluids [
18]. In spite of efforts, the elucidation of toxicity determinants of multi-walled carbon nanotubes (MWCNTs) remained limited. The research of nanoparticles in the automobile industry has focused on the release of nanoparticles by vehicle exhaust, brakes, catalytic converter and at road-tire abrasion processes [
19] but not on the MWCNTs generated in fires or car exhaust pipes. It was found that 1.5 wt% of MWCNTs enhanced significantly the mechanical properties of automobile bumpers. Less attention was paid to those MWCNTs and their aggregates that were released by accidents and aeroplane crashes [
20].
The risk of eye injuries in car accidents in the US has been confirmed among males, 15 to 19 years old [
21]. Corneal diseases, applications of nanotubes in drug-delivery and corneal disease management deserve special attention [
22]. Nevertheless, the effects on corneal injuries contaminated with MWCNTs and aggregates of MWCNTs are poorly characterized.
Limbal epithelial culture is used to further our understanding of limbal stem-cell biology, for the culture expansion of limbal stem cells for transplantation purposes in patients with limbal stem-cell deficiency [
23] or damage of the epithelial surface of eyes described in this paper. HuLi cells were regarded as stem cells [
24,
25]. Recent studies show that stem cell-derived extracellular vesicles (EVs) play a relevant role in stem cell-induced regeneration by reprogramming injured cells and inducing proregenerative pathways. EVs derived from mesenchymal stem cells are able to promote regeneration of damaged human corneal epithelial cells [
26].
We have established a cell line from the human limbal area to study the in vitro cell growth and response to the toxic effects of antibiotics used in ophthalmology [
3]. The cytotoxic effects were evaluated by monitoring the proliferation, measuring the cellular motility and following chromatin changes in various cell lines upon MWCNT treatment. Measurements applied long-term scanning microscopy and a perfusion platform that replaced the medium with test solutions, bypassed physical contact with the cell culture during experiments, and provided uninterrupted high time-resolution time-lapse photomicrography for an extended period of time. Genotoxicity specific chromatin changes characteristic to toxic effects were distinguished in human skin keratinocytes (HaCaT), human limbal cells (HuLi), colorectal adenocarcinoma (CaCO
2), murine squamous carcinoma (SCC) and Indian muntjac (IM) cell lines.
2. Materials and Methods
2.1. Materials
DABCO (1,4-diazobicyclo-(2,2,2)-octane), Penicillin-Streptomycin-Neomycin antibiotics (PSN-375963) were from Sigma-Aldrich, Budapest, Hungary. 2,6- diamino-2-phenylindole (DAPI) was the product of Braunschweig Chemie (Braunschweig, Germany). Dextran T-150 was purchased from Pharmacia-Biochemicals (Uppsala, Sweden). Colcemid (N-methyl-N-deacetyl-colchicine) was the product of Boehringer (Mannheim, Germany). To eliminate bacterial contamination sterile-filtered PSN antibiotics were used. Penicillin-Streptomycin-Neomycin antibiotics (PSN-375963) were from Sigma-Aldrich, Budapest, Hungary.
Dulbecco’s Modified Eagle’s Medium Nutrient Mixture (DMEM-HAM’S F12) (Sigma-Aldrich, Budapest, Hungary) was supplemented with 2 mM L-glutamine, 23 mM sodium bicarbonate, 10% Fetal Bovine Serum (FBS) (Hyclon, Logan, UT) and 1% PSN (Sigma-Aldrich, Budapest, Hungary). Multi-walled carbon nanotubes of 10-30 nm outer diameter (OD) and 10-30 µm length were provided by the producer Sun Innovations Inc (Fremont, CA, USA).
Nanotubes were suspended in sterile PBS, ultrasound-sonicated in an Ultrashall Reiniger Emmi 4 (EMAG AG, Mörfelden-Walldorf, Germany) ultrasonic homogenizer for 30 sec at room temperature. Nanotubes were homogenized again individually right before using the tube containing MWCNT in a Vortex shaker at 1000 rpm for a 2x5 sec to reduce the formation of macroaggregates.
MWCNTs here refer to those multi-walled carbon nanotubes (MWCNTs) that were used to treat limbal cell cultures at 5, 50, 100 and 500µg/ml concentrations and to follow the healing process of scratched murine eyes in vivo at 500µg/ml in sterile saline solution.
2.2. Methods
Kang et al. demonstrated that the uptake of sub-μm MWCNTs internalized easier through an energy-independent pathway [
27,
29]. Multi-walled carbon nanotubes 0.5–2 μm in length, 10–30 nm in our diameter were excluded from the interior of the cell [
28]. The poor dispersion in saline media resulted in the aggregation of MWCNTand suggested that they do not enter the much smaller cells.
2.2.1. Establishment of human limbal cell line
The corneoscleral limbal rim originated from an enucleated eye of a 56 years old female patient’s cadaver. The human limbal cell line used in the present investigation was described earlier [
29].
2.2.2. Trypan blue test
The trypan blue exclusion assay is a standard, inexpensive and fast method that needs only a small fraction of the cell population to distinguish viable cells from dead cells by light microscopy. The trypan blue inclusion does not differentiate between apoptosis and necrosis. Although apoptosis causes shrinkage and necrotic enlargement of dying cells, this is not necessarily seen by the trypan blue test, as the process of cell death may take place within minutes or last for days. The use of trypan blue is limited by the tiresome counting of each individual sample, by its subjective evaluation, by staining only necrotic or late apoptotic cells. The following simple protocol was used to determine cell viability by the trypan blue test [
30]:
1. Add 0.1 ml of 0.5% trypan blue in isotonic salt solution (saline, PBS) to 0.1 ml cells in the same solution.
2. Load the stained cells immediately on a hemacytometer, place the hemacytometer under the light microscope at low magnification (dilute if necessary with PBS).
3. Count the number of blue (dead) and white (live) stained cells, the sum of which gives the total cell number. Cell viability is calculated as the number of viable cells divided by the total number of cells within the grids of the hemacytometer.Trypan blue does not stain intact cells. Occasionally it may be observed that immediately after the addition of trypan blue permeability changes cause the slow infiltration of the stain and cells become first light blue then dark blue. Trypan blue stains dead (apoptotic and necrotic) cells dark blue.
2.2.3. Animal care
Inbred BALB/c mice were obtained from Charles River Animal Lab Kft, Budapest, Hungary. Both sexes of mice (10-12 weeks old, 20 ± 3 g of weight) were used in experiments. Animals have kept in PI plastic cages (425/135/120 mm, 573.75 cm
2) with mesh covers according to the guidelines of 2010/ 63/EU. Animals were fed with pelleted mouse chow (Purina, LabDiet, St. Louis, MO, USA) and tap water
ad libitum. Automated room illumination of 12 h light and 12 h dark cycles, and room temperatures between 22–25 °C were maintained. Animal experiments were carried out in our Experimental Animal Facility (reg. num.: III/3.-KÁT/2015) under the supervision of the Animal Care Committee, University of Debrecen. The experimental protocols were approved by the Animal Care Committee (Licence number: 2/2014 DEMAB). Animal experiments and care conformed to the general guidelines of the protection of the European Community (86/609/EEC) and special guidelines of BSL2 (200/54/EC 16/1). Human protection included respiratory protective equipment by observing the Health Protection Agency facemask guidelines and using the standard FFP2 equivalent to the N95 HEPA filter [
30].
2.2.4. In vitro and in vivo models mimicking corneal epithelial growth2.2.5. Treatment of limbal cell cultures with nanotubes
The stock mixture of MWCNTs carefully dispersed in PBS was diluted with 10% PBS containing growth medium (F12-Ham + 10% FBS + 1% PSN). The size measurement of aggregated MWCNTs was performed as described in the Methods using the ImageJ software program. The size distribution of aggregated nanotubes at 5, 50 and 100 µg/ml concentrations in the growth medium showed that at low concentration (5 µg/ml) the tendency of aggregation was lower, than at medium (50 µg/ml) and higher (100 µg/ml) nanoparticle concentrations. This tendency is accounted for by the strong non-polar character of nanotubes and strengthened by the presence of the growth medium primarily by the relatively high (10%) concentration of FBS. At the highest concentration (500 µg/ml) of MWCNTs, the size of their aggregates could not be measured.The size of the macroaggregates was selected by the progressive washing and trypsinization. At the highest (500 µg/ml) MWCNT concentration largest size reduction of macroaggregates was observed after the removal of the supernatant probably because the largest aggregates were floating and did not bind to the growth surface. These experiments were carried out in cell cultures at 20% confluency grown for 72 h in the presence of different concentrations of MWCNT corresponding to 5, 50, 100 or 500 µg/ml. The surface area of macroaggregates at these concentrations and after treatments (trypsin and osmotic shock) were given in µm2.
2.2.6. Time-lapse scanning (TLS) microscopy
Diodes emitting light at 940 nm (LED: 5 mm in diameter; 1.2 V, 50 mA, driven at 5 V using a serial 82 Ohm resistor) were used to illuminate cells while minimizing heat and phototoxicity. The 940 nm wavelength turned out to be an acceptable compromise to avoid phototoxicity and to maintain sufficient resolution power. Plan achromatic objectives (×10: 0.25 NA) (Carl Zeiss Jena, Germany) were used to secure a broad field of view to be imaged. Custom-modified 2 megapixel UVC camera (Asus Computer International, Fremont, CA, USA) boards with USB 2.0 connection served image detection. Cell cultures in glass-bottom dishes were placed under inverse microscopes, and photographs of cells were taken every minute. Ten images were collected, each within the 5-sec interval and averaged to minimize noise. Time-lapse microscopy images were collected every min during a 5-sec pulse of near-infrared -LED illumination and regarded as an optimal time resolution with the possibly lowest phototoxicity [
2,
3].
2.2.7. Image analysis
During time-lapse microscopy, the National Health Institute’s ImageJ software was used to analyze the image sequences (
https://fiji.sc). The image analysis method included:
(a) Image restoration and noise reduction: RGB image sequences were converted to an 8-bit grayscale image. Deflickering by using a sequence stack histogram served to avoid transient brightness changes between separated frames. Contrast and brightness were equalized based on the stack (sequence) histogram at 0.4% of the pixels saturated.
Fast Fourier-transformation and background subtraction. The background was reduced by bandpass filtering to exclude large structures down to 40 pixels and filtering small structures up to 3 pixels in size, and background extraction process using a rolling ball at a radius of 50 pixels.
(b) Segmentation: Image sequences were thresholded using a stacked histogram by keeping the information containing elements of the image sequence as foreground, and throwing the redundant pixels away by thresholding them into the background.
(c) Measurement: Thresholding results in a binary image were used for graphical representation.
2.2.8. Size distribution of MWCNT aggregates using time-lapse microscopy images
The determination of the size of aggregated CNTs was based on time-lapse scanning images obtained during cytotoxicity examinations described above. The evaluation of the images was performed employing the ImageJ software program. The Block Matching Parameters (Fiji J) helper plugin was applied for the measurements. Images were converted to the 8-bit formate applying the threshold function. After segmentation, only the aggregates of the binary images remained visible. Segmentation was followed by the analysis of the particles measuring the diameters of particles. Data were obtained in pixels. The microphotographs of the small squares of the Bürker chamber were used for calibration to express pixels in µm-s. The size of CNT aggregates was measured in pixels then converted to µm-s.
The scratch wound assay mimics cell behaviour during wound healing in vitro in a confluent cell layer [
32]. Control limbal cells in glass-bottom dish were grown in DMEM-F12 + 10% FBS +1% PSN in carbon dioxide incubator at 37°C at 5% CO
2 until confluency (~ 48 h) was attained. After reaching confluency the surface of the monolayer was scratched with a sterile 20 gauge needle and regeneration was traced by time-lapse photomicrography until the disrupted area was healed. White numbers at the bottom of panels indicate the time of photography in minutes taken from the beginning of time-lapse image analysis.
Time-lapse video microscopy combined with image analysis served to test different parameters including cell growth, reduction of the damaged surface, the regrowth of defect area, motility of cells in the monolayer, the viability of cells [
2,
28,
29]. Limbal cells in the presence of nanotube bundles were grown for the same time as the control population in the carbon dioxide incubator at 37°C at 5% CO
2. When confluency was reached the monolayer was scratched and further grown until the homogeneity of the culture was regained.
2.2.9. Fluorescence microscopy
Limbal stem cell growth mimicking corneal reepithelization, reversible permeabilization of cells, isolation of nuclei, the spread of nuclear structures and fluorescence microscopy have been described [
2]. The reason of studying the chromatin condensation patterns was to show that reepithelization process does not generate dead cells seen as large disrupted necrotic cells, or small apoptotic bodies. Briefly, two inverse microscopes, placed in a CO
2 incubator, were equipped with high-sensitivity digital cameras and connected to a dual image-acquisition computer system. Illumination was developed to minimize heat- and phototoxicity. Operation of the spectrally cold-white light emitting diodes was synchronized with image-acquisition periods. Frames were recorded every minute and the whole video sequence was converted to database form. The time of exposure was indicated in the right lower corner of each frame. Exposures were converted to video films by speeding up the projection to 30 exposures/seconds. The Carl Zeiss Photomicroscope II was used, with UV illuminating light produced by the XBO (150 W/1) vapour lamp.
2.2.10. Cell attachment, division and reattachment
Cells growth and division of individual HaCaT cells served as an example to explain the attachmemt and reattachment of dividing cell during TLM. Close to the tme of cell division healthy cells rounded up and detached from the monolayer. Cell division lastted for about 10 min. After detachment the cell rounding (20 min) was followed by the division and the appearance oftwo round daughter cells (30 min), their gradual separation (40-50 min), the attachment to the surface of the T-flask (60 min) [
33].
2.2.11. Chromatin changes during cell death
Generally known chromatin changes follow cellular distortions including rounding up of cells, detachment from the monolayer in cell cultures, blebbing, shrinkage, undergoing a so called apoptotic dance causing apoptosis or swelling and disruption of cells characteristic to necrosis [
34].
2.2.12. In vivo murine scratch model mimicking corneal reepithelization
Eight mice (4 males, 4 females) were anaesthetized with 50 mg/kg sodium pentobarbital i.p. and the cornea of one of their eyes was scratched with a 32 Gauge hypodermic needle. The first control group (2 mice) did not get any treatment only 2x10 µl saline was dropped into the eye. The eyes of the second control (2 animals) were scratched and saline eye drops were given that did not contain MWCNT. The eyes of the third group (4 mice) were scratched and the scratches were immediately treated with 2 x 10 µl eye drops of saline containing 500 µg/ml MWCNT. Mice were kept under regular breeding conditions. Photographs of the eyes were taken twelwe day as after treatment. In another experiment 12 days after 500 µg/ml, MWCNT treatment mice were euthanized with a 300 mg/kg intravenous injection of sodium pentobarbital. The eyes of this post mortem group were removed washed with saline and examined with a stereomicroscope at 20x magnification and photographed.
4. Discussion
Low concentrations of airborne nanotubes and their aggregates have not been tested for their ophthalmological impact although, they belong to daily damages. The release of huge amounts of airborne particles generated in fires, explosions, car exhaust pipes as well as their aggregation could cause scar formation and incomplete regeneration of the damaged cornea. Whereas fullerene soot containing single-walled carbon nanotubes (SWCNTs) caused no visible of health hazard [
37], others have observed the cytotoxicity of SWCNTs and apoptosis [
38], raising the question whether SWCNTs or MWCNTs exert higher cytotoxicity. It also needs to be explained why others found MWCNTs to interfere with cellular functions whereas in our study MWCNT macroaggregates caused no visible cell death.
Single–walled carbon nanotubes (CWNTs) due to their smaller size, large surface area and high reactivity permeate cellular membranes and attach to biological molecules, inhibit cell growth, induce DNA breakage and generate reactive oxygen species [
39]. The cytotoxicity of MWCNTs concerning functionalization suggested that pristine MWCNTs induced cell death but paradoxically functionalized MWCNTs were more genotoxic compared to their pristine form [
40]. In contrast, pristine CNTs were more toxic than oxidized CNTs [
41] Similarly, to our observations Coccini et al. [
42] have observed that the viability of astrocytoma and pneumocyte cells was unaffected by pristine and by functionalized MWCNTs up to 200 μg/ml of these nanotubes in samples incubated for 24 or 48 h.
When the clastogenic/genotoxic potential of functionalized and non-functionalized MWCNTs was investigated in murine bone marrow cells non-functional MWCNTs were toxic only at sufficiently high concentration, whereas functionalized MWCNTs had a higher clastogenic/genotoxic potential than the non-functionalized form of MWCNT [
43]. It was concluded that the lower cytotoxicity of MWCNTs than those of SWCNTs could make MWCNTs more suitable for medical applications [
44]. The picture that emerged from different functionalizations suggested that the toxicity of MWCNTs is related to their hydrophobicity [
45] in agreement with the view that polar pristine MWCNTs do not interact with giant unilamellar vesicles of cells, remain outside the cells and have little or no effect on the viability of these cells [
36]. Similarly, multi-walled carbon nanotube oxidation dependent keratinocyte cytotoxicity and skin inflammation were found by Palmer et al. [
10]. Similarly, it was found that HaCaT keratinocytes displayed increased cytotoxicity when exposed to MWCNTs with high levels of carboxylation [
10].
Interphase chromatin alterations are sensitive indicators of cytotoxicity at submicromolar and micromolar concentrations. The characteristic deformations and shrinkage of nuclei showed a close correlation between the frequency of micronucleus formation and the concentration of the genotoxic agent [
46]. Apoptotic agents are known to affect chromatin morphology in a genotoxicity specific manner [
46]. In general, nanomaterial cytotoxicity is composition-, size-, cell type- [
47] and time-dependent [
48]. As far as the cytotoxicity of MWCNTs is concerned, we have observed the reduced number of metaphase chromosomes beside initiating micronucleus formation but they did not cause significant cell death. Increased number of micronuclei was readily detectable in rapidly dividing interphase cells which may also result from clastogenic or aneugenic mechanisms. Micronucleus test is an umbrella term for many differing micronucleus tests [
36].
In vivo experiments by scratching the cornea of mice with a hypodermic needle (gauge 20) known as the scratch model and allowing reepithelization in the presence of MWCNTs particles confirmed in vitro results. More importantly, the in vivo test validated the applicability of time-lapse microscopy that was mimicking faithfully limbal cell regrowth under in vitro conditions.
5. Conclusions
Three types of ophthalmological experiments were carried out.
(
i) In our first study we have mimicked ocular injuries in vitro and have followed the regeneration of human limbal cells. time-lapse microscopy was in ophthalmology. During corneal damages, the healing process is regularly prevented by treating damaged eyes with antibiotics (chloramphenicol, rifampicin). We found that antibiotics delayed the in vitro regeneration of human limbal cells [
2].
(ii) The healing of unprotected damaged eyes in the presence of nanoparticles may extend the healing and is prone to ocular infection. We have selected those nanoparticles that are known for their mild antiseptic (oligodynamic) effect (silver and gold). Our results showed that limbal cell regeneration and chromatin toxicity were dose dependent. In spite of their lower size, Ag nanoparticles (10 nm) were less toxic than larger gold particles (100 nm) [
49].
(iii) The third aspect was dealing with gadolinium induced effect on the motility, adherence, and chromatin structure on mammalian cells including HuLi cells. Gadolinium complexes, endocytosed by macrophages and distributed to nuclei, cause apoptosis of macrophages preventing the regeneration of corneal damages [
50].
(iv) This paper is dealing with the regeneration of the damaged cornea in the presence of multi-walled carbon nanoparicles. The effect of nanotubes (10-30 µm in length and 10-30 nm in diameter) tested on limbal cell monolayer regeneration, corresponded to the observations of others on different cell lines that MWCNTs did not cause significant toxicity. The explanation to these observations could be that pristine MWCNTs díd do not interact with giant unilamellar vesicles of cells, remain outside the cells and have little or no effect on the viability of these cells [
51].
Eyes are exposed every day to low concentrations of airborne nanoparticles, among them nanotubes without notable ophthalmological consequences. However, the increased number of airborne particles upon accidents may cause corneal damages, incomplete regeneration and scar formation. Results confirmed that human limbal cell regrowth upon corneal trauma mimicked by the in vitro scratch model was slowed down. Low concentrations of nanomaterials including MWCNTs caused neither significant corneal irritation, nor cell death, nor pulmonary damages in rodents [
47].
Nevertheless, high concentrations of MWCNTs are prone to form aggregates in corneal wounds. These deposits have to be removed or at least minimized before other ophthalmic treatments. These observations are of medical importance and suggest that when the cornea is exposed to the sudden release of high concentrations of nanoparticles, due to their strong absorption they cannot be removed with ophthalmic fluids. Special coating for the removal of aggregated nanotubes with adherent materials deserves further investigation. Finally, in vivo results confirmed the validity of in vitro tests and suggested that time-lapse microscopy could be a useful model to follow the regeneration of cellular damages.
Figure 1.
The in vitro scratch model (to the left) and the in vivo scratch wound model (to the right) is shematically depicted. The in vitro model mimics cell behaviour during wound healing in a confluent cell layer [
3]. In the in vivo model the smudging of the damaged cornea was avoided during scratching by a sharply cut metal tip of a sterile hypodermic needle (average diameter 0.8-.0.9 mm). Modified with permission [
3]. Increased number of airborne particles upon accidents may cause corneal damages. The effect of multiwalled carbon nanotubes (MWCNT) (10-30 µm in length and 10-30 nm in diameter) was investigated on confluent monolayer culture of in vitro and in vivo on corneal wound healing. MWCNTs are prone to form aggregates and prevent to slow down limbal cells. The regeneration of corneal wounds caused by the scratch in the eyes of mice is shown at the right side of
Figure 1. The experiment was designed to confirm that human limbal cell regrowth mimicked by the in vitro scratch model of corneal trauma was slowed down by aggregates of MWCNTs. The size of the aggregates was proportional to the concentration of MWCNTs applied. In vivo results performed in mice confirmed in vitro tests and suggested that time-lapse microscopy is a useful means to follow the regeneration of cellular damages. These deposits have to be removed or at least minimized before corneal scar formation would take place.
Figure 1.
The in vitro scratch model (to the left) and the in vivo scratch wound model (to the right) is shematically depicted. The in vitro model mimics cell behaviour during wound healing in a confluent cell layer [
3]. In the in vivo model the smudging of the damaged cornea was avoided during scratching by a sharply cut metal tip of a sterile hypodermic needle (average diameter 0.8-.0.9 mm). Modified with permission [
3]. Increased number of airborne particles upon accidents may cause corneal damages. The effect of multiwalled carbon nanotubes (MWCNT) (10-30 µm in length and 10-30 nm in diameter) was investigated on confluent monolayer culture of in vitro and in vivo on corneal wound healing. MWCNTs are prone to form aggregates and prevent to slow down limbal cells. The regeneration of corneal wounds caused by the scratch in the eyes of mice is shown at the right side of
Figure 1. The experiment was designed to confirm that human limbal cell regrowth mimicked by the in vitro scratch model of corneal trauma was slowed down by aggregates of MWCNTs. The size of the aggregates was proportional to the concentration of MWCNTs applied. In vivo results performed in mice confirmed in vitro tests and suggested that time-lapse microscopy is a useful means to follow the regeneration of cellular damages. These deposits have to be removed or at least minimized before corneal scar formation would take place.
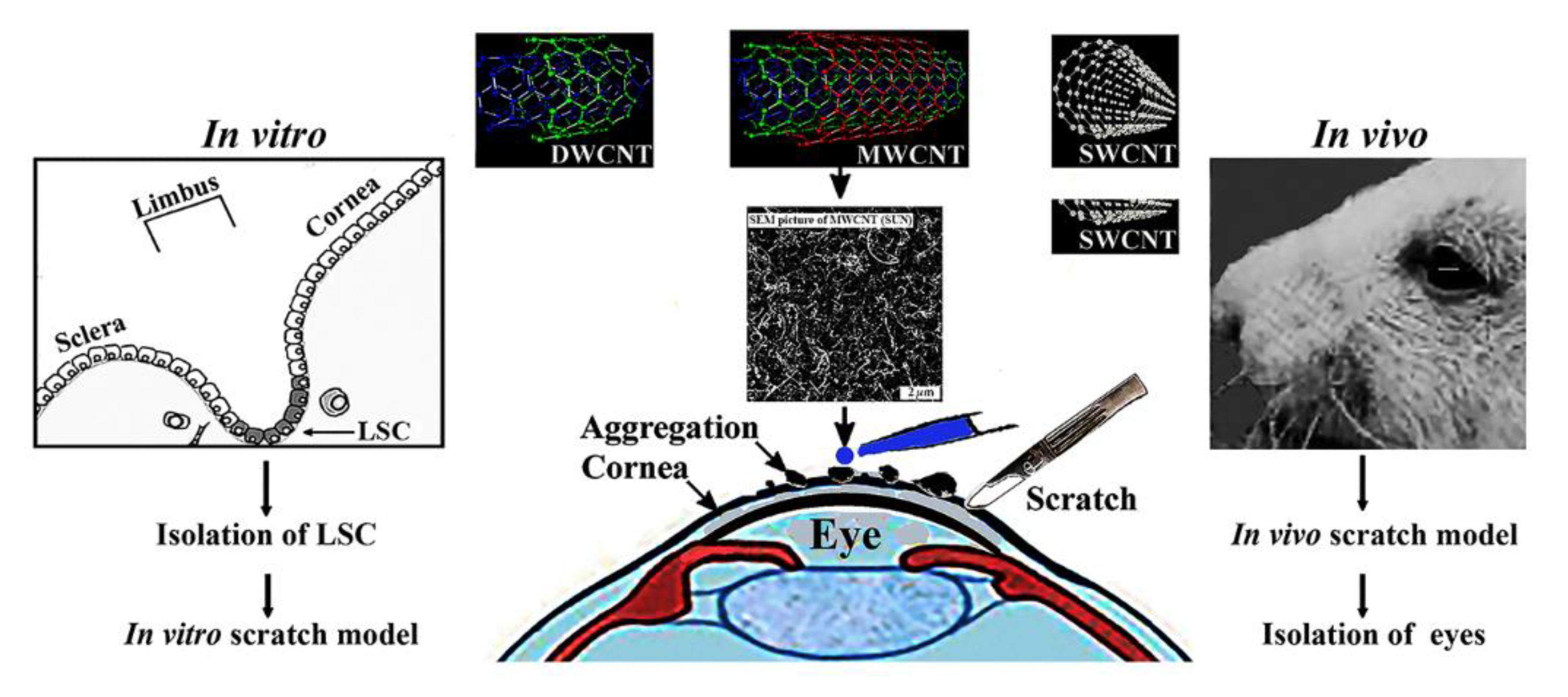
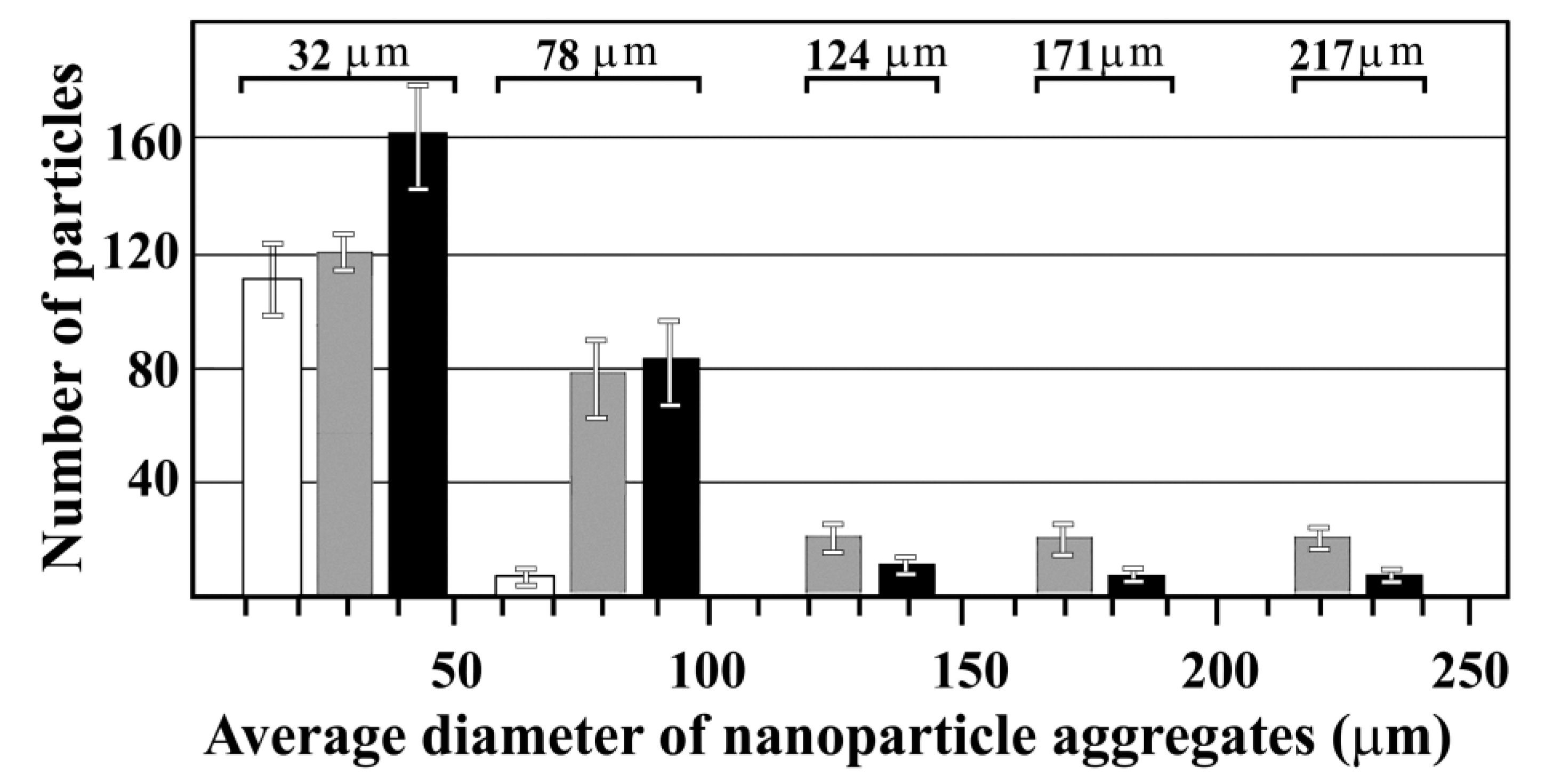
Figure 2.
The average diameter of MWCNTs suspended in PBS. Dispersion measurements were performed immediately after suspension, sonication and vortexing. MWCNT concentrations: 5 µg/ml (

),50 µg/ml (
)
, 100 µg/ml (

). Aggregation prevented the determination of the size of bundles at 500 µg/ml MWCNT concentration. It is important to note that this work does not deal with dispersed carbon nanotubes but with aggregated MWCNT bundles.
Figure 2.
The average diameter of MWCNTs suspended in PBS. Dispersion measurements were performed immediately after suspension, sonication and vortexing. MWCNT concentrations: 5 µg/ml (

),50 µg/ml (
)
, 100 µg/ml (

). Aggregation prevented the determination of the size of bundles at 500 µg/ml MWCNT concentration. It is important to note that this work does not deal with dispersed carbon nanotubes but with aggregated MWCNT bundles.
Figure 3.
Cytotoxicity of MWCNTs. A) The growth of four HuLi cell cultures was started at 20% confluency and grown for 60 h in the absence, in the presence of 5 µg/ml, 50 µg/ml, and 100 µg/ml MWCNT, respectively. Increasing confluencies are given in percentages. B) Regeneration of damaged limbal cell monolayer in the absence (control) and the presence of 5, 50, 100, and 500 µg/ml MWCNT. Distinguishable phases in the profile of the control curve are indicated by i, ii, iii and iv. C) Motility of limbal cells in the absence and presence of MWCNTs. Lowest motility during the regeneration of scratched surface in the absence of MWCNTs. Increased motility in the presence of MWCNT as a function of nanoparticle concentration (5-100 µg/ml). Resistance against monolayer regeneration at high (500 µg/ml) MWCNT.
Figure 3.
Cytotoxicity of MWCNTs. A) The growth of four HuLi cell cultures was started at 20% confluency and grown for 60 h in the absence, in the presence of 5 µg/ml, 50 µg/ml, and 100 µg/ml MWCNT, respectively. Increasing confluencies are given in percentages. B) Regeneration of damaged limbal cell monolayer in the absence (control) and the presence of 5, 50, 100, and 500 µg/ml MWCNT. Distinguishable phases in the profile of the control curve are indicated by i, ii, iii and iv. C) Motility of limbal cells in the absence and presence of MWCNTs. Lowest motility during the regeneration of scratched surface in the absence of MWCNTs. Increased motility in the presence of MWCNT as a function of nanoparticle concentration (5-100 µg/ml). Resistance against monolayer regeneration at high (500 µg/ml) MWCNT.
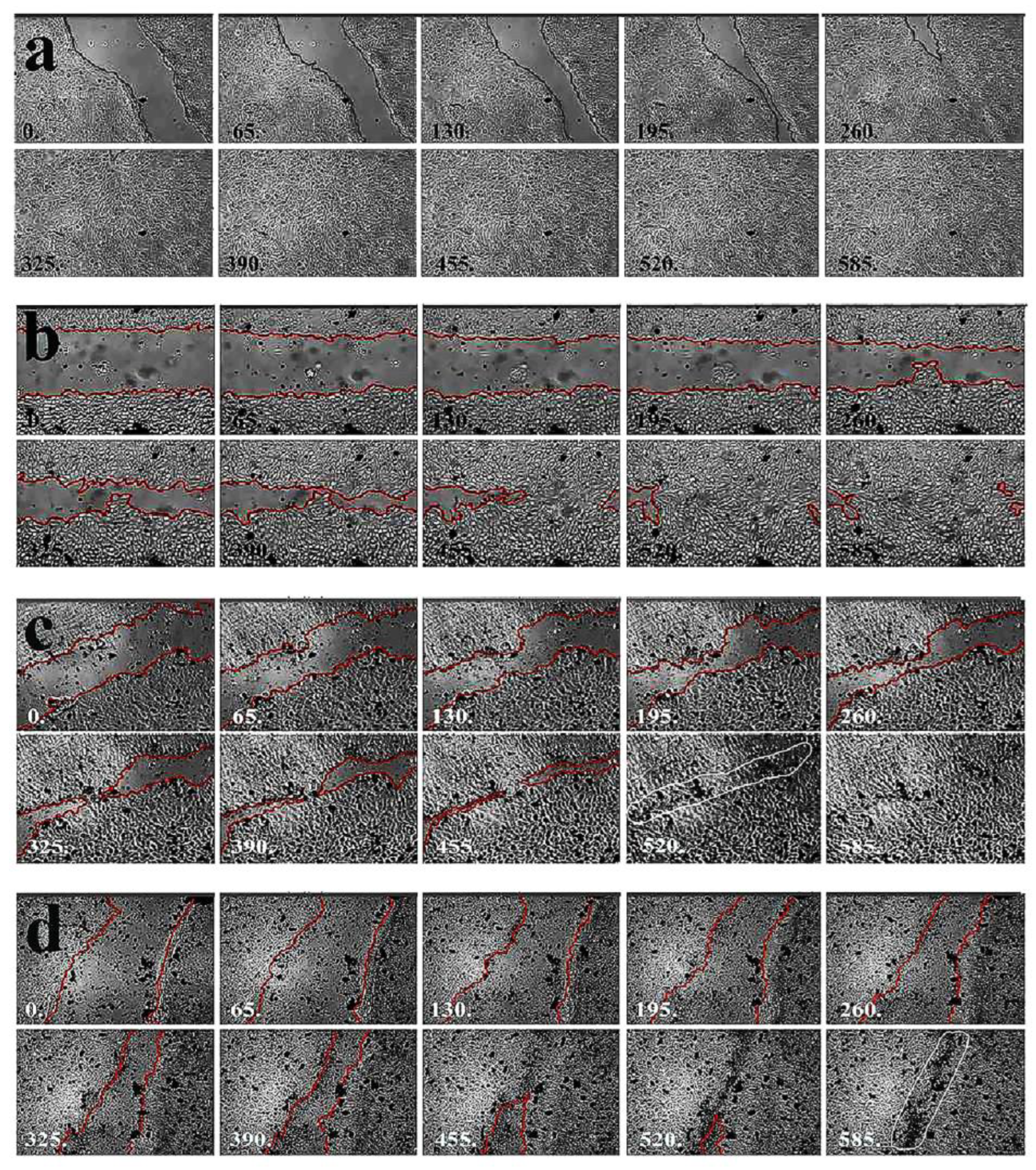
Figure 4.
Regeneration of damaged limbal cell monolayer in the absence of nanoparticles followed by time-lapse microscopy. After reaching confluency the surface of the monolayer was scratched with a sterile 20 gauge needle and regeneration was traced by time-lapse photomicrography until the disrupted area was healed. White numbers at the bottom of panels indicate the time of photography in minutes taken from the beginning of time-lapse image analysis. Scar formation is indicated by the surrounded area.
Figure 4.
Regeneration of damaged limbal cell monolayer in the absence of nanoparticles followed by time-lapse microscopy. After reaching confluency the surface of the monolayer was scratched with a sterile 20 gauge needle and regeneration was traced by time-lapse photomicrography until the disrupted area was healed. White numbers at the bottom of panels indicate the time of photography in minutes taken from the beginning of time-lapse image analysis. Scar formation is indicated by the surrounded area.
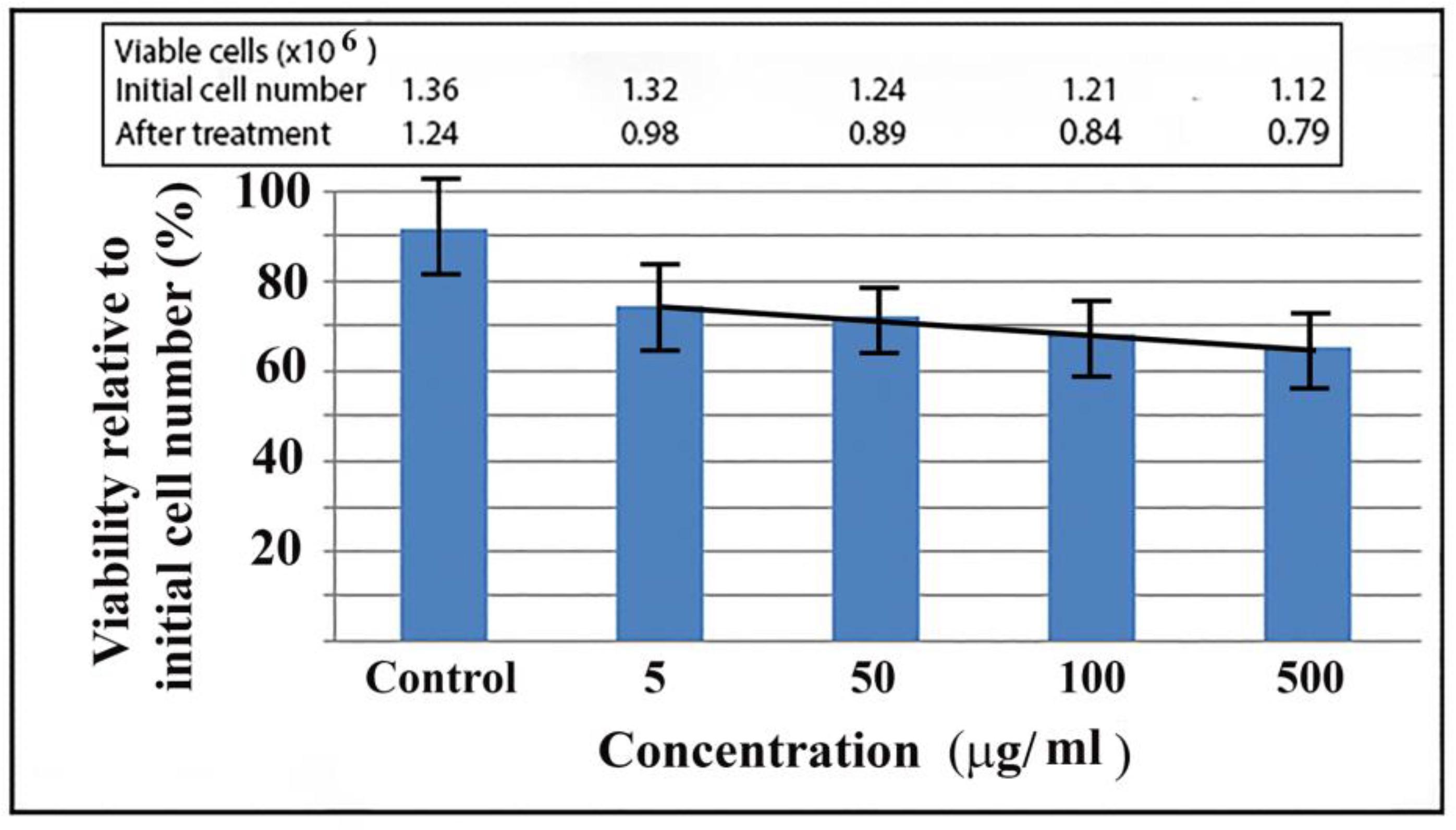
Figure 5.
Viability of HuLi cells in the presence of MWCNT. Cells were grown in the presence of different concentrations of nanoparticles for 18 h then subjected to trypan blue staining. Viable cells were expressed in percentages of initial cell numbers.
Figure 5.
Viability of HuLi cells in the presence of MWCNT. Cells were grown in the presence of different concentrations of nanoparticles for 18 h then subjected to trypan blue staining. Viable cells were expressed in percentages of initial cell numbers.
Figure 6.
Chromatin condensation in nuclei of limbal cells grown in the absence and the presence of MWNT. A) Control limbal cells were grown in T-25 flasks in DMEM-F12 +10% FBS + 1% PSN in carbon dioxide incubator at 37°C at 5% CO2, in the absence of nanotubes for 24 h, harvested, reversibly permeabilized and subjected to isolation of nuclei, isolation and visualization of chromatin structures as described earlier (left upper panels). Limbal cells were grown for 24 h in the presence of 5 µg/ml MWCNT, chromatin structures were isolated and visualized the same way (upper right panels). The growth of limbal cells in the presence of 50 µg/ml MWCNT, followed by isolation and visualization of chromatin structures (lower left panels). Limbal chromatin structures after treatment of cells with 100 µg/ml MWCNT (lower right panels). White arrows indicate the presence of micronuclei. Types of chromatin condensation intermediates: A-D, early decondensed chromatin; E-H, chromatin ribbon; F-L, chromatin bodies, the first recognizable chromosomes; M-P, the formation of metaphase chromosomes. Bars, 5 µm each.
Figure 6.
Chromatin condensation in nuclei of limbal cells grown in the absence and the presence of MWNT. A) Control limbal cells were grown in T-25 flasks in DMEM-F12 +10% FBS + 1% PSN in carbon dioxide incubator at 37°C at 5% CO2, in the absence of nanotubes for 24 h, harvested, reversibly permeabilized and subjected to isolation of nuclei, isolation and visualization of chromatin structures as described earlier (left upper panels). Limbal cells were grown for 24 h in the presence of 5 µg/ml MWCNT, chromatin structures were isolated and visualized the same way (upper right panels). The growth of limbal cells in the presence of 50 µg/ml MWCNT, followed by isolation and visualization of chromatin structures (lower left panels). Limbal chromatin structures after treatment of cells with 100 µg/ml MWCNT (lower right panels). White arrows indicate the presence of micronuclei. Types of chromatin condensation intermediates: A-D, early decondensed chromatin; E-H, chromatin ribbon; F-L, chromatin bodies, the first recognizable chromosomes; M-P, the formation of metaphase chromosomes. Bars, 5 µm each.
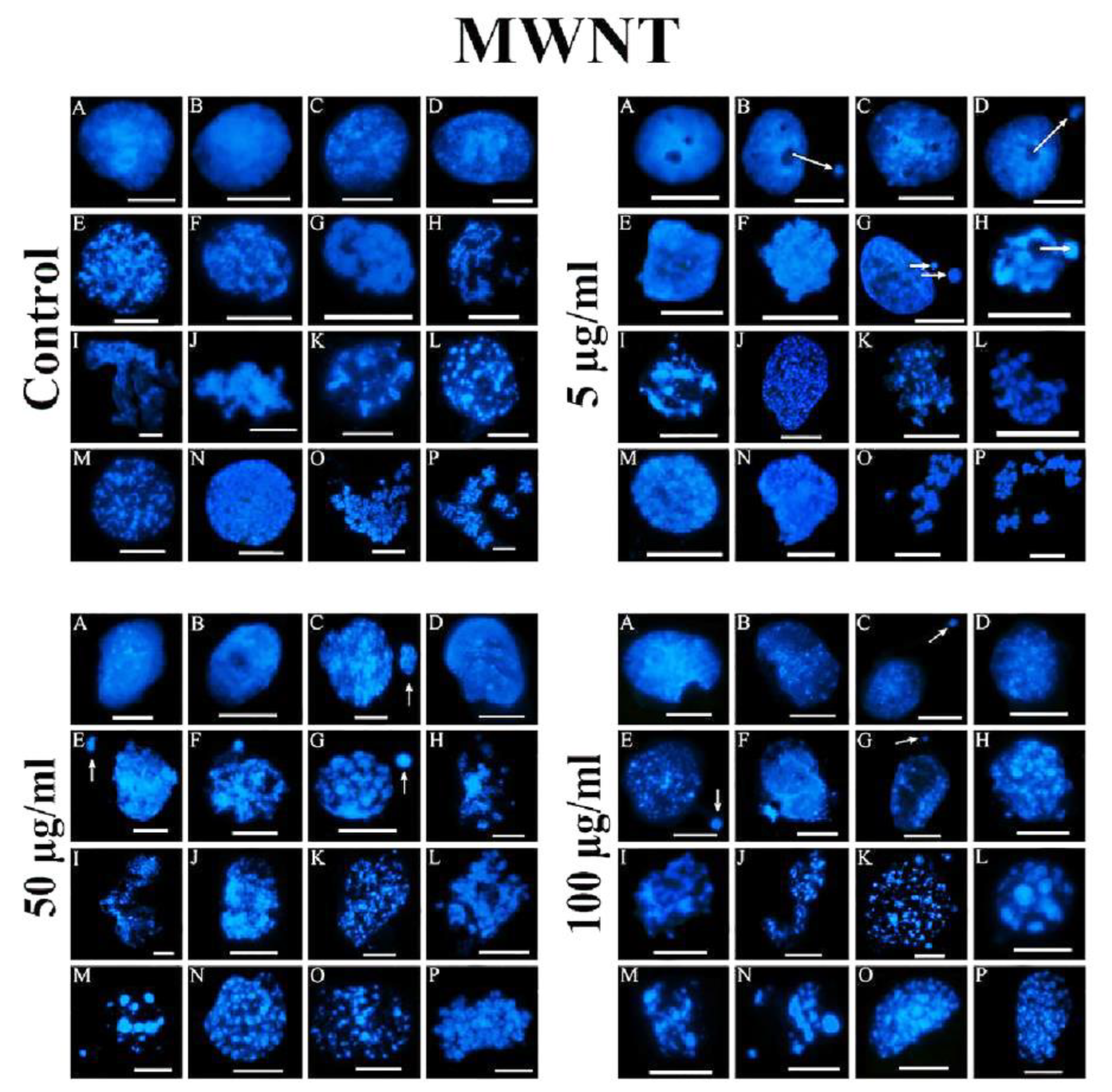
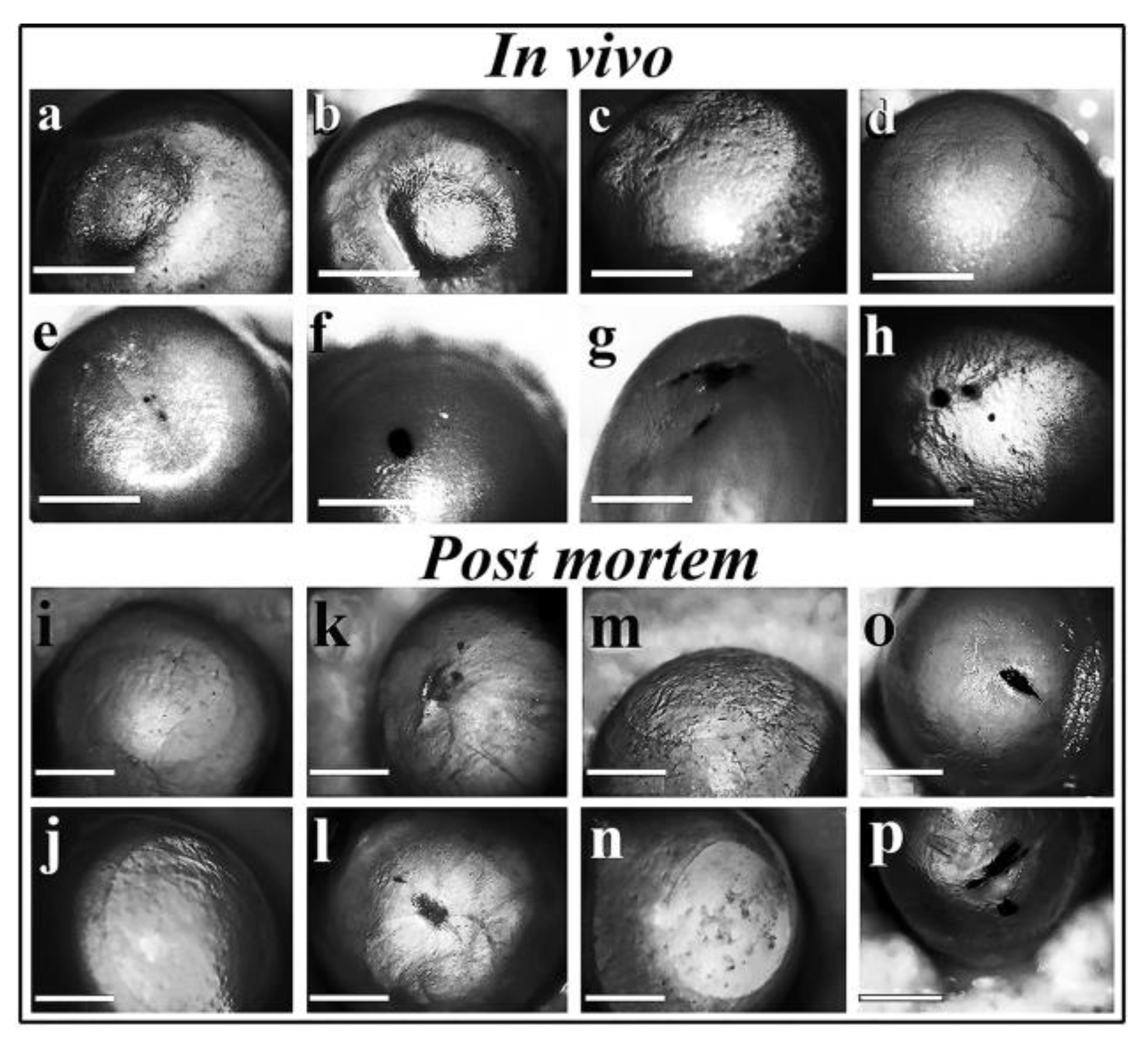
Figure 7.
Corneal reepithelization of damaged murine eyes in the absence and presence of MWCNT. Animal experiments were carried out to confirm or deny the toxicity of MWCNTs described in the Experimental Section. In vivo experiment: a, b) Control: dropping saline into the eyes of mice without any treatment. c, d) Reepithelization of scratched eyes in the absence of 500 µg/ml MWCNT particles. e-h) Prevention of reepithelization in the presence of 500 µg/ml MWCNT. Post mortem experiment: A similar experiment was carried but mice were euthanized, their eyes removed and washed with saline before photography. Control: saline drops alone (i, j), in vivo scratch and saline drops without regeneration and immediate photography (k, l), in vivo scratch, saline drops and regeneration (m, n), in vivo scratch and treatment with saline containing 500 µg/ml MWCNT (o, p). All photographs were taken 12 days after treatment except for k and l.
Figure 7.
Corneal reepithelization of damaged murine eyes in the absence and presence of MWCNT. Animal experiments were carried out to confirm or deny the toxicity of MWCNTs described in the Experimental Section. In vivo experiment: a, b) Control: dropping saline into the eyes of mice without any treatment. c, d) Reepithelization of scratched eyes in the absence of 500 µg/ml MWCNT particles. e-h) Prevention of reepithelization in the presence of 500 µg/ml MWCNT. Post mortem experiment: A similar experiment was carried but mice were euthanized, their eyes removed and washed with saline before photography. Control: saline drops alone (i, j), in vivo scratch and saline drops without regeneration and immediate photography (k, l), in vivo scratch, saline drops and regeneration (m, n), in vivo scratch and treatment with saline containing 500 µg/ml MWCNT (o, p). All photographs were taken 12 days after treatment except for k and l.
Table 1.
Spread of monolayer in the presence of different concentrations of MWCNT. Table 5. µg/ml MWCNT is given in µm2/min measured at the edges of 5.
Table 1.
Spread of monolayer in the presence of different concentrations of MWCNT. Table 5. µg/ml MWCNT is given in µm2/min measured at the edges of 5.
Monolayer
spread
(µm2/min) |
Control |
MWCNT (µg/ml) |
| 5 |
50 |
100 |
500 |
| in ROIs |
62.94
± 8.2 |
41.21
± 6.3 |
40.12
± 4.4 |
38.90
± 4.1 |
n.m. |

 ),50 µg/ml (
),50 µg/ml (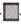 ), 100 µg/ml (
), 100 µg/ml ( ). Aggregation prevented the determination of the size of bundles at 500 µg/ml MWCNT concentration. It is important to note that this work does not deal with dispersed carbon nanotubes but with aggregated MWCNT bundles.
). Aggregation prevented the determination of the size of bundles at 500 µg/ml MWCNT concentration. It is important to note that this work does not deal with dispersed carbon nanotubes but with aggregated MWCNT bundles.
 ),50 µg/ml (
),50 µg/ml (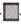 ), 100 µg/ml (
), 100 µg/ml ( ). Aggregation prevented the determination of the size of bundles at 500 µg/ml MWCNT concentration. It is important to note that this work does not deal with dispersed carbon nanotubes but with aggregated MWCNT bundles.
). Aggregation prevented the determination of the size of bundles at 500 µg/ml MWCNT concentration. It is important to note that this work does not deal with dispersed carbon nanotubes but with aggregated MWCNT bundles.